ABSTRACT
Many captive Asian elephant populations are not self-sustaining, possibly due in part to obesity-related health and reproductive issues. This study investigated relationships between estimated body composition and metabolic function, inflammatory markers, ovarian activity (females only) and physical activity levels in 44 Asian elephants (n=35 females, n=9 males). Deuterium dilution was used to measure total body water from which fat mass (FM) and fat-free mass (FFM) could be derived to estimate body composition. Serum was analyzed for progestagens and estradiol (females only), deuterium, glucose, insulin and amyloid A. Physical activity was assessed by an accelerometer placed on the elephant's front leg for at least 2 days. Relative fat mass (RFM) – the amount of fat relative to body mass – was calculated to take differences in body size between elephants into consideration. Body fat percentage ranged from 2.01% to 24.59%. Male elephants were heavier (P=0.043), with more FFM (P=0.049), but not FM (P>0.999), than females. For all elephants, estimated RFM (r=0.45, P=0.004) was positively correlated with insulin. Distance walked was negatively correlated with age (r=−0.46, P=0.007). When adjusted for FFM and age (P<0.001), non-cycling females had less fat compared with cycling females, such that for every 100 kg increase in FM, the odds of cycling were 3 times higher (P<0.001). More work is needed to determine what an unhealthy amount of fat is for elephants; however, our results suggest higher adiposity may contribute to metabolic perturbations.
KEY WORDS: Obesity, Body composition, Elephantidae, Reproduction, Walking
Highlighted Article: Investigation of the association between estimated body fat, health and activity in zoo Asian elephants suggests higher adiposity may contribute to metabolic perturbations, which may have additional down-stream health concerns.
INTRODUCTION
The Asian elephant (Elephas maximus Linnaeus 1758) is an endangered species, so captive breeding is one means of protecting it from extinction (Hoffmann et al., 2010; Conde et al., 2011). Worldwide, there are up to 16,000 Asian elephants under human care (e.g. zoos, circuses, logging and tourist camps) (Brown, 2019). However, many captive elephant populations are not self-sustaining, in part because of health and reproductive issues (Brown, 2000; Clubb and Mason, 2002; Lewis et al., 2010; Thitaram, 2012). Therefore, emphasis has been placed on understanding the underpinnings of morbidity and poor reproduction in this species (Keele and Ediger, 2011).
Previous studies suggest that some health and reproductive problems in zoo elephants may be related to excess adiposity. A body condition score (BCS) is a standard measure of obesity in elephants, and is based on visual assessment (Wemmer et al., 2006; Fernando et al., 2009; Treiber et al., 2012; Wijeyamohan et al., 2015; Morfeld et al., 2016). Elephants under human care appear to have higher BCSs (Schiffmann et al., 2018) and greater body mass compared with free-ranging elephants (Ange et al., 2001; Schiffmann et al., 2019a,b). In addition, possibly as a result of greater energy stores, zoo-born females begin cycling earlier compared with their wild counterparts (Sukumar, 2003; Glaeser et al., 2012; Holt et al., 2014), similar to girls with obesity reaching menarche at earlier ages (Biro et al., 2012). It has long been a concern that elephants in zoos are ‘obese’ (Clubb and Mason, 2002; Hatt and Clauss, 2006; Clubb et al., 2008; Mason et al., 2009). Recently, comprehensive surveys of North American and European zoos confirmed that over 65% and 56%, respectively, of adult Asian elephants are overweight or obese based on BCS measures (Morfeld et al., 2016; Schiffmann et al., 2018).
The link between obesity and co-morbidities, including metabolic perturbations, has been well established in mammals. For example, dogs with obesity have increased fasting concentrations of insulin, insulin peak response and overall total insulin secretion (Mattheeuws et al., 1984). Obesity is associated with insulin resistance in the horse (Vick et al., 2006) and type 2 diabetes in non-human primates (Wang et al., 2019; Chavez et al., 2009). Further, obesity and related metabolic perturbations may contribute to reproductive impairments (Vick et al., 2006; Clark et al., 1995). In elephants under human care, higher body condition metrics have often been associated with metabolic derangements in both African (Morfeld and Brown, 2016, 2017) and Asian elephants (Norkaew et al., 2018, 2019). The ratio of glucose to insulin (glucose:insulin) is an important metabolic measure, with a lower value indicating more insulin is required to clear similar concentrations of glucose from the blood. The glucose:insulin ratio has been shown to be negatively associated with BCS in US zoo elephants (Morfeld and Brown, 2017), and male (Norkaew et al., 2019) and female (Norkaew et al., 2018) Asian elephants in Thailand. In addition, US zoo African elephants, but not Asian elephants, with higher BCS and body mass index (BMI) are more likely to experience ovarian acyclicity (Morfeld and Brown, 2016; Freeman et al., 2009). Thus, higher body condition in elephants may be associated with health concerns.
The majority of elephant studies examining the relationship between obesity and metabolic health have been based on BCS, which is a subjective measure (Schiffmann et al., 2017) and may not reflect total adiposity (Chusyd et al., 2019). We recently showed that most Asian elephant BCS systems capture the elephant's body size rather than relative fat mass (RFM) (Chusyd et al., 2019). RFM is biologically more relevant than absolute fat mass (FM) as it accounts for differences in body size between elephants (i.e. two individuals both have 500 kg of FM, but the first individual weighs 2000 kg while the second weighs 4000 kg – they have the same absolute FM but different amounts of RFM and overall body composition). Also, fat is not only distributed under the skin (subcutaneous fat), which is what is being scored (plus musculature) when using BCS, but also distributed around the internal organs (visceral fat). Visceral fat, compared with subcutaneous fat, is associated with greater metabolic dysfunction (Chusyd et al., 2016). Thus, quantifying body composition, rather than relying on a visual assessment of external fat cover, will likely provide a clearer understanding of the relationship between adiposity and metabolic health. Using deuterium dilution, we previously estimated body composition in African elephants, and showed that RFM was correlated with metabolic measures (Chusyd et al., 2018). Further work is needed to understand how actual fatness relates to physiological function in Asian elephants.
There is a strong interest in the relationship between body composition and various health parameters in elephants. In this study, we evaluated relationships between adiposity and serum glucose, serum insulin, inflammatory markers, ovarian cyclicity status (females only) and activity levels in male and female zoo Asian elephants and compared results with those in African elephants. We hypothesized that greater amounts of relative adiposity would be positively associated with metabolic and inflammatory markers, and negatively associated with physical activity. In females, we hypothesized non-cycling elephants would have greater relative adiposity compared with cycling elephants.
MATERIALS AND METHODS
Animals
This study was approved by the Institutional Animal Care and Use Committees of the National Zoo, the University of Alabama at Birmingham (UAB) and participating zoos. A total of 35 female and nine male Asian elephants (≥8 years of age; Table 1) at nine zoos were studied. Female elephants were not pregnant, although three had calves aged 3 years or younger and were still lactating. Ovarian cyclicity status (cycling, non-cycling; follicular, luteal) was based on progestagen analyses of longitudinal serum samples using a validated assay (Brown et al., 2004), with data provided by participating zoos via personal communication. Males were not in musth according to keeper records, indicating a lack of physical signs (no temporal gland drainage, urine dribbling) and low testosterone concentrations.
Table 1.
Body composition of female and male Asian elephants
Deuterium dilution
As previously described (Chusyd et al., 2018), elephants were weighed to the nearest 1 or 5 pounds (0.45 or 2.27 kg), depending on the precision of the institution's scale. To determine background isotope enrichment, venous blood (∼9 ml) was collected from an ear or a leg vein before the deuterated water was administered. The site of blood collection depended on the institution's preference; however, for an individual elephant, blood samples were collected from only one anatomical location (i.e. always from the ear or always from the leg). Thereafter, a dose (99.9% atom percent excess) of deuterium oxide (0.05 ml D2O kg−1 of mass; DLM-4-1000, Cambridge Isotopes, Tewksbury, MA, USA) was administered orally using bread as the vehicle (White Mountain, Publix, Birmingham, AL, USA). Bread was weighed (to the nearest 0.01 g; Pioneer scale, Ohaus, Pine Brook, NJ, USA), deuterated water was added, and the bread was reweighed to determine the dose of deuterated water. Each elephant received four to six pieces of bread with approximately 40–50 g of deuterated water per piece. All zoos were asked not to provide elephants with their regular diet after the late afternoon of the day before deuterated water administration. All zoos complied with this request, except one zoo, as described below. It was not known at what time each individual elephant finished the food they were provided, and this likely varied among individuals both within and among zoos. The following morning, food was withheld until after the elephant received their bread with deuterated water. This was not possible to do at one zoo, which had seven elephants with body composition data, as elephants had access to food 24 h a day. Blood sampling continued at ∼24, 120, 240, 360 and 480 h after deuterium administration. Blood was centrifuged within 30 min of collection and the serum was aliquoted and frozen at a minimum of −20°C until shipped on dry ice overnight to UAB. Samples were kept in a frost-free −80°C freezer until analysis.
Isotope ratio mass spectroscopy (Finnigan Delta V Advantage, Thermo Fisher Scientific, Waltham, MA, USA) analysis was conducted by the UAB Nutrition Obesity Research Center's Metabolism Core with guidance and support from the Energetics Research Group at the University of Aberdeen. As previously described (Chusyd et al., 2018), the blood sample collected prior to deuterated water administration was used to determine the elephant's background 2H enrichment (i.e. naturally occurring 2H levels in the elephant reflective of their food and water sources), Ebg, while the serial blood draws, collected at ∼24, 120, 240, 360 and 480 h after deuterium administration, were used to calculate the elephant's initial 2H enrichment, Ei, i.e. the elephant's deuterium enrichment, above background levels, after the elephant received their deuterated water. This value cannot simply be determined by taking a blood sample immediately after dosing the elephant because it takes time for the deuterium to exchange with the hydrogens in the water molecules throughout the body. Therefore, this value was calculated based on the back-extrapolation method as described by Coward (1990). In brief, the enrichment of each sample, minus Ebg, was plotted, with time on the x-axis, and log(deuterium enrichment) on the y-axis. Then, based on these sample points, a best-fit line was constructed and back-extrapolated to time 0 (i.e. the y-intercept, when the elephant ingested its bolus of deuterated water). This value was reverted from log form and background enrichment was added back in to estimate the 2H enrichment at time 0, Ei. Ultimately, Ebg and Ei were used, in conjunction with the moles of deuterated water administered orally to the elephant, Molinj,, and the 2H enrichment of the deuterated water administered Einj, to calculate deuterium dilution space (Nd) (Eqns 1 and 2) (Speakman, 1997). Nd is considered to reflect total body water (TBW) content after accounting for deuterium exchanging with non-aqueous molecules (4% exchange rate) (Eqn 3), which is then converted to FFM by using the mammalian hydration constant (0.73) (Eqn 4). FM is then inferred by subtracting FFM from body mass (Eqn 5).
Body composition calculations
The following equations were used. First, Nd in moles was calculated:
| (1) |
where Molinj was calculated by dividing the amount of deuterated water administered by the molecular mass of deuterated water. To convert this to Nd in g:
| (2) |
Nd in g was then used to calculate TBW:
| (3) |
TBW was subsequently used to estimate body composition on the basis of the general allometric equation for mammals (Pace and Rathbun, 1945; Wang et al., 1999):
| (4) |
and FM was calculated as:
| (5) |
RFM was determined by the residual for each elephant when FM was regressed on body mass. As noted above, RFM refers to the amount of fat an elephant has after body mass is accounted for, as total FM and FFM normally increase with body size. For this reason, the magnitude of RFM, rather than overall FM, may be more biologically relevant when investigating potential health concerns associated with adiposity.
Morphometric information
Height of the elephant was determined by having the elephant stand next to a bollard inside the barn, and then a keeper would draw a line on the bollard that was approximately equivalent to the shoulder height of the elephant. After the elephant was no longer in the area, using a measuring tape, the distance between the ground and the mark on the bollard was recorded. This value represented the elephant's height. Body length of the elephant was determined by stretching a measuring tape along the side of the elephant from the base of the elephant's tail to the front of the trunk.
Serum analyses
Serum insulin was analyzed using a solid-phase, two-site bovine insulin enzyme immunoassay (EIA; 10-1201-01, Mercodia, Uppsala, Sweden) validated for elephants (Morfeld and Brown, 2016). Estradiol was measured in samples collected during the follicular phase of the cycle using an ultra-sensitive estradiol radioimmunoassay (DSL4800, Immunotech, Prague, Czech Republic). Samples with duplicate coefficients of variation (CVs) exceeding 10% were re-analyzed, so intra-assay EIA CVs were <10%.
Serum glucose was measured in singlicate by use of an automated glucose analyzer (Stanbio Sirrus, Stanbio Laboratories, Boerne, TX, USA). Serum amyloid A (SAA) was measured using an RX Daytona automated clinical chemistry analyzer (Randox Industries-US Ltd, Kearneysville, WV, USA) with commercially available reagents, calibrators (0.1–500 mg l−1) and two-level controls (Eiken Chemical Co. Ltd, Tokyo, Japan).
Monitoring physical activity
An accelerometer (Actigraph wGT3X, Actigraph, Pensacola, FL, USA) was placed in two industrial-strength plastic bags and then inside a waterproof protective case. The case was then inserted into a pouch on a customized bracelet (Delta Rigging, Hurst, TX, USA) placed on the front leg of each elephant (Fig. S1). The accelerometer was oriented such that the y positive axis was oriented up, and the z positive axis was oriented in the direction the elephant walked forward. The bracelet was worn for at least 2 days from the time the elephants left the barn in the morning until they were brought in at the end of the day (∼6–9 h day−1). Each elephant was also observed for a minimum of 20 min per observation period and the animal's activities documented.
Step counts were determined by graphing the raw z-axis data and counting each peak, which corresponded to a step. This technique was validated on a subset of the study population (n=22). Direct observations of steps were compared with the graphed peaks and found to be in agreement on the basis of a Bland Altman plot. The intraclass correlation coefficient (ICC) was in good agreement between directly observed steps and steps counted by the accelerometer [ICC (2,1)=0.987, P<0.0001].
A standardized walk test was implemented to determine average stride length per elephant. While each elephant walked a premeasured distance [58–200 feet (17.68–60.96 m) depending on institution] 3 times, starting and ending times and steps were recorded. A step equated to when the leg with the bracelet was lifted up, was moved forward along the z-axis, and then was placed down at a point different from the starting position. The distance of the standardized walk test divided by the number of steps taken to traverse the premeasured distance represented the average stride length and was averaged across the three tests per elephant. Average stride length was multiplied by the step count to determine total distance traveled. Because accelerometers were worn for different lengths of time, average distance traveled per hour was used in all analyses.
BCS
A single BCS was assigned to each elephant using the index created by Fernando et al. (2009). Scores for animals 201–228 were from an earlier study (Chusyd et al., 2019); additional elephants in this study were scored by D.E.C.
Dominance status
For females only, dominance status was determined based on keeper information, including whether an elephant was the matriarch of the herd.
Statistical analyses
Statistical analyses were performed using R statistical software (R version 3.5.2 and RStudio version 1.1.423) and were specified before examining data unless otherwise stated. Although 44 elephants were included in this study, six elephants (4 females, 2 males) were excluded from body composition statistical analyses because the amount of deuterated water ingested could not be determined and it was not possible to accurately calculate body composition. Calves derive most of their nutrients from the mother's milk until 3 years of age (Sukumar, 2003); therefore, body composition analyses were conducted with and without the three elephants with calves because the impact of lactation on hydration state is not known.
To test the hypothesis that adiposity predicts cycling status, we used generalized estimating equations (GEEs) to perform regression analysis of cyclicity status (cycling or not cycling) on FM with adjustment for FFM and age, with a random effect for zoo (n=35). GEE models were used because the dependent variable was binary, and to account for the random effect of residing in the same zoo. Thus, zoo ID was treated as a random effect in all models to adjust for correlation of factors related to residing in the same zoo. The assumption that the relationship between age and cycling status was linear was tested using a Box–Tidwell transformation test. This relationship was not significant (P>0.05); therefore, we can assume linearity between age and cycling status in this population. FM, FFM and age were included as continuous variables. The primary model was then conducted adjusted for FFM only. To further investigate the role of age, we determined a common age range in both cycling and non-cycling elephants (30–49 years of age), and then ran the primary GEE model adjusted for FFM (cycling: n=5; non-cycling: n=9). After we examined the data, we conducted secondary sensitivity analyses on the primary logistic model and included dominance status and whether the elephants were housed with male elephants. Dominance status was included as a dichotomized variable (female elephant was dominant or non-dominant), and then female elephants were characterized as not housed with male elephants (n=5), housed with males with direct contact (n=15), or housed with males without direct contact (n=15). Lastly, we examined the relationship between cycling status and RFM, unadjusted and adjusted for age. Although it is likely that males and females differ in terms of RFM, because of the small sample size for males, RFM was derived by regressing FM on body mass independent of sex.
To test the hypothesis that body composition is correlated with metabolic health and activity levels, Pearson correlations between estimated RFM, FM, FFM, age, body mass, glucose, insulin and distance walked were conducted. BCS was also included in correlation analyses. In other species, excess adipose tissue is known to be associated with an inflammatory state; thus, Spearman correlations were run between estimated RFM and FM and SAA. Spearman correlations were conducted because of the non-normal distribution of SAA. Partial correlations were conducted between FM and glucose and insulin, adjusted for FFM, between distance walked and glucose and insulin, adjusted for FFM, and between distance walked and estimated RFM and FM, adjusted for age. When correlations included only the variables age, body mass, glucose or insulin, the entire sample population was used (females: n=35; males: n=9); however, when correlations included body composition variables (FM, FFM, estimated RFM), sample size was n=31 for females, and n=7 for males. When correlations included distance walked, the sample size was n=32 for females, and n=7 for males. Although relative FM is generally the more biologically relevant measure, absolute FM was included in correlation analyses because of the general relationships between FM and glucose, insulin and certain inflammatory biomarkers.
To test the hypothesis that body composition and metabolic health differ between cycling and non-cycling elephants, t-tests were used to compare the means of age, body mass, FM, FFM, RFM, BCS, height, body length, glucose, insulin, estradiol and distance walked by cyclicity status. Wilcoxon tests were used to compare the means of SAA by cyclicity status because of its non-normal distribution. Fisher's exact test was used to compare the proportion of nulliparous elephants by cyclicity status.
To test the hypothesis that body composition and metabolic health differ between males and females, t-tests were used to compare the means of age, height, BCS and body length. Wilcoxon tests were used to compare the means of body mass, FM, FFM, RFM, glucose, insulin, distance walked and SAA because of their non-normal distributions.
To test species differences, the female Asian elephant data were then combined with female African elephant data from our previous study (Chusyd et al., 2018). This was done to provide a more comprehensive overview of the relationship between body composition and health in the Elephantidae family, as African and Asian elephants differ in susceptibility to various health concerns. With the data from both species combined, GEE models regressed cycling status on FM, adjusted for FFM, and adjusted for FFM and age, with zoo as a random effect. t-tests were conducted to determine whether there were species differences in the mean values of FM, glucose, insulin and SAA, while a linear regression model was used to investigate species differences in estimated RFM. Significance level was determined at P<0.05 (2-tailed).
Models with FM adjusted for FFM, and those with estimated RFM address the same necessity of accounting for the elephant's body size when examining relationships between FM and other variables of interest. In GEE models, we show analyses both ways, whereas estimated RFM is preferred for scatter plots where covariates are not possible.
RESULTS
Body mass and composition
For all elephants, body fat percentage averaged 9.75±4.85% (mean±s.d., range: 2.01–24.59%; n=38). Body fat percentage averaged 10.05±5.00% (2.01–24.59%; n=31) for females and 8.43±3.88% (4.23–14.15%; n=7) for males. Body composition was estimated based on the elephant's deuterium washout curve (Fig. S2A), which indicated that the deuterium equilibrated completely and rapidly with the rest of the body water, leading to single pool kinetics (Fig. S2B).
Descriptive statistics and tests for differences by sex are presented in Table 2 and Table S1. Males were younger (P=0.019), taller (P<0.001) and longer (P=0.015) than females. Males weighed more (P=0.043) and had more FFM than females (P=0.049), but there were no significant differences in FM by sex (P>0.999), indicating that females were relatively fatter than males. Indeed, females trended to have greater RFM compared with males (P=0.094). We did not find a significant difference in BCS between females and males (P=0.607).
Table 2.
Characteristics of the Asian elephant study sample

Body composition and ovarian cycling status
Descriptive statistics and tests for differences by cycling status are presented in Table 3 and Table S2. Non-cycling elephants were older than cycling elephants (P<0.001), had a lower BCS (P=0.017), and were more likely to be nulliparous (P=0.030). There was no significant difference between non-cycling and cycling elephants in terms of absolute FM (P=0.393) or FFM (P=0.146), but non-cycling elephants showed a trend towards having less RFM compared with cycling elephants (P=0.078).
Table 3.
Characteristics of the female Asian elephant study sample

Predictors of cycling status analyzed by GEE in females (n=31) are shown in Table 4 and Table S3. For the primary model, which was adjusted for FFM and age, for every 100 kg increase in FM per 100 kg of FFM, the elephant's odds of cycling were 3.00 times higher (P<0.001) (Fig. 1A). Similar odds were observed when examining the relationship between RFM and cycling status, adjusted for age (adjusted odds ratio, AOR: 2.36, P=0.020). The relationship between cycling status and FM remained significant when the model was adjusted for FFM only (AOR: 1.44, P=0.016), with similar results between cycling status and RFM (AOR: 1.60, P<0.001). Secondary sensitivity analyses were then conducted. After inclusion of male housing, dominance status and estradiol to the primary model (adjusted for FFM and age), FM remained significant (AOR: 2.83, P<0.001; AOR: 3.09, P<0.001; and AOR: 4.08, P=0.026, respectively). Body mass, unadjusted or adjusted for age, was not associated with cycling status (P=0.260; 0.340, respectively). Although non-cycling elephants tended to have less fat than cycling elephants, when adjusted for FFM and age, the estimate means did not reach significance (P=0.112) (Fig. 2). Patterns were similar after excluding elephants with calves under 3 years of age (P=0.060).
Table 4.
Odds ratios for FM and estimated RFM in generalized estimating equation (GEE) models to predict cycling status in Asian elephants

Fig. 1.

Predicted probability of cycling versus fat mass for Asian elephants. (A) Predicted probability of cycling, with FM unadjusted, adjusted by fat-free mass (FFM; at mean FFM=2991.0 kg), and adjusted by FFM and age (mean age 31.2 years). (B) Predicted probability of cycling by age, when the model was adjusted by FFM and age (at a mean FFM of 2991.0 kg). Data are shown for elephants of the mean study population age (31.2 years), those with ages above this mean (‘old’, mean age 43.7 years, n=17) and those with ages below this mean (‘young’, mean age 19.4 years, n=18).
Fig. 2.
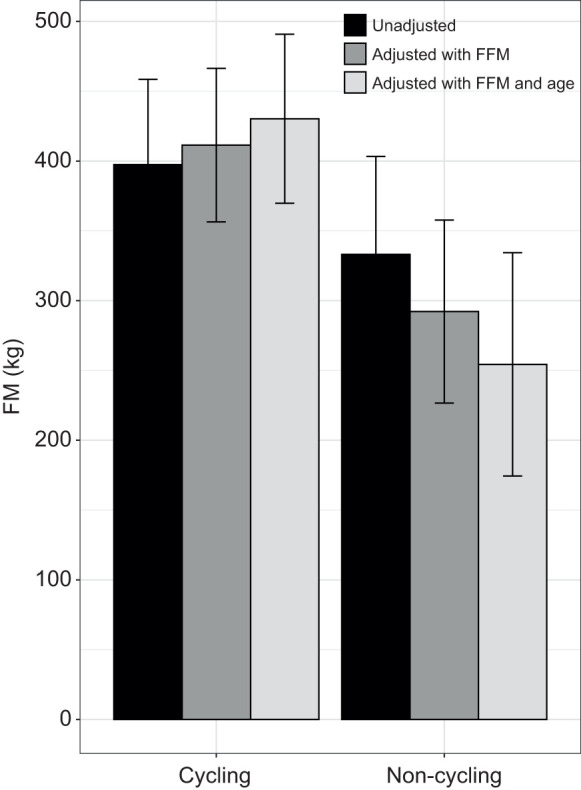
Estimated means for FM versus cycling status. Data are shown with FM unadjusted, adjusted by FFM, and adjusted by FFM and age for all female Asian elephants with body composition data. Cycling elephants: n=22; non-cycling elephants: n=13.
The relationship between the probability of cycling and FM was then further examined by age in two ways. First, a common age range for cycling and non-cycling elephants (30–49 years of age) was identified, and then the relationship between cycling status and FM adjusted for FFM in this age range was examined. The model did not converge because it had a perfect separation. The top five elephants with the most estimated RFM were all cycling elephants, while the rest of the elephants analyzed in this age group were not cycling. Within this age group, those elephants that were cycling had significantly higher adjusted FM (P=0.003), with an estimated RFM mean of 167.7 kg compared with −53.7 kg for non-cycling elephants. Second, we split the elephants into two categories, old (defined as above the mean age of 31.2 years) and young (defined as below the mean age) to graphically examine the relationship between the probability of cycling and FM adjusted for FFM by age (Fig. 1B). Older elephants needed more FM relative to FFM to have a higher likelihood of cycling (above 50%), whereas for younger elephants, their likelihood of cycling was already high regardless of their FM.
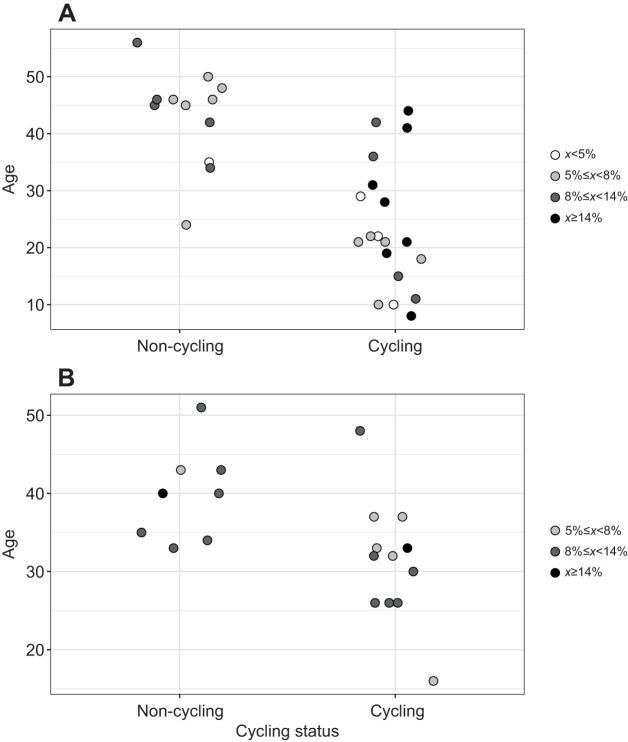
When Asian elephant data from the present study were combined with previous African elephant data (Chusyd et al., 2018), FM did not predict cycling status, adjusted for FFM and age (P=0.105), or FFM only (P=0.169), but RFM nearly reached significance (P=0.064). Nor did FM predict cycling status adjusted for FFM (P=0.210), when using the common age range of 30–49 years for the combined species data. Body composition distribution is graphically depicted by species in terms of age and cycling status in Fig. 3.
Fig. 3.
Body fat percentage according to cycling status and age. Data are shown for female Asian (A) and African (B) elephants. The highest body fat percentage (≥14%) represents elephants with the top 20% body fat percentage, which correlated with insulin values 1 s.d. above the mean. The lowest body fat percentage (<5%) represents elephants with the lowest 20% body fat percentage. The middle cutoff at 8% represents the median body fat percentage for this study population. Asian cycling elephants: n=19, Asian non-cycling elephants: n=12; African cycling elephants: n=12, African non-cycling elephants: n=8.
Body composition and estradiol concentration
Unadjusted estradiol was not a significant predictor of estimated RFM (P=0.081), nor was estradiol when adjusted for age (P=0.096). Because adipose tissue can contribute to circulating levels of estrogens, the relationship between FM and estradiol was examined. Estradiol, unadjusted and adjusted for age as a covariate in the model, did not predict FM (P=0.430, P=0.459, respectively) (Table S4). However, when excluding elephants with calves aged 3 years and younger, estradiol, unadjusted and adjusted for age as a covariate in the model, did predict FM (P=0.035, P=0.025, respectively) (Table S4).
Body composition, glucose, insulin, SAA and activity levels
Adipose tissue is known to contribute to glucose, insulin and some inflammatory biomarker concentrations; thus, correlations with RFM and absolute FM were conducted. While the correlations between estimated RFM (Fig. 4A) and FM with glucose were not significant (r=−0.08, P=0.654; r=−0.11, P=0.517, respectively), estimated RFM (Fig. 4B) and FM were positively correlated with insulin (r=0.45, P=0.004; r=0.36, P=0.025, respectively). There was no correlation between estimated RFM (Fig. 4C) or FM with SAA (ρ=−0.12, P=0.468; ρ=0.00, P=0.982, respectively). Estimated RFM (Fig. 4D) was not correlated with distance walked (r=−0.27, P=0.124), but estimated RFM had a marginally significant, negative relationship with distance walked when corrected for age (r=−0.33, P=0.064). Distance walked was not correlated with glucose (r=−0.06, P=0.722) or insulin (r=−0.25, P=0.125) (Fig. 4E), but was negatively associated with age (r=−0.35, P=0.028) (Fig. 4F). Glucose was positively correlated with insulin (r=0.32, P=0.035).
Fig. 4.
Correlations between relative fat mass, metabolic biomarkers and distance walked, and between distance walked and insulin and age in Asian elephants. (A–C) Relative fat mass (RFM) versus glucose (A), insulin (B) and serum amyloid A (SAA, mg dl−1; C). (D) RFM versus distance walked. (E,F) Distance walked in relation to insulin (E) and age (F). RFM was determined by the residual for each elephant when FM was regressed on body mass. Analyses were done with females and males together. Females are represented by black circles and males by gray circles. Different symbols are used for representative purposes only. Regression lines are included where correlations were significant. A–C: n=38; D–F: n=34.
There were no differences in the means of estimated RFM (P=0.090), FM (P=0.900), glucose (P=0.100) or SAA (P=0.600) between female Asian and African elephants. However, female Asian elephants had higher insulin concentrations compared with African elephants (P=0.008).
DISCUSSION
This study examined relationships between adiposity, glucose, insulin, SAA, ovarian cycle status and physical activity levels in zoo Asian elephants. Body fat percentage ranged from approximately 2% to 25% in our Asian elephant sample population, which was a greater range than previously observed in African elephants (5–16% body fat). There were no differences in fat measures between males and females or between species. In European zoos, BCSs in Asian and African elephants also were similar (Schiffmann et al., 2018), whereas in North American zoos, Asian elephants had comparatively higher BCSs than those of African elephants, and females had higher BCSs compared with males (Morfeld et al., 2016; Schiffmann et al., 2018), raising questions about how differences in zoo management affect body condition, adiposity and subsequent health consequences.
Our hypothesis that adiposity in Asian elephants would be positively correlated with metabolic status was supported by the finding of a positive correlation between estimated RFM and insulin concentration. In African elephants, estimated RFM correlated with glucose, and trended in the expected positive direction with insulin (P=0.128) (Chusyd et al., 2018). Adipose tissue is known to be associated with glucose–insulin regulation and expression. Higher adiposity can disrupt glucose homeostasis (Lindström, 2007; Yan et al., 2011a). Glucose is the preferred metabolic fuel for mammalian tissues and in part regulates the metabolism of other metabolic substrates (Grossman, 1986). In a healthy state, glucose concentration is tightly regulated by insulin, balancing the output of hepatic glucose with the uptake of glucose by the skeletal muscle, adipose tissue and liver (de Luca and Olefsky, 2006). In the elephant, glucose values typically range between 60 and 116 mg dl−1 (Fowler and Mikota, 2008). However, in a state of insulin resistance, glucose storage and metabolism are compromised. Concomitantly, there is an increase in blood glucose and a decrease in glucose storage, which can result in hyperglycemia. To prevent hyperglycemia and maintain normal glucose tolerance, compensatory hyperinsulinemia ensues. In the present study, insulin values ranged from 0.05 to 3.00 ng ml−1, while non-fasted insulin concentrations as high as 6.24 ng ml−1 (corrected units) have been reported in Asian elephants (Morfeld and Brown, 2017). The link between higher adiposity and insulin resistance has been well documented in other animals, such as the horse (Vick et al., 2007), pig (Sébert et al., 2005) and dog (German et al., 2009); therefore, it is reasonable to posit that this relationship may be present in elephants. Indeed, the majority of these elephants had greater than expected FM for their body mass (i.e. positive estimated RFM values). Thus, there should be concern that with continued adipose accrual, an elephant could develop a diabetic-like state and experience metabolic dysfunction.
Chronic inflammation is associated with obesity and obesity-related infertility in various species (Robker et al., 2011; Yan et al., 2011b; Jimenez-Gomez et al., 2013). However, our results do not support a relationship between inflammation and high adiposity or acyclicity. There were no significant differences in SAA values between cycling and non-cycling elephants, nor was SAA correlated with estimated RFM. The SAA reference interval for clinically healthy Asian elephants is 0–47.5 mg l−1 (Isaza et al., 2014). Based on that, five elephants between 8 and 47 years of age had elevated SAA levels: three females and two males. At the time of blood sampling, one elephant had a non-life-threatening abscess and the second highest SAA concentration. This injury may have contributed to her SAA levels (Rael et al., 2009), but the cause of higher SAA in the other animals is unknown. Regardless, SAA may not capture a low-grade inflammatory state associated with high adiposity, or the elephants in the present study may not have had sufficiently high levels of adiposity to induce a state of low-grade inflammation.
It has been suggested that elephants in zoos, because of spatial constraints, do not walk enough (Doyle and Roy, 2006; Poole and Granli, 2009). In the wild, elephants move for a variety of reasons, including resource acquisition and mate searching, with total distances traversed dependent on the habitat and season (i.e. wet versus dry season). Free-ranging African and Asian elephants have been observed to walk between 0.01 and 1.15 km on an hourly basis (McKay, 1973; Wyatt and Eltringham, 1974; Whitehouse and Schoeman, 2003; Slotow and Van Dyk, 2004), and, for Asian elephants, between 0.09 to 9.52 km on a daily basis (Bahar et al., 2018; Alfred et al., 2012). We found that elephants walked between 0.03 and 2.79 km on an hourly basis, which is similar to findings of previous studies (Leighty et al., 2009; Holdgate et al., 2016). Irrespective of why elephants walk, the simple act of walking may be beneficial, as physical activity is known to improve health, independent of weight loss (Di Blasio et al., 2014). Distance walked was nearly significant with RFM, such that increased walking may contribute to decreases in RFM. Therefore, it may be possible that voluntary walking is sufficient, it itself, to reduce RFM; however, previous studies have demonstrated that voluntary walking was not associated with lower BCS in elephants (Holdgate et al., 2016). Regardless, there are certainly other benefits for increased walking, and zoos should continue to maximize this activity. Increased activity is known to protect against a loss of physical function (Simonsick et al., 2005), and can reduce pain associated with arthritis (Roddy et al., 2005). Our results demonstrated that older elephants walk less, and it is likely that there is a high prevalence of foot and joint issues in this population (Csuti et al., 2008; Lewis et al., 2010). Therefore, an emphasis should be placed on getting older elephants moving.
Previous work has suggested that a high level of adiposity is a contributing factor to reproductive problems in elephants, as high BCS (Morfeld and Brown, 2016; Schiffmann et al., 2018), high BMI (Freeman et al., 2009) and greater body mass (Schiffmann et al., 2019c) have been associated with ovarian acyclicity. This relationship is typically observed in African elephants, which have rates of abnormal ovarian cycles exceeding 50% across all age categories, compared with Asian elephants where 27% exhibit no or irregular cycles, but most are older and post-reproductive (Brown et al., 2016). Thus, it was not surprising that there was a significant relationship between age and acyclicity in the Asian elephants of this study. However, the finding that non-cycling elephants, after adjustment for FFM and age, had less FM than did cycling elephants was not expected. Interestingly, despite high rates of ovarian cycle problems, there were no relationships between higher levels of adiposity (FM, RFM) and cyclicity status in African elephants (Chusyd et al., 2018). None of the elephants in our Asian or African elephant studies appeared to be excessively thin, and only two elephants appeared to be underweight based on visual observations (i.e. BCS=3–4 using Fernando BCS system), so ovarian cycle status speculatively may be related to where FM is deposited. Compared with subcutaneous fat, visceral fat is more closely associated with metabolic perturbations, which is thought to contribute to infertility (Diamanti-Kandarakis and Bergiele, 2001). Thus, the discord between findings in the present and previous studies relying on BCS may be due to differences in the methodologies used to assess body condition or the sample population.
We initially hypothesized that ovarian acyclicity in Asian elephants might be related to a disruption in the hormonal milieu. Brown and colleagues (2004) demonstrated that estradiol levels are approximately 3 times higher in non-cycling than in cycling Asian elephants. Decreases in estradiol alter body mass and distribution of fat (Geary and Lovejoy, 2008), while estradiol increases oxygen consumption (Wade et al., 1985), and decreases food intake (Wade, 1976). Further, ovariectomized rats, mice and cats have greater overall FM compared with amounts in age-matched controls (McElroy and Wade, 1987; Nguyen et al., 2004; Hong et al., 2009). However, we did not find higher estradiol concentrations in non-cycling elephants, nor did we observe a relationship between estradiol and estimated RFM.
The majority of acyclic Asian elephants are older (Brown et al., 2016; Brown, 2019; present study). Therefore, to further explore that relationship, we re-analyzed data by dividing elephants into ‘old’ or ‘young’ categories, i.e. above or below the mean age of this study population. Indeed, we found substantially different relationships with cycling status and FM adjusted for FFM between age groups. For any given amount of FM adjusted for FFM, young elephants' probability of cycling was higher than that of the older elephants. In addition, it appears that older elephants may require higher levels of FM relative to their body size to have a higher probability of cycling, while young elephants can cycle regardless of FM. Speculatively, this may reflect species-specific differences in the obligatory level of fat required for reproductive cycling to continue, with Asian elephants needing more RFM to cycle normally later in life, while it does not seem to matter in African elephants. Ultimately, it is unlikely that only one factor contributes to a non-cycling status, but rather a multitude of contributors, such as reproductive pathologies, nulliparous status and social factors, are important (Aupperle et al., 2008; Hermes et al., 2008; Dow et al., 2011).
Although deuterium has been used as a tracer of water to assess body composition for nearly 75 years (Schloerb et al., 1950), the deuterium dilution technique is relatively new for elephants. Like all measurement systems, there are inherent measurement errors. Body composition by deuterium dilution hinges on the proportion of water in FFM. Because the exact relationship is not known for the Asian elephant, we used the average mammalian hydration constant, for females and males. Thus, it may be possible that our body fat percentages are not the true values. Extreme body fat percentages may be attributed to the elephant having an extreme value, in addition to measurement error. But, because any disagreement in hydration constant would result in a linear transformation, we believe the ranking of the data, and the relative relationships and trends observed hold true. Some have postulated that water and bone mineral content may vary more in women than in men (Bunt et al., 1989; Vogel and Friedl, 1992), but the literature reviews do not support this notion (Fogelholm and van Marken Lichtenbelt, 1997; Chumlea et al., 2007). In addition, concerns have been raised about how gut contents may impact TBW in herbivores (Torbit et al., 1985). TBW is composed not only of the water in bodily tissues but also of the water found in the gut. Whether gut fill is assumed to be constant or variable, not accounting for gut fill will lead to an underestimate of body fat. If gut contents are assumed to be constant among individuals, this is similar to differences observed based on the hydration constant used (Wang et al., 1999), as we previously outlined (Chusyd et al., 2018), and this would not affect the ranking of the individuals. While non-parametric analyses would not be affected, inferences from parametric analyses might be. The variation in gut fill, in addition to gut moisture content, can be variable among individuals and can affect the results in magnitudes that are relevant. This is particularly true for lean animals. For example, the body fat percentage of animal ID 236 is estimated at 2%. If one assumes that animal's gut contents are 12% of body mass, with an assumed water in the gut contents of 82%, the animal's estimated body fat percentage increases to 3.5%, which is a relative percentage change of 55%. Such effects depend on variable differences (e.g. assumed water in gut contents ranging from 78% to 86%), and may change the ranking of individuals. Nevertheless, in our view, even with the deuterated water administered orally, based on the kinetic data, we believe the impact of variable gut fill overall was not a major factor in the body composition of elephants in this study. Specifically, if the gut fill was behaving as a separate dilution space, the deuterium washout curve characteristics would be different from those observed. A two-compartment model (gut fill being one compartment and the body being the second compartment) would lead to the washout curve having a kink due to separate kinetics of elimination of deuterium from the two compartments. Instead, what we observed was that each washout curve was linear, indicating the gut did not represent a separate pool. In addition, we used the back extrapolation method, rather than the plateau method, which minimizes dependence on a single post-dose measurement that would be susceptible to two-pool kinetics. Of course, reducing variation from any source is necessary, and thus deuterated water was administered in the morning prior to the elephants receiving their normal morning feeding regimen. However, elephants (n=7) at one zoo had ad libitum access to food. There were no clear patterns of FM values amongst these elephants compared with the average, suggesting whether food was withheld or not may not have had a significant effect on fat calculations in this study.
The use of the average mammalian hydration constant may not be appropriate in lactating elephants. Lactating mice, for example, have an approximately 10% greater TBW content than that of non-lactating mice (Król and Speakman, 2003), whereas no significant differences were observed in TBW between lactating and non-lactating cows (Martin and Ehle, 1986). The observed species differences are likely attributed to differences in body size. As the animal gets bigger, the effects of lactation get smaller owing to the scaling of milk production, metabolic rate and water turnover. Although the three females in our study with a calf aged 3 years or younger had comparatively higher rates of water turnover for body mass, they were all still within 2 s.d. of the mean (data not shown). Ultimately, the impact of lactation is not known, but the inclusion or exclusion of these females in the analyses did not alter the primary outcome results.
Obesity is a social creation to describe people with high levels of adiposity, and was originally developed based on mortality data (Dublin and Lotka, 1937). There are no data on the relationship between adiposity levels and mortality in elephants; thus, whether it is equally valuable to categorize elephants as being too fat (i.e. obese) still remains to be determined. Preliminarily, we attempted to identify an adiposity threshold in Asian elephants, whereby exceeding this value may increase an individual's risk for developing co-morbidities. Based on identifying individuals with an insulin value 1 s.d. above the mean (i.e. 1.43 ng ml−1), the adiposity threshold is 14% body fat and equated to elephants within the top 20% in body fat percentage. It should be noted that male elephants likely have a lower body fat percentage threshold, as seen in other species (Webb and Weaver, 1979; Kearns et al., 2002; Wells, 2007), particularly as male elephants had less relative FM and significantly more FFM compared with females. But because of the small male sample size, sexes were ultimately pooled for the adiposity threshold. An adiposity threshold may differ by species. Asian elephants had significantly higher insulin concentrations compared with African elephants, which is in agreement with previous publications (Morfeld and Brown, 2017; Chave et al., 2019). This suggests that Asian elephants may have a lower adiposity threshold, and be more susceptible to developing co-morbidities at similar adiposity levels compared with African elephants. More research is needed to determine whether our adiposity threshold is correct, or whether it should be used with future populations.
Another reasonable consideration is the elephant's evolutionary biology. In the wild, available food quality and quantity vary with season. This likely leads to a cyclicity in levels of adiposity, which may be accompanied by metabolic changes. Indeed, BCS have been observed to be lower in the dry season compared with the wet season in wild Asian elephants (Pokharel et al., 2017), whereas BCS in zoo elephants appears to remain steady throughout the year (Schiffmann et al., 2019a). Elephants in zoos are maintained on a constant nutritional plane, but it may be metabolically beneficial to fluctuate food quality throughout the year to mimic patterns in the wild. There are few data on this topic, however, so further research is warranted.
In conclusion, this study, in conjunction with our previous work in African elephants, suggests that higher adiposity can contribute to metabolic perturbations, but may not be related to abnormal reproductive cycles in zoo elephants. Although an attempt was made to establish an adiposity threshold, it must be emphasized that this is a new technique in elephants, the study involved a relatively small sample size, and there were no data on adiposity and mortality, so future work with larger sample sizes and greater ranges in body condition are needed. In addition, greater adiposity alone does not necessarily mean an individual is less healthy. An elephant's overall health (i.e. age, joint issues, metabolic dysfunction) should also be considered, in the context of their body fatness. Therefore, it may prove more useful in terms of understanding elephant morbidity and mortality to shift the focus from ‘ideal weight/adiposity’ to ‘dangerous weight/adiposity’ on a per elephant basis.
Acknowledgements
The authors thank Dr Barbara Gower, Maryellen Williams, Heather Hunter and Cindy Zeng at the UAB NORC's Metabolism Core for their assistance with hormone assays and mass spectroscopy, and Dr Katie Edwards, Steve Paris and Niki Boisseau at SCBI for inflammatory and estradiol analyses. The authors thank African Lion Safari, Cincinnati Zoo & Botanical Garden, Columbus Zoo & Aquarium, Fort Worth Zoo, Little Rock Zoo, Oklahoma City Zoo, Oregon Zoo, Santa Barbara Zoo and Saint Louis Zoo for their participation in this study. Specifically, a very big thank you to the zoos' elephant keepers and elephants, who made this study possible and enjoyable. A special thank you to the Birmingham Zoo and Pat Flora and his elephant team for their continued support, help and input with method improvement.
Footnotes
Competing interests
The authors declare no competing or financial interests.
Author contributions
Conceptualization: D.E.C., T.R.N., J.R.S., M.J., D.B.A., J.L.B.; Methodology: D.E.C., T.R.N., J.R.S., C.H.; Formal analysis: L.G., S.L.D., C.H., D.B.A.; Data curation: D.E.C.; Writing - original draft: D.E.C.; Writing - review & editing: D.E.C., T.R.N., L.G., S.L.D., J.R.S., C.H., M.J., D.B.A., J.L.B.; Supervision: T.R.N., M.J., D.B.A., J.L.B.; Project administration: D.E.C.
Funding
This work was supported in part by the Smithsonian Institution, the UAB Nutrition Obesity Research Center (P30DK056336), the Diabetes Research Center (P30DK079626), the Nathan Shock Center on Aging (P30AG050886), and the National Heart, Lung, and Blood Institute (T32HL105349 to D.E.C.). Deposited in PMC for release after 12 months.
Supplementary information
Supplementary information available online at https://jeb.biologists.org/lookup/doi/10.1242/jeb.219543.supplemental
References
- Alfred R., Ahmad A. H., Payne J., Williams C., Ambu L. N., How P. M. and Goossens B. (2012). Home range and ranging behaviour of Bornean elephant (Elephas maximus borneensis) females. PLoS ONE 7, e31400 10.1371/journal.pone.0031400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ange K., Crissey S. D., Doyle C., Lance K. and Hintz H. (2001). A survey of African (Loxodonta africana) and Asian (Elephas maximus) elephant diets and measured body dimensions compared to their estimated nutrient requirements. Proceedings of the Nutrition Advisory Group 4th Conference on Zoo and Wildlife Nutrition, Lake Buena Vista (ed. Edwards M. S., Lisi K. J., Schlegel M. L. and Bray R. E.). [Google Scholar]
- Aupperle H., Reischauer A., Bach F., Hildebrandt T., Göritz F., Jäger K., Scheller R., Klaue H.-J. and Schoon H.-A. (2008). Chronic endometritis in an Asian elephant (Elephas maximus). J. Zoo Wildl. Med. 39, 107-110. 10.1638/2006-0045.1 [DOI] [PubMed] [Google Scholar]
- Bahar A., Abu Kasim N. H. and Hambali K. (2018). Home range and movement patterns of Asian elephnat (Elephas maximus) in Gua Musang, Kelantan, Malaysia. Malay. Nat. J. 70: 221-232. [Google Scholar]
- Biro F. M., Greenspan L. C. and Galvez M. P. (2012). Puberty in girls of the 21st century. J. Pediatr. Adolesc. Gynecol. 25, 289-294. 10.1016/j.jpag.2012.05.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown J. L. (2000). Reproductive endocrine monitoring of elephants: an essential tool for assisting captive management. Zoo Biol. 19, 347-367. [DOI] [Google Scholar]
- Brown J. L. (2019). Update on comparative biology of elephants: factors affecting reproduction, health and welfare. Reprod. Sci. Anim. Conserv. 1200, 243-273. 10.1007/978-3-030-23633-5_9 [DOI] [PubMed] [Google Scholar]
- Brown J. L., Walker S. L. and Moeller T. (2004). Comparative endocrinology of cycling and non-cycling Asian (Elephas maximus) and African (Loxodonta africana) elephants. Gen. Comp. Endocrinol. 136, 360-370. 10.1016/j.ygcen.2004.01.013 [DOI] [PubMed] [Google Scholar]
- Brown J. L., Paris S., Prado-Oviedo N. A., Meehan C. L., Hogan J. N., Morfeld K. A. and Carlstead K. (2016). Reproductive health assessment of female elephants in North American zoos and association of husbandry practices with reproductive dysfunction in African elephants (Loxodonta africana). PLoS ONE 11, e0145673 10.1371/journal.pone.0145673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunt J. C., Lohman T. G. and Boileau R. A. (1989). Impact of total body water fluctuations on estimation of body fat from body density. Med. Sci. Sports Exerc. 21, 96-100. 10.1249/00005768-198902000-00017 [DOI] [PubMed] [Google Scholar]
- Chave E., Edwards K. L., Paris S., Prado N., Morfeld K. A. and Brown J. L. (2019). Variation in metabolic factors and gonadal, pituitary, thyroid, and adrenal hormones in association with musth in African and Asian elephant bulls. Gen. Comp. Endocrinol. 276, 1-13. 10.1016/j.ygcen.2019.02.005 [DOI] [PubMed] [Google Scholar]
- Chavez A. O., Gastaldelli A., Guardado-Mendoza R., Lopez-Alvarenga J. C., Leland M. M., Tejero M. E., Sorice G. P., Casiraghi F., Davalli A. M., Bastarrachea R. A. et al. (2009). Predictive models of insulin resistance derived from simple morphometric and biochemical indices related to obesity and the metabolic syndrome in baboons. Cardiovasc. Diabetol. 8, 22 10.1186/1475-2840-8-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chumlea W. C., Schubert C., Sun S. and Demerath E. (2007). A review of body water status and the effects of age and body fatness in children and adults. J. Nutr. Health Aging 11, 111. [PubMed] [Google Scholar]
- Chusyd D. E., Wang D., Huffman D. M. and Nagy T. R. (2016). Relationships between rodent white adipose fat pads and human white adipose fat depots. Front. Nutr. 3 10.3389/fnut.2016.00010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chusyd D. E., Brown J. L., Hambly C., Johnson M. S., Morfeld K. A., Patki A., Speakman J. R., Allison D. B. and Nagy T. R. (2018). Adiposity and reproductive cycling status in zoo African elephants. Obesity 26, 103-110. 10.1002/oby.22046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chusyd D. E., Brown J. L., Golzarri-Arroyo L., Dickinson S. L., Johnson M. S., Allison D. B. and Nagy T. R. (2019). Fat mass compared to four body condition scoring systems in the Asian elephant (Elephas maximus). Zoo Biol. 38, 424-433. 10.1002/zoo.21508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark A. M., Ledger W., Galletly C., Tomlinson L., Blaney F., Wang X. and Norman R. J. (1995). Weight loss results in significant improvement in pregnancy and ovulation rates in anovulatory obese women. Hum. Reprod. 10, 2705-2712. 10.1093/oxfordjournals.humrep.a135772 [DOI] [PubMed] [Google Scholar]
- Clubb R. and Mason G. (2002). A Review of the Welfare of Zoo Elephants in Europe. Horsham, UK: RSPCA. [Google Scholar]
- Clubb R., Rowcliffe M., Lee P., Mar K. U., Moss C. and Mason G. J. (2008). Compromised survivorship in zoo elephants. Science 322, 1649-1649. 10.1126/science.1164298 [DOI] [PubMed] [Google Scholar]
- Conde D. A., Flesness N., Colchero F., Jones O. R. and Scheuerlein A. (2011). An emerging role of zoos to conserve biodiversity. Science 331, 1390-1391. 10.1126/science.1200674 [DOI] [PubMed] [Google Scholar]
- Coward W. (1990). Calculation of pool sizes and flux rates. The Doubly Labelled Water Method: Technical Recommendations for Use in Humans. Report of an IDECG Expert Working Group 48-68.
- Csuti B., Sargent E. L. and Bechert U. S. (2008). The Elephant's Foot: Prevention and Care of Foot Conditions in Captive Asian and African Elephants. John Wiley & Sons. [Google Scholar]
- de Luca C. and Olefsky J. M. (2006). Stressed out about obesity and insulin resistance. Nat. Med. 12, 41-42. 10.1038/nm0106-41 [DOI] [PubMed] [Google Scholar]
- Di Blasio A., Izzicupo P., D'Angelo E., Melanzi S., Bucci I., Gallina S., Di Baldassarre A. and Napolitano G. (2014). Effects of patterns of walking training on metabolic health of untrained postmenopausal women. J. Aging Phys. Act 22, 482-489. 10.1123/JAPA.2013-0043 [DOI] [PubMed] [Google Scholar]
- Diamanti-Kandarakis E. and Bergiele A. (2001). The influence of obesity on hyperandrogenism and infertility in the female. Obes. Rev. 2, 231-238. 10.1046/j.1467-789X.2001.00041.x [DOI] [PubMed] [Google Scholar]
- Dow T., Holásková I. and Brown J. L. (2011). Results of the third reproductive assessment survey of North American Asian (Elephas maximus) and African (Loxodonta africana) female elephants. Zoo Biol. 30, 699-711. 10.1002/zoo.20377 [DOI] [PubMed] [Google Scholar]
- Doyle C. and Roy S. (2006). Comments of In Defense of Animals on USDA Docket No. APHIS-2006-0044 “Captive Elephant Welfare”, Citeseer, 1-101.
- Dublin L. I. and Lotka A. J. (1937). Twenty-Five Years of Health Progress. A Study of the Mortality Experience among the Industrial Policy-holders of the Metropolitan Life Insurance Company 1911 to 1935. Metropolitan Life Insurance Company. [Google Scholar]
- Fernando P., Janaka H., Ekanayaka S., Nishantha H. and Pastorini J. (2009). A simple method for assessing elephant body condition. Gajah 31, 29-31. [Google Scholar]
- Fogelholm M. and van Marken Lichtenbelt W. (1997). Comparison of body composition methods: a literature analysis. Eur. J. Clin. Nutr. 51, 495 10.1038/sj.ejcn.1600448 [DOI] [PubMed] [Google Scholar]
- Fowler M. and Mikota S. K. (2008). Biology, Medicine, and Surgery of Elephants. John Wiley & Sons. [Google Scholar]
- Freeman E. W., Guagnano G., Olson D., Keele M. and Brown J. L. (2009). Social factors influence ovarian acyclicity in captive African elephants (Loxodonta africana). Zoo Biol. 28, 1-15. 10.1002/zoo.20187 [DOI] [PubMed] [Google Scholar]
- Geary N. and Lovejoy J. (2008). Sex Differences in Energy Metabolism, Obesity, and Eating Behavior. Sex Differences in the Brain: from Genes to Behavior, pp. 253-274: Oxford University Press. [Google Scholar]
- German A. J., Hervera M., Hunter L., Holden S. L., Morris P. J., Biourge V. and Trayhurn P. (2009). Improvement in insulin resistance and reduction in plasma inflammatory adipokines after weight loss in obese dogs. Domest. Anim. Endocrinol. 37, 214-226. 10.1016/j.domaniend.2009.07.001 [DOI] [PubMed] [Google Scholar]
- Glaeser S. S., Hunt K. E., Martin M. S., Finnegan M. and Brown J. L. (2012). Investigation of individual and group variability in estrous cycle characteristics in female Asian elephants (Elephas maximus) at the Oregon Zoo. Theriogenology 78, 285-296. 10.1016/j.theriogenology.2012.01.014 [DOI] [PubMed] [Google Scholar]
- Grossman S. P. (1986). The role of glucose, insulin and glucagon in the regulation of food intake and body weight. Neurosci. Biobehav. Rev. 10, 295-315. 10.1016/0149-7634(86)90015-1 [DOI] [PubMed] [Google Scholar]
- Hatt J.-M. and Clauss M. (2006). Feeding Asian and African elephants Elephas maximus and Loxodonta africana in captivity. Int. Zoo Yearb. 40, 88-95. 10.1111/j.1748-1090.2006.00088.x [DOI] [Google Scholar]
- Hermes R., Saragusty J., Schaftenaar W., Göritz F., Schmitt D. L. and Hildebrandt T. B. (2008). Obstetrics in elephants. Theriogenology 70, 131-144. 10.1016/j.theriogenology.2008.04.003 [DOI] [PubMed] [Google Scholar]
- Hoffmann M., Hilton-Taylor C., Angulo A., Böhm M., Brooks T. M., Butchart S. H. M., Carpenter K. E., Chanson J., Collen B., Cox N. A. et al. (2010). The impact of conservation on the status of the world's vertebrates. Science 330, 1503-1509. 10.1126/science.1194442 [DOI] [PubMed] [Google Scholar]
- Holdgate M. R., Meehan C. L., Hogan J. N., Miller L. J., Soltis J., Andrews J. and Shepherdson D. J. (2016). Walking behavior of zoo elephants: Associations between GPS-measured daily walking distances and environmental factors, social factors, and welfare indicators. PLoS ONE 11, e0150331 10.1371/journal.pone.0150331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt W. V., Brown J. L. and Comizzoli P. (2014). Reproductive Sciences in Animal Conservation. Springer. [Google Scholar]
- Hong J., Stubbins R. E., Smith R. R., Harvey A. E. and Núñez N. P. (2009). Differential susceptibility to obesity between male, female and ovariectomized female mice. Nutr. J. 8, 11 10.1186/1475-2891-8-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaza R., Wiedner E., Hiser S. and Cray C. (2014). Reference intervals for acute phase protein and serum protein electrophoresis values in captive Asian elephants (Elephas maximus). J. Vet. Diagn. Invest. 26, 616-621. 10.1177/1040638714543923 [DOI] [PubMed] [Google Scholar]
- Jimenez-Gomez Y., Mattison J. A., Pearson K. J., Martin-Montalvo A., Palacios H. H., Sossong A. M., Ward T. M., Younts C. M., Lewis K., Allard J. S. et al. (2013). Resveratrol improves adipose insulin signaling and reduces the inflammatory response in adipose tissue of rhesus monkeys on high-fat, high-sugar diet. Cell Metab. 18, 533-545. 10.1016/j.cmet.2013.09.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kearns C. F., Mckeever K. H., Kumagai K. and Abe T. (2002). Fat-free mass is related to one-mile race performance in elite standardbred horses. Vet. J. 163, 260-266. 10.1053/tvjl.2001.0656 [DOI] [PubMed] [Google Scholar]
- Keele M. and Ediger N. (2011). AZA elephant master plan. AZA Publication, 10285.
- Król E. and Speakman J. R. (2003). Limits to sustained energy intake VII. Milk energy output in laboratory mice at thermoneutrality. J. Exp. Biol. 206, 4267-4281. 10.1242/jeb.00675 [DOI] [PubMed] [Google Scholar]
- Leighty K. A., Soltis J., Wesolek C. M., Savage A., Mellen J. and Lehnhardt J. (2009). GPS determination of walking rates in captive African elephants (Loxodonta africana). Zoo Biol. 28, 16-28. 10.1002/zoo.20199 [DOI] [PubMed] [Google Scholar]
- Lewis K. D., Shepherdson D. J., Owens T. M. and Keele M. (2010). A survey of elephant husbandry and foot health in North American zoos. Zoo Biol. 29, 221-236. 10.1002/zoo.20291 [DOI] [PubMed] [Google Scholar]
- Lindström P. (2007). The physiology of obese-hyperglycemic mice (ob/ob mice). Sci. World J. 7, 666-685. 10.1100/tsw.2007.117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin R. A. and Ehle F. R. (1986). Body composition of lactating and dry Holstein cows estimated by deuterium dilution. J. Dairy Sci. 69, 88-98. 10.3168/jds.S0022-0302(86)80373-3 [DOI] [PubMed] [Google Scholar]
- Mason G., Rowcliffe M., Mar K., Lee P., Moss C. and Clubb R. (2009). Fecundity and population viability in female zoo elephants: Problems and possible solutions. Anim. Welf. 18, 237-247. [Google Scholar]
- Mattheeuws D., Rottiers R., Kaneko J. J. and Vermeulen A. (1984). Diabetes mellitus in dogs: relationship of obesity to glucose tolerance and insulin response. Am. J. Vet. Res. 45, 98-103. [PubMed] [Google Scholar]
- McElroy J. F. and Wade G. N. (1987). Short- and long-term effects of ovariectomy on food intake, body weight, carcass composition, and brown adipose tissue in rats. Physiol. Behav. 39, 361-365. 10.1016/0031-9384(87)90235-6 [DOI] [PubMed] [Google Scholar]
- McKay G. M. (1973). Behavior and Ecology of the Asiatic Elephant in Southeastern Ceylon. Washington: Smithsonian Insitution Press. [Google Scholar]
- Morfeld K. A. and Brown J. L. (2016). Ovarian acyclicity in zoo African elephants (Loxodonta africana) is associated with high body condition scores and elevated serum insulin and leptin. Reprod. Fertil. Dev. 28, 640-647. 10.1071/RD14140 [DOI] [PubMed] [Google Scholar]
- Morfeld K. A. and Brown J. L. (2017). Metabolic health assessment of zoo elephants: Management factors predicting leptin levels and the glucose-to-insulin ratio and their associations with health parameters. PLoS ONE 12, e0188701 10.1371/journal.pone.0188701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morfeld K. A., Meehan C. L., Hogan J. N. and Brown J. L. (2016). Assessment of body condition in African (Loxodonta africana) and Asian (Elephas maximus) elephants in North American zoos and management practices associated with high body condition scores. PLoS ONE 11, e0155146 10.1371/journal.pone.0155146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen P. G., Dumon H. J., Siliart B. S., Martin L. J., Sergheraert R. and Biourge V. C. (2004). Effects of dietary fat and energy on body weight and composition after gonadectomy in cats. Am. J. Vet. Res. 65, 1708-1713. 10.2460/ajvr.2004.65.1708 [DOI] [PubMed] [Google Scholar]
- Norkaew T., Brown J. L., Bansiddhi P., Somgird C., Thitaram C., Punyapornwithaya V., Punturee K., Vongchan P., Somboon N. and Khonmee J. (2018). Body condition and adrenal glucocorticoid activity affects metabolic marker and lipid profiles in captive female elephants in Thailand. PLoS ONE 13, e0204965 10.1371/journal.pone.0204965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norkaew T., Brown J. L., Bansiddhi P., Somgird C., Thitaram C., Punyapornwithaya V., Punturee K., Vongchan P., Somboon N. and Khonmee J. (2019). Influence of season, tourist activities and camp management on body condition, testicular and adrenal steroids, lipid profiles, and metabolic status in captive Asian elephant bulls in Thailand. PLoS ONE 14, e0210537 10.1371/journal.pone.0210537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pace N. and Rathbun E. N. (1945). The body water and chemically combined nitrogen content in relation to fat content. J. Biol. Chem. 158, 685-691. [Google Scholar]
- Pokharel S. S., Seshagiri P. B. and Sukumar R. (2017). Assessment of season-dependent body condition scores in relation to faecal glucocorticoid metabolites in free-ranging Asian elephants. Conserv. Physiol. 5, cox039 10.1093/conphys/cox039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poole J. and Granli P. (2009). Mind and movement: Meeting the interests of elephants. In An Elephant in the Room: The Science and Well Being of Elephants in Captivity ( Forthman D. L., Kane F. L., Hancocks D. and Waldau P. F.), pp. 2-7. Center for Animals and Public Policy, Cummings School of Veterinary Medicine, Tufts University. [Google Scholar]
- Rael L. T., Bar-Or R., Salottolo K., Mains C. W., Slone D. S., Offner P. J. and Bar-Or D. (2009). Injury severity and serum amyloid A correlate with plasma oxidation-reduction potential in multi-trauma patients: a retrospective analysis. Scand. J. Trauma Resuscit. Emerg. Med. 17, 57 10.1186/1757-7241-17-57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robker R.L., Wu L.L.Y. and Yang X. (2011). Inflammatory pathways linking obesity and ovarian dysfunction. J. Reprod. Immunol. 88, 142-148. [DOI] [PubMed] [Google Scholar]
- Roddy E., Zhang W. and Doherty M. (2005). Aerobic walking or strengthening exercise for osteoarthritis of the knee? A systematic review. Ann. Rheum. Dis. 64, 544-548. 10.1136/ard.2004.028746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiffmann C., Clauss M., Hoby S. and Hatt J. M. (2017). Visual body condition scoring in zoo animals–composite, algorithm and overview approaches. J. Zoo Aquarium Res. 5, 1. [Google Scholar]
- Schiffmann C., Clauss M., Fernando P., Pastorini J., Wandler P., Ertl N., Hobys S. and Hatt J. M. (2018). Body condition scores of European zoo elephants (Elephas maximus and Loxodonta africana): Status quo and influencing factors. J. Zoo Aquarium Res. 6, 91-103. [Google Scholar]
- Schiffmann C., Clauss M., Hoby S., Codron D. and Hatt J. M. (2019a). Body Condition Scores (BCS) in European zoo elephants’ (Loxodonta africana and Elephas maximus) lifetimes–a longitudinal analysis. J. Zoo Aquarium Res. 7, 74-86. [Google Scholar]
- Schiffmann C., Clauss M., Hoby S. and Hatt J.-M. (2019b). Weigh and see—Body mass recordings versus body condition scoring in European zoo elephants (Loxodonta africana and Elephas maximus). Zoo Biol. 39, 97-108. 10.1002/zoo.21525 [DOI] [PubMed] [Google Scholar]
- Schiffmann C., Hatt J.-M., Hoby S., Codron D. and Clauss M. (2019c). Elephant body mass cyclicity suggests effect of molar progression on chewing efficiency. Mamm. Biol. 96, 81-86. 10.1016/j.mambio.2018.12.004 [DOI] [Google Scholar]
- Schloerb P. R., Hansen-Friis B. J., Edelman I. S., Solomon A. K. and Moore F. D. (1950). The measurement of total body water in the human subject by deuterium oxide dilution: with a consideration of the dynamics of deuterium distribution. J. Clin. Investig. 29: 1296-1310. 10.1172/JCI102366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sébert S. P., Lecannu G., Kozlowski F., Siliart B., Bard J. M., Krempf M. and Champ M. M.-J. (2005). Childhood obesity and insulin resistance in a Yucatan mini-piglet model: Putative roles of IGF-1 and muscle PPARs in adipose tissue activity and development. Int. J. Obes. 29, 324 10.1038/sj.ijo.0802823 [DOI] [PubMed] [Google Scholar]
- Simonsick E. M., Guralnik J. M., Volpato S., Balfour J. and Fried L. P. (2005). Just get out the door! Importance of walking outside the home for maintaining mobility: findings from the women's health and aging study. J. Am. Geriatr. Soc. 53, 198-203. 10.1111/j.1532-5415.2005.53103.x [DOI] [PubMed] [Google Scholar]
- Slotow R. and Van Dyk G. (2004). Ranging of older male elephants introduced to an existing small population without older males: Pilanesberg National Park. Koedoe 47, 91-104. 10.4102/koedoe.v47i2.82 [DOI] [Google Scholar]
- Speakman J. (1997). Doubly Labelled Water: Theory and Practice. Springer Science & Business Media. [Google Scholar]
- Sukumar R. (2003). The Living Elephants: Evolutionary Ecology, Behaviour, and Conservation. Oxford University Press. [Google Scholar]
- Thitaram C. (2012). Breeding management of captive Asian elephant (Elephas maximus) in range countries and zoos. Jpn. J. Zoo Wildl. Med. 7, 91-96. 10.5686/jjzwm.17.91 [DOI] [Google Scholar]
- Torbit S. C., Carpenter L. H., Alldredge A. W. and Swift D. M. (1985). Mule deer body composition: a comparison of methods. J. Wildl. Management 49, 86-91. 10.2307/3801850 [DOI] [Google Scholar]
- Treiber K., Reppert N. and Ward A. (2012). Transcutaneous Rump Ultrasound of Asian Elephants (Elephas maximus): Body fat, Body Condition and Body Weight. The 7th Crissey Zoological Nutrition Symposium, Raleigh, NC. [Google Scholar]
- Vick M. M., Sessions D. R., Murphy B. A., Kennedy E. L., Reedy S. E. and Fitzgerald B. P. (2006). Obesity is associated with altered metabolic and reproductive activity in the mare: effects of metformin on insulin sensitivity and reproductive cyclicity. Reprod. Fertil. Dev. 18, 609-617. 10.1071/RD06016 [DOI] [PubMed] [Google Scholar]
- Vick M. M., Adams A. A., Murphy B. A., Sessions D. R., Horohov D. W., Cook R. F., Shelton B. J. and Fitzgerald B. P. (2007). Relationships among inflammatory cytokines, obesity, and insulin sensitivity in the horse. J. Anim. Sci. 85, 1144-1155. 10.2527/jas.2006-673 [DOI] [PubMed] [Google Scholar]
- Vogel J. A. and Friedl K. E. (1992). Body fat assessment in women. Sports Med. 13, 245-269. 10.2165/00007256-199213040-00003 [DOI] [PubMed] [Google Scholar]
- Wade G. N. (1976). Sex hormones, regulatory behaviors, and body weight. Adv. Study Behav. 6, 201-279. 10.1016/S0065-3454(08)60085-6 [DOI] [Google Scholar]
- Wade G., Gray J. and Bartness T. (1985). Gonadal influences on adiposity. Int. J. Obes. 9, 83-92. [PubMed] [Google Scholar]
- Wang C., Xiao Y., Wang J., Hou N., Cui W., Hu X., Zeng F., Yuan Y., Ma D., Sun X. et al. (2019). Dynamic changes in insulin and glucagon during disease progression in rhesus monkeys with obesity-related type 2 diabetes mellitus. Diabetes Obes. Metab. 21, 1111-1120. 10.1111/dom.13624 [DOI] [PubMed] [Google Scholar]
- Wang Z., Deurenberg P., Wang W., Pietrobelli A., Baumgartner R. N. and Heysmfield S. B. (1999). Hydration of fat-free body mass: review and critique of a classic body-composition constant. Am. J. Clin. Nutr. 69, 833-841. 10.1093/ajcn/69.5.833 [DOI] [PubMed] [Google Scholar]
- Webb A. I. and Weaver B. M. Q. (1979). Body composition of the horse. Equine Vet. J. 11, 39-47. 10.1111/j.2042-3306.1979.tb01295.x [DOI] [PubMed] [Google Scholar]
- Wells J. C. K. (2007). Sexual dimorphism of body composition. Best Practice Res. Clin. Endocrinol. Metabol. 21, 415-430. 10.1016/j.beem.2007.04.007 [DOI] [PubMed] [Google Scholar]
- Wemmer C., Krishnamurthy V., Shrestha S., Hayek L.-A., Thant M. and Nanjappa K. A. (2006). Assessment of body condition in Asian elephants (Elephas maximus). Zoo Biol. 25, 187-200. 10.1002/zoo.20099 [DOI] [Google Scholar]
- Whitehouse A. M. and Schoeman D. S. (2003). Ranging behaviour of elephants within a small, fenced area in Addo Elephant National Park, South Africa. Afr. Zool. 38, 95-108. 10.1080/15627020.2003.11657197 [DOI] [Google Scholar]
- Wijeyamohan S., Treiber K., Schmitt D. and Santiapillai C. (2015). A visual system for scoring body condition of Asian elephants (Elephas maximus). Zoo Biol. 34, 53-59. 10.1002/zoo.21181 [DOI] [PubMed] [Google Scholar]
- Wyatt J. R. and Eltringham S. K. (1974). The daily activity of the elephant in the Rwenzori National Park, Uganda. Afr. J. Ecol. 12, 273-289. 10.1111/j.1365-2028.1974.tb01037.x [DOI] [Google Scholar]
- Yan X., Huang Y., Zhao J.-X., Long N. M., Uthlaut A. B., Zhu M.-J., Ford S. P., Nathanielsz P. W. and Du M. (2011a). Maternal obesity-impaired insulin signaling in sheep and induced lipid accumulation and fibrosis in skeletal muscle of offspring. Biol. Reprod. 85, 172-178. 10.1095/biolreprod.110.089649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan X., Huang Y., Wang H., Du M., Hess B. W., Ford S. P., Nathanielsz P. W. and Zhu M.-J. (2011b). Maternal obesity induces sustained inflammation in both fetal and offspring large intestine of sheep. Inflamm. Bowel Dis. 17, 1513-1522. 10.1002/ibd.21539 [DOI] [PMC free article] [PubMed] [Google Scholar]