Abstract
Introduction
COVID-19 is severely affecting countries globally and mortality is high. Xuebijing (XBJ) injection is widely used in the treatment of severe pneumonia and sepsis in China due to its anti-inflammatory effect and immunoregulation. This study investigated whether Xuebijing injection can prevent the cytokine storm and reduce the mortality from severe COVID-19.
Methods
This was a randomized, double-blinded trial in which 60 eligible patients were recruited from the First people's Hospital of Jingzhou from February 16 to March 25 in 2020. A total of 57 completed the trial, 3 dropped out. The treatment group received routine medication plus Xuebijing injection while the control group received routine medication plus saline.
Results
The secretion of interleukin-6(IL-6), interleukin-8(IL-8) and tumor necrosis factor-α(TNF-α) was suppressed significantly (P < 0.05) by Xuebijing. After 14 days treatment, lymphocyte levels in Xuebijing group was substantially higher than control, C-reactive protein (CRP) level in Xuebijing group was remarkably lower. The 28-day mortality was not significantly different between the two group. After 14 days of treatment, there were significant differences in the rate of mechanical ventilation, rate of septic shock, the proportion of patients severely affected who became critically ill, the duration of improvement of main clinical symptoms (P < 0.05) and the length of ICU hospitalization stay (P < 0.01) for the Xuebijing group compared with controls. No serious adverse reactions were identified in either group.
Conclusions
This study demonstrates that Xuebijing injection may suppress the cytokine storm in severe COVID-19 patients by regulating the secretion of pro- inflammatory cytokine IL-6, IL-8 and TNF -α. However, Xuebijing did not significantly reduce the 28-day mortality.
Keywords: COVID-19, Xuebijing injection, Cytokine storm, Pro-inflammatory cytokines, Immunomodulation, Randomized controlled trial
1. Introduction
In late December 2019, a lethal pneumonia was reported in Wuhan, China caused by a new severe acute respiratory syndrome coronavirus2 (SARS-CoV-2) variant. The novel virus was subsequently named as Corona Virus Disease 2019(COVID -19) by the World Health Organization [1]. The virus spread so rapidly and widely and as of 18 December 2020, a total of 73 575 202 confirmed cases and 1 656 317 deaths had been reported in 222 countries according to the daily report of the World Health Organization (https://www.who.int/emergencies/diseases/novel-coronavirus-2019) The pandemic continues to spread rapidly and globally. Patients with mild symptoms exhibit a fever and dry cough. The severe cases are more likely to develop dyspnoea, multiple organ dysfunction syndrome (MODS), irreversible coagulation disorders, uncorrectable metabolic acidosis, septic shock and acute respiratory distress syndrome (ARDS) [2], [3], [4]. Overall mortality for COVID-19 patients is about 4% [3,5], then this number reaches up to 60% for critically ill patients in the intensive care unit [6]. COVID-19 can be divided into four types according to the severity as follows: mild, moderate, severe and critical illness [7]. Patients with mild disease can become severe cases in a very short time. It has been reported that about 20% of COVID-19 patients may develop into severe cases [5] and severe COVID-19 patients have a high risk of mortality [2].
Cytokine storm (CS) is very common for severe COVID-19 patients and it means excessive and uncontrolled release of pro-inflammatory cytokines [5]. Inflammatory CS is associated with the progression and development of COVID-19. The level of serum cytokines in COVID-19 patients with ARDS is significantly increased, and it has been reported that the level of serum cytokines is positively correlated with mortality [8]. Accumulating evidence suggests that patients with severe COVID-19 might have a cytokine storm syndrome. A variety of pro-inflammatory cytokines, including interleukin-6(IL-6), interleukin-8(IL-8), and tumor necrosis factor-α(TNF-α), play a critical role in CS. CS can contribute to the development of ARDS which is the leading cause of death in patients with COVID-19 [9,10]. Currently, there is no approved treatment for COVID-19 [11]. Chinese clinical immunologists argue that traditional Chinese medicine and pharmacology, attributing to inhibition of excessive inflammation and immunoregulation, are required for the treatment of COVID-19 [5]. Growing evidence shows some traditional Chinese herbs and medicines may have an immunosuppressive effect [12]. Li et al reported that a Chinese patent medicine named Shuanghuanglian oral liquid combined with western medicine successfully cured three confirmed patients with COVID-19 in Wuhan [13]. Xuebijing (XBJ) injection is an intravenous herbal preparation made from five traditional Chinese medicines which are composed of Radix Paeoniae Rubra, Angelica Sinensis, Chuanxiong, Honghua (Flos Carthami) and Salvia Miltiorrhiza. Based on the clinical effectiveness and safety of XBJ injection in the treatment of infectious diseases such as severe pneumonia and sepsis [14], [15], [16], it was recommended as one of the treatment options for COVID-19 patients in the diagnosis and treatment guidelines which was issued by National Health Commission of the People`s Republic of China [12]. Network pharmacology suggests that XBJ may alleviate COVID-19 patients` inflammatory reaction through regulating some cytokines such as TNF [17]. So we conducted a prospective, randomized, double-blinded trial in order to investigate whether Xuebijing injection can suppress the cytokine storm and reduce the mortality of severe COVID-19 patients.
2. Materials and methods
2.1. Patients
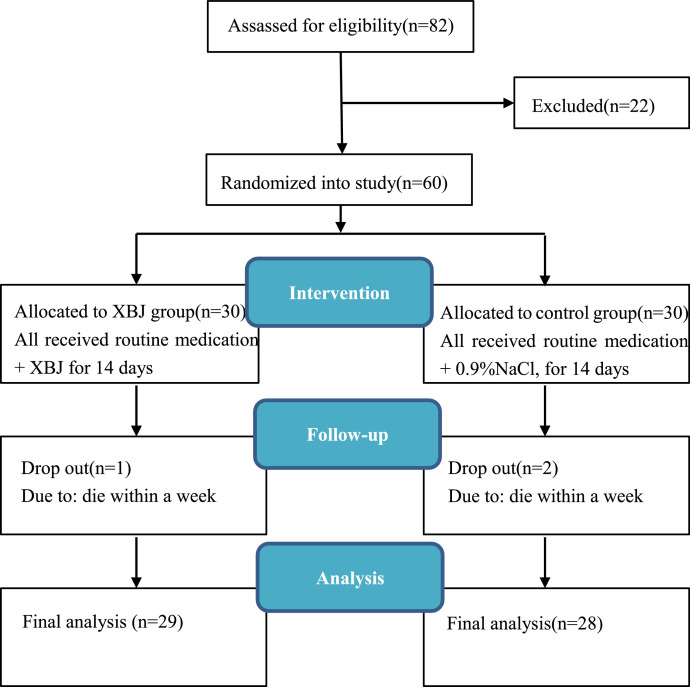
This study was a single-center, prospective, randomized, double-blinded trial. The trial was approved by the Medical Ethics Committee of the First People's Hospital of Jingzhou (No. Y20200211) and registered in the Chinese Clinical Trial Registry (http://www.chictr.org.cn; registration No. ChiCTR-2000030388.) A total of 60 eligible patients were recruited from the First people's Hospital of Jingzhou from February 16 to March 25 in 2020. The study design was illustrated in Fig. 1 .
Fig. 1.
Flow chart of the study design. XBJ, Xuebijing.
2.2. Inclusion criteria
-
1
Informed written consent from the patients themselves or their close relatives;
-
2
Age range from 18 to 80 years;
-
3Diagnostic criteria for novel coronavirus pneumonia in the diagnosis and treatment guidelines for severe COVID-19 patient (version sixth) developed by the National Health Committee of the People's Republic of China (http://www.nhc.gov.cn/).Those who met any of the following criterion were defined as severe-type:
-
1)dyspnea, respiratory rate≥30 times / min.
-
2)oxygen saturation less than 93%.
-
3)arterial partial pressure of oxygen (PaO2) / concentration of oxygen (FiO2) ≤300 mmHg. pulmonary imaging showed that patients with obvious progress of lesions more than 50% in 24-48 h.
-
1)
-
4
Laboratory-confirmed positive for SARS-CoV-2 by use of quantitative RT-PCR of throat swab samples.
-
5
Antiviral therapy with interferon-α inhalation.
-
6
Within 14 days of onset.
2.3. Exclusion criteria
-
1
The patient had a history of allergy to XBJ injection. The allergic reactions to XBJ injection were rash and anaphylactic shock.
-
2
Pregnant women or breastfeeding mothers.
-
3
Severe underlying disease, malignant tumor, hematopathy or AIDS included.
-
4
Patients with multiple organ dysfunction and unstable vital signs were expected to die within 48 hours.
-
5
Patients were enrolled in other clinical study.
-
6
Glucocorticoids were used during hospitalization.
2.4. Suspension criteria
-
1
Poor medical compliance.
-
2
A serious adverse event such as allergic shock occurred
-
3
Using of drugs or treatments that may affect the outcome. Antiviral drug such as lopinavir and ritonavir tablets and hydroxychloroquine, blood purification treatment.
-
4
If the patient died within one week after enrollment, the patient was classified as drop out case.
-
5
Voluntary withdrawal.
2.5. Trial design and Interventions
Of the 82 COVID-19 patients from Jingzhou, Hubei province, 60 met the eligibility criteria and agreed to take part. When the patients themselves or their close relatives signed the informed consent, these patients were assigned randomly to the XBJ or control group with a 1:1 ratio by a central randomization system. XBJ group received routine medication plus XBJ injection, and 50 ml XBJ injection was diluted with 100 ml normal saline to 150 ml, every 12 h for 60 min. The manufacturer of XBJ injection was the Tianjin Chase Sun Pharmaceutical Co., Ltd., Tianjin, China (lot number Z20040033). Control group patients received the same routine medication plus 150 ml saline. The routine medication included nutritional support, oxygen therapy, antiviral therapy with interferon-α inhalation, antibiotic agents, noninvasive and invasive ventilation if necessary. Both groups received the routine medication for 14 days starting within 24 h of enrollment.
2.6. Randomization
Randomization was generated centrally by a computerized random-number generator which drew up an allocation schedule for XBJ group and control group with a ratio of 1:1. Each group would then receive the corresponding treatment regimen.
2.7. Allocation concealment and blinding
Firstly the drug administrators received group information based on the above mentioned random number, then they dispensed the drug to the nurses, who made the mixture of drugs in a separate room. Photophobic brown color infusion bags and infusion set was prepared to ensure the same appearance.
The drug administrators and dispensing nurses were not involved in the data collection and analysis. Drug administrators and the investigators logged on the central randomization system using independent authority and accounts. The randomization system concealed group information for all the patient.
2.8. Outcome measures
The primary outcome was the level of peripheral blood lymphocyte and IL-6 on the 1st, 7th, 14th day of the enrollment, The secondary outcome measures were as follows: (1) 28-day follow-up mortality, the rate of mechanical ventilation, septic shock and conversion to the moderate type during 14 days of treatment. (2) Peripheral blood leukocytes, C-reactive protein (CRP), IL-8 and TNF-α on the 1st, 7th, 14th day of the enrollment. Sample collection was carried out before breakfast on the specific day. Serum samples were assayed with IL-6, IL-8, and TNF-α enzyme-linked immunosorbent assay (ELISA) kit respectively (Abcam; Cambridge, MA, USA). The CRP level was measured by the Cobas 6000 automatic immunochemiluminescence analyzer (Roche, Switzerland) (3) ICU hospitalization stay. (4) the duration of improvement of main clinical symptoms (5) Rash, allergic shock, abnormal function of liver and kidney and such adverse events were recorded throughout the trial.
2.9. Sample size and statistical analysis
The sample size was calculated based on the hypothesis that the effects of XBJ injection on severe COVID-19 patients would be similar to a previous study on severe pneumonia [18]. The sample size was estimated to be 60. The statistical software is SPSS version 20.0. Significance level at 0.05, two-sided. An unadjusted χ2 test was used for proportions for 28-day mortality. Two independent samples t-test was the analysis method for normal distribution data. P < 0.05 means statistical significance. Chi square test was used to analyze the categorical variables expressed as a percentage. Repeated measures analysis of variance was used to compare multiple groups of data. Descriptive statistics were used for demographics, baseline characteristics, and safety variables. All analyses were performed according to the Intention-To-Treat principle.
3. Results
3.1. Baseline characteristics of patients
A total of 82 COVID-19 patients were screened for eligibility and 60 met eligibility criteria. Using the central randomization system, participants were randomly divided into either XBJ or control group, 30 for each. 57 of them completed the trial. 1 case in XBJ group and 2 cases in control group died within one week and were classified as drop out cases (Fig. 1). Baseline characteristics of patients in both groups, such as gender, age, systolic blood pressure, respiratory rate, heart rate, comorbidities, use of antibiotics are summarized in Table 1 .
Table 1.
Comparison of demographic and basal clinical characteristics of patients between Xuebijing group and control group.
| Clinical characteristics | Xuebijing group (n = 29) | Control group (n = 28) | P value |
|---|---|---|---|
| Age, yr, mean (SD) | 60.26(15.62) | 56.35(18.28) | 0.286 |
| 18–50, n (%) | 12 (41.3) | 13 (46.4) | 0.872 |
| 50-80, n(%) | 17(58.6) | 15 (53.6) | 0.864 |
| Systolic blood pressure, mmHg, mean (SD) | 126.63 (22.36) | 126.52 (23.16) | 0.852 |
| Respiratory rate, breaths/min, mean (SD) | 29.25(6.42) | 29.91 (6.28) | 0.914 |
| Heart rate, beats/min, mean(SD) | 108.26 (19.65) | 110.32 (19.57) | 0.863 |
| Temperature,°C, mean (SD) | 38.26 (1.06) | 38.36 (1.04) | 0.936 |
| Pao2/Fio2, mean (SD) | 176.25 (55.06) | 178.34 (54.93) | 0.762 |
| Comorbidities, n (%) | |||
| Hypertension,n (%) | 12 (41.3) | 18(64.3) | 0.562 |
| Diabetes mellitus, n (%) | 3 (10.34) | 5(17.85) | 0.476 |
| Heart disease,n(%) | 2(6.9) | 1(3.57) | 0.554 |
| Cerebral infarction,n(%) | 3(10.34) | 2(7.14) | 0.640 |
| Leucocytes, 109 cells/L, mean (SD) | 7.37(4.42) | 5.80(2.56) | 0.126 |
| Lymphocyte, 109 cells/L, mean (SD) | 0.954(0.38) | 1.06(0.42) | 0.349 |
| C-reactive protein, mg/L, mean (SD) | 37.65(46.6) | 23.47(20.37) | 0.159 |
| IL-6,ng/ml,mean(SD) | 0.060(0.011) | 0.058(0.010) | 0.891 |
| IL-8,ng/ml,mean(SD) | 0.36(0.17) | 0.34(0.16) | 0.934 |
| TNF-α,pg/ml,mean(SD) | 38.42(10.12) | 37.85(10.46) | 0.736 |
| Total PSI score, mean (SD) | 118.47(35.32) | 120.36(32.16) | 0.692 |
| Systemic Inflammatory Response Syndrome score, mean (SD) | 3.12(0.66) | 3.08(0.71) | 0.964 |
| Antimicrobial treatment,n(%) | |||
| β-lactams enzyme inhibitors,n(%) | 8 (27.58) | 9 (32.14) | 0.947 |
| Quinolinones,n(%) | 4 (13.79) | 3 (10.71) | 0.731 |
| Fluconazole,n(%) | 2 (6.9) | 1 (3.57) | 0.554 |
| Carbapenem,n(%) | 3 (10.34) | 2 (7.14) | 0.640 |
| Mechanical ventilation, n (%) | |||
| IMV, n (%) | 0 | 0 | |
| NMV, n (%) | 4(13.79) | 5(17.85) | 0.583 |
| Intranasal oxygen (low flow), n (%) | 26(89.65) | 25(89.28) | 0.985 |
Abbreviations: IL-6, Interleukin-6; IL-8,Interleukin-8;TNF-α,tumor necrosis factor-α; PSI, Pneumonia severity index;IMV, invasive mechanical ventilation; NMV, noninvasive mechanical ventilation (including high flow supply and face mask).
3.2. Comparison of the rate of patients with ARDS, mechanical ventilation, septic shock and 28- day mortality between the two groups within 14 days of treatment
As shown in Table 2 , the incidence of ARDS and mechanical ventilation in XBJ group was lower than that for the XBJ group after 14 days of treatment (P < 0.05). Meanwhile, the septic shock rate of XBJ group was significantly lower than that of the control group within 14 days (P < 0.05). 28-day mortality was not significantly different between the two groups (1 [3.45%] died in the XBJ group vs 7 (25%) in the control group, although numerically higher in the control group; (P > 0.05), (Table 2).
Table 2.
Comparison of the rate of patients with ARDS, mechanical ventilation, septic shock and 28- day mortality between the two groups within 14 days for the Intention-to-Treat Populations.
| Variables | Xuebijing group (n = 29) | Control group (n = 28) | P value |
|---|---|---|---|
| ARDS,n (%) | 4(13.79) | 12(42.85) | 0.042 |
| Mechanical ventilation, n (%) | 3(10.34) | 13(46.42) | 0.024 |
| IMV, n (%) | 1(3.45) | 3(10.71) | 0.043 |
| NMV, n (%) | 2(6.9) | 10(35.71) | 0.016 |
| Septic shock, n(%) | 2(6.9) | 8(28.57) | 0.026 |
| 28-day mortality, n (%) | 1(3.45) | 7(25.00) | 0.557 |
Abbreviations: ARDS, acute respiratory distress syndrome; IMV, invasive mechanical ventilation; NMV, noninvasive mechanical ventilation (including high flow supply and face mask).
3.3. Comparison of improvement of clinical symptoms and clinical outcomes between the two groups
The results in Table 3 show that the proportion of patients in the XBJ group who became critically ill was lower than that of control group after 14 days treatment (P < 0.05) and the XBJ group were more or less likely to develop moderate illness compared with the control group (P < 0.05). As shown in Table 4 , duration of cardinal symptoms such as fever, cough, shortness of breath, fatigue in the XBJ group was shorter than that for the control group (P < 0.05). XBJ group also had a remarkably shorter duration in hospital in ICU in comparison with the control group (P < 0.01).
Table 3.
Comparison of the rate of clinical classification transformation in the 14 days between the two groups.
| Variables | Xuebijing group (n = 29) | Control group (n = 28) | P value |
|---|---|---|---|
| severe develop into critically ill cases, n (%) | 3(10.3) | 10(35.7) | 0.032 |
| severe develop into moderate cases, n (%) | 25(86.2) | 18(64.3) | 0.048 |
Table 4.
Comparison of clinical symptoms improvement, and the length of ICU stay between the two groups.
| Variables | Xuebijing group (n = 29) | Control group (n = 28) | P value |
|---|---|---|---|
| Fever, day, mean(SD) | 5.54(2.32) | 7.34(2.42) | 0.018 |
| Cough, day, mean(SD) | 7.47(1.68) | 9.46(2.35) | 0.045 |
| Shortness of breath, day, mean(SD) | 8.36(3.24) | 10.26(3.17) | 0.036 |
| Fatigue, day, mean(SD) | 6.67(2.76) | 12.16(2.74) | 0.019 |
| The length of ICU stay,day, mean(SD) | 8.42(2.26) | 10.72(3.64) | 0.004 |
Abbreviations: ICU, intensive care unit; SD: Standard Deviation.
3.4. Comparison of leukocyte, lymphocyte and C-reactive protein between the two groups
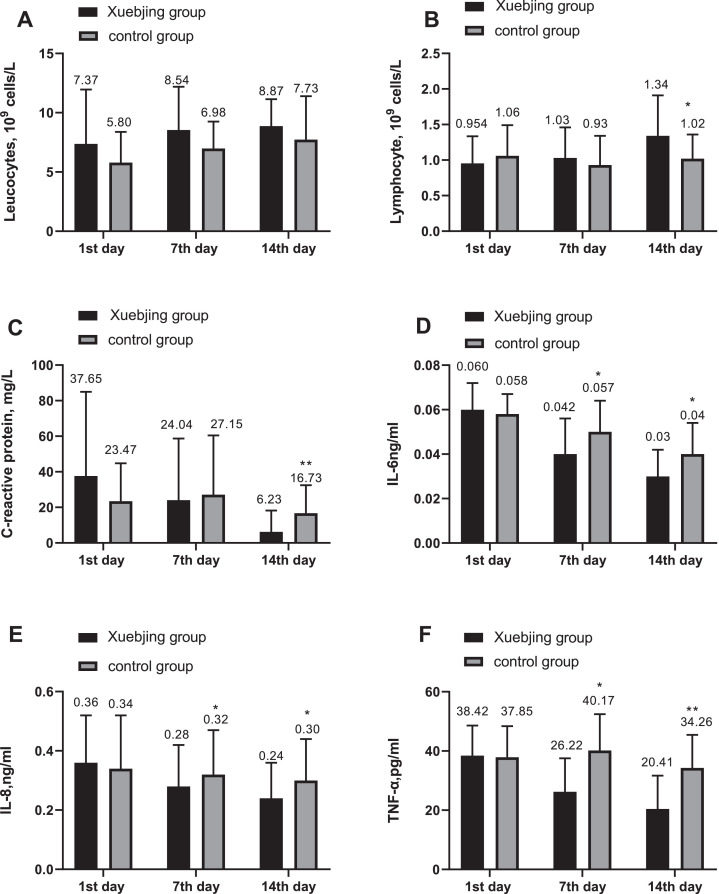
As shown in Fig. 2 A, the two groups have similar peripheral blood leukocyte count on the 1st day (7.37 ± 4.42 in XBJ group, and 5.80 ± 2.56 in control group;
Fig. 2.
*, (P < 0.05); **,(P < 0.01); Xuebijing injection had no significant effect on leucocytes; Xuebijing injection significantly elevated the levels of lymphocyte and lowered the levels of C-reactive protein on 14th day. The secretion of Interleukin-6, Interleukin-8 and tumor necrosis factor-α was suppressed significantly by Xuebijing injection on the 7th day and the 14th day.
P > 0.05). There was no significant difference in peripheral blood leucocyte count on the 7th and the 14th day in the Xuebijing group compared with the control group (Fig. 2A). No significant difference was observed in lymphocyte count between the two groups on the 7th day (P > 0.05), whereas the figure on the 14th day of enrollment was 1.34 ± 0.56 for XBJ group, and 1.02 ± 0.36 for control, with the difference being significant (P < 0.05 Fig. 2B). The level of CRP for XBJ group decreased and that of control group slightly increased on the 7th day with no significant difference between the two groups (P > 0.05), then the level of CRP on the 14th day of enrollment was 6.23 ± 11.86 for XBJ group, and 1.02 ± 0.36 for control group (P < 0.01 Fig. 2C).
3.5. Comparison of IL-6, IL-8 and TNF-α between the two groups
No significant differences were observed in the level of pro-inflammatory cytokines IL-6, IL-8 and TNF-α between the two groups on the 1st day (P > 0.05), then the level of IL-6 on the 7th day of enrollment was 0.042 ± 0.016 for XBJ group, and 0.057 ± 0.014 for control group (P < 0.05 Fig. 2D). In addition, the level of cytokines IL-6 declined on the 14th day of enrollment, 0.03 ± 0.013, 0.04 ± 0.014 for XBJ group and control one respectively (P < 0.05 Fig. 2D). Similarly, the level of IL-8 decreased on both the 7th and 14th day, with the difference being significant between the two groups (0.28 ± 0.14 vs 0.32 ± 0.15;0.24 ± 0.13 vs 0.30 ± 0.14 P < 0.05 respectively; Fig. 2E). Compared with the control group, TNF - α in Xuebijing group decreased significantly on the 7th and 14th day of enrollment, and the difference between the two groups was statistically significant (26.22 ± 11.32 vs 40.17 ± 12.25; P < 0.05; 20.41 ± 11.26 vs 34.26 ± 11.15; P < 0.01, respectively; Fig. 2F).
3.6. Safety evaluation
There were 5 cases of abnormal liver dysfunction, 3 cases of renal dysfunction, 2 cases of rash in the XBJ group and 3 cases of abnormal liver dysfunction, 4 cases renal dysfunction, 1 case of rash in the control group. Thus there were no obvious difference between the two groups(P > 0.05; Table 5 ). No anaphylactic shock occurred in both groups.
Table 5.
Comparison of adverse reactions between the two groups.
| Variables | Xuebijinggroup (n = 29) | Controlgroup (n = 28) | P value |
|---|---|---|---|
| abnormal liver function, cases,n (%) | 5(17.2) | 3(10.7) | 0.348 |
| renal dysfunction, cases, n (%) | 3(10.3) | 4(14.3) | 0.765 |
| rash, cases, n (%)anaphylactic shock n (%) | 2(6.9)0 | 1(3.6)0 | 0.554 |
4. Discussion
Globally the number of patients with COVID-19 is rapidly increasing and the number of deaths is rising. Some of the patients with mild illness develop rapidly into severe and critical illness, and die within in a short period of time. Approximately, 70% of COVID-19 patients suffered from ARDS and needed mechanical ventilation in ICU. Overall mortality of patients with COVID-19 is about 4% [3,5], while 28-day mortality for severe and critical patients reaches up to 60% [6]. Other research has used lymphocyte count and IL-6 level as the primary outcomes [19]. The results showed that XBJ injection did not significantly reduce the mortality rate, which may be related to the small sample size. Although 28-day mortality rate was not statistically significant, after XBJ injection intervention, the rates of ARDS and mechanical ventilation in patients with COVID-19 were obviously decreased, further XBJ injection also inhibited septic shock to a large extent. Moreover, the main inflammatory markers such as lymphocyte count and IL-6 level were evidently improved, which indicated that XBJ is effective for severe patients with COVID-19. According to Diagnosis and Treatment Protocol for COVID-19 released by National Health Commission of the People`s Republic of China (Version 6), COVID-19 can be divided into four types as follows: mild, moderate, severe and critical illness [7]. Compared with the control group, the number of severe patients deteriorating to critical illness in the XBJ group was less, and more severe patients developed into more moderate cases. The XBJ group also had a marked shorter hospital stay in ICU. This suggests that XBJ injection may be effective in preventing the progression of COVID-19 and improve the prognosis of patients.
According to relevant literature, the peripheral blood leukocytes of patients with COVID-19 are usually normal, the level of CRP in the peripheral blood of patients with COVID-19 increases significantly, whereas the lymphocyte count decreases greatly. Studies have shown that the severity of COVID-19 is positively correlated with CRP level and negatively correlate with lymphocyte level. Lymphopenia is a common feature and might be a critical factor associated with the mortality in the patients with COVID-19 [20,21]. Flow cytometry shows that peripheral blood T lymphocyte decreases and be activated. Lymphocytopenia is a marked feature for severe and critical COVID-19 patients. More than 80% of critical patients have lymphopenia. The degree of lymphocyte decreased reflects the severity of infection of SARS-CoV-2. There is a progressive decrease in lymphocytes in patients who died of COVID-19 [21]. Total lymphocyte count is an independent risk factor associated with disease progression in patients with COVID-19 [22,23]. Lymphocytopenia may be closely related to prognosis. Excessive inflammatory response caused by SARS-CoV-2 is regarded as a major cause of death in COVID-19 patients. This is related to lymphocytopenia and infiltration of monocytes into lung and other important organs [24], so that lymphopenia may be increasing the risk of death in those with COVID-19 infection. It seems that if the level of peripheral blood lymphocytes was restored, the prognosis of COVID-19 patients would be improved. So the results imply that XBJ injection have an inhibitory effect on the lymphocytopenia, indicating that XBJ injection might have the effect of regulating cellular immune function for severe COVID-19 patients.
There is also a positive correlation between CRP level and the risk of organ failure in critically ill COVID-19 patients [25]. A majority of the patients with severe COVID-19 in ICU have sustained high level of CRP [5], and the level of CRP can predict the severity of COVID-19 [[23], [26]]. Compared with conventional treatment, the expression of CRP for patients with severe COVID-19 was significantly inhibited by XBJ injection on the 14th day of enrollment, suggesting that the inflammatory response was under control.
TNF is considered to be the crucial cytokine in acute viral diseases. TNF is expressed by a variety of immune cells and it plays an important role in CS [9,27] Tumor necrosis factor alpha (TNF- α) plays a key role in acute inflammation which was induced by oxidative stress and synthetic reaction. TNF - α is produced by macrophages, monocytes, B cells and other tissues. Activation of TNF-α also leads to the production of IL-1 and IL-6. A large number of TNF - α were found in the tissues and plasma of patients with COVID-19. Anti TNF - α antibodies can reduce the early inflammatory response of COVID-19, so that it is a promising therapeutic option for COVID-19 patients [28]. Severe COVID-19 patients have immune dysfunction which was caused by CS. The decrease of lymphocytes may be due to the destruction of CS [5]. SARS-CoV-2 targeting mainly on lymphocyte may cause T cell consumption which is conducive to immune dysfunction. Qin C et al. proposed that serum IL-6, IL-8 and TNF-α levels are significantly higher in patients with severe COVID-19 than those of individuals with mild disease [29]. The CS is closely related to COVID-19 severity [29,30], and it is thought to be a primary cause of death from COVID-19 [24,31] IL-6 plays a key role in CS. IL-6 is secreted by endothelial cells under the stimulation of hypoxia and inflammatory cytokines such as bacterial lipopolysaccharide and TNF-α. High levels of IL-6 can activate vascular endothelial cells as well as coagulation pathway, and eventually leads to sepsis and MODS [32,33]. For severe COVID-19 patients, CS is closely related to death. IL-6 is regarded as a new target for COVID-19. If the signaling pathway of IL-6 is blocked, CS can be effectively inhibited. Then, the systemic inflammatory response can be prevented, contributing to the reduce of mortality [10,33,34]. In the present study, compared with conventional treatment, the expression of IL-6, IL-8 and TNF-a was substantially down regulated in patients with severe COVID-19 after the treatment with XBJ injection for two weeks, meanwhile the level of CRP significantly declined. Therefore, the present study suggests that CS is suppressed and systemic inflammatory response is under the control in patients with severe COVID-19.
The balance of pro-inflammatory and anti-inflammatory mechanisms is critical to maintain the immune homeostasis of the lung. Missing or abnormal for one or more of these regulatory mechanisms may lead to a CS. SARS-CoV-2 infection induces an excessive immune response and the high level of pro-inflammatory cytokines such as IL-6, IL-8 and TNF - α in patients with severe infection. The secretion of these proinflammatory cytokines leads to CS. Excessive cytokine release is considered to be the determinant of ARDS [9,27]. Prevention and mitigation of cytokine storm is considered as the key to save severe COVID-19 patients [31]. T cells play a critical role in antiviral immunity. Recent studies have demonstrated that SARS-CoV-2 viruses can induce reduction and functional exhaustion of T cell in COVID-19 patients, and it may be due to high levels of serum IL-6. TNF - α and IL-6 having a negative regulatory effect on T cell survival or proliferation [35]. After two weeks of intervention with XBJ injection, the levels of IL-6, IL-8 and TNF - α in patients with severe COVID-19 were remarkably reduced, and it indicated that XBJ injection may suppress the CS by inhibiting the reduction of T cells.(Figure3)
XBJ injection is a traditional Chinese medicine composed of Radix paeoniae rubra, Angelica sinensis, Chuanxiong, Honghua (Flos Carthami) and Salvia miltiorrhiza, etc [36]. The main ingredients of XBJ injection include amino acid, phenolic acid, flavonoid glycoside, elysine and phthalic acid ester. XBJ exerts effects on about 10 kinds of sepsis / inflammation pathways, and its 21 main active components participate in the regulation of 550 targets. Then XBJ injection possesses biological activity of promoting blood circulation and removing blood stasis and detoxifcation, therefore it is used in the treatment of sepsis with approval [15,37]. Basic research and clinical trials have confirmed that XBJ injection could suppress the secretion of proinflammatory cytokine IL-6, IL-8 and TNF-α, and alleviate the injury of severe pneumonia and sepsis [14,15,[37], [38], [39]]. Our result suggests that XBJ injection can also down regulate the expression of pro-inflammatory cytokines IL-6, IL-8 and TNF-α in severe COVID-19 patients.
This study was subject to some limitations. Firstly, XBJ injection is only available in China and treatment setting and protocol is different in other countries. Secondly, sample size of this study was relatively small and further studies including a larger number of patients with severe COVID-19 may be required to validate these findings in future.
In summary, it is suggested that XBJ injection may prevent severe COVID-19 patients against CS by regulating the secretion of pro-inflammatory cytokine IL-6, IL-8 and TNF - α.
Author Contributions
Zhijian Luo: Conceptualization, Project administration, Writing - original draft, Writing - review & editing. Wei Chen: Funding acquisition, Supervision, Writing - original draft, Writing - review & editing. Mingqing Xiang: Investigation. Hua Wang: Software. Wei Xiao: Formal analysis, Methodology. Cheng Xu: Formal analysis, Visualization. Yunkui Li: Methodology. Jie Min: Resources. Qiang Tu: Data curation, Validation, Writing - review & editing.
Financial support
No funding was received for the conduct of this study.
Declaration of Competing Interest
There was no any commercial involvement in the conduct of the study. The authors have no conflict of interests to declare.
Acknowledgements
Dr. Tang-Meng Guo, MD is acknowledged for his careful review of this manuscript, and precious comments for improvement.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.eujim.2021.101305.
Data availability – statement
Tables 3 and 4 are supplied as supplementary file and can be obtained from the author on request.
Appendix. Supplementary materials
References
- 1.He F., Deng Y., Li W. Coronavirus disease 2019: what we know. J. Med. Virol. 2020;92(7):719–725. doi: 10.1002/jmv.25766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huang D., Lian X., Song F., et al. Clinical features of severe patients infected with 2019 novel coronavirus: a systematic review and meta-analysis. Ann. Transl. Med. 2020;8(9):576. doi: 10.21037/atm-20-2124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J., et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huang C., Wang Y., Li X., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang W., Zhao Y., Zhang F., et al. The use of anti-inflammatory drugs in the treatment of people with severe coronavirus disease 2019 (COVID-19): the perspectives of clinical immunologists from China. Clin. Immunol. 2020;214 doi: 10.1016/j.clim.2020.108393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yang X., Yu Y., Xu J., et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir. Med. 2020;8(5):475–481. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chan K.W., Wong V.T., Tang S. COVID-19: an update on the epidemiological, clinical, preventive and therapeutic evidence and guidelines of integrative chinese-western medicine for the management of 2019 novel coronavirus disease. Am. J. Chin. Med. 2020;48(3):737–762. doi: 10.1142/S0192415X20500378. [DOI] [PubMed] [Google Scholar]
- 8.Ye Q., Wang B., Mao J. The pathogenesis and treatment of the `Cytokine Storm' in COVID-19. J. Infect. 2020;80:607–613. doi: 10.1016/j.jinf.2020.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Channappanavar R., Perlman S. Pathogenic human coronavirus infections: causes and consequences of cytokine storm and immunopathology. Semin. Immunopathol. 2017;39(5):529–539. doi: 10.1007/s00281-017-0629-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arnaldez F.I., O'Day S.J., Drake C.G., et al. The society for Immunotherapy of Cancer perspective on regulation of interleukin-6 signaling in COVID-19-related systemic inflammatory response. J. Immunother. Cancer. 2020;8(1) doi: 10.1136/jitc-2020-000930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shi Q., Zhou Q., Wang X., et al. Potential effectiveness and safety of antiviral agents in children with coronavirus disease 2019: a rapid review and meta-analysis. Ann. Transl. Med. 2020;8(10):624. doi: 10.21037/atm-20-3301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang Y., Islam M.S., Wang J., Li Y., Chen X. Traditional Chinese medicine in the treatment of patients infected with 2019-new coronavirus (SARS-CoV-2): a review and perspective. Int. J. Biol. Sci. 2020;16(10):1708–1717. doi: 10.7150/ijbs.45538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ni L., Zhou L., Zhou M., Zhao J., Wang DW. Combination of western medicine and Chinese traditional patent medicine in treating a family case of COVID-19. Front. Med. 2020;14(2):210–214. doi: 10.1007/s11684-020-0757-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang P., Song Y., Liu Z., et al. Xuebijing injection in the treatment of severe pneumonia: study protocol for a randomized controlled trial. Trials. 2016;17(1):142. doi: 10.1186/s13063-016-1282-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu S., Yao C., Zhang J., Yang Y., Qiu H. Investigators E.-.S.E.P. Efficacy of Xuebijing injection for sepsis (EXIT-SEP): protocol for a randomised controlled trial. BMJ Open. 2019;9(8) doi: 10.1136/bmjopen-2018-028664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Song Y., Yao C., Yao Y., et al. XuebiJing injection versus placebo for critically ill patients with severe community-acquired pneumonia: a randomized controlled trial. Crit. Care Med. 2019;47(9):e735–e743. doi: 10.1097/CCM.0000000000003842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang Y.L., Cui Q., Zhang D., et al. Efficacy of Xuebijing injection for the treatment of coronavirus disease 2019 via network pharmacology. Tradit. Med. Res. 2020;5(4):201–215. doi: 10.12032/TMR20200507178. [DOI] [Google Scholar]
- 18.Qi F., Liang Z.X., She D.Y., Yan G.T., Chen L.A. A clinical study on the effects and mechanism of xuebijing injection in severe pneumonia patients. J. Tradit. Chin. Med. 2011;31(1):46–49. doi: 10.1016/s0254-6272(11)60011-3. [DOI] [PubMed] [Google Scholar]
- 19.Qiu R., Zhao C., Liang T., et al. Core outcome set for clinical trials of COVID-19 based on traditional Chinese and Western medicine. Front. Pharmacol. 2020;11:781. doi: 10.3389/fphar.2020.00781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chan J.F., Yuan S., Kok K.H., et al. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet. 2020;395(10223):514–523. doi: 10.1016/S0140-6736(20)30154-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xu Z., Shi L., Wang Y., et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir. Med. 2020;8(4):420–422. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhou Y., Zhang Z., Tian J., Xiong S. Risk factors associated with disease progression in a cohort of patients infected with the 2019 novel coronavirus. Ann. Palliat. Med. 2020;9(2):428–436. doi: 10.21037/apm.2020.03.26. [DOI] [PubMed] [Google Scholar]
- 23.Liu X., Shi S., Xiao J., et al. Prediction of the severity of Corona Virus Disease 2019 and its adverse clinical outcomes. Jpn. J. Infect. Dis. 2020 doi: 10.7883/yoken.JJID.2020.194. [DOI] [PubMed] [Google Scholar]
- 24.Merad M., Martin J.C. Pathological inflammation in patients with COVID-19: a key role for monocytes and macrophages. Nat. Rev. Immunol. 2020;20:355–362. doi: 10.1038/s41577-020-0331-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hillas G., Vassilakopoulos T., Plantza P., Rasidakis A., Bakakos P. C-reactive protein and procalcitonin as predictors of survival and septic shock in ventilator-associated pneumonia. Eur. Respir. J. 2010;35(4):805–811. doi: 10.1183/09031936.00051309. [DOI] [PubMed] [Google Scholar]
- 26.Liu Y.P., Li G.M., He J., Liu Y., Li M., Zhang R., et al. Combined use of the neutrophil-to-lymphocyte ratio and CRP to predict 7-day disease severity in 84 hospitalized patients with COVID-19 pneumonia: a retrospective cohort study. Ann. Transl. Med. 2020;8:635. doi: 10.21037/atm-20-2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tisoncik J.R., Korth M.J., Simmons C.P., Farrar J., Martin T.R., Katze M.G. Into the eye of the cytokine storm. Microbiol. Mol. Biol. Rev. 2012;76(1):16–32. doi: 10.1128/MMBR.05015-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rizk J.G., Kalantar-Zadeh K., Mehra M.R., Lavie C.J., Rizk Y., Forthal D.N. Pharmaco-immunomodulatory therapy in COVID-19. Drugs. 2020;80:1267–1292. doi: 10.1007/s40265-020-01367-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Qin C., Zhou L., Hu Z., et al. Dysregulation of immune response in patients with COVID-19 in Wuhan, China. Clin. Infect. Dis. 2020 doi: 10.1093/cid/ciaa248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hui D., Zumla A. Severe acute respiratory syndrome: historical, epidemiologic, and clinical features. Infect. Dis. Clin. North Am. 2019;33(4):869–889. doi: 10.1016/j.idc.2019.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hu B., Huang S., Yin L. The cytokine storm and COVID-19. J. Med. Virol. 2020 doi: 10.1002/jmv.26232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tanaka T., Narazaki M., Kishimoto T. Immunotherapeutic implications of IL-6 blockade for cytokine storm. Immunotherapy. 2016;8(8):959–970. doi: 10.2217/imt-2016-0020. [DOI] [PubMed] [Google Scholar]
- 33.Zhang C., Wu Z., Li J.W., Zhao H., Wang GQ. Cytokine release syndrome in severe COVID-19: interleukin-6 receptor antagonist tocilizumab may be the key to reduce mortality. Int. J. Antimicrob. Agents. 2020;55(5) doi: 10.1016/j.ijantimicag.2020.105954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bennardo F., Buffone C., Giudice A. New therapeutic opportunities for COVID-19 patients with Tocilizumab: possible correlation of interleukin-6 receptor inhibitors with osteonecrosis of the jaws. Oral Oncol. 2020;106 doi: 10.1016/j.oraloncology.2020.104659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Diao B., Wang C., Tan Y., et al. Reduction and functional exhaustion of T cells in patients with coronavirus disease 2019 (COVID-19) Front. Immunol. 2020;11:827. doi: 10.3389/fimmu.2020.00827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang Q., Li J., Liang X., et al. The preventive effect of Chinese herbal preparation Xuebijing against hyperactive inflammation after hepato-pancreato-biliary surgery. Ann. Transl. Med. 2019;7(18):481. doi: 10.21037/atm.2019.07.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yin Q., Li C. Treatment effects of xuebijing injection in severe septic patients with disseminated intravascular coagulation. Evid. Based Complement Alternat. Med. 2014 doi: 10.1155/2014/949254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu M.W., Wang Y.H., Qian C.Y., Li H. Xuebijing exerts protective effects on lung permeability leakage and lung injury by upregulating Toll-interacting protein expression in rats with sepsis. Int. J. Mol. Med. 2014;34(6):1492–1504. doi: 10.3892/ijmm.2014.1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li T., Qian Y., Miao Z., et al. Xuebijing injection alleviates Pam3CSK4-induced inflammatory response and protects mice from sepsis caused by methicillin-resistant staphylococcus aureus. Front. Pharmacol. 2020;11:104. doi: 10.3389/fphar.2020.00104. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Tables 3 and 4 are supplied as supplementary file and can be obtained from the author on request.