Abstract
T follicular helper (Tfh) cells are a subset of CD4+ T cells that promote antibody production during vaccination. Conventional dendritic cells (cDCs) efficiently prime Tfh cells; however, conclusions regarding which cDC instructs Tfh cell differentiation have differed between recent studies. We found that these discrepancies might exist because of the unusual sites used for immunization in murine models, which differentially bias which DC subsets access antigen. We used intranasal immunization as a physiologically relevant route of exposure that delivers antigen to all tissue DC subsets. Using a combination of mice in which the function of individual DC subsets is impaired and different antigen formulations, we determined that CD11b+ migratory type 2 cDCs (cDC2s) are necessary and sufficient for Tfh induction. DC-specific deletion of the guanine nucleotide exchange factor DOCK8 resulted in an isolated loss of CD11b+ cDC2, but not CD103+ cDC1, migration to lung-draining lymph nodes. Impaired cDC2 migration or development in DC-specific Dock8 or Irf4 knockout mice, respectively, led to reduced Tfh cell priming, whereas loss of CD103+ cDC1s in Batf3−/− mice did not. Loss of cDC2-dependent Tfh cell priming impaired antibody-mediated protection from live influenza virus challenge. We show that migratory cDC2s uniquely carry antigen into the subanatomic regions of the lymph node where Tfh cell priming occurs—the T-B border. This work identifies the DC subset responsible for Tfh cell–dependent antibody responses, particularly when antigen dose is limiting or is encountered at a mucosal site, which could ultimately inform the formulation and delivery of vaccines.
INTRODUCTION
Vaccines are one of the most important medical interventions in global health. Rational designs are needed to extend vaccination efforts through antigen dose sparing, particularly during acute pandemic vaccine production (1). Because most vaccines protect the host via inducing antibodies, a clearer understanding of the cellular pathways that result in efficient antibody induction could facilitate this goal. Work over the past decade has comprehensively identified the T cells required for promoting effective antibody responses. T follicular helper (Tfh) cells promote long-lived, high-affinity antibody production by B cells (2, 3). It was recently shown that dendritic cells (DCs) were required for the first phase of Tfh cell initiation unless high antigen doses are used during immunization (4, 5). Yet, it remains unclear which DC subsets induce Tfh cell differentiation (2, 6); therefore, our goal was to identify which DC subset(s) initiates Tfh cell priming.
DCs are a heterogeneous population of cells. On the basis of lineage commitment, they are classified into plasmacytoid DCs, monocyte-derived DCs, and conventional/classical DCs (cDCs) (7); the latter group of cells uniquely express the transcription factor Zbtb46 and are primarily responsible for naïve T cell activation and Tfh cell induction (8, 9). cDCs can be further divided into two subsets based on ontogeny: type 1 cDCs (cDC1s) develop in a BATF3 [basic leucine zipper transcriptional factor, activating transcription factor (ATF)–like 3]–dependent and IRF8 (interferon regulatory factor 8)–dependent manner, whereas cDC2s require the transcription factor IRF4 (7). These two cDC populations differ in cell surface marker expression, cytokine production, and anatomic locations at steady state. Tissue cDCs survey for infection or host damage, which, if detected, induces their migration to draining lymph nodes (LNs). In contrast, LN-resident cDCs acquire antigen that drains via lymphatics or is carried into LNs by migratory tissue DCs. These distinctions make each DC subset specialized to drive particular T cell responses (7).
Which DC subset is best suited to induce Tfh cell responses? Approaches such as antibody-mediated antigen delivery to DC subsets revealed that targeting cDC subset either in the tissue or in the spleen leads to a robust Tfh cell response (10–12). However, these methods do not reflect the natural route or form of antigen encounter by a DC. Other studies have administered antigen via footpad or ear injection (13–16), which results in the rapid appearance of antigen in the draining LN (15, 17, 18). If antigen is delivered under these conditions, LN-resident DCs, in the absence of migratory DCs, are capable of inducing weak Tfh cell responses (13–15), although this finding was questioned by a recent study (18).
Given the requirement for DC migration in the induction of effective T helper cell responses (17, 19), we aimed to test whether Tfh cells also have specific requirements for migratory DCs. Alternatively, perhaps the method of immunization used in these studies biased the type of DC responsible for Tfh cell induction. To address these two possibilities, we used a natural route of antigen exposure via the nares. Despite the importance of inhalational sensitization both for beneficial states, such as vaccination, and pathological states, such as allergic responses, no study has evaluated which DCs in the lung or lung-draining LNs induce Tfh cells to inhaled antigens. We demonstrate that this method, unlike other routes of immunization (20), exposes all tissue DC subsets to antigen and does not force antigen into LNs via inoculation into a restricted tissue compartment. Using this immunization route in mice with specific DC subset deletion or paralysis, we conclude that migratory cDC2s are necessary and sufficient for Tfh cell priming to inhaled antigens. We further show that cDC2s express a unique constellation of chemokine receptors and, accordingly, are strategically positioned within LNs to direct Tfh differentiation. Identifying the primary cell responsible for an effective antibody response has direct implications on how we administer vaccines for efficient antibody responses.
RESULTS
Migratory DCs are required for inducing Tfh responses to subcutaneous antigens
Soluble antigen can drain to LNs, and this has been suggested as one mechanism by which LN-resident DCs acquire antigen and prime Tfh cells (13–15). However, these studies used a method of immunization (via footpad or ear pinna injection) that we found to deliver antigen within 30 min to LN phagocytic cell types, including all LN DC subsets and B cells (Fig. 1A). This is consistent with previous studies using low–molecular weight tracers demonstrating antigen in LNs within minutes of footpad injection (21, 22) or intra-auricular injection even after ear removal (18). Presumably, this is due to injection of a relatively large volume into a confined tissue space, thus forcing antigen into lymphatics via increased hydrostatic pressure (23) and bypassing the need for DC migration. Because B cells and DCs can compensate for each other in Tfh cell priming when antigen doses are high (4, 24), it is difficult to define the relative role of DCs and B cells with this form of immunization. Injection of the equivalent dose of antigen into less constrained spaces—such as subcutaneously, epicutaneously, or intraperitoneally—does not result in substantial antigen presence within 30 min (Fig. 1A), suggesting that active carriage via DCs is an essential route for antigen access to LNs (25). Therefore, we first addressed the role of migratory versus LN-resident DCs in Tfh cell priming using a subcutaneous immunization route in two mouse strains with impaired DC migration: Ccr7−/− and Dock8−/− mice (fig. S1A).
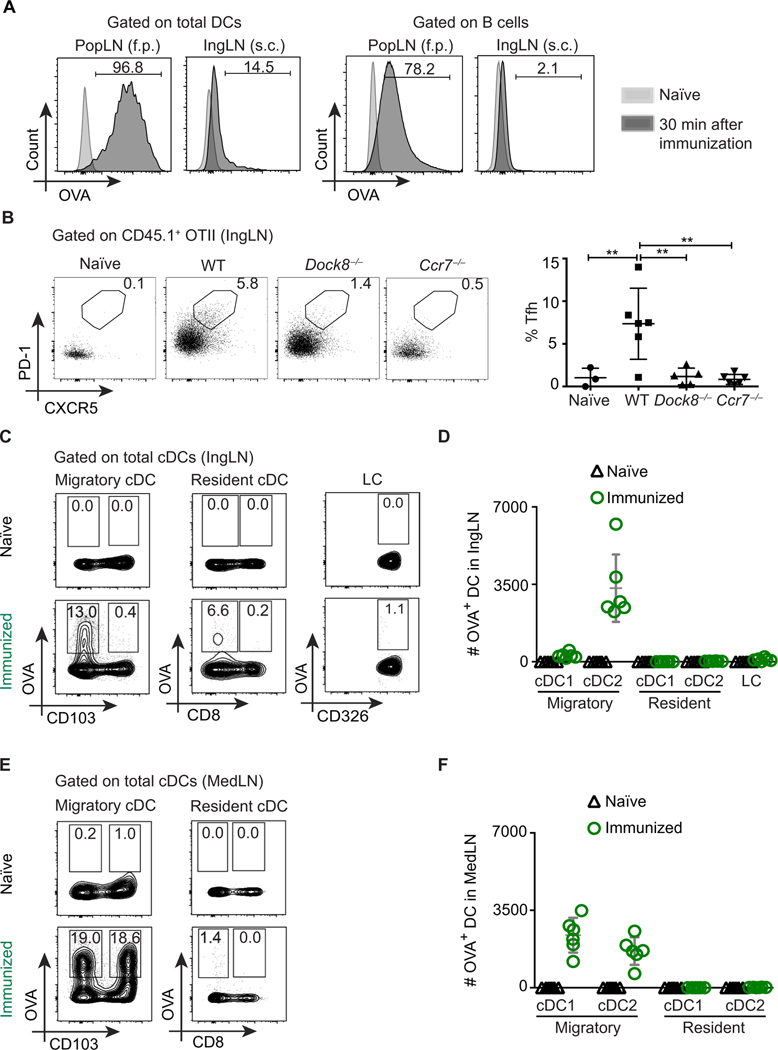
Fig. 1. Migratory cDCs are crucial for Tfh cell differentiation.
(A) WT mice were immunized with 50 μg of OVA–Alexa Fluor 647 (AF647) and 1 μg of LPS in each footpad (50 μl) or subcutaneously (s.c.) in the flank (100 μl); draining IngLNs or popliteal LNs (PopLNs) were analyzed 30 min after immunization. Overlays show DCs (left) and B cells (right) from immunized (dark gray) and naïve controls (light gray). f.p. footpad. (B) WT, Dock8−/−, and Ccr7−/− recipient mice were adoptively transferred with congenic OTII cells, immunized subcutaneously with OVA (10 μg per flank) and LPS (1 μg per flank), and analyzed 6 days later. PD-1, programmed cell death protein 1. Unimmunized WT mice were used as naïve controls. Representative data depicting the frequency of OTII Tfh cells (CD44+ PD-1+ CXCR5+) in IngLN of recipient mice (left) and cumulative data from individual mice (right) are shown. (C and D) Frequency (%) (C) and absolute counts (D) of OVA+ DC subsets from WT mice 18 hours after subcutaneous immunization with 50 μg of OVA-AF647 and 1 μg of LPS per flank (see fig. S1C). (E) Frequency (%) and (F) absolute counts of OVA+ DC subsets from WT mice 18 hours after intranasal immunization with 50 μg of OVA-AF647 and 1 μg of LPS per mouse (see fig. S2C). Data shown are representative of three to four independent experiments with two to six mice per group. Each symbol represents an individual mouse.
CCR7 is the major chemokine receptor that directs DC migration from peripheral tissues into LNs, and in its absence T cell activation is impaired (19). However, LN architecture is disrupted in Ccr7−/− mice; therefore, we also chose to study Tfh cell activation in Dock8−/− mice. DOCK8 (dedicator of cytokinesis 8) is a member of the DOCK family of atypical guanine nucleotide exchange factors that regulate the activity of Rho-family GTPases. Dock8-deficient mice and patients have impaired long-lived high-affinity antibody production (26, 27). We and others previously reported that Dock8-deficient mice have a DC migration defect (28, 29) without substantial disruption of secondary lymphoid organ structure (30). However, deficiency of either Dock8 or Ccr7 impairs T cell function in an intrinsic manner (19, 31, 32). Thus, to study the impact of DCs on Tfh cell responses, we used a model system in which differentiation of adoptively transferred wild-type (WT) ovalbumin (OVA)–specific CD4+ T cells (OTII cells) was evaluated 6 days after antigen exposure. This early time point gauges the first phase of Tfh cell differentiation, one that is DC-dependent (5, 33); in contrast, later stages of Tfh development/maintenance in most models require cognate B cells (33, 34). Further, we used a high antigen dose to potentially promote passive antigen drainage to LNs and LN-resident DC access to antigen. This higher dose overcame the CD4+ T cell activation defect caused by loss of DC migration in Dock8-deficient mice (fig. S1B) (29). However, there was a profound loss of PD-1+ CXCR5+ Tfh cells in the draining LN 6 days after subcutaneous immunization in Ccr7-deficient and Dock8-deficient mice (Fig. 1B), suggesting that migratory DCs are required for Tfh cell differentiation.
Subcutaneous versus intranasal immunization delivers antigen to different DC subsets
Migratory cDCs from the skin can be further divided into CD103+ cDC1s and CD11b+ cDC2s. Similarly, LN-resident cDCs can be divided into CD8αα+ cDC1s and CD11b+ cDC2s (fig. S1C). An additional population of non-cDCs, Langerhans cells (LCs), exists uniquely in the skin and can transport antigen to draining LNs (35). To trace which DCs contain antigen after subcutaneous immunization, we used a fluorescently tagged protein antigen (OVA–Alexa Fluor 647) administered subcutaneously with an adjuvant, followed by evaluation of DC subsets in the draining inguinal LNs (IngLNs) 18 hours later. Most of the antigen+ DCs in the IngLN were migratory cDCs (Fig. 1, C and D). Very few LN-resident cDCs were OVA+, and, as expected, few LCs contained antigen at this early time point. Although migratory DCs contained most of the injected antigens, almost all antigens were concentrated in CD103-negative cDCs (Fig. 1, C and D), which we confirmed were CD11b+ cDC2s (fig. S1, C and D), consistent with a previous report using photoconvertible tracking of DC subsets (36). Analysis of cDC subsets within digested skin biopsies from naïve mice demonstrated a more than 10-fold difference in number of cDC1s and cDC2s (fig. S1E). Therefore, given the scarcity of dermal CD103+ cDC1s, subcutaneous immunization likely engages considerably fewer skin cDC1 compared with cDC2, potentially limiting the utility of this immunization route to study the nature of the DC subset responsible for Tfh cell induction.
To answer whether other routes of immunization target both migratory cDCs, we administered fluorescently tagged antigen via an intranasal route to WT mice (37) and assessed antigen uptake by pulmonary DC subsets (fig. S2A). A comparable number of pulmonary cDC1s and cDC2s contained soluble antigen (fig. S2B). We then examined antigen+ DC subsets in the lung-draining mediastinal LN (MedLN; fig. S2C) 18 hours after immunization. In contrast to subcutaneous route, intranasal immunization leads to comparable antigen+ migratory cDC1 and cDC2 subsets in the draining MedLN (Fig. 1, E and F). As in the IngLNs, most of the antigen was present in migratory, but not LN-resident, cDCs of the MedLN of WT mice even up to 3 days after immunization (fig. S2D). Therefore, intranasal, but not subcutaneous, immunization allows comparison of the role of migratory cDC1s versus cDC2s in priming Tfh cells to soluble antigens.
Dock8−/− mice have a cDC2 subset–specific migration defect
In previous work, we and others found an almost complete loss of antigen transport to skin-draining LNs in mice lacking Dock8 (Fig. 2A, top) (29); however, our current finding suggests that these studies only truly evaluated the ability of cDC2s to migrate. Using intranasal administration of fluorescent antigen, we next asked whether one or both cDC subsets failed to migrate to MedLNs in Dock8−/− mice. Despite normal frequencies of cDC subsets at steady state in LNs and lungs of Dock8−/− mice (fig. S3A) and antigen+ pulmonary cDC1 and cDC2 after immunization (Fig. 2B), migratory cDC2s failed to carry antigen to MedLNs (Fig. 2A, bottom). However, cDC1 migration in Dock8−/− mice was not impaired, unlike Ccr7−/− mice, which have a loss of all DC migration (Fig. 2A, bottom). Alveolar macrophages, which also express CD11c, were not altered in frequency (5.9 ± 0.5% of CD24−MHCIIloB220−TCR− versus 4.0 ± 0.3% in Dock8−/− lungs). As a control, we used Batf3−/− mice, which have a defect in cDC1 development (7), including in the lung (fig. S3A), and therefore demonstrated the expected loss of antigen+ cDC1 migration to MedLN (Fig. 2A). In contrast, the frequency of antigen+ cDC2s in both the MedLNs and IngLNs of Batf3−/− mice was not impaired (Fig. 2A and fig. S3B).
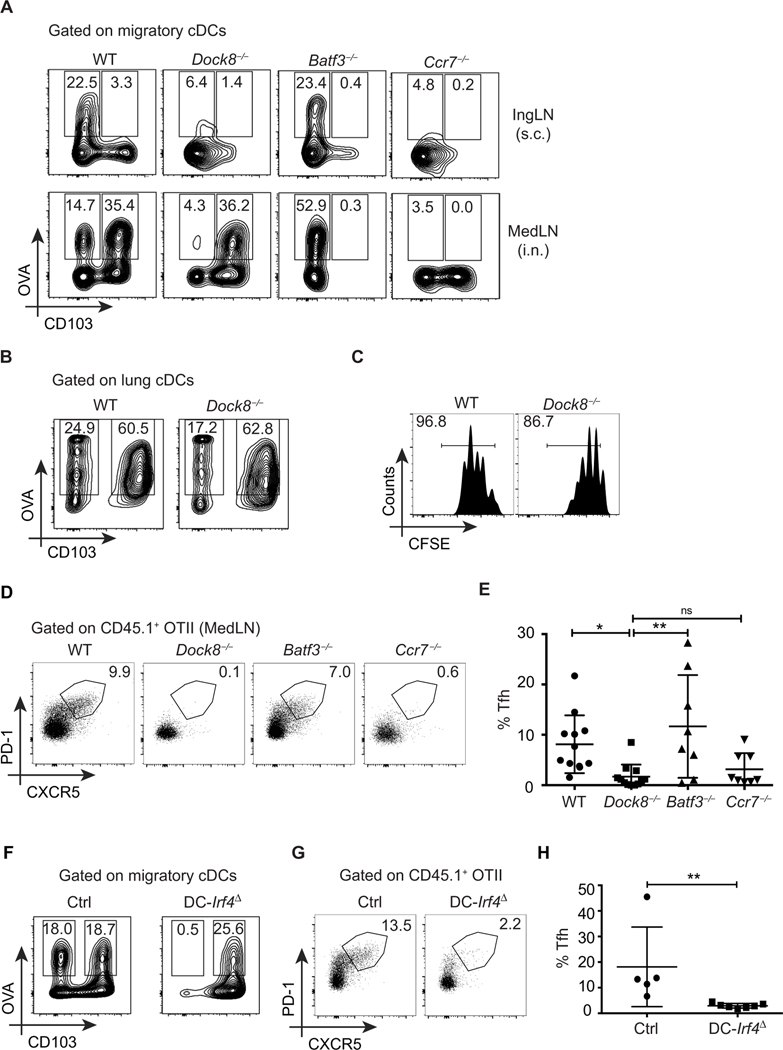
Fig. 2. Migratory cDC2s, not cDC1s, are necessary for Tfh cell induction.
(A) Frequency (%) of OVA+ migratory cDCs in WT, Dock8−/−, Batf3−/−, and Ccr7−/− 18 hours after immunization with OVA-AF647 and LPS administered either subcutaneously (IngLN; top) or intranasally (i.n.) (MedLN; bottom), as in Fig 1 (C and E, respectively). (B) Frequency of OVA+ lung cDCs in WT and Dock8−/− mice 6 hours after intranasal immunization with 50 μg of OVA-AF647 and 1 μg of LPS. Data shown are gated on cDCs, as in fig. S2A. (C) WT and Dock8−/− recipient mice were adoptively transferred with CFSE-labeled OTII cells, immunized intranasally with 10 μg of OVA and 1 μg of LPS, and analyzed for T cell proliferation 3 days later. (D) Congenic OTII cells were adoptively transferred into WT, Dock8−/−, Batf3−/−, and Ccr7−/− recipient mice, immunized intranasally with 10 μg of OVA and 1 μg of LPS, and analyzed 6 days later. Representative data depicting the frequency of OTII Tfh cells (CD44+ PD-1+ CXCR5+) in MedLNs of recipient mice are shown. (E) Pooled analyses of frequencies of OTII Tfh cells as in (D) from four independent experiments. (F) Frequency (%) of OVA+ migratory cDCs in DC-Irf4Δ and control (Ctrl) mice (Irf4fl/fl Itgax-Cre−) immunized intranasally with 50 μg of OVA-AF647 and 1 μg of LPS, as in (A). (G) DC-Irf4Δ and control mice were adoptively transferred with congenic OTII cells, immunized with 10 μg of OVA and 1 μg of LPS intranasally, and analyzed 6 days later for OTII Tfh cells, as in (D). (H) Pooled analyses of frequencies of OTII Tfh cells as in (G) from two independent experiments. Data shown are representative of two to four independent experiments with two to three mice per group.
Irf4-deficient mice are reported to have impaired development and migration of CD11b+ cDC2s (7, 38). To identify whether defective migration in Dock8−/− mice was a result of reduced Irf4 expression, we analyzed WT and Dock8−/− bone marrow–derived DCs (BMDCs) for expression of Irf4. Dock8−/− BMDCs have a normal expression of Irf4 as compared to WT BMDCs (fig. S3C). Further, in vitro activation with lipopolysaccharide (LPS) (the same adjuvant used in vivo for these studies) did not induce significantly more apoptosis in Dock8-deficient BMDCs after 24 hours of stimulation (fig. S3D). Over the same time frame in vivo, Dock8-deficient cDC2s failed to reach draining LNs, suggesting that this was not due to a survival defect. Therefore, DOCK8 selectively regulates the migration but not the development, survival, or antigen uptake of tissue cDC2s in an IRF4-independent manner, leaving cDC1 function intact. Consistent with these findings, T cell activation to inhaled antigens in Dock8−/− mice was only mildly impaired in the MedLN, as assessed by proliferation of adoptively transferred OTII cells (Fig. 2C). Therefore, intranasal immunization of Dock8-deficient versus Batf3-deficient mice enabled us to test the relative role of migratory cDC2s versus cDC1s in Tfh priming, respectively.
Migratory DCs are required for inducing Tfh responses to inhaled antigen
We used Dock8−/−, Batf3−/−, and Ccr7−/− mice, as well as mice with DC-specific deletion of Irf4, to delineate the roles of the two migratory cDC subsets in driving Tfh cell responses to inhaled antigens. Dock8-deficient patients and mice have impaired antibody responses (26, 27), including after intranasal immunization (fig. S3E), which has been primarily ascribed to impaired B cell function (26). It is not known whether this could also in part be due to defective priming of Tfh cells. Both Ccr7−/− and Dock8−/− mice failed to induce significant Tfh cell differentiation of WT OTII cells (Fig. 2, D and E). On the other hand, Batf3−/− mice had no defect in Tfh cell induction to inhaled antigen (Fig. 2, D and E), demonstrating that migratory cDC1s are dispensable for priming Tfh cells.
Together, these results suggest that migratory cDC2s are required for instructing Tfh cell differentiation; to test this in a second model, we generated a DC-selective Irf4 deletion using Itgax-Cre (CD11cCre) strain (subsequently referred to as DC-Irf4Δ mice) (7, 38). In line with previous reports, DC-Irf4Δ mice have impaired cDC2 development that leads to a selective loss of antigen+ cDC2s in the MedLN (Fig. 2F and fig. S3F). However, because of leakiness of the Irf4 conditional allele in B cells and T cells (even in Cre− mice; fig. S3G) (38) and the crippling of early germinal center (GC) B cell responses in the absence of Irf4, we limited our analyses to early Tfh differentiation in WT transferred T cells. Further, we excluded mice in which >20% of blood lymphocytes were green fluorescent protein–positive (Irf4-deleted) for experiments. As observed in Dock8-deficient mice, DC-Irf4Δ mice also have defective Tfh cell priming (Fig. 2, G and H). Therefore, using a second mouse strain, we confirmed that cDC2s are required to initiate Tfh cell differentiation.
Migratory cDC2s are necessary for Tfh-induced antibody responses
We next asked whether cDC2 migration was necessary for Tfh-driven high-affinity antibody production. Randall et al. demonstrated that Dock8 deficiency reduced the ability of B cells to form immune synapses, impairing late-stage antibody production (26). Because B cells are required for reinforcing the Tfh cell program (5, 33, 34), the loss of Tfh cells and OVA-specific immunoglobulin G1 (IgG1) in Dock8−/− mice could be due to defective signals from B cells instead of impaired cDC2 migration. To address this, we generated mice in which Dock8 could be depleted in a cell-specific manner (fig. S4A). Crossing Dock8flox/− mice with CD11cCre mice led to specific deletion of Dock8 in DCs (DC-Dock8Δ mice; fig. S4B). Unlike Dock8−/− mice (26, 29), DC-Dock8Δ mice had equivalent frequencies of splenic marginal zone B cells and T cells as control mice, suggesting that these cell types are not affected (fig. S4, C and D).
Similar to Dock8−/− mice, DC-Dock8Δ mice had a cDC2-specific migration defect, whereas cDC1 migration was intact (Fig. 3, A and B, and fig. S4E). Again, we did not observe significant antigen+ LN-resident cDC populations in either control or DC-Dock8Δ mice (Fig. 3B). DC-Dock8Δ mice immunized with inhaled antigen demonstrated significant loss of antigen-specific Tfh cells as compared to WT and control Dock8flox/− mice (Fig. 3, C and D, and fig. S4F). Loss of OVA+ CD11b+ cDC2s in immunized DC-Dock8Δ did not impair OTII activation or proliferation (fig. S4G) or effector T helper 1 (TH1) differentiation (Fig. 3E), which was comparable to previous results using a similar model (39). Therefore, the absence of migratory cDC2s impairs Tfh differentiation without inducing a pan-CD4+ T cell activation defect.
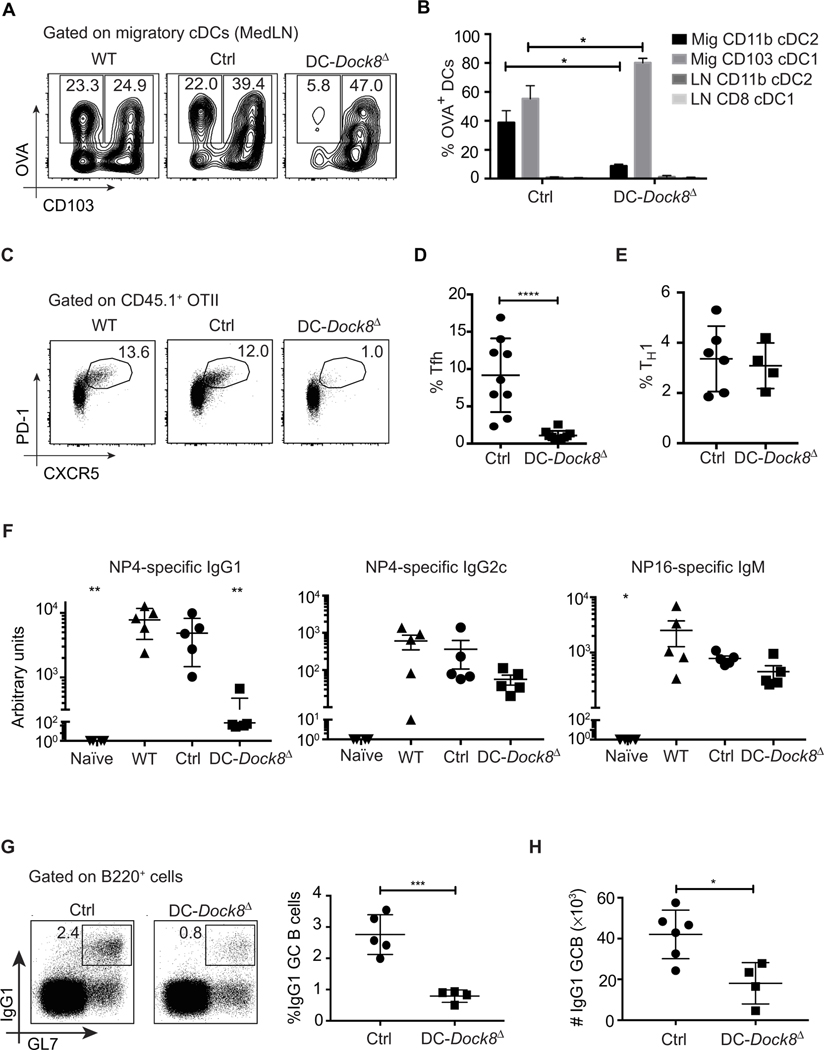
Fig. 3. DC-specific deletion of DOCK8 results in a cDC2-specific migration defect and a failure to prime Tfh cells.
(A and B) Representative data show the frequency of OVA+ migratory cDC subsets in WT, DC-Dock8Δ, and control mice (Dock8fl/− Itgax-Cre−) 18 hours after intranasal immunization with 50 μg of OVA-AF647 and 1 μg of LPS. (B) Distribution of OVA+ cells among cDC subsets in MedLNs of DC-Dock8Δ or control mice immunized as in (A). Mig, migratory cDCs; LN, LN-resident cDCs as in fig. S2C. (C) Frequency (%) of OTII Tfh cells (CD44+ PD-1+ CXCR5+) in MedLNs of WT, DC-Dock8Δ, and control mice immunized intranasally with 10 μg of OVA and 1 μg of LPS and analyzed 6 days later. (D) Pooled analyses of frequencies of OTII Tfh cells as in (C) from three independent experiments. (E) Frequencies of interferon-γ+ cells of transferred OTII cells in MedLN of mice immunized as in (C). (F) WT, DC-Dock8Δ, and control (Itgax-Cre−) mice were immunized and boosted intranasally with 10 μg of NP16-OVA (first immunization contained 1 μg of LPS). Unimmunized WT mice served as naïve controls. NP4-specific serum IgG1, IgG2c, and NP16-specific serum IgM from mice 21 days after intranasal immunization are shown. *P < 0.05 and **P < 0.01 in comparison to WT. (G) Representative data depicting the frequency of IgG1+ GL7+ GC B cells in MedLNs of DC-Dock8Δ or control mice 6 days after intranasal immunization with NP16-OVA and LPS. Pooled analyses of frequencies of IgG1+ GL7+ B cells are shown on the right. (H) Absolute counts of IgG1+ GL7+ B cells in MedLNs of DC-Dock8Δ or control mice immunized as in (G). Data are a representative of two (A, B, and F) or three (C to E, G, and H) independent experiments with three to six mice per group. Each symbol (D to H) represents an individual mouse.
Previous work has shown that early (e.g., day 6) Tfh cell induction after immunization is DC-dependent, whereas after this time point, B cells are required to reinforce Tfh differentiation (33, 40–42). Further, CD11c is expressed in a fraction of activated B cells (33), and DOCK8 loss has been shown to impair GC B cell responses, although at time points (days 7 to 11) after we typically evaluate for Tfh induction (26, 43). To confirm that the Tfh cell defect in DC-Dock8Δ mice was due to cDC2 loss and not due to off-target Dock8 deletion in activated B cells (33), we created mixed bone marrow chimeras using DC-Dock8Δ and Zbtb46-DTR (diphtheria toxin receptor) (7) donors. During diphtheria toxin treatment, cDCs in the chimeric mice are Dock8-deficient but in the presence of a WT complement of B cells. Again, we observed a significant impairment in Tfh cell differentiation of adoptively transferred OTII cells (fig. S4H). These results demonstrate that, although lung migratory CD103+ DCs are sufficient for T cell activation (Figs. 2C and 3E and fig. S4G), cDC2s are crucial for induction of the Tfh cell fate.
The hapten-protein conjugate system NP-OVA [(4-hydroxy-3-nitrophenyl)-acetyl–OVA] can be used to study T cell–dependent affinity maturation (44). Loss of Tfh cells in DC-Dock8Δ mice was associated with reduced high-affinity IgG1 production and class-switched GC B cells after intranasal immunization (Fig. 3, F to H). Isotypes driven by TH1 or T-independent mechanisms, such as IgG2c and IgM, respectively (45), were only modestly impaired in DC-Dock8Δ mice (Fig. 3F), and IgE was not significantly induced in any group (60.2 ± 27.3 ng/ml of total IgE in control versus 31.3 ± 38.3 in DC-Dock8Δ and 26.8 ± 16.4 in naïve mice). Therefore, migration of pulmonary cDC2s to LNs is required for priming Tfh cells and driving subsequent high-affinity IgG1 production.
Migratory cDC2s are sufficient for the first step of Tfh cell priming
To test whether migratory cDC2s are sufficient to drive Tfh cell responses, we administered 1.5-μm fluorescently tagged OVA-encapsulated microparticles intranasally, which cannot passively drain into MedLNs (37, 46), to WT, Dock8−/−, and Batf3−/− mice. Fluorescent microparticles were phagocytosed and transported to MedLN by both migratory cDC2s and cDC1s but were not present in resident LN DCs (Fig. 4A). Intranasal administration of OVA microparticles was sufficient to induce antigen-specific T cell activation in the MedLN (Fig. 4B) and resulted in comparable expansion of adoptively transferred OTII cells in WT, Batf3−/−, and Dock8−/− mice (Fig. 4C). However, only WT and Batf3−/− mice developed a significant Tfh cell population to these beads, whereas Dock8−/− mice demonstrated a profound loss of Tfh cells (Fig. 4D). Given the lack of cDC1s in Batf3−/− mice and the inability of antigen-encapsulated microparticles to freely drain to LNs, the presence of a substantial Tfh population in Batf3−/− mice demonstrated that migratory cDC2s are sufficient to induce the Tfh cell fate.
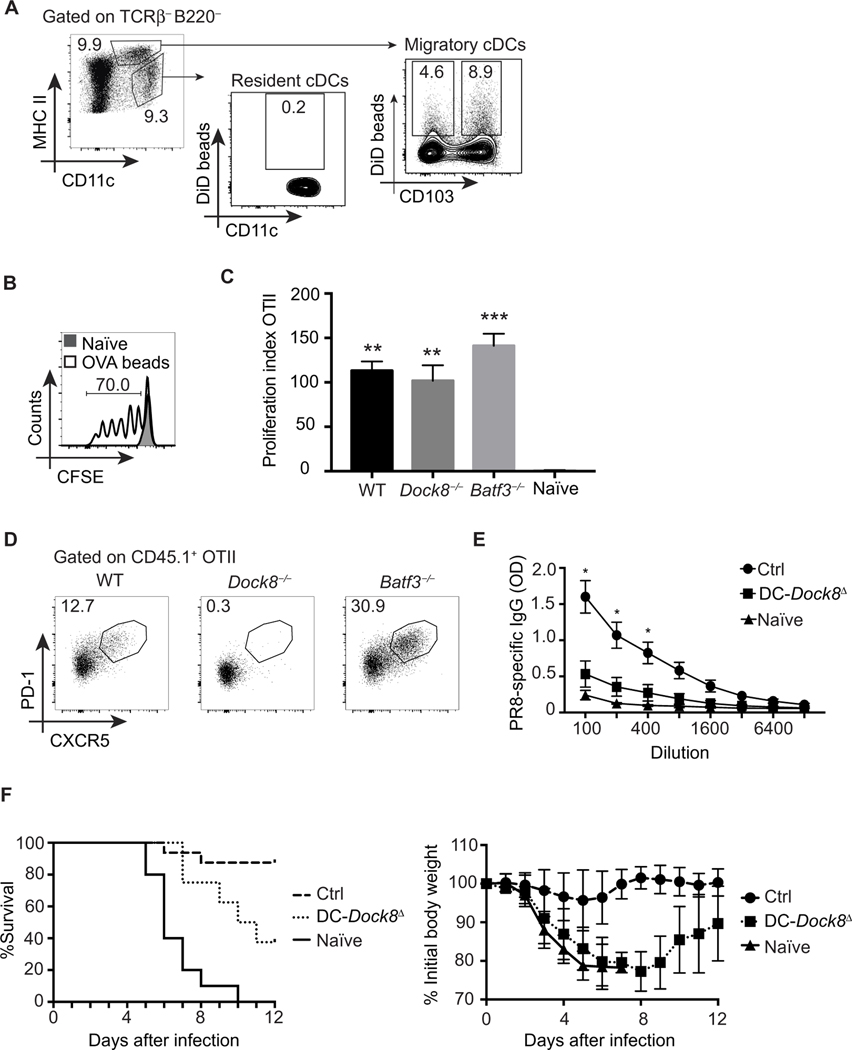
Fig. 4. Migratory cDC2s are sufficient for inducing Tfh cells to inhaled antigens.
(A) Representative data showing the frequency of nanoparticle-containing migratory and LN-resident cDCs in WT mice 18 hours after intranasal immunization with DiD-encapsulated PLGA nanoparticles (1.5 μm in diameter; 500 μg per mouse) and 1 μg of LPS. MHC II, major histocompatibility complex class II. (B) Representative data depicting frequency of proliferation of CFSE-labeled OTII cells in MedLNs of WT mice 3 days after intranasal immunization either with saline (naïve) or with OVA-encapsulated nanoparticles with 1.5-μm-diameter OVA-encapsulated nanoparticles (500 μg of beads per mouse; OVA concentration, 50 μg/mg beads) and 1 μg of LPS. (C) Recipient WT, Dock8−/−, and Batf3−/− mice were adoptively transferred with congenic OTII cells and immunized intranasally with OVA-encapsulated nanoparticles (1.5 μm in diameter; 500 μg per mouse) and 1 μg of LPS. MedLNs were analyzed 6 days later for OTII cell expansion. Representative data depicting the proliferation index of OTII cells among total CD44+ CD4+ T cells with respect to naïve controls are shown. Proliferation index was calculated as the ratio of % CD45.1 OTII of total CD44+ CD4+ T cells from immunized/naïve mice. **P < 0.01, ***P < 0.001 as compared to naïve. (D) Frequency of OTII Tfh cells (CD44+ PD-1+ CXCR5+) in MedLNs of WT, Dock8−/−, and Batf3−/− recipient mice adoptively transferred with congenic OTII cells, immunized with OVA-encapsulated nanoparticles and LPS intranasally as in (C), and analyzed 6 days later. (E) DC-Dock8Δ and control (Itgax-Cre− Dock8fl/fl) mice were intranasally immunized with 100 μg and boosted twice with 50 μg of inactivated influenza A/PR/8/34. Unimmunized WT mice served as naïve controls. PR8-sp ecific serum IgG from mice 21 days after first immunization is shown. *P < 0.05 as compared to naïve and DC-Dock8Δ. OD, optical density. (F) DC-Dock8Δ and control mice were immunized with inactivated PR8, as in (E), followed by intranasal challenge with 3000 plaque-forming units of PR8. Data pooled from two independent experiments with 8 to 15 mice per group show frequency of survival (left) and weight loss (right) in mice after challenge. Data are representative of three (A to E) independent experiments with three to eight mice per group.
Using an intranasal vaccination model of inactivated PR8 influenza A (A/PR/8/34) in DC-Dock8Δ mice, we tested whether loss of migratory cDC2s affected antibody-mediated protection from a live viral challenge (47). Consistent with our results using OVA, we observed a significant reduction in influenza-specific antibody production in DC-Dock8Δ mice (Fig. 4E). Neutralizing antibodies protect immunized mice from a lethal challenge of influenza, which we observed in control mice (Fig. 4F). However, naïve and DC-Dock8Δ mice exhibited morbidity (weight loss) and mortality, demonstrating that cDC2-dependent Tfh cell priming engendered antibody protection from a live viral challenge.
Migratory cDC1 and cDC2 express different patterns of homing molecules
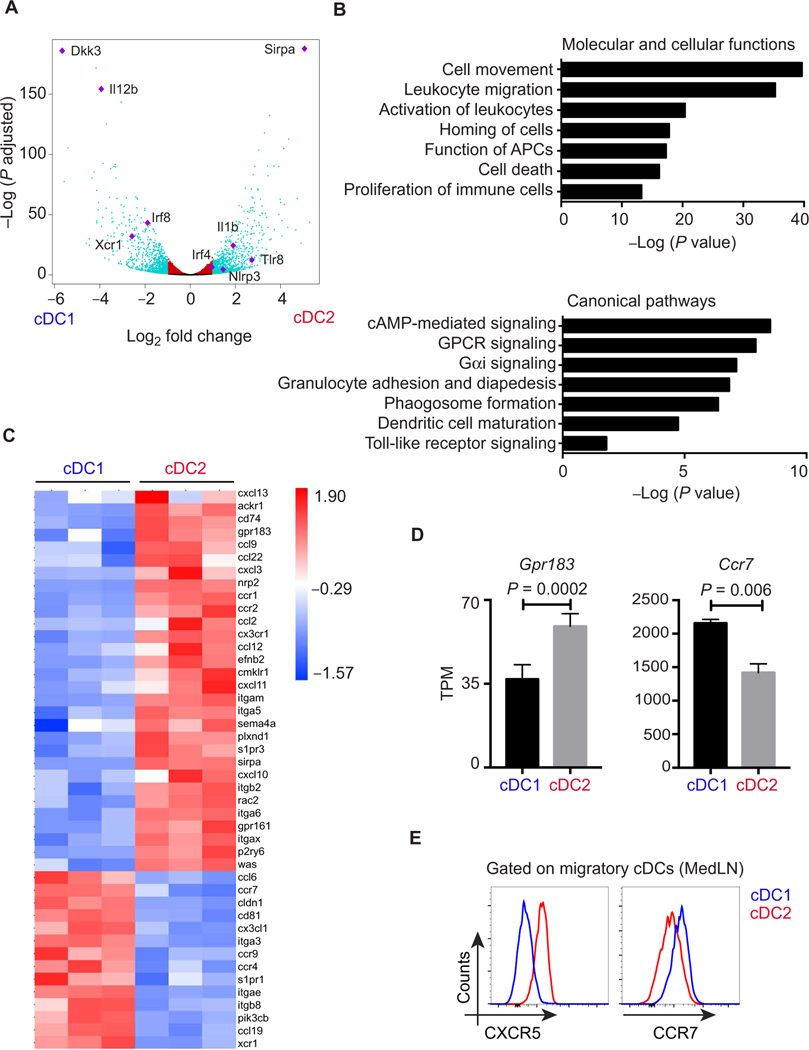
What is unique about tissue migratory cDC2s that enable them to initiate Tfh cell differentiation? It is known that the cytokine interleukin-2 (IL-2) is detrimental to the induction of Tfh cells (48). A recent study by Li and colleagues demonstrated that CD25 expression by CD4+ splenic cDC2s was required for Tfh-regulated B cell responses to sheep red blood cells (RBCs) by creating an “IL-2–free” zone (9). Because this study did not evaluate CD25 on migratory cDC1s, we compared the expression of CD25 on both migratory and LN-resident pulmonary cDC1s and cDC2s after intranasal immunization. Migratory cDC2s expressed higher levels of CD25 (fig. S5, A and B). However, we could not find evidence to support a role for CD25 on pulmonary DCs in promoting antibody responses, and therefore, we screened for other differentially expressed genes between the two cDC subsets using transcriptional profiling. We performed RNA sequencing (RNA-seq) on sorted cDC1s and cDC2s from pooled MedLNs 18 hours after intranasal immunization. A total of 1574 genes were differentially expressed by more than twofold between cDC1s and cDC2s in lung-draining LNs. Supporting the accuracy of the sorting and sequencing results, markers characteristic of each subset across multiple tissues including Xcr1 and Sirpa, transcription factors selectively required for each subset such as Irf8 and Irf4, cytokines such as IL-12, and numerous pattern recognition receptors were found to be significantly different (Fig. 5A). However, Ingenuity Pathway Analysis (IPA) revealed that the top four most significantly different canonical pathways were all related to cell movement, which correlated with the most significant pathway listed under molecular and cellular functions (Fig. 5B). In particular, a signature of chemokine, chemokine receptor, and integrin expression distinguished the two DC subsets (Fig. 5C). Specifically, cDC1s had higher levels of Ccr7 expression by RNA-seq (Fig. 5D), reverse transcription polymerase chain reaction (fig. S5C), and flow cytometry staining (Fig. 5E). Conversely, migratory cDC2s in MedLNs 18 hours after immunization expressed higher levels of chemokine receptors, such as Gpr183 (Ebi2) and S1pr3, and less Ccr7 than cDC1s (Fig. 5, C and D). GPR183 (G protein–coupled receptor 183) was recently shown to not only affect Tfh development in a T cell–intrinsic fashion but also guide splenic cDC2s to the T-B border (9, 49). The reciprocal expression of S1PR1 (sphingosine 1-phosphate receptor 1) and S1PR3 could also potentially contribute to distinct anatomic microdomain organization of cDC subsets. As compared with cDC1s, cDC2s also express higher levels of the CXCR5 ligand Cxcl13, indicating that cDC2s also produce chemokines that could attract nascent pre-Tfh cells (Fig. 5C and fig. S5C). We confirmed that CXCR5 surface expression was restricted to cDC2s by flow cytometry (Fig. 5E and fig. S5, D and E). This pattern of higher CXCR5 and lower CCR7 on cDC2s as compared with cDC1s should position cDC2s at the edge of the paracortex and adjacent to B cell follicles, rather than centrally in the CCR7 ligand–rich T cell zone (50). Because Tfh cell priming has been shown to occur in the interfollicular zone/T-B border (T cell regions adjacent to B cell follicles), we next examined localization of migratory DC subsets in the MedLNs.
Fig. 5. Migratory cDC2s express unique migratory signature distinct from cDC1s.
(A to D) RNA-seq analyses of migratory cDC1s (CD11c+MHChiCD24+CD103+) and cDC2s (CD11c+MHChiCD24+CD11b+) isolated from pooled MedLN of WT mice 18 hours after intranasal immunization with 1 μg of LPS. (A) Volcano plot showing statistical significance against fold change between cDC1s and cDC2s. Teal dots, P ≤ 0.01 and fold change of ≥2; red dots, P ≤ 0.01 and fold change of <2; orange dots, P > 0.01 and fold change of ≥2; black dots, P > 0.01 and fold change of <2. Genes of interest are indicated in purple. (B) IPA showing significant pathways in the “Molecular and cellular functions” and “Canonical pathways” categories. cAMP, cyclic adenosine monophosphate; GPCR, G protein–coupled receptor. (C) Heat map showing the expression of selected key molecules normalized to row mean. (D) Relative expression as indicated by TPM (transcripts per kilobase million) of Gpr183 and Ccr7 on migratory cDC1 and cDC2s from RNA-seq data. Adjusted P values are shown. (E) Flow cytometry histograms show an overlay of CXCR5 and CCR7 expression on migratory cDC subsets in MedLNs (gating as in fig. S2C) 18 hours after intranasal immunization with 1 μg of LPS. Data representative of three to four independent experiments (n = 2 to 3 mice per group).
Migratory cDC2s are appropriately positioned within LNs to promote Tfh induction
During early stages of Tfh cell differentiation, T cells coexpress CCR7 and CXCR5; the balance of these chemokine receptors promotes developing Tfh cell residence in a subanatomic region of secondary lymphoid organs called the T-B cell border and interfollicular zone (34, 50). This region, which contains a low density of both T cells and B cells, is outside of the B cell follicles and the central T cell zone. We used filter-dense and multiparameter confocal microscopy to simultaneously visualize T cells, B cells, and migratory DC subsets within the MedLN (51). We defined the outer T-B border (nearest the B cell follicles) by the zone where T cell (CD3+) staining ended and the inner T-B border (nearest the T cell zone) by where B cell (B220+) staining ended (fig. S6A). These two borders define an area of overlap between the two lymphocyte populations and were used to blindly score where DC subsets were located.
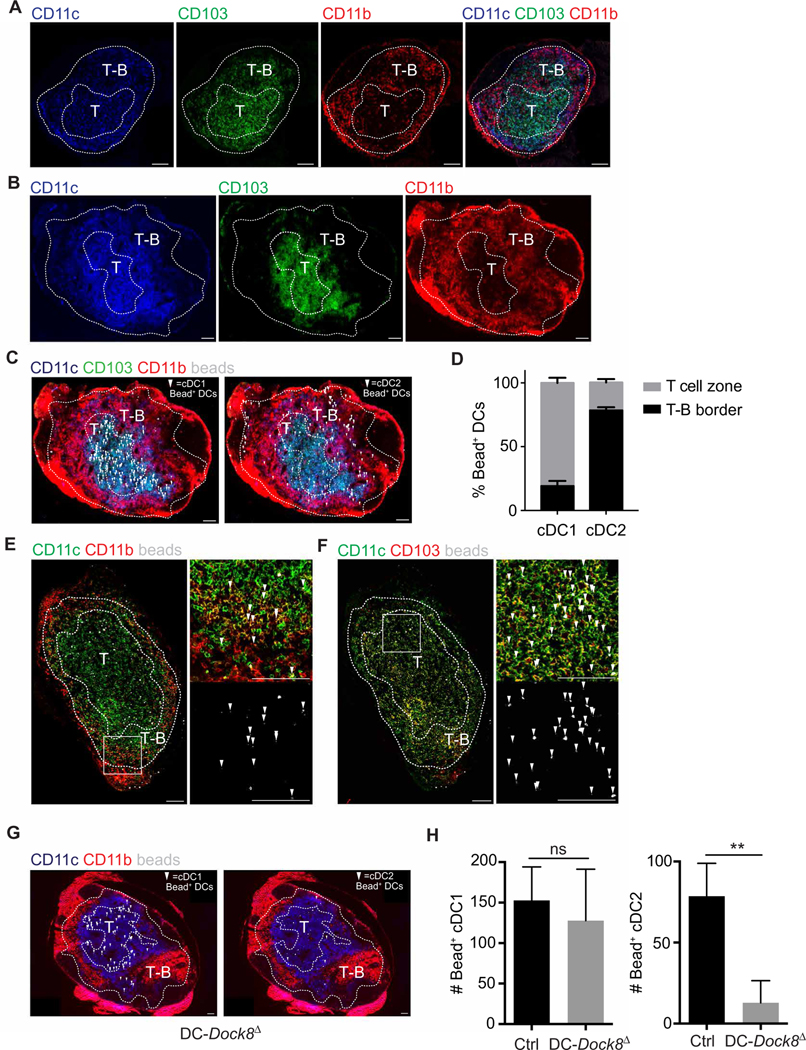
In MedLNs of immunized mice, we observed a concentration of CD11c+CD11b+ cDC2s in the T-B border, whereas CD11c+CD103+ cDC1s were concentrated in the T cell zone (Fig. 6, A and B). However, CD11b is expressed on multiple cell types, including monocytes and LN-resident cDC2s. To identify the lung-derived migratory DC subsets present in the MedLN after intranasal immunization, we administered fluorescent OVA microparticles, which do not free-drain, to WT mice and imaged MedLN 18 hours later. Bead+ DCs in the T-B border were CD11b+ DCs, whereas most in the T cell zone were CD103+ DCs (Fig. 6, B to D, and fig. S6B). Higher magnification of each subanatomic region of a draining MedLN confirmed the predominance of bead-containing cDC1s in the T cell zone and cDC2s in the T-B border (Fig. 6, E and F, and fig. S6, C to D). These data suggest that migratory CD11b+ cDC2s express the appropriate chemotactic receptors to home to the T-B border and induce Tfh cells for robust antibody responses.
Fig. 6. Migratory cDC2s are positioned in the T-B border poised to promote Tfh cell induction.
(A to G) T cell zone (T) and T-B border (T-B) definitions from the same MedLN section are shown in fig S6. Arrows indicate bead+ cDCs. (A and B) Immunofluorescence images of MedLN from WT mice 18 hours after intranasal immunization either with LPS (A) or with LPS plus yellow-green 1.0-μm beads (B). (C) Immunofluorescence images indicating bead+ migratory cDC1s (CD11c+ CD103+; left) and cDC2s (CD11c+ CD11b+; right) in MedLN from WT mice after immunization as in (B). (D) Quantitation of bead+ cDC1s and cDC2s in T-B border and T cell zone of MedLN after immunization as in (B). (E and F) Immunofluorescence images with close-ups indicating bead+ migratory cDC2s in the T-B border (E) and bead+ migratory cDC1s in the T cell zone (F) in MedLN from WT mice after immunization as in (B). (G) Immunofluorescence images indicating bead+ migratory cDC1s (CD11c+ CD103+; left) and cDC2s (CD11c+ CD11b+; right) in MedLN from DC-DOCK8Δ (Dock8fl/− ItgaxCre+) mice after immunization as in (B). (H) Quantitation of bead+ cDC1s and cDC2s in MedLN from control (Dock8fl/− ItgaxCre−) or DC-Dock8Δ (Dock8fl/− ItgaxCre+) mice after immunization as in (B). Data are representative of two independent experiments with two to four mice per group. Scale bars, 100 μm.
Given the lack of Tfh cell–dependent humoral immunity in DC-Dock8Δ mice associated with the loss of cDC2 migration, we predicted that these mice should have a deficit of bead+ pulmonary cDC2s in the T-B border of MedLN after immunization. Although we observed a similar number of bead+ cDC1s in the T cell zone of control and DC-Dock8Δ mice, bead+ cDC2s were significantly reduced in DC-Dock8Δ mice (Fig. 6, G and H, and fig. S6, E and F). This confirms our observations by flow cytometry that Dock8 deficiency impairs migration of cDC2s but not cDC1s, thus abrogating the presence of antigen-carrying DCs from the lung to the T-B border, resulting in a failure to induce Tfh cell priming and Tfh-dependent protective antibody responses.
Together, our study demonstrates that, unlike LN-resident cDCs, lung CD11b+ cDC2s have access to inhaled antigen, and unlike migratory cDC1s, they express the right balance of CCR7 and CXCR5 to position adjacent to B cell follicles in MedLNs. Therefore, we conclude that migratory cDC2s are ideally situated in lung-draining LNs to prime Tfh cell responses to inhaled antigens.
DISCUSSION
DCs are critical for generating the first step of a vaccine response, namely, Tfh cell priming (2, 3, 5). We aimed to address two related questions about the nature of the DC capable of Tfh cell priming: (i) Is migration of tissue-scanning DCs required? And (ii) which subset of DC is capable of instructing Tfh cell differentiation? DC migration is a central step regulating whether antigen, particularly at limiting doses, is presented to naïve T cells. However, recent work suggested that Tfh cell priming only required LN-resident DCs (13–15). We show that the route of immunization affects which cells can present antigen and, therefore, the requirement for DC migration in Tfh cell priming. These findings potentially help reconcile disparate observations of antigen presentation by DCs in LNs minutes to hours after footpad or ear pinna injections but not flank injection, although all of these sites are considered subcutaneous immunizations (13, 15, 17). Our data demonstrate that when antigen is not forced into lymphatics, DC migration is required for Tfh cell priming and the subsequent antibody-mediated protection after inhalational immunization.
DC-specific Dock8-deficient mice provided a unique model to selectively block the function of cDC2s. Loss of antigen+ cDC2s in the MedLN of Dock8−/− mice was due to neither impaired development nor defective phagocytic capacity, but rather a failure to migrate from the lungs. In a previous study, we found that Dock8−/− mice displayed a similar selective cDC2 migration defect within the spleen (30). Ongoing studies are addressing how DOCK8 selectively regulates tissue cDC2 but not cDC1 migration. Patients with mutations in Dock8 present with a hyper IgE syndrome but simultaneously have impaired production of antibodies to vaccines (31, 43). The hyper IgE state has been ascribed to heightened TH2 responses observed in these patients. Multiple strains of Dock8 deficiency (including our own) do not recapitulate the hyper IgE state (26). Therefore, the pathogenesis of the hyper IgE state in Dock8-deficient patients remains to be elucidated. Nevertheless, preventing cDC2 migration in DC-Dock8Δ mice or deleting cDC2s in DC-Irf4Δ mice abrogated Tfh cell responses to inhaled antigens, consistent with our previous work and that of others showing that deletion or paralysis of splenic cDC2s abrogates the antibody response to RBCs (8, 30, 52). This was not due to a pan-CD4+ T cell activation defect because TH1 priming was unimpaired in DC-Dock8Δ mice. In contrast to the DC-Dock8Δ mice, deletion of migratory and LN-resident cDC1s in Batf3−/− mice does not impair Tfh cell responses to inhaled antigens. Together, these data demonstrate that, although multiple DC populations can induce CD4+ T cell activation, Tfh cell differentiation and the associated antibody response require migratory cDC2s (Fig. 7). This is consistent with a recent report on DC subsets in the spleen after sheep RBC immunization (9). We also propose that migratory cDC2s are sufficient for the first step of Tfh cell priming. Using antigen-encapsulated beads, which LN-resident DCs cannot access, and Batf3-deficient mice to eliminate cDC1s, we isolated antigen presentation to migratory cDC2s and observed robust Tfh cell induction. Further, although DC-Dock8Δ mice have intact antigen transport to MedLNs by cDC1s, any possible transfer to LN-resident DCs does not seem sufficient to induce Tfh cells to soluble or particulate antigen. Therefore, these data support a model in which migratory cDC2s are sufficient for the first phase of Tfh priming.
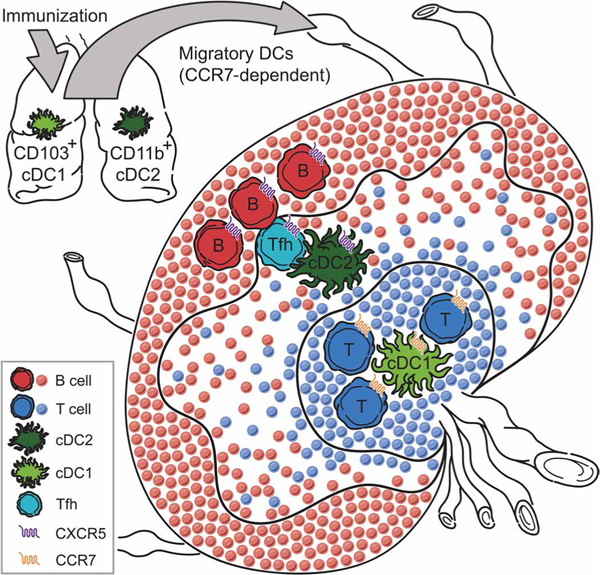
Fig. 7. Model of Tfh cell priming by cDC2s in the LN.
On encountering antigens in the airways, pulmonary cDCs migrate to the MedLNs, a process dependent on CCR7. cDC2s express lower levels of CCR7 and higher levels of CXCR5 as compared to cDC1s, which positions cDC2s between the CXCL13hi B cell follicles and the CCL-19hi T cell area—the T-B border. We propose that the T-B border is where Tfh differentiation occurs because both cDC2- and B cell–derived signals are present.
To identify potentially unique characteristics of cDC2s that might enable Tfh cell differentiation, we performed RNA-seq of MedLN cDC1s and cDC2s after intranasal immunization. Unexpectedly, we did not identify differential expression of ligands known to drive Tfh cell differentiation, including OX40L, ICOSL (inducible T cell costimulator ligand), or CD25. The latter was recently identified as a mechanism by which the inhibitory Tfh cytokine IL-2 could be quenched by DCs during Tfh cell priming (9); we observed cDC2-specific expression of CD25 yet could not find evidence for a DC-specific role of CD25 for antibody production. B cells, which also express high levels of CD25 upon activation (53), could also be positioned to induce an IL-2–free zone during Tfh formation. This possibility requires further investigation. Instead, we found that cDC2s expressed a unique pattern of chemokine receptors, similar to Tfh cells, which should position them proximal to B cell follicles (Fig. 7). Conversely, cDC1s expressed higher levels of CCR7, which should position them distal to B cell follicles. Consistent with these gene expression profiles, we observed cDC1 and cDC2 migration from the lung into distinct regions of the T cell–rich areas of the MedLNs–CXCR5-expressing cDC2s to the T-B border and cDC1s to CCR7 ligand–rich regions of the T cell zone. This pattern matches what we and others have described for the two cDC subsets in the splenic white pulp (30, 49) and in skin-draining LNs at steady state (13). Expression of CXCR5 on activated DCs has been observed during helminth infection, and loss of CXCR5-based homing of DCs impaired TH2 priming (54). The models used in our study rely on type 1 immunizations, and therefore, given that we show selective CXCR5 expression on cDC2s, it remains to be determined whether a type 2 immunization induces a similar pattern of cDC1 and cDC2 segregation within the LN. Recent work has observed a similar pattern of DC subset segregation in human LNs (55). In this T cell– and B cell–rich region of LNs, we propose that cDC2s drive Tfh cell differentiation by establishing a niche conducive for nascent Tfh cell interaction with both appropriate DC- and B cell–derived signals.
This work helps define the nature of the pulmonary DC that efficiently induces Tfh cell priming and thereby has implications on our understanding of mucosal immune responses to infection and could potentially guide vaccination practices. Our study emphasizes that the route of antigen delivery substantially affects the type of antigen-presenting cells that can present antigen. Given our findings, current intramuscular vaccine strategies might be inefficient because they do not target cDC2s, simply because of the route of injection. Human studies of intradermal injection showed equivalent antibody titers to intramuscular injection at 1/10 the dose of antigen (1). Work to compare the form and route of antigen delivered during various types of vaccination strategies could reveal approaches that will aid dose-sparing efforts and optimize vaccine efficacy.
MATERIALS AND METHODS
Study design
The study aimed to identify the subset of DCs that drive Tfh responses to inhaled antigens. Experiments included WT, transgenic, and knockout mouse strains (C57BL/6 background). All mice used were between 6 and 10 weeks old and were age- and sex-matched for each experiment. Analysis included DC, T cell, and B cell phenotyping. Experiments included intranasal, subcutaneous, or footpad immunizations and retro-orbital route for adoptive transfers. Counts for bead+ migratory cDCs in immunofluorescence studies were blinded. Experiment replications are included in the figure legends.
In vivo DC migration
Mice were immunized intranasally, subcutaneously (per flank), or by footpad with 50 μg of OVA–Alexa Fluor 647 (Molecular Probes) and 1 μg of LPS (Invivogen) or with 500 μg of DiD-OVA–encapsulated 1.5-μm PLGA (poly lactic-co-glycolic acid) nanoparticles and 1 μg of LPS, as indicated. DiD (1,1′-dioctadecyl-3,3,3′,3′-tetramethylindodicarbocyanine perchlorate) is a lipophilic tracer from Invitrogen. MedLNs, IngLNs, and popliteal LNs were harvested 18, 48, or 72 hours after immunization (as indicated), minced, and digested with collagenase IV (1 mg/ml; Sigma-Aldrich) for 40 min at 37°C. Single-cell suspensions were prepared, stained, and then analyzed on an LSRII (BD Biosciences) or MACSQuant (Miltenyi Biotec) flow cytometer.
In vivo T cell proliferation and Tfh or TH1 cell analysis
OTII cells were prepared from the spleen and LNs of OTII TCR (T cell receptor) transgenic mice by negative selection using the EasySep CD4+ T Cell Isolation kit (STEMCELL Technologies) according to the manufacturer’s instructions. For T cell proliferation assays, OTII cells were labeled with 2 μM carboxyfluorescein diacetate succinimidyl ester (CFSE) before transfer. A total of 106 purified OTII cells were transferred into mice by retro-orbital injection. Mice were intranasally or subcutaneously immunized 24 hours later with 10 μg of OVA (Sigma-Aldrich) and 1 μg of LPS (Invivogen), as indicated. MedLN or IngLN was harvested 3 days (T cell proliferation) or 6 days after immunization (Tfh and TH1 cell analysis), and single-cell suspensions were prepared, stained, and then analyzed on an LSRII flow cytometer (BD Biosciences). For the OVA-encapsulated beads experiments, 500 μg of OVA-encapsulated PLGA particles with a mean diameter of 1.5 μm and OVA concentration of 50 μg/mg beads (gifted by T. Fahmy, Yale University) was administered intranasally with 1 μg of LPS (Invivogen) to mice that received OTII cells the previous day. Tfh cell analysis was performed as described above.
Statistical analysis
All statistical analyses were performed using GraphPad Prism software. Data were analyzed with the unpaired Student t test using Welch’s correction or one-way analysis of variance (ANOVA) and Tukey post hoc analysis. The Kaplan-Meier method was used to analyze survival data. All graphs show mean (±SD); ns, not significant; *P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001.
Supplementary Material
Fig. S1. Skin migratory cDC2s transport most of the antigen to draining LNs after subcutaneous immunization.
Fig. S2. Intranasal immunization targets antigen to both migratory cDC subsets.
Fig. S3. Dock8 deficiency is not associated with impaired lung or MedLN DC development or survival.
Fig. S4. Generation and characterization of DC-Dock8Δ mice.
Fig. S5. cDC2 characteristics suggest a unique localization within MedLNs.
Fig. S6. Definition of T cell zone and T-B border in MedLN.
Acknowledgments:
DiD-OVA–encapsulated PLGA nanoparticles were a gift from T. Fahmy (Yale University), and A/PR/8/34 was a gift from A. Iwasaki (Yale University). We thank M. Firla for the technical assistance, M. Wimsatt for the illustration, and J. Craft for the critical review of the manuscript.
Funding: This work was supported by R01 AI108829 (to S.C.E.), The Hartwell Foundation Individual Biomedical Research Award (to S.C.E.), Hood Foundation Child Health Research Award (to S.C.E.), 1R21AI110776-01 (to A.W.), and the Agency for Science, Technology, and Research Singapore (to B.Z.).
Footnotes
Competing interests: P.F. and U.Y. are shareholders and cofounders of Kromnigon AB. All other authors declare that they have no competing interests.
Data and materials availability: RNA-seq data have been submitted to the Gene Expression Omnibus database repository (accession number: GSE103956).
SUPPLEMENTARY MATERIALS
immunology.sciencemag.org/cgi/content/full/2/18/eaam9169/DC1
REFERENCES AND NOTES
- 1.Kenney RT, Frech SA, Muenz LR, Villar CP, Glenn GM, Dose sparing with intradermal injection of influenza vaccine. N. Engl. J. Med 351, 2295–2301 (2004). [DOI] [PubMed] [Google Scholar]
- 2.Crotty S, T follicular helper cell differentiation, function, and roles in disease. Immunity 41, 529–542 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vinuesa CG, Linterman MA, Yu D, MacLennan ICM, Follicular helper T cells. Annu. Rev. Immunol 34, 335–368 (2016). [DOI] [PubMed] [Google Scholar]
- 4.Dahlgren MW, Gustafsson-Hedberg T, Livingston M, Cucak H, Alsén S, Yrlid U, Johansson-Lindbom B, T follicular helper, but not Th1, cell differentiation in the absence of conventional dendritic cells. J. Immunol 194, 5187–5199 (2015). [DOI] [PubMed] [Google Scholar]
- 5.Goenka R, Barnett LG, Silver JS, O’Neill PJ, Hunter CA, Cancro MP, Laufer TM, Cutting edge: Dendritic cell-restricted antigen presentation initiates the follicular helper T cell program but cannot complete ultimate effector differentiation. J. Immunol 187, 1091–1095 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ballesteros-Tato A, Randall TD, Priming of T follicular helper cells by dendritic cells. Immunol. Cell Biol 92, 22–27 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Durai V, Murphy KM, Functions of murine dendritic cells. Immunity 45, 719–736 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Calabro S, Gallman A, Gowthaman U, Liu D, Chen P, Liu J, Krishnaswamy JK, Nascimento MSL, Xu L, Patel SR, Williams A, Tormey CA, Hod EA, Spitalnik SL, Zimring JC, Hendrickson JE, Stowell SR, Eisenbarth SC, Bridging channel dendritic cells induce immunity to transfused red blood cells. J. Exp. Med 213, 887–896 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li J, Lu E, Yi T, Cyster JG, EBI2 augments Tfh cell fate by promoting interaction with IL-2-quenching dendritic cells. Nature 533, 110–114 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lahoud MH, Ahmet F, Kitsoulis S, Wan SS, Vremec D, Lee C-N, Phipson B, Shi W, Smyth GK, Lew AM, Kato Y, Mueller SN, Davey GM, Heath WR, Shortman K, Caminschi I, Targeting antigen to mouse dendritic cells via Clec9A induces potent CD4 T cell responses biased toward a follicular helper phenotype. J. Immunol 187, 842–850 (2011). [DOI] [PubMed] [Google Scholar]
- 11.Shin C, Han J-A, Koh H, Choi B, Cho Y, Jeong H, Ra J-S, Sung PS, Shin E-C, Ryu S, Do Y, CD8α− dendritic cells induce antigen-specific T follicular helper cells generating efficient humoral immune responses. Cell Rep. 11, 1929–1940 (2015). [DOI] [PubMed] [Google Scholar]
- 12.Yao C, Zurawski SM, Jarrett ES, Chicoine B, Crabtree J, Peterson EJ, Zurawski G, Kaplan DH, Igyártó BZ, Skin dendritic cells induce follicular helper T cells and protective humoral immune responses. J. Allergy Clin. Immunol 136, 1387–1397.e7 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gerner MY, Torabi-Parizi P, Germain RN, Strategically localized dendritic cells promote rapid T cell responses to lymph-borne particulate antigens. Immunity 42, 172–185 (2015). [DOI] [PubMed] [Google Scholar]
- 14.Kumamoto Y, Hirai T, Wong PW, Kaplan DH, Iwasaki A, CD301b+ dendritic cells suppress T follicular helper cells and antibody responses to protein antigens. Elife 5, e17979 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Woodruff MC, Heesters BA, Herndon CN, Groom JR, Thomas PG, Luster AD, Turley SJ, Carroll MC, Trans-nodal migration of resident dendritic cells into medullary interfollicular regions initiates immunity to influenza vaccine. J. Exp. Med 211, 1611–1621 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Anandasabapathy N, Feder R, Mollah S, Tse S-W, Longhi MP, Mehandru S, Matos I, Cheong C, Ruane D, Brane L, Teixeira A, Dobrin J, Mizenina O, Park CG, Meredith M, Clausen BE, Nussenzweig MC, Steinman RM, Classical Flt3L-dependent dendritic cells control immunity to protein vaccine. J. Exp. Med 211, 1875–1891 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Itano AA, McSorley SJ, Reinhardt RL, Ehst BD, Ingulli E, Rudensky AY, Jenkins MK, Distinct dendritic cell populations sequentially present antigen to CD4 T cells and stimulate different aspects of cell-mediated immunity. Immunity 19, 47–57 (2003). [DOI] [PubMed] [Google Scholar]
- 18.Levin C, Bonduelle O, Nuttens C, Primard C, Verrier B, Boissonnas A, Combadière B, Critical role for skin-derived migratory DCs and Langerhans cells in TFH and GC responses after intradermal immunization. J. Invest. Dermatol 137, 1905–1913 (2017). [DOI] [PubMed] [Google Scholar]
- 19.Ohl L, Mohaupt M, Czeloth N, Hintzen G, Kiafard Z, Zwirner J, Blankenstein T, Henning G, Förster R, CCR7 governs skin dendritic cell migration under inflammatory and steady-state conditions. Immunity 21, 279–288 (2004). [DOI] [PubMed] [Google Scholar]
- 20.Tang H, Cao W, Kasturi SP, Ravindran R, Nakaya HI, Kundu K, Murthy N, Kepler TB, Malissen B, Pulendran B, The T helper type 2 response to cysteine proteases requires dendritic cell-basophil cooperation via ROS-mediated signaling. Nat. Immunol 11, 608–617 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gretz JE, Norbury CC, Anderson AO, Proudfoot AEI, Shaw S, Lymph-borne chemokines and other low molecular weight molecules reach high endothelial venules via specialized conduits while a functional barrier limits access to the lymphocyte microenvironments in lymph node cortex. J. Exp. Med 192, 1425–1440 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gonzalez SF, Lukacs-Kornek V, Kuligowski MP, Pitcher LA, Degn SE, Kim Y-A, Cloninger MJ, Martinez-Pomares L, Gordon S, Turley SJ, Carroll MC, Capture of influenza by medullary dendritic cells via SIGN-R1 is essential for humoral immunity in draining lymph nodes. Nat. Immunol 11, 427–434 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grigorova IL, Panteleev M, Cyster JG, Lymph node cortical sinus organization and relationship to lymphocyte egress dynamics and antigen exposure. Proc. Natl. Acad. Sci. U.S.A 107, 20447–20452 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Deenick EK, Chan A, Ma CS, Gatto D, Schwartzberg PL, Brink R, Tangye SG, Follicular helper T cell differentiation requires continuous antigen presentation that is independent of unique B cell signaling. Immunity 33, 241–253 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kool M, Pétrilli V, De Smedt T, Rolaz A, Hammad H, van Nimwegen M, Bergen IM, Castillo R, Lambrecht BN, Tschopp J, Cutting edge: Alum adjuvant stimulates inflammatory dendritic cells through activation of the NALP3 inflammasome. J. Immunol 181, 3755–3759 (2008). [DOI] [PubMed] [Google Scholar]
- 26.Randall KL, Lambe T, Johnson AL, Treanor B, Kucharska E, Domaschenz H, Whittle B, Tze LE, Enders A, Crockford TL, Bouriez-Jones T, Alston D, Cyster JG, Lenardo MJ, Mackay F, Deenick EK, Tangye SG, Chan TD, Camidge T, Brink R, Vinuesa CG, Batista FD, Cornall RJ, Goodnow CC, Dock8 mutations cripple B cell immunological synapses, germinal centers and long-lived antibody production. Nat. Immunol 10, 1283–1291 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang Q, Jing H, Su HC, Recent advances in DOCK8 immunodeficiency syndrome. J. Clin. Immunol 36, 441–449 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Harada Y, Tanaka Y, Terasawa M, Pieczyk M, Habiro K, Katakai T, Hanawa-Suetsugu K, Kukimoto-Niino M, Nishizaki T, Shirouzu M, Duan X, Uruno T, Nishikimi A, Sanematsu F, Yokoyama S, Stein JV, Kinashi T, Fukui Y, DOCK8 is a Cdc42 activator critical for interstitial dendritic cell migration during immune responses. Blood 119, 4451–4461 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Krishnaswamy JK, Singh A, Gowthaman U, Wu R, Gorrepati P, Nascimento MSL, Gallman D. Liu, Rhebergen AM, Calabro S, Xu L, Ranney P, Srivastava A, Ranson M, Gorham JD, McCaw Z, Kleeberger SR, Heinz LX, Müller AC, Bennett KL, Superti-Furga G, Henao-Mejia J, Sutterwala FS, Williams A, Flavell RA, Eisenbarth SC, Coincidental loss of DOCK8 function in NLRP10-deficient and C3H/HeJ mice results in defective dendritic cell migration. Proc. Natl. Acad. Sci. U.S.A 112, 3056–3061 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Calabro S, Liu D, Gallman A, Nascimento MSL, Yu Z, Zhang T-T, Chen P, Zhang B, Xu L, Gowthaman U, Krishnaswamy JK, Haberman AM, Williams A, Eisenbarth SC, Differential intrasplenic migration of dendritic cell subsets tailors adaptive immunity. Cell Rep. 16, 2472–2485 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tangye SG, Pillay B, Randall KL, Avery DT, Phan TG, Gray P, Ziegler JB, Smart JM, Peake J, Arkwright PD, Hambleton S, Orange J, Goodnow CC, Uzel G, Casanova J-L, Lugo Reyes SO, Freeman AF, Su HC, Ma CS, Dedicator of cytokinesis 8–deficient CD4+ T cells are biased to a TH2 effector fate at the expense of TH1 and TH17 cells. J. Allergy Clin. Immunol 139, 933–949 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Janssen E, Tohme M, Hedayat M, Leick M, Kumari S, Ramesh N, Massaad MJ, Ullas S, Azcutia V, Goodnow CC, Randall KL, Qiao Q, Wu H, Al-Herz W, Cox D, Hartwig J, Irvine DJ, Luscinskas FW, Geha RS, A DOCK8-WIP-WASp complex links T cell receptors to the actin cytoskeleton. J. Clin. Invest 126, 3837–3851 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Baumjohann D, Preite S, Reboldi A, Ronchi F, Ansel KM, Lanzavecchia A, Sallusto F, Persistent antigen and germinal center B cells sustain T follicular helper cell responses and phenotype. Immunity 38, 596–605 (2013). [DOI] [PubMed] [Google Scholar]
- 34.Kerfoot SM, Yaari G, Patel JR, Johnson KL, Gonzalez DG, Kleinstein SH, Haberman M, Germinal center B cell and T follicular helper cell development initiates in the interfollicular zone. Immunity 34, 947–960 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Igyártó BZ, Kaplan DH, Antigen presentation by Langerhans cells. Curr. Opin. Immunol 25, 115–119 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tomura M, Hata A, Matsuoka S, Shand FHW, Nakanishi Y, Ikebuchi R, Ueha S, Tsutsui H, Inaba K, Matsushima K, Miyawaki A, Kabashima K, Watanabe T, Kanagawa O, Tracking and quantification of dendritic cell migration and antigen trafficking between the skin and lymph nodes. Sci. Rep 4, 6030 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Desch AN, Randolph GJ, Murphy K, Gautier EL, Kedl RM, Lahoud MH, Caminschi I, Shortman K, Henson PM, Jakubzick CV, CD103+ pulmonary dendritic cells preferentially acquire and present apoptotic cell–associated antigen. J. Exp. Med 208, 1789–1797 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schlitzer A, McGovern N, Teo P, Zelante T, Atarashi K, Low D, Ho AWS, See P, Shin PS Wasan, Hoeffel G, Malleret B, Heiseke A, Chew S, Jardine L, Purvis HA, Hilkens CMU, Tam J, Poidinger M, Stanley ER, Krug AB, Renia L, Sivasankar B, Ng LG, Collin M, Ricciardi-Castagnoli P, Honda K, Haniffa M, Ginhoux F, IRF4 transcription factor-dependent CD11b+ dendritic cells in human and mouse control mucosal IL-17 cytokine responses. Immunity 38, 970–983 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chiu B-C, Stolberg VR, Chensue SW, Mononuclear phagocyte-derived IL-10 suppresses the innate IL-12/IFN-γ axis in lung-challenged aged mice. J. Immunol 181, 3156–3166 (2008). [DOI] [PubMed] [Google Scholar]
- 40.Baumjohann D, Okada T, Ansel KM, Cutting edge: Distinct waves of BCL6 expression during T follicular helper cell development. J. Immunol 187, 2089–2092 (2011). [DOI] [PubMed] [Google Scholar]
- 41.Cannons JL, Qi H, Lu KT, Dutta M, Gomez-Rodriguez J, Cheng J, Wakeland EK, Germain RN, Schwartzberg PL, Optimal germinal center responses require a multistage T cell:B cell adhesion process involving integrins, SLAM-associated protein, and CD84. Immunity 32, 253–265 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Poholek AC, Hansen K, Hernandez SG, Eto D, Chandele A, Weinstein JS, Dong X, Odegard JM, Kaech SM, Dent AL, Crotty S, Craft J, In vivo regulation of Bcl6 and T follicular helper cell development. J. Immunol 185, 313–326 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jabara HH, Mcdonald DR, Janssen E, Massaad MJ, Ramesh N, Borzutzky A, Rauter I, Benson H, Schneider L, Baxi S, Recher M, Notarangelo LD, Wakim R, Dbaibo G, Dasouki M, Al-Herz W, Barlan I, Baris S, Kutukculer N, Ochs HD, Plebani A, Kanariou M, Lefranc G, Reisli I, Fitzgerald KA, Golenbock D, Manis J, Keles S, Ceja R, Chatila TA, Geha RS, DOCK8 functions as an adaptor that links TLR-MyD88 signaling to B cell activation. Nat. Immunol 13, 612–620 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Takahashi Y, Dutta PR, Cerasoli DM, Kelsoe G, In situ studies of the primary immune response to (4-hydroxy-3-nitrophenyl)acetyl. V. Affinity maturation develops in two stages of clonal selection. J. Exp. Med 187, 885–895 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Miyauchi K, Sugimoto-Ishige A, Harada Y, Adachi Y, Usami Y, Kaji T, Inoue K, Hasegawa H, Watanabe T, Hijikata A, Fukuyama S, Maemura T, Okada-Hatakeyama M, Ohara O, Kawaoka Y, Takahashi Y, Takemori T, Kubo M, Protective neutralizing influenza antibody response in the absence of T follicular helper cells. Nat. Immunol 17, 1447–1458 (2016). [DOI] [PubMed] [Google Scholar]
- 46.Jakubzick C, Helft J, Kaplan TJ, Randolph GJ, Optimization of methods to study pulmonary dendritic cell migration reveals distinct capacities of DC subsets to acquire soluble versus particulate antigen. J. Immunol. Methods 337, 121–131 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pang IK, Ichinohe T, Iwasaki A, IL-1R signaling in dendritic cells replaces pattern-recognition receptors in promoting CD8+ T cell responses to influenza A virus. Nat. Immunol 14, 246–253 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ray JP, Staron MM, Shyer JA, Ho P-C, Marshall HD, Gray SM, Laidlaw BJ, Araki K, Ahmed R, Kaech SM, Craft J, The interleukin-2-mTORc1 kinase axis defines the signaling, differentiation, and metabolism of T helper 1 and follicular B helper T cells. Immunity 43, 690–702 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lu E, Dang EV, McDonald JG, Cyster JG, Distinct oxysterol requirements for positioning naïve and activated dendritic cells in the spleen. Sci. Immunol 2, eaal5237 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Haynes NM, Allen CDC, Lesley R, Ansel KM, Killeen N, Cyster JG, Role of CXCR5 and CCR7 in follicular Th cell positioning and appearance of a programmed cell death gene-1high germinal center-associated subpopulation. J. Immunol 179, 5099–5108 (2007). [DOI] [PubMed] [Google Scholar]
- 51.Kijani S, Yrlid U, Heyden M, Levin M, Borén J, Fogelstrand P, Filter-dense multicolor microscopy. PLOS ONE 10, e0119499 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yi T, Li J, Chen H, Wu J, An J, Xu Y, Hu Y, Lowell CA, Cyster JG, Splenic dendritic cells survey red blood cells for missing self-CD47 to trigger adaptive immune responses. Immunity 43, 764–775 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Waldmann TA, Goldman CK, Robb RJ, Depper JM, Leonard WJ, Sharrow SO, Bongiovanni KF, Korsmeyer SJ, Greene WC, Expression of interleukin 2 receptors on activated human B cells. J. Exp. Med 160, 1450–1466 (1984). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.León B, Ballesteros-Tato A, Browning JL, Dunn R, Randall TD, Lund FE, Regulation of TH2 development by CXCR5+ dendritic cells and lymphotoxin-expressing B cells. Nat. Immunol 13, 681–690 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Granot T, Senda T, Carpenter DJ, Matsuoka N, Weiner J, Gordon CL, Miron M, Kumar BV, Griesemer A, Ho S-H, Lerner H, Thome JJC, Connors T, Reizis B, Farber DL, Dendritic cells display subset and tissue-specific maturation dynamics over human life. Immunity 46, 504–515 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Skin migratory cDC2s transport most of the antigen to draining LNs after subcutaneous immunization.
Fig. S2. Intranasal immunization targets antigen to both migratory cDC subsets.
Fig. S3. Dock8 deficiency is not associated with impaired lung or MedLN DC development or survival.
Fig. S4. Generation and characterization of DC-Dock8Δ mice.
Fig. S5. cDC2 characteristics suggest a unique localization within MedLNs.
Fig. S6. Definition of T cell zone and T-B border in MedLN.