Abstract
Background
Upon SARS-CoV-2 infection, most individuals develop neutralizing antibodies and T-cell immunity. However, some individuals reportedly remain SARS-CoV-2 PCR positive by pharyngeal swabs weeks after recovery. Whether viral RNA in these persistent carriers is contagious and stimulates SARS-CoV-2-specific immune responses is unknown.
Methods
This cohort study was conducted between April 3rd–July 9th 2020, recruiting COVID-19 recovered individuals that were symptom-free for at least 14 days. We collected serum for SARS-CoV-2-specific total Ig, IgA and IgM detection by ELISA, pharyngeal swabs (two time points) for ddPCR and PBMCs for anti-SARS-CoV-2 CD8 T-cell dextramer analyses.
Findings
We enrolled 203 post-symptomatic participants with a previous RT-PCR-verified SARS-CoV-2 infection. At time point 1, a median of 23 days (range 15–44) after recovery, 26 individuals (12⋅8%) were PCR positive. At time point 2, 90 days (median, range 85–105) after recovery, 5 (5⋅3%) were positive. There was no difference in SARS-CoV-2 antibody levels between the PCR negative and positive group. The persistent PCR positive group however, had SARS-CoV-2-specific CD8 T-cell responses of significantly increased breadth and magnitude. Assisted contact tracing among persistent PCR positive individuals revealed zero new COVID-19 diagnoses among 757 close contacts.
Interpretation
Persistent pharyngeal SARS-CoV-2 PCR positivity in post-symptomatic individuals is associated with elevated cellular immune responses and thus, the viral RNA may represent replicating virus. However, transmission to close contacts was not observed indicating that persistent PCR positive individuals are not contagious at the post-symptomatic stage of the infection.
Keywords: SARS-CoV-2, Persistent PCR positive, Immunology, Antibodies, CD8 T cell responses, Transmission, Contact tracing
1. Research in context
1.1. Evidence before this study
We searched on PubMed on November 10th, 2020, with the terms ”SARS-CoV-2”, “COVID-19”, “coronavirus”, “persistent PCR positive”, ”viral load”, ”immune response.” We found several papers focusing on the immune responses in SARS-CoV-2 infection. Some studies assessed antibody responses and/or clinical characteristics in persistent RT-PCR positive patients. One of these studies did not assess antigen-specific responses and included four patients only. Another study only assessed antibody responses but not cellular responses. A few studies were based on clinical characteristics of hospitalized patients with persistent positive RT-PCR tests and relating antibody responses. We further found four small observational cohort studies on hospitalized adults and one Letter to the Editor describing the prevalence of persistent RT-PCR positivity among >7000 patients discharged from hospital, not including any immunological analyses. We found 2 case reports on persistent SARS-CoV-2 infected patients and two studies on convalescent plasma donors, all of which were not conducted as observational cohort studies. We found 2 case reports of immunocompromised patients being RT-PCR and outgrowth positive for up to 70 days. No studies that combined data on persistent RT-PCR positive individuals with analyses of both antigen-specific cellular and humoral immune responses were identified. Also, we found no observational cohort studies where the composition of the cohort was reflecting the grades of COVID-19 severity as is observed in vivo, with 80% of infected individuals recovering at home and 20% hospitalized, hereof 5% critically ill.
1.2. Added value of this study
The question of continuing PCR positivity versus risk of transmission is important to address. We found that persistent PCR positive individuals had an ongoing cellular anti-SARS-CoV-2 response in contrast to PCR negative individuals, indicative of persistent viral replication. Serum levels of immunoglobulins were equal between PCR positive versus negative individuals. We further did assisted contact tracing among the persistent PCR positive individuals and found that these individuals were not transmitting at the post-symptomatic stage of infection.
1.3. Implications of all the available evidence
Several other viral infections like Zika virus, Ebola virus and Measles are known to cause persistent or prolonged viral shedding. For some of these, e.g. Zika, the potential transmission period can be very long. SARS-CoV-2 is to be placed among these types of long-term detectable viruses. The results of this study support the current COVID-19 management where infected individuals can discontinue self-isolation ≥48 h after recovery. For individuals who are persistently or intermittently PCR positive there is however a hypothetical risk of recurrent COVID-19 in case of immune suppression (e.g. iatrogenic), which should be considered by clinicians. Evidence from this and several other studies show, that seroconversion and enhanced CD8 T-cell activity is not able to completely eliminate mucosal virus in some individuals. The molecular basis for this phenomenon is not yet understood, why further in-depth virological and molecular immunological studies needs to be undertaken to clarify potential consequences, who is at risk and the full extend of persistent PCR positive testing period.
2. Introduction
Novel coronavirus, SARS-CoV-2, has elicited a global health crisis with overwhelming consequences for healthcare, societies and economics [1]. According to the World Health Organization, the pandemic has affected more than 200 countries, infected 80 million people and claimed millions of lives by December 2020 [2]. Persistent SARS-CoV-2 RNA shedding has been described, but the nature of this phenomenon is not fully understood, as most reports are anecdotal and lack in-depth immunological analyses [3], [4], [5], [6], [7]. Two case reports have indicated that seroconversion does not necessarily lead to elimination of viral RNA from the pharyngeal mucosa with cases being RT-PCR positive up to 63 days [8] and 104 days after symptom onset despite having neutralizing antibodies [9]. Xu et al followed a cohort of 113 hospitalized patients with a median duration of 17 days of RNA positivity and assessed clinical risk factors associated with prolonged RNA detection [7]. However, the role of antigen-specific CD8 T-cell responses in combination with antibodies in persistent SARS-CoV-2 RNA carriers is largely unknown. Further, it is unclear if viral RNA represents contagious virus in post-symptomatic individuals who remain SARS-CoV-2 PCR positive. This gap in our understanding of COVID-19 has important implications. For persistent PCR positive individuals it is essential to be informed if there is a transmission risk and when to safely discontinue self-isolation. Additionally, it is essential for health care personnel to provide instructions regarding hygiene and physical distance to patients and their contacts after hospital discharge. Lastly, health care workers themselves are at increased risk of COVID-19 [10,11]. Thus, a potential post-symptomatic transmission risk is important to prevent, as they work with vulnerable populations.
Our objective was to investigate immunological responses and viral persistence upon SARS-CoV-2 infection. Here, we report a cohort study of 203 convalescent individuals with a history of RT-PCR verified SARS-CoV-2 infection.
3. Methods
3.1. Study design
The study was conducted at the Department of Infectious Diseases at Aarhus University Hospital, Denmark from April 3rd–July 9th 2020. Inclusion criteria: Age >18 years, documented SARS-CoV-2 RT-PCR positive within the preceding 12 weeks, fully recovered from COVID-19 (defined as no ongoing COVID-19 symptoms, except loss of sense of smell/taste and cognitive deficits, which are symptoms equivalent to Long-COVID-19 [12]), and able to give informed consent. Exclusion criteria: Current febrile illness, immunosuppressive treatment/known immunodeficiency and pregnancy. Participants were invited for a visit at time point 1 (minimum 14 days after full recovery and maximum 12 weeks after first positive SARS-CoV-2 RT-PCR) and time point 2 (optional) for a 2nd swab test, 6–10 weeks after time point 1. Clinical data on baseline characteristics, comorbidities, symptoms and duration of COVID-19 were collected to assess if certain parameters predicted persistent SARS-CoV-2 positive PCR testing. Participants were allocated in 5 groups according to severity of COVID-19 history (Table 1).
Table. 1.
Demographic and Clinical Characteristics
| Characteristic | n = 203 |
|---|---|
| Age, y | 46⋅8 (20⋅6–79⋅4) |
| Female sex | 92 (45⋅3%) |
| Race | |
| Asian | 2 (1⋅0%) |
| Caucasian | 199 (98⋅0%) |
| Other | 2 (1⋅0%) |
| Smoking status | |
| Never | 133 (65⋅5%) |
| Former | 61 (29⋅6%) |
| Current | 9 (3⋅9%) |
| BMI | |
| BMI 18⋅5–24⋅9 | 91 (44⋅8%) |
| BMI 25–29⋅9 | 75 (36⋅5%) |
| BMI > 30 | 37 (17⋅7%) |
| Duration of COVID-19 symptoms, days | 14 (0–68) |
| Average number of symptomsa | 6 (0–12) |
| Time from symptom onset to time point 1, days | 45 (18–76) |
| COVID-19 Disease Severity Scale | |
| 1) Outpatient, no limitation of daily activities | 17 (8⋅4%) |
| 2) Outpatient, limitation of daily activities | 152 (74⋅9%) |
| 3) Hospitalized, no oxygen supplement | 9 (4⋅4%) |
| 4) Hospitalized, oxygen supplement | 18 (8⋅8%) |
| 5) Hospitalized, ICU | 7 (3⋅4%) |
| No. of participants with comorbiditiesb | 83 (40⋅8%) |
Data are n (%) or median (range).
COVID-19 symptoms registered: Nasal congestion, fever, cough, sputum production, dyspnoea, sore throat, headache, fatigue, myalgia, diarrhoea, nausea, loss of smell and/or taste, dizziness, rash, other.
Diseases/diagnoses/conditions registered or self-reported within 10 years of inclusion date
3.2. Ethics
The study was approved by The National Health Ethics Committee (case number 1-10-72-76-20) and the Danish Data Protection Agency (case number not applicable). Each participant provided written informed consent before any study activities.
3.3. Total Ig, IgM and IgA detection
Serum levels of anti-SARS-CoV-2 antibodies were detected by semi-quantitative ELISA.
IgA antibodies were measured using the Euroimmun Anti-SARS-CoV-2 IgA ELISA (Euroimmun Medizinische Labordiagnostika AG, Lübeck, Germany, #El 2606-9601 A), according to manufacturer's instructions and as previously described elsewhere [13,14]. The Euroimmun ELISA assay has demonstrated high sensitivity and specificity for IgA detection (90% and 93%, respectively) [13].
Serum IgM and total Ig against SARS-CoV-2 receptor binding domain (RBD) were measured using the Wantai SARS-CoV-2 IgM and SARS-CoV-2 Ab ELISA kits (Beijing Wantai Biological Pharmacy Enterprise Co., Beijing, China, Cat. No. WS-1196 and WS-1096) as previously described and according to manufacturer's instructions [13]. Sensitivity for the Wantai ELISA kit is reported to be 90–100% and the specificity 100% [13,15]. All serum samples were run in duplicates.
3.4. ddPCR for SARS-CoV-2
SARS-CoV-2 was detected from pharyngeal swabs, using ddPCR with primers and probe targeting the nucleocapsid; Forward: 5’-GACCCCAAAATCAGCGAAAT-3’(LGC Biosearch Technologies), Reverse: 5’-TCTGGTTACTGCCAGTTGAATCTG-3’(LCG Biosearch Technologies), probe: 5’-FAM-ACC CCG CAT TAC GTT TGG TGG ACC BHQ1-3’ (nCOV_N1 probe, IDT) published by the CDC. Primers and probes targeting the human gene RPP30 was used as internal control to detect background sample material; Forward: 5’-GATTTGGACCTGCGAGCG-3’ (IDT), Reverse: 5’-GCGGCTGTCTCCACAAGT-3’ (IDT) and Probe: 5’-6FAM-CTGACCTGAAGGCTCT-3’ (Applied Biosystems, UK). For RNA extraction, media from the swabs were collected and spun for 1 h at 21.000 x g at 4°C, supernatant was discarded and pellet dissolved in 200 μL TBS. RNA was extracted using QIAamp Viral RNA Mini Kit (Qiagen) according to manufacturer's instruction and eluted in 70 μL buffer AVE. ddPCR was performed using Bio-Rad One-step RT ddPCR advanced kit according to the instructions. Samples were assayed in duplicates for SARS-CoV-2 with 10 μL RNA in each reaction and a final concentration of 1000 nM primer and 250 nM probe. Droplets were generated using the QX200 Droplet generator (Bio-Rad) and amplified in a C1000 Touch Thermal Cycler (Bio-Rad) under the following conditions; 25°C for 3 min, 50°C for 1 h, 40 cycles of 95°C for 30 s and 55°C for 1 min, lastly 98°C for 10 min and infinite hold on 12°C. Subsequently droplets were read in a QX200 droplet reader (Bio-Rad) and analysis was performed in QuantaSoftTM analysis software (Bio-Rad). Total concentration per sample was calculated based on total N1 concentration per reaction. Non-template-control plates were run with nuclease-free water with a total of 176 wells to determine background signal yielding false positive events. False positive events were found with 1 droplet/well in a total of 14 wells out of 176. To avoid false positive quantified signals in study-samples, positive outcomes were defined as ≥3 events per duplicate wells.
3.5. HLA-A2 typing and dextramer staining by flow cytometry
For HLA-A2 typing cryopreserved PBMCs were thawed, stained at room temperature for 20 min with HLA-A2 (clone BB7.2, BioLegend Cat# 343328, RRID: AB_2721564) or matching isotype control (Biolegend #400356) and acquired on a five-laser Fortessa flow cytometer. Overall, 113 participants were HLA-A2 positive, from which we analyzed 106 samples. The dextramer stains were then performed on the HLA-A2 positive samples as previously described [16,17]. PBMCs were incubated at room temperature for 30 min with the following SARS-CoV-2 dextramers (all from Immundex): A*0201/TLACFVLAAV-PE (WB3848-PE), A*0201/GMSRIGMEV-FITC (WB5751-FITC), A*0201/LLLDRLNQL-APC (WB5762-APC), A*0201/ILLNKHIDA-PE (WB5848-PE), A*0201/RLNEVAKNL-FITC (WB5750-FITC), A*0201/YLQPRTFLL-APC (WB5824-APC), A*0201/VLNDILSRL-PE (WB5823-PE), A*0201/NLNESLIDL-FITC (WB5850-FITC), A*0201/FIAGLIAIV-APC (WB5825-APC), human corona virus 229E dextramer A*0201/LLLNCLWSV-PE (WB3513-PE), or positive/negative control dextramers: A*0201/NLVPMVATV-PE (WB2132-PE, Pos. Control, CMV), A*0201/NLVPMVATV-FITC (WB2132-FITC, Pos. Control, CMV), A*0201/NLVPMVATV-APC (WB2132-APC, Pos. Control, CMV), A*0201/Neg. Control-PE (WB2666-PE), A*0201/Neg. Control-FITC (WB2666-FITC), A*0201/Neg. Control-APC (WB2666-APC). Cells were washed and stained with viability dye (Zombie Violet, Biolegend, #423114) and CD8 (Clone RPA-T8, BD, #563795) and acquired on a five-laser Fortessa flow cytometer. Data was analyzed using FlowJo (Version 10.7.1).
3.6. Assisted contact tracing
Guidelines in Denmark dictates self-isolation until 48 h after symptom resolution [18]. Assisted contact tracing was done to determine if persistent post-symptomatic viral RNA positivity is associated with COVID-19 transmission. We instructed individuals from the PCR positive group, to record each person they had been into close contact with (<2 m for >15 min) from >48 h after recovery and until the time point 1 study visit. Contacts (colleagues, household contacts, private contacts) that were infected during the symptomatic phase were excluded. Contacts where the index person had been wearing a face mask/visor and one-time contacts where the index person was not capable of knowing the future outcome were all excluded.
3.7. Statistical analyses
Given the explorative nature of the study, no sample size calculations were performed. Numbers of enrolled participants were based on the number of eligible study subjects within the time frame of the study. Graphs and data analyses were performed using GraphPad Prism 7.0 and STATA 13.1. Specific statistical tests to assess changes from baseline are called out in the figure legends. Test for normality distribution was by Shapiro-Wilk test. Mann-Whitney U test or Students t-test were used for comparison between groups as appropriate. Spearman rank correlation coefficient was used to assess significant correlations.
3.8. Role of the funding source
The funding source had no role in the study design, collection, analysis, and interpretation of data, writing of the paper or the decision to submit the paper for publication.
4. Results
4.1. Cohort and study visits
We recruited 203 convalescent individuals with a history of a positive RT-PCR test for SARS-CoV-2 during acute infection. Ninety-two (45⋅3%) of the participants were females. There was no significant difference in age between males and females. Baseline and clinical characteristics are shown in Table 1. At time point 1, all participants had fully recovered from COVID-19 symptoms (with the exception of loss of smell and/or taste and cognitive deficits). Median time since onset of symptoms was 45 days (range: 18–76) and median time since recovery was 31 days (range: 14–61). An additional pharyngeal swab was done for 93 of the 203 participants 4 months (time point 2) after onset of symptoms (median 108 days, range 68–127 for all 93 participants). COVID-19 severity (Table 1) in this cohort varied from grade 1 (8⋅4%) and 2 (74⋅9%) (both ambulatory) to requiring hospital admission without/with supplementary oxygen (4⋅4% and 8⋅8%) and intensive care unit treatment (3⋅4%).
4.2. Persistent SARS-CoV-2 PCR positivity after recovery of acute infection
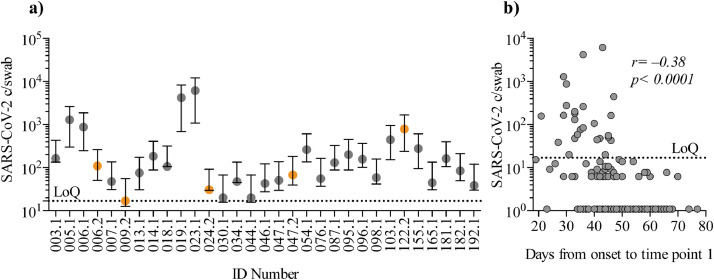
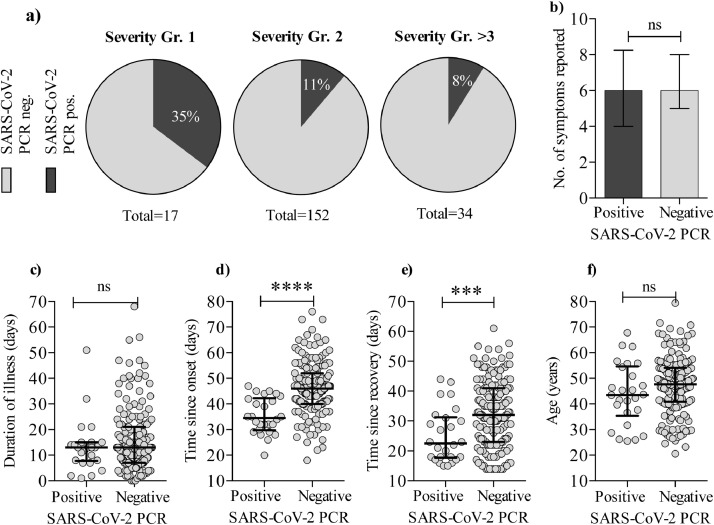
At time point 1, 26 individuals (12⋅8% of 203) were positive for SARS-CoV-2 a median of 23 days (range 15–44) after full recovery. Moreover, 5 individuals (5⋅3% of 93 individuals) were positive at time point 2 up to 105 days after recovery (median, range 85–105, for the 5 individuals) (Fig. 1a). Only 2 of the 5 (ID 006 and 047) were positive on both time points. The remaining 3 individuals testing positive only at time point 2, did not experience re-onset of COVID-19-like symptoms from time point 1 to time point 2. In total, 29 individuals (14⋅3%) tested positive several weeks–months after recovery. Timespan from onset of symptoms until time point 1 and viral copy numbers were inversely correlating (Spearman's rank correlation coefficient: p < 0⋅0001, r = −0⋅38 Fig. 1b). Further, a higher proportion of participants with grade 1, mild/asymptomatic COVID-19 illness had a positive PCR test at time point 1, compared to severity groups 2 or >3 (moderate illness, hospitalized or in ICU) (Fig. 2a). There was no difference in the number of symptoms reported (e.g. headache, fever, cough etc.) between the PCR positive and negative group (Fig. 2b) and no difference in the duration of illness (Fig. 2c). We found, that for the negative vs. positive group, there was a significantly longer timespan from onset of illness until sample collection (Mann-Whitney U test: p < 0⋅0001) (Fig. 2d). Also, timespan from recovery until sample collection was longer for the PCR negative vs. PCR positive group (Mann-Whitney U test: p = 0⋅001) (Fig. 2e). In contrast to previous reports, we did not find an association between age and persistent viral RNA detection (Fig. 2f)[5]. In conclusion, while the fraction of SARS-CoV-2 PCR positive individuals declined over time for the entire cohort, we detected SARS-CoV-2 RNA for as long as 109 days after onset of COVID-19. We found that participants in COVID-19 disease severity group 1 were more likely to be persistently PCR positive.
Fig. 1.
SARS-CoV-2 PCR copy number at time point 1 and 2. a) Positive SARS-CoV-2 copies/swab (c/swab) for the cohort at first and second visit. X-axis show ID numbers and visit time points. Time point 1 is grey and time point 2 is orange. Y-axis depicts detected SARS-CoV-2 c/swab as measured by digital droplet PCR (log10). Only positive values with copy number above limit of quantification (LoQ) is shown. Error bars are 95% confidence intervals. b) Correlation between time from symptom onset to sample collection on time point 1 (days) and copy number per swab. X-axis depicts time from onset. Y-axis show SARS-CoV-2 c/swab (log10). LoQ was determined to ≥ 3 events (see materials and methods section). Statistical analyses were by Spearman´s correlation coefficient, and the analyses included all data points.
Fig. 2.
Clinical characteristics of persistent SARS-CoV-2 positivity. PCR outcome vs. severity of COVID-19 disease. a) Pie charts depicting the proportion of participants who had a positive PCR on time point 1 in each on the COVID-19 severity groups (as described in table 1). b) Graph shows the number of symptoms reported during illness for the PCR positive vs. PCR negative group. c) The duration of COVID-19 illness for the PCR positive vs. PCR negative group. d) Time since symptom onset (Mann-Whitney U test: p < 0⋅0001) and e) time since recovery (Mann-Whitney U test: p = 0⋅001) for the PCR negative vs. positive group. f) Age distribution for the PCR positive vs. PCR negative group. Error bars are shown as median with IQR. Statistical comparisons were by Mann Whitney U test. (ns: not significant. *: p < 0⋅05; **: p < 0⋅01; ***: p < 0⋅001; ****: p < 0⋅0001).
4.3. Seroconversion does not affect viral RNA shedding
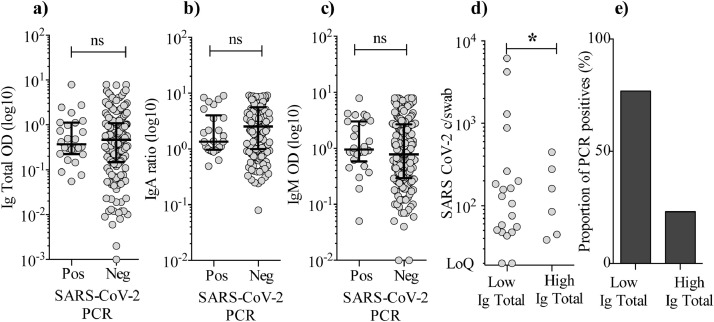
Next, we analyzed serum levels of SARS-CoV-2-specific total Ig, IgA, and IgM. We found that 202 individuals (99⋅5%) had seroconverted at time point 1, a median of 45 days after symptom onset. Surprisingly, there was no difference in the levels of total Ig, IgA or IgM in the PCR positive vs. PCR negative group (Fig. 3a–c). We divided the PCR positive individuals at time point 1 in two groups based on the level of total Ig, IgM, and IgA, respectively. The groups consisted of the 50% highest (n = 102) and the 50% lowest (n = 101), total Ig, IgM, and IgA (Fig. 3d–e). We found that the “high total Ig group” had a significantly lower SARS-CoV-2 copies/swab level and fewer PCR positives (6 individuals) compared to the “low total Ig group” (20 individuals) (Mann-Whitney U test: p = 0⋅014). For IgA and IgM, the trend was similar but not statistically significant. Thus, we conclude that seroconversion does not eliminate viral RNA from the pharyngeal mucosa, although a higher SARS-CoV-2-specific total Ig level may be associated with lower likelihood of persistent shedding.
Fig. 3.
Levels of SARS-CoV-2-specific total Ig, IgA, and IgM and the relation to PCR results. a–c) Levels of SARS-CoV-2-specific total immunoglobulins (Ig), IgA, and IgM measured in serum for participants tested PCR positive vs. negative at the time point 1 visit (median of 45 days after onset of illness). Total Ig is shown as OD values (sample diluted 1:100), IgA shown as ratio against standard, IgM shown as OD (diluted 1:11). Signal was read at 450 nm with reference measurements at 650 nm. Error bars are shown as median with IQR. Statistical comparisons were by Mann-Whitney U test. d) SARS-CoV-2 RNA c/swab for the 50% lowest (n = 101) total Ig group were compared to the copy number in the 50% highest total Ig group (n = 102). Only data points with a value above the LoQ (≥3 events by ddPCR) are depicted in the graph. Statistical analyses included all data points and were by Mann-Whitney U test (Ig total low vs. Ig total high; p = 0⋅014). e) Proportions of PCR positive individuals in the low total Ig group and high total Ig group.
4.4. Contacts of persistent post-symptomatic SARS-CoV-2 PCR positive individuals
To assess if persistent post-symptomatic viral RNA shedding leads to increased risk of transmission, we conducted assisted contact tracing for each PCR positive individual. The number of close contacts varied greatly (Table 2), as some individuals were working from home or isolated themselves in fear of transmitting the virus, while most returned to work in e.g. primary health care settings like elderly nursing homes. Among 757 close contacts, zero new COVID-19 infections were identified for a period of 23 days (median) after recovery, corresponding to a transmission risk of 0–0⋅13% for developing symptomatic COVID-19 among close contacts of persistent PCR positive individuals. Therefore we conclude that fully recovered individuals with persistent viral RNA shedding are unlikely to be a significant source of SARS-CoV-2 transmission.
Table. 2.
Contact Tracing Overview
| ID | SARS-CoV-2 c/swab | Household contactsa | Work-related contactsa | Private contactsa | Total | Infected contacts |
|---|---|---|---|---|---|---|
| 003 | 163 | 2 | 0 | 0 | 2 | 0 |
| 005 | 1293 | 3 | 0 | 0 | 3 | 0 |
| 006 | 878 | 4 | 21 | 13 | 38 | 0 |
| 007 | 48 | 2 | 11 | 9 | 22 | 0 |
| 013 | 76 | 0 | 0 | 10 | 10 | 0 |
| 014 | 183 | 1 | 0 | 3 | 4 | 0 |
| 018 | 106 | 3 | 0 | 15 | 18 | 0 |
| 019 | 4202 | 1 | 5 | 12 | 18 | 0 |
| 023 | 6145 | 1 | 0 | 12 | 13 | 0 |
| 030 | 20 | 0 | 0 | 2 | 2 | 0 |
| 034 | 48 | 0 | 0 | 2 | 2 | 0 |
| 044 | 20 | 1 | 0 | 52 | 53 | 0 |
| 046 | 43 | 2 | 15 | 20 | 37 | 0 |
| 047 | 51 | 4 | 1 | 7 | 12 | 0 |
| 054 | 262 | 5 | 103 | 5 | 113 | 0 |
| 076 | 55 | 5 | 5 | 0 | 10 | 0 |
| 087 | 131 | 0 | 68 | 28 | 96 | 0 |
| 095 | 200 | 1 | 2 | 0 | 3 | 0 |
| 096 | 157 | 0 | 0 | 4 | 4 | 0 |
| 098 | 59 | 1 | 40 | 7 | 48 | 0 |
| 103 | 447 | 4 | 42 | 15 | 61 | 0 |
| 155 | 277 | 2 | 2 | 0 | 4 | 0 |
| 165 | 45 | 4 | 71 | 10 | 85 | 0 |
| 181 | 162 | 1 | 23 | 0 | 24 | 0 |
| 182 | 85 | 4 | 37 | 15 | 56 | 0 |
| 192 | 39 | 4 | 9 | 10 | 23 | 0 |
| Total | 757 | 0 |
Only contacts that were not symptomatically or documented infected during the index persons' acute phase of the infection are included in the table
4.5. Breadth and magnitude of CD8 T-cell responses are increased in persistent PCR positive individuals
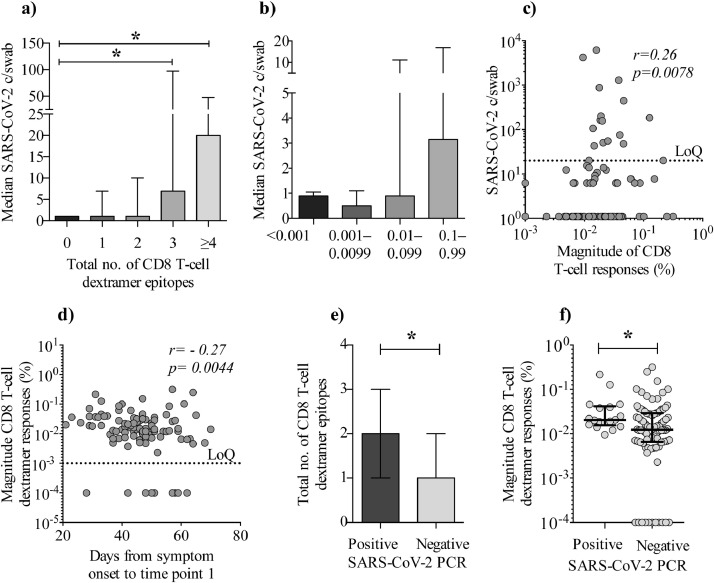
We analyzed SARS-CoV-2-specific CD8 T-cell responses using a dextramer stain investigating nine different CD8 T cell epitopes. We found that individuals with a higher pharyngeal viral load also had increased breadth and magnitude of the SARS-CoV-2-specific CD8 T-cell responses (Fig. 4a–b). Further, we found that the magnitude of the CD8 response correlated with SARS-CoV-2 copies/swab (Spearman's rank correlation coefficient: p = 0⋅0078, r = 0⋅26), suggesting that a higher viral load maintains a CD8 T-cell response (Fig. 4c). Also, we found a negative correlation (Spearman's rank correlation coefficient: p = 0⋅0044, r = −0⋅27) between the magnitude of the CD8 T-cell response and time from symptom onset to sample collection, indicating that the CD8 T-cell response wanes over time (Fig. 4d) comparable to what was observed for pharyngeal viral load (Fig. 1b). Lastly, we found that persistently PCR positive individuals had increased breadth and magnitude of the SARS-CoV-2-specific CD8 T-cell response compared to PCR negative individuals (Fig. 4e–f). Based on the data, we find evidence that persistent PCR positive individuals exhibits elevated SARS-CoV-2-specific CD8 T-cell immune responses.
Fig. 4.
Increased breadth and magnitude of CD8 T-cell responses in persistent PCR positive individuals. CD8 T-cell dextramer responses vs. SARS-CoV-2 PCR c/swab. a) The x-axis depicts the breadth of CD8 T-cell dextramer responses for HLA-A2-positive part of the cohort (n = 106), where ≥4 equals 4–7 epitopes. Y-axis shows the median copy number of SARS-CoV-2 copies per swab (c/swab) (Mann-Whitney U test: p = 0⋅02 and 0⋅03). b) X-axis depicts median SARS-CoV-2 c/swab. At the y-axis, individuals are grouped according to the magnitude of the accumulated/total CD8 T-cell dextramer responses (n = 106). c) X-axis depicts absolute SARS-CoV-2 c/swab and y-axis the magnitude of CD8 T-cell dextramer responses (Spearman's rank correlation coefficient: p = 0⋅0078, r = 0⋅26). d) Graph depicts the association between timespan from symptom onset until time point 1 (x-axis) vs. the magnitude of CD8 T-cell dextramer responses (y-axis) (Spearman's rank correlation coefficient: p = 0⋅0044, r = −0⋅27). e) Breadth of the CD8 T-cell responses shown as the total number of epitopes (y-axis) detected in PCR positive vs. PCR negative individuals (x-axis) (Mann-Whitney U test: p = 0⋅02). f) Magnitude of CD8 T-cells response (y-axis) for PCR positive individuals vs. PCR negative individuals (p = 0⋅01). Error bars are shown as median with IQR. Statistical comparisons were by Mann Whitney U test (*: p < 0⋅05). Correlations were Spearman's rank correlation coefficient.
5. Discussion
Our finding that a total of 14⋅3% of the participants were repeatedly PCR positive for a prolonged period after recovery is in line with previous data [5,7,19]. However, the duration of this period is greater than any previously reported (up to 109 days after onset for this cohort). Further, during follow-up, a subset of participants had remarkably high viral loads (103 copies per swab) which is comparable to what is found in symptomatic/acutely affected individuals [20] despite our sampling being more than 4 weeks after symptoms onset.
In contrast to previous studies, which have focused on the fraction of infected who are severely ill [4,[6], [7], [8], [9],21], our cohort consisted mainly of non-hospitalized individuals, which also represents the majority of COVID-19 cases [22,23]. Thus, the data presented here is representative for the general population and the results have high external validity.
During SARS-CoV-2 infection, most individuals develop varying levels of neutralizing antibodies, with higher titers observed in severe cases [24], [25], [26], [27], [28]. Additionally, studies demonstrate that SARS-CoV-2-specific T-cell immunity is also induced [29,30] and some SARS-CoV-2 exposed individuals, even develop T-cell responses in the absence of antibodies. Also, previously SARS-CoV-1 infected individuals possess preserved memory T cells which are responsive to SARS-CoV-2 years after the 2003-SARS outbreak [29,30]. Thus, T cells are likely crucial for the protection against and eradication of SARS-CoV-2. Interestingly, we found that on a cohort level, seroconversion did not affect SARS-CoV-2 RNA shedding, as individuals with high antibody levels were also observed in the PCR positive group. We also observed that individuals with immunoglobulin levels around or below the limit of detection were found in the PCR negative group. Therefore, seroconversion is not a requisite leading to complete elimination of viral RNA. On the contrary, we found that levels of SARS-CoV-2-specific CD8 T cells were increased in PCR positive individuals. We consider this finding important, for the following reasons; It has been suggested that persistent PCR positive signals originate from nucleic acid remnants [31] and also shown that SARS-CoV-2 outgrowth cultures can only be established if a corresponding RT-PCR Ct value is below 24 [32], implying that above this threshold, in vitro infectivity is insignificant. We used ddPCR, which is more sensitive and allows for an absolute quantitation of copy numbers as compared to RT-PCR[20]. As opposed to RT-PCR, ddPCR is shown to have a sensitivity of 100% in detecting SARS-CoV-2 [33]. Moreover, the RT-PCR assay is implemented with several different protocols and approaches around the world, why the Ct values are not reproducible and comparable across laboratories, machines or sample techniques [34]. We did not perform outgrowth culture of virus, as this also has certain limitations when viral copy numbers are relatively low. Pharyngeal swabs also collect mucosal neutralizing IgA, which can opsonize viral particles and thus bias the outcome of a swab-based culture. Further, the minimal amount of virus necessary in a biological specimen to initiate and propagate infection in vitro remains undefined making it even harder for in vivo projections. Given that RNA is labile and based on the anti-SARS-CoV-2 CD8 T cell data presented here, we propose that virus from persistent PCR positive individuals is in fact viable and/or intact, but that other factors, like e.g. mucosal IgA, prevents transmission [35]. Our observation, that SARS-CoV-2 is detectable months after recovery is in line with one other study, which recovered nucleic acids in the gut of convalescent individuals, associated with ongoing antibody evolution [36]. This antigen carrier state could become clinically significant if persistent PCR positive individuals are subsequently immunosuppressed [19,37].
Data on other virus infections (MERS-CoV, SARS-CoV-1, Ebola virus, Zika virus, Measles) demonstrate that viral shedding may be detectable for weeks–months after recovery despite ongoing antiviral immune responses and therefore this phenomenon is not unique to SARS-CoV-2 [38], [39], [40], [41]. An interesting perspective could be whether persistent viral RNA shedding leads to more durable immunity in these individuals. Follow-up studies addressing this issue are needed.
We show that individuals with persistent virus are not contagious, as we performed extensive assisted contact tracing and were unable to identify a single case of transmission from these post-symptomatic carriers. A limitation is that we did not test the contacts of the index persons and by our exclusion of one-time contacts.
Another limitation to our conclusion, is that we cannot definitively rule out, that the 3 individuals who only tested positive at time point 2 were not in fact re-infections. We do however find the possibility of re-infections unlikely, because studying risk of transmission in a Danish setting has been optimal for the following reasons; Danish authorities have not encouraged the use of facemasks until July 31st, and thus not during the time of this study. The use of them in public has been completely absent. Further, testing for COVID-19 is free and widely available – even without symptoms or referral. During April–June 2020, R0 has been below or equal to 1 in Denmark [42] and comprehensive measures were taken by authorities to prevent community transmission [43]. Re-infection is a very rare event – even in high-prevalence areas [44]. With low prevalence of COVID-19, new infections or debut of COVID-19 symptoms in the closest relations were efficiently traced, detected and tested. We find it unlikely that transmission events resulting in symptomatic infection would go unnoticed. Asymptomatic infections may however have gone undocumented. Our conclusion, that post-symptomatic persistent virus is not transmitted in vivo is in line with previous studies suggesting, that most transmissions occur in the pre-symptomatic stage [45]. We therefore conclude, that SARS-CoV-2 RNA can be recovered in pharyngeal mucosa ≥105 days after recovery. Further, insufficient levels of circulating SARS-CoV-2-specific antibodies do not explain this persistent viral RNA shedding. SARS-CoV-2 RNA detection is characterized by an increased anti-SARS-CoV-2-specific CD8 T-cell response suggestive of low-level viral persistence resulting in ongoing immune stimulation.
6. Contributors
LKV, SFN, MT, MHP, OSS, MHS, LØ contributed to study design, data collection, analyses and interpretation. LKV handled all legal and ethical permissions, participants’ logistics and recruitment, performed the literature search, created figures and tables. JFH, JDG, and RA contributed to participant recruitment, data collection, and clinical management. RO, MHP, SFN, GSF, IM AFA, MMT, CVK, SDA contributed to experiments, analyses, and data interpretation. LKV, MT and OSS wrote the first manuscript draft. All authors read and approved the final version of the manuscript. All authors had access to the raw data, which was verified by LKV, SFN, MT, GSF, IM, RO and MHS. All authors reviewed and approved the final version of the paper.
7. Data Sharing
Individual participant data cannot be made available due to EU Data Protection Regulations (GDPR). A limited and completely anonymized version of the dataset can be obtained upon request. Study protocols, including laboratory protocols will be available upon request. Proposals should be directed to linvib@rm.dk or marttols@rm.dk
Funding
Funding was from the Danish Ministry for Research and Education and The Danish Innovation Fund.
Declaration of Competing Interest
The authors report no conflict of interest.
Acknowledgement
We thank all study participants who devoted their time for the research and kindly donated their biological material. We thank staff at The Clinical Research Unit, Dept. of Infectious Diseases, Aarhus University Hospital for assistance with participant recruitment, logistics, laboratory assistance and productive discussions. This study was supported by a grant from the Danish Ministry for Research and Education (grant# 0238-00001B) and The Danish Innovation Fund (grant# 0208-00018B).
References
- 1.Zhou P, Yang X-L, Wang X-G, Hu B, Zhang L, Zhang W. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization. COVID-19 weekly epidemiological update. World Heal Organ. 2020 December 27. [Google Scholar]
- 3.Wu Yongjian, Guo C, Tang L, Hong Z, Zhou J, Dong X. Prolonged presence of SARS-CoV-2 viral RNA in faecal samples. Lancet Gastroenterol Hepatol. 2020;5:434–435. doi: 10.1016/S2468-1253(20)30083-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wu J, Liu X, Liu J, Liao H, Long S, Zhou N. Coronavirus Disease 2019 test results after clinical recovery and hospital discharge among patients in China. JAMA Netw Open. 2020;3 doi: 10.1001/jamanetworkopen.2020.9759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hartman WR, Hess AS, Connor J. Persistent viral RNA shedding after COVID-19 symptom resolution in older convalescent plasma donors. Transfusion. 2020:1–3. doi: 10.1111/trf.15927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang B, Liu S, Dong Y, Zhang L, Zhong Q, Zou Y. Positive rectal swabs in young patients recovered from coronavirus disease 2019 (COVID-19) J Infect. 2020;81:e49–e52. doi: 10.1016/j.jinf.2020.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xu K, Chen Y, Yuan J, Yi P, Ding C, Wu W. Factors associated with prolonged viral RNA shedding in patients with Coronavirus Disease 2019 (COVID-19) Clin Infect Dis. 2020;71:799–806. doi: 10.1093/cid/ciaa351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu W-D, Chang S-Y, Wang J-T, Tsai M-J, Hung C-C, Chia-Lin H. Prolonged virus shedding even after seroconversion in a patient with COVID-19. J Infect. 2020;81:329–331. doi: 10.1016/j.jinf.2020.03.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Molina LP, Chow S-K, Nickel A, Love JE. Prolonged detection of severe acute respiratory syndrome Coronavirus 2 (SARS-CoV-2) RNA in an obstetric patient with antibody seroconversion. Obstet Gynecol. 2020:1–4. doi: 10.1097/AOG.0000000000004086. [DOI] [PubMed] [Google Scholar]
- 10.Galán I, Velasco M, Casas L, Goyanes J, Rodríguez-Caravaca G, Losa JE. SARS-CoV-2 seroprevalence among all workers in a teaching hospital in Spain: unmasking the risk. MedRxiv. 2020:1–32. [Google Scholar]
- 11.Celebi G, Piskin N, Beklevic¸ AC, Altunay Y, Keles AS, Tüz MA. Specific risk factors for SARS-CoV-2 transmission among health care workers in a university hospital. Am J Infect Control. 2020:1–6. doi: 10.1016/j.ajic.2020.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sudre CH, Murray B, Varsavsky T, Graham MS, Penfold RS, Bowyer RC, et al. Attributes and predictors of Long-COVID: analysis of COVID cases and their symptoms collected by the Covid Symptoms Study App. MedRxiv 2020.
- 13.Lassaunière R, Frische A, Harboe Z, Nielsen AC, Fomsgaard A, Krogfelt K. Evaluation of nine commercial SARS-CoV-2 immunoassays. MedRxiv. 2020:1–15. [Google Scholar]
- 14.Beavis KG, Matushek SM, Precy A, Abeleda F, Bethel C, Hunt C. Evaluation of the EUROIMMUN Anti-SARS-CoV-2 ELISA Assay for detection of IgA and IgG antibodies. J Clin Virol. 2020;129:1–3. doi: 10.1016/j.jcv.2020.104468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brochot E, Demey B, Handala L, Duverlie G, Castelain S, François C. Comparison of different serological assays for SARS-CoV-2 in real life. J Clin Virol. 2020;130 doi: 10.1016/j.jcv.2020.104569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lai J, Tan WJ, Too CT, Choo JAL, Wong LH, Mustafa FB. Targeting Epstein-Barr virus-transformed B lymphoblastoid cells using antibodies with T-cell receptor-like specificities. Blood. 2016;128:1396–1407. doi: 10.1182/blood-2016-03-707836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dolton G, Lissina A, Skowera A, Ladell K, Tungatt K, Jones E. Comparison of peptide-major histocompatibility complex tetramers and dextramers for the identification of antigen-specific T cells. Clin Exp Immunol. 2014;177:47–63. doi: 10.1111/cei.12339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.The Danish Health Authority. https://www.sst.dk/dk/en/English/Corona-eng/FAQ. Accessed July 27, 2020.
- 19.Avanzato VA, Matson MJ, Seifert SN, Pryce R, Williamson BN, Anzick SL. Case Study: Prolonged infectious SARS-CoV-2 shedding from an asymptomatic immunocompromised cancer patient. Cell. 2020 doi: 10.1016/j.cell.2020.10.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yu F, Yan L, Wang N, Yang S, Wang L, Tang Y. Quantitative Detection and Viral Load Analysis of SARS-CoV-2 in Infected Patients. Clin Infect Dis. 2020;71:793–798. doi: 10.1093/cid/ciaa345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Burke RM, Midgley CM, Dratch A, Fenstersheib M, Haupt T, Holshue M. Active monitoring of persons exposed to patients with confirmed COVID-19 - United States. MMWR Morb Mortal Wkly Rep. 2020;69:245–246. doi: 10.15585/mmwr.mm6909e1. January-February 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eythorsson E, Helgason D, Ingvarsson RF, Bjornsson HK, Olafsdottir LB, Bjarnadottir V. Clinical spectrum of coronavirus disease 2019 in Iceland: population based cohort study. BMJ. 2020;371:1–9. doi: 10.1136/bmj.m4529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu Z, McGoogan JM., Characteristics of and Important Lessons from the Coronavirus Disease COVID-19) Outbreak in China: summary of a report of 72314 cases from the Chinese Center for Disease Control and Prevention. JAMA - J Am Med Assoc 2020. 2019;323:1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 24.Robbiani DF, Gaebler C, Muecksch F, Lorenzi JCC, Wang Z, Cho A. Convergent antibody responses to SARS-CoV-2 in convalescent individuals. Nature. 2020;584:437–442. doi: 10.1038/s41586-020-2456-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rogers TF, Zhao F, Huang D, Beutler N, Abbott RK, Ricketts J. Isolation of potent SARS-CoV-2 neutralizing antibodies and protection from disease in a small animal model. Science. 2020;369:956–963. doi: 10.1126/science.abc7520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wu Y, Wang F, Shen C, Peng W, Li D, Zhao C. A noncompeting pair of human neutralizing antibodies block COVID-19 virus binding to its receptor ACE2. Science. 2020;368:1274–1278. doi: 10.1126/science.abc2241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ju B, Zhang Q, Ge X, Wang R, Yu J, Shan S. Human neutralizing antibodies elicited by SARS-CoV-2 infection. Nature. 2020:1–21. doi: 10.1038/s41586-020-2380-z. [DOI] [PubMed] [Google Scholar]
- 28.Wu F, Wang A, Liu M, Wang Q, Chen J, Xia S. Neutralizing antibody responses to SARS-CoV-2 in a COVID-19 recovered patient cohort and their implications. MedRxiv. 2020:1–10. [Google Scholar]
- 29.Sekine T, Perez-Potti A, Rivera-Ballesteros O, Strålin K, Gorin J-B, Olsson A. Robust T cell immunity in convalescent individuals with asymptomatic or mild COVID-19. Cell. 2020:1–37. doi: 10.1016/j.cell.2020.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Le Bert N, Tan AT, Kunasegaran K, Tham CYL, Hafezi M, Chia A. SARS-CoV-2-specific T cell immunity in cases of COVID-19 and SARS, and uninfected controls. Nature. 2020;584:457–584. doi: 10.1038/s41586-020-2550-z. [DOI] [PubMed] [Google Scholar]
- 31.Atkinson B, Petersen E. SARS-CoV-2 shedding and infectivity. The Lancet Corresp. 2020:1339–1340. doi: 10.1016/S0140-6736(20)30868-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bullard J, Dust K, Funk D, Strong JE, Alexander D, Garnett L. Predicting infectious SARS-CoV-2 from diagnostic samples. Clin Infect Dis. 2020:1–11. doi: 10.1093/cid/ciaa638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dang Y, Liu N, Tan C, Feng Y, Yuan X, Fan D. Comparison of qualitative and quantitative analyses of COVID-19 clinical samples. Clin Chim Acta. 2020;510:613–616. doi: 10.1016/j.cca.2020.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rhoads D, Peaper DR, She RC, Nolte FS, Wojewoda CM, Anderson NW. College of American Pathologists (CAP) Microbiology Committee Perspective: Caution Must Be Used in Interpreting the Cycle Threshold (Ct) Value. Clin Infect Dis. 2020:1–2. doi: 10.1093/cid/ciaa1199. [DOI] [PubMed] [Google Scholar]
- 35.Faustini SE, Jossi SE, Perez-Toledo M, Shields A, Allen JD, Watanabe Y. Detection of antibodies to the SARS-CoV-2 spike glycoprotein in both serum and saliva enhances detection of infection. MedRxiv. 2020:1–17. [Google Scholar]
- 36.Gaebler C, Wang Z, Lorenzi JCC, Muecksch F, Finkin S, Tokuyama M, et al. Evolution of Antibody Immunity to SARS-CoV-2. BioRxiv 2020. [DOI] [PMC free article] [PubMed]
- 37.Helleberg M, Niemann CU, Moestrup KS, Kirk O, Lebech AM, Lane C. Persistent COVID-19 in an immunocompromised patient temporarily responsive to two courses of remdesivir therapy. J Infect Dis. 2020;222:1103–1107. doi: 10.1093/infdis/jiaa446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Oh M, Park WB, Choe PG, Choi S-J, Kim J-I, Jeesoo Chae BSSSP. Viral Load Kinetics of MERS Coronavirus Infection. N Engl J Med. 2016;375:1303. doi: 10.1056/NEJMc1511695. [DOI] [PubMed] [Google Scholar]
- 39.Sissoko D, Duraffour S, Kerber R, Kolie JS, Beavogui AH, Camara AM. Persistence and clearance of Ebola virus RNA from seminal fluid of Ebola virus disease survivors: a longitudinal analysis and modelling study. Lancet Glob Heal. 2017;5:e80–e88. doi: 10.1016/S2214-109X(16)30243-1. [DOI] [PubMed] [Google Scholar]
- 40.Paz-Bailey G, Rosenberg ES, Doyle K, Munoz-Jordan J, Santiago GA, Klein L. Persistence of Zika virus in body fluids – Final report. N Engl J Med. 2018;379:1234–1243. doi: 10.1056/NEJMoa1613108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lin WHW, Kouyos RD, Adams RJ, Grenfell BT, Griffin DE. Prolonged persistence of measles virus RNA is characteristic of primary infection dynamics. Proc Natl Acad Sci U S A. 2012;109:14989–14994. doi: 10.1073/pnas.1211138109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Statens Serum Institut. https://www.ssi.dk/aktuelt/nyheder/2020/reproduktionstallet-har-ligget-under-eller-omkring-1-siden-slutningen-af-april 2020:Accessed July 27, 2020.
- 43.Bundgaard H, Bundgaard JS, Raaschou-Pedersen DET, von Buchwald C, Todsen T, Norsk JB. Effectiveness of adding a mask recommendation to other public health measures to prevent sars-cov-2 infection in Danish mask wearers. Ann Intern Med. 2020:1–10. doi: 10.7326/M20-6817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Raddad LJA, Chemaitelly H, Malek JA, Ahmed AA, Mohamoud YA, Younuskunju S. Assessment of the risk of SARS-CoV-2 reinfection in an intense re-exposure setting. Clinl Infect Dis. 2020 doi: 10.1093/cid/ciaa1846. ciaa1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Oran DP, Topol EJ. Prevalence of Asymptomatic SARS-CoV-2 Infection - a narrative Review. Ann Intern Med. 2020:1–6. doi: 10.7326/M20-3012. [DOI] [PMC free article] [PubMed] [Google Scholar]