ABSTRACT
Mutations in the RNA helicase DDX3 have emerged as a frequent cause of intellectual disability in humans. Because many individuals carrying DDX3 mutations have additional defects in craniofacial structures and other tissues containing neural crest (NC)-derived cells, we hypothesized that DDX3 is also important for NC development. Using Xenopus tropicalis as a model, we show that DDX3 is required for normal NC induction and craniofacial morphogenesis by regulating AKT kinase activity. Depletion of DDX3 decreases AKT activity and AKT-dependent inhibitory phosphorylation of GSK3β, leading to reduced levels of β-catenin and Snai1: two GSK3β substrates that are crucial for NC induction. DDX3 function in regulating these downstream signaling events during NC induction is likely mediated by RAC1, a small GTPase whose translation depends on the RNA helicase activity of DDX3. These results suggest an evolutionarily conserved role of DDX3 in NC development by promoting AKT activity, and provide a potential mechanism for the NC-related birth defects displayed by individuals harboring mutations in DDX3 and its downstream effectors in this signaling cascade.
KEY WORDS: DDX3, Neural crest (NC), AKT, GSK3β, Wnt signaling, Xenopus
Summary: To model human birth defects caused by DDX3X mutations, we show that Xenopus DDX3 induces neural crest via the AKT-GSK3β axis, which promotes Wnt signaling and Snai1 stability.
INTRODUCTION
Neural crest (NC) cells are multipotent stem cells that are induced to form at the neural plate border (the border of neural plate and epidermis; NPB) during vertebrate gastrulation. After the induction and subsequent maintenance, NC cells migrate out of the closing neural tube to specific destinations, where they differentiate into various types of cells and contribute to many tissues, such as the craniofacial structures, dental tissues, the peripheral nervous system, pigment cells and cardiac tissues (Bronner and Simões-Costa, 2016; Simões-Costa and Bronner, 2015; Stuhlmiller and García-Castro, 2012). In humans, impaired NC development can lead to birth defects that are collectively called neurocristopathies, including craniofacial disorders, congenital heart diseases and pigment defects (Dubey and Saint-Jeannet, 2017; Vega-Lopez et al., 2018).
Nearly all of our knowledge on the induction of NC was obtained from studies using Xenopus and other non-mammalian vertebrates such as chicks, owing to technical difficulties that have been encountered in mice (Barriga et al., 2015; Betters et al., 2018). These studies have uncovered a two-stage process for NC induction. First, the interplay of multiple signaling pathways activates the expression of transcription factors such as Pax3, Zic1 and Msx1 (the ‘NPB specifiers’), which induce NPB formation. These NPB specifiers subsequently induce other transcription factors, including Snai2, FoxD3 and Sox9 (the ‘NC specifiers’), to specify the NC fate within the NPB (Bronner and Simões-Costa, 2016; Simões-Costa and Bronner, 2015; Stuhlmiller and García-Castro, 2012). A major signaling pathway that induces the NC in non-mammalian vertebrates is canonical Wnt/β-catenin signaling (hereinafter referred to as ‘Wnt signaling’), which is required for both the initial formation of the NPB and the subsequent specification of the NC (Li et al., 2018; Prasad et al., 2019). Similarly, the induction of NC from human embryonic stem (ES) cells or induced pluripotent stem (iPS) cells also requires the activation of Wnt signaling (Gomez et al., 2019; Leung et al., 2016; Menendez et al., 2011; Mica et al., 2013), indicating that Wnt function in NC induction is conserved during vertebrate evolution.
The DEAD/H box RNA helicase DDX3 plays important roles in many cellular processes, including cell cycle regulation, differentiation, survival and apoptosis, primarily by regulating ATP-dependent RNA metabolism, such as RNA splicing, nuclear export and translation (He et al., 2018; Heerma van Voss et al., 2017; Sharma and Jankowsky, 2014). DDX3 contains a helicase ATP-binding domain and a helicase C-terminal domain, both of which are evolutionarily conserved and required for its RNA helicase activity. In addition, DDX3 has been shown to regulate Wnt signaling by stimulating the casein kinase CK1ε to phosphorylate the adaptor protein Dishevelled, resulting in enhanced β-catenin nuclear import. This mechanism is independent of the RNA helicase activity of DDX3, as mutants with the ATP-binding domain and part of the helicase C-terminal domain deleted can still activate Wnt signaling (Cruciat et al., 2013). There are two functional DDX3 homologs in humans, DDX3X and DDX3Y, which are localized on the X and Y chromosome, respectively. Although the two gene products share high sequence similarity, they have distinct expression patterns and function. DDX3Y is translated specifically in the testes and is necessary for male fertility, whereas DDX3X is expressed ubiquitously and accounts for most, if not all, DDX3 function in somatic tissues (Kotov et al., 2017; Snijders Blok et al., 2015). In rodents, there is at least one additional homolog of DDX3 that is actively expressed. The tissue specificity of DDX3X and DDX3Y is also different in rodents and primates, suggesting that these genes may have evolved differently in the two mammalian orders (Chang and Liu, 2010; Matsumura et al., 2019). In contrast, Xenopus tropicalis has only one ddx3 homolog, which is localized on an autosome, and the encoded protein is 91% identical to human DDX3X. Similar to human DDX3X, the X. tropicalis ddx3 mRNA is expressed ubiquitously throughout early development (Cruciat et al., 2013). Thus, X. tropicalis may be a suitable model for studying DDX3 function in somatic tissue development.
Recently, de novo mutations in DDX3X were reported to be a common cause of intellectual disability in humans (https://ddx3x.org) (Dikow et al., 2017; Kellaris et al., 2018; Lennox et al., 2020; Nicola et al., 2019; Snijders Blok et al., 2015; Wang et al., 2018). These mutations were found primarily in females, and there are strong indications that some of them lead to embryonic lethality in males (Nicola et al., 2019; Snijders Blok et al., 2015). Most of these mutations were predicted to result in decreased DDX3X activity, and the defects in female patients may be caused by haploinsufficiency or dominant-negative effects of the mutants; partial X inactivation of the DDX3X gene may also contribute to the phenotypes (Garieri et al., 2018; Lennox et al., 2020; Snijders Blok et al., 2015). Individuals with DDX3X mutations display various defects in central nervous system (CNS) development, including intellectual disability, autism spectrum disorder, microcephaly and corpus callosum hypoplasia. Most patients also have craniofacial disorders, ranging from mild dysmorphic facial features to severe oral-facial clefts (Dikow et al., 2017; Kellaris et al., 2018; Lennox et al., 2020; Nicola et al., 2019; Snijders Blok et al., 2015; Wang et al., 2018). Other common symptoms include congenital heart diseases, pigment defects and hearing/visual impairment (Snijders Blok et al., 2015; Wang et al., 2018); a recent report also suggests that these patients have high rates of neuroblastoma: a rare NC-derived childhood tumor (Lennox et al., 2020). Because this spectrum of non-CNS symptoms is typically caused by impaired NC development (Dubey and Saint-Jeannet, 2017; Vega-Lopez et al., 2018), we hypothesized that DDX3X mutations can lead to not only CNS defects but also neurocristopathies.
In this study, we have investigated the roles of DDX3 in NC development using X. tropicalis as a model. Knockdown (KD) of DDX3 in X. tropicalis embryos led to inhibited NC induction and hypoplasia in craniofacial cartilage. Wild-type human DDX3X, but not an RNA helicase-dead mutant, rescued the NC induction defects caused by DDX3 KD. In both X. tropicalis embryos and human cells, DDX3 KD reduced endogenous AKT activity and Wnt signaling. The latter could be attributed to decrease in AKT-mediated GSK3β phosphorylation and inhibition, which resulted in enhanced degradation of β-catenin. In addition, Snai1, another GSK3β substrate, was also reduced post-transcriptionally. The defects in NC induction caused by DDX3 KD could be rescued by a constitutively active form of AKT, or by co-expression of β-catenin and Snai1. Finally, DDX3 KD led to decreased levels of RAC1, a known downstream target of DDX3 and upstream activator of AKT, and exogenous RAC1 also rescued the craniofacial phenotypes caused by DDX3 KD in X. tropicalis embryos. Collectively, our data suggest a crucial role for DDX3 in regulating the AKT-GSK3β signaling axis through RAC1, and provide a mechanistic explanation for the similar neurocristopathies that have been observed in individuals with mutations in DDX3X, RAC1 or AKT-related genes.
RESULTS
DDX3 is required for normal NC induction and craniofacial morphogenesis in X. tropicalis embryos
We first performed in situ hybridization to determine the expression of ddx3 during early Xenopus development. As reported previously (Cruciat et al., 2013), ddx3 is expressed ubiquitously in the animal half of the embryos at early gastrula stages (Fig. S1A,A′). The expression was primarily found in the dorsal ectoderm at the end of gastrulation (Fig. S1B,B′), and in the neural plate with relatively high levels at the NPB during neurulation (Fig. S1C,D). At tailbud stages, ddx3 mRNA was detected in the head and somites (Fig. S1E). Thus, the expression of ddx3 is consistent with its potential function in CNS and NC development. To understand the roles of DDX3 in NC development and craniofacial morphogenesis, we used a new transgenic X. tropicalis line expressing enhanced green fluorescent protein (eGFP) driven by the snai2 promoter/enhancer. The strong eGFP expression in the differentiating cranial NC lineage allows direct observation of craniofacial cartilage development in live snai2:eGFP embryos (Li et al., 2019). Injection of a well-characterized translation-blocking DDX3 morpholino (MO), which targets the 5′-untranslated region (5′-UTR) of the X. tropicalis ddx3 mRNA (Cruciat et al., 2013), led to hypoplasia of craniofacial cartilage structures on the injected side, when compared with the uninjected side (Fig. 1A). The same phenotype was obtained with a second MO that targets a different site (MO DDX3-2), confirming that this phenotype is specific for DDX3 KD (Fig. 1A). These results suggest that, similar to human DDX3X, Xenopus DDX3 is also important for craniofacial morphogenesis.
Fig. 1.
DDX3 is necessary for normal NC induction and craniofacial cartilage development. (A) One anterodorsal (D1) blastomere of eight-cell stage snai2:eGFP embryos was injected with the indicated MO (1.5 ng each). Embryos were cultured to stage ∼46 and imaged for eGFP expression. (B,C) Wild-type X. tropicalis embryos were injected in one blastomere at the two-cell stage with the indicated MO (6 ng each) and mRNA encoding wild-type human DDX3X or encoding the AAA mutant (200 pg each), cultured to stage ∼12.5, and processed for in situ hybridization for the indicated markers. Representative embryos are shown in ventral (A) or dorsal (B,C) view with anterior at the top; injected side is indicated with a red asterisk. CT, control; WT, wild type; n, number of embryos scored. ***P<0.001; NS, not significant (χ2 tests). Scale bars: 500 μm.
To assess the effects of DDX3 KD on early NC development, we examined the expression of NPB and NC specifiers at the end of gastrulation (stage ∼12.5), when the induction of cranial NC is nearly complete. KD of DDX3 reduced the expression of snai2 and sox9, two NC specifiers, indicating that NC induction was inhibited (Fig. 1B,C). Interestingly, different NPB specifiers responded differently to DDX3 KD: although both pax3 and msx1 were downregulated, zic1 expression remained largely unchanged and was slightly expanded in some embryos (Fig. 1B). The expansion of zic1 was probably secondary to the reduction in PAX3, as KD of PAX3 causes a similar effect (Sato et al., 2005). Consistent with these results, injection of a plasmid encoding X. tropicalis DDX3 expanded the expression of snai2 and msx1 (Fig. S2). Because loss of either Pax3 or Msx1 can block subsequent NC specification (Monsoro-Burq et al., 2005), we conclude that DDX3 depletion inhibits NC induction by interfering with proper NPB formation, although we cannot rule out the possibility that DDX3 also regulates NC specification directly. Co-injection of wild-type human DDX3X mRNA restored the expression of all markers to normal levels (Fig. 1B,C), further confirming the specificity of the MO and conservation of DDX3 function in NC induction between frogs and humans. Nearly all missense DDX3X mutations associated with human birth defects affect evolutionarily conserved residues within the two RNA helicase domains, and some of these mutations have been shown to disrupt the RNA helicase activity of DDX3X (Lennox et al., 2020). We therefore tested whether DDX3 RNA helicase activity is needed for NC induction. As shown in Fig. 1C, the S382A/T384A (‘AAA’) mutant of human DDX3X, which cannot unwind RNA but retains the ATPase activity (Chao et al., 2006), did not rescue sox9 expression in DDX3 morphants, suggesting that the RNA helicase activity of DDX3 is important for its function in NC induction.
DDX3 can regulate β-catenin levels through an RNA helicase-dependent mechanism
DDX3 is known to be a key regulator of Wnt/β-catenin signaling (Cruciat et al., 2013), and the differential responses of NPB specifiers to DDX3 KD (Fig. 1B) are consistent with previous reports showing that Wnt signaling induces the expression of pax3 and msx1 but not zic1 (de Crozé et al., 2011; Hong and Saint-Jeannet, 2007; Li et al., 2009). To investigate directly the roles of DDX3 in Wnt signaling during NC induction, we took advantage of a transgenic Wnt reporter line (Tran et al., 2010). Using this reporter line, we showed recently that there is strong endogenous Wnt signaling at the NPB during NC induction (Li et al., 2018). As expected, this Wnt activity was inhibited by KD of DDX3 (Fig. 2A). The transcription factor GBX2 is a direct Wnt target that mediates β-catenin-induced pax3 and msx1 expression during Xenopus NC induction (Li et al., 2009). Similarly, when human ES and iPS cells are induced to differentiate into NC cells, gbx2 is expressed before the NPB specifiers such as pax3 and msx1 in a β-catenin-dependent manner (Leung et al., 2016). In embryos injected with the DDX3 MO (‘morphants’), there was a reduction of gbx2 at stage ∼12 that can be rescued by the mRNA encoding wild-type human DDX3X (Fig. 2B). Conversely, ectopic DDX3 expanded the expression of gbx2 (Fig. S2), further supporting an important role for DDX3 in Wnt signaling during NC induction.
Fig. 2.
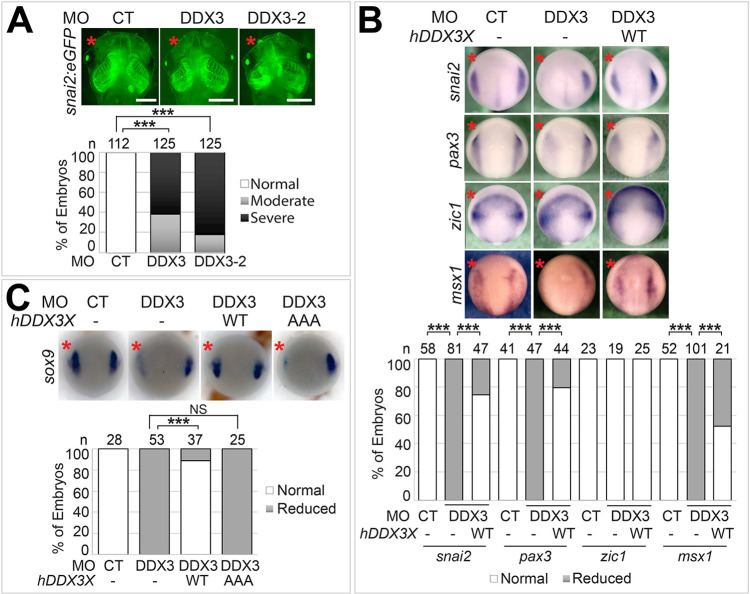
DDX3 stabilizes β-catenin and activates Wnt signaling. Wnt reporter (A) or wild-type (B) embryos were injected into one blastomere at the two-cell stage with the indicated MO (6 ng each) and mRNA (200 pg), cultured to stage ∼12.5 (A) or ∼12 (B), and imaged for eGFP expression (A) or processed for in situ hybridization for gbx2 (B). Representative embryos are shown in dorsal view with anterior at the top. n, number of embryos scored. **P<0.01; ***P<0.001; NS, not significant (χ2 tests). (C-G) HEK293T cells were treated with DMSO or 10 µM RK-33 for 24 h (C,F), or transfected with the indicated siRNA (200 nM, D,E,G). In F and G, a plasmid encoding wild-type β-catenin or the stabilized mutant (Stab; 1 µg per well for 6-well plates), as indicated, was also transfected for 48 h. Cell lysates of three biological replicates were processed for TOP/FOPFLASH assays (C,D,F,G), or western blot analyses using the indicated antibodies (E). aβ-cat, active (unphosphorylated) β-catenin. Injected side is indicated with a red asterisk. *P<0.05; **P<0.01; ***P<0.001; NS, not significant (unpaired t-tests). (C,D,F,G) Data are mean±s.e.m. Scale bars: 250 μm.
Activation of the Wnt signaling pathway starts with binding of the soluble Wnt ligands to cell-surface Frizzled receptors and LRP5/6 co-receptors, which leads to inhibition of glycogen synthase kinase 3 β (GSK3β)-mediated β-catenin phosphorylation and degradation. Consequently, β-catenin accumulates and enters the nucleus, where it interacts with LEF/TCF family transcription factors to control target gene expression (Loh et al., 2016; Nusse and Clevers, 2017). DDX3 has been shown to regulate Wnt signaling through an RNA helicase-independent mechanism (Cruciat et al., 2013). However, when compared with wild-type human DDX3X, the AAA mutant had decreased activity in rescuing gbx2 expression in DDX3 morphants (Fig. 2B). In the transgenic Wnt reporter embryos, this mutant also did not significantly restore the reduced Wnt activity caused by DDX3 KD (Fig. S3), suggesting that DDX3 RNA helicase activity is involved in Wnt signaling. This is consistent with previous studies showing that RK-33, a selective DDX3 RNA helicase inhibitor, inhibits Wnt signaling in multiple types of cancer cells (Heerma van Voss et al., 2017, 2015; Tantravedi et al., 2018). Because cancer cells usually harbor many mutations, which could interfere with the action of DDX3, we tested the effects of RK-33 in HEK293T cells, which do not contain any major mutations despite being somewhat tumorigenic (Stepanenko and Dmitrenko, 2015). As in cancer cells, RK-33 inhibits endogenous Wnt signaling in HEK293T cells; a similar effect was observed with a DDX3X siRNA (Fig. 2C,D). Together, these results point to an important role of DDX3 RNA helicase activity in Wnt signaling and NC induction.
We next examined if DDX3 can regulate Wnt signaling via a mechanism that is different from what was described previously. Interestingly, in both X. tropicalis DDX3 morphants and HEK293T cells treated with DDX3X siRNA, we detected decreased levels of total and active (unphosphorylated) β-catenin (Figs 2E, 3B,C), which could not be explained by the helicase-independent mechanism that affects β-catenin nuclear import only (Cruciat et al., 2013). To test whether DDX3 function in Wnt signaling is dependent on β-catenin degradation, we compared the effects of DDX3X siRNA on Wnt signal activities induced by wild-type β-catenin and a stabilized mutant that cannot be phosphorylated by GSK3β. As shown in Fig. 2F,G, KD of DDX3X or treatment with RK-33 effectively reduced the Wnt activity induced by wild-type β-catenin but not the stabilized mutant, suggesting that DDX3 can activate Wnt signaling by stabilizing β-catenin.
Fig. 3.
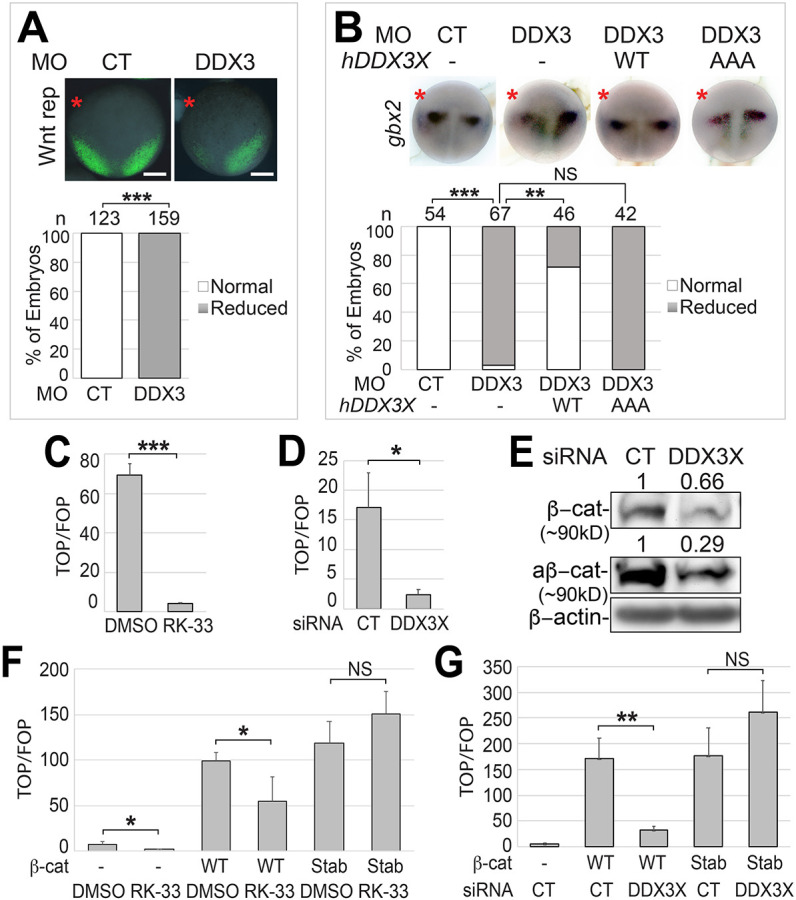
KD of DDX3 reduces AKT activity, AKT-mediated GSK3β phosphorylation and ectopically expressed Snai1. (A,D,E,G) HEK293T cells were transfected with the indicated siRNA (200 nM) for 48 h (A,D,G), or treated with DMSO or 10 µM RK-33 for 24 h (E). In G, a plasmid encoding HA-tagged Snai1 (1 µg per well for six-well plates) was also transfected. (B,F) Embryos were injected at the one-cell stage with the indicated MO (12 ng each; 50 pg mRNA encoding HA-tagged Snai1 was co-injected in F), and cultured to stage ∼12.5. Cell or embryo lysates were processed for western blot analyses using the indicated antibodies. (C) Summary of the results of three independent experiments shown in B. Density of the target protein was normalized against that of β-actin, and fold change (DDX3 MO versus control MO) is shown. *P<0.05; **P<0.01 (unpaired t-tests). Data are mean±s.e.m.
Depletion of DDX3 leads to reduced AKT activity and AKT-dependent GSK3β phosphorylation
The stability of β-catenin is mainly controlled by GSK3β-mediated phosphorylation, and GSK3β activity can be regulated by upstream Wnt ligand-receptor interaction or other signal inputs. Among the latter, the serine/threonine kinase AKT can inhibit GSK3β activity via direct phosphorylation of the Ser9 residue (Cross et al., 1995; Fukumoto et al., 2001; Naito et al., 2005; Sharma et al., 2002). DDX3 has been shown to promote AKT activity in colon cancer cells, and mutations that cause gain of AKT activity in humans and mice lead to overgrowth phenotypes in the CNS and NC, which are opposite to those caused by loss of DDX3 function (Fig. 1) (Akgumus et al., 2017; Butler et al., 2005; Rivière et al., 2012; Wu et al., 2016). In addition, AKT and its upstream activator phosphatidylinositol 3-kinase (PI3K) regulate NC development in Xenopus and zebrafish, although the exact roles of PI3K and AKT in NC induction remain controversial (Bahm et al., 2017; Ciarlo et al., 2017; Figueiredo et al., 2017; Geary and LaBonne, 2018; Pegoraro et al., 2015). We therefore asked whether DDX3 KD interferes with the AKT-GSK3β signaling axis. AKT is activated through two consecutive phosphorylation events, the first occurring at Thr308 and the second at Ser473 (Manning and Toker, 2017). In HEK293T cells, siRNA-mediated KD of DDX3X inhibited both phosphorylation events without affecting total AKT levels (Fig. 3A). Similarly, MO-mediated DDX3 KD also blocked Ser473 phosphorylation in X. tropicalis (Fig. 3B,C); our pThr308 antibody did not pick up the endogenous pThr308 signal in frog embryos. Consistent with the reduced AKT activity, the phosphorylation of GSK3β at Ser9, but not the total GSK3β levels, was also reduced upon DDX3X KD or treatment with RK-33 in HEK293T cells (Fig. 3D,E). Because phosphorylation at Ser9 inhibits GSK3β activity, these results provide an explanation for the decrease in β-catenin level when DDX3 activity was reduced (Figs 2E, 3B,C). Besides β-catenin, the transcription factor Snai1 is also phosphorylated by GSK3β and subsequently targeted to degradation (Yook et al., 2005; Zhou et al., 2004). To test whether DDX3, which promotes the inhibitory phosphorylation of GSK3β, can stabilize Snai1, we expressed exogenous HA-tagged Snai1 in both X. tropicalis embryos and HEK293T cells. KD of DDX3 resulted in a clear reduction of the exogenous Snai1 protein in both cases (Fig. 3F,G), indicating that DDX3 upregulates Snai1 post-transcriptionally. Together, our data suggest that DDX3 is required for AKT activation and downstream GSK3β inhibition, leading to stabilization of β-catenin and Snai1 in Xenopus embryos and human cells.
AKT is a key regulator of Wnt/β-catenin signaling and NC induction
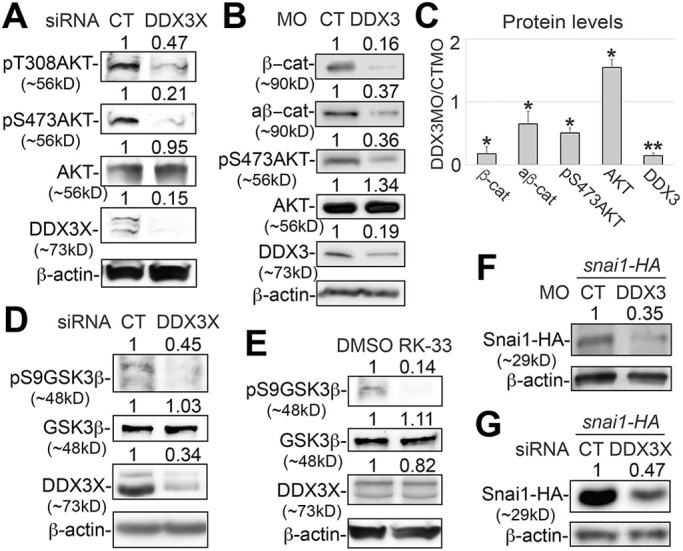
The function of PI3K-AKT signaling in NC induction is controversial. Although treatment of gastrula-stage X. laevis embryos with a PI3K inhibitor reduces the expression of NC markers, a constitutively active form of PI3K can also reduce NC markers induced by Pax3 and Zic1 in isolated X. laevis animal caps, which are thought to contain pluripotent stem cells (Buitrago-Delgado et al., 2015; Geary and LaBonne, 2018; Pegoraro et al., 2015). However, PI3K has AKT-independent function (Faes and Dormond, 2015), and the mechanisms underlying the possible roles of AKT in NC induction remain largely unknown. To address these issues, we first determined the patterns of NPB and NC markers in embryos treated with AKT inhibitor IV (AKTi), which blocks AKT activity downstream of PI3K (Kau et al., 2003; Wang et al., 2006). Because inhibition of AKT activity at earlier stages caused gastrulation defects, we cultured the embryos in AKTi from stage ∼10 to ∼12.5. This transient inhibition of AKT activity reduced the expression of NC specifiers snai2 and sox9, as well as the NPB specifier msx1, but not the other NPB specifier zic1 (Fig. 4A). These effects are similar to those caused by DDX3 KD (Fig. 1B,C). As discussed above, msx1 but not zic1 is induced by Wnt signaling, and AKT can stabilize β-catenin by directly inhibiting GSK3β activity. We therefore hypothesized that AKT is required for activating the Wnt pathway: one of the most important signaling pathways in NC induction. To test this hypothesis directly, we treated the Wnt reporter embryos with AKTi, and indeed observed a reduction in the endogenous Wnt activity at the NPB (Fig. 4B). A similar reduction was obtained with the injection of an mRNA encoding a dominant-negative AKT mutant (dnAKT; Fig. 4C), confirming that AKT is a key regulator of Wnt signaling during NC induction.
Fig. 4.
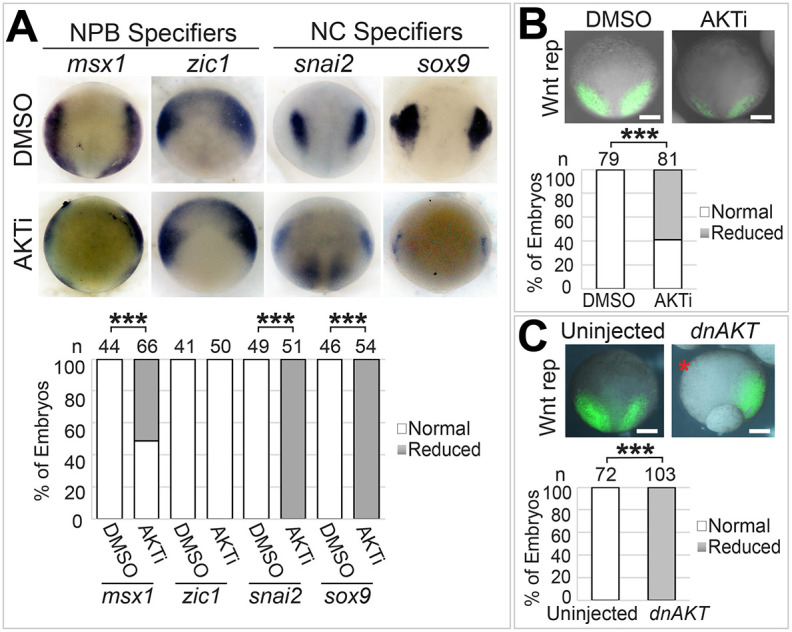
AKT activity is required for Wnt signaling and NC induction. (A,B) Wild-type (A) or Wnt reporter (B) embryos were treated with 20 µM AKTi or DMSO from stage ∼10 to ∼12.5, and processed for in situ hybridization for the indicated markers (A) or imaged for eGFP expression (B). (C) Wnt reporter embryos were injected in one blastomere at the two-cell stage with dnAKT mRNA (50 pg), cultured to stage ∼12.5 and imaged for eGFP expression. Representative embryos are shown in dorsal view with anterior at the top. Injected side is indicated with a red asterisk. n, number of embryos scored. ***P<0.001 (χ2 tests). Scale bars: 250 μm.
DDX3 induces the NC by regulating AKT and the GSK3β substrates β-catenin and Snai1
To establish the relationship between DDX3 and its downstream targets in NC induction, we carried out a series of rescue experiments. AKT is recruited to the plasma membrane, where it is activated, through the interaction of its pleckstrin homology (PH) domain and the phosphorylated lipid products of PI3K (Manning and Toker, 2017). Replacement of the PH domain by a myristoylation signal results in a constitutively active form of AKT (caAKT) that functions independently of PI3K (Kohn et al., 1996). We found that overexpression of caAKT induced Wnt activity in HEK293T cells, and this activity was not affected by DDX3X KD (Fig. S4). This is in line with our hypothesis that AKT functions downstream of DDX3 to activate Wnt signaling. We tested further whether caAKT can rescue the phenotypes caused by DDX3 KD in X. tropicalis embryos. Indeed, co-injection of the caAKT mRNA restored snai2 expression and Wnt signaling at the NPB in DDX3 morphants (Fig. 5A,B), confirming the roles of AKT in mediating DDX3 function in Wnt signaling and NC induction. Although AKT is known to protect cells from apoptosis and promote survival (Manning and Cantley, 2007), a previous study shows that KD of PFKFB4, which is required for AKT activation, does not cause apoptosis in Xenopus embryos until stage ∼14 (Pegoraro et al., 2015). Similarly, we were unable to detect any apparent increase in apoptosis in stage ∼12.5 DDX3 morphants using terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assays (Fig. S5). These data, together with the lack of reduction in zic1 level at the NPB (Fig. 1B), indicate that the loss of NC markers upon DDX3 KD is not caused by death of NC cells or their precursors but is a bona fide inhibition of NC induction. We have shown that KD of DDX3 reduced the inhibitory phosphorylation of GSK3β at Ser9, and have identified β-catenin and Snai1 as two GSK3β substrates whose levels are regulated by DDX3 (Figs 2E and 3B-G). Because both of these GSK3β substrates are essential for NC induction in Xenopus (Aybar et al., 2003; LaBonne and Bronner-Fraser, 1998), we tested whether they are downstream effectors of DDX3 in NC induction. Ectopic expression of either β-catenin or Snai1 could partially rescue the NPB specifier msx1 and the NC specifier sox9, and a combination of both GSK3β substrates resulted in a nearly complete rescue (Fig. 5C). Based on these results, we conclude that DDX3 functions in NC induction by regulating AKT as well as β-catenin and Snai1.
Fig. 5.
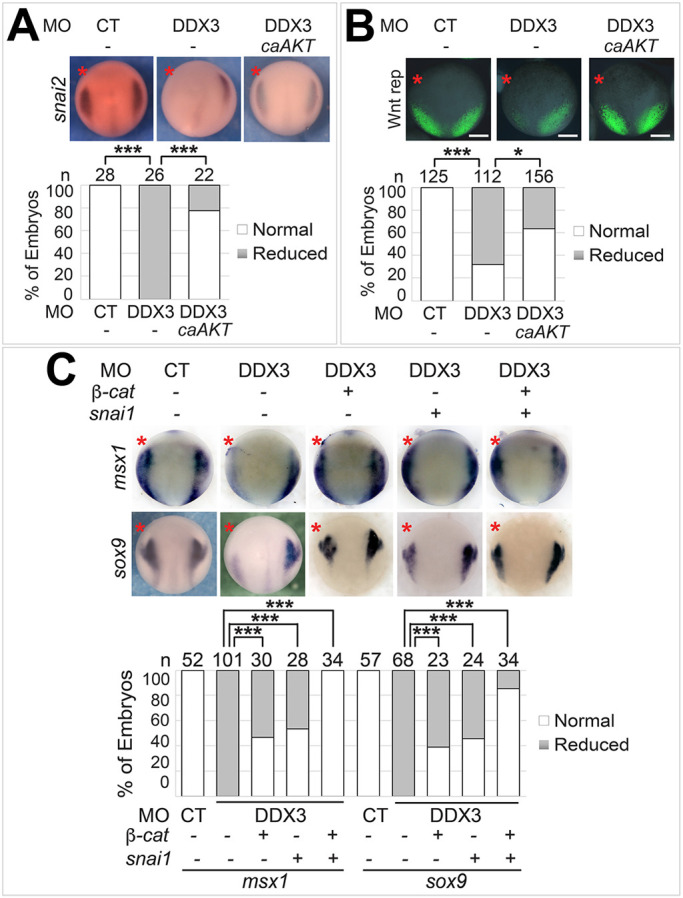
DDX3 induces the NC through AKT, β-catenin and Snai1. Wild-type (A,C) or Wnt reporter (B) embryos were injected in one blastomere at the two-cell stage with the indicated MO (6 ng each), mRNA (50 pg for caAKT and 100 pg for Snai1) and plasmid (10 pg for β-catenin), cultured to stage ∼12.5, and processed for in situ hybridization for the indicated markers (A,C) or imaged for eGFP expression (B). Representative embryos are shown in dorsal view with anterior at the top. Injected side is indicated with a red asterisk. n, number of embryos scored. *P<0.05; ***P<0.001 (χ2 tests). Scale bars: 250 μm.
RAC1 is likely an immediate downstream effector of DDX3 in NC induction
We next asked how DDX3 activates AKT and the downstream signaling events. DDX3 can promote translational initiation of mRNAs with long and structured 5′-UTR through its RNA helicase activity, a function that is conserved from yeast to humans (Guenther et al., 2018; Sen et al., 2015). One of these mRNAs encodes RAC1, which can mediate DDX3 function in other physiological processes in mammals (Chen et al., 2015, 2016; Ku et al., 2018). Notably, RAC1 can activate AKT through its downstream effector p21-activated kinase (PAK) (Higuchi et al., 2008). In Xenopus embryos, a dominant-negative mutant of RAC1 reduces, whereas a constitutively active mutant expands, the expression of NC specifiers (Broders-Bondon et al., 2007). We therefore examined whether RAC1 functions downstream of DDX3 in Xenopus. To do this, we obtained an X. tropicalis rac1 cDNA with a 257-nucleotide 5′-UTR. Although the 5′-UTR of X. tropicalis rac1 did not show any significant sequence similarity to that of human RAC1, the prediction using the Mfold algorithm suggests that they contain similar secondary structures that are energetically stable (Fig. S6). Thus, the mechanism of translational regulation of RAC1 may be conserved. As expected, treatment of HEK293T cells with RK-33 or DDX3X siRNA reduced the levels of endogenous RAC1 (Fig. 6A,B). Although a similar ∼90% reduction in RAC1 protein was detected in X. tropicalis embryos with DDX3 KD (Fig. 6C,D), there was only a minimal decrease of rac1 mRNA in the same batches of injected embryos, as shown by quantitative reverse transcription polymerase chain reaction (RT-qPCR) using two separate pairs of primers (Fig. 6E), indicating that DDX3 also regulates RAC1 primarily through post-transcriptional mechanisms in X. tropicalis. Finally, we generated an X. tropicalis rac1 mRNA without the 5′-UTR, which did not cause any apparent defects when injected alone into an anterodorsal blastomere of eight-cell stage snai2:eGFP embryos (Fig. S7). Co-injection of this mRNA with DDX3 MO rescued the craniofacial defects (Fig. 6F), suggesting that RAC1 mediates DDX3 function in NC development.
Fig. 6.
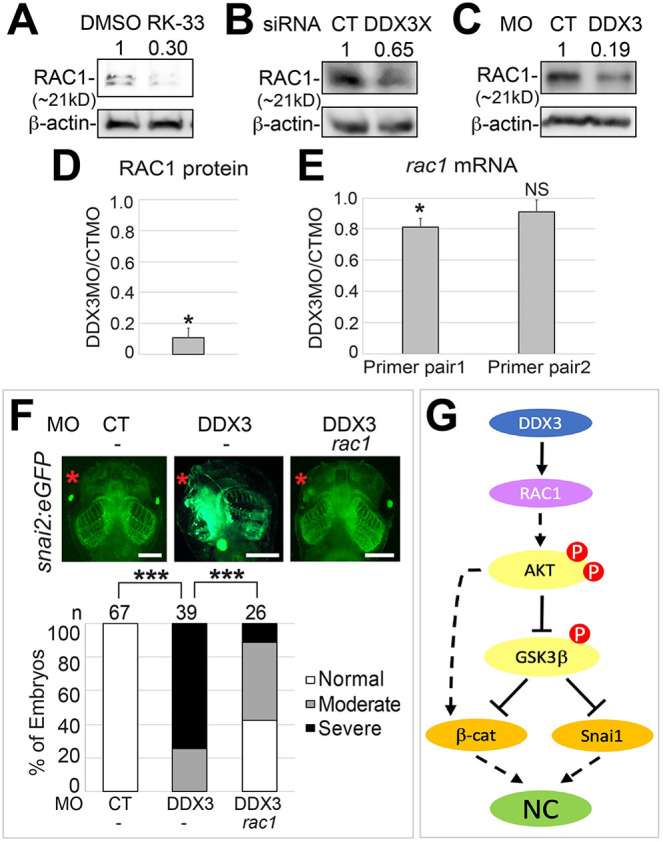
RAC1 is a downstream effector of DDX3 in NC induction. (A,B) HEK293T cells were treated with RK-33 (A) or transfected with the indicated siRNA (B) as in Fig. 3. Cell lysates were processed for western blotting for RAC1. (C) Embryo lysates shown in Fig. 3B were reblotted for RAC1. Results of three independent experiments are summarized in D. Density of RAC1 was normalized against that of β-actin, and fold change (DDX3 MO versus control MO) is shown. *P<0.05 (unpaired t-test). (E) The same batches of injected embryos in C and D (three biological replicates) were processed for RT-qPCR using two pairs of primers for rac1 mRNA. Results were normalized against gapdh mRNA (internal control). *P<0.05; NS, not significant (unpaired t-tests). (D,E) Data are mean±s.e.m. (F) One anterodorsal (D1) blastomere of eight-cell stage snai2:eGFP embryos was injected with the indicated MO (1.5 ng each) and mRNA (100 pg). Embryos were cultured to stage ∼46 and imaged for eGFP expression. Representative embryos are shown in ventral view with anterior at the top. n, number of embryos scored. ***P<0.001 (χ2 tests). (G) A model for DDX3 function in regulating downstream signaling during NC induction. Scale bars: 500 μm.
DISCUSSION
First discovered in 2015, mutations in DDX3 have drawn a great deal of research interest as a frequent cause of intellectual disability in humans (Dikow et al., 2017; Kellaris et al., 2018; Lennox et al., 2020; Nicola et al., 2019; Snijders Blok et al., 2015; Wang et al., 2018). Although nearly all published studies on individuals with DDX3 mutations are focused on the CNS defects, we noted that the majority of these individuals also have abnormalities in craniofacial structures and/or other NC-derived tissues, which have not been addressed previously. We therefore hypothesized that DDX3 plays important roles in NC development, and provide here the first evidence supporting this hypothesis. Based on our data, we propose that the RNA helicase activity of DDX3 is required for efficient translation of RAC1, which activates AKT to phosphorylate and inhibit GSK3β. The inhibition of GSK3β leads to stabilization of β-catenin and Snai1: two proteins essential for NC induction (Fig. 6G).
Although DDX3 has been shown to activate Wnt signaling through an RNA helicase-independent mechanism (Cruciat et al., 2013), several lines of evidence suggest the existence of an additional helicase-dependent mechanism. Missense DDX3X mutations associated with human birth defects cause decrease in RNA helicase activity, and assays with zebrafish embryos indicate that these mutations impair the ability of DDX3X to activate Wnt signaling (Lennox et al., 2020; Snijders Blok et al., 2015). Furthermore, RK-33, a selective inhibitor of DDX3 RNA helicase activity, reduces Wnt signaling in several cancer cell lines (Bol et al., 2015; Tantravedi et al., 2018). Similarly, RK-33 inhibits Wnt signaling in HEK293T cells in our reporter assays, and the helicase-dead AAA mutant did not efficiently rescue the reduced expression of the NC marker sox9 or the Wnt target gene gbx2 in DDX3 morphants (Figs 1C, 2B,C). In addition, we uncovered a helicase-dependent effect of DDX3 KD on β-catenin levels, which is consistent with the downregulation of AKT activity. Thus, our data suggest that DDX3 can also regulate Wnt signaling through its RNA helicase activity.
The RNA helicase activity of DDX3 is needed for the efficient translation of some mRNAs with highly structured 5′-UTR (Guenther et al., 2018; Sen et al., 2015). A known direct target of DDX3 in mammals is RAC1 (Chen et al., 2015, 2016; Ku et al., 2018), which is extremely conserved throughout vertebrate evolution (100% identical between human and X. tropicalis orthologs). Interestingly, mutations in RAC1 cause craniofacial disorders and other potential NC and CNS defects in humans, which are highly similar to those defects caused by DDX3X mutations (Reijnders et al., 2017). In line with these observations, rac1 is expressed in the NC and CNS in Xenopus embryos, and has been implicated in Xenopus NC induction (Broders-Bondon et al., 2007; Lucas et al., 2002). KD of DDX3 leads to drastically reduced RAC1 protein but not mRNA levels in X. tropicalis embryos, and exogenous RAC1 rescues the craniofacial defects caused by DDX3 KD (Fig. 6C-F), suggesting that RAC1 mediates DDX3 function in NC induction. RAC1 can activate AKT through its immediate downstream effector PAK (Higuchi et al., 2008). Among the vertebrate PAK family members, four (PAK1-4) contain a CDC42/RAC interaction/binding (CRIB) motif within the N-terminal regulatory domain, which binds and inhibits the C-terminal kinase domain when PAK is inactive. The direct interaction of the CRIB motif with activated RAC1 leads to dissociation of the regulatory domain from the kinase domain and activation of PAK (Zhao and Manser, 2012). The freed kinase domain of PAK1 can serve as a scaffold to bind AKT to facilitate its membrane localization and activation through a kinase-independent mechanism (Higuchi et al., 2008), but a separate study suggests that PAK1 activates AKT by phosphorylating AKT directly at Ser473 (Mao et al., 2008). Hence, it would be of interest to test whether PAK also functions downstream of DDX3 in NC induction and, if so, whether this function is dependent on PAK kinase activity.
Previous studies suggested that the PI3K-AKT pathway can either promote or inhibit NC induction (Ciarlo et al., 2017; Figueiredo et al., 2017; Geary and LaBonne, 2018; Pegoraro et al., 2015). Here, we show that direct inhibition of AKT activity reduces endogenous Wnt signaling at the NPB, causing altered expression of NPB and NC markers that resembles loss of DDX3 or Wnt signaling (Fig. 4A-C). These data are more in line with the model that the PI3K-AKT pathway is required for NC induction (Ciarlo et al., 2017; Figueiredo et al., 2017; Pegoraro et al., 2015), and indicate that one mechanism through which AKT functions in NC induction is by regulating Wnt signaling, a major signaling pathway that is crucial for NC induction. It should be noted that the results shown by Geary and LaBonne were mainly obtained from gain-of-function assays in dissected animal caps (Geary and LaBonne, 2018), and are difficult to compare with the in vivo studies in Xenopus and zebrafish published by the other groups (Ciarlo et al., 2017; Figueiredo et al., 2017; Pegoraro et al., 2015). However, our results do not contradict the model that excessive PI3K/AKT activities inhibit NC induction. In fact, we found that high levels of ectopic DDX3 can cause reduction in NPB and NC markers (data not shown), suggesting that stringently controlled AKT activity is required for proper NC induction.
AKT-mediated phosphorylation of GSK3β at Ser9 inhibits GSK3β activity, leading to the stabilization of certain GSK3β substrates (Cross et al., 1995; Zhou et al., 2004). Although some studies show that AKT promotes Wnt signaling, likely by inhibiting GSK3β and stabilizing β-catenin (Fukumoto et al., 2001; Naito et al., 2005; Sharma et al., 2002), others suggest that AKT and Wnt act on two separate pools of GSK3β and do not crosstalk with each other (Ding et al., 2000; Ng et al., 2009). Besides inhibiting GSK3β, AKT has also been shown to activate Wnt signaling through other mechanisms. For example, PI3K/AKT signaling can upregulate the transcriptionally active β-catenin (containing unphosphorylated Ser37 and Thr41) in HEK293T and other cell lines, possibly by inducing the phosphatase PP2A to dephosphorylate these two residues (Persad et al., 2016). Thus, AKT may mediate DDX3-induced Wnt signaling through GSK3β-dependent and -independent mechanisms.
Snai1, another substrate of GSK3β, is generally believed to be stabilized upon AKT-mediated phosphorylation of GSK3β (Zhou et al., 2004). Here, we show that KD of DDX3 results in decreased levels of ectopically expressed Snai1 (Fig. 3F,G), and that ectopic Snai1 and β-catenin cooperatively rescue the NC induction defects caused by DDX3 KD in X. tropicalis embryos (Fig. 5C). These data suggest that DDX3-regulated AKT-GSK3β signaling axis induces NC through Snai1 in addition to β-catenin. Currently, most protocols for inducing the NC from human pluripotent stem cells include GSK3β inhibition, which is thought to activate Wnt signaling through β-catenin stabilization (Gomez et al., 2019; Leung et al., 2016; Menendez et al., 2011; Mica et al., 2013). In light of our results, it would be of interest to examine whether part of the NC induction abilities of GSK3β inhibitors can be attributed to the stabilization of Snai1.
Consistent with our model that DDX3, RAC1, AKT and β-catenin function in the same signaling cascade to regulate NC induction (Fig. 6G), mutations in RAC1, CTNNB1 (encoding β-catenin) and genes that directly control AKT activity (including AKT1, AKT3, PIK3R2, PIK3CA and PTEN) can all lead to craniofacial disorders and other potential neurocristopathies, such as pigment defects, in humans (Akgumus et al., 2017; Butler et al., 2005; Kharbanda et al., 2017; Reijnders et al., 2017; Rivière et al., 2012; Tucci et al., 2014). Our current efforts are therefore focused on investigating the effects of human mutations in DDX3X and these target genes on downstream signaling and NC induction. In addition, it is worth noting that individuals carrying mutations in these genes also display highly similar CNS defects, including intellectual disability, autism spectrum disorder, micro/macrocephaly and corpus callosum hypo/hyperplasia (Akgumus et al., 2017; Butler et al., 2005; Kharbanda et al., 2017; Reijnders et al., 2017; Rivière et al., 2012; Snijders Blok et al., 2015; Tucci et al., 2014; Wang et al., 2018). Hence, it is tempting to test whether the signaling cascade that we propose here (Fig. 6G) is also essential for neurogenesis and CNS development. Our study may lay the foundation for future work to understand the pathophysiology of the birth defects caused by mutations that interfere with this signaling cascade.
MATERIALS AND METHODS
Plasmids and reagents
Constructs encoding dnAKT (Addgene #9031) (Ramaswamy et al., 1999), caAKT (Addgene #10841) (Kohn et al., 1996), FLAG-tagged human β-catenin (Addgene #16828) (Kolligs et al., 1999) and HA-tagged human Snai1 (Addgene #31697) (Kajita et al., 2004) were purchased from Addgene. Full-length cDNA clones for human DDX3X (Accession: BC011819), human RAC1 (BC050687) and X. tropicalis gbx2 (Accession: NM_001011472) were from GE-Dharmacon. The cDNAs for human DDX3X and RAC1 were subcloned into a pCS2+ expression vector with an in-frame HA tag, and the S382A/T384A (‘AAA’) mutants of DDX3 were generated by PCR using the mutagenesis primers CCACACTATGATGTTTGCTGCTGCTTTTCCTAAGGAAATAC (forward) and GTATTTCCTTAGGAAAAGCAGCAGCAAACATCATAGTGTGG (reverse). Constructs for the expression of X. laevis β-catenin and Snai1 and for in situ hybridization for snail2, sox9, foxd3, zic1 and msx1 were obtained in previous studies (Li et al., 2018; Wei et al., 2012, 2010). In vitro transcription was carried out as described to generate in situ hybridization probes and mRNA transcripts (Sive et al., 2000). Pharmacological inhibition of AKT and DDX3X was performed with AKT Inhibitor IV (Calbiochem 124011) and RK-33 (Selleckchem S8246), respectively. Antibodies that were used in this study include mouse anti-myc (DSHB 9E10, 1:500), mouse anti-HA (Sigma-Aldrich H9658, 1:1000), rabbit anti-DDX3 (Abcam ab151965, 1:1000), mouse anti-RAC1 (Abcam ab33186, 1:1000), rabbit anti-β-catenin (Sigma C2206, 1:2000), rabbit anti-active-β-catenin (Cell Signaling Technology 8814, 1:2000), mouse anti-GSK3β (Santa Cruz sc-53931, 1:1000), rabbit anti-piS9-GSK3β (Invitrogen PA1-4688, 1:1000), rabbit anti-AKT1 (Aviva Systems Biology AVARP06008_P050, 1:1000), rabbit anti-pS473-AKT (Cell Signaling Technology 4060S, 1:1000), rabbit anti-Xenopus-pS473-AKT (Cell Signaling Technology 9271S, 1:1000) and rabbit anti-pT308-AKT (Cell Signaling Technology 9275, 1:1000). Secondary antibodies that were used include HRP-conjugated rabbit anti-mouse (Sigma-Aldrich A9044, 1:7500) and goat anti-rabbit (Sigma-Aldrich A0545, 1:10,000). Mouse anti-β-actin (Sigma-Aldrich A5316, 1:15,000) was used as a loading control.
Animals and embryo manipulation
Wild-type X. tropicalis frogs were purchased from NASCO. The transgenic X. tropicalis Wnt reporter frogs were provided courtesy of Dr Kris Vleminckx (Flanders Institute for Biotechnology, Belgium), and the transgenic X. tropicalis snai2:eGFP line was generated as described previously (Li et al., 2019). Methods involving live animals were carried out in accordance with the guidelines and regulations approved and enforced by the Institutional Animal Care and Use Committees at West Virginia University and University of Delaware. Embryo were collected and injected with PLI-100A microinjectors (Harvard Apparatus) as described previously (Li et al., 2018). Morpholinos DDX3 (5′-CCGTGATTGATGCTT-3′) and DDX3-2 (5′-TTTCCACGGCCACATGACTCATAAC-3′) were designed and generated by Gene Tools. Alexa Fluor 555 (Invitrogen; for direct phenotype observation) or 488 (Invitrogen; for in situ hybridization) was co-injected as a lineage tracer. Injected embryos were sorted by the co-injected lineage tracer and cultured in 0.1× MBS to desired stages, when the embryos were imaged directly or processed for in situ hybridization, western blotting or RT-qPCR. Bright-field and fluorescence imaging was carried out using a Zeiss Axiozoom v16 epifluorescence microscope, and images of embryos were taken with an AxioCam MRc Rev3 camera. Western blotting and RT-qPCR were carried out as described below.
Cell culture and transfection
HEK293T cells (ATCC), recently authenticated and tested for contamination, were cultured in DMEM (ATCC) supplemented with 10% fetal bovine serum (Gibco) at 37°C with 5% CO2. Cells were transfected with DDX3 or control siRNA (Dharmacon J-006874-06-0002 and D-001810-01-05) at 50% confluency or with plasmids at 70% confluency, using Lipofectamine 3000 (Invitrogen). For experiments that used both siRNA and plasmid, siRNA was transfected first, the media were changed and the plasmid was transfected on the following day. For TOP/FOPFLASH assays, cell were transfected and luciferase assays were carried out as described previously (Wei et al., 2010).
In situ hybridization and TUNEL assays
Embryos collected at desired stages were fixed in 4% paraformaldehyde for 24 h at 4°C, and in situ hybridization was conducted subsequently as described previously (Sive et al., 2000). TUNEL assays were carried out for fixed embryos using terminal transferase (New England BioLabs) and digoxigenin-11-dUTP (Roche) as described previously (Shi et al., 2009). lacZ mRNA was co-injected as a lineage tracer, and β-galactosidase activity was detected using 1 mg/ml Red-Gal (Millipore) in PBS buffer containing 5 mM K3Fe(CN)6, 5 mM K4Fe(CN)6, and 2 mM MgCl2.
Western blotting and RT-qPCR analyses
For western blotting, embryos or cells were lysed in ice-cold RIPA lysis buffer (Fisher Scientific) as described previously (Li et al., 2018). Approximately 15 µg protein from HEK293T cell lysates or 50 embryos equivalent was loaded in each lane of 12% SDS-polyacrylamide gels. Blots were detected with HRP-conjugated antibodies and chemiluminescence substrates (GE Healthcare) using a Bio-Rad ChemiDoc or LI-COR Odyssey imager. For RT-qPCR experiments, total RNA was isolated from 10 embryos using the RNeasy Mini Kit (Qiagen), and RNA quality was assessed by electrophoresis. Total RNA was reverse transcribed into cDNA using the iScript cDNA Synthesis Kit (Bio-Rad Laboratories) with DNase I (Qiagen) treatment. Quantitative PCR was performed on the Quant Studio 6 Flex (Applied Biosystems) using the qMAX Green Low Rox qPCR Mix (Accuris) under the following conditions: a 2 m initial denaturation at 95°C, followed by 40 cycles of 5 s at 95°C and 30 s at 60°C. The following primer sets were used for the quantitative PCR: rac1 first pair, CTTGGAATGCTCTGCCCTTA (forward) and TCTCTTTCGCTTCTTGACTGG (reverse); rac1 second pair, ACAGGAGGACTACGACAGATTA (forward) and GGTACCACTTAGCACGAACAT (reverse); gapdh, GTGACACTCATTCCTCCATCTT (forward) and GCTGTAGCCACACTCGTTAT (reverse). All samples were assayed in triplicate and data were processed using the Comparative CT Method (Schmittgen and Livak, 2008).
Phenotype scoring and statistics
Embryos were scored by comparing the injected side with the uninjected side of the same embryos. The percentage of normal and reduced phenotypes were calculated for injected embryos obtained from multiple independent experiments, and χ2-squared tests were performed to compare the percentage of embryos with normal phenotypes in different treatment groups. For craniofacial phenotypes, injected snai2-eGFP embryos were allowed to develop to stage ∼46 and scored for defects in head cartilage structures. Images of head cartilages (eGFP) were taken with a Zeiss Axiozoom.v16 epifluorescence microscope.
Supplementary Material
Acknowledgements
We thank Dr Kris Vleminckx for providing the transgenic Wnt reporter frogs, and Drs William Sellers, Richard Roth, Eric Fearon and Paul Wade for providing plasmids through Addgene.
Footnotes
Competing interests
The authors declare no competing or financial interests.
Author contributions
Conceptualization: M.P., Y.Y.Y., S.W.; Methodology: M.P., X.X., C.L., Y.S., J.L., Y.Y.Y., S.W.; Validation: M.P., X.X., C.L., Y.S.; Formal analysis: M.P., X.X., C.L., Y.S., N.Y., J.L.; Investigation: M.P., X.X., C.L., Y.S., N.Y., J.L.; Resources: Y.Y.Y.; Data curation: M.P., X.X., N.Y., J.L.; Writing - original draft: M.P., X.X., C.L., Y.S., S.W.; Writing - review & editing: M.P., Y.Y.Y., S.W.; Visualization: M.P., X.X., C.L., Y.S., N.Y., J.L., S.W.; Supervision: Y.Y.Y., S.W.; Project administration: S.W.; Funding acquisition: Y.Y.Y., S.W.
Funding
This work was supported by the National Institutes of Health (R01GM114105 and R01DE029802 to S.W.; P20GM104316 to S.W. and Y.Y.Y.; R35GM133560 and P01HL032262 to Y.Y.Y.). Deposited in PMC for release after 12 months.
Supplementary information
Supplementary information available online at https://dev.biologists.org/lookup/doi/10.1242/dev.184341.supplemental
Peer review history
The peer review history is available online at https://dev.biologists.org/lookup/doi/10.1242/dev.184341.reviewer-comments.pdf
References
- Akgumus G., Chang F. and Li M. M. (2017). Overgrowth syndromes caused by somatic variants in the phosphatidylinositol 3-kinase/AKT/mammalian target of rapamycin pathway. J. Mol. Diagn. 19, 487-497. 10.1016/j.jmoldx.2017.04.001 [DOI] [PubMed] [Google Scholar]
- Aybar M. J., Nieto M. A. and Mayor R. (2003). Snail precedes slug in the genetic cascade required for the specification and migration of the Xenopus neural crest. Development 130, 483-494. 10.1242/dev.00238 [DOI] [PubMed] [Google Scholar]
- Bahm I., Barriga E. H., Frolov A., Theveneau E., Frankel P. and Mayor R. (2017). PDGF controls contact inhibition of locomotion by regulating N-cadherin during neural crest migration. Development 144, 2456-2468. 10.1242/dev.147926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barriga E. H., Trainor P. A., Bronner M. and Mayor R. (2015). Animal models for studying neural crest development: is the mouse different? Development 142, 1555-1560. 10.1242/dev.121590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betters E., Charney R. M. and Garcia-Castro M. I. (2018). Early specification and development of rabbit neural crest cells. Dev. Biol. 444, S181-S192. 10.1016/j.ydbio.2018.06.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bol G. M., Vesuna F., Xie M., Zeng J., Aziz K., Gandhi N., Levine A., Irving A., Korz D., Tantravedi S. et al. (2015). Targeting DDX3 with a small molecule inhibitor for lung cancer therapy. EMBO Mol. Med. 7, 648-669. 10.15252/emmm.201404368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broders-Bondon F., Chesneau A., Romero-Oliva F., Mazabraud A., Mayor R. and Thiery J. P. (2007). Regulation of XSnail2 expression by Rho GTPases. Dev. Dyn. 236, 2555-2566. 10.1002/dvdy.21273 [DOI] [PubMed] [Google Scholar]
- Bronner M. E. and Simões-Costa M. (2016). The neural crest migrating into the twenty-first century. Curr. Top. Dev. Biol. 116, 115-134. 10.1016/bs.ctdb.2015.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buitrago-Delgado E., Nordin K., Rao A., Geary L. and LaBonne C. (2015). NEURODEVELOPMENT. Shared regulatory programs suggest retention of blastula-stage potential in neural crest cells. Science 348, 1332-1335. 10.1126/science.aaa3655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler M. G., Dasouki M. J., Zhou X. P., Talebizadeh Z., Brown M., Takahashi T. N., Miles J. H., Wang C. H., Stratton R., Pilarski R. et al. (2005). Subset of individuals with autism spectrum disorders and extreme macrocephaly associated with germline PTEN tumour suppressor gene mutations. J. Med. Genet. 42, 318-321. 10.1136/jmg.2004.024646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang T.-C. and Liu W.-S. (2010). The molecular evolution of PL10 homologs. BMC Evol. Biol. 10, 127 10.1186/1471-2148-10-127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao C.-H., Chen C.-M., Cheng P.-L., Shih J.-W., Tsou A.-P. and Lee Y.-H. W. (2006). DDX3, a DEAD box RNA helicase with tumor growth-suppressive property and transcriptional regulation activity of the p21waf1/cip1 promoter, is a candidate tumor suppressor. Cancer Res. 66, 6579-6588. 10.1158/0008-5472.CAN-05-2415 [DOI] [PubMed] [Google Scholar]
- Chen H.-H., Yu H.-I., Cho W.-C. and Tarn W.-Y. (2015). DDX3 modulates cell adhesion and motility and cancer cell metastasis via Rac1-mediated signaling pathway. Oncogene 34, 2790-2800. 10.1038/onc.2014.190 [DOI] [PubMed] [Google Scholar]
- Chen H.-H., Yu H.-I. and Tarn W.-Y. (2016). DDX3 modulates neurite development via translationally activating an RNA regulon involved in Rac1 activation. J. Neurosci. 36, 9792-9804. 10.1523/JNEUROSCI.4603-15.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciarlo C., Kaufman C. K., Kinikoglu B., Michael J., Yang S., D'Amato C., Blokzijl-Franke S., den Hertog J., Schlaeger T. M., Zhou Y. et al. (2017). A chemical screen in zebrafish embryonic cells establishes that Akt activation is required for neural crest development. eLife 6, e29145 10.7554/eLife.29145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross D. A. E., Alessi D. R., Cohen P., Andjelkovich M. and Hemmings B. A. (1995). Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase B. Nature 378, 785-789. 10.1038/378785a0 [DOI] [PubMed] [Google Scholar]
- Cruciat C.-M., Dolde C., de Groot R. E. A., Ohkawara B., Reinhard C., Korswagen H. C. and Niehrs C. (2013). RNA helicase DDX3 is a regulatory subunit of casein kinase 1 in Wnt-beta-catenin signaling. Science 339, 1436-1441. 10.1126/science.1231499 [DOI] [PubMed] [Google Scholar]
- de Crozé N., Maczkowiak F. and Monsoro-Burq A. H. (2011). Reiterative AP2a activity controls sequential steps in the neural crest gene regulatory network. Proc. Natl. Acad. Sci. USA 108, 155-160. 10.1073/pnas.1010740107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dikow N., Granzow M., Graul-Neumann L. M., Karch S., Hinderhofer K., Paramasivam N., Behl L.-J., Kaufmann L., Fischer C., Evers C. et al. (2017). DDX3X mutations in two girls with a phenotype overlapping Toriello-Carey syndrome. Am. J. Med. Genet. A 173, 1369-1373. 10.1002/ajmg.a.38164 [DOI] [PubMed] [Google Scholar]
- Ding V. W., Chen R.-H. and McCormick F. (2000). Differential regulation of glycogen synthase kinase 3β by insulin and Wnt signaling. J. Biol. Chem. 275, 32475-32481. 10.1074/jbc.M005342200 [DOI] [PubMed] [Google Scholar]
- Dubey A. and Saint-Jeannet J.-P. (2017). Modeling human craniofacial disorders in Xenopus. Curr. Pathobiol. Rep. 5, 79-92. 10.1007/s40139-017-0128-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faes S. and Dormond O. (2015). PI3K and AKT: unfaithful partners in cancer. Int. J. Mol. Sci. 16, 21138-21152. 10.3390/ijms160921138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figueiredo A. L., Maczkowiak F., Borday C., Pla P., Sittewelle M., Pegoraro C. and Monsoro-Burq A. H. (2017). PFKFB4 control of AKT signaling is essential for premigratory and migratory neural crest formation. Development 144, 4183-4194. 10.1242/dev.157644 [DOI] [PubMed] [Google Scholar]
- Fukumoto S., Hsieh C.-M., Maemura K., Layne M. D., Yet S.-F., Lee K.-H., Matsui T., Rosenzweig A., Taylor W. G., Rubin J. S. et al. (2001). Akt participation in the Wnt signaling pathway through Dishevelled. J. Biol. Chem. 276, 17479-17483. 10.1074/jbc.C000880200 [DOI] [PubMed] [Google Scholar]
- Garieri M., Stamoulis G., Blanc X., Falconnet E., Ribaux P., Borel C., Santoni F. and Antonarakis S. E. (2018). Extensive cellular heterogeneity of X inactivation revealed by single-cell allele-specific expression in human fibroblasts. Proc. Natl. Acad. Sci. USA 115, 13015-13020. 10.1073/pnas.1806811115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geary L. and LaBonne C. (2018). FGF mediated MAPK and PI3K/Akt Signals make distinct contributions to pluripotency and the establishment of Neural Crest. eLife 7, e33845 10.7554/eLife.33845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez G. A., Prasad M. S., Sandhu N., Shelar P. B., Leung A. W. and García-Castro M. I. (2019). Human neural crest induction by temporal modulation of WNT activation. Dev. Biol. 449, 99-106. 10.1016/j.ydbio.2019.02.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guenther U.-P., Weinberg D. E., Zubradt M. M., Tedeschi F. A., Stawicki B. N., Zagore L. L., Brar G. A., Licatalosi D. D., Bartel D. P., Weissman J. S. et al. (2018). The helicase Ded1p controls use of near-cognate translation initiation codons in 5′ UTRs. Nature 559, 130-134. 10.1038/s41586-018-0258-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Y., Zhang D., Yang Y., Wang X., Zhao X., Zhang P., Zhu H., Xu N. and Liang S. (2018). A double-edged function of DDX3, as an oncogene or tumor suppressor, in cancer progression (Review). Oncol. Rep. 39, 883-892. 10.3892/or.2018.6203 [DOI] [PubMed] [Google Scholar]
- Heerma van Voss M. R., Vesuna F., Trumpi K., Brilliant J., Berlinicke C., de Leng W., Kranenburg O., Offerhaus G. J., Bürger H., van der Wall E. et al. (2015). Identification of the DEAD box RNA helicase DDX3 as a therapeutic target in colorectal cancer. Oncotarget 6, 28312-28326. 10.18632/oncotarget.4873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heerma van Voss M. R., van Diest P. J. and Raman V. (2017). Targeting RNA helicases in cancer: the translation trap. Biochim. Biophys. Acta Rev. Cancer 1868, 510-520. 10.1016/j.bbcan.2017.09.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higuchi M., Onishi K., Kikuchi C. and Gotoh Y. (2008). Scaffolding function of PAK in the PDK1-Akt pathway. Nat. Cell Biol. 10, 1356-1364. 10.1038/ncb1795 [DOI] [PubMed] [Google Scholar]
- Hong C.-S. and Saint-Jeannet J.-P. (2007). The activity of Pax3 and Zic1 regulates three distinct cell fates at the neural plate border. Mol. Biol. Cell 18, 2192-2202. 10.1091/mbc.e06-11-1047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kajita M., McClinic K. N. and Wade P. A. (2004). Aberrant expression of the transcription factors snail and slug alters the response to genotoxic stress. Mol. Cell. Biol. 24, 7559-7566. 10.1128/MCB.24.17.7559-7566.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kau T. R., Schroeder F., Ramaswamy S., Wojciechowski C. L., Zhao J. J., Roberts T. M., Clardy J., Sellers W. R. and Silver P. A. (2003). A chemical genetic screen identifies inhibitors of regulated nuclear export of a Forkhead transcription factor in PTEN-deficient tumor cells. Cancer Cell 4, 463-476. 10.1016/S1535-6108(03)00303-9 [DOI] [PubMed] [Google Scholar]
- Kellaris G., Khan K., Baig S. M., Tsai I.-C., Zamora F. M., Ruggieri P., Natowicz M. R. and Katsanis N. (2018). A hypomorphic inherited pathogenic variant in DDX3X causes male intellectual disability with additional neurodevelopmental and neurodegenerative features. Hum. Genomics 12, 11 10.1186/s40246-018-0141-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kharbanda M., Pilz D. T., Tomkins S., Chandler K., Saggar A., Fryer A., McKay V., Louro P., Smith J. C., Burn J. et al. (2017). Clinical features associated with CTNNB1 de novo loss of function mutations in ten individuals. Eur. J. Med. Genet. 60, 130-135. 10.1016/j.ejmg.2016.11.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohn A. D., Takeuchi F. and Roth R. A. (1996). Akt, a pleckstrin homology domain containing kinase, is activated primarily by phosphorylation. J. Biol. Chem. 271, 21920-21926. 10.1074/jbc.271.36.21920 [DOI] [PubMed] [Google Scholar]
- Kolligs F. T., Hu G., Dang C. V. and Fearon E. R. (1999). Neoplastic transformation of RK3E by mutant β-catenin requires deregulation of Tcf/Lef transcription but not activation of c-myc expression. Mol. Cell. Biol. 19, 5696-5706. 10.1128/MCB.19.8.5696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotov A. A., Olenkina O. M., Godneeva B. K., Adashev V. E. and Olenina L. V. (2017). Progress in understanding the molecular functions of DDX3Y (DBY) in male germ cell development and maintenance. Biosci. Trends 11, 46-53. 10.5582/bst.2016.01216 [DOI] [PubMed] [Google Scholar]
- Ku Y.-C., Lai M.-H., Lo C.-C., Cheng Y.-C., Qiu J.-T., Tarn W.-Y. and Lai M.-C. (2018). DDX3 participates in translational control of inflammation induced by infections and injuries. Mol. Cell. Biol. 39, e00285-18 10.1128/MCB.00285-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaBonne C. and Bronner-Fraser M. (1998). Neural crest induction in Xenopus: evidence for a two-signal model. Development 125, 2403-2414. [DOI] [PubMed] [Google Scholar]
- Lennox A. L., Hoye M. L., Jiang R., Johnson-Kerner B. L., Suit L. A., Venkataramanan S., Sheehan C. J., Alsina F. C., Fregeau B., Aldinger K. A. et al. (2020). Pathogenic DDX3X mutations impair RNA metabolism and neurogenesis during fetal cortical development. Neuron 106, 404-420.e8. 10.1016/j.neuron.2020.01.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung A. W., Murdoch B., Salem A. F., Prasad M. S., Gomez G. A. and García-Castro M. I. (2016). WNT/β-catenin signaling mediates human neural crest induction via a pre-neural border intermediate. Development 143, 398-410. 10.1242/dev.130849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B., Kuriyama S., Moreno M. and Mayor R. (2009). The posteriorizing gene Gbx2 is a direct target of Wnt signalling and the earliest factor in neural crest induction. Development 136, 3267-3278. 10.1242/dev.036954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J., Perfetto M., Neuner R., Bahudhanapati H., Christian L., Mathavan K., Bridges L. C., Alfandari D. and Wei S. (2018). Xenopus ADAM19 regulates Wnt signaling and neural crest specification by stabilizing ADAM13. Development 145, dev158154 10.1242/dev.158154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J., Perfetto M., Materna C., Li R., Thi Tran H., Vleminckx K., Duncan M. K. and Wei S. (2019). A new transgenic reporter line reveals Wnt-dependent Snai2 re-expression and cranial neural crest differentiation in Xenopus. Sci. Rep. 9, 11191 10.1038/s41598-019-47665-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loh K. M., van Amerongen R. and Nusse R. (2016). Generating cellular diversity and spatial form: Wnt signaling and the evolution of multicellular animals. Dev. Cell 38, 643-655. 10.1016/j.devcel.2016.08.011 [DOI] [PubMed] [Google Scholar]
- Lucas J. M., Nikolic I. and Hens M. D. (2002). cDNA cloning, sequence comparison, and developmental expression of Xenopus rac1. Mech. Dev. 115, 113-116. 10.1016/S0925-4773(02)00117-X [DOI] [PubMed] [Google Scholar]
- Manning B. D. and Cantley L. C. (2007). AKT/PKB signaling: navigating downstream. Cell 129, 1261-1274. 10.1016/j.cell.2007.06.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning B. D. and Toker A. (2017). AKT/PKB signaling: navigating the network. Cell 169, 381-405. 10.1016/j.cell.2017.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao K., Kobayashi S., Jaffer Z. M., Huang Y., Volden P., Chernoff J. and Liang Q. (2008). Regulation of Akt/PKB activity by P21-activated kinase in cardiomyocytes. J. Mol. Cell. Cardiol. 44, 429-434. 10.1016/j.yjmcc.2007.10.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumura T., Endo T., Isotani A., Ogawa M. and Ikawa M. (2019). An azoospermic factor gene, Ddx3y and its paralog, Ddx3x are dispensable in germ cells for male fertility. J. Reprod. Dev. 65, 121-128. 10.1262/jrd.2018-145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menendez L., Yatskievych T. A., Antin P. B. and Dalton S. (2011). Wnt signaling and a Smad pathway blockade direct the differentiation of human pluripotent stem cells to multipotent neural crest cells. Proc. Natl. Acad. Sci. USA 108, 19240-19245. 10.1073/pnas.1113746108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mica Y., Lee G., Chambers S. M., Tomishima M. J. and Studer L. (2013). Modeling neural crest induction, melanocyte specification, and disease-related pigmentation defects in hESCs and patient-specific iPSCs. Cell Rep. 3, 1140-1152. 10.1016/j.celrep.2013.03.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monsoro-Burq A.-H., Wang E. and Harland R. (2005). Msx1 and Pax3 cooperate to mediate FGF8 and WNT signals during Xenopus neural crest induction. Dev. Cell 8, 167-178. 10.1016/j.devcel.2004.12.017 [DOI] [PubMed] [Google Scholar]
- Naito A. T., Akazawa H., Takano H., Minamino T., Nagai T., Aburatani H. and Komuro I. (2005). Phosphatidylinositol 3-kinase-Akt pathway plays a critical role in early cardiomyogenesis by regulating canonical Wnt signaling. Circ. Res. 97, 144-151. 10.1161/01.RES.0000175241.92285.f8 [DOI] [PubMed] [Google Scholar]
- Ng S. S., Mahmoudi T., Danenberg E., Bejaoui I., de Lau W., Korswagen H. C., Schutte M. and Clevers H. (2009). Phosphatidylinositol 3-kinase signaling does not activate the wnt cascade. J. Biol. Chem. 284, 35308-35313. 10.1074/jbc.M109.078261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicola P., Blackburn P. R., Rasmussen K. J., Bertsch N. L., Klee E. W., Hasadsri L., Pichurin P. N., Rankin J., Raymond F. L., Clayton-Smith J. et al. (2019). De novo DDX3X missense variants in males appear viable and contribute to syndromic intellectual disability. Am. J. Med. Genet. A 179, 570-578. 10.1002/ajmg.a.61061 [DOI] [PubMed] [Google Scholar]
- Nusse R. and Clevers H. (2017). Wnt/β-catenin signaling, disease, and emerging therapeutic modalities. Cell 169, 985-999. 10.1016/j.cell.2017.05.016 [DOI] [PubMed] [Google Scholar]
- Pegoraro C., Figueiredo A. L., Maczkowiak F., Pouponnot C., Eychène A. and Monsoro-Burq A. H. (2015). PFKFB4 controls embryonic patterning via Akt signalling independently of glycolysis. Nat. Commun. 6, 5953 10.1038/ncomms6953 [DOI] [PubMed] [Google Scholar]
- Persad A., Venkateswaran G., Hao L., Garcia M. E., Yoon J., Sidhu J. and Persad S. (2016). Active β-catenin is regulated by the PTEN/PI3 kinase pathway: a role for protein phosphatase PP2A. Genes Cancer 7, 368-382. 10.18632/genesandcancer.128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad M. S., Charney R. M. and García-Castro M. I. (2019). Specification and formation of the neural crest: Perspectives on lineage segregation. Genesis 57, e23276 10.1002/dvg.23276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramaswamy S., Nakamura N., Vazquez F., Batt D. B., Perera S., Roberts T. M. and Sellers W. R. (1999). Regulation of G1 progression by the PTEN tumor suppressor protein is linked to inhibition of the phosphatidylinositol 3-kinase/Akt pathway. Proc. Natl. Acad. Sci. USA 96, 2110-2115. 10.1073/pnas.96.5.2110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reijnders M. R. F., Ansor N. M., Kousi M., Yue W. W., Tan P. L., Clarkson K., Clayton-Smith J., Corning K., Jones J. R., Lam W. W. K. et al. (2017). RAC1 missense mutations in developmental disorders with diverse phenotypes. Am. J. Hum. Genet. 101, 466-477. 10.1016/j.ajhg.2017.08.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivière J.-B., Mirzaa G. M., O'Roak B. J., Beddaoui M., Alcantara D., Conway R. L., St-Onge J., Schwartzentruber J. A., Gripp K. W., Nikkel S. M. et al. (2012). De novo germline and postzygotic mutations in AKT3, PIK3R2 and PIK3CA cause a spectrum of related megalencephaly syndromes. Nat. Genet. 44, 934-940. 10.1038/ng.2331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato T., Sasai N. and Sasai Y. (2005). Neural crest determination by co-activation of Pax3 and Zic1 genes in Xenopus ectoderm. Development 132, 2355-2363. 10.1242/dev.01823 [DOI] [PubMed] [Google Scholar]
- Schmittgen T. D. and Livak K. J. (2008). Analyzing real-time PCR data by the comparative C(T) method. Nat. Protoc. 3, 1101-1108. 10.1038/nprot.2008.73 [DOI] [PubMed] [Google Scholar]
- Sen N. D., Zhou F., Ingolia N. T. and Hinnebusch A. G. (2015). Genome-wide analysis of translational efficiency reveals distinct but overlapping functions of yeast DEAD-box RNA helicases Ded1 and eIF4A. Genome Res. 25, 1196-1205. 10.1101/gr.191601.115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma D. and Jankowsky E. (2014). The Ded1/DDX3 subfamily of DEAD-box RNA helicases. Crit. Rev. Biochem. Mol. Biol. 49, 343-360. 10.3109/10409238.2014.931339 [DOI] [PubMed] [Google Scholar]
- Sharma M., Chuang W. W. and Sun Z. (2002). Phosphatidylinositol 3-kinase/Akt stimulates androgen pathway through GSK3β inhibition and nuclear β-catenin accumulation. J. Biol. Chem. 277, 30935-30941. 10.1074/jbc.M201919200 [DOI] [PubMed] [Google Scholar]
- Shi Y., Zhao S., Li J. and Mao B. (2009). Islet-1 is required for ventral neuron survival in Xenopus. Biochem. Biophys. Res. Commun. 388, 506-510. 10.1016/j.bbrc.2009.08.017 [DOI] [PubMed] [Google Scholar]
- Simões-Costa M. and Bronner M. E. (2015). Establishing neural crest identity: a gene regulatory recipe. Development 142, 242-257. 10.1242/dev.105445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sive H. L., Grainger R. M. and Harland R. M. (2000). Early Development of Xenopus Laevis. A Laboratory Manual. Cold Spring Harbor Laboratory Press. [Google Scholar]
- Snijders Blok L., Madsen E., Juusola J., Gilissen C., Baralle D., Reijnders M. R. F., Venselaar H., Helsmoortel C., Cho M. T., Hoischen A. et al. (2015). Mutations in DDX3X are a common cause of unexplained intellectual disability with gender-specific effects on Wnt signaling. Am. J. Hum. Genet. 97, 343-352. 10.1016/j.ajhg.2015.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stepanenko A. A. and Dmitrenko V. V. (2015). HEK293 in cell biology and cancer research: phenotype, karyotype, tumorigenicity, and stress-induced genome-phenotype evolution. Gene 569, 182-190. 10.1016/j.gene.2015.05.065 [DOI] [PubMed] [Google Scholar]
- Stuhlmiller T. J. and García-Castro M. I. (2012). Current perspectives of the signaling pathways directing neural crest induction. Cell. Mol. Life Sci. 69, 3715-3737. 10.1007/s00018-012-0991-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tantravedi S., Vesuna F., Winnard P. T., Martin A., Lim M., Eberhart C. G., Berlinicke C., Raabe E., van Diest P. J. and Raman V. (2018). Targeting DDX3 in medulloblastoma using the small molecule inhibitor RK-33. Transl. Oncol. 12, 96-105. 10.1016/j.tranon.2018.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran H. T., Sekkali B., Van Imschoot G., Janssens S. and Vleminckx K. (2010). Wnt/β-catenin signaling is involved in the induction and maintenance of primitive hematopoiesis in the vertebrate embryo. Proc. Natl. Acad. Sci. USA 107, 16160-16165. 10.1073/pnas.1007725107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucci V., Kleefstra T., Hardy A., Heise I., Maggi S., Willemsen M. H., Hilton H., Esapa C., Simon M., Buenavista M.-T. et al. (2014). Dominant β-catenin mutations cause intellectual disability with recognizable syndromic features. J. Clin. Invest. 124, 1468-1482. 10.1172/JCI70372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vega-Lopez G. A., Cerrizuela S., Tribulo C. and Aybar M. J. (2018). Neurocristopathies: new insights 150 years after the neural crest discovery. Dev. Biol. 444, S110-S143. 10.1016/j.ydbio.2018.05.013 [DOI] [PubMed] [Google Scholar]
- Wang G., Barrett J. W., Stanford M., Werden S. J., Johnston J. B., Gao X., Sun M., Cheng J. Q. and McFadden G. (2006). Infection of human cancer cells with myxoma virus requires Akt activation via interaction with a viral ankyrin-repeat host range factor. Proc. Natl. Acad. Sci. USA 103, 4640-4645. 10.1073/pnas.0509341103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Posey J. E., Rosenfeld J. A., Bacino C. A., Scaglia F., Immken L. D., Harris J. M., Hickey S. E., Mosher T. M., Slavotinek A. et al. (2018). Phenotypic expansion in DDX3X - a common cause of intellectual disability in females. Ann. Clin. Transl. Neurol 5, 1277-1285. 10.1002/acn3.622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei S., Xu G., Bridges L. C., Williams P., White J. M. and DeSimone D. W. (2010). ADAM13 induces cranial neural crest by cleaving class B Ephrins and regulating Wnt signaling. Dev. Cell 19, 345-352. 10.1016/j.devcel.2010.07.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei S., Xu G., Bridges L. C., Williams P., Nakayama T., Shah A., Grainger R. M., White J. M. and Desimone D. W. (2012). Roles of ADAM13-regulated Wnt activity in early Xenopus eye development. Dev. Biol. 363, 147-154. 10.1016/j.ydbio.2011.12.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu D.-W., Lin P.-L., Cheng Y.-W., Huang C.-C., Wang L. and Lee H. (2016). DDX3 enhances oncogenic KRAS-induced tumor invasion in colorectal cancer via the β-catenin/ZEB1 axis. Oncotarget 7, 22687-22699. 10.18632/oncotarget.8143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yook J. I., Li X.-Y., Ota I., Fearon E. R. and Weiss S. J. (2005). Wnt-dependent regulation of the E-cadherin repressor snail. J. Biol. Chem. 280, 11740-11748. 10.1074/jbc.M413878200 [DOI] [PubMed] [Google Scholar]
- Zhao Z.-S. and Manser E. (2012). PAK family kinases: physiological roles and regulation. Cell Logist. 2, 59-68. 10.4161/cl.21912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou B. P., Deng J., Xia W., Xu J., Li Y. M., Gunduz M. and Hung M.-C. (2004). Dual regulation of Snail by GSK-3β-mediated phosphorylation in control of epithelial-mesenchymal transition. Nat. Cell Biol. 6, 931-940. 10.1038/ncb1173 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.