Abstract
Background:
Allogeneic hematopoietic cell transplantation is indicated for refractory hematologic malignancy and some non-malignant disorders. Survival is limited by recurrent malignancy and organ toxicity.
Objective:
We determined whether survival has improved over the last decade and noted impediments to better outcomes.
Design:
We compared cohorts undergoing transplantation during 2003-2007 and 2013-2017. Survival outcome measures were analyzed, along with transplant-related complications.
Setting:
A center performing allogeneic transplant procedures.
Participants:
All recipients of first allogeneic transplantation during 2003-2007 and 2013-2017.
Interventions:
Patients received a conditioning regimen then infusion of donor hematopoietic cells, then immunosuppressive drugs and antimicrobial approaches to infection control.
Measurements:
Day-200 non-relapse mortality (NRM), recurrence or progression of malignancy, relapse-related mortality, and overall mortality, adjusted for co-morbidity scores, source of donor cells, donor type, age, disease severity, conditioning regimen, patient/donor sex, and Cytomegalovirus serostatus.
Results:
During 2003-2007 and 2013-2017, 1148 and 1131 patients received their first transplants. Over the decade, decreases were seen in the adjusted hazards of day-200 NRM (hazard ratio (HR) 0.66), relapse of malignancy (HR=0.76), relapse-related mortality (HR=0.69), and overall mortality (HR=0.66). The degree of reduction in overall mortality was similar for patients who received either myeloablative or reduced-intensity conditioning and for patients transplanted from a matched-sibling donor or an unrelated donor. Reductions were also seen in the frequency of jaundice, renal insufficiency, mechanical ventilation, high-level Cytomegalovirus viremia, gram-negative bacteremia, invasive mold infection, acute and chronic GVHD, and prednisone exposure.
Limitations:
Cohort studies cannot determine causality. Current disease severity criteria were not available for 2003-2007 patients.
Conclusions:
We document a substantial improvement in survival and reduction in complications following allogeneic transplantation. Relapse of malignancy remains the largest obstacle to better survival outcomes.
Keywords: Marrow transplantation, hematopoietic cell transplantation, hematologic malignancy, liver disease, kidney disease, pulmonary disease, infectious complications, graft-vs.-host disease, mortality, survival, comparative outcomes research
Introduction
The inception of allogeneic hematopoietic cell transplantation some 50 years ago was attended by considerable morbidity and mortality. In 2010, we published results comparing outcomes in a cohort of patients transplanted between 1993-97 with a cohort transplanted a decade later and showed that the rates of non-relapse mortality (NRM), relapse, and overall mortality had substantially dropped (1). Reports from other transplant centers noted similar improvements (2-5). The reasons for improved outcomes were reductions in the frequency of virtually every morbid complication of allogeneic transplant.
Since the time of our last publication on transplant outcomes (1), we and others have continued to effect further improvements in the outcome of allogeneic transplantation. These efforts include attempts to refine donor selection, modulate the intensity of the preparative regimen in hopes of reducing toxicity without sacrificing antitumor effects, improve GVHD prevention and treatment, and apply more effective anti-viral and anti-fungal strategies. While none of these efforts has as yet been proven to have a major impact on overall mortality when studied in isolation, we sought to determine whether the overall trajectory of improved outcome witnessed from the mid-1990’s to the mid-2000’s has been sustained over the last decade.
Accordingly, we now compare the rates of day-200 NRM, recurrent malignancy, relapse-related mortality, and overall mortality in 1131 allograft patients from 2013-2017, to 1148 patients transplanted during 2003-2007. We also analyzed the frequency and severity of acute and chronic GVHD; hepatic, kidney, pulmonary and infectious complications; and exposure to prednisone during the post-transplant period.
Methods
Patient selection.
All recipients of first allogeneic transplantation from 2003-2007 and 2013-2017, except those who had undergone previous autologous transplantation, were evaluated under an Institutional Review Board-approved protocol. Data for this analysis of outcomes and complications was gathered from five sources: Our Center’s Master Patient Database, for core data elements; specialty databases (for example, reduced intensity conditioning protocols); hospital databases for procedures; review of electronic medical records of individual patients; and data collected by our Long-Term Follow-Up group which follows all discharged patients at six months, 1 year, and yearly after the date of transplant.
Technique of allogeneic transplantation.
The basic technique for molecular typing of HLA was constant over the study period, but there were subtle changes in the methodology of unrelated donor selection, including alterations in which mismatches we considered “allowable” as well as the inclusion of HLA-DP typing in the more recent cohort. All patients received a conditioning regimen followed by infusion of donor hematopoietic cells. Myeloablative conditioning regimens generally contained cyclophosphamide 120 mg/kg with either busulfan or 12-13.2 Gray total body irradiation. Reduced-intensity regimens included 2-4.5 Gray total body irradiation with or without fludarabine. Recipients were given immunosuppressive drugs, usually a calcineurin inhibitor plus methotrexate or mycophenolate mofetil, or sirolimus/calcineurin inhibitor regimens, or regimens that included post-transplant cyclophosphamide (PTCY) or antithymocyte globulin (ATG) or abatacept (6) to prevent GVHD (Table 1). Prophylaxis for infections included low-dose acyclovir; trimethoprim / sulfamethoxazole or dapsone or atovaquone; and an antifungal agent (7). In both periods, our practice included anti-mold drugs such as voriconazole for patients with pre-transplant mold infections or pulmonary nodules; fluoroquinolone prophylaxis for patients with neutropenia; and pre-emptive therapy with ganciclovir or foscarnet for patients with CMV antigenemia or DNAemia. Screening of blood samples for CMV infection was with antigenemia during 2003-2006, and with DNA during 2007 and 2013-2017 (8). Ursodiol was given to all patients as prophylaxis against cholestasis.
Table 1. Patient Characteristics.
Additional patient characteristics can be found in the Appendix, Supplementary Table 1.
| Characteristic | 2003-2007 (n=1148) |
2013-2017 (n=1131) |
|---|---|---|
| Age at transplant | ||
| Median age (range) | 47.2 (0.4 - 78.9) | 50.0 (0.1 – 80.9) |
| <10 years | 91 (8%) | 126 (11%) |
| ≥10 – <30 years | 198 (17%) | 181 (16%) |
| ≥30 – <50 years | 356 (31%) | 259 (23%) |
| ≥50 – <70 years | 487 (42%) | 481 (43%) |
| ≥70 years | 16 (1%) | 84 (7%) |
| Diagnosis | ||
| Aplastic anemia | 39 (3%) | 54 (5%) |
| Acute lymphocytic leukemia | 166 (14%) | 202 (18%) |
| Acute myeloid leukemia | 459 (40%) | 427 (38%) |
| Chronic lymphocytic leukemia | 32 (3%) | 19 (2%) |
| Chronic myeloid leukemia | 104 (9%) | 32 (3%) |
| Hodgkin’s lymphoma | 3 (<1%) | 0 |
| Myelodysplastic syndrome | 230 (20%) | 274 (24%) |
| Multiple myeloma | 3 (<1%) | 1 (<1%) |
| Non-Hodgkin’s lymphoma | 60 (5%) | 29 (3%) |
| Other | 52 (5%) | 93 (8%) |
| Disease Severity* | ||
| Low | 174 (15%) | 149 (13%) |
| Intermediate | 622 (54%) | 823 (73%) |
| High | 352 (31%) | 159 (14%) |
| Augmented Hematopoietic Cell Transplant Co-morbidity Index Scores† | 0 = 114 (10%) | 0 = 74 (7%) |
| 1-3 = 567 (49%) | 1-3 = 448 (40%) | |
| 4-6 = 360 (31%) | 4-6 = 411 (36%) | |
| 7-9 = 93 (8%) | 7-9 = 172 (15%) | |
| ≥10 = 14 (1%) | ≥10 = 29 (3%) | |
| Mean 3.2 | Mean 4.0 | |
| Median 3.0 | Median 4.0 | |
| Donor | ||
| HLA-identical sibling | 429 (37%) | 277 (24%) |
| Mismatched sibling or non-sibling relative | 43 (4%) | 63 (6%) |
| Unrelated | 676 (59%) | 791 (70%) |
| Hematopoietic Cell Source | ||
| Bone marrow | 227 (20%) | 173 (15%) |
| Peripheral blood hematopoietic cells | 871 (76%) | 803 (71%) |
| Bone marrow and either peripheral blood or cord blood cells | 1 (<1%) | 4 (<1%) |
| Cord blood | 49 (4%) | 151 (13%) |
| Conditioning Intensity | ||
| Reduced-intensity | 257 (22%) | 383 (34%) |
| Myeloablative‡ | 117 (10%) | 582 (51%) |
| High-dose myeloablative§ | 774 (67%) | 166 (15%) |
| GVHD prophylaxis** | ||
| CNI+MTX ± ATG | 712 (62%) | 424 (37%) |
| CNI+MMF ± ATG | 342 (30%) | 407 (36%) |
| CNI+Sirolimus+MMF/MTX | 27 (2%) | 147 (13%) |
| CNI+PTCY regimens | 15 (1%) | 77 (7%) |
| CNI+MTX+abatacept (6) | 0 | 41 (4%) |
| Other regimens | 52 (5%) | 35 (3%) |
Abbreviations: HLA, human leukocyte antigen; CNI, calcineurin inhibitor; MTX, methotrexate; ATG, antithymocyte globulin; MMF, mycophenolate mofetil; PTCY, post-transplant cyclophosphamide at 50 mg/kg x 2 doses.
Experts in each hematologic disease category reviewed clinical and laboratory data of patients with hematologic malignancy, including pathology of marrow samples, and classified each patient according to these risk categories.
Augmented Hematopoietic Cell Transplant Co-morbidity Index scores included all risk factors with the exception of serum ferritin.
Myeloablative regimens included cyclophosphamide plus total body irradiation ≤12 Gray; targeted busulfan plus cyclophosphamide; and fludarabine plus busulfan or treosulfan.
High-dose myeloablative regimens included cyclophosphamide plus total body irradiation >12 Gray; busulfan, cyclophosphamide, and total body irradiation; and non-targeted busulfan plus cyclophosphamide.
During 2003-207 and 2013-2017, 153/1148 (13%) and 107/1131 (9%) received ATG during conditioning therapy, respectively.
Co-morbidities that were present before transplant.
A score based on the Augmented Hematopoietic Cell Transplantation Co-Morbidity Index (HCT-CI) was calculated for each patient in both eras, with the single exception of pre-transplant serum ferritin levels, which were infrequently measured during 2003-2007 (9,10).
Clinical assessments and definition of terms
Primary outcome measures.
Day-200 NRM was defined as death before day-200 after transplant that was not preceded by recurrent or progressive malignancy. Relapse-related mortality (RRM) was defined as death that was preceded by a relapse or progression of malignancy. Data for overall mortality, relapse or progression of malignancy, and RRM reflect events as of the date of last contact before the database was locked on March 1, 2019, and all events that occurred by last contact were considered for each of these endpoints.
Complications involving the liver, kidneys, and lungs through day 100.
Liver and kidney injury were assessed by total serum bilirubin and serum creatinine concentrations and use of renal replacement therapy (1). Severe lung injury was defined by the need for mechanical ventilation via an endotracheal tube.
Viral, bacterial, and fungal infections through day 100.
Cytomegalovirus infection was defined as the presence of viral pp65 antigen (2003-2006) or DNA in plasma after 2006; CMV disease was defined by international criteria (11). Patients with one or more positive blood cultures for gram-negative organisms were considered to have gram-negative bacteremia. Invasive fungal infections were classified by international criteria (12). Only fungal infections that were proven or probable were included in this analysis.
Acute GVHD.
The peak stage of gut and liver GVHD and the peak severity of acute GVHD were graded for all patients in both eras by PJM (1,13). GVHD grading was determined by retrospective chart review. Midlevel providers reported the extent of body surface involved with rash in the medical record. Nurses reported stool volumes for hospitalized patients in the medical record. Staging of gut involvement accounted for estimates of urine mixed with stool. Stage 1 gut GVHD was assigned for outpatients who had diarrhea attributed to GVHD, under the premise that sustained stool volumes greater than 1000 mL per day would prompt hospitalization. Grades 2, 3, and 4 GVHD respectively indicate mild, moderate and severe peak GVHD manifestations. National Institutes of Health diagnostic criteria were used to define chronic GVHD cases (14,15). Some patients in the 2003-2007 cohort had their chronic GVHD diagnosis confirmed retrospectively from medical records.
Prednisone initial dosing and exposure.
The dose of prednisone in mg/kg upon initiation of therapy was noted, and a dose of zero was assigned to patients who were never treated with prednisone. Prednisone exposure (in mg/kg) across time was expressed as the average of all values that were available from first dose to last dose, normalized by the time from prednisone start to the earliest of their last prednisone dose or day of death.
Statistical analyses.
Endpoints of primary interest were overall survival, relapse or progression, RRM, and day-200 NRM. The probability of overall survival was estimated by the method of Kaplan and Meier. Probabilities of relapse or progression of malignancy, RRM, and day-200 NRM were summarized using cumulative incidence estimates, where relapse was viewed as a competing risk for NRM, NRM a competing risk for relapse, and NRM a competing risk for RRM. Cox regression was used to compare the cause-specific hazards of failure between cohorts for RRM, day-200 NRM, and relapse or progression with competing-risk failures being censored. Two-sided p-values from regression models were estimated from the Wald test, and no adjustments were made for these four comparisons. All models were adjusted for source of donor hematopoietic cells (G-CSF mobilized blood vs. bone marrow vs. cord blood), donor type (HLA-identical sibling vs. unrelated vs. HLA-nonidentical sibling or non-sibling relative), severity of disease (low vs. intermediate vs. high), conditioning intensity, patient/donor sex, patient/donor CMV serostatus, the augmented HCT-CI score (modeled as a continuous linear variable), and patient age (modeled as a continuous linear variable). Adjusted estimates of day-200 NRM, relapse, and overall mortality were obtained by averaging modeled survival functions obtained from the Cox regression model across all covariate vectors observed in the later era with coefficients estimated from the regression model and the baseline hazard associated with each era (16). Further details on statistical methods are contained in the Appendix, including a tabular summary (Appendix Table 1) of regression methods for the primary outcomes as well as methods used to explore secondary endpoints consisting of major complications associated with mortality, namely organ dysfunction, infection, and GVHD. SAS version 9.4 was used for all analyses.
The current analysis focuses on 2003-7 and 2013-17 cohorts, but for purposes of reference to the 1993-1997 cohort, Appendix Figure 1 provides a visual representation of unadjusted overall mortality for all three cohorts.
Role of the funding source.
The application of allogeneic hematopoietic cell transplantation to treatment of patients with refractory hematologic malignancy, and all of the research emanating from transplantation procedures, was supported by grants from the National Institutes of Health and individual awards. National Institutes of Health grants included CA18029, CA15704, CA78902, HL36444, HL088201, HL088021, HL096831, DK063038, HL122173, and HL108307. Mohamed Sorror was also supported by Research Scholar Grant No. RSG-13-084-01-CPHPS from the American Cancer Society; and by Patient-Centered Outcome Research Institute Contract No. CE-1304-7451. None of these funding sources had an influence on the analysis of transplant outcomes as reported here.
Results
Patient characteristics.
Table 1 and Appendix Table 2 display demographic, disease, and transplant characteristics, including augmented HCT-CI scores (9,10). Notable differences between the two eras were more recent inclusion of older patients, fewer patients with high-risk malignancy, more patients with high scores for HCT-CI, greater use of unrelated and HLA-mismatched donors (including cord blood), and a shift away from high-dose myeloablative conditioning regimens in favor of slightly less intensive (but still myeloablative) conditioning and reduced-intensity regimens. For GVHD prophylaxis, newer agents (sirolimus, PTCY, abatacept) were used more frequently (6,18-20).
Outcome measures: Day-200 NRM, relapse of malignancy, relapse-related mortality, and overall mortality.
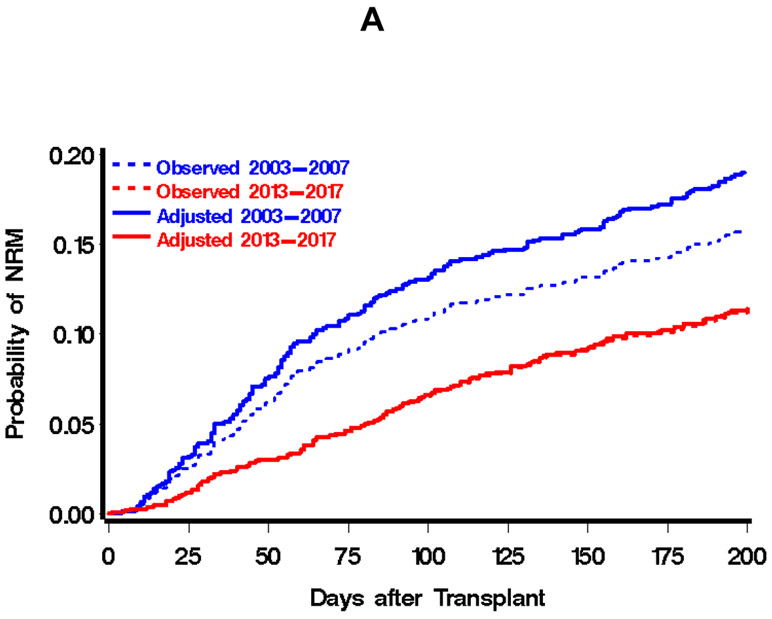
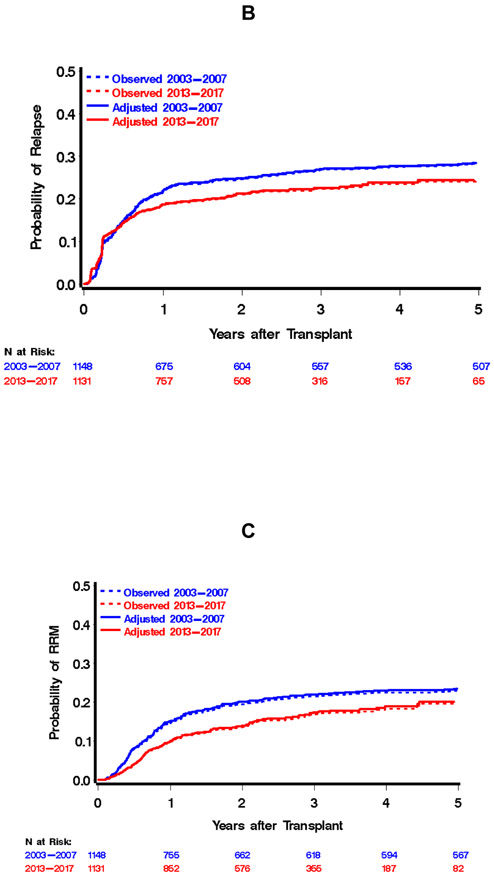
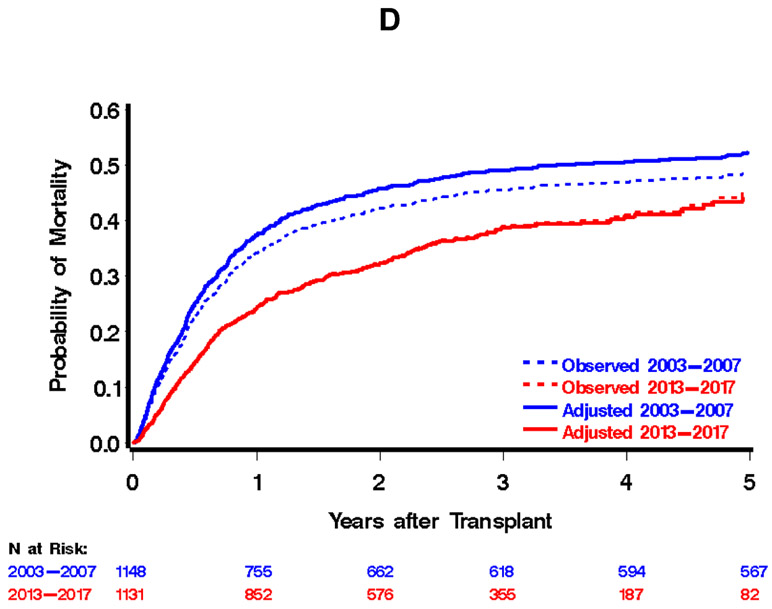
There was a total of 308 events for day-200 NRM, with point estimates of 16% and 11% in the earlier and later cohorts, respectively. This contributed to statistically significant reductions in the hazard of day-200 NRM (unadjusted hazard ratio 0.69, 95% confidence interval (CI) 0.55 to 0.87). By last contact, we had observed a total of 592 relapses or progressions of malignancy, with 1-, 2-, and 3-year estimates of 41%, 47% and 51%, respectively, in the 2003-2007 cohort and 33%, 40%, and 44%, respectively, in the 2013-2017 cohort. This contributed to an unadjusted hazard ratio for relapse or progression of 0.79 (95% CI 0.67 to 0.93). There were 1071 deaths observed by last contact, with 1-, 2-, and 3-year estimates of overall mortality in the 2003-2007 cohort of 34%, 42%, and 45%, respectively. In the 2013-2017 cohort, these estimates were 24%, 32%, and 39%, respectively, with an unadjusted hazard ratio for overall mortality of 0.77 (95% CI 0.68 to 0.88). There were 493 relapse-related mortality events by last contact, with 1-, 2-, and 3-year estimates of 15%, 20%, and 21%, respectively, in the 2003-2007 era and 10%, 14%, and 17%, respectively, in the 2013-2017 era.
The magnitude of reduction in overall mortality across eras was similar regardless of the intensity of conditioning regimens (HR=0.60 and HR=0.65 for ablative and reduced-intensity conditioning, respectively). Moreover, the degree of reduction in the hazard of mortality between time periods was similar for HLA-matched sibling and unrelated donor allografts (HR=0.62 for HLA-matched sibling, HR=0.63 for unrelated donor). While each of these outcomes has seen improvements since 1993-1997 (see Appendix Figure 1 for unadjusted estimates of overall mortality for each of the three cohorts), the largest improvement across the three decades has been in day-200 NRM: a reduction in the hazard of day-200 NRM from 2003 to 2007 compared to1993-1997 (HR=0.40 (1)) and HR=0.66 for 2013-2017 compared to 2003-2007. While not as large, we have also witnessed a reduction in relapse or progression in 2003-2007 compared to 1993-1997 (HR=0.79) and a reduction in 2013-2017 compared to 2003-2007 (HR=0.76). Relapse-related mortality was not assessed in our original report (1), but the reduction in 2013-2017 compared to 2003-2007 was comparable to that for both overall and non-relapse mortality.
Complications associated with mortality: organ dysfunction, infection, acute GVHD, and chronic GVHD.
Liver disease.
Post-transplant elevation of total serum bilirubin ≥68.4 μmol/L (4 mg/dL) was less frequent during 2013-2017 than during 2003-2007 (11% vs. 20%, Table 3). Figure 2 (top panel) shows fitted average daily total serum bilirubin values for 2003-2007 vs. 2013-2017; the means of these averages were 23.9 vs. 17.1 μmol/L (1.4 vs. 1.0 mg/dL). Extreme hyperbilirubinemia (total serum bilirubin ≥171 μmol/L (10 mg/dL)) occurred in 6% and 3% of patients in 2003-2007 and 2013-2017, respectively (Table 3). Hyperbilirubinemia ≥171 μmol/L (10 mg/dL) developed in 29 patients during 2013-2017, caused mostly by cholestatic liver disease (21 patients). Development of severe Sinusoidal Obstruction Syndrome (treated with defibrotide) occurred in 20 (2%) patients during 2003-2007 and in 5 (0.4%) patients during 2013-2017. As noted previously (21), extreme hyperbilirubinemia before day 100 was an ominous prognostic sign, as such patients had a dramatically increased hazard of day-200 NRM relative to patients who were not deeply jaundiced (hazard ratio 33.85) (Table 4). The frequency of stage 3-4 liver GVHD was low in both eras (Table 3).
Table 3.
Comparison of the frequency of organ toxicity, infection, acute and chronic GVHD, and prednisone exposure after transplant between two eras, overall and by intensity of the conditioning regimen.*
| Event | Frequency of event among all patients |
Frequency of event among patients who received myeloablative conditioning therapy |
Frequency of event among patients who received reduced intensity conditioning therapy |
||||||
|---|---|---|---|---|---|---|---|---|---|
| 2003- 2007 (n=1148) |
2013-2017 (n=1131) |
Adjusted HR/OR (95% CI, p- value) |
2003- 2007 (n=891) |
2013- 2017 (n=748) |
Adjusted HR/OR (95% CI, p-value) |
2003- 2007 (n=257) |
2013- 2017 (n=383) |
Adjusted HR/OR (95% CI, p- value) |
|
| Liver dysfunction through day 100 | |||||||||
| Peak total serum bilirubin ≥68.4 μmol/L (4 mg/dL) | 233 (20%) | 123 (11%) | 0.54 (0.39-0.73) | 198 (22%) | 88 (12%) | 0.41 (0.30-0.57) | 35 (14%) | 35 (9%) | 0.69 (0.40-1.20) |
| Peak total serum bilirubin ≥171 μmol/L (10 mg/dL) | 64 (6%) | 29 (3%) | 0.43 (0.24-0.78) | 55 (6%) | 24 (3%) | 0.46 (0.26-0.80) | 9 (4%) | 5 (1%) | 0.25 (0.06-0.94) |
| Stage 3-4 liver GVHD† | 25 (2%) | 13 (1%) | 0.47 (0.20-1.15) | 22 (2%) | 12 (2%) | 0.39 (0.18-0.87) | 3 (1%) | 1 (<1%) | --- |
| Stage 4 liver GVHD† | 2 (<1%) | 6 (<1%) | --- | 2 (2%) | 5 (<1%) | --- | 0 | 1 (<1%) | --- |
| Acute Kidney Injury through day 100 | |||||||||
| Creatinine 2-times baseline | 384 (33%) | 254 (22%) | 0.51 (0.42-0.63) | 294 (33%) | 150 (20%) | 0.43 (0.33-0.56) | 90 (35%) | 104 (27%) | 0.69 (0.47-1.01) |
| Creatinine 3-times baseline | 115 (10%) | 67 (6%) | 0.49 (0.35-0.69) | 93 (10%) | 41 (5%) | 0.41 (0.27-0.63) | 22 (9%) | 26 (7%) | 0.78 (0.41-1.51) |
| Renal Replacement Therapy | 58 (5%) | 39 (3%) | 0.61 (0.39-0.95) | 50 (6%) | 29 (4%) | 0.58 (0.35-0.97) | 8 (3%) | 10 (3%) | 0.90 (0.32-2.55) |
| Respiratory failure through day 100 | |||||||||
| Mechanical ventilation | 131 (11%) | 77 (7%) | 0.49 (0.35-0.67) | 104 (12%) | 51 (7%) | 0.46 (0.31-0.69) | 27 (11%) | 26 (7%) | 0.59 (0.31-1.10) |
| Infections through day 100 | |||||||||
| CMV infection‡ | 74/104 (71%) | 501/679 (74%) | 1.15 (0.88–1.52) | 47/69 (68%) | 329/443 (74%) | 1.31 (0.90–1.90) | 27/35 (77%) | 172/236 (73%) | 1.01 (0.65–1.55) |
| CMV infection >250 IU/mL‡ | 48/104 (46%) | 288/679 (42%) | 0.78 (0.55–1.10) | 27/69 (39%) | 175/443 (40%) | 0.87 (0.53–1.42) | 21/35 (60%) | 113/236 (48%) | 0.71 (0.42–1.18) |
| CMV infection >1000 IU/mL‡ | 26/104 (46%) | 138/679 (20%) | 0.46 (0.28–0.74) | 14/69 (20%) | 83/443 (19%) | 0.45 (0.22–0.94) | 12/35 (34%) | 55/236 (23%) | 0.50 (0.25–1.01) |
| CMV disease§ | 34/660 (5%) | 24/679 (4%) | 0.61 (0.32-1.14) | 26/504 (5%) | 15/443 (3%) | 0.55 (0.27-1.11) | 8/156 (5%) | 9/236 (4%) | 0.65 (0.23-1.88) |
| Gram-negative bacteremia | 129 (11%) | 74 (7%) | 0.42 (0.29-0.60) | 89 (10%) | 55 (7%) | 0.62 (0.43-0.91) | 40 (16%) | 19 (5%) | 0.26 (0.14-0.47) |
| Invasive mold infection | 80 (7%) | 42 (4%) | 0.55 (0.33-0.92) | 64 (7%) | 28 (4%) | 0.33 (0.20-0.54) | 16 (6%) | 14 (4%) | 0.41 (0.19-0.89) |
| Invasive Candida infection | 10 (1%) | 6 (1%) | --- | 10 (1%) | 3 (<1%) | --- | 0 | 3 (1%) | --- |
| Acute and chronic GVHD | |||||||||
| Grades 2-4 | 815 (71%) | 784 (69%) | 0.80 (0.63-1.02) | 650 (73%) | 554 (74%) | 0.83 (0.64-1.06) | 165 (64%) | 230 (60%) | 0.72 (0.49-1.05) |
| Grades 3-4 | 161 (14%) | 135 (12%) | 0.63 (0.46-0.86) | 123 (14%) | 103 (14%) | 0.75 (0.55-1.03) | 38 (15%) | 32 (8%) | 0.51 (0.29-0.89) |
| Grade 4 | 27 (2%) | 40 (4%) | 1.38 (0.74-2.57) | 19 (2%) | 33 (4%) | 1.67 (0.89-3.17) | 8 (3%) | 7 (2%) | --- |
| Stage 2-4 gut GVHD∥ | 119 (10%) | 126 (11%) | 0.84 (0.60-1.17) | 91 (10%) | 95 (13%) | 1.02 (0.73-1.44) | 28 (11%) | 31 (8%) | 0.69 (0.38-1.25) |
| Stage 3-4 gut GVHD∥ | 73 (6%) | 69 (6%) | 0.77 (0.50-1.18) | 55 (6%) | 54 (7%) | 0.92 (0.59-1.42) | 18 (7%) | 15 (4%) | 0.58 (0.27-1.25) |
| Chronic GVHD | 500 (44%) | 327 (29%) | 0.40 (0.33-0.48) | 381 (43%) | 204 (27%) | 0.40 (0.33-0.49) | 119 (46%) | 123 (32%) | 0.58 (0.44-0.77) |
| Prednisone – initial dose and exposure | |||||||||
| 2003- 2007 (n=1148) |
2013- 2017 (n=1131) |
Comparison of mean ranks |
2003- 2007 (n=891) |
2013- 2017 (n=748) |
Comparison of mean ranks |
2003- 2007 (n=257) |
2013- 2017 (n=383) |
Comparison of mean ranks |
|
| Initial prednisone dose (mg/kg)¶ | |||||||||
| Mean | 0.83 | 0.58 | p<0.0001 | 0.85 | 0.62 | p<0.0001 | 0.76 | 0.49 | p<0.0001 |
| Median | 0.96 | 0.56 | 0.97 | 0.76 | 0.94 | 0.46 | |||
| Range | 0 – 4.00 | 0 – 2.45 | 0 - 4.00 | 0 - 2.45 | 0 - 2.33 | 0 - 2.00 | |||
| Prednisone exposure (mg/kg)** | |||||||||
| Mean | 0.08 | 0.05 | p<0.0001 | 0.77 | 0.46 | p=0.04 | 0.07 | 0.06 | p=0.0007 |
| Median | 0.01 | 0.008 | 0.01 | 0.009 | 0.009 | 0.005 | |||
| Range | 0 – 3.96 | 0 – 2.0 | 0 – 3.96 | 0 - 2 | 0 - 2 | 0 - 2 | |||
Abbreviations: GVHD, graft-versus-host disease; HR, hazard ratio; OR, odds ratio; CI, confidence interval; CMV, cytomegalovirus
Change over the decade is expressed as a hazard ratio (HR), odds ratio (OR), or adjusted mean difference, as calculated by regression models adjusted for source of donor hematopoietic cells, donor type, disease severity, patient/donor sex, patient/donor CMV serostatus, patient age, conditioning regimen intensity, and HCT-Comorbidity Index scores (see Methods).
Liver stage 1, total serum bilirubin 34.2-49.6 μmol/L (2-2.9 mg/dL); stage 2, 51.3-100.9 μmol/L (3-5.9 mg/dL); stage 3, 102.6-254.8 μmol/L (6-14.9 mg/dL); stage 4, ≥256.5 μmol/L (15 mg/dL).
Among CMV-seropositive patients whose screening for CMV infection was with CMV DNA in serum.
Among CMV-seropositive patients.
Gut stage 1, diarrhea 500-999 mL/day or biopsy-proven upper gut involvement; stage 2, diarrhea 1000-1499 mL/day; stage 3, diarrhea 1500-1999 mL/day; stage 4, diarrhea > 2000 mL or severe abdominal pain with or without ileus.
Considering all patients, with zero assigned to patients who never received prednisone and with p-values based on adjusted comparison of the ranks, using linear regression.
Estimated average daily prednisone dose in mg/kg, with p-values based on adjusted comparison of the ranks of the averages, using linear regression.
Figure 2.

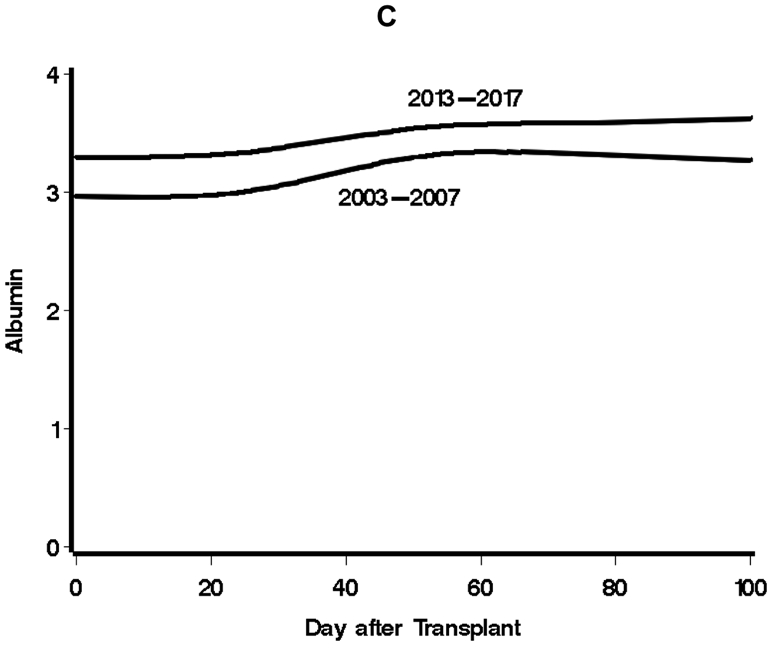
Comparison of daily laboratory values in conventional units for total serum bilirubin (A), serum creatinine (B), and serum albumin (C), 2003-2007 vs. 2013-2017. Restricted cubic spline curves are fit to the observed data (see Appendix, Supplementary Statistical Methods). Factors for conversion to SI units are 17.1 for total serum bilirubin, 88.4 for serum creatinine, and 10 for serum albumin.
Table 4.
Complications of HCT during the first 100 days and their attendant Day-200 Non-Relapse Mortality Hazard Ratios, 2013-2017.
| Complications of allogeneic hematopoietic cell transplant during 2013-2017 |
Number of patients* |
Hazard Ratio for Day-200 Non- Relapse Mortality (95% CI) |
|---|---|---|
| Peak total serum bilirubin ≥68.4 μmol/L (4 mg/dL) | 123 | 11.94 (8.16 – 17.47) |
| Peak total serum bilirubin ≥171 μmol/L (10 mg/dL) | 29 | 33.85 (20.06 – 57.11) |
| Serum creatinine 2-times baseline | 254 | 6.63 (4.60 – 9.57) |
| Serum creatinine 3-times baseline | 67 | 15.47 (9.97 – 24.01) |
| Renal Replacement Therapy | 39 | 20.80 (13.03 – 33.22) |
| Mechanical ventilation | 77 | 15.85 (10.72 – 23.44) |
| Jaundice + Renal Replacement Therapy + Mechanical ventilation | 19 | 36.70 (19.31 – 69.77) |
| Any CMV infection† | 501 | 1.23 (0.73 – 2.08) |
| CMV DNA >250 IU/mL† | 288 | 2.33 (1.42 – 3.84) |
| CMV DNA >1000 IU/mL† | 138 | 2.82 (1.68 – 4.75) |
| CMV disease b | 24 | 2.00 (0.72 – 5.56) |
| Gram negative bacteremia | 74 | 5.16 (3.22 – 8.24) |
| Invasive mold infection | 42 | 9.73 (5.83 – 16.22) |
| GVHD grade 2-4 | 784 | 1.45 (0.95 – 2.22) |
| GVHD grade 3-4 | 135 | 5.29 (3.59 – 7.81) |
See Table 3, Frequency of events among all patients transplanted during 2013-2017.
Among CMV-seropositive patients only.
Kidney injury.
During the 2013-2017 era, the frequency of acute kidney injury was lower than that in the 2003-2007 cohort, with doubling of baseline serum creatinine occurring in 22% and 33% of patients, respectively; tripling of baseline serum creatinine occurred in 6% and 10% of patients, respectively. Figure 2 (middle panel) shows fitted average daily serum creatinine values to day 100 for 2003-2007 vs. 2013-2017; the means of these averages were 89.3 vs. 79.6 μmol/L (1.01 vs. 0.90 mg/dL). In the current era, 3% of patients received renal replacement therapy, compared to 5% during 2003-2007 (Table 3). The need for renal replacement therapy before day 100 was an ominous prognostic sign, as the hazard of day-200 NRM for such patients was 20.80-fold higher than that for patients who did not require renal replacement therapy before day 100 (Table 4).
Pulmonary complications.
The frequency of respiratory failure as measured by need for endotracheal mechanical ventilation was 7% in the most recent cohort, compared to 11% during 2003-2007 (Table 3). The need for mechanical ventilation before day 100 was a poor prognostic sign, with a 15.85-fold increase in the hazard of day-200 NRM compared to those who were not mechanically ventilated (Table 4).
Multiorgan failure.
Survival was nil among 1990s transplant recipients who developed jaundice, respiratory failure, and renal insufficiency requiring dialysis (22). Among patients transplanted during the 2013-2017 era, there were 19 who developed this combination of events, of whom 15 died of non-relapse causes by day 200. The hazard of day-200 NRM associated with this triad of complications was 36.70 times that among patients who did not experience all three (Table 4).
Infections.
The hazard of any CMV infection was not lower in the 2013-2017 cohort, but the hazards of higher levels of CMV viremia (by DNA >1000 IU/mL) were less in the 2013-2017 cohort of CMV seropositive patients (Table 3). The occurrence of higher levels of CMV viremia were also associated with an increased risk of day-200 NRM among patients in the 2013-2017 cohort (Table 4). The hazards of developing bacteremia with a gram-negative organism declined from 11% to 7% and invasive mold infection declined from 7% to 4% (Table 3). The magnitude of declines in the hazard of these infections was similar between the entire cohort and patients who received myeloablative conditioning regimens. The incidence of invasive Candida infections remained low (roughly 1%) across the two periods. of invasive Candida infections remained low (roughly 1%) across the two periods.
Acute GVHD.
The frequency of peak grade 3-4 acute GVHD was 14% in 2003-2007 and 12% in 2013-2017, and after adjustment the odds ratio was 0.63 (0.46-0.86, Table 3). The hazard ratio for day-200 NRM associated with the occurrence of grade 3-4 acute GVHD in the 2013-2017 cohort was 5.29 (Table 4). Figure 2 (bottom panel) shows fitted average daily serum albumin values for each era, as falls in serum albumin can be a surrogate for protein-losing enteropathy after transplant (23). For 2003-2007 vs. 2013-2017, the means of these averages were 31.8 vs. 34.9 g/L (3.18 vs. 3.49 g/dL), suggesting less gastrointestinal protein loss in the more recent era.
Prednisone exposure.
Initial doses of prednisone for acute GVHD in the 2013-2017 era were significantly lower than during 2003-2007 (mean initial dose 0.58 vs. 0.83 mg/kg, Table 3), a result of a change in practice toward steroid-sparing dosing (24,25). The estimated average daily prednisone dose (normalized to days survived as noted in Methods) was also significantly reduced in patients transplanted during the current era, from 0.0757 during 2003-2007, to 0.0510 mg/kg (Table 3).
Chronic GVHD.
The adjusted hazard of developing chronic GVHD was reduced by 60% in 2013-2017 relative to 2003-2007 (Table 3).
Discussion
We and others have previously reported substantial improvement in the outcomes of patients undergoing allogeneic hematopoietic cell transplantation from the 1990s through the early 2000s (1-5). The primary question now is whether further improvement in outcomes could be demonstrated, or whether a survival plateau had been reached. These data demonstrate further improvement in survival, with a 34% reduction in overall mortality compared to 2003-2007 after consideration of factors that reflect older and sicker patients treated in recent years (9,10). The degree of reduction in overall mortality was similar for patients who received either myeloablative or reduced-intensity conditioning as well as for patients transplanted from a matched-sibling vs. an unrelated donor. Even without adjusting for confounding factors such as increased age and co-morbidities, there was still a significant drop in overall mortality and day-200 NRM in the more recent era.
The reasons for improved survival lie mostly in a decline in the rate of day-200 NRM but also to a reduced hazard of relapse or progression of hematologic malignancy. NRM is largely a function of organ damage from the conditioning regimen, infections during immune suppression, and more severe acute GVHD. We saw improvements in each of these areas. Ascribing direct cause-effect relationships in outcomes to recent changes in practice and improved outcomes is not possible in cohort comparison studies. However, we can speculate on why we continue to see such improvement in outcomes. Our transplant practice has transitioned away from high-dose myeloablative conditioning therapies to less toxic myeloablative regimens and to reduced-intensity conditioning. Reducing the intensity of conditioning may reduce the incidence of acute GVHD (26), which may in turn result in less immune suppression and fewer infections. We speculate that reduction in the intensity of conditioning regimens is partly responsible for falls in the frequency of hyperbilirubinemia, kidney failure, respiratory failure, and infections.
In the most recent cohort, we did not see any apparent change in overall CMV infection but the hazard of higher-level viremia (DNAemia >1000 IU/mL, associated with increased mortality (8)) was reduced by 54%. Substantial reductions in gram-negative bacteremia and invasive mold infections were also seen. Multiple factors may have contributed to this fall, including the use of less intensive preparative regimens, the availability and use of improved anti-fungal drugs as prophylaxis for patients at high risk, and, perhaps most important, substantially reduced prednisone doses to treat GVHD. Our prednisone dosing practices changed when a randomized trial showed that, when combined with topical oral glucocorticoid, lower initial doses of prednisone for patients with good-prognosis GVHD were safe and as effective as higher-dose prednisone (24). Patients whose GVHD prophylaxis included sirolimus also had significantly less prednisone exposure (20).
Less grade 3-4 acute GVHD and chronic GVHD were seen in the more recent cohort. The avoidance of the most toxic preparative regimens may have played a part, and we have been exploring newer methods for GVHD avoidance, including the use of post-transplant cyclophosphamide, sirolimus, partial T cell depletion, and novel combinations of immunosuppressive drugs (6,18-20, 27). The drop in severe acute and chronic GVHD may represent the aggregate outcome of these efforts. A concern of improved GVHD control is an increase in post-transplant recurrent malignancy. Fortunately, we did not witness an increase in disease recurrence, and, in fact, saw a reduction, even after adjustment for disease severity.
We cannot dismiss the possibility that the improvements are the result of better patient selection. However, several factors associated with an increased risk of poor outcome were more frequent in the 2013-2017 cohort, reducing this possibility. We have also attempted to diminish this complicating factor by adjusting for factors such as age, disease status, and co-morbidity scores in our analysis. We must note, however, that other less easily definable factors may have escaped notice, and consideration of such factors could reduce the magnitude of the reported differences. For example, we were unable to compare current molecular and genomic markers predictive of outcome in hematologic malignancy with the 2003-2007 cohort, when such tests were not available.
Over the last 25 years, the frequency of day-200 NRM has progressively declined (from 30% to 16%, and now 11%). Relapse rates have also declined with the result that overall survival from allogeneic transplantation has further improved. These improvements likely result from the accumulation of many individually incremental advances in conditioning therapy, GVHD prophylaxis, prednisone dosing, infection control, and supportive care. Data showing better survival after allogeneic transplantation are encouraging, but the results are only relative to an earlier era. In absolute terms, the frequency of overall mortality during 2013-2017 was 40%, and this number will obviously increase with further follow-up. Prevention and treatment of the causes of NRM are within reach, by focusing on complications with the greatest mortality hazard (organ failure, infection, more severe GVHD) (Table 4) (28-33). The risk of mortality related to relapse or progression has also decreased across these eras, but not to the same degree as have reductions in NRM. The more difficult path to further improvements in survival lies in prevention of post-transplant relapse or progression of hematologic malignancy.
Supplementary Material
Figure 1.
Comparison of outcomes of hematopoietic cell transplantation, 2003-2007 vs. 2013-2017 cohorts. Day-200 non-relapse mortality is displayed in A, relapse or progression of malignancy in B, relapse-related mortality in C, and overall mortality in D. Dotted lines in each figure represent the unadjusted estimates of the appropriate outcome, solid lines represent adjusted estimates. Adjusted estimates were derived by assuming a population in the 2003-2007 cohort that is consistent with the 2013-2017 population with respect to source of stem cells, donor type, disease severity, patient/donor sex, patient/donor CMV serostatus, comorbidity index, patient age, and conditioning intensity. Abbreviations: NRM, Non-Relapse Mortality; RRM, Relapse-Related Mortality.
Table 2.
Comparison of outcomes after transplant between two eras, overall and by intensity of the conditioning regimen.
| Frequency of outcomes among all patients |
Frequency of outcomes among patients who received myeloablative conditioning therapy |
Frequency of outcomes among patients who received reduced intensity conditioning therapy |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| 2003- 2007 (n=1148) |
2013- 2017 (n=1131) |
Adjusted HR (95% CI, p- value) |
2003- 2007 (n=891) |
2013- 2017 (n=748) |
Adjusted HR (95% CI, p-value) |
2003- 2007 (n=257) |
2013- 2017 (n=383) |
Adjusted HR (95% CI, p- value) |
|
| Day-200 non-relapse mortality | 181 (16%)* | 127 (11%) | 0.66 (0.48-0.89, p=0.008) | 144 (16%) | 84 (11%) | 0.60 (0.45-0.82) | 37 (14%)* | 43 (11%) | 0.65 (0.41–1.05) |
| Relapse or progression | 348† | 244 | 0.76 (0.61-0.94, p=0.011) | 261 | 153 | 0.74 (0.59-0.92) | 87 | 96 | 0.78 (0.56-1.08) |
| Relapse-related mortality | 307 | 186 | 0.69 (0.54-0.87, p=0.002) | 230 | 115 | 0.63 (0.49-0.81) | 77 | 71 | 0.66 (0.45-0.96) |
| Overall morality | 653† | 418 | 0.66 (0.56-0.78, p<0.001) | 480 | 263 | 0.65 (0.55-0.78) | 173 | 15 | 0.67 (0.52-0.86) |
These numbers are smaller than those previously reported for the 2003-2007 cohort (1). Upon further review, five patients who received reduced intensity conditioning had relapsed before day-200.
These numbers are larger than those previously reported for the 2003-2007 cohort, reflecting the extended period of time during which patients were at risk for relapse/progression and mortality.
Acknowledgements:
We are grateful to the many physicians, nurses, physician assistants, pharmacists, social workers, and support staff who cared for our patients during this decade and to the patients who participated in our ongoing clinical research.
George McDonald and Ted Gooley had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Brenda Sandmaier and Rainer Storb were responsible for data related to patients who received reduced-intensity conditioning therapy. Marco Mielcarek was responsible for data analysis related to prednisone exposure. Mohamed Sorror was responsible for data related to the Hematopoietic Cell Transplantation Co-Morbidity Index. Steven Pergam and Michael Boeckh were responsible for the integrity of data related to infectious disease endpoints. Guang-Shing Cheng was responsible for data related to mechanical ventilation after transplant. Sangeeta Hingorani was responsible for data related to renal insufficiency and dialysis/hemofiltration after transplant. Mary Flowers and Stephanie Lee were responsible for data related to chronic GVHD. Fred Appelbaum and Joachim Deeg were responsible for classification of underlying hematologic disorders with regard to risk assessment. Paul Martin determined acute GVHD stages and grades for all patients in the two cohorts and was responsible for the integrity of these data. Gary Schoch was responsible for extracting data from our longitudinal database that contains demographic information, laboratory results and clinical endpoints. All co-authors (whose affiliations are listed on the Title Page) reviewed the manuscript for accuracy and concur with its submission.
Funding sources:
Our research was supported by grants from the National Institutes of Health (CA18029, CA15704, CA78902, HL36444, HL088201, HL088021, HL096831, DK063038, HL122173, HL108307). Mohamed Sorror was also supported by Research Scholar Grant No. RSG-13-084-01-CPHPS from the American Cancer Society; and by Patient-Centered Outcome Research Institute Contract No. CE-1304-7451.
References
- 1.Gooley TA, Chien JW, Pergam SA, et al. Reduced mortality after allogeneic hematopoietic-cell transplantation. N Engl J Med 2010;363:2091–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Remberger M, Ackefors M, Berglund S, et al. Improved survival after allogeneic hematopoietic stem cell transplantation in recent years. A single-center study. Biol Blood Marrow Transplant 2011;17:1688–97. [DOI] [PubMed] [Google Scholar]
- 3.Giebel S, Labopin M, Holowiecki J, et al. Outcome of HLA-matched related allogeneic hematopoietic stem cell transplantation for patients with acute leukemia in first complete remission treated in Eastern European centers. Better results in recent years. Annals of Hematology 2009;88:1005–13. [DOI] [PubMed] [Google Scholar]
- 4.Horan JT, Logan BR, Agovi-Johnson MA, et al. Reducing the risk for transplantation-related mortality after allogeneic hematopoietic cell transplantation: how much progress has been made? Journal of Clinical Oncology 2011;29:805–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Spees LP, Martin PL, Kurtzberg J, et al. Reduction in mortality after umbilical cord blood transplantation in children over a 20-year period (1995-2014). Biol Blood Marrow Transplant 2019;25:756–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.ClinicalTrials.gov Identifier NCT01743131, Abatacept as GVHD Prophylaxis Phase 2. [Google Scholar]
- 7.Ljungman P, Snydman D, Boeckh M., ed. Transplant Infections. 4 ed. Switzerland: Springer International Publishing; 2016. [Google Scholar]
- 8.Green ML, Leisenring W, Xie H, et al. Cytomegalovirus viral load and mortality after haemopoietic stem cell transplantation in the era of pre-emptive therapy: a retrospective cohort study. The Lancet Haematology 2016;3:e119–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vaughn JE, Storer BE, Armand P, et al. Design and Validation of an Augmented Hematopoietic Cell Transplantation-Comorbidity Index Comprising Pretransplant Ferritin, Albumin, and Platelet Count for Prediction of Outcomes after Allogeneic Transplantation. Biol Blood Marrow Transplant 2015;21:1418–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Elsawy M, Storer BE, Milano F, et al. Prognostic Performance of the Augmented Hematopoietic Cell Transplantation-Specific Comorbidity/Age Index in Recipients of Allogeneic Hematopoietic Stem Cell Transplantation from Alternative Graft Sources. Biol Blood Marrow Transplant 2019;25:1045–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ljungman P, Boeckh M, Hirsch HH, et al. Definitions of Cytomegalovirus Infection and Disease in Transplant Patients for Use in Clinical Trials. Clin Infect Dis 2017;64:87–91. [DOI] [PubMed] [Google Scholar]
- 12.De Pauw B, Walsh TJ, Donnelly JP, et al. Revised definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Clin Infect Dis 2008;46:1813–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Przepiorka D, Weisdorf D, Martin P, et al. 1994 Consensus Conference on Acute GVHD Grading. Bone Marrow Transplant 1995;15:825–8. [PubMed] [Google Scholar]
- 14.Filipovich AH, Weisdorf D, Pavletic S, et al. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. Diagnosis and staging working group report. Biology of Blood & Marrow Transplantation 2005;11:945–56. [DOI] [PubMed] [Google Scholar]
- 15.Jagasia MH, Greinix HT, Arora M, et al. National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease: I. The 2014 Diagnosis and Staging Working Group report. Biol Blood Marrow Transplant 2015;21:389–401.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Storer BE, Gooley TA, Jones MP. Adjusted estimates for time-to-event endpoints. Lifetime Data Analysis 2008;14:484–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Heinzl H, Kaider A. Gaining more flexibility in Cox proportional hazards regression models with cubic spline functions. Computer Methods and Programs in Biomedicine 1997;54:201–8. [DOI] [PubMed] [Google Scholar]
- 18.Mielcarek M, Furlong T, O'Donnell PV, et al. Posttransplantation cyclophosphamide for prevention of graft-versus-host disease after HLA-matched mobilized blood cell transplantation. Blood 2016;127:1502–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kanakry CG, Bolanos-Meade J, Kasamon YL, et al. Low immunosuppressive burden after HLA-matched related or unrelated BMT using posttransplantation cyclophosphamide. Blood 2017;129:1389–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sandmaier BM Kornblit B, Storer BE, et al. Effectiveness of addition of sirolimus to cyclosporine and mycophenolate mofetil-based graft-versus-host disease prophylaxis after unrelated nonmyeloablative hematopoietic cell transplantation: A multicentre phase III randomized trial. Lancet Haematology 2019;in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gooley TA, Rajvanshi P, Schoch HG, McDonald GB. Serum bilirubin levels and mortality after myeloablative allogeneic hematopoietic cell transplantation. Hepatology 2005;41:345–52. [DOI] [PubMed] [Google Scholar]
- 22.Rubenfeld GD, Crawford SW. Withdrawing life support from mechanically ventilated recipients of bone marrow transplants: a case for evidence-based guidelines. Ann Intern Med 1996;125:625–33. [DOI] [PubMed] [Google Scholar]
- 23.Rezvani AR, Storer BE, Storb R, et al. Decreased serum albumin as a biomarker for severe acute graft-versus-host disease after reduced-intensity allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant 2011;17:1594–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mielcarek M, Furlong T, Storer BE, et al. Effectiveness and safety of lower dose prednisone for initial treatment of acute graft-versus-host disease: a randomized controlled trial. Haematologica 2015;100:842–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McDonald GB. How I treat acute graft-versus-host disease of the gastrointestinal tract and the liver. Blood 2016;127:1544–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hill GR, Crawford JM, Cooke KR, Brinson YS, Pan L, Ferrara JL. Total body irradiation and acute graft-versus-host disease: the role of gastrointestinal damage and inflammatory cytokines. Blood 1997;90:3204–13. [PubMed] [Google Scholar]
- 27.Bleakley M, Heimfeld S, Loeb KR, et al. Outcomes of acute leukemia patients transplanted with naive T cell-depleted stem cell grafts. The Journal of Clinical Investigation 2015;125:2677–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hingorani S Renal Complications of Hematopoietic-Cell Transplantation. N Engl J Med 2016;374:2256–67. [DOI] [PubMed] [Google Scholar]
- 29.Saillard C, Darmon M, Bisbal M, et al. Critically ill allogenic HSCT patients in the intensive care unit: a systematic review and meta-analysis of prognostic factors of mortality. Bone Marrow Transplant 2018;53:1233–41. [DOI] [PubMed] [Google Scholar]
- 30.Seo S, Yu J, Jenkins IC, et al. Diagnostic and Prognostic Plasma Biomarkers for Idiopathic Pneumonia Syndrome after Hematopoietic Cell Transplantation. Biol Blood Marrow Transplant 2018;24:678–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Blazar BR, MacDonald KPA, Hill GR. Immune regulatory cell infusion for graft-versus-host disease prevention and therapy. Blood 2018;131:2651–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fredricks DN. The gut microbiota and graft-versus-host disease. The Journal of Clinical Investigation 2019;129:1808–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhou X, O’Dwyer DN, Xia M, Miller HK, Chan PR, Trulik K, et al. First-onset herpesviral infection and lung injury in allogeneic hematopoietic cell transplantation. American Journal of Respiratory and Critical Care Medicine 2019;200:63–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.