Abstract
Heart failure (HF) post-myocardial infarction (MI) presents with increased vulnerability to monomorphic ventricular tachycardia (mmVT). To appropriately evaluate new therapies for infarct-mediated reentrant arrhythmia in the preclinical setting, chronologic characterization of the preclinical animal model pathophysiology is critical. This study aimed to evaluate the rigor and reproducibility of mmVT incidence in a rodent model of HF. We hypothesize a progressive increase in the incidence of mmVT as the duration of HF increases. Adult male Sprague-Dawley rats underwent permanent left coronary artery ligation or SHAM surgery and were maintained for either 6 or 10 wk. At end point, SHAM and HF rats underwent echocardiographic and invasive hemodynamic evaluation. Finally, rats underwent electrophysiologic (EP) assessment to assess susceptibility to mmVT and define ventricular effective refractory period (ERP). In 6-wk HF rats (n = 20), left ventricular (LV) ejection fraction (EF) decreased (P < 0.05) and LV end-diastolic pressure (EDP) increased (P < 0.05) compared with SHAM (n = 10). Ten-week HF (n = 12) revealed maintenance of LVEF and LVEDP (P > 0.05), (P > 0.05). Electrophysiology studies revealed an increase in incidence of mmVT between SHAM and 6-wk HF (P = 0.0016) and ERP prolongation (P = 0.0186). The incidence of mmVT and ventricular ERP did not differ between 6- and 10-wk HF (P = 1.0000), (P = 0.9831). Findings from this rodent model of HF suggest that once the ischemia-mediated infarct stabilizes, proarrhythmic deterioration ceases. Within the 6- and 10-wk period post-MI, no echocardiographic, invasive hemodynamic, or electrophysiologic changes were observed, suggesting stable HF. This is the necessary context for the evaluation of experimental therapies in rodent HF.
NEW & NOTEWORTHY Rodent model of ischemic cardiomyopathy exhibits a plateau of inducible monomorphic ventricular tachycardia incidence between 6 and 10 wk postinfarction.
Keywords: adverse remodeling, ischemia, monophasic action potential, rigor and reproducibility, ventricular tachycardia
INTRODUCTION
Heart failure (HF) is increasingly prevalent in the United States and incurs substantial patient morbidity and economic burden (1). HF patients can be divided into three classes based on their ejection fraction (EF), with preserved EFs remaining above 50%, reduced EFs falling below 40%, and midrange EFs between the two cutoff values. A subclass of HF patients with reduced EFs has been described as those with recovered EFs, which were once below 40% but have since recovered (2).
With respect to HF with reduced EF, coronary atherosclerosis remains the dominant pathophysiologic mechanism. Occurring over decades, progressive narrowing of a single coronary system or multiple coronary vessels manifests clinically as an acute myocardial infarction. The ischemic insult leads to adverse ventricular remodeling, specifically replacement of granulation tissue by myofibroblast-derived type 1 collagen bundles and compensatory hypertrophy in the noninfarcted myocardium.
Although the two phenomena are certainly related, it should not be assumed that structural remodeling is equivalent with electrical remodeling (3). After the initial proarrhythmic phase, facilitated by spontaneous depolarizations of metabolically compromised cardiac cells, the chronic proarrhythmic phase begins and remains. This chronic phase is facilitated by both the scar tissue with islands of surviving cardiomyocytes, as well as the ischemia-associated dysregulation of membrane-bound potassium channels and calcium ATPases (4) leading to repolarization heterogeneity that overwhelms the intrinsic cardiac repolarization reserve (5).
Cardiac scar burden, in either the atria or in the ventricles, increases susceptibility to reentrant arrhythmias. Structural scar burden imposed by myocardial infarction combines with the electrical repolarization dysregulation to produce arrhythmogenic “substrate.” In the ventricle, these two components of substrate enable the creation of a relatively stable reentrant tachyarrhythmia called monomorphic ventricular tachycardia (mmVT). The term monomorphic describes the activation of a single reentrant circuit within the myocardium that involves both electrically remodeled myocardium as well as structurally remodeled scar tissue. A less organized, more unstable form of VT, namely polymorphic VT, and ventricular fibrillation have no defined circuit path length and thus are more likely to cause insurmountable hemodynamic instability to both the compromised coronaries and the systemic arteries. Clinically, the odds of arrhythmogenic sudden cardiac death (SCD) for a HF patient are highest in the peri-infarct period and reperfusion period and increase cumulatively as the duration of HF increases (6, 7).
Pharmacologic treatment (8) and targeted ablation [both invasive catheter (9) and noninvasive vest (10, 11) approaches] are routinely used clinically to suppress substrates and mitigate SCD risk. However, it is common to develop unpleasant or even life-threatening toxicities to antiarrhythmic drugs (12) or to experience arrhythmia recurrence postablation (13). The third and final treatment option available is implantable cardioverter defibrillator.
Implantable cardioverter defibrillators, created in 1980 (14), are used to treat SCD in patients who exhibit either form of VT or ventricular fibrillation. These devices, paired with the criteria established by the Multicenter Unsustained Tachycardia Trial (15) and broadened by the Sudden Cardiac Death in Heart Failure Trial and the Multicenter Automatic Defibrillator Implantation Trial (16), have decreased the number of patients that have succumbed to SCD from ventricular tachyarrhythmia. However, nearly one-third of HF patients who receive a defibrillator receive inappropriate shocks during their lifetime (17), and for other patient populations like end-stage renal disease patients, defibrillator utilization is futile (18).
Given the shortcomings of these three treatment options to suppress arrhythmogenic substrate and prevent SCD, novel treatment options that disrupt the current treatment paradigm are needed. The current Food and Drug Administration regulatory pathway portends that these future experimental therapies will undergo evaluation in a preclinical animal model that recapitulates a certain component of the human pathophysiology. To facilitate the next generation of disruptive therapies that better mitigate arrhythmogenic SCD in HF (19), preclinical animal models must be characterized with increased precision so as to minimize ineffective clinical translation. A recent consensus statement urged increased rigor and reproducibility of experimental models of myocardial infarction (20).
The Sprague-Dawley rodent model of ischemic HF has been utilized by investigators for decades with strong clinical correlation (21–23). However, to our knowledge there are no data characterizing the model’s chronologic arrhythmogenesis, specifically related to ventricular reentry in the setting of HF with reduced ejection fraction. Furthermore, there are no data correlating these findings to what is seen clinically.
With respect to electrical remodeling and proarrhythmic deterioration, multiweek studies to evaluate ventricular arrhythmia in Sprague-Dawley rats with HF have been completed (24, 25); however, none of them focused on inducible reentrant arrhythmia but rather triggered electrical activity. With respect to structural remodeling, magnetic resonance imaging and histopathology support cessation of compensatory fibrosis between 2 and 4 wk post-myocardial infarction (MI) in rats (26, 27). This paucity of data regarding concomitant electrical and structural remodeling for substrate-driven reentrant mmVT in rodent HF justifies 10 wk post-MI as a long-term evaluation. In our laboratory, we are investigating experimental therapies in the Sprague-Dawley HF model at both the 6 wk post-MI time point as well as at the 10-wk post-MI time point. It is for this reason that these specific time points were selected for this study.
In the present report, in vivo cardiac electrophysiology (EP) studies are performed in ischemic HF rats to evaluate the incidence of substrate-driven reentrant mmVT. We hypothesize that this model of HF will exhibit a progressively increased incidence of mmVT as the duration of HF increases.
METHODS
Heart Failure Induction
All rats enrolled in this study received humane care in compliance with protocols approved by the Institutional Animal Care and Use Committee in the University of Arizona Animal Care Program and also in compliance with the National Institutes of Health’s Guide for the Care and Use of Laboratory Animals (8th ed.). Adult male Sprague-Dawley rats (Envigo, Indianapolis, IN) 6 to 8 wk of age were randomized to one of three groups: SHAM, 6-wk HF, or 10-wk HF. The HF rats underwent permanent left coronary artery ligation for myocardial infarction and HF induction as previously described (21, 28, 29).
In brief, rats (244–283 g) underwent induction using 3–5% volatile isoflurane in 100% oxygen (1 L/min) before visual oropharyngeal intubation and mechanical ventilation at ∼2.3 mL per stroke at ∼90 strokes/min (Harvard Apparatus, Holliston, MA). Next, rats received an anesthetic cocktail of 50 mg/kg ketamine, 5 mg/kg xylazine, 1 mg/kg acepromazine, and 0.5 mg/kg atropine (along with 10 mg/kg ip 2% lidocaine HCl to prevent ventricular arrhythmias. A left-sided thoracotomy expressed the heart from the mediastinum and a 5-0 TiCron braided polyester ligature (Covidien, Minneapolis, MN) was tightly fastened around the proximal left coronary artery for occlusion. The suture was tied in a knot and left to maintain permanent coronary occlusion.
After the chest was closed, rats were transferred from the surgical table to a heated recovery pad with a ventilator. Successful HF induction was defined as a minimum decrease in left ventricular (LV) EF to 40% during the screening echocardiographic study at three wk post-MI. The imaging studies were performed at 3 wk post-MI because after 3 wk, the majority of maladaptive structural remodeling is complete (17, 30). A LVEF of 40% was selected as the cutoff for HF rats in reference to the clinical definition of HF but also accounting for the hyperdynamic physiology of rodent ventricles, relative to human EF. These successfully infarcted rats were randomized to either the 6-wk timeline or the 10-wk timeline and maintained on standard chow and water ad libitum.
SHAM-operated rats were simultaneously enrolled in the study and underwent the same surgical schedule, minus coronary artery ligation, to serve as appropriate controls. SHAM rats were maintained for 6 wk to follow the recommendations set out by our Institutional Animal Care and Use Committee and also comply with the National Institutes of Health’s Guide for the Care and Use of Laboratory Animals. In an effort to responsibly reduce the overall number of animals used in this study, we elected to only pursue a 6 wk post-MI SHAM cohort given that no change in cardiac function is expected in SHAM rats living in standardized living conditions. An uninfarcted SHAM rat at any time point, either 6 wk post-MI or 10 wk post-MI, should suffice as an adequate negative control for this focused study of HF-associated mmVT. Furthermore, no changes were observed in cardiac function for the 6 wk post-MI SHAM cohort as measured by serial echocardiography (data not shown).
Echocardiography
In addition to undergoing the screening echocardiography study at 3 wk post-MI for HF cohort assignment, rats also underwent a final echo study between 1 and 3 days before the terminal hemodynamic and electrophysiologic studies to quantify cardiac function at 6 wk post-MI.
Rats were anesthetized with 2–3% isoflurane in 100% oxygen (1 L/min) and placed supine on a warming pad with dorsal paw electrodes. Transthoracic echocardiography was performed with a 13- to 25-MHz linear transducer on a dedicated rodent system (Vevo2100, FUJIFILM VisualSonics, Toronto, ON, Canada) by an operator blinded to the group identity within 3 days of the terminal study, as is standard operating procedure in our laboratory (26, 28, 31).
Parasternal short-axis and long-axis images were collected along with two-chamber apical views to visualize the anterior, lateral, antero-lateral, inferior, and posterior walls. All parameters of interest related to left ventricular mechanical shortening, volumes, diameters, and wall thicknesses.
Invasive Hemodynamics
Invasive hemodynamic data were obtained by a surgeon blinded to the group identity during the two-part terminal study (1: Invasive Hemodynamics followed by 2: Cardiac Electrophysiology Study). Rats were anesthetized with an intraperitoneal saline suspension (100 mg/mL) of Inactin (125 mg/kg), which is “a long-lasting rodent anesthetic with minimal effects on cardiovascular tone” (Sigma-Aldrich, St. Louis, MO), volume loaded with a 3-mL subcutaneous bolus of lactated Ringer’s solution, placed on a heated surgical table, intubated, and ventilated as previously described (26, 28, 31).
A three-French solid-state micromanometer-tipped catheter (ADInstruments, Colorado Springs, CO) was equilibrated before insertion into the right carotid artery, exposed via right neck cutdown, and advanced into the left ventricular cavity. Data were digitized at a rate of 1,000 Hz for ∼15 min after catheter placement to allow for hemodynamic stabilization before ventricular manipulation and data collection.
In Vivo Cardiac Electrophysiology
After invasive hemodynamic analysis, rats underwent cardiac electrophysiology (EP) evaluation as described previously (28, 29). In brief, rats immediately underwent a second median sternotomy after the micromanometer was retracted from the left ventricular cavity to the right common carotid artery. Any cardiac adhesions were dissected to expose the epicardial surface. No antiarrhythmic compounds were utilized at any point during the EP evaluation.
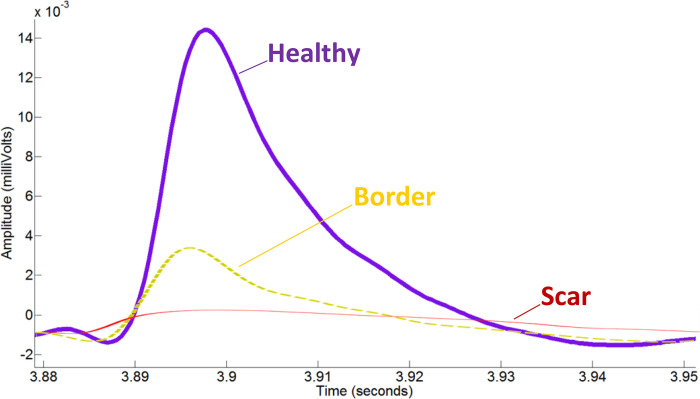
Monophasic action potentials were collected from the epicardium using a bipolar concentric microelectrode (MicroProbes for Life Science, Gaithersburg, MD) and filtered using a MP150 system with MCE100C amplifier modules (BIOPAC Systems Inc., Goleta, CA) before integration into a two-dimensional electroanatomic colormap (24 equidistant points arranged in a rectangle collected sequentially, 40 mm for 6 columns by 30 mm for 4 rows). The mapped epicardium spanned the anterior-most portion of the right ventricle, over the anterior left ventricle, to the middle of the left ventricular free wall (28). Consistency in mapped epicardium was ensured by comparable surgical windows of equal length and retractor placement. Healthy (≥7.2 mV)-Border (7.2 > x ≥ 2.8 mV)-Scar (<2.8 mV) cutoff values for monophasic action potential amplitude were calculated empirically in a previously published study (28). Although monophasic action potential amplitude has been described to be inferior to bipolar voltage electrogram amplitude for quantifying scar burden, it is noninferior with respect to detecting the three subtypes of tissue found in infarcted myocardium and has high spatial resolution for characterizing the two-dimensional distribution of the tissue subtypes (28).
A three-lead surface electrocardiogram was obtained while programmed electrical stimulation protocols were performed to induce mmVT (MATLAB, Natick, MA) (29). Capture threshold in volts was determined in healthy epicardial myocardium before a S1-S2 drivetrain was initiated to induce sustained ventricular tachyarrhythmia with two-times the capture threshold. Eight equidistant stimuli (S1) with one extrastimulus (S2) were utilized in all drivetrains. The S1-S1 interval was set so as to be slightly faster than the intrinsic rhythm (∼10 beats/min greater than the rat’s sedated heart rate) and the drivetrain was triggered to initiate slightly before a P wave so as to allow for complete ventricular repolarization before pacing.
Pacing started at an arbitrarily long S1-S2 interval to characterize subsequent ventricular capture morphology on the surface electrocardiogram. The drivetrain was executed three times, and then the S1-S2 interval was trimmed sequentially by 5 ms until arrhythmia occurred and/or until failure to capture. The longest S1-S2 interval that failed to consistently capture the heart and produce uniform repolarization waveforms was reported as the minimum value of the ventricular effective refractory window. The pacing electrode for all S1-S2 drivetrains was placed on the anterior epicardium in the visually determined border region (peripheral to the infarcted tissue, on visually viable myocardium). Often, this location resided near the junction of the right ventricle and left ventricle.
Sustained mmVT in rats has been defined as greater than fifteen consecutive premature ventricular complexes at any rate exceeding 350 beats/min (31).
Statistical Analysis
Data are expressed as means ± SE. An alpha level of 0.05 was set as the upper boundary for statistical significance. Differences between groups were determined by either one-way ANOVA and Tukey’s honestly significant difference post hoc testing or by Kruskal-Wallis one-way ANOVA on ranks and Dunn’s method, if the Shapiro-Wilk normality test or equal variance test was failed. The incidence of inducible mmVT was compared using Fisher’s exact test.
RESULTS
Heart Failure Induction
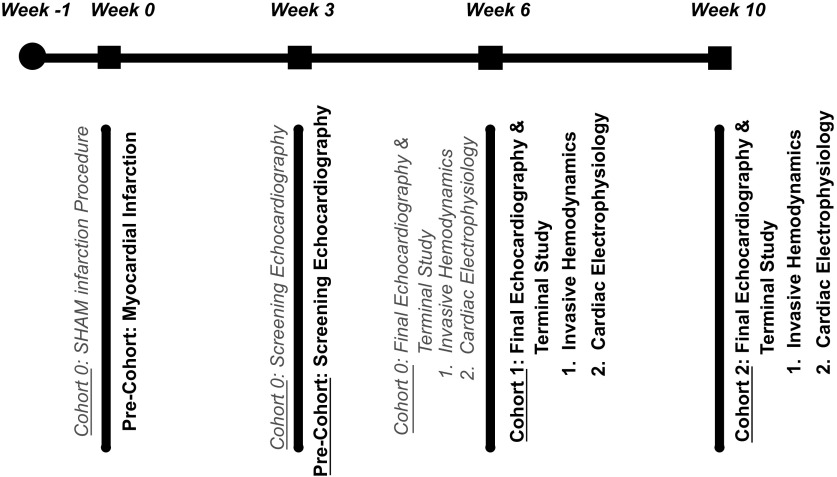
A schematic of the study timeline is provided in Fig. 1. The survival rate 48 h after myocardial infarction was 60%. HF rats were randomized to either the 6-wk arm or the 10-wk arm. Three SHAM rats died within 48 h of the surgery; the remaining SHAM rats survived to the completion of the study.
Figure 1.
Study timeline. A graphical display of the study design. All rats were acclimated upon arrival for 1 wk (week 1) until myocardial infarction (MI; sample size = 62). SHAM rats (cohort 0; gray text) (sample size = 10) underwent a left thoracotomy without left coronary artery ligation (week 0) to serve as appropriate surgical controls. Left ventricular ejection fraction below 40% was confirmed via screening echocardiography (week 3) for surviving infarcted precohort rats (sample size = 32) before random assignment to either cohort 1 (6-wk HF; sample size = 20) or cohort 2 (10-week HF; sample size = 12). Cohort 1 was survived for 6 wk post-MI (week 6), and cohort 2 was survived for 10 wk post-MI (week 10). At each respective end point, rats underwent a final echocardiographic study before returning to the surgical table for their terminal study. The terminal study began with invasive hemodynamic assessment and was completed after the cardiac electrophysiology study. Overall survival post-MI was 60%, and 5 rats were excluded for ejection fractions above 40%.
Echocardiography
The screening echo session occurred at 3 wk post-MI to assess cardiac dysfunction subsequent to the MI. Of the rats that survived the infarction, nearly all of them (n = 32) were successfully induced into HF as evidenced by a LVEF below 40% (35 ± 3%). Only five rats were excluded from HF cohort assignment as their LVEFs were not sufficiently low.
The final echo session occurred at the study end point, 6 wk post-MI. Compared with SHAM rats (n = 10), 6-wk HF rats (n = 20) had impaired LV function and an increase in systolic and diastolic geometric parameters consistent with maladaptive LV remodeling (Table 1). LVEF decreased (27 ± 4 vs. 73 ± 3%, P < 0.05) along with fractional shortening (14 ± 2 vs. 44 ± 3%, P < 0.05). LV internal diameter increased in both systole (9.0 ± 0.4 vs. 4.4 ± 0.3 mm, P < 0.05) and diastole (10.3 ± 0.3 vs. 7.9 ± 0.2 mm, P < 0.05). LV volume in 6-wk HF also increased in both systole (464 ± 34 vs. 93 ± 13 µL, P < 0.05) and diastole (616 ± 31 vs. 338 ± 15 µL, P < 0.0001). Finally, LV anterior wall thickness decreased both in systole (1.6 ± 0.1 vs. 3.1 ± 0.2 mm, P < 0.05) and diastole (1.3 ± 0.1 vs. 1.8 ± 0.1 mm, P = 0.0058).
Table 1.
Echocardiography reveals no difference between 6- and 10-wk HF
| n | EF, % | FS, % | LVID:s, mm | LVID:d, mm | Vol:s, mL | Vol:d, mL | AW:s, mm | AW:d, mm | ||
|---|---|---|---|---|---|---|---|---|---|---|
| SHAM | 10 | 73 ± 3 | 44 ± 3 | 4.4 ± 0.3 | 7.9 ± 0.2 | 93 ± 13 | 337 ± 15 | 3.1 ± 0.2 | 1.8 ± 0.1 | |
| HF-6 wk | 20 | 27 ± 4* | 14 ± 2* | 9.0 ± 0.4* | 10.3 ± 0.3* | 464 ± 34* | 616 ± 31* | 1.6 ± 0.1* | 1.3 ± 0.1* | |
| HF-10 wk | 11 | 34 ± 5* | 18 ± 3* | 8.4 ± 0.5* | 10.2 ± 0.3* | 404 ± 51* | 591 ± 40* | 1.6 ± 0.1* | 1.3 ± 0.1* |
Summary of the echocardiographic results (means ±SE; n = number of rats in sample size) for the left ventricles of SHAM rats, 6-wk heart failure (HF) rats, and 10-wk HF rats. Echo data were collected within 3 days of the terminal invasive hemodynamic and electrophysiologic study. EF, ejection fraction; FS, fractional shortening; LVID:s/d, left ventricular internal diameter in systole/diastole; Vol:s/d, left ventricular volume in systole/diastole; AW:s/d, ventricular anterior wall thickness in systole/diastole. While overt differences are seen between SHAM and 6-wk HF (*P < 0.05, ANOVA-Tukey’s honestly significant difference or Kruskal-Wallis ANOVA on ranks and Dunn’s method), as well as between SHAM and 10-wk HF (*P < 0.05), no differences are observed between 6-wk HF and 10-wk HF.
Compared with 6-wk HF rats, 10-wk HF rats (n = 11) had no statistically significant changes in echocardiographic parameters (Table 1).
Invasive Hemodynamics
Six-week HF rats (n = 20) had an increase in LV end-diastolic pressure (25 ± 2 vs. 6 ± 1 mmHg, P < 0.05), a decrease in LV systolic pressure (110 ± 4 vs. 140 ± 4 mmHg, P = 0.0003), and a decrease in LV peak-developed pressure (121 ± 8 vs. 180 ± 4 mmHg, P < 0.05) compared with SHAM rats (n = 10) (Table 2). LV ±dP/dt decreased (4,477 ± 251 vs. 7,612 ± 299 mmHg/s, P < 0.0001) and (−3,031 ± 202 vs. −7,554 ± 242 mmHg/s, P < 0.05), and Tau increased (31.7 ± 2.3 vs. 18.6 ± 0.5 ms, P < 0.05) compared with SHAM rats. Heart rate also decreased in 6-wk HF rats compared with SHAM (248 ± 5 vs. 286 ± 7 beats/min, P = 0.0001).
Table 2.
Hemodynamics reveals no difference between 6- and 10-wk HF
| n | Heart Rate, beats/min | EDP, mmHg | SP, mmHg | (+)dP/dt, mmHg/s | (−)dP/dt, mmHg/s | Tau, ms | PDP, mmHg | |
|---|---|---|---|---|---|---|---|---|
| SHAM | 10 | 286 ± 7 | 6 ± 1 | 140 ± 4 | 7,612 ± 299 | −7,554 ± 242 | 18.6 ± 0.5 | 180 ± 5 |
| HF-6 wk | 20 | 248 ± 5* | 25 ± 2* | 110 ± 4* | 4,477 ± 251* | −3,031 ± 202* | 31.7 ± 2.3* | 121 ± 8* |
| HF-10 wk | 12 | 246 ± 5* | 22 ± 2* | 122 ± 6* | 5,084 ± 308* | −3204 ± 381* | 34.1 ± 2.2* | 129 ± 7* |
Summary of the invasive hemodynamic results (means ±SE; n = number of rats in sample size) from SHAM rats, 6-wk heart failure (HF) rats, and 10-wk HF rats. Hemodynamic data were collected at the terminal procedure, immediately before electrophysiologic assessment. EDP, left ventricular end-diastolic pressure; SP, left ventricular systolic pressure; (+)/(−)dP/dt, positive/negative change in ventricular pressure with respect to time; Tau, left ventricular relaxation time constant; PDP, left ventricular peak developed pressure. While overt differences are seen between SHAM and 6-wk HF (*P < 0.05, ANOVA-Tukey’s honestly significant difference or Kruskal-Wallis ANOVA on ranks and Dunn’s Method), as well as between SHAM and 10-wk HF (*P < 0.05), no differences are observed between 6-wk HF and 10-wk HF.
Comparison of 10-wk HF rats (n = 12) to 6-wk HF rats revealed no difference in invasive hemodynamic parameters (Table 2).
In Vivo Cardiac Electrophysiology
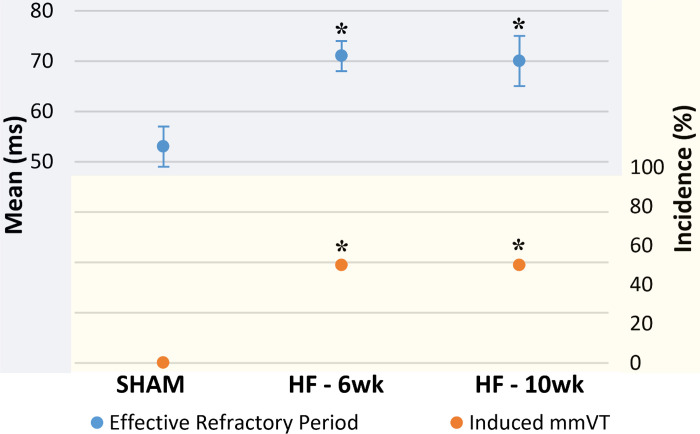
Two dimensional electroanatomic colormap generation with monophasic action potential amplitude was successfully performed in a portion of each group (Figs. 2 and 3). SHAM rats (n = 15) exhibited no inducible mmVT (0/15, 0%) and a short effective refractory period (ERP; 53 ± 4 ms) (Figs. 4 and 5). Six-week HF rats (n = 20) had an increase in mmVT (10/20, 50 vs. 0%, P = 0.0016) and a prolongation of the ventricular ERP (71 ± 3 vs. 53 ± 4 ms, P = 0.0042). Ten-week HF rats (n = 10) exhibited no difference with respect to the incidence of inducible mmVT (5/10, 50 vs. 50%, P = 1.0000) and no change in the ventricular ERP (70 ± 5 vs. 71 ± 3 ms, P = 0.9831). Episodes of mmVT in the HF rats either persisted indefinitely (beyond 5 s), with no major alteration in cycle length or waveform morphology, or spontaneously terminated within ∼5 cycles after the 15 consecutive depolarizations necessary to be classified as a sustained episode. Rats that exhibited indefinitely persisting mmVT were converted back to normal sinus rhythm with anti-tachycardia pacing, which exhibited a perfect success rate. These rats were converted back to sinus to continue the S1-S2 drivetrain so as to quantify ventricular ERP.
Figure 2.
Epicardial monophasic action potentials are of excellent quality. Three epicardial monophasic action potential tracings from a single 10-wk heart failure (HF) rat. The tracings highlight the substantial difference in monophasic action potential amplitude in millivolts (y-axis) between healthy myocardium, border tissue, and scar tissue, likely due to the decreased number of viable cardiomyocytes. A difference can also be observed in the monophasic action potential duration to 90% repolarization (x-axis). Differences in the waveform slope, particularly in the 2nd and 3rd phase, are thought to be due to impaired potassium efflux associated with ischemic remodeling.
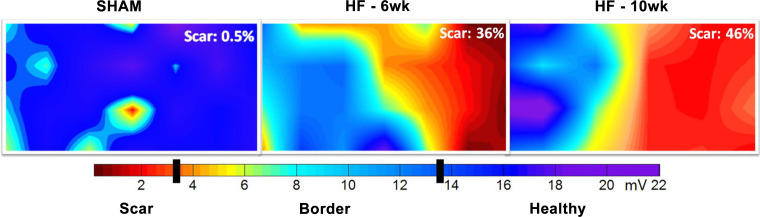
Figure 3.
Two-dimensional electroanatomic colormaps reveal comparable substrate. Epicardial monophasic action potential amplitude colormaps from 3 randomly selected rats, each representing its respective group namely SHAM, 6-wk heart failure (HF), or 10-wk HF. The colormaps reveal a normal epicardium in SHAM, with a single area of red highlighting the sensitivity of monophasic action potential amplitude (MAPA) in characterizing the epicardium. The 6-wk HF colormap shows gross MAPA defects on the right, which corresponds to the left coronary artery myocardial territory. The HF-10-wk map exhibits maintenance of the electrical infarct with a widening of the border region but improvement in MAPA millivoltage in the scar. Although scar burden is different between these 2 randomly selected HF rats representing the 6-wk end point and the 10-wk end point, the qualities of the substrates are similar and the hemodynamic and echocardiographic group averages support the notion that the degree of myocardial infarction-mediated HF was comparable between the 2 groups.
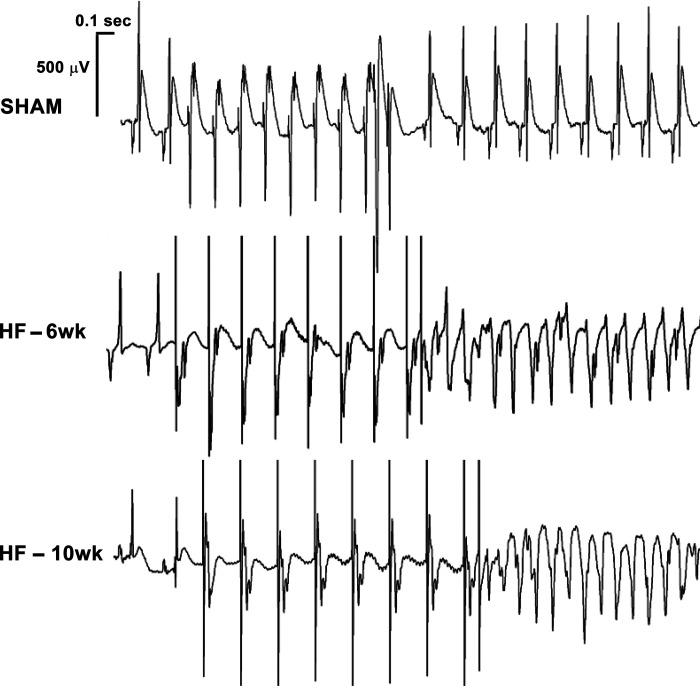
Figure 4.
Programmed electrical stimulation-induced ventricular tachycardia in heart failure (HF). Surface electrocardiogram (lead II) tracings from individual rats representing each group. Each tracing begins with 2 cardiac cycles of intrinsic electrical activity, followed by the programmed electrical stimulation (PES) drivetrain of 8 S1 stimulations and 1 premature S2 stimulation. The resulting rhythm is two premature ventricular contractions followed by a brief delay and then spontaneous return to normal sinus rhythm for the SHAM rat, monomorphic ventricular tachycardia (mmVT) for the HF-6-wk rat, and a comparable mmVT for the HF-10wk rat. Scale bars are provided before the SHAM tracing. For SHAM and HF 6-wk tracings, the P wave observed during intrinsic electrical activity can be observed during PES.
Figure 5.
Plateau of both ventricular tachycardia and effective refractory period. A graph summarizing the electrophysiologic results for incidence of inducible monomorphic ventricular tachycardia (mmVT) and ventricular effective refractory period (ERP; means ±SE. Changes between SHAM (n = 15) and 6-wk heart failure (HF) (n = 20) include an increase in inducible mmVT and a prolongation of the ventricular ERP. These changes do not continue between 6 wk and 10 wk of HF (n = 10). *P < 0.01 vs. SHAM (ERP: ANOVA-Tukey; mmVT: Fisher’s exact).
Capture threshold did not differ enough between SHAM (0.70 ± 0.13 V) and 6-wk HF (1.00 ± 0.12 V), SHAM and 10-wk HF (0.84 ± 0.12 V) or 6-wk HF and 10-wk HF to warrant any pairwise comparisons (P = 0.343).
DISCUSSION
In this study, we compared the incidence of inducible mmVT in a rat HF model at 6- and 10 wk post-MI (Fig. 1). Induction of HF was defined by changes in echocardiography (Table 1), invasive hemodynamics (Table 2), electroanatomic mapping (Figs. 2 and 3), and incidence of inducible mmVT (Figs. 4 and 5). Six-week HF rats exhibited an expected increase in the incidence of inducible mmVT and a prolongation of ERP but no difference in capture threshold voltage compared with SHAM. 10-wk HF rats met statistical significance in echocardiographic, invasive hemodynamic, and EP datasets versus SHAM but lacked any statistical distinction versus 6-wk HF (Tables 1 and 2; Fig. 4). This is consistent with a previous study that describes hemodynamic stabilization in the 5 to 10 wk post-MI in a Sprague-Dawley rat model (32).
While rodent cardiac EP is certainly distinct from human cardiac EP (33), it is an appropriate model with respect to clinical translation. Previous publications from our laboratory have described this HF model’s propensity to clinically relevant arrhythmias and electromechanical uncoupling, taking the form of monomorphic and polymorphous mmVT, electrical and mechanical alternans, and pulseless electrical activity (29).
The incidence of induced mmVT increases between SHAM and 6-wk HF (Figs. 4 and 5). This increase may be out of proportion to what is seen clinically (34), potentially due to increased collateral circulation (35) and timely percutaneous coronary intervention for patients with acute MI. The plateau of inducible mmVT between 6 and 10 wk is attributed to the remodeling process likely completing before 6 wk post-MI. The remodeled myocardium, both the compensatory cardiomyocyte hypertrophy and the myofibroblast-mediated scar, may convey no additional arrhythmogenic risk after 6 wk of permanent ischemia in HF rats. This is consistent with canine models of HF (36) and with clinical findings, where the early convalescent phase imparts increased SCD risk that lowers upon conversion to the chronic phase after 1 yr (37).
While the clinical definition of sustained mmVT is a rapid ventricular rhythm exceeding 100 beats/min that persists for over 30 s, this definition for rodents is extrapolated to account for the substantially higher resting heart rate (∼250 beats/min) and subsequently excessively high mmVT rate (500+ beats/min). For humans, the minimum number of premature ventricular complexes totals 50 (100 beats/min for 30 s). For a rodent with a VT rate of 840 beats/min (29), 50 complexes would be reached in nearly 3 s. Utilizing a cutoff value of 15 premature ventricular complexes is sufficiently sensitive to stratify SHAM and HF rats (38–40) and also allows for distinguishing of acute ischemia-related transient ectopy versus stable and persistent infarct-related reentry.
We hypothesized that this rodent model of HF would exhibit a progressively increased incidence of mmVT as the duration of HF increased. The data reveal a plateau of inducible mmVT between the two HF cohorts. Although this result does not support our hypothesis, it does not diminish the potential impact of a future experimental therapy for mmVT or SCD that has undergone preclinical evaluation in a Sprague-Dawley HF model (19). The knowledge that structural remodeling and proarrhythmic deterioration are quiescent between 6 and 10 wk post-MI are the necessary background to properly interpret the efficacy of any novel antiarrhythmic therapy applied to this model in that time frame. Clinically, the odds of arrhythmogenic SCD in HF are thought to increase cumulatively as the duration of HF increases, however, that does not seem to be recapitulated in this rodent HF model between 6- and 10 wk post-MI. Nonetheless, this animal model still holds value for studying interventions in hemodynamically stable HF with stably reentrant tachyarrhythmia, which describes many optimally managed HF patients.
Of note is the relatively large voltage that is necessary to entrain the rat hearts, compared with what is necessary to entrain human hearts. Although the rat heart mass is smaller than the human heart mass, the voltage required for capture in both species is comparable. When programmed electrical stimulation is performed on a patient, it is most often 1) utilizing an endocardial approach, which decreases the distance between the pacing electrodes and the conduction pathways; 2) targeting their pacing electrodes near the conduction pathways so as to further minimize resistance; and 3) using larger electrodes than are used in rats as the human heart’s size easily accommodates larger electrodes. These factors of capture threshold and other currently unknown factors may be the explanation for this observed phenomenon.
Rodent left coronary artery occlusion model for HF is an aggressive model that creates large partially transmural to fully transmural infarcts in the range of 30% LV involvement, predominately in the free wall (28). These infarcts resulted in a survival rate of 60% for HF rats. Utilizing a model of permanent coronary occlusion yields a relatively high mortality rate, as compared with ischemia-reperfusion methodologies (20). Nonetheless, appropriately sized cohorts with narrow ventricular function error margins were feasible.
Sedated heart rate was found to be depressed in both HF cohorts as compared with the SHAM cohort (Table 2). This finding is consistent with previous studies from our laboratory (29, 41). The mechanism of this depressed heart rate is currently unknown.
Limitations
This translational study set out to evaluate the strength of correlation between rat and human cardiac electrophysiology, specifically via identification of the incidence of inducible mmVT in HF rats at progressive time points. It is necessary to acknowledge that utilizing an animal model like swine whose ion channel isoform expression, electromechanical coupling via calcium handling, and cardiovascular anatomy better replicate what is seen clinically would carry greater translational value with fewer limitations. Degeneration of induced mmVT to ventricular fibrillation or arrhythmogenic sudden cardiac death was not observed in this rodent study, although it may have been if a larger animal model of HF was utilized instead of rats.
In addition, the method of HF induction for the rats, permanent left coronary artery occlusion by surgical means, is certainly distinct from the mechanism of HF induction observed clinically. In humans, slowly progressing atherosclerosis of the coronary arteries results in ischemic heart disease that sustains islands of living myocardium in heterogeneously distributed scar tissue (i.e., nontransmural) (42, 43). Additionally, after an acute MI, coronary artery blood flow can eventually be reestablished with percutaneous coronary intervention. These characteristics are not present in HF models induced by permanent coronary artery ligation and thus are a limitation of any coronary occlusion animal model’s ability to recapitulate the clinical phenomenon of HF post-MI. Nonetheless, the mmVT exhibited by this rodent HF model likely propagates by the same reentrant circuit mechanism that is observed in human HF patients.
Finally, rats 6 to 8 wk in age were utilized to study MI and HF. Though seemingly young, it is important to account for the average lifespan of the Sprague-Dawley rat: 3 yr (30). One human year is the equivalent of roughly 14 rat days, and their developmental timeline is drastically accelerated compared with mankind’s. Thus their age at MI corresponds to their young adult stage. Regardless, the ability of this rat model to recapitulate human cardiovascular pathophysiology from ischemic insult has been long established.
Conclusions
The development of HF after MI correlates with increased ventricular scar burden. This scar tissue, in combination with the remaining viable myocardium, creates a substrate that predisposes to reentrant ventricular tachyarrhythmias, namely mmVT. Clinically, the odds of arrhythmogenic SCD increase cumulatively as the duration of HF increases. Whether this finding held true for a rodent model of permanent coronary occlusion had yet to be documented. In characterizing the chronologic progression of reentrant arrhythmogenesis observed in this model, the efficacy of experimental therapeutic interventions can be interpreted more appropriately.
We hypothesized that this rat model of HF would exhibit increased reentrant tachyarrhythmia during HF progression, specifically with an increase in the incidence of inducible mmVT over time. These findings do not support this hypothesis but do support the continued utilization of rodent HF models to describe cardiac pathophysiology and investigate novel therapies for mmVT and SCD.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grant T32-HL-007249-43, WARMER Research Foundation, Sarver Heart Center, and University of Arizona.
DISCLOSURE
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHORS CONTRIBUTIONS
J.J.L., E.J., and S.G. conceived and designed research; I.R.C. performed experiments; I.R.C. analyzed data; I.R.C., T.M., M.D.H., E.J., and S.G. interpreted results of experiments; I.R.C. prepared figures; I.R.C. drafted manuscript; I.R.C., T.M., M.D.H., J.J.L., and S.G. edited and revised manuscript; I.R.C., T.M., M.D.H., J.J.L., E.J., and S.G. approved final version of manuscript.
ACKNOWLEDGMENTS
We extend a special thank you to Sherry Daugherty, Maribeth Stansifer, Grace Gorman, Mary Kaye Pierce, and Mark Borgstrom for technical assistance, and Dr. Clyde Yancy for revisions.
REFERENCES
- 1.Benjamin EJ, Muntner P, Alonso A, Bittencourt MS, Callaway CW, Carson AP , et al. Heart Disease and Stroke Statistics-2019 updated: a report from the American Heart Association. Circulation 139: e56–e528, 2019. [Erratum in Circulation 141: e33, 2020]. [DOI] [PubMed] [Google Scholar]
- 2.Basuray A, French B, Ky B, Vorovic E, Olt C, Sweitzer NK, Cappola TP, Fang JC. Heart failure with recovered ejection fraction: clinical description, biomarkers, and outcomes. Circulation 129: 2380–2387, 2014. doi: 10.1161/CIRCULATIONAHA.113.006855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cutler MJ, Rosenbaum DS, Dunlap ME. Structural and electrical remodeling as therapeutic targets in heart failure. J Electrocardiol 40: S1–S7, 2007. [DOI] [PubMed] [Google Scholar]
- 4.Ebinger MW, Krishnan S, Schuger CD. Mechanisms of ventricular arrhythmias in heart failure. Curr Heart Fail Rep 2: 111–117, 2005. doi: 10.1007/s11897-005-0018-y. [DOI] [PubMed] [Google Scholar]
- 5.Roden DM, Abraham RL. Refining repolarization reserve. Heart Rhythm 8: 1756–1757, 2011. doi: 10.1016/j.hrthm.2011.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alenazy B, Tharkar S, Kashour T, Alhabib KF, Alfaleh H, Hersi A. In-hospital ventricular arrhythmia in heart failure patients: 7 year follow-up of the multi-centric HEARTS registry. ESC Heart Fail 6: 1283–1290, 2019. doi: 10.1002/ehf2.12525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moss AJ, Greenberg H, Case RB, Zareba W, Hall WJ, Brown MW, Daubert JP, McNitt S, Andrews ML, Elkin AD. Long-term clinical course of patients after termination of ventricular tachyarrhythmia by an implanted defibrillator. Circulation 110: 3760–3765, 2004. doi: 10.1161/01.CIR.0000150390.04704.B7. [DOI] [PubMed] [Google Scholar]
- 8.Koplan BA, Stevenson WG. Ventricular tachycardia and sudden cardiac death. Mayo Clin Proc 84: 289–297, 2009. [Erratum in Mayo Clin Proc 84: 483, 2009]. doi: 10.4065/84.3.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lopez EM, Malhotra R. Ventricular tachycardia in structural heart disease. J Innov Cardiac Rhythm Manage 10: 3762–3773, 2019. doi: 10.19102/icrm.2019.100801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cuculich PS, Schill MR, Kashani R, Mutic S, Lang A, Cooper D, Faddis M, Gleva M, Noheria A, Smith TW, Hallahan D, Rudy Y, Robinso CG. Noninvasive cardiac radiation for ablation of ventricular tachycardia. N Engl J Med 377: 2325–2336, 2017. doi: 10.1056/NEJMoa1613773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wei C, Qian P, Tedrow U, Mak R, Zei PC. Non-invasive stereotactic radioablation: a new option for the treatment of ventricular arrhythmias. Arrhythm Electrophysiol Rev 8: 285–293, 2020. doi: 10.15420/aer.2019.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Katz AM Selectivity and toxicity of antiarrhythmic drugs: molecular interactions with ion channels. Am J Med 104: 179–P195, 1998. doi: 10.1016/S0002-9343(97)00388-4. [DOI] [PubMed] [Google Scholar]
- 13.Tokuda M, Kojodjojo P, Tung S, Tedrow UB, Nof E, Inada K, Koplan BA, Michaud GF, John RM, Epstein LM, Stevenson WG. Acute failure of catheter ablation for ventricular tachycardia due to structural heart disease: causes and significance. J Am Heart Assoc 2: e000072, 2013. doi: 10.1161/JAHA.113.000072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van Welsenes GH, Borleffs CJ, van Rees JB, Atary JZ, Thijssen J, van der Wall EE, Schalij MJ. Improvements in 25 years of implantable cardioverter defibrillator therapy. Neth Heart J 19: 24–30, 2011. doi: 10.1007/s12471-010-0047-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Klein HU, Reek S. The MUSTT study: evaluating testing and treatment. J Interv Card Electrophysiol 4: 45–50, 2000. doi: 10.1023/A:1009862028599. [DOI] [PubMed] [Google Scholar]
- 16.Klein H, Auriccho A, Reek S, Geller C. New primary prevention trials of sudden cardiac death in patients with left ventricular dysfunction: SCD-HEFT and MADIT-II. Am J Cardiol 11: 91D–97D, 1999. doi: 10.1016/s0002-9149(99)00040-5. [DOI] [PubMed] [Google Scholar]
- 17.Hajduk AM, Gurwitz JH, Tabada G, Masoudi FA, Magid DJ, Greenlee RT, Sung SH, Cassidy-Bushrow AE, Liu TI, Reynolds K, Smith DH, Fiocchi F, Goldberg R, Gill TM, Gupta N, Peterson PN, Schuger C, Vidaillet H, Hammill SC, Allore H, Go AS, Cardiovascular Research Network Longitudinal Study of Implantable Cardioverter Defibrillators. Influence of multimorbidity on burden and appropriateness of implantable cardioverter-defibrillator therapies. J Am Geriatr Soc 67: 1370–1378, 2019. [Erratum in J Am Geriatr Soc 67: 2430, 2019]. doi: 10.1111/jgs.15839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jukema JW, Timal RJ, Rotmans JI, Hensen LC, Buiten MS, de Bie MK, Putter H, Zwinderman AH, van Erven L, Straaten MJ, Hommes N, Gabreels B, Dorp WV, Dam BV, Herzog CA, Schalij MJ, Rabelink TJ, ICD2 Trial Investigators. Prophylactic use of implantable cardioverter-defibrillators in the prevention of sudden cardiac death in dialysis patients. Circulation 139: 2628–2638, 2019. doi: 10.1161/CIRCULATIONAHA.119.039818. [DOI] [PubMed] [Google Scholar]
- 19.Chinyere IR, Hutchinson M, Moukabary T, Koevary JW, Juneman E, Goldman S, Lancaster JJ. Modulating the infarcted ventricle’s refractoriness with an epicardial biomaterial. J Invest Med. In press. doi: 10.1136/jim-2020-001486. [DOI] [PubMed] [Google Scholar]
- 20.Lindsey ML, Bolli R, Canty JM, Jr, Du XJ, Frangogiannis NG, Frantz S, Gourdie RG, Holmes JW, Jones SP, Kloner RA, Lefer DJ, Liao R, Murphy E, Ping P, Przyklenk K, Recchia FA, Longacre LS, Ripplinger CM, Van Eyk JE, Heusch G. Guidelines for experimental models of myocardial ischemia and infarction. Am J Physiol Heart Circ Physiol 314: H812–H838, 2018. doi: 10.1152/ajpheart.00335.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lancaster JJ, Sanchez P, Repetti G, Juneman E, Pandey A, Chinyere IR, Moukabary T, Lahood N, Daugherty S, Goldman S. Human induced pluripotent stem cell derived cardiomyocyte patch in rats with heart failure. Ann Thorac Surg 108: 1169–1177, 2019. doi: 10.1016/j.athoracsur.2019.03.099. [DOI] [PubMed] [Google Scholar]
- 22.Milavetz JJ, Raya TW, Johnson CS, Morkin E, Goldman S. Survival after myocardial infarction in rats: captopril versus losartan. J Am Coll Cardiol 27: 714–719, 1996. doi: 10.1016/0735-1097(95)00506-4. [DOI] [PubMed] [Google Scholar]
- 23.Pfeffer JM, Pfeffer MA, Braunwald E. Influence of chronic captopril therapy on the infarcted left ventricle of the rat. Circ Res 57: 84–95, 1985. doi: 10.1161/01.RES.57.1.84. [DOI] [PubMed] [Google Scholar]
- 24.Lucero CM, Andrade DC, Toledo C, Díaz HS, Pereyra KV, Diaz-Jara E, Schwarz KG, Marcus NJ, Retamal MA, Quintanilla RA, Rio RD. Cardiac remodeling and arrhythmogenesis are ameliorated by administration of Cx43 mimetic peptide Gap27 in. Sci Rep 10: 6878, 2020. doi: 10.1038/s41598-020-63336-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lyon AR, Bannister ML, Collins T, Pearce E, Sepehripour AH, Dubb SS, Garcia E, O’Gara P, Liang L, Kohlbrenner E, Hajjar RJ, Peters NS, Poole-Wilson PA, Macleod KT, Harding SE. SERCA2a gene transfer decreases sarcoplasmic reticulum leak and reduces ventricular arrhythmias in a model of chronic heart failure. Circ Arrhythm Electrophysiol 4: 362–372, 2011. doi: 10.1161/CIRCEP.110.961615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kellar RS, Lancaster JJ, Thai HM, Juneman E, Johnson NM, Byrne HG, Stansifer M, Arsanjani R, Baer M, Bebbington C, Flashner M, Yarranton G, Goldman S. Antibody to granulocyte macrophage colony-stimulating factor reduces the number of activated tissue macrophages and improves left ventricular function after myocardial infarction in a rat coronary artery ligation model. J Cardiovasc Pharmacol 57: 568–574, 2011. doi: 10.1097/FJC.0b013e318213258b. [DOI] [PubMed] [Google Scholar]
- 27.Stuckey DJ, Carr CA, Tyler DJ, Aasum E, Clarke K. Novel MRI method to detect altered left ventricular ejection fraction and filling patterns in rodent models of disease. Magn Reson Med 60: 582–587, 2008. doi: 10.1002/mrm.21677. [DOI] [PubMed] [Google Scholar]
- 28.Chinyere IR, Hutchinson M, Moukabary T, Lancaster JJ, Goldman S, Juneman E. Monophasic action potential amplitude for substrate mapping. Am J Physiol Heart Circ Physiol 317: H667–H673, 2019. doi: 10.1152/ajpheart.00225.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chinyere IR, Moukabary T, Goldman S, Juneman E. Electrical and mechanical alternans during ventricular tachycardia with moderate heart failure. J Electrocardiol 51: 33–37, 2018. doi: 10.1016/j.jelectrocard.2017.09.002. [DOI] [PubMed] [Google Scholar]
- 30.Sengupta P The laboratory rat: relating its age with human’s. Int J Prev Med 4: 624–630, 201 3. [PMC free article] [PubMed] [Google Scholar]
- 31.Weigand K, Witte R, Moukabary T, Chinyere IR, Lancaster J, Pierce MK, Goldman S, Juneman E. In vivo electrophysiological study of induced ventricular tachycardia in intact rat model of chronic ischemic heart failure. IEEE Trans Biomed Eng 64: 1393–1399, 2017. doi: 10.1109/TBME.2016.2605578. [DOI] [PubMed] [Google Scholar]
- 32.Krzemiński TF, Nożyński JK, Grzyb J, Porc M. Wide-spread myocardial remodeling after acute myocardial infarction in rat. features for heart failure progression. Vascul Pharmacol 48: 100–108, 2008. doi: 10.1016/j.vph.2008.01.002. [DOI] [PubMed] [Google Scholar]
- 33.Oh JG, Kho C, Hajjar RJ, Ishikawa K. Experimental models of cardiac physiology and pathology. Heart Fail Rev 24: 601–615, 2019. doi: 10.1007/s10741-019-09769-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tran HV, Ash AS, Gore JM, Darling CE, Kiefe CI, Goldberg RJ. Twenty-five year trends (1986-2011) in hospital incidence and case-fatality rates of ventricular tachycardia and ventricular fibrillation complicating acute myocardial infarction. Am Heart J 208: 1–10, 2019. doi: 10.1016/j.ahj.2018.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pohl T, Seiler C, Billinger M, Herren E, Wustmann K, Mehta H, Windecker S, Eberli FR, Meier B. Frequency distribution of collateral flow and factors influencing collateral channel development. functional collateral channel measurement in 450 patients with coronary artery disease. J Am Coll Cardiol 38: 1872–1878, 2001. doi: 10.1016/S0735-1097(01)01675-8. [DOI] [PubMed] [Google Scholar]
- 36.Garan H, Ruskin JN, McGovern B, Grant G. Serial analysis of electrically induced ventricular arrhythmias in a canine model of myocardial infarction. J Am Coll Cardiol 5: 1095–1106, 1985. doi: 10.1016/S0735-1097(85)80010-3. [DOI] [PubMed] [Google Scholar]
- 37.Myerburg RJ, Junttila MJ. Sudden cardiac death caused by coronary artery disease. Circulation 125: 1043–1052, 2012. doi: 10.1161/CIRCULATIONAHA.111.023846. [DOI] [PubMed] [Google Scholar]
- 38.Jiao KL, Li YG, Zhang PP, Chen RH, Yu Y. Effects of valsartan on ventricular arrhythmia induced by programmed electrical stimulation in rats with myocardial infarction. J Cell Mol Med 16: 1342–1351, 2012. doi: 10.1111/j.1582-4934.2011.01502.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lee TM, Chen CC, Chang NC. Granulocyte colony-stimulating factor increases sympathetic reinnervation and the arrhythmogenic response to programmed electrical stimulation after myocardial infarction in rats. Am J Physiol Heart Circ Physiol 297: H512–H522, 2009. doi: 10.1152/ajpheart.00077.2009. [DOI] [PubMed] [Google Scholar]
- 40.Lee TM, Chen CC, Lin MS, Chang NC. Effect of endothelin receptor antagonists on ventricular susceptibility in postinfarcted rats. Am J Physiol Heart Circ Physiol 294: H1871–H1879, 2008. doi: 10.1152/ajpheart.01129.2007. [DOI] [PubMed] [Google Scholar]
- 41.Sanchez P, Lancaster JJ, Weigand K, Mohran SA, Goldman S, Juneman E. Doppler Assessment of diastolic function reflect the severity of injury in rats with chronic heart failure. J Card Fail 23: 753–761, 2017. doi: 10.1016/j.cardfail.2017.08.446. [DOI] [PubMed] [Google Scholar]
- 42.Nakahara S, Tung R, Ramirez RJ, Gima J, Wiener I, Mahajan A, Boyle NG, Shivkumar K. Distribution of late potentials within infarct scars assessed by ultra high-density mapping. Heart Rhythm 7: 1817–1824, 2010. doi: 10.1016/j.hrthm.2010.07.032. [DOI] [PubMed] [Google Scholar]
- 43.Nakahara S, Vaseghi M, Ramirez RJ, Fonseca CG, Lai CK, Finn JP, Mahajan A, Boyle NG, Shivkumar K. Characterization of myocardial scars: electrophysiological imaging correlates in a porcine infarct model. Heart Rhythm 8: 1060–1067, 2011. doi: 10.1016/j.hrthm.2011.02.029. [DOI] [PubMed] [Google Scholar]