Abstract
This study investigated the impact of HFpEF on neuromuscular fatigue and peripheral hemodynamics during small muscle mass exercise not limited by cardiac output. Eight HFpEF patients (NYHA II-III, ejection-fraction: 61 ± 2%) and eight healthy controls performed dynamic knee extension exercise (80% peak workload) to task failure and maximal intermittent quadriceps contractions (8 × 15 s). Controls repeated knee extension at the same absolute intensity as HFpEF. Leg blood flow (QL) was quantified using Doppler ultrasound. Pre/postexercise changes in quadriceps twitch torque (ΔQtw; peripheral fatigue), voluntary activation (ΔVA; central fatigue), and corticospinal excitability were quantified. At the same relative intensity, HFpEF (24 ± 5 W) and controls (42 ± 6 W) had a similar time-to-task failure (∼10 min), ΔQtw (∼50%), and ΔVA (∼6%). This resulted in a greater exercise-induced change in neuromuscular function per unit work in HFpEF, which was significantly correlated with a slower QL response time. Knee extension exercise at the same absolute intensity resulted in an ∼40% lower QL and greater ΔQtw and ΔVA in HFpEF than in controls. Corticospinal excitability remained unaltered during exercise in both groups. Finally, despite a similar ΔVA, ΔQtw was larger in HFpEF versus controls during isometric exercise. In conclusion, HFpEF patients are characterized by a similar development of central and peripheral fatigue as healthy controls when tested at the same relative intensity during exercise not limited by cardiac output. However, HFpEF patients have a greater susceptibility to neuromuscular fatigue during exercise at a given absolute intensity, and this impairs functional capacity. The patients’ compromised QL response to exercise likely accounts, at least partly, for the patients’ attenuated fatigue resistance.
NEW & NOTEWORTHY The susceptibility to neuromuscular fatigue during exercise is substantially exaggerated in individuals with heart failure with a preserved ejection fraction. The faster rate of fatigue development is associated with the compromised peripheral hemodynamic response characterizing these patients during exercise. Given the role of neuromuscular fatigue as a factor limiting exercise, this impairment likely accounts for a significant portion of the exercise intolerance typical for this population.
Keywords: exercise intolerance, fatigue, heart failure, hemodynamics
INTRODUCTION
Although heart failure has historically been associated with a reduced ejection fraction (HFrEF), approximately one-half of all patients exhibit a preserved ejection fraction ≥50% (HFpEF) (1). Of importance is that despite significant pathophysiological differences (for review, see Ref. 2), patients with HFrEF and HFpEF exhibit similar morbidity and mortality (1) and severe exercise intolerance (3–5). Although this phenomenon has been extensively investigated in patients with HFrEF (6, 7), the mechanisms responsible for the impaired exercise tolerance in patients with HFpEF are not fully understood.
In addition to the expected central (i.e., cardiac) abnormalities in HFpEF (3, 8), recent studies have revealed significant impairments in numerous peripheral mechanisms that determine skeletal muscle oxygen transport and utilization in these patients (4, 9–12). Because oxygen supply and utilization are key determinants of neuromuscular fatigue (13), these peripheral impairments could exacerbate the development of fatigue during physical activity and explain, at least in part, the compromised exercise tolerance in patients with HFpEF (8–10). However, a thorough assessment of the impact of HFpEF on the response to exercise in terms of both the peripheral hemodynamics and the development of neuromuscular fatigue is currently lacking.
Exercise-induced neuromuscular fatigue, defined as a reversible decrease in the torque or power-generating capacity of a muscle or muscle group (14), compromises exercise tolerance in both health (15) and disease (16). This temporary impairment can result from neuronal changes within the central nervous system causing a decrease in neural activation of the muscle (i.e., central fatigue) and/or biochemical changes at or distal to the neuromuscular junction causing an attenuated contractile response to neural input (i.e., peripheral fatigue) (17). Central fatigue can be evaluated by quantifying the exercise-induced fall in a participant’s ability to voluntarily activate a muscle (14), whereas peripheral fatigue can be assessed by quantifying the exercise-induced fall in muscle twitch torque (18). Importantly, as voluntary activation is critically dependent on descending neural input reaching skeletal muscle, the integrity and excitability of the motor pathway linking the brain with the exercising muscle, termed the corticospinal pathway, is vital and has been considered a potential contributor to central fatigue (19). Alterations in the excitability of the corticospinal pathway can be assessed with transcranial magnetic stimulation (20). Such comprehensive assessments of both central and peripheral fatigue have yet to be performed in patients with HFpEF and could provide significant insight into the exercise intolerance exhibited by this population.
Therefore, it was the goal of this investigation to evaluate the impact of HFpEF on the development of neuromuscular fatigue and peripheral hemodynamics during exercise. To minimize the potentially confounding effect of the patients’ cardiac dysfunction, a single-joint exercise, which minimally taxes the heart, was utilized rather than whole body exercise (21). Specifically, given the task specificity of fatigue, both patients with HFpEF and healthy controls performed submaximal dynamic single-leg knee extension exercise as well as maximal, intermittent, isometric quadriceps contractions. It was hypothesized that, compared with healthy controls, HFpEF patients would develop greater neuromuscular fatigue (both central and peripheral) and exhibit attenuated peripheral hemodynamics during exercise.
METHODS
Participants
HFpEF participants were identified by a retrospective review of electronic medical records of patients then attending the general cardiac clinic at the University of Utah and the Salt Lake City Veterans Affairs Medical Center. Patient identification was determined by the following criteria: 1) clinical manifestation of heart failure (exertional dyspnea or orthopnea combined with signs of edema or jugular venous distention), 2) one or more hospitalizations for heart failure in the previous 12 mo and/or B-type natriuretic peptide ≥100 pg/mL, 3) a left ventricular ejection fraction ≥50%, and 4) evidence of diastolic dysfunction on echocardiogram (lateral wall E/e′ of >10 with a lateral wall e′ of <10). Exclusion for HFpEF included uncontrolled systolic blood pressure, significant valvular heart disease or unstable heart failure, the presence of a pacemaker, evidence of compensated HFrEF, changes in medications within 3 mo, body mass index (BMI) ≥50 kg/m2, and orthopedic limitations precluding exercise. Initially, 45 patients who had a clinical diagnosis of HFpEF were screened, from which 15 patients met the inclusion criteria to participate in this exercise-based study. Eight of these patients (6 males, 2 females) tolerated the procedures required for the assessment of neuromuscular function and completed the study. To serve as controls, eight sex- and age-matched healthy participants were enrolled from the general population around Salt Lake City, UT. During enrollment, participants completed a general health questionnaire to determine whether they were free from overt cardiopulmonary disease or were currently taking medications that could influence cardiopulmonary function. Indication of disease or medication use of this nature was determined to be an exclusion criterion. All subjects were nonsmokers and not regularly exercising. Descriptive subject characteristics are presented in Table 1. Description of comorbidities, echocardiography, and current medications for the HFpEF patients are presented in Table 2. Written, informed consent was obtained from all participants before their inclusion in the study. The Institutional Review Boards of the University of Utah and the Salt Lake City Veterans Affairs Medical Center approved all protocols.
Table 1.
Subject characteristics
| Control | HFpEF | |
|---|---|---|
| Age, yr | 67 ± 4 | 64 ± 3 |
| Height, cm | 174 ± 2 | 173 ± 7 |
| Weight, kg | 76 ± 14 | 114 ± 28* |
| BMI, kg/m2 | 25 ± 5 | 38 ± 9* |
| Physical activity | ||
| Sedentary, min/day | 1,196 ± 81 | 1,273 ± 105 |
| Light, min/day | 102 ± 40 | 90 ± 65 |
| Moderate, min/day | 64 ± 26 | 16 ± 14* |
| Vigorous, min/day | 7 ± 13 | 0 ± 0 |
| Steps/day, counts/day | 7,597 ± 1,972 | 3,688 ± 2,011* |
| Hematological characteristics | ||
| RBC, M/μL | 5 ± 1 | 5 ± 3 |
| Hemoglobin, g/dL | 15 ± 1 | 15 ± 3 |
| Hematocrit, % | 44 ± 4 | 45 ± 6 |
| Glucose, mg/dL | 86 ± 11 | 135 ± 54 |
| Cholesterol, mg/dL | 186 ± 19 | 165 ± 62 |
| Triglycerides, mg/dL | 74 ± 9 | 229 ± 133* |
| HDL, mg/dL | 60 ± 14 | 40 ± 11* |
| LDL, mg/dL | 115 ± 32 | 85 ± 48 |
Values are means ± SD; n = 8 control and 8 HFpEF. BMI, body mass index; HDL, high-density lipoprotein; HFpEF, heart failure with a preserved ejection fraction; LDL, low-density lipoprotein; RBC, red blood cells. *Significant difference between groups, P < 0.05.
Table 2.
HFpEF clinical characteristics and medications
| Disease-Related Characteristics | Normal Reference | |||
|---|---|---|---|---|
| NYHA Class II | 4 (50%) | |||
| NYHA Class III | 4 (50%) | |||
| Atrial fibrillation | 2 (25%) | |||
| CAD | 3 (38%) | |||
| COPD | 1 (13%) | |||
| Diabetes | 2 (25%) | |||
| Hypertension | 4 (50%) | |||
| Six-min walk distance, m | 387 ± 43 | |||
| B-type natriuretic peptide, pg/mL | 132 ± 55 | |||
| Echocardiography | ||||
| Ejection fraction, % | 61 ± 5 | ≥55 | ||
| LV IVSd, cm | 1.1 ± 0.2 | 0.6 | 1.1 | |
| LV PWd, cm | 1.1 ± 0.1 | 0.6 | 0.9 | |
| LV ID diastole, cm | 4.8 ± 0.6 | 3.9 | 5.3 | |
| LV ID systole, cm | 3.2 ± 0.5 | 2.0 | 4.0 | |
| LA ESV index, mL/m2 | 39 ± 12 | <34 | ||
| TR gradient, mmHg | 44 ± 8 | |||
| Peak E wave, cm/s | 95 ± 20 | ≤50 | ||
| Peak A wave, cm/s | 72 ± 28 | |||
| E/A ratio | 1.6 ± 0.8 | 0.8–2.0 | ||
| E′ lateral wall, cm/s | 8 ± 1 | >10 | ||
| E′ septal wall, cm/s | 6 ± 1 | >7 | ||
| E/E′ lateral | 12 ± 3 | <13 | ||
| E/E′ septal | 17 ± 4 | <15 | ||
| E/E′ ratio (average) | 14 ± 3 | <14 | ||
| Mitral E-wave deceleration, ms | 236 ± 60 | |||
| Peak TR velocity, m/s | 3.1 ± 0.4 | <2.8 | ||
| Medications | ||||
| β-Receptor blockers | 4/8 (50% | |||
| ACEi or ARB | 5/8 (63%) | |||
| Loop diuretics | 4/8 (50%) | |||
| Statin | 2/8 (25%) | |||
| Nitrates | 2/8 (25%) | |||
| Calcium channel blockers | 5/8 (63%) |
Values are means ± SD or %group; n = 8 HFpEF. ACEi, angiotensin-converting enzyme iinhibitor; ARB, angiotensin receptor blocker; CAD, coronary artery disease; COPD, chronic obstructive pulmonary disease; HFpEF, heart failure with preserved ejection fraction; NYHA, New York Heart Association; LA ESV, left atrium end systolic volume; LV ID, left ventricular internal diameter; LV IVSd, left ventrical interventricular septum diameter; LV PWd, left ventricular posterior wall diameter; TR, tricuspid regurgitation.
Experimental Procedures
During preliminary visits, anthropometric measurements were collected, and participants were familiarized with the exercise and procedures to be performed in both experimental protocol A (dynamic single-leg knee extension exercise) and protocol B (maximal isometric single-leg knee extensions). All exercise sessions were separated by ≥24 h and performed on the right leg. Quadriceps fatigue was quantified by the pre- to postexercise decrease in neuromuscular function. Alterations in the excitability of the corticospinal pathway were assessed pre- to postexercise with femoral nerve and transcranial magnetic stimulations (20, 22, 23).
Protocol A: dynamic single-leg knee extension exercise.
The first visit consisted of a maximal incremental single-leg knee extensor exercise test on a custom-made knee extensor ergometer. Subjects were asked to maintain a consistent cadence at 60 rpm throughout all testing procedures. Peak work rate (Wpeak) and oxygen consumption (V̇o2peak) were determined by increasing the work rate from an unloaded state by 5–10 W/min until task failure (an rpm drop below 50 for >5 s despite verbal encouragement). Wpeak was defined as the work rate during the last stage completed, and V̇o2peak was the average O2 consumption during the last minute of exercise. During the second and third visits, participants performed familiarization trials at 80% Wpeak to task failure (Tlim). During the fourth visit, considered the experimental day, participants performed the 80% Tlim exercise and, subsequently, exercise-induced neuromuscular quadriceps fatigue, and changes in corticospinal excitability were assessed (Fig. 1A). The controls returned for a fifth visit, in which they matched the absolute work rate and exercise duration of the patients with HFpEF on a person-to-person exercise performance basis (e.g., the control with the highest Wpeak was matched with the patient with HFpEF with the highest Wpeak, etc.). Prior to all 80% Tlim exercise and the assessment of baseline neuromuscular function, participants performed a 1-min unloaded warm-up on the knee extensor ergometer.
Figure 1.
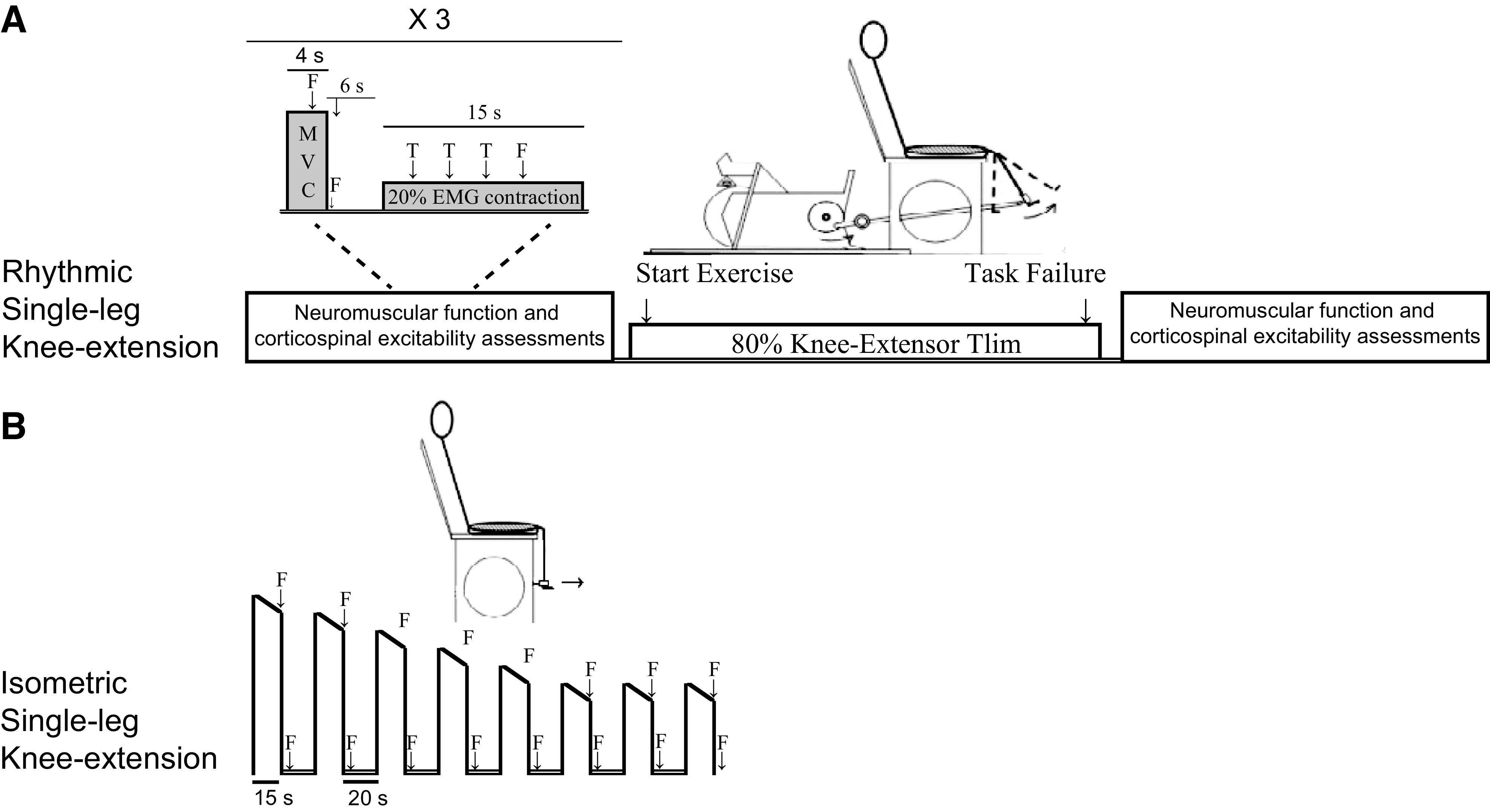
Schematic illustration of the 2 exercise protocols. Protocol A included the assessment of neuromuscular and corticospinal function before and after exercise. In protocol B, fatigue was quantified during exercise using femoral nerve stimulations (F). EMG, electromyography; MVC, maximal voluntary quadriceps contraction; T, transcranial magnetic stimulation; Tlim, time-to-task failure: 20% EMG contraction, 20% of the EMG attained during the preexercise MVC.
Protocol B: maximal isometric single-leg knee extension.
Prior to exercise and the assessment of baseline neuromuscular function, participants performed a standardized warm-up consisting of ten 5-s contractions at 20% of the participant’s maximal voluntary quadriceps contraction (MVC) force, which was determined in preliminary visits. Participants then performed eight 15-s intermittent MVCs, each followed by a 20-s rest period (Fig. 1B).
General Measurements
Physical activity level and 6-min walk test.
Participants wore an accelerometer (GTIM; Actigraph, Pensacola, FL) for 7 consecutive days after completing the study to measure physical activity, as previously described (24). Additionally, during the second preliminary visit, HFpEF patients performed a 6-min walk test (25).
Ventilation, pulmonary gas exchange, perceived exertion, and cardiovascular measurements.
Ventilation and pulmonary gas exchange were measured at rest and during exercise with a metabolic cart (Innocor; Innovision, Odense, Denmark). A rating of perceived exertion was obtained at the end of each minute during exercise using Borg’s Category Ratio 10 scale (26). Heart rate (HR) was measured with a 12-lead echocardiogram, whereas stroke volume (SV) and mean arterial pressure (MAP) were determined via finger photoplethsmography (Finapres Medical Systems, Amsterdam, The Netherlands), as previously described (27). Cardiac output was calculated as the product of HR and SV. Systemic vascular conductance was calculated as the quotient of cardiac output and MAP.
Electromyography.
The electromyography (EMG) signals of the vastus lateralis were obtained, as previously described (28). EMG signals were amplified 1,000 times (Neurolog Systems, Digitimer Ltd., Welwyn Garden City, Hertfordshire, UK), band-pass filtered (20–1,000 Hz, NL-844; Digitimer Ltd.), and converted from analog to digital at a sampling rate of 2,000 Hz using a 16-bit Micro 1401 mk-II and Spike2 data collection software (Cambridge Electronic Design Ltd., Cambridgeshire, UK) running custom-written scripts. During the dynamic knee extension exercise, EMG was analyzed as previously described (27, 28) and normalized to the signal obtained during the first minute of exercise.
Femoral nerve stimulation.
Femoral stimulation of the motor nerve was performed as previously described (27, 28). Briefly, the femoral nerve was stimulated using a constant-current stimulator (voltage 400 V, model DS7AH; Digitimer Ltd., Welwyn Garden City, Hertfordshire, UK). Stimulation intensity was increased by 20-mA increments (200-µs pulse width) until the size of the evoked twitch and compound muscle action potential (M-wave) demonstrated no further increase [i.e., maximal M-wave (Mmax)] at rest, which was then confirmed during a 50% MVC. Stimulation intensity was then set at 130% of Mmax intensity.
Transcranial magnetic stimulation.
Transcranial magnetic stimulation of the left motor cortex was performed with a concave double-cone coil (Magstim 200; Magstim Co. Ltd, Whitland, UK) to evoke a short-latency EMG response, termed motor-evoked potentials (MEP), in the right leg, as previously described (27, 28). Briefly, after determination of optimal positioning of the coil (posterior to anterior direction of current flow), stimulator intensity was set at 120% of the resting motor threshold (i.e., the stimulation intensity that evokes a MEP in at least 4 of 7 stimulations): control, 53 ± 4%; HFpEF, 57 ± 8%; P = 0.86. To quantify exercise-induced changes in corticospinal excitability, participants received three stimulation sets before and, again, after exercise, consisting of three transcranial magnetic stimulations and one femoral nerve stimulation. These stimulations were randomized during a constant quadriceps contraction equating to 20% of the EMG during a preexercise MVC (Fig. 1A) (29).
Neuromuscular quadriceps function and torque acquisition.
To examine exercise-induced quadriceps fatigue, neuromuscular knee extensor function of the exercising leg was assessed before and immediately after exercise (time to postexercise measures, protocol A: controls 38 ± 7 s, HFpEF 35 ± 7 s, P = 0.51; protocol B: controls 9 ± 2 s, HFpEF 10 ± 2 s, P = 0.58). Participants were seated upright and connected to a calibrated load cell (MLP 300; Transducer Techniques, Inc. Temecula, CA) to measure torque, as previously described (27). The assessment of quadriceps function included three MVCs. Femoral nerve stimulation was triggered during each MVC and 2 s into the rest phase to quantify potentiated quadriceps twitch torque (Qtw) and calculate quadriceps voluntary activation during the protocol (18, 27). The maximal rate of torque development and peak relaxation rate were analyzed for each Qtw, as previously described (27). As an estimate of the susceptibility to fatigue, exercise-induced changes in Qtw, and voluntary activation were normalized to the amount of work performed (e.g., ΔQtw/kJ; protocol A).
Leg blood flow.
Femoral blood flow, reported as a minute average, was measured at rest and during exercise for visit 1 (maximal dynamic knee extension exercise) and visit 4 (Tlim exercise), using a Logic 7 ultrasound (General Electric Medical Systems, Milwaukee, WI), as previously described (24). Leg vascular conductance was calculated as femoral blood flow/MAP.
Mean response time.
The overall time course of the femoral blood flow responses for protocol A was assessed as a mean response time (MRT; time constant + time delay) analyzed from a single transition from unloaded knee extensions to the 80% work rate (achieved target workload within ∼3–4 s). Markers were synced within the data acquisition system with the Doppler ultrasound clips for the start and end of each bout. Leg blood flow data were analyzed in 12-s bins for the final minute of unloaded knee extension exercise and for the duration of the 80% Tlim bout. These bins were not averaged, and any missing data points were excluded from analysis. Because of the lack of multiple transitions and higher temporal resolution, only the MRT is reported to describe the overall time course of the blood flow response, as it is less affected by these limitations. The MRT was determined using the monoexponential function y(t) = y(b) + A (1 – e−(t – TD)/τ), where y is femoral blood flow, t is time, b is the unloaded exercise value, A is the amplitude of the exponential response, and τ is the time constant of the exponential response (30).
Data and Statistical Analysis
All data were stored and analyzed offline using Spike2 data acquisition software. The area for each MEP and Mmax was measured and averaged over the three stimulation sets. To examine changes in the excitability of corticospinal pathway and account for potential changes within the muscle, MEPs were normalized to Mmax (22, 23).
Two-way mixed-model ANOVAs (group × work rate and group × time) were performed for the comparison of leg blood flow during the maximal dynamic knee extension exercise test and the Tlim trial, respectively. Additionally, two-way mixed-model ANOVAs were performed to asses changes in neuromuscular function (MVC, Qtw, and voluntary activation) and second-by-second cardiovascular changes during isometric exercise. The area under the curve was analyzed for the comparison of cardiovascular changes during isometric exercise. A priori statistical comparisons were performed between the HFpEF and control groups for both the absolute and relative and work rate conditions. Group differences for descriptive characteristics, pre- to postexercise changes in neuromuscular function (MVC, Qtw, voluntary activation, maximal rate of torque development, and peak relaxation rate), corticospinal excitability (Mmax, normalized MEP), blood flow mean response time, and end-exercise metabolic and ventilatory data were determined by independent group t tests when normality was met or Mann-Whitney U tests when normality was not met. The correlation between fatigability (e.g., ΔQtw/kJ) and blood flow mean response time was assessed via a Pearson product-moment correlation. α was set at 0.05. All data are reported as means ± SD.
RESULTS
Baseline Neuromuscular and Cardiovascular Function
Baseline neuromuscular function was not different between experimental days [i.e., protocol A (dynamic knee extension) and protocol B (isometric knee extension)] in the control (MVC: ∼154 Nm, P = 0.76; Qtw: ∼42 Nm, P = 0.98; voluntary activation: ∼98%, P = 0.17; maximal rate of torque development: ∼1,000 Nm·s, P = 0.46; peak relaxation rate: ∼395 Nm·s, P = 0.74) or HFpEF group (MVC: ∼144 Nm, P = 0.99; Qtw: ∼42 Nm, P = 0.49; voluntary activation: ∼94%, P = 0.97; maximal rate of torque development: ∼900 Nm·s, P = 0.58; peak relaxation rate: ∼360 Nm·s, P = 0.53). Although resting MVC, Qtw, maximal rate of torque development, and peak relaxation rate were not different between groups (P > 0.38), baseline voluntary activation was significantly lower in the patients with HFpEF compared with controls (P < 0.05).
Baseline cardiovascular variables were not different between experimental days in the control (HR: ∼68 beats/min, P = 0.69; SV: ∼83 mL, P = 0.80; CO: ∼5.5 L/min, P = 0.97; MAP: ∼94 mmHg, P = 0.11) or HFpEF group (HR: ∼74 beats/min, P = 0.32; SV: ∼82 mL, P = 0.16; CO: ∼6.0 L/min P = 0.16; MAP: ∼99 mmHg, P = 0.93). Additionally, there were no significant group effects for any cardiovascular variable at baseline (HR: P = 0.16; SV: P = 0.31; CO: P = 0.89; MAP: P = 0.16).
Protocol A: Dynamic Single-Leg Knee Extension Exercise
Incremental exercise test.
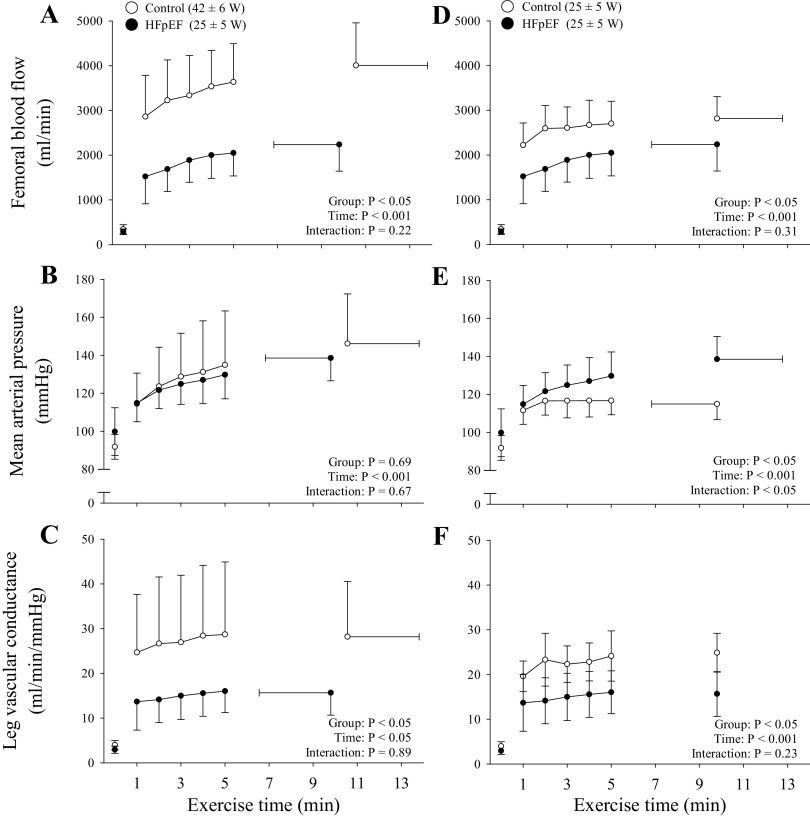
At baseline, femoral blood flow (296 ± 74 vs. 262 ± 99 mL/min, P = 0.44) and leg vascular conductance (3 ± 1 vs. 3 ± 1 mL·min−1·mmHg−1; P = 0.34) were not different between controls and patients with HFpEF. However, femoral blood flow and leg vascular conductance were lower in the patients with HFpEF compared with controls (group, F1,14 > 11.4, P < 0.05; time, F1,14 > 16.6, P < 0.001). Specifically, the patients with HFpEF exhibited a significantly attenuated femoral blood flow compared with controls at 0 W (1,408 ± 229 vs. 1,061 ± 296 mL/min), 10 W (1,752 ± 203 vs. 1,299 ± 412 mL/min), and 20 W (2,166 ± 282 vs. 1,560 ± 486 mL/min). Leg vascular conductance was also attenuated in HFpEF compared with controls at 10 W (16 ± 2 vs. 12 ± 3 mL·min−1·mmHg−1, P < 0.05), and 20 W (19 ± 2 vs. 13 ± 4 mL·min−1·mmHg−1, P < 0.05). Finally, Wpeak was ∼42% lower in patients with HFpEF (31 ± 8 W) compared with the controls (53 ± 6 W, P < 0.01). Consequently, VE (62 ± 13 vs. 41 ± 17 L/min), V̇o2 (1.2 ± 0.2 vs. 0.9 ± 0.2 L/min), and V̇co2 (1.5 ± 0.3 vs. 1.0 ± 0.1 L/min) were significantly lower at the end of the incremental knee extension exercise test in HFpEF compared with controls.
Exercise at the same relative intensity to task failure.
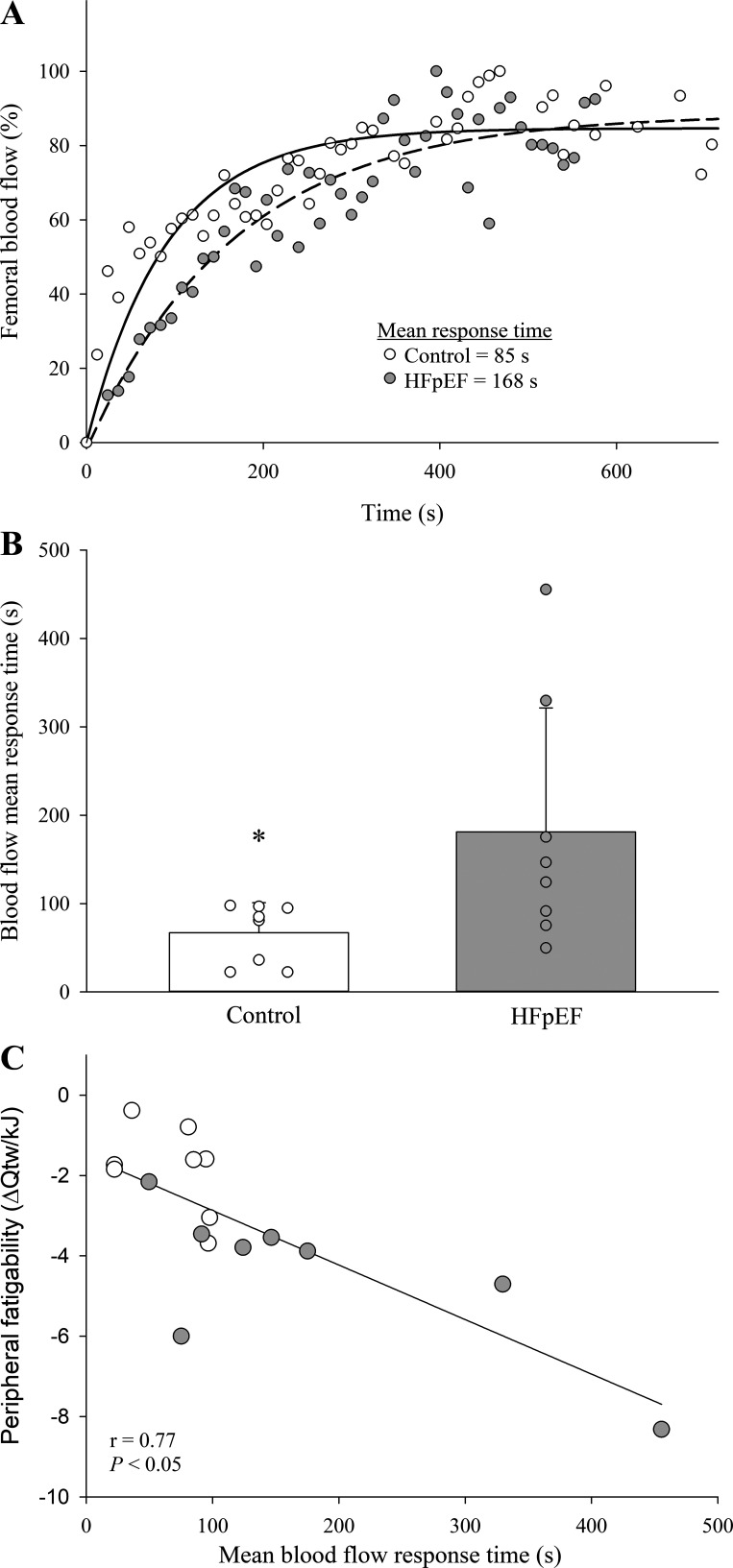
Calculated coefficients of variation for the time-to-task failure during dynamic knee extension were 8 and 12% in the controls and the patients with HFpEF, respectively. Whereas the control group completed more total work during the Tlim trial (29 ± 11 kJ vs. 15 ± 8 kJ, P < 0.05), endurance time-to-task failure was not different from the patients with HFpEF (control: 668 ± 198 s vs. HFpEF: 606 ± 209 s; P = 0.55). VL EMG progressively increased to task failure; however, the increase in EMG (∼100%, P = 0.93) was not different between the controls and patients with HFpEF. During dynamic knee extension at the same relative exercise intensity, femoral blood flow (group, F1,14 = 28.4, P < 0.001; time, F1,14 = 17.3, P < 0.05) and leg vascular conductance (group, F1,14 = 4.85, P < 0.05; time, F1,14 = 3.90, P < 0.05) were lower at each time point in HFpEF compared with controls (Fig. 2, A and C). HFpEF patients also displayed a longer femoral blood flow mean response time compared with the controls (Fig. 3). Additional cardiovascular and metabolic data collected during dynamic knee extension are presented in Table 3.
Figure 2.
Femoral blood flow (A and D), mean arterial pressure (B and E), and leg vascular conductance (C and F) in control participants (n = 8) and patients with heart failure with preserved ejection fraction (HFpEF; n = 8) during dynamic knee extension exercise at the same relative intensity (A–C) and the same absolute (D–F) work rate. Main effects were determined via 2-way mixed-model ANOVAs (group × time). Data are presented as means ± SD.
Figure 3.
A and B: representative (A) and group mean (B) data for the femoral blood flow mean response time in healthy controls (n = 8) and patients with heart failure with preserved ejection fraction (HFpEF; n = 8). C: correlation between peripheral fatigability [i.e., change in quadriceps twitch torque (Qtw) from baseline per unit of work] and the mean response time of femoral blood flow for these participants. A Mann-Whitney U test was utilized to determine differences between groups (B), and a Pearson product-moment correlation was used to assess the correlation between the mean response time and fatigability (C). Data are presented as means ± SD. *Significantly different from HFpEF, P < 0.05.
Table 3.
Cardiopulmonary and metabolic measures during the final minute of rhythmic knee extension performed at the same relative intensity and the same absolute work rate
| Control (80% Wpeak) | HFpEF (80% Wpeak) | Control (Matched for Work Rate with HFpEF) | |
|---|---|---|---|
| Power output, W | 43 ± 6* | 24 ± 6 | 24 ± 6 |
| Exercise time, min | 11.2 ± 3.4 | 10.1 ± 1.4 | 10.1 ± 1.4 |
| HR, beats/min | 130 ± 13* | 99 ± 20 | 85 ± 9 |
| ΔHR, beats/min | 63 ± 12* | 29 ± 14 | 24 ± 6 |
| SV, mL/beat | 82 ± 31 | 94 ± 24 | 98 ± 23 |
| ΔSV, mL/beat | 1 ± 33 | 5 ± 33 | 2 ± 15 |
| CO, L/min | 10.6 ± 3.2 | 10.2 ± 3.3 | 8.3 ± 1.7 |
| ΔCO, L/min | 5.2 ± 2.6 | 3.1 ± 2.0 | 2.4 ± 1.1 |
| MAP, mmHg | 146 ± 26 | 139 ± 12 | 115 ± 20* |
| ΔMAP, mmHg | 55 ± 25 | 38 ± 17 | 27 ±12 |
| V̇E, L/min | 75 ± 20* | 48 ± 15 | 35 ± 7* |
| V̇o2, L/min | 1.4 ± 0.2* | 1.0 ± 0.2 | 0.8 ± 0.2* |
| V̇o2, kg·mL−1·min−1 | 18.2 ± 2.3* | 9.2 ± 3.1 | 9.4 ± 1.8 |
| V̇co2, L/min | 1.5 ± 0.2* | 1.1 ± 0.2 | 0.8 ± 0.2* |
| RER | 1.07 ± 0.10 | 1.06 ± 0.05 | 0.97 ± 0.08* |
| V̇E/V̇o2 | 50 ± 12 | 42 ± 8 | 40 ± 8 |
| V̇E/V̇co2 | 46 ± 9 | 40 ± 5 | 41 ± 6 |
| RPE | 10 ± 0 | 10 ± 0 | 4 ± 3* |
Values are means ± SD; n = 8 control and 8 HFpEF. Δ, change from baseline to end-exercise for a given variable; CO, cardiac output; HFpEF, heart failure with preserved ejection fraction; HR, heart rate; MAP, mean arterial pressure; RER, respiratory exchange ratio; RPE, rating of perceived effort; SV, stroke volume; V̇co2, carbon dioxide production; V̇E, minute ventilation; V̇E/V̇co2, ventilatory equivalent for CO2; V̇E/V̇o2, ventilatory equivalent for O2; V̇o2, oxygen consumption; *Significant difference from HFpEF, P < 0.05.
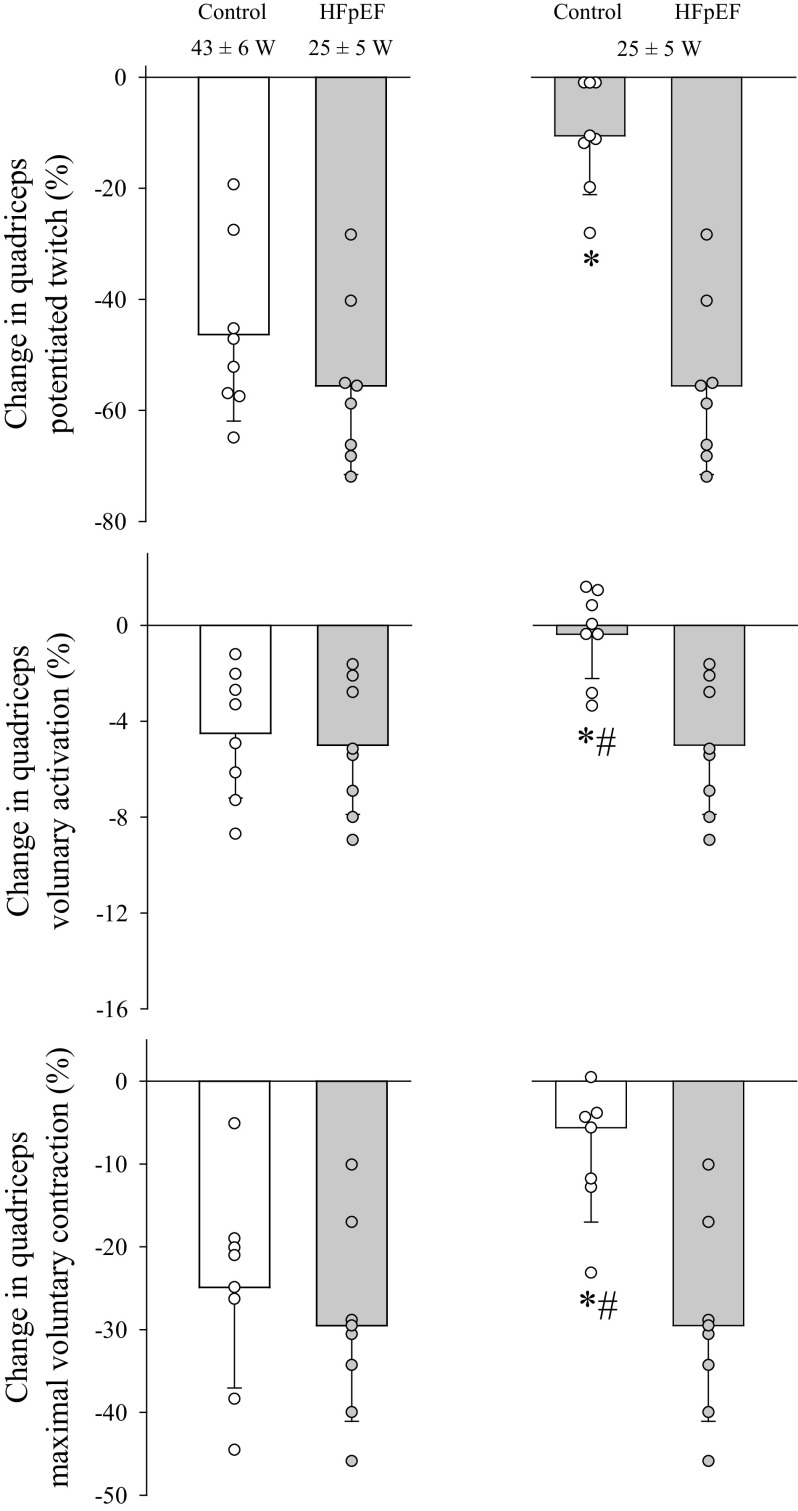
The exercise-induced changes in neuromuscular function due to dynamic knee extension at the same relative exercise intensity to task failure are illustrated in Fig. 4 (left). Considering the amount of work performed, HFpEF demonstrated an approximately threefold greater exercise-induced decrease in Qtw (4.3 ± 1.6 vs. 1.6 ± 0.8 Nm/kJ, P < 0.05) and voluntary activation (0.4 ± 0.3 vs. 0.2 ± 0.1%/kJ, P < 0.05) per unit of work compared with controls. The change in Qtw per unit of work was significantly correlated with the mean response time for femoral blood flow (r = 0 .77; Fig. 3). Additionally, maximal rate of torque development (−67 ± 11 vs. −47 ± 17%, P < 0.05) and peak relaxation rate (−61 ± 14 vs. −37 ± 11%, P < 0.05) were diminished to a greater extent in the patients with HFpEF compared with the controls. Pre- and postexercise Mmax were not different between the patients with HFpEF (29 ± 9 vs. 30 ± 9 μV·s, P = 0.31) and controls (31 ± 6 vs. 31 ± 5 μV·s, P = 0.82). MEPs (normalized to Mmax) remained unchanged from pre- to postexercise in patients with HFpEF (31 ± 6 vs. 30 ± 5%, P = 0.15) and controls (35 ± 5 vs. 33 ± 5%, P = 0.72).
Figure 4.
Pre- to postexercise changes in neuromuscular function in control (open bars; n = 8) and patients with heart failure with preserved ejection fraction (HFpEF; filled bars; n = 8) performed at a given relative exercise intensity (left) or absolute work rate (right). Group differences were determined by independent group t tests. Data are presented as means ± SD. #Not significantly changed from preexercise, P > 0.2; *significantly different from HFpEF, P < 0.05.
Exercise at the same absolute work rate and for the same duration.
By design, for this comparison, knee extension exercise time and work rate were matched for the HFpEF and control groups. VL EMG was not different from the first minute of exercise to the end of exercise in controls (P = 0.69), whereas it increased by 95 ± 26% (P < 0.05) during exercise in the patients with HFpEF. Femoral blood flow was consistently ∼30% lower in the patients with HFpEF than the controls (time, F1,14 = 19.06, P < 0.001; group, F1,14 = 7.2, P < 0.05; Fig. 2D). Furthermore, leg vascular conductance was consistently ∼35% lower in HFpEF compared with controls (time, F1,14 = 10.35, P < 0.05; group, F1,14 = 7.82, P < 0.05; Fig. 2E). Additional cardiovascular and metabolic data collected during dynamic knee extension are presented in Table 3.The exercise-induced changes in neuromuscular function due to dynamic knee extension at the same absolute work rate are reflected in Fig. 4 (right). The exercise-induced decrease in Qtw (4.3 ± 1.6 vs. 0.7 ± 0.6 Nm/kJ, P < 0.05) and voluntary activation (0.4 ± 0.3 %/kJ vs. 0.1 ± 0.1%/kJ, P < 0.05) per unit of work was greater in the patients with HFpEF compared with the controls. The greater degree of peripheral fatigue in HFpEF was also reflected in the pre- to postexercise change in the within-twitch variables, maximal rate of torque development (−67 ± 11 vs. −12 ± 11%, P < 0.05), and peak relaxation rate (−61 ± 14 vs. −8 ± 14%, P < 0.05), both of which fell to a lesser extent in the controls. Pre- and postexercise Mmax were similar in the patients with HFpEF (29 ± 9 vs. 30 ± 9 μV·s, P = 0.31) and the controls (37 ± 3 s vs. 37 ± 3 μV·s, P = 0.65). Additionally, normalized MEPs (normalized to Mmax) remained unchanged from pre- to postexercise in the patients with HFpEF (31 ± 6 vs. 30 ± 5%, P = 0.15) and the controls (35 ± 5 vs. 35 ± 7 mV, P = 0.95).
Protocol B: Maximal Isometric Single-Leg Knee Extension
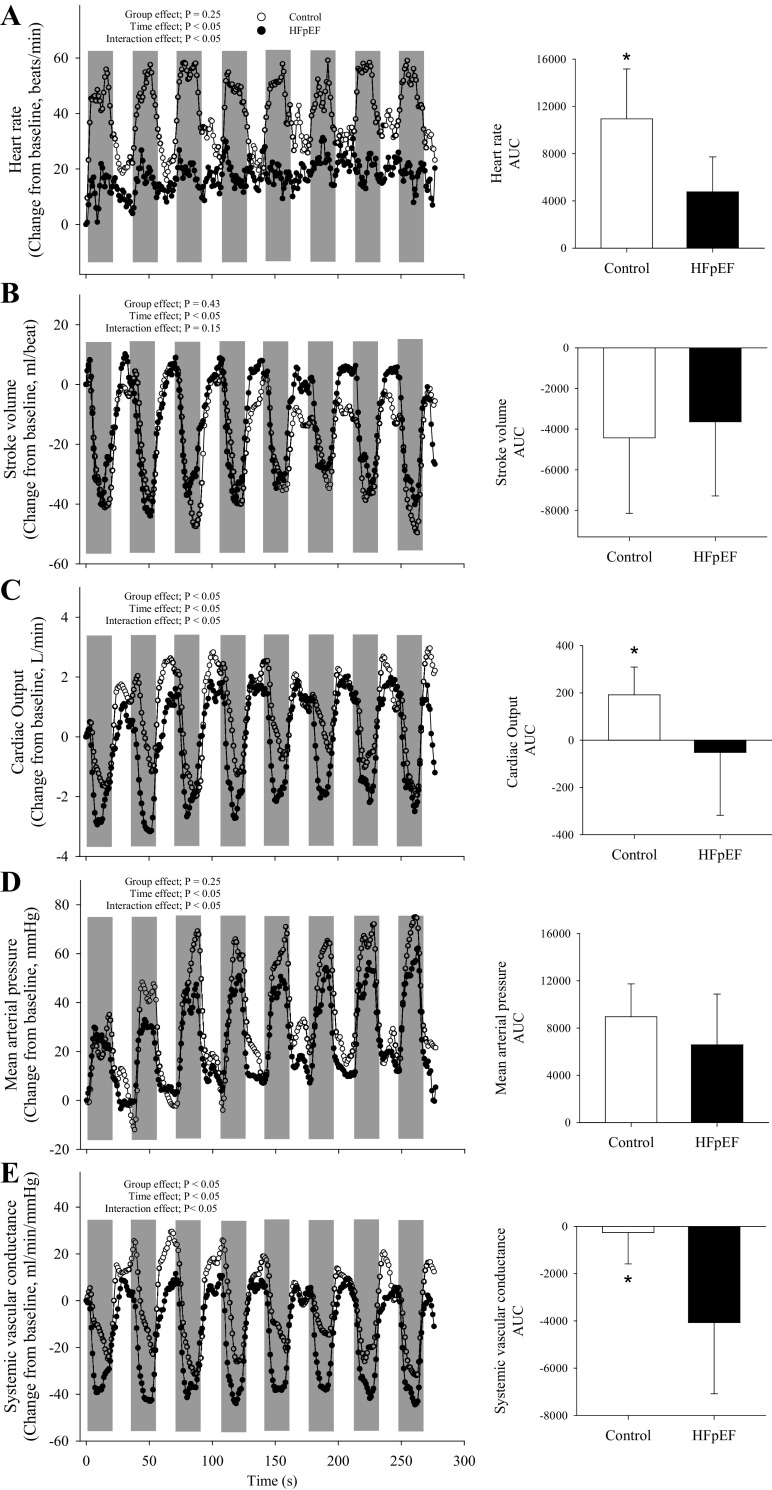
The cardiovascular responses to the isometric protocol are illustrated in Fig. 5. MVC torque at the beginning of exercise was not different between groups and progressively declined throughout the protocol. There was a significant interaction between MVC (interaction, F1,13 = 3.25, P < 0.05) and the number of contractions due to the greater fall in MVC torque impulse in the patients with HFpEF compared with the controls (Fig. 6E). Furthermore, Qtw was not different between groups at the beginning of exercise, but fell to a greater extent across contractions in the patients with HFpEF compared with the controls (interaction, F1,14 = 2.62, P < 0.05; Fig. 6A). In contrast, voluntary activation was not different throughout the protocol in both groups (group, F1,14 = 3.53, P = 0.08; time, F1,14 = 0.55, P = 0.79; interaction, F1,14 = 1.12, P = 0.36; Fig. 6C). Finally, maximal rate of torque development (−53 ± 17 vs. −27 ± 17%, P < 0.01) and peak relaxation rate (−51 ± 17 vs. −26 ± 20%, P < 0.05) were impaired to a greater extent in the patients with HFpEF compared with controls.
Figure 5.
Second-by-second plots of heart rate (A), stroke volume (B), cardiac output (C), mean arterial pressure (D), and systemic vascular conductance (E) for controls (open bars; n = 8) and patients with heart failure with preserved ejection fraction (HFpEF; filled bars; n = 8). Shaded regions represent the maximal 15-s isometric contraction. Main effects were determined via 2-way mixed-model ANOVAs (group × time); independent group t tests were used to determined group differences for the area under the curve for each variable (A–E; presented in bar graph at the right). Data are presented as means ± SD, except that the SD was omitted in the across-time figures for clarity. *Significant difference between groups, P < 0.05.
Figure 6.
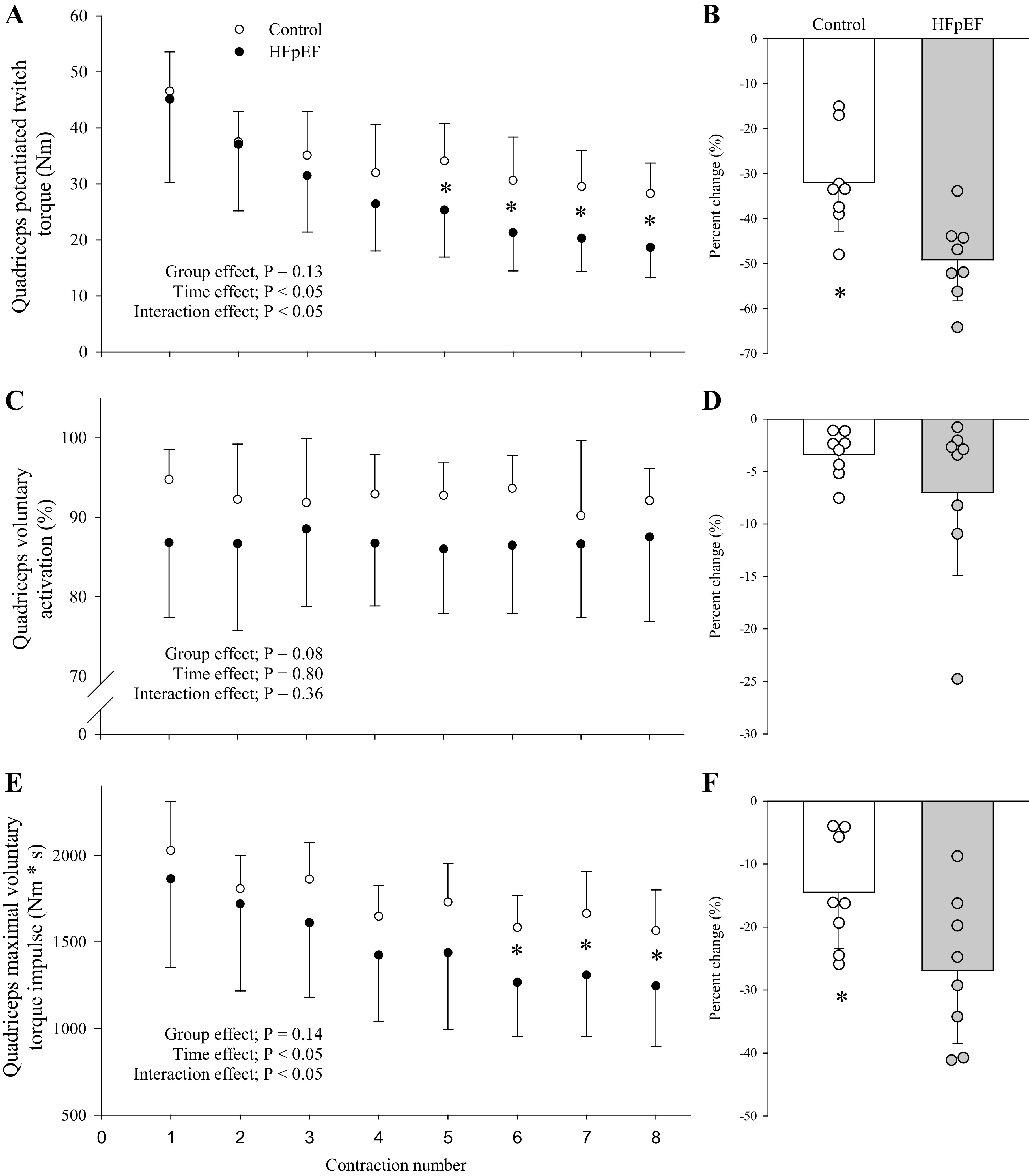
Development of central and peripheral fatigue during the intermittent isometric exercise protocol (A, C, and E) and the ensuing pre- to postexercise change (B, D, and F) in neuromuscular function for controls (n = 8) and patients with heart failure with preserved ejection fraction (HFpEF; n = 8). Main effects were determined via 2-way mixed-model ANOVAs (group × time) for A, C, and E; independent group t tests were used to assess group differences in the pre- to postexercise change in neuromuscular function (B, D, and F). Data are presented as means ± SD. *Significant difference between groups, P < 0.05.
DISCUSSION
This study sought to quantify the impact of HFpEF on the development of neuromuscular fatigue and the peripheral hemodynamic response to exercise involving only a small muscle mass and minimally taxing the heart. The primary findings were threefold. 1) During exercise performed at the same relative intensity, patients with HFpEF developed an equivalent degree of end-exercise peripheral and central fatigue as the healthy controls, indicating similar fatigue mechanisms associated with task failure between groups; 2) however, when exercise was performed at the same absolute intensity, patients with HFpEF demonstrated a much greater susceptibility to both central and peripheral fatigue, which is a key cause of functional impairment; and 3) a major mechanism underlying this functional impairment appears to be deranged peripheral hemodynamics, including a reduced leg blood flow and vascular conductance. Taken together, these findings reveal that patients with HFpEF are characterized by an increased susceptibility to neuromuscular fatigue during work commensurate with activities of daily living and that a compromised peripheral hemodynamic response to exercise likely accounts, at least in part, for this impairment. Given the role of neuromuscular fatigue as a factor that limits exercise, this disease-related impairment accounts, at least in part, for the exercise intolerance characterizing patients with HFpEF.
Dynamic Single-Leg Knee Extension Exercise
Time-to-task failure during dynamic knee extension exercise performed at the same relative exercise intensity (80% Wpeak) was not different in the patients with HFpEF and controls and resulted in an equivalent magnitude of end-exercise central and peripheral fatigue (Fig. 4). These similarities were evident despite the substantially lower work rate performed by the patients with HFpEF (24 vs. 43 W) and, therefore, indirectly indicate a greater susceptibility to fatigue during exercise at the same external load and functional demands in the patients. The additional experiments, in which both groups performed the same work (i.e., ∼24 W for ∼10 min), further highlighted this impairment and revealed a three- to fourfold greater degree of end-exercise peripheral fatigue in the patients with HFpEF compared with controls. Because fatigue is considered a major factor responsible for limiting physical activity (15), the previously documented exercise intolerance in patients with HFpEF (3–5) may, therefore, at least partially, be explained by a greater susceptibility to neuromuscular fatigue in this population.
During dynamic exercise, oxygen supply to skeletal muscle is a significant determinant of endurance capacity and the development of peripheral fatigue (13), with oxygen supply being limited by both central (i.e., cardiac output) and peripheral (i.e., blood flow and arterial oxygen content) hemodynamic factors (31). Because this study aimed to examine a potential link between peripheral hemodynamic abnormalities and neuromuscular fatigue, single-leg dynamic knee extension exercise, which is not limited by cardiac output (21), was selected as the exercise modality. Indeed, by utilizing this approach, cardiac output during dynamic knee extension exercise at the same absolute work rate was not different between the patients with HFpEF and the controls (Table 3). However, femoral blood flow and leg vascular conductance were, at any given work rate, significantly attenuated in the patients compared with the controls (Fig. 2, right). This observation corroborates the only other study examining the impact of HFpEF on limb blood flow during exercise (12) and suggests, given the similar hemoglobin concentration and arterial oxygen saturation (21) between groups (Table 1), that convective O2 supply was lower in the patients. Furthermore, and novel to the current investigation, HFpEF patients also demonstrated, compared with controls, a slower mean response time for blood flow (Fig. 3). Although little is currently known about the underlying mechanisms determining these hemodynamic abnormalities in HFpEF, an exaggerated exercise pressor reflex (32, 33), resulting in higher muscle sympathetic nervous activity, may play a role in this apparent impairment.
The compromised convective O2 supply was likely a key determinant (13) of the increased susceptibility to peripheral fatigue in HFpEF compared with controls. Specifically, compromised convective O2 supply during exercise at a given workload increases the reliance on nonoxidative sources for energy production and facilitates the accumulation of intramuscular metabolites (34) known to cause peripheral fatigue (i.e., Pi and H+) (35, 36). Indeed, a recent study utilizing 31P-MRS and plantar flexion exercise reported that the exercise-induced accumulation of these fatigue metabolites occurs at a greater rate in HFpEF patients compared with healthy controls (37). The significant negative correlation between the mean response time for blood flow and peripheral fatigue in the present study (Fig. 3) further supports the hypothesis suggesting convective O2 supply limitation as a key determinant of the heightened susceptibility to fatigue in patients with HFpEF. However, other disease-related changes in muscle characteristics, such as the shift from fatigue-resistant oxidative skeletal muscle toward fatigue-prone glycolytic muscle (4), the slower O2 uptake kinetics (38), and impaired muscle O2 diffusion (9, 10) and utilization (11), potentially also contributed to the difference in the susceptibility to peripheral fatigue in HFpEF and controls.
Patients with HFpEF also demonstrated a greater susceptibility to central fatigue. Although it is plausible that the disease itself may have a direct impact on various central fatigue-related processes within the central nervous system, it is also likely that the neural feedback from the exercising quadriceps muscle plays a key role in this observation. Specifically, group III/IV muscle afferents, which respond to mechanical stress and metabolic perturbations within exercising muscle (39), project to various sites within the central nervous system (29, 40), facilitate the development of central fatigue (17, 29), and limit aerobic exercise performance (41). Given the tight relationship between intramuscular metabolic perturbation and peripheral fatigue (35), group III/IV muscle afferent feedback was likely augmented in the patients (characterized by greater peripheral fatigue) compared with the controls, which may, at least in part, account for the substantially greater degree of end-exercise central fatigue (29).
Although the exact relevance of corticospinal excitability as a contributor to central fatigue remains to be determined (17, 29), it has been suggested that changes in corticospinal excitability can influence the amount of neural input from higher brain areas to skeletal muscle and, therefore, alter voluntary movements and muscle activation (19). The current study examined the impact of HFpEF on exercise-induced changes in the efficacy of the central motor pathway to relay neural signals. However, since single-joint exercise had no effect on the net excitability of the corticospinal pathway in either the patients with HFpEF or the controls, this mechanism can be excluded as a potential cause of the greater degree of end-exercise central fatigue in the patients.
Isometric Single-Leg Knee Extension
Because the development of fatigue during exercise is highly task specific (42), this study evaluated whether the compromised fatigue resistance observed in the patients with HFpEF during the dynamic exercise is also evident during a different form of small muscle mass exercise, i.e., maximal, intermittent, isometric quadriceps contractions. Interestingly, in contrast to the difference in maximal work rate during dynamic knee extension exercise, the initial maximal quadriceps torque-generating capacity was not different between groups. Thus, the isometric protocol began at the same absolute force production and the same relative exercise intensity for the patients with HFpEF and the controls. Although the relative intensity remained, per design, the same during the task (i.e., maximal), the overall torque voluntarily generated throughout the protocol was lower in HFpEF compared with controls (Fig. 6), indirectly indicating a greater susceptibility to fatigue in the patients. Indeed, although voluntary activation remained unchanged throughout the protocol in both groups, the exercise-induced decrease in MVC and Qtw was significantly greater in HFpEF compared with controls (Fig. 6). The invariant voluntary activation during this exercise modality supports previous studies documenting that central fatigue is minimal during maximal, intermittent, isometric exercise in healthy individuals (43) and suggests that HFpEF does not impact this phenomenon. Therefore, the compromised fatigue resistance in the patients with HFpEF during this type of exercise is predominantly accounted for by the exaggerated development of peripheral fatigue.
Interestingly, in contrast to the dynamic knee extension protocol, disease-related differences in the central hemodynamic response to exercise were evident during the isometric protocol. Specifically, whereas cardiac output increased from rest in the controls, there was no such increase in the patients with HFpEF (Fig. 5). Given the known sensitivity to left ventricular afterload in heart failure (44, 45), the patients’ lack of an increase was likely secondary to the substantial fall in systemic vascular conductance, which remained unaltered in the controls (Fig. 5). In addition, potential decreases in β-receptor sensitivity could have blunted the patients’ chronotropic response (46) and also contributed to their compromised cardiac output response. Finally, it should be noted that the assessment of any peripheral hemodynamic differences could not be achieved due to the difficulty of attaining and interpreting accurate blood flow measurements during maximal, intermittent, isometric contractions.
Experimental Considerations and Limitations
An important feature of this investigation was the inclusion of exercise tasks performed at both relative and absolute intensities. Although each modality offers a particular set of information, their combination provides comprehensive insights on the impact of HFpEF on the exercise-induced development of fatigue and fatigue resistance. Specifically, the similar level of neuromuscular fatigue at task failure following the relative-intensity trial in patients and controls (Fig. 4) suggests that HFpEF does not alter the tolerance for fatigue. Combined with the similar time-to-task failure in both groups, this indicates that the patients’ severe exercise intolerance is not simply due to a symptom-limited compromised ability to stress the neuromuscular system. Indeed, exercise is not relinquished before the attainment of a degree of neuromuscular fatigue comparable with that seen in healthy individuals, i.e., the so called “critical threshold of fatigue” (47). However, because these similarities occurred at an ∼40% lower workload in the patients, these observations document the impact of HFpEF on fatigue resistance. The findings from the absolute-intensity trial, characterized by a substantially larger degree of end-exercise fatigue in HFpEF (Fig. 4), reflect the patients’ impaired fatigue response to a given external load and highlight the functional relevance for tasks associated with daily living (e.g., handling a flight of stairs).
Typical of this population, the current HFpEF patients were characterized by an overall lower physical activity and fitness level and exhibited various comorbidities, including diabetes, hypertension, and obesity (Table 2), potential influences that were not present in the controls. Although neither obesity (48) nor leg muscle mass (49) influences the hyperemic response to dynamic exercise, both diabetes (50) and hypertension (51, 52) can blunt peripheral hemodynamics. As such, it cannot be excluded that these factors, whether individually or in combination with HFpEF, may have contributed to the findings of this study. It is, however, important to consider that these comorbidities are not only hallmark characteristics of HFpEF but may be necessary for the etiology of HFpEF itself (53). Although an ideal control group for isolating the impact of HFpEF on neuromuscular function would have presented with some combination of the aforementioned comorbidities, we contend that our comparison with healthy controls encompasses the whole disease of HFpEF, including comorbidities and lifestyle habits that may be crucial for establishing the proinflammatory state leading to the myocardial remodeling characteristic of HFpEF (53). It should be acknowledged that HFpEF patients who qualify (based on safety reasons) and volunteer to participate in an exercise-based study likely represent a specific subgroup of the population, an inevitable fact that limits generalizability to the general HFpEF population.
Finally, patients did not abstain from taking prescribed medications, and, although considered to be optimally medicated, the potential for a drug-related influence on the observed cardiovascular responses and/or fatigue measures cannot be excluded. It could, for example, be argued that β-blockers or calcium-channel blockers may have suppressed cardiac output and, subsequently, the peripheral hemodynamic response to exercise. On the contrary, the vasorelaxant and antiadrenergic effect of angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers might have facilitated vascular conductance and cardiac output (by decreasing afterload). This could have masked the effect of β-blockers on cardiac output and potentially also ameliorated the full impact of HFpEF on the hemodynamic response to exercise. However, the similar cardiac output responses to dynamic knee extension exercise of a given absolute and relative intensity in HFpEF and controls argues against an overall significant drug-related impact. Regardless, since the inclusion of medicated patients is sometimes criticized, it is important to emphasize that this approach is clinically relevant, as it offers insight on the functional impact of optimally treated HFpEF.
Conclusions
Patients with HFpEF are characterized by an increased susceptibility to central and peripheral fatigue during single-joint exercise at a given absolute amount of work, i.e., work commensurate with activities of daily living. The patients’ compromised peripheral hemodynamic response to exercise likely accounts, at least in part, for the attenuated fatigue resistance observed in the HFpEF population. Given the role of neuromuscular fatigue as a factor that limits exercise, this disease-related impairment likely accounts, at least in part, for the exercise intolerance characterizing patients with HFpEF.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grants HL-116579 and HL-139451 and Department of Veterans Affairs Grant E6910-R.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
J.C.W. and M.A. conceived and designed research; J.C.W., T.S.T., T.J.H., J.R.G., R.S.R., P.K., and J.N.N. performed experiments; J.C.W., R.M.B., and A.D.B. analyzed data; J.C.W., M.A., R.M.B., and A.D.B. interpreted results of experiments; J.C.W. and M.A. prepared figures; J.C.W. and M.A. drafted manuscript; J.C.W., M.A., T.S.T., T.J.H., J.R.G., P.K., R.M.B., A.D.B., J.N.N., and R.S.R. edited and revised manuscript; J.C.W., M.A., T.S.T., T.J.H., J.R.G., P.K., R.M.B., A.D.B., J.N.N., and R.S.R. approved final version of manuscript.
REFERENCES
- 1.Owan TE, Hodge DO, Herges RM, Jacobsen SJ, Roger VL, Redfield MM. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med 355: 251–259, 2006. doi: 10.1056/NEJMoa052256. [DOI] [PubMed] [Google Scholar]
- 2.Reddy YN, Borlaug BA. Heart failure with preserved ejection fraction. Curr Probl Cardiol 41: 145–188, 2016. doi: 10.1016/j.cpcardiol.2015.12.002. [DOI] [PubMed] [Google Scholar]
- 3.Borlaug BA, Melenovsky V, Russell SD, Kessler K, Pacak K, Becker LC, Kass DA. Impaired chronotropic and vasodilator reserves limit exercise capacity in patients with heart failure and a preserved ejection fraction. Circulation 114: 2138–2147, 2006. doi: 10.1161/CIRCULATIONAHA.106.632745. [DOI] [PubMed] [Google Scholar]
- 4.Kitzman DW, Nicklas B, Kraus WE, Lyles MF, Eggebeen J, Morgan TM, Haykowsky M. Skeletal muscle abnormalities and exercise intolerance in older patients with heart failure and preserved ejection fraction. Am J Physiol Heart Circ Physiol 306: H1364–H1370, 2014. doi: 10.1152/ajpheart.00004.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Parthasarathy HK, Pieske B, Weisskopf M, Andrews CD, Brunel P, Struthers AD, MacDonald TM. A randomized, double-blind, placebo-controlled study to determine the effects of valsartan on exercise time in patients with symptomatic heart failure with preserved ejection fraction. Eur J Heart Fail 11: 980–989, 2009. doi: 10.1093/eurjhf/hfp120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Poole DC, Richardson RS, Haykowsky MJ, Hirai DM, Musch TI. Exercise limitations in heart failure with reduced and preserved ejection fraction. J Appl Physiol (1985) 124: 208–224, 2018. doi: 10.1152/japplphysiol.00747.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wilson JR, Mancini DM, Dunkman WB. Exertional fatigue due to skeletal muscle dysfunction in patients with heart failure. Circulation 87: 470–475, 1993. doi: 10.1161/01.CIR.87.2.470. [DOI] [PubMed] [Google Scholar]
- 8.Haykowsky MJ, Brubaker PH, John JM, Stewart KP, Morgan TM, Kitzman DW. Determinants of exercise intolerance in elderly heart failure patients with preserved ejection fraction. J Am Coll Cardiol 58: 265–274, 2011. doi: 10.1016/j.jacc.2011.02.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bhella PS, Prasad A, Heinicke K, Hastings JL, Arbab-Zadeh A, Adams-Huet B, Pacini EL, Shibata S, Palmer MD, Newcomer BR, Levine BD. Abnormal haemodynamic response to exercise in heart failure with preserved ejection fraction. Eur J Heart Fail 13: 1296–1304, 2011. doi: 10.1093/eurjhf/hfr133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dhakal BP, Malhotra R, Murphy RM, Pappagianopoulos PP, Baggish AL, Weiner RB, Houstis NE, Eisman AS, Hough SS, Gd L. Mechanisms of exercise intolerance in heart failure with preserved ejection fraction: the role of abnormal peripheral oxygen extraction. Circ Heart Fail 8: 286–294, 2015. doi: 10.1161/CIRCHEARTFAILURE.114.001825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kitzman DW, Little WC, Brubaker PH, Anderson RT, Hundley WG, Marburger CT, Brosnihan B, Morgan TM, Stewart KP. Pathophysiological characterization of isolated diastolic heart failure in comparison to systolic heart failure. JAMA 288: 2144–2150, 2002. doi: 10.1001/jama.288.17.2144. [DOI] [PubMed] [Google Scholar]
- 12.Lee JF, Barrett-O'Keefe Z, Nelson AD, Garten RS, Ryan JJ, Nativi-Nicolau JN, Richardson RS, Wray DW. Impaired skeletal muscle vasodilation during exercise in heart failure with preserved ejection fraction. Int J Cardiol 211: 14–21, 2016. doi: 10.1016/j.ijcard.2016.02.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Amann M, Calbet JA. Convective oxygen transport and fatigue. J Appl Physiol (1985) 104: 861–870, 2008. doi: 10.1152/japplphysiol.01008.2007. [DOI] [PubMed] [Google Scholar]
- 14.Gandevia SC Spinal and supraspinal factors in human muscle fatigue. Physiol Rev 81: 1725–1789, 2001. doi: 10.1152/physrev.2001.81.4.1725. [DOI] [PubMed] [Google Scholar]
- 15.Amann M, Dempsey JA. Locomotor muscle fatigue modifies central motor drive in healthy humans and imposes a limitation to exercise performance. J Physiol 586: 161–173, 2008. doi: 10.1113/jphysiol.2007.141838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gagnon P, Saey D, Vivodtzev I, Laviolette L, Mainguy V, Milot J, Provencher S, Maltais F. Impact of preinduced quadriceps fatigue on exercise response in chronic obstructive pulmonary disease and healthy subjects. J Appl Physiol (1985) 107: 832–840, 2009. doi: 10.1152/japplphysiol.91546.2008. [DOI] [PubMed] [Google Scholar]
- 17.Taylor JL, Amann M, Duchateau J, Meeusen R, Rice CL. Neural contributions to muscle fatigue: from the brain to the muscle and back again. Med Sci Sports Exerc 48: 2294–2306, 2016. doi: 10.1249/MSS.0000000000000923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Merton PA Voluntary strength and fatigue. J Physiol 123: 553–564, 1954. doi: 10.1113/jphysiol.1954.sp005070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Martin PG, Smith JL, Butler JE, Gandevia SC, Taylor JL. Fatigue-sensitive afferents inhibit extensor but not flexor motoneurons in humans. J Neurosci 26: 4796–4802, 2006. doi: 10.1523/JNEUROSCI.5487-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barker AT, Jalinous R, Freeston IL. Non-invasive magnetic stimulation of human motor cortex. Lancet 325: 1106–1107, 1985. doi: 10.1016/S0140-6736(85)92413-4. [DOI] [PubMed] [Google Scholar]
- 21.Esposito F, Mathieu-Costello O, Shabetai R, Wagner PD, Richardson RS. Limited maximal exercise capacity in patients with chronic heart failure partitioning the contributors. J Am Coll Cardiol 55: 1945–1954, 2010. doi: 10.1016/j.jacc.2009.11.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Day BL, Dressler D, Maertens de Noordhout A, Marsden CD, Nakashima K, Rothwell JC, Thompson PD. Electric and magnetic stimulation of human motor cortex: surface EMG and single motor unit responses. J Physiol 412: 449–473, 1989. [Erratum in J Physiol 1990 Nov; 430: 617]. doi: 10.1113/jphysiol.1989.sp017626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rothwell JC, Thompson PD, Day BL, Boyd S, Marsden CD. Stimulation of the human motor cortex through the scalp. Exp Physiol 76: 159–200, 1991. doi: 10.1113/expphysiol.1991.sp003485. [DOI] [PubMed] [Google Scholar]
- 24.Groot HJ, Trinity JD, Layec G, Rossman MJ, Ives SJ, Richardson RS. Perfusion pressure and movement-induced hyperemia: evidence of limited vascular function and vasodilatory reserve with age. Am J Physiol Heart Circ Physiol 304: H610–619, 2013. doi: 10.1152/ajpheart.00656.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cahalin LP, Mathier MA, Semigran MJ, Dec GW, DiSalvo TG. The six-minute walk test predicts peak oxygen uptake and survival in patients with advanced heart failure. Chest 110: 325–332, 1996. doi: 10.1378/chest.110.2.325. [DOI] [PubMed] [Google Scholar]
- 26.Borg GA Psychophysical bases of perceived exertion. Med Sci Sports Exerc 14: 377–381, 1982. [PubMed] [Google Scholar]
- 27.Weavil JC, Hureau TJ, Thurston TS, Sidhu SK, Garten RS, Nelson AD, McNeil CJ, Richardson RS, Amann M. Impact of age on the development of fatigue during large and small muscle mass exercise. Am J Physiol Regul Integr Comp Physiol 315: R741–R750, 2018. doi: 10.1152/ajpregu.00156.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Weavil JC, Sidhu SK, Mangum TS, Richardson RS, Amann M. Intensity-dependent alterations in the excitability of cortical and spinal projections to the knee extensors during isometric and locomotor exercise. Am J Physiol Regul Integr Comp Physiol 308: R998–1007, 2015. doi: 10.1152/ajpregu.00021.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sidhu SK, Weavil JC, Mangum TS, Jessop JE, Richardson RS, Morgan DE, Amann M. Group III/IV locomotor muscle afferents alter motor cortical and corticospinal excitability and promote central fatigue during cycling exercise. Clin Neurophysiol 128: 44–55, 2017. doi: 10.1016/j.clinph.2016.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fukuba Y, Ohe Y, Miura A, Kitano A, Endo M, Sato H, Miyachi M, Koga S, Fukuda O. Dissociation between the time courses of femoral artery blood flow and pulmonary VO2 during repeated bouts of heavy knee extension exercise in humans. Exp Physiol 89: 243–253, 2004. doi: 10.1113/expphysiol.2003.026609. [DOI] [PubMed] [Google Scholar]
- 31.Saltin B Capacity of blood flow delivery to exercising skeletal muscle in humans. Am J Cardiol 62: 30E–35E, 1988. doi: 10.1016/S0002-9149(88)80007-9. [DOI] [PubMed] [Google Scholar]
- 32.Amann M, Venturelli M, Ives SJ, Morgan DE, Gmelch B, Witman MA, Groot HJ, Walter Wray D, Stehlik J, Richardson RS. Group III/IV muscle afferents impair limb blood in patients with chronic heart failure. Int J Cardiol 174: 368–375, 2014. doi: 10.1016/j.ijcard.2014.04.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Roberto S, Mulliri G, Milia R, Solinas R, Pinna V, Sainas G, Piepoli MF, Crisafulli A. Hemodynamic response to muscle reflex is abnormal in patients with heart failure with preserved ejection fraction. J Appl Physiol (1985) 122: 376–385, 2017. doi: 10.1152/japplphysiol.00645.2016. [DOI] [PubMed] [Google Scholar]
- 34.Hogan MC, Richardson RS, Haseler LJ. Human muscle performance and PCr hydrolysis with varied inspired oxygen fractions: a 31P-MRS study. J Appl Physiol (1985) 86: 1367–1373, 1999. doi: 10.1152/jappl.1999.86.4.1367. [DOI] [PubMed] [Google Scholar]
- 35.Blain GM, Mangum TS, Sidhu SK, Weavil JC, Hureau TJ, Jessop JE, Bledsoe AD, Richardson RS, Amann M. Group III/IV muscle afferents limit the intramuscular metabolic perturbation during whole body exercise in humans. J Physiol 594: 5303–5315, 2016. doi: 10.1113/JP272283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sundberg CW, Hunter SK, Trappe SW, Smith CS, Fitts RH. Effects of elevated H(+) and Pi on the contractile mechanics of skeletal muscle fibres from young and old men: implications for muscle fatigue in humans. J Physiol 596: 3993–4015, 2018. doi: 10.1113/JP276018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Weiss K, Schar M, Panjrath GS, Zhang Y, Sharma K, Bottomley PA, Golozar A, Steinberg A, Gerstenblith G, Russell SD, Weiss RG. Fatigability, exercise intolerance, and abnormal skeletal muscle energetics in heart failure. Circ Heart Fail 10, 2017. doi: 10.1161/CIRCHEARTFAILURE.117.004129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hearon CM Jr, Sarma S, Dias KA, Hieda M, Levine BD. Impaired oxygen uptake kinetics in heart failure with preserved ejection fraction. Heart 105: 1552–1558, 2019. doi: 10.1136/heartjnl-2019-314797. [DOI] [PubMed] [Google Scholar]
- 39.Kaufman MP, Rybicki KJ. Discharge properties of group III and IV muscle afferents: their responses to mechanical and metabolic stimuli. Circ Res 61: I60–65, 1987. [PubMed] [Google Scholar]
- 40.Liu JZ, Shan ZY, Zhang LD, Sahgal V, Brown RW, Yue GH. Human brain activation during sustained and intermittent submaximal fatigue muscle contractions: an FMRI study. J Neurophysiol 90: 300–312, 2003. doi: 10.1152/jn.00821.2002. [DOI] [PubMed] [Google Scholar]
- 41.Hureau TJ, Weavil JC, Thurston TS, Wan HY, Gifford JR, Jessop JE, Buys MJ, Richardson RS, Amann M. Pharmacological attenuation of group III/IV muscle afferents improves endurance performance when oxygen delivery to locomotor muscles is preserved. J Appl Physiol (1985) 127: 1257–1266, 2019. doi: 10.1152/japplphysiol.00490.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Enoka RM Mechanisms of muscle fatigue: central factors and task dependency. J Electromyogr Kinesiol 5: 141–149, 1995. doi: 10.1016/1050-6411(95)00010-W. [DOI] [PubMed] [Google Scholar]
- 43.Callahan DM, Foulis SA, Kent-Braun JA. Age-related fatigue resistance in the knee extensor muscles is specific to contraction mode. Muscle Nerve 39: 692–702, 2009. doi: 10.1002/mus.21278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kitzman DW, Higginbotham MB, Cobb FR, Sheikh KH, Sullivan MJ. Exercise intolerance in patients with heart failure and preserved left ventricular systolic function: failure of the Frank-Starling mechanism. J Am Coll Cardiol 17: 1065–1072, 1991. doi: 10.1016/0735-1097(91)90832-T. [DOI] [PubMed] [Google Scholar]
- 45.Melenovsky V, Hwang SJ, Lin G, Redfield MM, Borlaug BA. Right heart dysfunction in heart failure with preserved ejection fraction. Eur Heart J 35: 3452–3462, 2014. doi: 10.1093/eurheartj/ehu193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sarma S, Stoller D, Hendrix J, Howden E, Lawley J, Livingston S, Adams-Huet B, Holmes C, Goldstein DS, Levine BD. Mechanisms of chronotropic incompetence in heart failure with preserved ejection fraction. Circ Heart Fail 13: e006331, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hureau TJ, Romer LM, Amann M. The ‘‘sensory tolerance limit’: a hypothetical construct determining exercise performance? Eur J Sport Sci 18: 13–24, 2018. doi: 10.1080/17461391.2016.1252428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Limberg JK, De Vita MD, Blain GM, Schrage WG. Muscle blood flow responses to dynamic exercise in young obese humans. J Appl Physiol (1985) 108: 349–355, 2010. doi: 10.1152/japplphysiol.00551.2009. [DOI] [PubMed] [Google Scholar]
- 49.Garten RS, Groot HJ, Rossman MJ, Gifford JR, Richardson RS. The role of muscle mass in exercise-induced hyperemia. J Appl Physiol (1985) 116: 1204–1209, 2014. doi: 10.1152/japplphysiol.00103.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kingwell BA, Formosa M, Muhlmann M, Bradley SJ, McConell GK. Type 2 diabetic individuals have impaired leg blood flow responses to exercise: role of endothelium-dependent vasodilation. Diabetes Care 26: 899–904, 2003. doi: 10.2337/diacare.26.3.899. [DOI] [PubMed] [Google Scholar]
- 51.Nyberg M, Jensen LG, Thaning P, Hellsten Y, Mortensen SP. Role of nitric oxide and prostanoids in the regulation of leg blood flow and blood pressure in humans with essential hypertension: effect of high-intensity aerobic training. J Physiol 590: 1481–1494, 2012. doi: 10.1113/jphysiol.2011.225136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sidhu SK, Weavil JC, Rossman MJ, Jessop JE, Bledsoe AD, Buys MJ, Supiano MS, Richardson RS, Amann M. Exercise pressor reflex contributes to the cardiovascular abnormalities characterizing: hypertensive humans during exercise. Hypertension 74: 1468–1475, 2019. doi: 10.1161/HYPERTENSIONAHA.119.13366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Paulus WJ, Tschope C. A novel paradigm for heart failure with preserved ejection fraction: comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J Am Coll Cardiol 62: 263–271, 2013. doi: 10.1016/j.jacc.2013.02.092. [DOI] [PubMed] [Google Scholar]