ABSTRACT
Autophagy is deregulated in many cancers and represents an attractive target for therapeutic intervention. However, the precise contributions of autophagy to metastatic progression, the principle cause of cancer-related mortality, is only now being uncovered. While autophagy promotes primary tumor growth, metabolic adaptation and resistance to therapy, recent studies have unexpectedly revealed that autophagy suppresses the proliferative outgrowth of disseminated tumor cells into overt and lethal macrometastases. These studies suggest autophagy plays unexpected and complex roles in the initiation and progression of metastases, which will undoubtedly impact therapeutic approaches for cancer treatment. Here, we discuss the intricacies of autophagy in metastatic progression, highlighting and integrating the pleiotropic roles of autophagy on diverse cell biological processes involved in metastasis.
KEY WORDS: Autophagy, Selective Autophagy, Cancer, Metastasis
Summary: A discussion of the diverse cell biological roles of autophagy that promote and suppress metastatic progression in cancer.
Introduction
Macroautophagy (hereafter termed autophagy) is a lysosomal degradation pathway that removes superfluous, damaged or toxic cytosolic proteins, organelles and pathogens. Autophagy requires over 30 highly conserved autophagy-related genes (ATGs) that coordinate the formation of double-membrane vesicles, termed autophagosomes, which encapsulate cytosolic materials and ultimately fuse with the lysosome for degradation (Bento et al., 2016). As a result, cargo degraded via autophagy is broken down into molecular building blocks that are released back to the cytosol and repurposed for cellular anabolism (Kaur and Debnath, 2015). Initially viewed as a bulk, non-selective degradative process in response to limiting nutrients, mounting evidence suggests exquisite specificity in autophagic cargo selection mediated by both the ubiquitin-targeting activities of the autophagy receptors and spatiotemporal control of autophagosome biogenesis (Zaffagnini and Martens, 2016).
Autophagy plays dual roles in cancer. On the one hand, the genetic loss of essential components of the autophagy machinery facilitates cancer initiation due to mitochondrial damage, elevated oxidative stress and genomic instability (Qu et al., 2003; Takamura et al., 2011; Yue et al., 2003). On the other hand, autophagic degradation in established tumors promotes their ability to metabolically adapt to limiting nutrients and hypoxic conditions, thereby supporting growth and aggressiveness (Guo et al., 2013; Rao et al., 2014; Strohecker et al., 2013; Wei et al., 2011; Yang et al., 2014). Moreover, an increasing body of evidence suggests that tumor cell autophagy can regulate immune recognition of established tumors (Box 1). Indeed, recent clinical and pre-clinical findings have rekindled interest in autophagy inhibition for cancer therapy (Amaravadi et al., 2019). For example, multiple studies recently demonstrated that combined inhibition of autophagy and the mitogen-activated protein kinase (MAPK) pathway, using both pharmacological or genetic approaches, elicits potent synergistic cytotoxicity in multiple mutant RAS-driven models of lung and pancreatic cancer (Bryant et al., 2019; Kinsey et al., 2019; Lee et al., 2019).
Box 1. Tumor cell autophagy and immune recognition.
Research over the past decade has elucidated the multifaceted role of autophagy in distinct immune cell types, as detailed in other reviews (Jiang et al., 2019; Levine et al., 2011). Early studies investigating autophagy suggested that, during tumor progression, autophagy-deficient tumors exhibit elevated necrosis and immune infiltrate (Degenhardt et al., 2006; Wei et al., 2011). In hypoxic tumor cells, loss of autophagy promotes susceptibility to cytotoxic T cell (CTL)-mediated lysis, and autophagy induction protects tumor cells from CTL recognition via regulation of signal transducer and activator of transcription 3 (STAT3) activation (Noman et al., 2011). Recently, a genome-wide CRISPR screen utilizing diverse tumor models revealed tumor cell autophagy as a top determinant of CTL evasion, and loss of autophagy conferred increased sensitivity to interferon-γ (IFNγ)- and tumor necrosis factor (TNF)-induced cell death (Lawson et al., 2020). Similarly, autophagy induction in hypoxic tumor cells impairs natural killer (NK) cell-mediated killing via autophagic degradation of granzyme B (Baginska et al., 2013) and connexin 43 (Tittarelli et al., 2015), the latter of which controls stability of the immunological synapse between tumor cells and NK cells. Further studies demonstrate that impairment of autophagy via genetic and pharmacological approaches can enhance effects of anti-PD-L1 and -PD1 (also known as CD274 and PDCD1, respectively) immunotherapies (Noman et al., 2020). However, this is not universally observed across a wide spectrum of tumor models (Starobinets et al., 2016). Moreover, autophagy can also function to promote anti-tumor immune responses. Autophagy enhances the secretion of high mobility group box 1 (HMGB1) from dying cells (Thorburn et al., 2009), which in turn, activates toll-like receptor 4 (TLR4) on dendritic cells to enhance adaptive antitumor responses (Apetoh et al., 2007). Additionally, autophagy supplies the lysosome with ATP, which is released via lysosomal exocytosis in dying tumor cells and potentiates the recruitment and activation of tumoral immune populations (Wang et al., 2013b). Thus, tumor cell autophagy can both facilitate immune evasion as well as initiate immune responses to dying cells. As autophagy inhibitors are further developed as cancer therapeutics, it will be crucial to understand how these compounds influence both tumor cells and immune populations.
Despite these advances in our understanding of autophagy in primary tumor progression and response to therapy, the impact of autophagy on metastasis, the primary cause of cancer-related mortality, is still being unraveled. Metastasis encompasses a multistep process in which tumor cells leave the primary tumor by intravasating into hematogenous circulation, travel as single cells or cell clusters, extravasate into foreign organs and regain proliferative potential to colonize secondary sites (Lambert et al., 2017). Tumor cells must navigate a wide array of stresses during metastasis, including migration through diverse microenvironments, anchorage-independent survival, adaptation to nutrient deprivation and hypoxia, and survival at foreign tissue sites (Nikolaou and Machesky, 2020; Senft and Ronai, 2016). While in vitro studies demonstrate that tumor cells utilize autophagy to adapt and survive in response to these stressors (Kroemer et al., 2010; Mathew et al., 2007), recent in vivo studies paint a more complex picture of autophagy during metastasis, demonstrating that autophagy can either promote or restrict metastasis depending on the specific metastatic stage (Fig. 1). In this Review, we discuss these nuanced roles of autophagy in metastasis, with a focus on breast and mammary cancer models, and how they influence current efforts to therapeutically target this pathway to combat cancer progression.
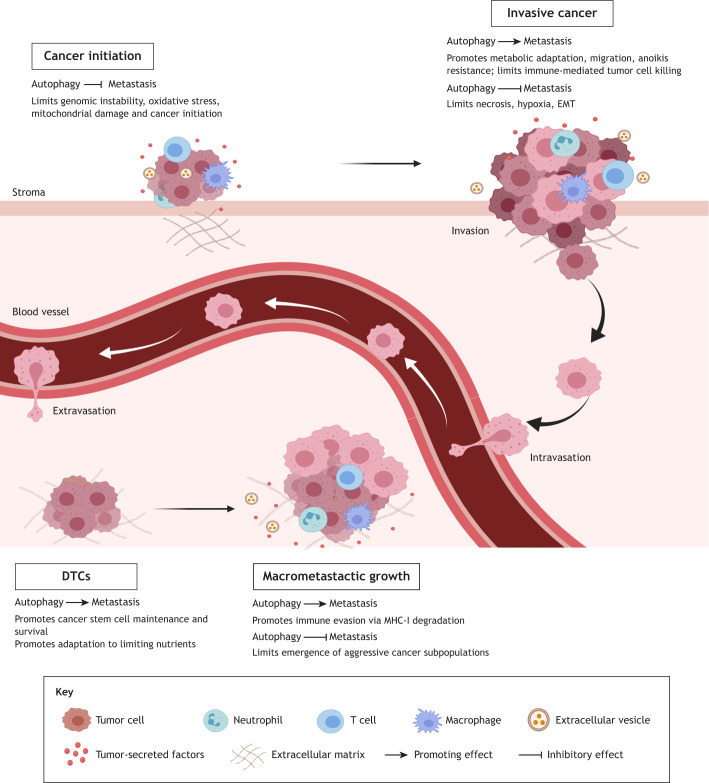
Fig. 1.
Stage-specific roles for autophagy during metastatic progression. Pre-clinical evidence demonstrates that autophagy can both suppress and promote tumor progression at distinct steps of the metastatic cascade. Autophagy hinders cancer initiation, but critically supports the growth and metabolic fitness of advanced primary tumors. In primary tumors, autophagy limits hypoxic and necrotic cell death, which might influence critical tumor–stroma interactions that initiate metastatic spread. Moreover, tumor cells utilize autophagy for migration, invasion and anoikis resistance. Upon initial seeding of distant metastatic sites, disseminated tumor cells (DTCs) utilize autophagy to facilitate dormant and quiescent cell survival, which may facilitate clinically undetectable metastatic disease resistant to therapeutic intervention. As DTCs erupt into a proliferative growth phase, autophagy can limit the emergence of aggressive subpopulations of tumor cells with high proliferative potential. Finally, autophagy selectively degrades MHC-I, which is crucial for immune recognition of tumor cells.
Effects of autophagy on metastatic cell biology
Research to date demonstrates that autophagy regulates numerous metastatic cellular properties (Fig. 2), thereby impacting several discrete steps during the metastatic cascade; these include (1) migration, invasion and epithelial-to-mesenchymal transition (EMT), (2) resistance to detachment-induced cell death, (3) metabolic fitness and adaptation, and (4) maintenance of dormancy and cancer stem cells (CSCs), as discussed below.
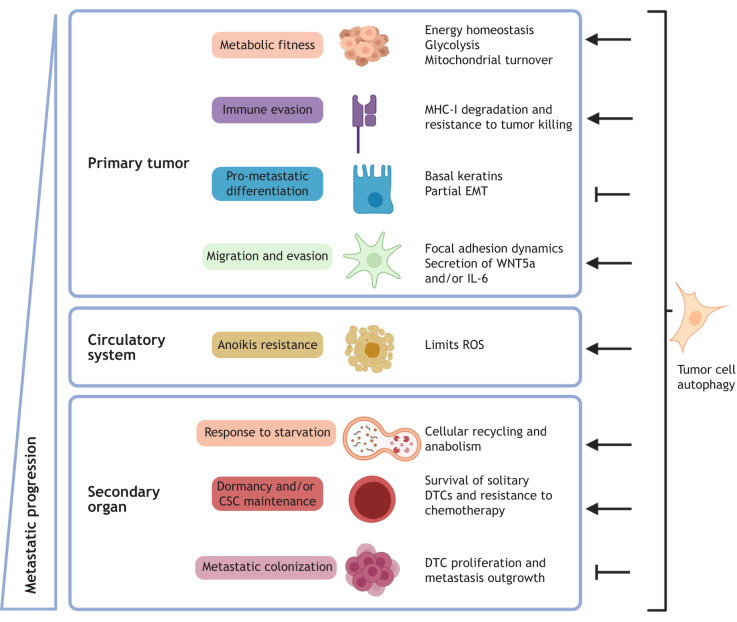
Fig. 2.
Autophagic regulation of tumor cell biological functions that influence metastatic progression. Autophagy supports multiple pro-metastatic cellular functions in primary tumors and during the early stages of metastasis, namely metabolic recycling and homeostasis, invasion and migration, and resistance to anoikis and chemotherapy, as well as cancer stem cell survival and fitness (arrows). In contrast, especially during the late stage of metastasis, such as colonization, autophagy has been found to be anti-metastatic by restricting the proliferation of disseminated tumor cells and inhibiting pro-metastatic differentiation programs (inhibitory arrows). How these paradoxical autophagy-regulated phenotypes coalesce during in vivo metastasis remains an area of intense investigation and clinical significance.
Migration, invasion and EMT
For metastasis to ensue, cancer cells require increased motility to invade and escape from the primary tumor site, as well as to extravasate into foreign organs (Hanahan and Weinberg, 2011; Langley and Fidler, 2011). Several studies demonstrate that autophagy promotes the migratory capacity of tumor cells (Kenific et al., 2016; Kim et al., 2016; Li et al., 2013; Sharifi et al., 2016), although this is not universally the case (Dower et al., 2017; Görgülü et al., 2019; Peng et al., 2013; Qiang et al., 2014). Impaired migration and invasion in autophagy-deficient tumor cells is at least partly due to perturbed disassembly of focal adhesions at the leading edge of migrating cells (Kenific et al., 2016; Sharifi et al., 2016). Moreover, autophagy promotes the coordinate secretion of the pro-invasive factors interleukin 6 (IL-6), matrix metalloproteinase-2 (MMP2) and Wnt family member 5a (WNT5A), suggesting that paracrine effects of autophagy are important for invasive capacity (Lock et al., 2014). Interestingly, the autophagy machinery also specifies cargo loading and biogenesis of secreted extracellular vesicles (EVs), a critical component in establishing a pro-metastatic environment in secondary organs (Costa-Silva et al., 2015; Leidal et al., 2020; Murrow et al., 2015; Peinado et al., 2012). Whether EVs containing autophagy-regulated cargo potentiate the migratory capacity or colonization efficiency of metastatic tumor cells remains an important area of future investigation.
EMT is a pro-metastatic process in which epithelial cells acquire mesenchymal features, including loss of polarity, spindle cell morphology and enhanced motility. Studies suggest that autophagy can either promote or restrict the ability of cancer cells to undergo EMT. For example, the loss of the essential autophagy proteins ATG3 and ATG7 impairs starvation-induced EMT in hepatocellular cancer cells (Li et al., 2013). In contrast, the knockdown of uncoordinated 51-like kinase 2 (ULK2) induces autophagy, which promotes both EMT and migration in lung cancer cells (Kim et al., 2016). Moreover, loss of essential ATGs can revert EMT phenotypes, a phenomenon called mesenchymal-to-epithelial transition (MET), in RAS-transformed mammary cancer cells (Lock et al., 2014). Nevertheless, other studies have found that autophagy deficiency promotes EMT and increased migration in squamous and gastric tumor cells (Qiang et al., 2014; Qin et al., 2015). Intriguingly, in several of these studies, autophagy was found to control the levels of critical EMT-inducing transcription factors. For example, genetic loss of ATG-encoding genes promotes p62 (also known as SQSTM1)-dependent stabilization of the EMT-promoting transcription factor twist family bHLH transcription factor 1 (TWIST1), which facilitates EMT phenotypes in vitro (Qiang et al., 2014). Similarly, in breast cancer cells, the activation of autophagy by death effector domain-containing DNA-binding protein (DEDD) prevents EMT by promoting selective autophagic degradation of TWIST, as well as snail family transcriptional repressor 1 (SNAI1, also known as Snail), another EMT-inducing transcription factor (Lv et al., 2012). Overall, these results illustrate the spectrum of interconnections between autophagy, EMT and tumor cell migration and invasion, all of which dictate the context-dependent effects of autophagy on early metastatic progression.
Anoikis resistance
Detachment of cells from their surrounding extracellular matrix (ECM) typically results in anoikis, a form of programmed cell death caused by loss of integrin-mediated survival cues (Frisch and Francis, 1994). Metastatic cancer cells encounter prolonged anoikis-inducing conditions during hematogenous transit and entry into secondary organs, prior to the restoration of robust ECM attachments (Paoli et al., 2013; Taddei et al., 2012). Both normal and tumor cells upregulate autophagy during matrix detachment, and impaired autophagy promotes anoikis and decreases the ability of residual cells to recover and grow upon reattachment (Chen et al., 2017; Fung et al., 2008; Peng et al., 2013). Moreover, autophagic regulation of anoikis can be observed during organotypic growth in 3D culture, in which mammary cells undergo spontaneous apoptosis upon loss of ECM contacts within the luminal space of growing acini (Chen et al., 2013). Interestingly, the pro-survival role of autophagy during ECM detachment is oncogene specific, as mammary cells transformed with prevalent human oncogenic mutations in phosphatidylinositol-4,5-biphosphate 3-kinase catalytic subunit α (PIK3CA), but not HRAS, are sensitive to detachment-induced autophagy in 3D morphogenesis assays (Chen et al., 2013; Lock et al., 2014). At the molecular level, ECM detachment triggers the activation of a PERK (EIF2AK3)–eIF2a–ATF4–CHOP (DDIT3) pathway that concomitantly upregulates expression of the critical autophagy proteins Beclin-1 and LC3B (MAP1LC3B), and suppresses reactive oxygen species (ROS) formation, allowing cells to resist anoikis for extended periods of time (Avivar-Valderas et al., 2011). Thus, PERK performs dual functions during ECM detachment, promoting robust survival through induction of autophagy genes and an unfolded protein response to temporarily arrest cell growth (Brewer and Diehl, 2000). Furthermore, PERK activation promotes autophagy by activating the AMP-activated protein kinase (AMPK) catalytic subunits α1 and α2 (also known as PRKAA1 and PRKAA2), which in turn, inhibits downstream mammalian target of rapamycin complex 1 (mTORC1) signaling, thereby releasing the mTORC1-mediated inhibition of the autophagy initiation machinery (Avivar-Valderas et al., 2013). Nevertheless, mTORC1-independent pathways also mediate detachment-induced autophagy, such as the activation of the IκB kinase (IKK) complex in response to the loss of α3β1 integrin function in mammary epithelial cells (Chen and Debnath, 2013). Although detachment-induced autophagy is now considered a principal mechanism of anoikis resistance, whether it promotes metastatic progression remains less certain. Notably, in vivo models of hepatocellular carcinoma demonstrate that autophagy promotes anoikis-resistance and ascites formation, highlighting the importance of this pathway in a physiological setting (Peng et al., 2013). Thus, autophagy may promote metastasis by imparting survival advantages to disseminating tumor cells as they navigate environments that are deprived of ECM–integrin contact en route to secondary organs.
Metabolic fitness and adaptation
During transit to and upon arrival at secondary organs, cancer cells must metabolically adapt to changing microenvironments. Stage-specific metabolic plasticity is a commonly observed feature of metastatic tumor cells (Lehuédé et al., 2016). For example, circulating breast cancer cells exhibit elevated mitochondrial biogenesis and oxidative phosphorylation (OXPHOS) compared to their isogenic counterparts from both the primary tumor and established pulmonary metastases (LeBleu et al., 2014). Specific tissue microenvironments direct metabolic adaptation during metastasis, with metastatic tumor cells relying more heavily on glycolytic metabolism when they reside in liver relative to bone and lung (Dupuy et al., 2015). Given the ability of autophagy to degrade mitochondria, termed mitophagy, a tempting hypothesis is that cancer cells employ mitophagy to control the balance between OXPHOS and glycolytic metabolism during dissemination and metastatic colonization. Accumulation of damaged mitochondria in autophagy-deficient cancer cells results in elevated ROS, which can potentiate cancer initiation and shift the balance from OXPHOS to glycolytic (Warburg) metabolism (Chourasia et al., 2015; Guo et al., 2011; Takamura et al., 2011). Moreover, mammary cancer cells with impaired mitophagy display enhanced metastatic capacity, underscoring the contribution of autophagy in regulating the metabolic state of metastatic tumor cells (Chourasia et al., 2015). Interestingly, autophagy-deficient cells with accumulated damaged mitochondria adapt to excessive ROS by inducing nuclear factor erythroid 2 like 2 (NRF2; also known as NFE2L2)-mediated antioxidant transcriptional programs that are mediated by the accumulation of the autophagy receptor p62 (Fan et al., 2010; Komatsu et al., 2010; Lau et al., 2010). Taken together, these studies support the notion that impaired autophagy might limit the ability of cancer cells to upregulate OXPHOS in the circulatory environment due to decreased mitochondrial integrity, but the resultant shift to glycolytic metabolism and the induction of NRF2-driven antioxidant programs in these cells might facilitate the subsequent outgrowth of disseminated tumor cells.
Upon arrival at the metastatic organ, cancer cells encounter harsh, nutrient-limited environments prior to the establishment of a tumor-supportive microenvironment. Accordingly, cancer cells can switch to nonconventional energy sources mediated by autophagic-lysosomal nutrient scavenging pathways (Lawrence and Zoncu, 2019). During starvation, AMPK directly associates with UNC-51-like autophagy activating kinase 1 (ULK1; also known as ATG1), an upstream component of the autophagy machinery, resulting in ULK1 phosphorylation and a signaling cascade leading to productive autophagic flux (Egan et al., 2011; Kim et al., 2011). In conjunction with recycling of cytosolic components by autophagy, cancer cells also utilize macropinocytosis to endocytose bulk protein from the extracellular milieu, degrading them in the lysosome to fuel tumor growth when conventional nutrients are limiting (Commisso et al., 2013). Interestingly, autophagy and macropinocytosis are interconnected as they are both regulated by upstream mTOR and AMPK signaling (Florey and Overholtzer, 2019), suggesting that the coordinated regulation of these two processes promotes cancer cell viability and growth prior to the creation of tumor-supportive environments in early metastases.
CSC survival and maintenance
Autophagy is essential for normal maintenance and differentiation of hematopoietic, adipose, bone, neural and muscle stem cells (Fiacco et al., 2016; Li et al., 2018; Singh et al., 2009; Tang and Rando, 2014; Wang et al., 2013a; Warr et al., 2013). Akin to their normal counterparts, CSCs or tumor-initiating cells comprise small subpopulations within a tumor and, owing to their capacity to self-renew and differentiate into multiple cell types, CSCs are proposed to initiate tumor progression at secondary metastatic sites (Malanchi et al., 2012). CSCs can remain quiescent, but viable, when they stop dividing, which at a population level, is termed dormancy. Overall, CSCs display high metastatic potential because of their differentiation properties, invasiveness and resistance to conventional therapies (Hen and Barkan, 2020; Li et al., 2007; Yeh and Ramaswamy, 2015).
Autophagy is strongly associated with CSC survival and maintenance and thus tumor aggressiveness (Nazio et al., 2019; Smith and Macleod, 2019). Autophagy has been shown to positively regulate the CSC-like phenotype in breast cancer by maintaining breast CSCs, identified by CD44+ CD24−/low surface expression, in vitro; RNAi-mediated depletion of LC3B or ATG12 reduced the number of epithelial cells and favored CD24+ cells in human breast ductal carcinoma in situ, demonstrating that autophagy is functionally required for the maintenance of breast CSCs (Cufí et al., 2011). In a genome-wide mammosphere-based screen for CSC function, ATG4 was identified as a regulator of this CD44+ CD24−/low cell population and their ability to form mammospheres in vitro (Wolf et al., 2013); interestingly, a second study found that ATG4 similarly controlled stem-like properties in gastric tumor cells via regulation of Notch signaling in an autophagy-independent fashion (Yang et al., 2016). Not surprisingly, CD44 has been correlated with increased metastatic invasiveness, and TGF-β, which promotes EMT and tumor cell motility, and also induces autophagy and stemness (Comen et al., 2011). Similarly, in MDA-MD-468, a triple-negative breast cancer cell line that depends on autophagy for survival, autophagy promoted IL-6 secretion, which was required for the CD44+ CD24−/low phenotype and mammosphere formation (Maycotte et al., 2015). Finally, in mouse mammary cancer models driven by the polyoma middle T oncogene (MMTV-PyMT), the autophagy regulator FIP200 (also known as RB1CC1) was genetically required for the tumor-initiating potential of two different CSC populations, ALDH-active luminal CSCs and mesenchymal CD29hi CD61+ CSCs (Yeo et al., 2016). Although we focus here on breast cancer, the importance of autophagy in CSC maintenance and function has been demonstrated in other cancer types, as detailed in other reviews (Nazio et al., 2019).
Stage-specific effects of autophagy on metastatic progression in vivo
Pre-clinical evidence to date suggests that autophagic control of metastasis in vivo is nuanced and context-dependent, with evidence that autophagy both supports and restricts metastasis at different steps in the metastatic cascade (Fig. 1). Again, we focus on human breast and mouse mammary cancer models as a paradigm; however, additional work has been conducted in other cancer types (Caino et al., 2013; Görgülü et al., 2019; Li et al., 2016, 2019; Maes et al., 2014; Peng et al., 2013; Qiang et al., 2014; Qin et al., 2015; Yang et al., 2016).
Early work in the well-characterized MMTV-PyMT-driven mammary tumor model on the FVB/N background demonstrated that genetic loss of Fip200, a critical regulator of autophagy induction, potently attenuated primary tumor growth and resulted in decreased spontaneous pulmonary metastases (Wei et al., 2011). Similar findings on reduced primary tumor growth and metastasis were found in our recent study utilizing orthotopic transplantation of MMTV-PyMT cells deficient for Atg12 or Atg5 in C57BL/6 mice (Marsh et al., 2020). In contrast, autophagy-deficient tumors displayed increased spontaneous metastasis compared to autophagy-competent counterparts following excision of size-matched primary tumors, highlighting the importance of disentangling the effects of autophagy on primary tumor versus metastasis phenotypes (Marsh et al., 2020). The potential discrepancies between these studies may be due to enhanced susceptibility to tumorigenesis in the FVB/N mouse genetic background relative to in C57BL/6 mice (Davie et al., 2007). Additional studies utilizing distinct models underscore the cell-type-dependent effects of autophagy on metastasis. Specifically, Atg5 or Atg7 knockdown in monoclonal 4T1 mammary cancer cells did not impair primary tumor growth but led to decreased numbers of spontaneous pulmonary and hepatic metastases. This effect was not seen in experimental metastasis assays, suggesting autophagy inhibition impaired tumor cell dissemination from the primary tumor (Sharifi et al., 2016). Similarly, knockdown of Beclin1 or administration of the anti-malarial lysosomal inhibitor chloroquine (CQ) in 4T1 cells reduced spontaneous metastasis but did not impair primary tumor growth (Barnard et al., 2016). However, CQ administration after primary tumor resection or in experimental metastasis assays with 4T1 or B16-F10 melanoma had no effect on metastatic burden, providing further support that autophagy primarily promotes early metastatic dissemination in these models (Barnard et al., 2016). A separate study corroborated that CQ did not impact B16-F10 metastasis, whereas Atg5 knockdown decreased experimental metastasis, revealing divergent effects of CQ and genetic autophagy inhibition in this melanoma line (Maes et al., 2014). In contrast, studies using MDA-MB-231 human breast cancer cells harboring a hypoxia-inducible dominant-negative mutation of ULK1 have demonstrated that hypoxia-dependent loss of autophagy did not impair primary tumor growth, but increased spontaneous pulmonary and hepatic metastases, a result similarly observed in experimental metastasis models, once again broaching that autophagy restricts the late stages of metastasis in these cells (Dower et al., 2017).
During metastasis, disseminated tumor cells often remain dormant and undetectable at metastatic sites for protracted periods of time, before erupting into proliferative growth cycles and forming macrometastatic lesions. This process of outgrowth of disseminated tumor cells into clinically lethal metastasis, termed metastatic colonization, is now viewed as a key rate-limiting step in the metastatic cascade (Lambert et al., 2017). Understanding the mechanisms by which these cells emerge from dormancy has profound implications for patients; in breast cancer, metastatic recurrence can occur decades after primary tumor removal (Sosa et al., 2014). Because autophagy is upregulated in dormant cancer cells and controls the maintenance and survival of CSCs in vitro, whether autophagy regulates the emergence from dormancy and metastatic colonization in in vivo physiological settings represents an issue of immense biological and clinical significance. In recent years, several studies have tried to address this question. The first utilized a dormant-to-proliferative switch model in which pulmonary fibrosis induces otherwise dormant D2.OR mammary cancer cells to become proliferative (Vera-Ramirez et al., 2018). In this context, the acute administration of hydroxychloroquine (HCQ) immediately after tumor cell inoculation into the pulmonary environment decreased proliferative outgrowth compared to vehicle-treated controls in immune-compromised animals, indicating that autophagy is important for the survival and early expansion of disseminated dormant tumor cells in vivo. In contrast, HCQ treatment after this proliferative switch had occurred under fibrotic conditions had minimal impact. Interestingly, decreased autophagic flux was associated with emergence from dormancy under fibrotic conditions, potentially underlying the lack of effect of HCQ during the later stages of colonization (Vera-Ramirez et al., 2018). A second study demonstrated the pro-metastatic effects of autophagy inhibition in D2.OR cells under non-fibrotic conditions in syngeneic animals (La Belle Flynn et al., 2019). Atg3 knockdown in this context elicited the emergence of proliferative, stem-like metastatic cells marked by elevated levels of a key glycolysis regulator, 6-phosphofructo-2-kinase/fructose-2,6-biphosphatase 3 (PFKFB3), suggesting a heterogeneous response to autophagy inhibition that gives rise to aggressive subpopulations (La Belle Flynn et al., 2019). A third study employing adriamycin-induced dormant breast cancer cells found that stable impairment of autophagy mediated by knockdown of Atg5, but not transient blockade of autophagy with CQ, resulted in both escape from dormancy and earlier metastatic recurrence (Aqbi et al., 2018). In this study, autophagy-deficient metastases exhibited higher frequencies of proliferating polyploid-like cells, indicating that the loss of autophagy promotes genomic instability and macrometastatic outgrowth. Finally, in the MMTV-PyMT model, the impairment of tumor cell autophagy by conditional genetic deletion of Atg5 or Atg12 after cells have seeded the pulmonary environment resulted in highly proliferative, basal-like subpopulations and increased metastatic outgrowth (Marsh et al., 2020). Similar results were found upon Atg12 knockdown in polyclonal 4T1 experimental metastasis models, suggesting that heterogeneity in the response to autophagy inhibition might impart distinct functional consequences (Marsh et al., 2020). Additionally, enforced autophagy induction by knockdown of Rubicon (RUBCN), an established negative regulator of autophagy, decreased the emergence of aggressive basal populations, thereby attenuating macrometastatic outgrowth (Marsh et al., 2020). The emergence of aggressive subpopulations of tumor cells downstream of autophagy inhibition occurs across a broad range of cancer cell types (Towers et al., 2019). Taken together, these findings suggest that autophagy promotes early stages of metastasis, including dissemination and survival in circulation, as well as survival and maintenance of dormant tumor cells. However, during later stages of metastasis, autophagy attenuates metastatic colonization by restricting the emergence of aggressive cancer cell subpopulations and suppressing macrometastatic outgrowth.
Selective autophagy receptors and metastasis
Autophagy sequesters and degrades large portions of the cellular proteome (Zhang and Ghaemmaghami, 2016). Research over the past decade has revealed exquisite selectivity for autophagic cargo (Zaffagnini and Martens, 2016). This selectivity is mediated by a related group of proteins termed autophagy receptors, which share common features including ubiquitin-binding domains and LC3-interacting regions (LIRs). Thus, autophagy receptors bring the ubiquitylated cargos into proximity with LC3 on the growing autophagosomal membrane for their eventual degradation (Box 2) (Johansen and Lamark, 2020). Interestingly, recent work demonstrates that autophagy receptor binding to cargo is sufficient to recruit the autophagosome initiation complex and to stimulate the formation of the isolation membrane in a spatiotemporal manner (Ravenhill et al., 2019; Turco et al., 2019; Vargas et al., 2019), suggesting that the recognition of cargo by autophagy receptors can precede autophagosome biogenesis in particular contexts. Upon fusion of the autophagosome with the lysosome, autophagy receptors are degraded alongside ubiquitylated cargo (Johansen and Lamark, 2020). Remarkably, an important consequence of autophagy inhibition includes cytosolic accumulation of autophagy receptors, which can function as critical signaling scaffolds. Moreover, accumulation of specific autophagy receptors (i.e. p62 and NBR1) induces aggregate formation and phase separation (Kirkin et al., 2009; Sun et al., 2018; Zaffagnini et al., 2018), which might sequester key signaling modulators away from canonical signaling partners, thus impacting downstream pathways or leading to novel signaling interactions within the aggregate. Thus, the modulation of autophagy may regulate tumorigenesis and metastasis through multiple, non-mutually exclusive, routes. How these functions of autophagy receptors impact cancer progression and metastasis is only starting to be deciphered (Fig. 3).
Box 2. The autophagy receptors p62 and NBR1.
Selective autophagy is mediated by a group of proteins termed autophagy receptors, including p62 (also called sequestosome 1; SQSTM1), NBR1, OPTN, NDP52, BNIP3, TAX1BP1 and NCOA4 (Johansen and Lamark, 2020). Among these, p62 and NBR1, two evolutionarily related proteins with ubiquitous expression in humans, are the best studied for their impact in human disease (Aguet et al., 2017). p62 and NBR1 possess similar domain structures, including a N-terminal PB1 domain, a ZZ type zinc-finger domain, a LC3-interacting region (LIR) and a C-terminal ubiquitin-binding domain (UBD) (Kirkin and Rogov, 2019). During autophagy, cargo recognition is mediated by binding of the UBD to ubiquitylated cargo and interaction with LC3 family proteins on the autophagosomal membrane via LIRs (Johansen and Lamark, 2020). Phosphorylation of S403 and S409 on the p62 UBD enhances an otherwise poor avidity for ubiquitin chains and promotes autophagic cargo recognition and clearance (Lim et al., 2015; Matsumoto et al., 2011; Matsumoto et al., 2015). Similarly, phosphorylation of LIR domains can promote binding affinity for LC3 family proteins (Wild et al., 2011). p62 and NBR1 both polymerize independently and cooperatively via PB1 domains, a process which promotes formation of phase-separated aggregates and autophagic degradation of cargo (Bjørkøy et al., 2005; Sun et al., 2018; Svenning et al., 2011; Zaffagnini et al., 2018). Formation of phase-separated condensates require ubiquitin chains, and elevated monoubiquitin can inhibit their formation (Zaffagnini et al., 2018). Interestingly, p62-positive condensates require NBR1 for their formation, and overexpression of NBR1 prevents autophagic clearance of condensates and promotes activation of NRF2 by p62 (Kirkin et al., 2009; Sánchez–Martín et al., 2020). Although these findings highlight the importance of autophagy receptors in mediating phase separation, the consequences of these emerging properties for tumorigenesis remain obscure. Finally, NBR1 possesses distinct domains compared with p62, including a coiled-coil domain that is important for dimerization, a FW domain and an amphipathic helix adjacent to the UBD (Kirkin and Rogov, 2019). However, the functional importance of these domains in cancer biology remains unclear. Overall, future studies are needed to uncover how these autophagy receptors govern distinct signaling events during cancer progression, both in the setting of autophagy competence and deficiency.
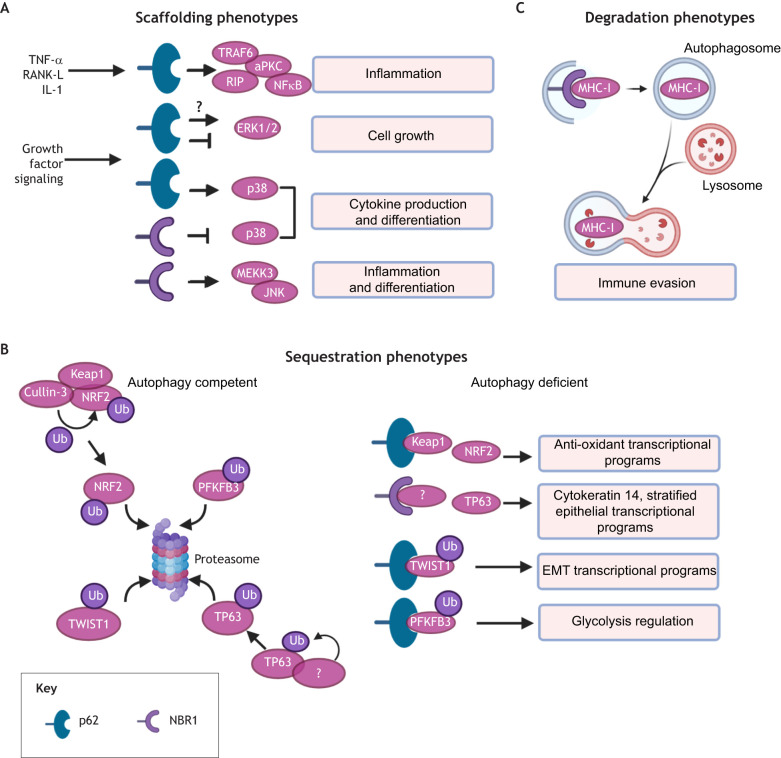
Fig. 3.
Pleiotropic functions of autophagy receptors in cancer. Selective autophagy is mediated by receptors, such as p62 and NBR1, which recognize ubiquitylated substrates and target them to the autophagosome for degradation. As a result, autophagy receptors are themselves degraded via autophagy. (A) Upon inhibition of autophagy, p62 and NBR1 accumulate within cells and act as scaffolds for components that are critical for MAPK and NFκB signaling cascades. (B) Elevated levels of cytosolic autophagy receptors in autophagy-deficient cells may also stabilize pro-metastatic transcription factors (NRF2, TP63 and TWIST1) or metabolic enzymes (PFKFB3) by sequestrating molecules that would normally promote the proteasomal degradation of these metastasis-promoting factors in autophagy-competent cells. (C) In autophagy-competent cells, NBR1 selectively removes MHC-I from the cell surface for autophagic degradation, thereby facilitating tumor cell immune evasion. aPKC, atypical protein kinase C; Ub, ubiquitin.
The most well-studied and archetypal autophagy receptor is p62, which mediates pleiotropic functions upon accumulation in autophagy-deficient cells. p62 acts as a scaffold to potentiate pro-tumorigenic, inflammatory NFκB signaling downstream of RANK-L (also known as TNFSF11), TNF and IL-1 stimulation by binding TRAF6 and RIP (also known as RIPK1) and enhancing activation of atypical protein kinase C (Fig. 3A) (Durán et al., 2004; Sanz, 1999, 2000). Moreover, several studies demonstrate that p62 promotes NFκB signaling and growth of primary tumors (Duran et al., 2008; Mathew et al., 2009; Wei et al., 2014), although it is unclear whether similar pathways promote metastases. Additionally, p62 antagonizes ERK1 and ERK2 (also known as MAPK3 and MAPK1, respectively) MAPK signaling, a key driver of cancer cell proliferation, by directly binding to ERK1/2 and sequestering it away from the downstream MEK1/2 kinases (MAP2K1 and MAP2K2) (Fig. 3A) (Rodriguez et al., 2006). In contrast, p62 promotes p38 family MAPK signaling and downstream cytokine production, as well as differentiation gene expression programs, suggesting that p62 may serve to balance functional outcomes of MAPK activation (Fig. 3A) (Kawai et al., 2007; Sudo et al., 2000). In addition to providing a scaffold for key signaling pathways, p62 can also prevent the proteasomal degradation of important antioxidants, EMT and metabolic effectors (Fig. 3B). Notably, p62 accumulation stabilizes NRF2, a transcription factor that mediates antioxidant gene expression during oxidative stress, by preventing the KEAP1-dependent recruitment of the E3-ubiqiutin ligase cullin-3 to NRF2 (Fig. 3B) (Komatsu et al., 2010). Additionally, by preventing the degradation of TWIST1, overexpression of p62 promotes both primary tumor and metastatic growth in vivo (Fig. 3B) (Qiang et al., 2014). Similarly, p62 accumulation prevents proteasomal degradation of PFKFB3, a critical glycolysis mediator, and promotes the outgrowth of otherwise dormant metastatic tumor cells (Fig. 3B) (La Belle Flynn et al., 2019). Overall, the level of p62 controls key signaling programs, and its accumulation upon autophagy inhibition confers important pro-tumorigenic functions that likely impact metastatic progression in vivo.
Recently, important roles for NBR1, which is closely related to p62, have been delineated in cellular differentiation and cancer. Early studies demonstrated that NBR1 directly interacts with activated p38 MAPK family proteins, and mice expressing a NBR1 truncation mutant exhibit aberrant p38 MAPK signaling that mediates increased osteoblast differentiation and bone formation (Fig. 3A) (Whitehouse et al., 2010). Later, NBR1 was found to interact with MEKK3 (also known as MAP3K3) via its PB1 domain to induce JNK MAPK signaling in myeloid cells, which promoted polarization of M2 macrophages and obesity-induced inflammation in adipose tissue (Fig. 3A) (Hernandez et al., 2014). In breast cancer models, NBR1 is both necessary and sufficient for pulmonary metastatic colonization and the acquisition of aggressive, basal differentiation traits (i.e. cytokeratin 14 expression) downstream of the basal epithelial transcription factor TP63, in disseminated tumor cells (Fig. 3B) (Marsh et al., 2020). Moreover, we found that accumulation of NBR1 is required for both pro-metastatic differentiation and macrometastatic outgrowth of autophagy-deficient tumor cells, suggesting that NBR1 is responsible for the generation of pro-metastatic tumor cell subpopulations that arise when autophagy is impaired, and that this effect is functionally independent of its role in selective autophagic degradation (Marsh et al., 2020). Finally, in pancreatic cancer models, NBR1 is required for autophagy-dependent translocation of major histocompatibility complex, class I (MHC-I) from the plasma membrane to the lysosome, which elicits immune evasion and subsequent tumor growth in vivo (Fig. 3C) (Yamamoto et al., 2020). Overall, these results point to NBR1 as a novel regulator of a spectrum of differentiation states in tumor cells and suggest that its accumulation potentiates tumor metastasis.
In addition, the mitophagy receptor BNIP3 has been shown to be critical for mammary tumor progression (Chourasia et al., 2015). Loss of BNIP3 increased ROS and HIF1α expression in primary tumor cells and induced a shift from OXPHOS to glycolytic metabolism, ultimately leading to increased spontaneous metastasis in autochthonous models. Interestingly, loss of BNIP3 in the stromal compartment had no effect on metastatic progression in this model, suggesting a tumor-cell-intrinsic role for mitophagy during metastasis (Chourasia et al., 2015). As therapeutic modulation of autophagy in cancer continues to evolve, it will be important to consider the effects of these strategies on autophagy receptor abundance and associated functions when evaluating their potential utility in preventing and treating metastasis.
Therapeutically targeting autophagy in metastasis
In addition to its role in tumorigenesis and metastasis, autophagy induction can be cytoprotective to cancer cells against commonly used cancer treatments, including a wide array of FDA-approved drugs (Rebecca and Amaravadi, 2016). Indeed, autophagy is implicated in therapeutic resistance in multiple cancers, including chemo-resistance to genotoxic therapies, kinase inhibitors, androgen-ablation therapy and targeted therapies (Cook et al., 2014; Frassanito et al., 2016; Mulcahy Levy et al., 2017; Nguyen et al., 2014; Wang and Wu, 2014; Wei et al., 2013; Yan et al., 2016). Potential mechanisms include the effects of autophagy on CSC plasticity, the expression of multi-drug resistance genes, and evasion of anoikis and immune recognition (Amaravadi et al., 2019). Because chemotherapeutic treatments can induce autophagy in cancer cells, combining cytotoxic drugs with autophagy inhibitors can augment their chemosensitivity and also target refractory cells, including CSCs (Sui et al., 2013). This concept prompted the first clinical trials of autophagy inhibitors, repurposing the anti-malarial CQ and its less toxic form hydroxychloroquine (HCQ) (see Table 1) for a wide spectrum of advanced tumors. With over 60 clinical trials either completed or ongoing, several striking responses and prolonged stable disease in patients with colon cancer, myeloma, melanoma and renal cell carcinoma provide evidence to support regimens that include anti-malarials (Table 1). However, CQ and HCQ only indirectly inhibit autophagy; their primary target is proposed to be palmitoyl-protein thioesterase 1 (PPT1), an enzyme involved in removal of fatty acyl groups during endolysosomal degradation (Rebecca et al., 2019). Since PPT1 itself is required for tumor growth, it is unclear whether the efficacy of CQ or HCQ treatment can be attributed to classical autophagy inhibition, to broader effects on the lysosome, or both. Furthermore, CQ may facilitate autophagy-independent mechanisms to reduce cancer cell invasion, intravasation and tumor hypoxia, as well as the number of circulating tumor cells (Maes et al., 2014). Specifically, it has been posited that, in endothelial cells, Notch1 functions downstream of CQ, but not genetic Atg deletion, to partially prevent metastasis by promoting vessel normalization, thereby improving the delivery and efficacy of chemotherapy and perhaps immune cell infiltration (Maes et al., 2014).
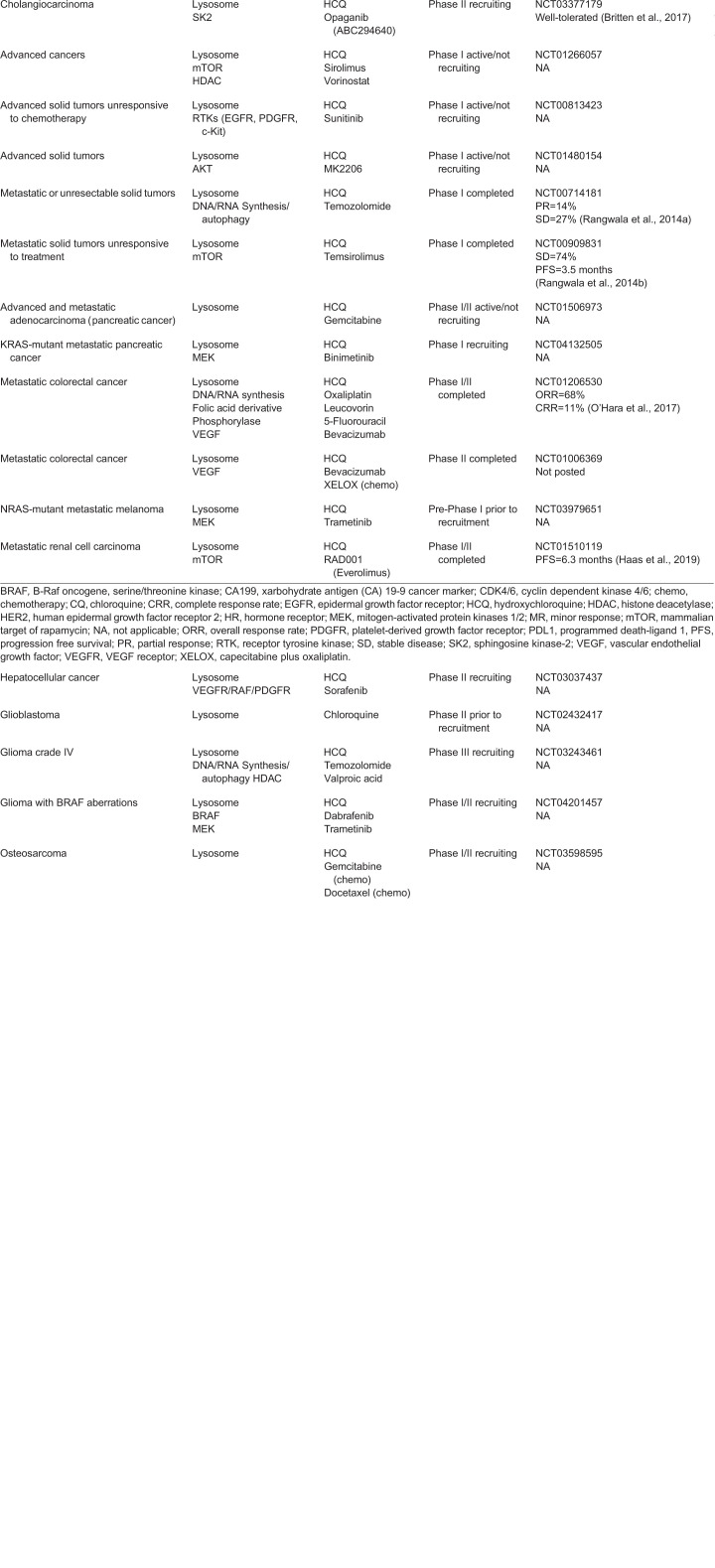
Table 1.
Current status of clinical trials inhibiting autophagy using CQ or HCQ in various cancers
With regard to metastasis, four clinical trials specifically designed to investigate the effect of HCQ in metastatic malignancies have reported encouraging results (Table 1). Partial responses or prolonged stable disease for these HCQ combinations have been reported in metastatic colorectal cancer (O'Hara et al., 2017), metastatic renal cell carcinoma (Amato et al., 2009) and metastatic melanoma (Rangwala et al., 2014a,b). Furthermore, certain patients showed an increase in the autophagy markers LC3 and p62 in peripheral blood mononuclear cells and exhibited increased intact autophagosomes within the cytosol as determined by electron microscopy (O'Hara et al., 2017). From a cell biological standpoint, these results highlight a key difference in the mechanism of action between anti-malarial-mediated lysosomal inhibition versus genetic ablation of autophagosome formation. In response to HCQ, autophagy receptors, including p62 and NBR1, are postulated to be sequestered inside undigested autophagosomes rather than accumulating in the cytoplasm. Accordingly, this lack of accumulation of autophagy receptors in the cytosol will not trigger adverse downstream signaling events that promote metastatic behavior. In support of this argument, the anti-malarial CQ does not impact metastatic outgrowth or pro-metastatic differentiation in contrast to genetic ablation of early autophagy genes in mammary cancer models (Marsh et al., 2020). Hence, such results might ameliorate concerns that HCQ treatment in cancer patients will promote metastasis.
Nevertheless, HCQ has associated toxicity risks and fails to inhibit autophagy in acidic environments (pH≤6.8) in melanoma, colon carcinoma and osteosarcoma likely due to reduced cellular uptake (Pellegrini et al., 2014; Shi et al., 2017). This has motivated the development of next-generation autophagy inhibitors, and several small molecules have already emerged as intriguing tool compounds in the preclinical setting. For instance, ROC325, Lys05, DC661 and DQ661 are second-generation analogs of HCQ that show enhanced lysosome inhibition, as well as potent antitumor activity, as single agents both in vitro and in vivo (Carew and Nawrocki, 2017; Carew et al., 2017; Nicastri et al., 2018; Rebecca et al., 2019; White, 2012). In addition, several molecules have been developed that target earlier steps in autophagosome formation, either directly or indirectly. For example, Spautin-1 blocks autophagy by inhibiting the deubiquitylation activities of C-terminal hydrolase 10 (USP10) and 13 (USP13), leading to increased ubiquitination and proteasomal degradation of the class III phosphoinositide 3-kinase (PI3K) complex [e.g. VPS34 (PIK3C3), Beclin-1, ATG14L, VPS15 (PIK3R4) and UVRAG], which is necessary for early stages of autophagosome formation (Donner, 2011; Liu et al., 2011). Additionally, Spautin-1 synergizes with clinically relevant cancer therapies (Shao et al., 2014). Another molecule, SAR405, which targets kinase vacuolar sorting protein 18 (VPS18) and VPS34, two key components of class III PI3K complex, impairs vesicle trafficking between late endosomes and the lysosome. When combined with the mTOR inhibitor everolimus, SAR40 impairs proliferation in renal cancer cells (Pasquier et al., 2015; Ronan et al., 2014). Other VPS34 inhibitors with clinical potential include SB02024 and Compound 13 (Dyczynski et al., 2018; Pasquier, 2015). In addition, the small-molecule SBI-0206965 inhibits the serine/threonine kinase ULK1 in the core autophagy pathway, synergizing with the mTOR inhibitor rapamycin to reduce viability of lung cancer and glioblastoma cells (Egan et al., 2015). Owing to off-target effects of SBI-0206965, the more specific compound ULK101 has been developed and is under investigation (Martin et al., 2018). NSC185058 is an ATG4B inhibitor that suppresses tumor growth in osteosarcoma both in vitro and in vivo (Akin et al., 2014), and new, more potent, ATG4B-targeting compounds, such as S130 and FMK-9a, have been reported (Chu et al., 2018; Fu et al., 2019). Finally, several inhibitors of the phosphoinositide lipid kinase, FYVE-type zinc finger-containing kinase (PIKFYVE), which controls endolysosomal membrane trafficking, such as apilimod, have been developed; however, as PIKFYVE acts at multiple intracellular locations apart from autophagosomes and lysosomes, further studies are needed to scrutinize whether their mechanism of action is specifically due to targeting autophagy (Gayle et al., 2017; Sharma et al., 2019). Moreover, it remains to be determined whether any of these preclinical drug candidates will be effective in preventing or treating metastatic disease, or, alternatively, whether they harbor any long-term risks of enhancing metastasis in cancer patients.
Concluding perspectives
Despite substantial progress in our understanding of autophagy in cancer, we still have much to learn about the precise biological roles of autophagy in metastasis and how selective autophagy receptors contribute to late-stage cancer progression. Although initial clinical trials employing repurposed anti-malarials have garnered some encouraging results, it remains unclear whether these effects arise from impaired tumor cell autophagy, dysregulated lysosomal homeostasis or broader effects on the tumor microenvironment. Considering the tumor-suppressive effects of autophagy, researchers and clinicians should continue to scrutinize whether it is appropriate to inhibit autophagy in cancer treatment; specifically, studies are needed to establish whether therapeutic ‘windows of opportunity’ exist in which autophagy can be specifically targeted in tumors without untoward effects on metastasis. As novel and more-specific compounds targeting autophagy are developed, it will also be important to consider the stage of metastatic progression at which autophagy is being targeted in patients and recognize the downstream effects of autophagy receptor-mediated phenotypes in order to efficiently combat metastatic disease.
Acknowledgements
The review is dedicated to the memories of Professors Beth Levine (University of Texas Southwestern) and Zena Werb (UCSF), two exceptional women in science, and incredible mentors and luminaries in the fields of autophagy and metastasis, respectively. Figures were created with BioRender.com.
Footnotes
Competing interests
J.D. is a Scientific Advisory Board Member for Vescor Therapeutics, LLC. T.M. is employed by Casma Therapeutics.
Funding
Support for research on autophagy and metastasis to J.D. includes grants from the National Cancer Institute (CA201849, CA126792, CA213775), the Department of Defense BCRP (W81XWH-11-1-0130), Samuel Waxman Cancer Research Foundation, and Mark Foundation for Cancer Research (Endeavor Award). B.T. is supported by a Pancreas Center Pilot Project Grant Award and a Hellman Family Award for Early Career Faculty from the University of California San Francisco (UCSF). T.M. received fellowship support from the National Cancer Institute (F31CA217015). Deposited in PMC for release after 12 months.
References
- Aguet F., Brown A. A., Castel S. E., Davis J. R., He Y., Jo B., Mohammadi P., Park Y. S., Parsana P., Segrè A. V. et al. (2017). Genetic effects on gene expression across human tissues. Nature 550, 204-213. 10.1038/nature24277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akin D., Wang S. K., Habibzadegah-Tari P., Law B., Ostrov D., Li M., Yin X.-M., Kim J.-S., Horenstein N. and Dunn W. A (2014). A novel ATG4B antagonist inhibits autophagy and has a negative impact on osteosarcoma tumors. Autophagy 10, 2021-2035. 10.4161/auto.32229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amaravadi R. K., Kimmelman A. C. and Debnath J (2019). Targeting autophagy in cancer: recent advances and future directions. Cancer Discov. 9, 1167-1181. 10.1158/2159-8290.CD-19-0292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amato R. J., Jac J., Giessinger S., Saxena S. and Willis J. P (2009). A phase 2 study with a daily regimen of the oral mTOR inhibitor RAD001 (everolimus) in patients with metastatic clear cell renal cell cancer. Cancer 115, 2438-2446. 10.1002/cncr.24280 [DOI] [PubMed] [Google Scholar]
- Apetoh L., Ghiringhelli F., Tesniere A., Obeid M., Ortiz C., Criollo A., Mignot G., Maiuri M. C., Ullrich E., Saulnier P. et al. (2007). Toll-like receptor 4–dependent contribution of the immune system to anticancer chemotherapy and radiotherapy. Nat. Med. 13, 1050-1059. 10.1038/nm1622 [DOI] [PubMed] [Google Scholar]
- Aqbi H. F., Tyutyunyk-Massey L., Keim R. C., Butler S. E., Thekkudan T., Joshi S., Smith T. M., Bandyopadhyay D., Idowu M. O., Bear H. D. et al. (2018). Autophagy-deficient breast cancer shows early tumor recurrence and escape from dormancy. Oncotarget 9, 22113-22122. 10.18632/oncotarget.25197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avivar-Valderas A., Salas E., Bobrovnikova-Marjon E., Diehl J. A., Nagi C., Debnath J. and Aguirre-Ghiso J. A (2011). PERK Integrates autophagy and oxidative stress responses to promote survival during extracellular matrix detachment. Mol. Cell. Biol. 31, 3616-3629. 10.1128/MCB.05164-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avivar-Valderas A., Bobrovnikova-Marjon E., Alan Diehl J., Bardeesy N., Debnath J. and Aguirre-Ghiso J. A (2013). Regulation of autophagy during ECM detachment is linked to a selective inhibition of mTORC1 by PERK. Oncogene 32, 4932-4940. 10.1038/onc.2012.512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baginska J., Viry E., Berchem G., Poli A., Noman M. Z., van Moer K., Medves S., Zimmer J., Oudin A., Niclou S. P. et al. (2013). Granzyme B degradation by autophagy decreases tumor cell susceptibility to natural killer-mediated lysis under hypoxia. Proc. Natl. Acad. Sci. USA 110, 17450-17455. 10.1073/pnas.1304790110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnard R. A., Regan D. P., Hansen R. J., Maycotte P., Thorburn A. and Gustafson D. L (2016). Autophagy inhibition delays early but not late-stage metastatic disease. J. Pharmacol. Exp. Ther. 358, 282-293. 10.1124/jpet.116.233908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bento C. F., Renna M., Ghislat G., Puri C., Ashkenazi A., Vicinanza M., Menzies F. M. and Rubinsztein D. C (2016). Mammalian autophagy: how does it work? Annu. Rev. Biochem. 85, 685-713. 10.1146/annurev-biochem-060815-014556 [DOI] [PubMed] [Google Scholar]
- Bjørkøy G., Lamark T., Brech A., Outzen H., Perander M., Øvervatn A., Stenmark H. and Johansen T (2005). p62/SQSTM1 forms protein aggregates degraded by autophagy and has a protective effect on huntingtin-induced cell death. J. Cell Biol. 171, 603-614. 10.1083/jcb.200507002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boone B. A., Bahary N., Zureikat A. H., Moser A. J., Normolle D. P., Wu W.-C., Singhi A. D., Bao P., Bartlett D. L., Liotta L. A. et al. (2015). Safety and biologic response of pre-operative autophagy inhibition in combination with gemcitabine in patients with pancreatic adenocarcinoma. Ann. Surg. Oncol. 22, 4402-4410. 10.1245/s10434-015-4566-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewer J. W. and Diehl J. A (2000). PERK mediates cell-cycle exit during the mammalian unfolded protein response. Proc. Natl. Acad. Sci. USA 97, 12625-12630. 10.1073/pnas.220247197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britten C. D., Garrett-Mayer E., Chin S. H., Shirai K., Ogretmen B., Bentz T. A., Brisendine A., Anderton K., Cusack S. L., Maines L. W. et al. (2017). A phase I study of ABC294640, a first-in-class sphingosine kinase-2 inhibitor, in patients with advanced solid tumors. Clin. Cancer Res. 23, 4642-4650. 10.1158/1078-0432.CCR-16-2363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant K. L., Stalnecker C. A., Zeitouni D., Klomp J. E., Peng S., Tikunov A. P., Gunda V., Pierobon M., Waters A. M., George S. D. et al. (2019). Combination of ERK and autophagy inhibition as a treatment approach for pancreatic cancer. Nat. Med. 25, 628-640. 10.1038/s41591-019-0368-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caino M. C., Chae Y. C., Vaira V., Ferrero S., Nosotti M., Martin N. M., Weeraratna A., O'Connell M., Jernigan D., Fatatis A. et al. (2013). Metabolic stress regulates cytoskeletal dynamics and metastasis of cancer cells. J. Clin. Invest. 123, 2907-2920. 10.1172/JCI67841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carew J. S. and Nawrocki S. T (2017). Drain the lysosome: development of the novel orally available autophagy inhibitor ROC-325. Autophagy 13, 765-766. 10.1080/15548627.2017.1280222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carew J. S., Espitia C. M., Zhao W., Han Y., Visconte V., Phillips J. and Nawrocki S. T (2017). Disruption of autophagic degradation with ROC-325 antagonizes renal cell carcinoma pathogenesis. Clin. Cancer Res. 23, 2869-2879. 10.1158/1078-0432.CCR-16-1742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen N. and Debnath J (2013). IκB kinase complex (IKK) triggers detachment-induced autophagy in mammary epithelial cells independently of the PI3K-AKT-MTORC1 pathway. Autophagy 9, 1214-1227. 10.4161/auto.24870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen N., Eritja N., Lock R. and Debnath J (2013). Autophagy restricts proliferation driven by oncogenic phosphatidylinositol 3-kinase in three-dimensional culture. Oncogene 32, 2543-2554. 10.1038/onc.2012.277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J. L., David J., Cook-Spaeth D., Casey S., Cohen D., Selvendiran K., Bekaii-Saab T. and Hays J. L (2017). Autophagy induction results in enhanced anoikis resistance in models of peritoneal disease. Mol. Cancer Res. 15, 26-34. 10.1158/1541-7786.MCR-16-0200-T [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chourasia A. H., Tracy K., Frankenberger C., Boland M. L., Sharifi M. N., Drake L. E., Sachleben J. R., Asara J. M., Locasale J. W., Karczmar G. S. et al. (2015). Mitophagy defects arising from BNip3 loss promote mammary tumor progression to metastasis. EMBO Rep. 16, 1145-1163. 10.15252/embr.201540759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu J., Fu Y., Xu J., Zheng X., Gu Q., Luo X., Dai Q., Zhang S., Liu P., Hong L. et al. (2018). ATG4B inhibitor FMK-9a induces autophagy independent on its enzyme inhibition. Arch. Biochem. Biophys. 644, 29-36. 10.1016/j.abb.2018.03.001 [DOI] [PubMed] [Google Scholar]
- Comen E., Norton L. and Massagué J (2011). Clinical implications of cancer self-seeding. Nat. Rev. Clin. Oncol. 8, 369-377. 10.1038/nrclinonc.2011.64 [DOI] [PubMed] [Google Scholar]
- Commisso C., Davidson S. M., Soydaner-Azeloglu R. G., Parker S. J., Kamphorst J. J., Hackett S., Grabocka E., Nofal M., Drebin J. A., Thompson C. B. et al. (2013). Macropinocytosis of protein is an amino acid supply route in Ras-transformed cells. Nature 497, 633-637. 10.1038/nature12138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook K. L., Warri A., Soto-Pantoja D. R., Clarke P. A., Cruz M. I., Zwart A. and Clarke R (2014). Chloroquine inhibits autophagy to potentiate antiestrogen responsiveness in ER+ breast cancer. Clin. Cancer Res. 20, 3222-3232. 10.1158/1078-0432.CCR-13-3227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa-Silva B., Aiello N. M., Ocean A. J., Singh S., Zhang H., Thakur B. K., Becker A., Hoshino A., Mark M. T., Molina H. et al. (2015). Pancreatic cancer exosomes initiate pre-metastatic niche formation in the liver. Nat. Cell Biol. 17, 816-826. 10.1038/ncb3169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cufí S., Vazquez-Martin A., Oliveras-Ferraros C., Martin-Castillo B., Vellon L. and Menendez J. A (2011). Autophagy positively regulates the CD44+ CD24−/low breast cancer stem-like phenotype. Cell Cycle 10, 3871-3885. 10.4161/cc.10.22.17976 [DOI] [PubMed] [Google Scholar]
- Davie S. A., Maglione J. E., Manner C. K., Young D., Cardiff R. D., MacLeod C. L. and Ellies L. G (2007). Effects of FVB/NJ and C57Bl/6J strain backgrounds on mammary tumor phenotype in inducible nitric oxide synthase deficient mice. Transgenic Res. 16, 193-201. 10.1007/s11248-006-9056-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degenhardt K., Mathew R., Beaudoin B., Bray K., Anderson D., Chen G., Mukherjee C., Shi Y., Gélinas C., Fan Y. et al. (2006). Autophagy promotes tumor cell survival and restricts necrosis, inflammation, and tumorigenesis. Cancer Cell 10, 51-64. 10.1016/j.ccr.2006.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donner A. (2011). Deubiquitinating p53. Nat. Chem. Biol. 7, 856-856. 10.1038/nchembio.723 [DOI] [Google Scholar]
- Dower C. M., Bhat N., Wang E. W. and Wang H.-G (2017). Selective reversible inhibition of autophagy in hypoxic breast cancer cells promotes pulmonary metastasis. Cancer Res. 77, 646-657. 10.1158/0008-5472.CAN-15-3458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupuy F., Tabariès S., Andrzejewski S., Dong Z., Blagih J., Annis M. G., Omeroglu A., Gao D., Leung S., Amir E. et al. (2015). PDK1-dependent metabolic reprogramming dictates metastatic potential in breast cancer. Cell Metab. 22, 577-589. 10.1016/j.cmet.2015.08.007 [DOI] [PubMed] [Google Scholar]
- Duran A., Linares J. F., Galvez A. S., Wikenheiser K., Flores J. M., Diaz-Meco M. T. and Moscat J (2008). The signaling adaptor p62 is an important NF-κB mediator in tumorigenesis. Cancer Cell 13, 343-354. 10.1016/j.ccr.2008.02.001 [DOI] [PubMed] [Google Scholar]
- Durán A., Serrano M., Leitges M., Flores J. M., Picard S., Brown J. P., Moscat J. and Diaz-Meco M. T (2004). The atypical PKC-interacting protein p62 is an important mediator of RANK-activated osteoclastogenesis. Dev. Cell 6, 303-309. 10.1016/S1534-5807(03)00403-9 [DOI] [PubMed] [Google Scholar]
- Dyczynski M., Yu Y., Otrocka M., Parpal S., Braga T., Henley A. B., Zazzi H., Lerner M., Wennerberg K., Viklund J. et al. (2018). Targeting autophagy by small molecule inhibitors of vacuolar protein sorting 34 (Vps34) improves the sensitivity of breast cancer cells to Sunitinib. Cancer Lett. 435, 32-43. 10.1016/j.canlet.2018.07.028 [DOI] [PubMed] [Google Scholar]
- Egan D. F., Shackelford D. B., Mihaylova M. M., Gelino S., Kohnz R. A., Mair W., Vasquez D. S., Joshi A., Gwinn D. M., Taylor R. et al. (2011). Phosphorylation of ULK1 (hATG1) by AMP-activated protein kinase connects energy sensing to mitophagy. Science 331, 456-461. 10.1126/science.1196371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egan D. F., Chun M. G. H., Vamos M., Zou H., Rong J., Miller C. J., Lou H. J., Raveendra-Panickar D., Yang C.-C., Sheffler D. J. et al. (2015). Small molecule inhibition of the autophagy kinase ULK1 and identification of ULK1 substrates. Mol. Cell 59, 285-297. 10.1016/j.molcel.2015.05.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan W., Tang Z., Chen D., Moughon D., Ding X., Chen S., Zhu M. and Zhong Q (2010). Keap1 facilitates p62-mediated ubiquitin aggregate clearance via autophagy. Autophagy 6, 614-621. 10.4161/auto.6.5.12189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiacco E., Castagnetti F., Bianconi V., Madaro L., De Bardi M., Nazio F., D'Amico A., Bertini E., Cecconi F., Puri P. L. et al. (2016). Autophagy regulates satellite cell ability to regenerate normal and dystrophic muscles. Cell Death Differ. 23, 1839-1849. 10.1038/cdd.2016.70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Florey O. and Overholtzer M (2019). Macropinocytosis and autophagy crosstalk in nutrient scavenging. Philos. Trans. R. Soc. B Biol. Sci. 374, 20180154 10.1098/rstb.2018.0154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frassanito M. A., De Veirman K., Desantis V., Marzo L. Di, Vergara D., Ruggieri S., Annese T., Nico B., Menu E., Catacchio I. et al. (2016). Halting pro-survival autophagy by TGFβ inhibition in bone marrow fibroblasts overcomes bortezomib resistance in multiple myeloma patients. Leukemia 30, 640-648. 10.1038/leu.2015.289 [DOI] [PubMed] [Google Scholar]
- Frisch S. and Francis H (1994). Disruption of epithelial cell-matrix interactions induces apoptosis. J. Cell Biol. 124, 619-626. 10.1083/jcb.124.4.619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Y., Hong L., Xu J., Zhong G., Gu Q., Gu Q., Guan Y., Zheng X., Dai Q., Luo X. et al. (2019). Discovery of a small molecule targeting autophagy via ATG4B inhibition and cell death of colorectal cancer cells in vitro and in vivo. Autophagy 15, 295-311. 10.1080/15548627.2018.1517073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fung C., Lock R., Gao S., Salas E. and Debnath J (2008). Induction of autophagy during extracellular matrix detachment promotes cell survival. Mol. Biol. Cell 19, 797-806. 10.1091/mbc.e07-10-1092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gayle S., Landrette S., Beeharry N., Conrad C., Hernandez M., Beckett P., Ferguson S. M., Mandelkern T., Zheng M., Xu T. et al. (2017). Identification of apilimod as a first-in-class PIKfyve kinase inhibitor for treatment of B-cell non-Hodgkin lymphoma. Blood 129, 1768-1778. 10.1182/blood-2016-09-736892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Görgülü K., Diakopoulos K. N., Ai J., Schoeps B., Kabacaoglu D., Karpathaki A.-F., Ciecielski K. J., Kaya-Aksoy E., Ruess D. A., Berninger A. et al. (2019). Levels of the autophagy-related 5 protein affect progression and metastasis of pancreatic tumors in mice. Gastroenterology 156, 203-217.e20. 10.1053/j.gastro.2018.09.053 [DOI] [PubMed] [Google Scholar]
- Guo J. Y., Chen H.-Y., Mathew R., Fan J., Strohecker A. M., Karsli-Uzunbas G., Kamphorst J. J., Chen G., Lemons J. M. S., Karantza V. et al. (2011). Activated Ras requires autophagy to maintain oxidative metabolism and tumorigenesis. Genes Dev. 25, 460-470. 10.1101/gad.2016311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo J. Y., Karsli-Uzunbas G., Mathew R., Aisner S. C., Kamphorst J. J., Strohecker A. M., Chen G., Price S., Lu W., Teng X. et al. (2013). Autophagy suppresses progression of K-ras-induced lung tumors to oncocytomas and maintains lipid homeostasis. Genes Dev. 27, 1447-1461. 10.1101/gad.219642.113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas N. B., Appleman L. J., Stein M., Redlinger M., Wilks M., Xu X., Onorati A., Kalavacharla A., Kim T., Zhen C. J. et al. (2019). Autophagy inhibition to augment mTOR inhibition: a phase I/II trial of everolimus and hydroxychloroquine in patients with previously treated renal cell carcinoma. Clin. Cancer Res. 25, 2080-2087. 10.1158/1078-0432.CCR-18-2204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanahan D. and Weinberg R. A (2011). Hallmarks of cancer: the next generation. Cell 144, 646-674. 10.1016/j.cell.2011.02.013 [DOI] [PubMed] [Google Scholar]
- Hen O. and Barkan D (2020). Dormant disseminated tumor cells and cancer stem/progenitor-like cells: similarities and opportunities. Semin. Cancer Biol. 60, 157-165. 10.1016/j.semcancer.2019.09.002 [DOI] [PubMed] [Google Scholar]
- Hernandez E. D., Lee S. J., Kim J. Y., Duran A., Linares J. F., Yajima T., Müller T. D., Tschöp M. H., Smith S. R., Diaz-Meco M. T. et al. (2014). A macrophage NBR1-MEKK3 complex triggers JNK-mediated adipose tissue inflammation in obesity. Cell Metab. 20, 499-511. 10.1016/j.cmet.2014.06.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang G.-M., Tan Y., Wang H., Peng L., Chen H.-T., Meng X.-J., Li L.-L., Liu Y., Li W.-F. and Shan H (2019). The relationship between autophagy and the immune system and its applications for tumor immunotherapy. Mol. Cancer 18, 17 10.1186/s12943-019-0944-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansen T. and Lamark T (2020). Selective autophagy: ATG8 family proteins, LIR motifs and cargo receptors. J. Mol. Biol. 432, 80-103. 10.1016/j.jmb.2019.07.016 [DOI] [PubMed] [Google Scholar]
- Kaur J. and Debnath J (2015). Autophagy at the crossroads of catabolism and anabolism. Nat. Rev. Mol. Cell Biol. 16, 461-472. 10.1038/nrm4024 [DOI] [PubMed] [Google Scholar]
- Kawai K., Saito A., Sudo T. and Osada H (2007). Specific regulation of cytokine-dependent p38 MAP kinase activation by p62/SQSTM1. J. Biochem. 143, 765-772. 10.1093/jb/mvn027 [DOI] [PubMed] [Google Scholar]
- Kenific C. M., Stehbens S. J., Goldsmith J., Leidal A. M., Faure N., Ye J., Wittmann T. and Debnath J (2016). NBR1 enables autophagy-dependent focal adhesion turnover. J. Cell Biol. 212, 577-590. 10.1083/jcb.201503075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J., Kundu M., Viollet B. and Guan K.-L (2011). AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Nat. Cell Biol. 13, 132-141. 10.1038/ncb2152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y. H., Baek S. H., Kim E. K., Ha J. M., Jin S. Y., Lee H. S., Ha H. K., Song S. H., Kim S. J., Shin H. K. et al. (2016). Uncoordinated 51-like kinase 2 signaling pathway regulates epithelial-mesenchymal transition in A549 lung cancer cells. FEBS Lett. 590, 1365-1374. 10.1002/1873-3468.12172 [DOI] [PubMed] [Google Scholar]
- Kinsey C. G., Camolotto S. A., Boespflug A. M., Guillen K. P., Foth M., Truong A., Schuman S. S., Shea J. E., Seipp M. T., Yap J. T. et al. (2019). Protective autophagy elicited by RAF→MEK→ERK inhibition suggests a treatment strategy for RAS-driven cancers. Nat. Med. 25, 620-627. 10.1038/s41591-019-0367-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkin V. and Rogov V. V (2019). A diversity of selective autophagy receptors determines the specificity of the autophagy pathway. Mol. Cell 76, 268-285. 10.1016/j.molcel.2019.09.005 [DOI] [PubMed] [Google Scholar]
- Kirkin V., Lamark T., Sou Y.-S., Bjørkøy G., Nunn J. L., Bruun J.-A., Shvets E., McEwan D. G., Clausen T. H., Wild P. et al. (2009). A role for NBR1 in autophagosomal degradation of ubiquitinated substrates. Mol. Cell 33, 505-516. 10.1016/j.molcel.2009.01.020 [DOI] [PubMed] [Google Scholar]
- Komatsu M., Kurokawa H., Waguri S., Taguchi K., Kobayashi A., Ichimura Y., Sou Y.-S., Ueno I., Sakamoto A., Tong K. I. et al. (2010). The selective autophagy substrate p62 activates the stress responsive transcription factor Nrf2 through inactivation of Keap1. Nat. Cell Biol. 12, 213-223. 10.1038/ncb2021 [DOI] [PubMed] [Google Scholar]
- Kroemer G., Mariño G. and Levine B (2010). Autophagy and the integrated stress response. Mol. Cell 40, 280-293. 10.1016/j.molcel.2010.09.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Belle Flynn A., Calhoun B. C., Sharma A., Chang J. C., Almasan A. and Schiemann W. P. (2019). Autophagy inhibition elicits emergence from metastatic dormancy by inducing and stabilizing Pfkfb3 expression. Nat. Commun. 10, 3668 10.1038/s41467-019-11640-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert A. W., Pattabiraman D. R. and Weinberg R. A (2017). Emerging biological principles of metastasis. Cell 168, 670-691. 10.1016/j.cell.2016.11.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langley R. R. and Fidler I. J (2011). The seed and soil hypothesis revisited-The role of tumor-stroma interactions in metastasis to different organs. Int. J. Cancer 128, 2527-2535. 10.1002/ijc.26031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau A., Wang X.-J., Zhao F., Villeneuve N. F., Wu T., Jiang T., Sun Z., White E. and Zhang D. D (2010). A noncanonical mechanism of Nrf2 activation by autophagy deficiency: direct interaction between Keap1 and p62. Mol. Cell. Biol. 30, 3275-3285. 10.1128/MCB.00248-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence R. E. and Zoncu R (2019). The lysosome as a cellular centre for signalling, metabolism and quality control. Nat. Cell Biol. 21, 133-142. 10.1038/s41556-018-0244-7 [DOI] [PubMed] [Google Scholar]
- Lawson K. A., Sousa C. M., Zhang X., Kim E., Akthar R., Caumanns J. J., Yao Y., Mikolajewicz N., Ross C., Brown K. R. et al. (2020). Functional genomic landscape of cancer-intrinsic evasion of killing by T cells. Nature 586, 120-126. 10.1038/s41586-020-2746-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeBleu V. S., O'Connell J. T., Gonzalez Herrera K. N., Wikman H., Pantel K., Haigis M. C., de Carvalho F. M., Damascena A., Domingos Chinen L. T., Rocha R. M. et al. (2014). PGC-1α mediates mitochondrial biogenesis and oxidative phosphorylation in cancer cells to promote metastasis. Nat. Cell Biol. 16, 992-1003. 10.1038/ncb3039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C.-S., Lee L. C., Yuan T. L., Chakka S., Fellmann C., Lowe S. W., Caplen N. J., McCormick F. and Luo J (2019). MAP kinase and autophagy pathways cooperate to maintain RAS mutant cancer cell survival. Proc. Natl. Acad. Sci. USA 116, 4508-4517. 10.1073/pnas.1817494116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehuédé C., Dupuy F., Rabinovitch R., Jones R. G. and Siegel P. M (2016). Metabolic plasticity as a determinant of tumor growth and metastasis. Cancer Res. 76, 5201-5208. 10.1158/0008-5472.CAN-16-0266 [DOI] [PubMed] [Google Scholar]
- Leidal A. M., Huang H. H., Marsh T., Solvik T., Zhang D., Ye J., Kai F., Goldsmith J., Liu J. Y., Huang Y.-H. et al. (2020). The LC3-conjugation machinery specifies the loading of RNA-binding proteins into extracellular vesicles. Nat. Cell Biol. 22, 187-199. 10.1038/s41556-019-0450-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine B., Mizushima N. and Virgin H. W (2011). Autophagy in immunity and inflammation. Nature 469, 323-335. 10.1038/nature09782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F., Tiede B., Massagué J. and Kang Y (2007). Beyond tumorigenesis: cancer stem cells in metastasis. Cell Res. 17, 3-14. 10.1038/sj.cr.7310118 [DOI] [PubMed] [Google Scholar]
- Li J., Yang B., Zhou Q., Wu Y., Shang D., Guo Y., Song Z., Zheng Q. and Xiong J (2013). Autophagy promotes hepatocellular carcinoma cell invasion through activation of epithelial–mesenchymal transition. Carcinogenesis 34, 1343-1351. 10.1093/carcin/bgt063 [DOI] [PubMed] [Google Scholar]
- Li L., Chen H., Gao Y., Wang Y.-W., Zhang G.-Q., Pan S.-H., Ji L., Kong R., Wang G., Jia Y.-H. et al. (2016). Long noncoding RNA MALAT1 promotes aggressive pancreatic cancer proliferation and metastasis via the stimulation of autophagy. Mol. Cancer Ther. 15, 2232-2243. 10.1158/1535-7163.MCT-16-0008 [DOI] [PubMed] [Google Scholar]
- Li H., Li D., Ma Z., Qian Z., Kang X., Jin X., Li F., Wang X., Chen Q., Sun H. et al. (2018). Defective autophagy in osteoblasts induces endoplasmic reticulum stress and causes remarkable bone loss. Autophagy 14, 1726-1741. 10.1080/15548627.2018.1483807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S., Song Y., Quach C., Guo H., Jang G.-B., Maazi H., Zhao S., Sands N. A., Liu Q., In G. K. et al. (2019). Transcriptional regulation of autophagy-lysosomal function in BRAF-driven melanoma progression and chemoresistance. Nat. Commun. 10, 1693 10.1038/s41467-019-09634-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim J., Lachenmayer M. L., Wu S., Liu W., Kundu M., Wang R., Komatsu M., Oh Y. J., Zhao Y. and Yue Z (2015). Proteotoxic stress induces phosphorylation of p62/SQSTM1 by ULK1 to regulate selective autophagic clearance of protein aggregates. PLoS Genet. 11, e1004987 10.1371/journal.pgen.1004987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J., Xia H., Kim M., Xu L., Li Y., Zhang L., Cai Y., Norberg H. V., Zhang T., Furuya T. et al. (2011). Beclin1 controls the levels of p53 by regulating the deubiquitination activity of USP10 and USP13. Cell 147, 223-234. 10.1016/j.cell.2011.08.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lock R., Kenific C. M., Leidal A. M., Salas E. and Debnath J (2014). Autophagy-dependent production of secreted factors facilitates oncogenic RAS-driven invasion. Cancer Discov. 4, 466-479. 10.1158/2159-8290.CD-13-0841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lv Q., Wang W., Xue J., Hua F., Mu R., Lin H., Yan J., Lv X., Chen X. and Hu Z.-W (2012). DEDD interacts with PI3KC3 to activate autophagy and attenuate epithelial-mesenchymal transition in human breast cancer. Cancer Res. 72, 3238-3250. 10.1158/0008-5472.CAN-11-3832 [DOI] [PubMed] [Google Scholar]
- Maes H., Kuchnio A., Peric A., Moens S., Nys K., De Bock K., Quaegebeur A., Schoors S., Georgiadou M., Wouters J. et al. (2014). Tumor vessel normalization by chloroquine independent of autophagy. Cancer Cell 26, 190-206. 10.1016/j.ccr.2014.06.025 [DOI] [PubMed] [Google Scholar]
- Malanchi I., Santamaria-Martínez A., Susanto E., Peng H., Lehr H.-A., Delaloye J.-F. and Huelsken J (2012). Interactions between cancer stem cells and their niche govern metastatic colonization. Nature 481, 85-89. 10.1038/nature10694 [DOI] [PubMed] [Google Scholar]
- Malhotra J., Jabbour S., Orlick M., Riedlinger G., Guo Y., White E. and Aisner J (2019). Phase Ib/II study of hydroxychloroquine in combination with chemotherapy in patients with metastatic non-small cell lung cancer (NSCLC). Cancer Treat. Res. Commun. 21, 100158 10.1016/j.ctarc.2019.100158 [DOI] [PubMed] [Google Scholar]
- Marsh T., Kenific C. M., Suresh D., Gonzalez H., Shamir E. R., Mei W., Tankka A., Leidal A. M., Kalavacherla S., Woo K. et al. (2020). Autophagic degradation of NBR1 restricts metastatic outgrowth during mammary tumor progression. Dev. Cell 52, 591-604.e6. 10.1016/j.devcel.2020.01.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin K. R., Celano S. L., Solitro A. R., Gunaydin H., Scott M., O'Hagan R. C., Shumway S. D., Fuller P. and MacKeigan J. P (2018). A potent and selective ULK1 inhibitor suppresses autophagy and sensitizes cancer cells to nutrient stress. iScience 8, 74-84. 10.1016/j.isci.2018.09.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathew R., Karantza-Wadsworth V. and White E (2007). Role of autophagy in cancer. Nat. Rev. Cancer 7, 961-967. 10.1038/nrc2254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathew R., Karp C. M., Beaudoin B., Vuong N., Chen G., Chen H.-Y., Bray K., Reddy A., Bhanot G., Gelinas C. et al. (2009). Autophagy suppresses tumorigenesis through elimination of p62. Cell 137, 1062-1075. 10.1016/j.cell.2009.03.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto G., Wada K., Okuno M., Kurosawa M. and Nukina N (2011). Serine 403 phosphorylation of p62/SQSTM1 regulates selective autophagic clearance of ubiquitinated proteins. Mol. Cell 44, 279-289. 10.1016/j.molcel.2011.07.039 [DOI] [PubMed] [Google Scholar]
- Matsumoto G., Shimogori T., Hattori N. and Nukina N (2015). TBK1 controls autophagosomal engulfment of polyubiquitinated mitochondria through p62/SQSTM1 phosphorylation. Hum. Mol. Genet. 24, 4429-4442. 10.1093/hmg/ddv179 [DOI] [PubMed] [Google Scholar]
- Maycotte P., Jones K. L., Goodall M. L., Thorburn J. and Thorburn A (2015). Autophagy supports breast cancer stem cell maintenance by regulating IL6 secretion. Mol. Cancer Res. 13, 651-658. 10.1158/1541-7786.MCR-14-0487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulcahy Levy J. M., Zahedi S., Griesinger A. M., Morin A., Davies K. D., Aisner D. L., Kleinschmidt-DeMasters B., Fitzwalter B. E., Goodall M. L., Thorburn J., (2017). Autophagy inhibition overcomes multiple mechanisms of resistance to BRAF inhibition in brain tumors. Elife 6, e19671 10.7554/eLife.19671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murrow L., Malhotra R. and Debnath J (2015). ATG12–ATG3 interacts with Alix to promote basal autophagic flux and late endosome function. Nat. Cell Biol. 17, 300-310. 10.1038/ncb3112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nazio F., Bordi M., Cianfanelli V., Locatelli F. and Cecconi F (2019). Autophagy and cancer stem cells: molecular mechanisms and therapeutic applications. Cell Death Differ. 26, 690-702. 10.1038/s41418-019-0292-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen H. G., Yang J. C., Kung H.-J., Shi X.-B., Tilki D., Lara P. N., DeVere White R. W., Gao A. C. and Evans C. P (2014). Targeting autophagy overcomes Enzalutamide resistance in castration-resistant prostate cancer cells and improves therapeutic response in a xenograft model. Oncogene 33, 4521-4530. 10.1038/onc.2014.25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicastri M. C., Rebecca V. W., Amaravadi R. K. and Winkler J. D (2018). Dimeric quinacrines as chemical tools to identify PPT1, a new regulator of autophagy in cancer cells. Mol. Cell. Oncol. 5, e1395504 10.1080/23723556.2017.1395504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolaou S. and Machesky L. M (2020). The stressful tumour environment drives plasticity of cell migration programmes, contributing to metastasis. J. Pathol. 250, 612-623. 10.1002/path.5395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noman M. Z., Janji B., Kaminska B., Van Moer K., Pierson S., Przanowski P., Buart S., Berchem G., Romero P., Mami-Chouaib F. et al. (2011). Blocking hypoxia-induced autophagy in tumors restores cytotoxic T-Cell activity and promotes regression. Cancer Res. 71, 5976-5986. 10.1158/0008-5472.CAN-11-1094 [DOI] [PubMed] [Google Scholar]
- Noman M. Z., Parpal S., Van Moer K., Xiao M., Yu Y., Viklund J., De Milito A., Hasmim M., Andersson M., Amaravadi R. K. et al. (2020). Inhibition of Vps34 reprograms cold into hot inflamed tumors and improves anti–PD-1/PD-L1 immunotherapy. Sci. Adv. 6, eaax7881 10.1126/sciadv.aax7881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Hara M. H., Karasic T. B., Vasilevskaya I., Redlinger M., Loaiza-Bonilla A., Teitelbaum U. R., Giantonio B. J., Damjanov N., Reiss K. A., Rosen M. A. et al. (2017). Phase II trial of the autophagy inhibitor hydroxychloroquine with FOLFOX and bevacizumab in front line treatment of metastatic colorectal cancer. J. Clin. Oncol. 35, 3545-3545. 10.1200/JCO.2017.35.15_suppl.3545 [DOI] [Google Scholar]
- Paoli P., Giannoni E. and Chiarugi P (2013). Anoikis molecular pathways and its role in cancer progression. Biochim. Biophys. Acta Mol. Cell Res. 1833, 3481-3498. 10.1016/j.bbamcr.2013.06.026 [DOI] [PubMed] [Google Scholar]
- Pasquier B. (2015). SAR405, a PIK3C3/Vps34 inhibitor that prevents autophagy and synergizes with MTOR inhibition in tumor cells. Autophagy 11, 725-726. 10.1080/15548627.2015.1033601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasquier B., El-Ahmad Y., Filoche-Rommé B., Dureuil C., Fassy F., Abecassis P.-Y., Mathieu M., Bertrand T., Benard T., Barrière C. et al. (2015). Discovery of (2 S)-8-[(3 R)-3-methylmorpholin-4-yl]-1-(3-methyl-2-oxobutyl)-2-(trifluoromethyl)-3,4-dihydro-2 H -pyrimido[1,2- a ]pyrimidin-6-one: a novel potent and selective inhibitor of Vps34 for the treatment of solid tumors. J. Med. Chem. 58, 376-400. 10.1021/jm5013352 [DOI] [PubMed] [Google Scholar]
- Patel S., Hurez V., Nawrocki S. T., Goros M., Michalek J., Sarantopoulos J., Curiel T. and Mahalingam D (2016). Vorinostat and hydroxychloroquine improve immunity and inhibit autophagy in metastatic colorectal cancer. Oncotarget 7, 59087-59097. 10.18632/oncotarget.10824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peinado H., Alečković M., Lavotshkin S., Matei I., Costa-Silva B., Moreno-Bueno G., Hergueta-Redondo M., Williams C., García-Santos G., Ghajar C. M. et al. (2012). Melanoma exosomes educate bone marrow progenitor cells toward a pro-metastatic phenotype through MET. Nat. Med. 18, 883-891. 10.1038/nm.2753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellegrini P., Strambi A., Zipoli C., Hägg-Olofsson M., Buoncervello M., Linder S. and De Milito A (2014). Acidic extracellular pH neutralizes the autophagy-inhibiting activity of chloroquine. Autophagy 10, 562-571. 10.4161/auto.27901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng Y.-F., Shi Y.-H., Ding Z.-B., Ke A.-W., Gu C.-Y., Hui B., Zhou J., Qiu S.-J., Dai Z. and Fan J (2013). Autophagy inhibition suppresses pulmonary metastasis of HCC in mice via impairing anoikis resistance and colonization of HCC cells. Autophagy 9, 2056-2068. 10.4161/auto.26398 [DOI] [PubMed] [Google Scholar]
- Qiang L., Zhao B., Ming M., Wang N., He T.-C., Hwang S., Thorburn A. and He Y.-Y (2014). Regulation of cell proliferation and migration by p62 through stabilization of Twist1. Proc. Natl. Acad. Sci. USA 111, 9241-9246. 10.1073/pnas.1322913111 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Qin W., Li C., Zheng W., Guo Q., Zhang Y., Kang M., Zhang B., Yang B., Li B., Yang H. et al. (2015). Inhibition of autophagy promotes metastasis and glycolysis by inducing ROS in gastric cancer cells. Oncotarget 6, 39839-39854. 10.18632/oncotarget.5674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu X., Yu J., Bhagat G., Furuya N., Hibshoosh H., Troxel A., Rosen J., Eskelinen E.-L., Mizushima N., Ohsumi Y. et al. (2003). Promotion of tumorigenesis by heterozygous disruption of the beclin 1 autophagy gene. J. Clin. Invest. 112, 1809-1820. 10.1172/JCI20039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rangwala R., Leone R., Chang Y. C., Fecher L. A., Schuchter L. M., Kramer A., Tan K.-S., Heitjan D. F., Rodgers G., Gallagher M. et al. (2014a). Phase I trial of hydroxychloroquine with dose-intense temozolomide in patients with advanced solid tumors and melanoma. Autophagy 10, 1369-1379. 10.4161/auto.29118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rangwala R., Chang Y. C., Hu J., Algazy K. M., Evans T. L., Fecher L. A., Schuchter L. M., Torigian D. A., Panosian J. T., Troxel A. B. et al. (2014b). Combined MTOR and autophagy inhibition: phase I trial of hydroxychloroquine and temsirolimus in patients with advanced solid tumors and melanoma. Autophagy 10, 1391-1402. 10.4161/auto.29119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao S., Tortola L., Perlot T., Wirnsberger G., Novatchkova M., Nitsch R., Sykacek P., Frank L., Schramek D., Komnenovic V. et al. (2014). A dual role for autophagy in a murine model of lung cancer. Nat. Commun. 5, 3056 10.1038/ncomms4056 [DOI] [PubMed] [Google Scholar]
- Ravenhill B. J., Boyle K. B., von Muhlinen N., Ellison C. J., Masson G. R., Otten E. G., Foeglein A., Williams R. and Randow F (2019). The cargo receptor NDP52 initiates selective autophagy by recruiting the ULK complex to cytosol-invading bacteria. Mol. Cell 74, 320-329.e6. 10.1016/j.molcel.2019.01.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebecca V. W. and Amaravadi R. K (2016). Emerging strategies to effectively target autophagy in cancer. Oncogene 35, 1-11. 10.1038/onc.2015.99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebecca V. W., Nicastri M. C., Fennelly C., Chude C. I., Barber-Rotenberg J. S., Ronghe A., McAfee Q., McLaughlin N. P., Zhang G., Goldman A. R. et al. (2019). PPT1 promotes tumor growth and is the molecular target of chloroquine derivatives in cancer. Cancer Discov. 9, 220-229. 10.1158/2159-8290.CD-18-0706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez A., Durán A., Selloum M., Champy M.-F., Diez-Guerra F. J., Flores J. M., Serrano M., Auwerx J., Diaz-Meco M. T. and Moscat J (2006). Mature-onset obesity and insulin resistance in mice deficient in the signaling adapter p62. Cell Metab. 3, 211-222. 10.1016/j.cmet.2006.01.011 [DOI] [PubMed] [Google Scholar]
- Ronan B., Flamand O., Vescovi L., Dureuil C., Durand L., Fassy F., Bachelot M.-F., Lamberton A., Mathieu M., Bertrand T. et al. (2014). A highly potent and selective Vps34 inhibitor alters vesicle trafficking and autophagy. Nat. Chem. Biol. 10, 1013-1019. 10.1038/nchembio.1681 [DOI] [PubMed] [Google Scholar]
- Sánchez-Martín P., Sou Y., Kageyama S., Koike M., Waguri S. and Komatsu M. (2020). NBR 1-mediated p62-liquid droplets enhance the Keap1-Nrf2 system. EMBO Rep. 21, e48902 10.15252/embr.201948902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanz L. (1999). The interaction of p62 with RIP links the atypical PKCs to NF-kappa B activation. EMBO J. 18, 3044-3053. 10.1093/emboj/18.11.3044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanz L. (2000). The atypical PKC-interacting protein p62 channels NF-kappa B activation by the IL-1-TRAF6 pathway. EMBO J. 19, 1576-1586. 10.1093/emboj/19.7.1576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott E. C., Maziarz R. T., Spurgeon S. E., Medvedova E., Gajewski J., Reasor-Heard S., Park B., Kratz A., Thomas G. V., Loriaux M. et al. (2017). Double autophagy stimulation using chemotherapy and mTOR inhibition combined with hydroxychloroquine for autophagy modulation in patients with relapsed or refractory multiple myeloma. Haematologica 102, e261-e265. 10.3324/haematol.2016.162321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senft D. and Ronai Z. A (2016). Adaptive stress responses during tumor metastasis and dormancy. Trends in Cancer 2, 429-442. 10.1016/j.trecan.2016.06.004 [DOI] [PubMed] [Google Scholar]
- Shao S., Li S., Qin Y., Wang X., Yang Y., Bai H., Zhou L., Zhao C. and Wang C (2014). Spautin-1, a novel autophagy inhibitor, enhances imatinib-induced apoptosis in chronic myeloid leukemia. Int. J. Oncol. 44, 1661-1668. 10.3892/ijo.2014.2313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharifi M. N., Mowers E. E., Drake L. E., Collier C., Chen H., Zamora M., Mui S. and Macleod K. F (2016). Autophagy promotes focal adhesion disassembly and cell motility of metastatic tumor cells through the direct interaction of paxillin with LC3. Cell Rep. 15, 1660-1672. 10.1016/j.celrep.2016.04.065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma G., Guardia C. M., Roy A., Vassilev A., Saric A., Griner L. N., Marugan J., Ferrer M., Bonifacino J. S. and DePamphilis M. L (2019). A family of PIKFYVE inhibitors with therapeutic potential against autophagy-dependent cancer cells disrupt multiple events in lysosome homeostasis. Autophagy 15, 1694-1718. 10.1080/15548627.2019.1586257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi T.-T., Yu X.-X., Yan L.-J. and Xiao H.-T (2017). Research progress of hydroxychloroquine and autophagy inhibitors on cancer. Cancer Chemother. Pharmacol. 79, 287-294. 10.1007/s00280-016-3197-1 [DOI] [PubMed] [Google Scholar]
- Singh R., Xiang Y., Wang Y., Baikati K., Cuervo A. M., Luu Y. K., Tang Y., Pessin J. E., Schwartz G. J. and Czaja M. J (2009). Autophagy regulates adipose mass and differentiation in mice. J. Clin. Invest. 119, 3329-3339. 10.1172/JCI35541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith A. G. and Macleod K. F (2019). Autophagy, cancer stem cells and drug resistance. J. Pathol. 247, 708-718. 10.1002/path.5222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sosa M. S., Bragado P. and Aguirre-Ghiso J. A (2014). Mechanisms of disseminated cancer cell dormancy: an awakening field. Nat. Rev. Cancer 14, 611-622. 10.1038/nrc3793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starobinets H., Ye J., Broz M., Barry K., Goldsmith J., Marsh T., Rostker F., Krummel M. and Debnath J (2016). Antitumor adaptive immunity remains intact following inhibition of autophagy and antimalarial treatment. J. Clin. Invest. 126, 4417-4429. 10.1172/JCI85705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strohecker A. M., Guo J. Y., Karsli-Uzunbas G., Price S. M., Chen G. J., Mathew R., McMahon M. and White E (2013). Autophagy sustains mitochondrial glutamine metabolism and growth of BrafV600E-driven lung tumors. Cancer Discov. 3, 1272-1285. 10.1158/2159-8290.CD-13-0397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudo T., Maruyama M. and Osada H (2000). p62 Functions as a p38 MAP kinase regulator. Biochem. Biophys. Res. Commun. 269, 521-525. 10.1006/bbrc.2000.2333 [DOI] [PubMed] [Google Scholar]
- Sui X., Chen R., Wang Z., Huang Z., Kong N., Zhang M., Han W., Lou F., Yang J., Zhang Q. et al. (2013). Autophagy and chemotherapy resistance: a promising therapeutic target for cancer treatment. Cell Death Dis. 4, e838-e838. 10.1038/cddis.2013.350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun D., Wu R., Zheng J., Li P. and Yu L (2018). Polyubiquitin chain-induced p62 phase separation drives autophagic cargo segregation. Cell Res. 28, 405-415. 10.1038/s41422-018-0017-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svenning S., Lamark T., Krause K. and Johansen T (2011). Plant NBR1 is a selective autophagy substrate and a functional hybrid of the mammalian autophagic adapters NBR1 and p62/SQSTM1. Autophagy 7, 993-1010. 10.4161/auto.7.9.16389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taddei M., Giannoni E., Fiaschi T. and Chiarugi P (2012). Anoikis: an emerging hallmark in health and diseases. J. Pathol. 226, 380-393. 10.1002/path.3000 [DOI] [PubMed] [Google Scholar]
- Takamura A., Komatsu M., Hara T., Sakamoto A., Kishi C., Waguri S., Eishi Y., Hino O., Tanaka K. and Mizushima N (2011). Autophagy-deficient mice develop multiple liver tumors. Genes Dev. 25, 795-800. 10.1101/gad.2016211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang A. H. and Rando T. A (2014). Induction of autophagy supports the bioenergetic demands of quiescent muscle stem cell activation. EMBO J. 33, 2782-2797. 10.15252/embj.201488278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorburn J., Horita H., Redzic J., Hansen K., Frankel A. E. and Thorburn A (2009). Autophagy regulates selective HMGB1 release in tumor cells that are destined to die. Cell Death Differ. 16, 175-183. 10.1038/cdd.2008.143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tittarelli A., Janji B., Van Moer K., Noman M. Z. and Chouaib S (2015). The selective degradation of synaptic connexin 43 protein by hypoxia-induced autophagy impairs natural killer cell-mediated tumor cell killing. J. Biol. Chem. 290, 23670-23679. 10.1074/jbc.M115.651547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towers C. G., Fitzwalter B. E., Regan D., Goodspeed A., Morgan M. J., Liu C.-W., Gustafson D. L. and Thorburn A (2019). Cancer cells upregulate NRF2 Signaling to adapt to autophagy inhibition. Dev. Cell 50, 690-703.e6. 10.1016/j.devcel.2019.07.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turco E., Witt M., Abert C., Bock-Bierbaum T., Su M.-Y., Trapannone R., Sztacho M., Danieli A., Shi X., Zaffagnini G. et al. (2019). FIP200 claw domain binding to p62 promotes autophagosome formation at ubiquitin condensates. Mol. Cell 74, 330-346.e11. 10.1016/j.molcel.2019.01.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vargas J. N. S., Wang C., Bunker E., Hao L., Maric D., Schiavo G., Randow F. and Youle R. J (2019). Spatiotemporal control of ULK1 activation by NDP52 and TBK1 during selective autophagy. Mol. Cell 74, 347-362.e6. 10.1016/j.molcel.2019.02.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vera-Ramirez L., Vodnala S. K., Nini R., Hunter K. W. and Green J. E (2018). Autophagy promotes the survival of dormant breast cancer cells and metastatic tumour recurrence. Nat. Commun. 9, 1944 10.1038/s41467-018-04070-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogl D. T., Stadtmauer E. A., Tan K.-S., Heitjan D. F., Davis L. E., Pontiggia L., Rangwala R., Piao S., Chang Y. C., Scott E. C. et al. (2014). Combined autophagy and proteasome inhibition. Autophagy 10, 1380-1390. 10.4161/auto.29264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J. and Wu G. S (2014). Role of autophagy in cisplatin resistance in ovarian cancer cells. J. Biol. Chem. 289, 17163-17173. 10.1074/jbc.M114.558288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C., Liang C.-C., Bian Z. C., Zhu Y. and Guan J.-L (2013a). FIP200 is required for maintenance and differentiation of postnatal neural stem cells. Nat. Neurosci. 16, 532-542. 10.1038/nn.3365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Martins I., Ma Y., Kepp O., Galluzzi L. and Kroemer G (2013b). Autophagy-dependent ATP release from dying cells via lysosomal exocytosis. Autophagy 9, 1624-1625. 10.4161/auto.25873 [DOI] [PubMed] [Google Scholar]
- Warr M. R., Binnewies M., Flach J., Reynaud D., Garg T., Malhotra R., Debnath J. and Passegué E (2013). FOXO3A directs a protective autophagy program in haematopoietic stem cells. Nature 494, 323-327. 10.1038/nature11895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei H., Wei S., Gan B., Peng X., Zou W. and Guan J.-L (2011). Suppression of autophagy by FIP200 deletion inhibits mammary tumorigenesis. Genes Dev. 25, 1510-1527. 10.1101/gad.2051011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei Y., Zou Z., Becker N., Anderson M., Sumpter R., Xiao G., Kinch L., Koduru P., Christudass C. S., Veltri R. W. et al. (2013). EGFR-mediated beclin 1 phosphorylation in autophagy suppression, tumor progression, and tumor chemoresistance. Cell 154, 1269-1284. 10.1016/j.cell.2013.08.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei H., Wang C., Croce C. M. and Guan J.-L (2014). p62/SQSTM1 synergizes with autophagy for tumor growth in vivo. Genes Dev. 28, 1204-1216. 10.1101/gad.237354.113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- White E. (2012). Deconvoluting the context-dependent role for autophagy in cancer. Nat. Rev. Cancer 12, 401-410. 10.1038/nrc3262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitehouse C. A., Waters S., Marchbank K., Horner A., McGowan N. W. A., Jovanovic J. V., Xavier G. M., Kashima T. G., Cobourne M. T., Richards G. O. et al. (2010). Neighbor of Brca1 gene (Nbr1) functions as a negative regulator of postnatal osteoblastic bone formation and p38 MAPK activity. Proc. Natl. Acad. Sci. USA 107, 12913-12918. 10.1073/pnas.0913058107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wild P., Farhan H., McEwan D. G., Wagner S., Rogov V. V., Brady N. R., Richter B., Korac J., Waidmann O., Choudhary C. et al. (2011). Phosphorylation of the autophagy receptor optineurin restricts salmonella growth. Science 333, 228-233. 10.1126/science.1205405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf J., Dewi D. L., Fredebohm J., Müller-Decker K., Flechtenmacher C., Hoheisel J. D. and Boettcher M (2013). A mammosphere formation RNAi screen reveals that ATG4A promotes a breast cancer stem-like phenotype. Breast Cancer Res. 15, R109 10.1186/bcr3576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto K., Venida A., Yano J., Biancur D. E., Kakiuchi M., Gupta S., Sohn A. S. W., Mukhopadhyay S., Lin E. Y., Parker S. J. et al. (2020). Autophagy promotes immune evasion of pancreatic cancer by degrading MHC-I. Nature 581, 100-105. 10.1038/s41586-020-2229-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Y., Xu Z., Dai S., Qian L., Sun L. and Gong Z (2016). Targeting autophagy to sensitive glioma to temozolomide treatment. J. Exp. Clin. Cancer Res. 35, 23 10.1186/s13046-016-0303-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang A., Rajeshkumar N. V., Wang X., Yabuuchi S., Alexander B. M., Chu G. C., Von Hoff D. D., Maitra A. and Kimmelman A. C (2014). Autophagy is critical for pancreatic tumor growth and progression in tumors with p53 alterations. Cancer Discov. 4, 905-913. 10.1158/2159-8290.CD-14-0362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S.-W., Ping Y.-F., Jiang Y.-X., Luo X., Zhang X., Bian X.-W. and Yu P.-W (2016). ATG4A promotes tumor metastasis by inducing the epithelial-mesenchymal transition and stem-like properties in gastric cells. Oncotarget 7, 39279-39292. 10.18632/oncotarget.9827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh A. C. and Ramaswamy S (2015). Mechanisms of cancer cell dormancy--another hallmark of cancer? Cancer Res. 75, 5014-5022. 10.1158/0008-5472.CAN-15-1370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeo S. K., Wen J., Chen S. and Guan J.-L (2016). Autophagy differentially regulates distinct breast cancer stem-like cells in murine models via EGFR/Stat3 and Tgf /Smad signaling. Cancer Res. 76, 3397-3410. 10.1158/0008-5472.CAN-15-2946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue Z., Jin S., Yang C., Levine A. J. and Heintz N (2003). Beclin 1, an autophagy gene essential for early embryonic development, is a haploinsufficient tumor suppressor. Proc. Natl. Acad. Sci. USA 100, 15077-15082. 10.1073/pnas.2436255100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaffagnini G. and Martens S (2016). Mechanisms of selective autophagy. J. Mol. Biol. 428, 1714-1724. 10.1016/j.jmb.2016.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaffagnini G., Savova A., Danieli A., Romanov J., Tremel S., Ebner M., Peterbauer T., Sztacho M., Trapannone R., Tarafder A. K. et al. (2018). p62 filaments capture and present ubiquitinated cargos for autophagy. EMBO J. 37, e98308 10.15252/embj.201798308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang T. and Ghaemmaghami S (2016). Global analysis of cellular protein flux quantifies the selectivity of basal autophagy. Autophagy 12, 1411-1412. 10.1080/15548627.2016.1190891 [DOI] [PMC free article] [PubMed] [Google Scholar]