ABSTRACT
Background:
Pediatric anxiety and restlessness may create issues and difficulties in performing accurate diagnostic studies even noninvasive ones, such as radiological imaging. There are some agents that will help to get this goal. This study aimed to compare the intranasal effect of dexmedetomidine (DEX) and midazolam (MID) for sedation parameters of children undergoing computerized tomography (CT) imaging.
Materials and Methods:
A double-blind clinical trial was conducted on 162 eligible children who underwent CT imaging. These patients were divided into two groups including MID (n = 81) with dose of 0.3 mg.kg and DEX (n = 81) with dose of 3 μg.kg, which was consumed intranasally. The mean blood pressure (MBP), respiratory rate (RR), heart rate (HR), and oxygen saturation (O2Sat) in children were recorded. Then, time of initiation, level of sedation, and duration effect of medication were measured at 0, 10, 20, and 30 min. Parents and clinician satisfaction score was asked. All data were analyzed using the Statistical Package for the Social Sciences (SPSS) software by t test and chi-square test.
Results:
Decreasing in MBP and HR was higher in DEX group than MID group (P < 0.001), whereas decrease of O2Sat in MID group was higher than DEX group (0.009). Starting time of sedation (22.72 ± 11.64 vs. 33.38 ± 10.17, P = 0.001) was lower in DEX group. Parents (P < 0.001) and physician (P < 0.001) satisfaction score was higher in DEX group than the MID group.
Conclusion:
Using 3 μg/kg intranasal DEX for sedation of 1–6-year-old children was a suitable method to undergo noninvasive studies such as CT imaging. Intranasal DEX is superior to MID due to higher sedation satisfactory, faster starting effect of sedation, and lower side effects and complications. Nevertheless, in children with hemodynamic instability DEX is not an appropriate choice.
KEYWORDS: Computerized tomography imaging, dexmedetomidine, intranasal, midazolam
INTRODUCTION
Pediatric anxiety and restlessness may create issues and difficulties in performing careful and accurate diagnostic studies even noninvasive ones, such as radiological imaging.[1,2,3] During the emergence of general anesthesia, hallucinations, delusions, and confusion revealed by fear, anxiety, agitation, restlessness, and involuntary physical movement caused change in both pre- and postoperative behaviors.[1,3] Postoperative delirium occurs in 12%–18% of all children who undergo general anesthesia.[1] Children do not allow to perform diagnostic procedures correctly due to lack of calmness and excessive movement. It is estimated that the failure rate of diagnostic imaging in children varies from 10% to 20%.[4] In a large prospective study in children aged 4 years old, who underwent magnetic resonance imaging (MRI) or computerized tomography (CT) imaging along with sedation, revealed that sedation was inadequate in 16% of children and diagnostic imaging failed in 7% of time.[4]
Over 65% of the pediatric population experience significant anxiety during the induction of anesthesia for diagnostic studies.[1,3] Sedative medications can be prescribed in several ways such as taking by mouth (PO), trans rectally, intravenous or muscular injections, transmucosal administration (such as intranasal).[5] Intravenous injection is complicated with small and narrow veins, even having skilled personnel as well as higher failure of sedation due to unconscious resistance of children and the anxiety of their parents are the important point in diagnostic procedures for children in the emergency room.[6] Therefore, the process of diagnosing in emergency situations might be significantly delayed.[7] Nevertheless, the goals of the American Academy of Pediatrics (AAP) are to protect patient’s safety, minimize physical, pain, and psychological damage, and control anxiety and behavior of children.[5]
Currently, various medications are used alone or in combination with one another to achieve these goals. However, none of those or mode of administration are completely safe and reliable. The ideal sedative agent should be a medication with a faster starting effect, low side effects, no adverse breathing or hemodynamics effect, adequate length of action, high safety, and rapid recovery.[8,9,10] Therefore, numerous studies compared different agents such as dexmedetomidine (DEX), propofol, pentobarbital, midazolam (MID), chloral hydrate, ketamine, and nitrous oxide alone or in combination with one another.[8,11,12,13,14,15]
MID is one of the most common medications that is being used in pediatrics intranasally. MID is a benzodiazepine family, which contains an imidazole ring. This is a water-soluble agent and quickly absorbed from the rectum, nose, and inner mucosa.[16,17] On the contrary, MID is a potent anticonvulsant, which usually is administered intravenously as well as intramuscularly.[13] Other forms of administration, such as intranasally, rectally, or buccal injection, may be a substitute method for this matter.
DEX is a newer agent that is a selective α-2 agonist and acts as a central agent in brain and spinal cord.[1] Although its primary function is sedation, mediated via inhibition of the locus coeruleus, it also has some analgesic effects mediated at the spinal cord level via inhibition of the release of substance.[18] It also has several different administrative options such as intranasal technique.[11] One of the most important features of DEX is low chance of respiratory depression and little effect on the upper airway morphology.[19] In addition, DEX seems to affect upper airway morphology less than other sedative agents, which may make it an attractive option for pediatric with a history of, airway obstruction during sedation, such as patients with obstructive sleep apnea.[18] Therefore, in children with obstructive sleep apnea, with higher risk of upper airway obstruction during sedation, DEX is a very suitable choice.[5,19]
According to the difference in efficacy and side effect of DEX and MID in sedation, this study aimed to comparing the intranasal injection effect of DEX and MID in sedation parameters in 1–6-year-old children who required CT scan imaging.
MATERIALS AND METHODS
A double-blind clinical trial was conducted in Kashani and Al-Zahra Hospitals, Isfahan University of Medical Science, in Isfahan, Iran between 2107 and 2018. Our study population were all children who underwent CT imaging as diagnostic study and they were referred to emergency department of Kashani and Al-Zahra Hospitals in Isfahan.
The required number of samples for this study was calculated based on sample size formula at 95% confidence level, power study 80%, and according to the results of previous studies (34). Based on these parameters, 162 eligible subjects were the minimum sample size. The eligible samples were recruited based on the convenience sampling method and randomly assigned into two groups (n1 = n2 = 81) by block randomization method [Figure 1]. The block size was 4 and therefore, each block containing four subjects based on the statistical combinations and two subjects assigned to group A (DEX), and two subjects assigned to group B (MID). Informed consent was taken from the parents of all patients and the study protocol was approved by the ethics committee of Isfahan University of Medical Sciences by Ir.mui.rec.1396.2.076 Code.
Figure 1.
CONSORT 2010 flow diagram
Inclusion criteria in our study were children’s age range from 1 to 6 years who need CT imaging and signing the informed consent by the parents. However, exclusion criteria were (1) having an over-the-top sensitivity to benzodiazepines and interleukins and α-agonists, (2) alertness level less than 15 based on Glasgow’s coma benchmark, (3) having weak and unstable vital signs such as instability in blood pressure, and (4) lung diseases.
Two agents including 0.3 mg/kg MID (Exir, Borujerd, Iran) and 3 μg/kg DEX (Hospira, Ohio, USA) prepared daily by a nurse and placed in the sachet by label A or B. Those agents were prescribed interanasally by a 2-cc syringe (G 22, Avapezeshk, Tehran, Iran). After decoding of blinding, it was revealed that group A received nasal doses of DEX and group B received MID, respectively [Figure 1]. Both agents were used by Intranasal Mucosal Atomization Device (IMAD), and its name is MAD Nasal 300 (Teleflex Medical, Morrisville, USA) with a specific dose of 0.3 mg/kg for MID and 3 μg/kg for DEX, which were prescribed intranasal. The injection of medication is carried out with the patient in lying down position. In each nostril of nose, up to 1mL of the medication can be injected. Five minutes after injection of medication on the nostrils, the patient could be placed in sitting position.
Measurements
Patient’s demographic characteristics including age, sex, and past medical history were collected. Baseline measurements were included, for example, BP, respiratory rate (RR), heart rate (HR), and the percentage of oxygen (O2sat). The effect time of sedation, the level of anesthesia (sedation), duration of the effect, and hemodynamic parameters were measured at times 0, 10, 20, and 30 min, respectively. Duration of the sedation effect (beginning of sedation to return to complete consciousness) was calculated and recorded. In addition, at 0, 10, 20, and 30 min after administration of medications, the MBP, RR, HR, and O2 sat were measured in both groups.
The Ramsay Sedation Scale (RSS)[20] was used to measure anesthetic depth after the onset of sedation was initiated. The sedation level was measured at 0, 10, 20, and 30 min after administration. The Ramsay sedation score varied between 0 and 5 and the score is zero = restlessness, 1 = complete awakening, 2 = poor sleepiness, 3 = often sleepy, but wakes up by sound, 4 = sleep, wake up with painful stimulation, and 5 = deep sleep, which does not wake up with painful stimulation. Reaching of anesthesia depth equal to 3 was considered desirable which patient was more relaxed. Patients did not have any movement, did not follow any verbal commands, but wakes up with physical stimulation.
The satisfaction score was asked as level of relaxation during the test to parents and their therapist by a self-rated questionnaire. The satisfactory score varied from (0 = weak, 1 = mild, 2 = moderate, and 3 = good), in which zero was considered as complete dissatisfaction and 3 was as the highest level of satisfaction.
Statistical analysis
The collected data were entered into Statistical Package for the Social Sciences (SPSS) software, version 20 and analyzed by descriptive statistics such as mean, standard deviation, frequency, and percentage of hemodynamic parameter changes. Independent t test was used to compare two group factors such as age, satisfactory score, and the starting effect (between medication injection to appropriate sedation), procedure (time between medication injection to CT imaging), and recovery time. Moreover, chi-square test was used to compare two groups in sex distribution and change in hemodynamic parameters. The significance level was considered as less than 0.05.
RESULTS
A double-blinded clinical study was conducted on 144 patients including 98 boys (68.1%) and 46 girls (31.9%). Our results showed that there was no significant difference between two groups [Table 1] according to sex distribution (P = 0.059), patient age (P = 0.126), and patient weight (P = 0.759).
Table 1.
Comparison of the baseline characteristics of patients in two groups study
| Variables | Dexmedetomidine (n = 78) n(%) | Midazolam (n = 65) n(%) | P Value | |
|---|---|---|---|---|
| Patient gender | Male | 48 (60.8) | 50 (76.9) | 0.059 |
| Female | 31 (39.2) | 15 (23.1) | ||
| Patient age (month) | 27.67 ± 16.37 | 32.17 ± 18.58 | 0.126 | |
| Weight (kg) | 12.97 ± 3.88 | 12.78 ± 3.16 | 0.759 | |
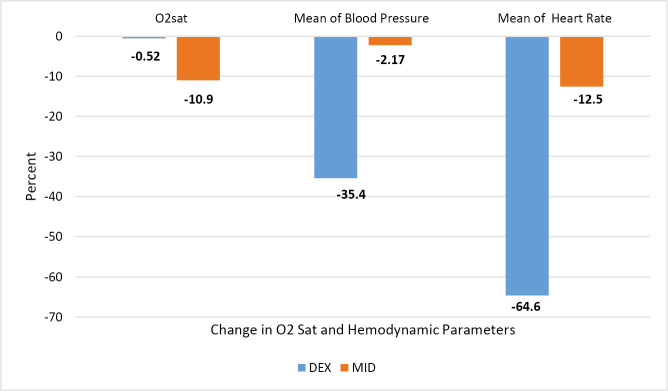
The chi-square test [Table 2] showed that seven patients (10.9%) in MID group have decrease in O2 sat and was different with DEX group (P = 0.009). Figure 2 shows that decrease in mean of blood pressure (more than 10 mm Hg) was occurred in 28 patients (35.4%) in DEX group and was statistically significant with MID group (P < 0.001). Moreover, the HR decrease was higher in DEX group than MID (64.6% vs. 0%, respectively) (P < 0.001).
Table 2.
Change (decrease) in Spo2, and hemodynamic parameters including mean of blood pressure and pulse (heart rate) from baseline to 30th minute after medication administration
| Variables | Dexmedetomidine (n = 78) | Midazolam (n = 65) | P Value |
|---|---|---|---|
| Spo2 | 0 | 7(10.9) | 0.009 |
| Mean of blood pressure (more than 10 mm Hg) | 28(35.4) | 0 | <0.001 |
| Mean of pulse (heart rate) | 51(64.6) | 0 | <0.001 |
Figure 2.
Percent change (decrease) in hemodynamic parameters in 0–30 min after medical administration
Our results [Table 3] revealed that the starting effect for sedation was faster in DEX group (22.72 ± 11.64 vs. 33.38 ± 10.17, P = 0.001). Nevertheless, there was no statistically difference in recovery time between two groups (P = 0.580). The parents and physician satisfaction score was higher in DEX group than the MID group (P < 0.001 in both groups), but there was no significant difference between two groups regarding to the patient cooperation score and was 2.86 ± 0.373 and 2.92 ± 0.311 in DEX and MID groups, respectively (P = 0.067).
Table 3.
Comparison of outcomes of sedation between patients of two groups study
| Variables | Dexmedetomidine (n = 78) | Midazolam (n = 65) | P Value |
|---|---|---|---|
| Starting effect (between medication injection to appropriate sedation) | 22.72 ± 11.64 | 33.38 ± 10.17 | 0.001 |
| Recovery time | 44.63 ± 18.37 | 42.79 ± 8.81 | 0.580 |
| Patient cooperation score | 2.86 ± 0.373 | 2.92 ± 0.311 | 0.067 |
| Parents satisfaction score | 2.95 ± 0.221 | 1.86 ± 0.89 | <0.001 |
| Physician satisfaction score | 2.92 ± 0.311 | 1.69 ± 0.75 | <0.001 |
Side effect and complications
Although 22 patients (15.3%) required repeated dose and remained nonresponsive, changing of medication method was happened in 31 patients (21.5%). Based on chi-square test, a significant difference was observed between two groups regarding to these consequences. The incidence of the repeated dose was 4 patients (5.1%) in DEX group and 18 patients (37.7%) in MID groups (P < 0.001). Moreover, the change of drug due to nonresponse to medication was none in DEX group and 47.7% (31 patients) in MID group (P < 0.001).
DISCUSSION
Our results showed that the mean of sedation was shorter in DEX group than MID group. Moreover, higher decrease in HR and more than 10 mm Hg decrease in MBP were observed in 35.4% of patients in DEX group. DEX has sedative, analgesic, and anxiolytic effects and could decrease overall anesthetic requirements by reducing the sympathetic outflow in response to painful stimulation.[1]
According to starting effect of sedative agents and satisfactory sedation, DEX was superior to MID in premedication of children aged between 1 and 6 years. Other studies showed similar results. Yuen et al.[10] using 1 μg/kg intranasal DEX provided favorable sedation in children aged between 2 and 12 years and the satisfactory sedation rate at the time of agent cannulation was 62% for intranasal DEX. Keles et al.[14] study revealed that oral DEX (2 µg/kg) is related with higher satisfactory sedation and lower emergence delirium as compared to MID (0.5 mg/kg). Berkenbosch et al.[21] study revealed that using 0.5–1.0 µg/kg DEX over 5–10 min produced effective sedation in children undergoing noninvasive procedures. Gahi et al.[1] study showed that intranasal 3 μg/kg DEX is more effective than oral MID (0.5 mg·kg) for sedation of 1–6 years’ children who are undergoing the CT imaging. However, a review study showed that there is no scientific evidence identifying effectiveness of preoperative intranasal DEX, compared with oral MID, for prevention of emergence delirium in pediatric patient population. Moreover, other study showed that patients sedated with DEX caused longer time to discharge from hospital, and total admit time in comparison with sedative agents.[22]
DEX has shorter half-life[1,2] and caused faster starting effect. The effectiveness of DEX in pediatric sedation for undergoing MRI is demonstrated.[11] Moreover, the impact of DEX on respiratory status and airway patency and tone is very less than other sedative agents.[12] However, other studies showed a significant improvement in the quality of CT imaging and MRI performed using general anesthesia than those who were sedated.[4,11,23]
In our study, there was no statistically difference in recovery time and results were the same between two groups. In fact, the target or deeper depth of sedation is varied according to the imaging method (CT imaging vs. MRI and nuclear medicine imaging) as well as the characteristics of the patient.[5,8] In CT imaging, the child’s sedative process is modest due to existence of a modern multifunction scanner and allows the operator to take immediate photographs, whereas to carry out long-term methods, child must sleep, which may take up to 1h of sleep.[8]
Parents and physician satisfaction score was higher in DEX group than the MID group, but there was no significant difference between the two groups regarding to patients’ cooperation. A review study showed that DEX is a promising alternative to MID in procedural sedation usage due to more comforting during the procedure for the patient and clinician with similar safety.[19] However, sedation caused by DEX is very similar to normal sleep, and electroencephalogram (EEG) of patients under sedation has a pattern similar to normal sleep in stages two and three.[11,15,24]
Postanesthetic cognitive and psychomotor behavioral change due to emergence delirium at postoperative might cause a risk of injury for patients and health-care personnel.[1] The main goal of premedication in pediatric anesthesia is decrease of anxiety before and after the procedure and smooth induction of anesthesia. Based on recent studies, anxiety during in medication procedures is related to aggressive reactions, increased distress, postoperative behavioral changes, and postoperative agitation.[2,14,25,26] Therefore, psychological factors are related to lower satisfaction from medication procedures in patients and physicians. Our results showed that using DEX is related with higher satisfaction score in patients and physicians. In addition, administration intranasal medication of DEX increases the success rate of a satisfactory procedure.[12]
There is a significant difference between the two groups in repetition of dose and changing the medication. The repeated dose was needed in 22 patients (15.3%) including four patients (5.1%) in DEX group and 18 patients (37.7%) in MID groups. The change in method due to nonresponse to medication was 0% in DEX group and 47.7% (31 patients) in MID group. Nevertheless, bradycardia and hypotension are the most common reported complication of DEX.[5,19] Moreover, our results showed that the decreasing O2sat was higher in MID group than DEX group. MID is the most common drug which is used for pediatric anesthesia due to some beneficial effects in children including good sedation, fast onset, and limited duration of action and some side effects such as restlessness, decrease in respiratory function, paradoxical reaction, and cognitive impairment.[2] Side effects and complications of three different regimes of MID medication, including oral alone, nasal, and combination of both together, were compared in 650 cases by Gentz et al.[13] study. Its results showed that successful rate of sedations was 80% and the planned treatment was completed in over 85% of cases. It also revealed that nausea and or vomiting, dysphoria, or hiccups were occurred in about 10% of cases. All three regimens were effective with minimal postoperative complications but oral MID alone yielded the best effect. However, in emergency situations, intravenous injection is considered as the most appropriate method for transferring sufficient amounts of benzodiazepines in a short time.[21]
Certainly, due to lack of instrument for intranasal spray, the appropriate response decreased, especially in MID. Moreover, decline in blood pressure and HR is one of the side effects of medication during sedation, especially in DEX and therefore, decision on a therapeutic intervention is difficult for the physicians. Very likely large number of patients in future studies will provide more detailed and defined facts.
CONCLUSION
Using 3 μg/kg intranasal DEX for sedation of 1–6-year-old children was a suitable method in children undergoing noninvasive procedures such as CT imaging. Intranasal DEX was superior to MID regarding to more sedation satisfactory in patients and clinicians and faster starting effect for procedure with lower side effect and complication. Nevertheless, in children with hemodynamic instability DEX is not an appropriate agent. Repeated dose or replacement of sedative agent was lower in DEX than MID group. However, it seems that DEX will produce better sedation and smooth induction of anesthesia than MID for pediatric premedication. Finally, more studies with larger patient pool will be needed to compare the combination of MID and DEX with each of them alone.
Ethical policy and institutional review board statement
Authors confirm that the ethical standards recommended by the Helsinki Declaration have been met.
Financial support and sponsorship
This study was financially supported by Isfahan University of Medical Sciences.
Conflicts of interest
There are no conflicts of interest.
Acknowledgement
The authors are very grateful for children and their parents who participated in study and for research Vic chancellor of Isfahan University of Medical Sciences for financial support.
REFERENCES
- 1.FitzSimons J, Bonanno LS, Pierce S, Badeaux J. Effectiveness of preoperative intranasal dexmedetomidine, compared with oral midazolam, for the prevention of emergence delirium in the pediatric patient undergoing general anesthesia: a systematic review. JBI Database System Rev Implement Rep. 2017;15:1934–51. doi: 10.11124/JBISRIR-2016-003096. [DOI] [PubMed] [Google Scholar]
- 2.Bhat R, Santhosh MC, Annigeri VM, Rao RP. Comparison of intranasal dexmedetomidine and dexmedetomidine-ketamine for premedication in pediatrics patients: a randomized double-blind study. Anesth Essays Res. 2016;10:349–55. doi: 10.4103/0259-1162.172340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bonanno LS, Pierce S, Badeaux J, FitzSimons JJ. Effectiveness of preoperative intranasal dexmedetomidine compared with oral midazolam for the prevention of emergence delirium in pediatric patients undergoing general anesthesia: a systematic review protocol. JBI Database System Rev Implement Rep. 2016;14:70–9. doi: 10.11124/JBISRIR-2016-003059. [DOI] [PubMed] [Google Scholar]
- 4.Malviya S, Voepel-Lewis T, Eldevik OP, Rockwell DT, Wong JH, Tait AR. Sedation and general anaesthesia in children undergoing MRI and CT: adverse events and outcomes. Br J Anaesth. 2000;84:743–8. doi: 10.1093/oxfordjournals.bja.a013586. [DOI] [PubMed] [Google Scholar]
- 5.Coté CJ, Wilson S. American Academy of Pediatrics; American Academy of Pediatric Dentistry. Guidelines for monitoring and management of pediatric patients before, during, and after sedation for diagnostic and therapeutic procedures. Pediatrics. 2016;143(6):e20191000. doi: 10.1542/peds.2019-1000. [DOI] [PubMed] [Google Scholar]
- 6.Bellolio MF, Puls HA, Anderson JL, Gilani WI, Murad MH, Barrionuevo P, et al. Incidence of adverse events in paediatric procedural sedation in the emergency department: a systematic review and meta-analysis. BMJ Open. 2016;6:e011384. doi: 10.1136/bmjopen-2016-011384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ramalho CE, Bretas PMC, Schvartsman C, Reis AG. Sedation and analgesia for procedures in the pediatric emergency room. J Pediatr (Rio J) 2017;93:2–18. doi: 10.1016/j.jped.2017.07.009. [DOI] [PubMed] [Google Scholar]
- 8.Sury M, Bullock I, Rabar S, Demott K Guideline Development Group. Sedation for diagnostic and therapeutic procedures in children and young people: summary of NICE guidance. BMJ. 2010;341:c6819. doi: 10.1136/bmj.c6819. [DOI] [PubMed] [Google Scholar]
- 9.Ghali AM, Mahfouz AK, Al-Bahrani M. Preanesthetic medication in children: a comparison of intranasal dexmedetomidine versus oral midazolam. Saudi J Anaesth. 2011;5:387–91. doi: 10.4103/1658-354X.87268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yuen VM, Hui TW, Irwin MG, Yao TJ, Wong GL, Yuen MK. Optimal timing for the administration of intranasal dexmedetomidine for premedication in children. Anaesthesia. 2010;65:922–9. doi: 10.1111/j.1365-2044.2010.06453.x. [DOI] [PubMed] [Google Scholar]
- 11.Fang H, Yang L, Wang X, Zhu H. Clinical efficacy of dexmedetomidine versus propofol in children undergoing magnetic resonance imaging: a meta-analysis. Int J Clin Exp Med. 2015;8:11881–9. [PMC free article] [PubMed] [Google Scholar]
- 12.Cozzi G, Norbedo S, Barbi E. Intranasal dexmedetomidine for procedural sedation in children, a suitable alternative to chloral hydrate. Paediatr Drugs. 2017;19:107–11. doi: 10.1007/s40272-017-0217-5. [DOI] [PubMed] [Google Scholar]
- 13.Gentz R, Casamassimo P, Amini H, Claman D, Smiley M. Safety and efficacy of 3 pediatric midazolam moderate sedation regimens. Anesth Prog. 2017;64:66–72. doi: 10.2344/anpr-64-02-04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Keles S, Kocaturk O. Comparison of oral dexmedetomidine and midazolam for premedication and emergence delirium in children after dental procedures under general anesthesia: a retrospective study. Drug Des Devel Ther. 2018;12:647–53. doi: 10.2147/DDDT.S163828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reynolds J, Rogers A, Capehart S, Manyang P, Watcha MF. Retrospective comparison of intranasal dexmedetomidine and oral chloral hydrate for sedated auditory brainstem response exams. Hosp Pediatr. 2016;6:166–71. doi: 10.1542/hpeds.2015-0152. [DOI] [PubMed] [Google Scholar]
- 16.Blumer S, Peretz B, Zisman G, Ratson T. Effect of sedation with midazolam and time to discharge among pediatric dental patients. J Clin Pediatr Dent. 2017;41:384–7. doi: 10.17796/1053-4628-41.5.384. [DOI] [PubMed] [Google Scholar]
- 17.Khodadad A, Aflatoonian M, Jalilian R, Babaei N, Motamed F, Ebrahime Soltani A, et al. Comparison of oral midazolam with intravenous midazolam for sedation children during upper gastrointestinal endoscopy. Acta Med Iran. 2016;54:576–82. [PubMed] [Google Scholar]
- 18.Tobias JD, Cravero JP. Procedural sedation for infants, children, and adolescents. Elk Grove Village, IL: American Academy of Pediatrics; 2019. [Google Scholar]
- 19.Barends CR, Absalom A, van Minnen B, Vissink A, Visser A. Dexmedetomidine versus midazolam in procedural sedation. A systematic review of efficacy and safety. Plos One. 2017;12:e0169525. doi: 10.1371/journal.pone.0169525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sessler CN, Grap MJ, Ramsay MA. Evaluating and monitoring analgesia and sedation in the intensive care unit. Crit Care. 2008;12:S2. doi: 10.1186/cc6148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Berkenbosch JW, Wankum PC, Tobias JD. Prospective evaluation of dexmedetomidine for noninvasive procedural sedation in children. Pediatr Crit Care Med. 2005;6:435–9; quiz 440. doi: 10.1097/01.PCC.0000163680.50087.93. [DOI] [PubMed] [Google Scholar]
- 22.Behrle N, Birisci E, Anderson J, Schroeder S, Dalabih A. Intranasal dexmedetomidine as a sedative for pediatric procedural sedation. J Pediatr Pharmacol Ther. 2017;22:4–8. doi: 10.5863/1551-6776-22.1.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ghai B, Jain K, Saxena AK, Bhatia N, Sodhi KS. Comparison of oral midazolam with intranasal dexmedetomidine premedication for children undergoing CT imaging: a randomized, double-blind, and controlled study. Paediatr Anaesth. 2017;27:37–44. doi: 10.1111/pan.13010. [DOI] [PubMed] [Google Scholar]
- 24.Miller JW, Divanovic AA, Hossain MM, Mahmoud MA, Loepke AW. Dosing and efficacy of intranasal dexmedetomidine sedation for pediatric transthoracic echocardiography: a retrospective study. Can J Anaesth. 2016;63:834–41. doi: 10.1007/s12630-016-0617-y. [DOI] [PubMed] [Google Scholar]
- 25.Schmidt AP, Valinetti EA, Bandeira D, Bertacchi MF, Simões CM, Auler JO., Jr Effects of preanesthetic administration of midazolam, clonidine, or dexmedetomidine on postoperative pain and anxiety in children. Paediatr Anaesth. 2007;17:667–74. doi: 10.1111/j.1460-9592.2006.02185.x. [DOI] [PubMed] [Google Scholar]
- 26.Yuen VM, Hui TW, Irwin MG, Yuen MK. A comparison of intranasal dexmedetomidine and oral midazolam for premedication in pediatric anesthesia: a double-blinded randomized controlled trial. Anesth Analg. 2008;106:1715–21. doi: 10.1213/ane.0b013e31816c8929. [DOI] [PubMed] [Google Scholar]