Abstract
Ethnopharmacological relevance
The coronavirus disease 2019 (COVID-19) has formed a global pandemic since late 2019. Benefitting from the application experience of Chinese Medicine (CM) for influenza and SARS, CM has been used to save patients at the early stage of COVID-19 outbreak in China.
Aim of the study
In order to evaluate the efficacy and safety of CM, and compare with Western Medicine (WM) for COVID-19, we conducted a retrospective case series study based on the patients in Wuhan Jinyintan Hospital, Wuhan, China.
Methods
The inclusion and exclusion criteria of data extraction were set for this retrospective study. All patients who were admitted by the Wuhan Jinyintan Hospital between January 17th and February 25th 2020 were considered. In addition, patients enrolled met the severe defined by the guidelines released by the National Health Commission of the People's Republic of China. In these cases included in the study, CM or WM treatment was selected according to the wishes of the patients at the beginning of hospitalization. The patients in CM group were treated with Huashi Baidu granule (137 g po, bid) combined with the injections of Xiyanping (100 mg iv, bid), Xuebijing (100 ml iv, bid) and Shenmai (60 ml iv, qd) according to the syndrome of epidemic toxin blocking the lung in the theory of Traditional Chinese Medicine. The WM group received antiviral therapy (including abidor capsule 0.2 g po, tid; Lopinavir–Ritonavir tablets, 500 mg po, bid), antibiotics (such as cefoperazone 2 g iv, bid; moxifloxacin hydrochloride tablets, 0.4 g po, qd) or corticosteroid therapy (such as methylprednisolone succinate sodium 40 mg iv, qd; prednisone, 30 mg po, qd). In addition, patients in both groups received routine supportive treatment, including oxygen inhalation, symptomatic therapy, and/or human intravenous immunoglobulin, and/or serum albumin, and treatment for underlying diseases. The clinical outcomes were evaluated based on changes related with clinical manifestations, computer tomography (CT) scan images, and laboratory examinations before and after the treatment.
Results
55 severe COVID-19 patients, with 23 in CM group and 32 in WM group, were included for analyzed. There was no case of death, being transferred to ICU, or receiving invasive mechanical ventilation in two groups during hospitalization. The median time of SARS-CoV-2 RNA clearance in CM and WM group were 12 days and 15.5 days respectively, the ratio of nucleic acid negative conversion of CM group at different follow-up time points was significantly higher than that of WM group (HR: 2.281, P = 0.018). Further, the chest CT imaging showed more widely lung lesion opacity absorbed in the CM group. The high sensitivity C-reactive protein and serum ferritin decreased significantly in the CM group (P<0.05). There was no significant difference in adverse events in terms of liver function and renal function between the two groups.
Conclusion
Based on this retrospective analysis from Wuhan Jinyintan Hospital, CM has better effects in SARS-CoV-2 RNA clearance, promoting lung lesion opacity absorbed and reducing inflammation in severe COVID-19 patients, which is effective and safe therapy for treating severe COVID-19 and reducing mortality.
Keywords: Severe COVID-19, SARS-CoV-2, Chinese medicine, Huashi Baidu granule
Graphical abstract
1. Introduction
In December 2019, a sudden epidemic of pneumonia occurred in Wuhan, China. The etiologic agent of the pneumonia was reported to be the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), and the disease was officially named coronavirus disease 2019 (COVID-19) on February 11th, 2020 (Zhu N et al., 2019; Huang et al., 2020; Zhang et al., 2020; Xin et al., 2020; WHO a, 2020). The COVID-19 is characterized by influenza-like symptoms, including fever, cough, severe acute respiratory distress syndrome and, in some cases, death (Li et al., 2020a, Li et al., 2020b). The World Health Organization (WHO) described the ongoing epidemic as a pandemic on March 11th, 2020 (WHO b, 2020). As of October 30th, 2020, a total of 44 888 869 cases and 1 178 475 deaths had been documented globally (WHO c, 2020). The COVID-19 pandemic has become a great threat to global health. (Arshad Ali et al., 2020). Unfortunately, there is still no efficacy and safety therapy.
Chinese Medicine (CM) has been used to prevent and treat new outbreaks of infectious diseases for thousands of years (Li et al., 2020a, Li et al., 2020b). With the application experience of CM for influenza and SARS (Li et al., 2016; Wu et al., 2020; Leung, 2007; Ho et al., 2007; Chen and Nakamura, 2004; Jia and Gao, 2003; Lau et al., 2005; Liu et al., 2012), CM was applied for the treatment of COVID-19 at the beginning of the epidemic of COVID-19 in Wuhan.
China National Medical Task Force of Traditional Chinese Medicine (TCM) for COVID-19 had been formed by the State Administration of Traditional Chinese Medicine of the People's Republic of China (SATCM) on January 24th, 2020. The member of China National Medical Team of Traditional Chinese medicine for COVID-19 (CNMGTCM-COVID19), including medical staff from the emergency and respiratory departments of Guanganmen Hospital and Xiyuan Hospital who have experienced in SARS treatment, are the member of this Task Force.
The protocol had been issued by the National Health Commission (NHC) of the People's Republic of China (PRC) on January 15th, 2020, which has been revised periodically, and practiced throughout China. CNMGTCM-COVID-19 from China Academy of Chinese Medical Sciences took the lead in using CM to treat patients with severe COVID-19 in Wuhan Jinyintan Hospital which is the largest of an infectious disease hospital in Wuhan.
In order to objectively reflect the efficacy and safety of CM on COVID-19 at that time, CNMGTCM-COVID-19 conducted a retrospective review to evaluate the efficacy and safety of CM, comparing with that of WM treatment. The results indicated that CM has better effects in SARS-CoV-2 RNA clearance, promoting lung lesion opacity absorbed and reducing inflammation in severe COVID-19 patients, which is effective and safe therapy for treating severe COVID-19 and reducing mortality.
We also wish the data from this assessment can provide reference to the healthcare providers and a choice for COVID-19 patients.
2. Materials and methods
2.1. Patients
This study was a retrospective case series study, the enrolled patients were met the following inclusion and exclusion criteria.
Inclusion criteria including 1) all patients were admitted between January 17th, 2020 and February 25th, 2020; 2) all patients had complete medical records during their hospitalization. 3) all patients were over 18 years old; 4) all patients were with laboratory confirmed SARS-Cov-2 RNA infection; 5) all patients met the severe defined by the “Diagnosis and Treatment Protocol for Novel Coronavirus Pneumonia” released by NHC of the PRC (NHC, 2020); 6) all patients were treated in the wards in charge of the CNMGTCM-COVID-19 in Wuhan Jinyintan Hospital; 7) CM or WM treatment was selected according to the wishes of the patients at the beginning of hospitalization, and both of them met the treatment protocol defined by the “Diagnosis and Treatment Protocol for Novel Coronavirus Pneumonia” released by NHC of the PRC (NHC, 2020); 8) the clinical manifestation of the patients treated with CM were consistent with the syndrome of epidemic toxin blocking the lung in the theory of TCM.
Exclusion criteria including 1) mild or critical cases of COVID-19; 2) patients who need emergency surgery or other intensive care; 3) mental illness; 4) pregnant or lactating women; 5) patients participating in clinical trials for other intervention.
Patients were discharged if two repeated tests of SARS-CoV-2 virus polymerase chain reaction (PCR) test of pharyngeal swabs turned out to be negative, and the chest lesion in computed tomography (CT) was improved.
2.2. Drug administration
The patients in CM group were treated with Huashi Baidu granule (137 g po, bid) combined with the injections of Xiyanping (100 mg iv, bid), Xuebijing (100 ml iv, bid) and Shenmai (60 ml iv, qd) according to the syndrome of epidemic toxin blocking the lung in the theory of TCM.
The composition of Huashi Baidu granule is as follows: Ephedrae Herba (Shengmahuang), 6 g; Armeniacae Semen (Xingren), 9 g; Gypsum Fibrosum (Shengshigao), 15 g; Glycyrrhizae Radix (Gancao), 3 g; Pogostemonis Herba (Huoxiang), 10 g; Magnoliae Officmalis Cortex (Houpo), 10 g; Atractlodis Rhizoma (Cangzhu), 15 g; Tsaoko Fructus (Caoguo), 10 g; Pinellinae Rhizoma Praeparatum (Fabanxia), 9 g; Poria (Fuling), 15 g; Rhei Radix et Rhizoma (Shengdahuang), 5 g; Astmgali Radix (Shenghuangqi), 10 g; Descurainiae Semen (Tinglizi), 10 g; Paeoniae Radix rubra (Chishao), 10 g.
Xiyanping injection (SFDA approval number Z20026249, 50mg/piece) was made by sulfonation process of andrographis B extracted from Andrographis Herba (Chuanxinlian), which was provided by Jiangxi Qingfeng Pharmaceutical Co., Ltd. (Ganzhou, China).
Xuebijing injection (SFDA approval number Z20040033 for 10ml/piece) is a combination of five herbs extraction, which are Carthamus tinctorius Linn (Honghua), Paeoniae Radix rubra (Chishao), Chuanxiong Rhizoma (Chuanxiong), Angelicae Sinensis Radix (Danggui), and Salviae Miltiorrhizae (Danshen), which was provided by Tianjin Chase Sun Pharmaceutical Co. Ltd. (Tianjin, China).
Shenmai injection (SFDA approval number Z33020020 for 20ml/piece) was made from Red Ginseng (Hongshen) and Radix Ophiopogonis (Maidong), which was provided by Chiatai Qingchunbao Pharmaceutical Co., Ltd. (Hangzhou, China).
The WM group received antiviral therapy (including abidor capsule 0.2 g po, tid; Lopinavir–Ritonavir tablets, 500 mg po, bid), antibiotics (such as cefoperazone, 2 g iv, bid; moxifloxacin hydrochloride tablets, 0.4 g po, qd) or corticosteroid therapy (such as methylprednisolone succinate sodium 40 mg iv, qd; prednisone, 30 mg po, qd). Supportive therapy in both groups included oxygen inhalation, symptomatic treatment, and/or human intravenous immunoglobulin, and/or serum albumin, and treatment for underlying diseases.
2.3. Assessment
In view of the maximum median time of persistent SARS-CoV-2 RNA infection in the two groups before the implementation of the temporary ward reconstruction plan on March 5th and a systematic review on COVID-19 length of hospital stay (Rees et al., 2020), the clinical treatment period was set at 16 days,the clinical observation period was set at 27 days. The data collected at the time of admission were baseline data. The outcome indexes about the efficacy and safety were collected from the baseline (admission) of patients to the end of 16 days of intervention, including: 1)discharge from hospital; 2) transfer to ICU; 3) the use of invasive mechanical ventilation; 4) number of death; 5) the time of viral clearance; 6) changes of the major symptoms; 7) chest CT findings; 8) laboratory results from baseline.
CT findings were semi-quantitated based on the sign and range of pneumonia lesions on admission and throughout the observation period as an objective indicators of inflammation. All patients’ chest CT images were independently evaluated by two radiologists. When the diagnosis was inconsistent, they reached a reasonable conclusion after discussion and/or consultation. The optimized semi-quantitative scoring system for CT signs was established basing on the previous research methods (Zhao et al., 2020; Ajlan et al., 2014). Briefly, seven CT signs included in this study: ground-glass opacities (GGO), consolidation, mixed GGO with consolidation, bronchial wall thickening, reticulation, sub-pleural bands, and bronchiectasis. Presence one of any of the lesion features is scored as 1, and absence is scored as 0. Secondly, the lesion distribution in the lung is scored according to the following rule: The bilateral lung lobes are divided into 6 areas, the upper, middle, and lower. The upper-middle boundary in the CT axial section is defined at the level of the tracheal bifurcation, and the middle-lower boundary was the maximum transverse section of the right pulmonary vein. Each lung area is scored visually according to the infiltrative range of lesion, which is divided into 0–4 points. Absence of inflammatory lesion was scored as 0, inflammatory area below 25% was scored as 1, between 26% to 50% as 2, between 51% to 75% as 3, and between 76% to 100% as 4. A higher total score for a patient indicated that his/her pneumonia was worse.
The changes of liver and kidney function test results and the occurrence of adverse events were observed as safety outcomes.
2.4. Statistical analysis
The primary analysis in our study was descriptive or inferential statistics, and survival analysis. The continuous variables are expressed as medians (and interquartile range, IQR), and t or Wilcoxon rank sum test was used for inter-group comparison; The categorical variables are presented as frequency and percentage, and Chi-square or Fisher's exact test was used for comparison between groups. Paired t/sign rank or McNemar/McNemar-Bowker test was used within a group. Analysis of covariance or Mantel-Haenszel test was used for baseline correction. The non-nucleic acid negative conversion curve or rate was estimated using the non-parametric Kaplan-Meier method, and was compared between groups by log-rank test, giving the median time for viral shedding and 95% confidence intervals (CIs). The stratified log-rank test and Cox proportional hazards model were used to correct the baseline. In this study, R packages Stats, Rcompanion and survival in version-3.6.2 and SPSS 20.0 were utilized for statistical analysis and verification.
3. Results
3.1. Demographic characteristics
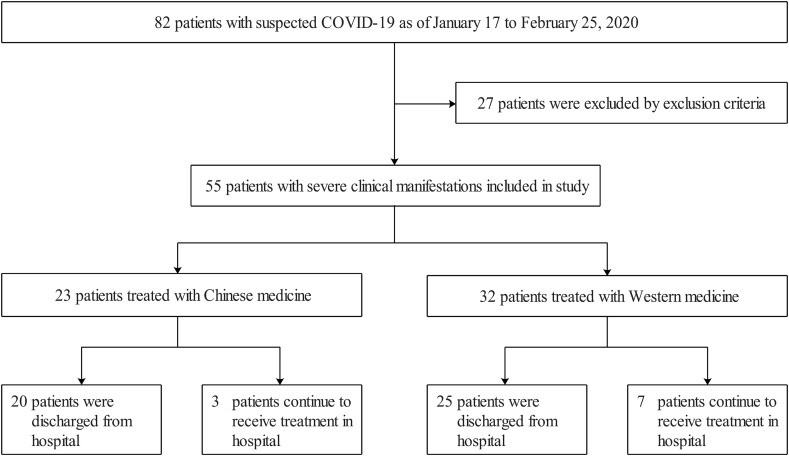
A total of 55 patients met the study conditions (Fig. 1 ). The age ranged from 26 to 77 years old, with a median age of 58 years old (IQR, 43.50-66.50). The CM group included 23 patients, including 12 males and 11 females. The age ranged from 31 to 77 years old, with a median age of 56 years old (IQR, 43–64). In CM group, 10 patients (43.5%) had underlying diseases (Table 1 ). The WM group had 32 patients, including 17 males and 15 females. The age ranged from 26 to 73 years old, with a median of 61.50 years old (IQR, 45.75- 68.00). In WM group, 19 patients (61.3%) had underlying diseases (Table 1). The baseline data of laboratory measurements of the two groups are shown in Table 1.
Fig. 1.
Flow of participants through the study.
Table 1.
Demographic and clinical characteristics of the patients at baseline.
| Characteristic | WM (N=32) | CM (N=23) | Total(N=55) | P value |
|---|---|---|---|---|
| Sex—n/N (%) | >0.999 | |||
| Male | 17/32(53.1) | 12/23(52.2) | 29/55(52.7) | |
| Female | 15/32(46.9) | 11/23(47.8) | 26/55(47.3) | |
| Age (yr)—Median (IQR) | 61.50(45.75-68.00) | 56.00(43.00-64.00) | 58.00(43.50-66.50) | 0.232 |
| Coexisting conditions—n/N (%) | 0.307 | |||
| Yes | 19/31(61.3) | 10/23(43.5) | 29/54(53.7) | |
| Cardiovascular disease | 11/32(34.4) | 2/23(8.7) | 13/55(23.6) | 0.059 |
| Digestive disease | 3/32(9.4) | 1/23(4.3) | 4/55(7.3) | 0.856 |
| Endocrine disease | 6/32(18.8) | 2/23(8.7) | 8/55(14.5) | 0.512 |
| Cancer | 1/32(3.1) | 3/23(13.0) | 4/55(7.3) | 0.384 |
| Nervous system disease | 0/32(0.0) | 0/23(0.0) | 0/55(0.0) | -- |
| Respiratory disease | 1/32(3.1) | 1/23(4.3) | 2/55(3.6) | >0.999 |
| Body temperature, Median (IQR)—(°C) | 36.60(36.40-36.82) | 36.70(36.40-37.05) | 36.60(36.40-36.95) | 0.329 |
| Heart Rate—Median(IQR) | 84.00(77.25-96.00) | 96.00(89.00-101.00) | 90.00(80.00-99.50) | 0.007 |
| Breathing rate—Median (IQR) | 21.00(20.00-22.00) | 22.00(21.50-23.00) | 22.00(20.00-22.00) | 0.029 |
| Systolic blood pressure (mmHg)—Median(IQR) | 125.50(119.00-135.25) | 128.00(122.00-140.50) | 127.00(120.00-138.00) | 0.534 |
| Diastolic blood pressure(mmHg)—Median(IQR) | 80.50(71.50-86.00) | 85.00(79.50-91.00) | 82.00(76.00-90.00) | 0.07 |
| White blood cell count (×10∧9/L)—n/N (%) | 0.969 | |||
| <4 | 4/32(12.5) | 3/23(13.0) | 7/55(12.7) | |
| 4-10 | 27/32(84.4) | 19/23(82.6) | 46/55(83.6) | |
| >10 | 1/32(3.1) | 1/23(4.3) | 2/55(3.6) | |
| Lymphocyte count (×10∧9/L)—n/N (%) | 0.373 | |||
| <1.1 | 19/32(59.4) | 10/23(43.5) | 29/55(52.7) | |
| 1.1-3.2 | 13/32(40.6) | 13/23(56.5) | 26/55(47.3) | |
| >3.2 | 0/32(0.0) | 0/23(0.0) | 0/55(0.0) | |
| Lymphocyte percentage (%)—n/N (%) | 0.36 | |||
| <20-50 | 17/32(53.1) | 9/23(39.1) | 26/55(47.3) | |
| 20-50 | 14/32(43.8) | 14/23(60.9) | 28/55(50.9) | |
| >20-50 | 1/32(3.1) | 0/23(0.0) | 1/55(1.8) | |
| Neutrophil count/lymphocyte count—n/N (%) | > 0.999 | |||
| <1.6 | 0/32(0.0) | 0/23(0.0) | 0/55(0.0) | |
| 1.6-1.9 | 4/32(12.5) | 3/23(13.0) | 7/55(12.7) | |
| >1.9 | 28/32(87.5) | 20/23(87.0) | 48/55(87.3) | |
| Erythrocyte sedimentation rate (mm/h)—n/N (%) | > 0.999 | |||
| 0-20 | 5/30(16.7) | 4/22(18.2) | 9/52(17.3) | |
| >20 | 25/30(83.3) | 18/22(81.8) | 43/52(82.7) | |
| High sensitivity C-reactive protein (mg/L) —n/N (%) | > 0.999 | |||
| 0-3 | 11/32(34.4) | 8/23(34.8) | 19/55(34.5) | |
| >3 | 21/32(65.6) | 15/23(65.2) | 36/55(65.5) | |
| Ferritin (ng/mL)—n/N (%) | 0.91 | |||
| <21.8 | 0/30(0.0) | 0/23(0.0) | 0/53(0.0) | |
| 21.8-274.66 | 5/30(16.7) | 5/23(21.7) | 10/53(18.9) | |
| >274.66 | 25/30(83.3) | 18/23(78.3) | 43/53(81.1) | |
| D dimer (ug/mL) —n/N (%) | 0.452 | |||
| 0-1.5 | 24/30(80.0) | 21/23(91.3) | 45/53(84.9) | |
| > 0-1.5 | 6/30(20.0) | 2/23(8.7) | 8/53(15.1) | |
| Interleukin-6 (pg/mL) —n/N (%) | 0.664 | |||
| 0-7 | 10/28(35.7) | 6/23(26.1) | 16/51(31.4) | |
| >7 | 18/28(64.3) | 17/23(73.9) | 35/51 (68.6) | |
| Aspartate aminotransferase (U/L) —n/N (%) | 0.817 | |||
| <13 | 0/32(0.0) | 0/23 (0.0) | 0/55 (0.0) | |
| 13-35 | 23/32(71.9) | 15/23 (65.2) | 38/55 (69.1) | |
| >35 | 9/32(28.1) | 8/23 (34.8) | 17/55 (30.9) | |
| Alanine aminotransferase (U/L) —n/N (%) | 0.938 | |||
| <7 | 0/32 (0.0) | 0/23 (0.0) | 0/55 (0.0) | |
| 7-40 | 21/32 (65.6) | 14/23 (60.9) | 35/55 (63.6) | |
| >40 | 11/32 (34.4) | 9/23 (39.1) | 20/55 (36.4) | |
| Creatine kinase (U/L) —n/N (%) | 0.075 | |||
| <40 | 6/32 (18.8) | 1/23 (4.3) | 7/55 (12.7) | |
| 40-200 | 25/32 (78.1) | 18/23 (78.3) | 43/55 (78.2) | |
| >200 | 1/32 (3.1) | 4/23 (17.4) | 5/55 (9.1) | |
| High sensitivity troponin (pg/mL)—n/N (%) | - | |||
| 0-28 | 27/27 (100.0) | 23 (100.0) | 50 (100.0) | |
| >28 | 0/27(0.0) | 0/23(0.0) | 0/50(0.0) | |
| Myoglobin (ng/mL) —n/N (%) | 0.86 | |||
| 0-146.9 | 27/28 (96.4) | 21 (91.3) | 48 (94.1) | |
| >146.9 | 1/28 (3.6) | 2 (8.7) | 3 (5.9) | |
| Lactate dehydrogenase (U/L) —n/N (%) | 0.509 | |||
| <120 | 0/32 (0.0) | 0/23 (0.0) | 0/55 (0.0) | |
| 120-250 | 14/32 (43.8) | 13/23 (56.5) | 27/55 (49.1) | |
| >250 | 18/32 (56.2) | 10/23 (43.5) | 28/55 (50.9) | |
| Amyloid A (mg/L)—n/N (%) | 0.222 | |||
| <0 | 0/29(0.0) | 0/20(0.0) | 0/49(0.0) | |
| 0-10 | 7/29(24.1) | 9/20(45.0) | 16/49(32.7) | |
| >10 | 22/29(75.9) | 11/20(55.0) | 33/49(67.3) |
Note. CM denotes Chinese medicine; WM Western medicine; IQR interquartile range.
In the cases included in this retrospective study, there was no patient of both groups died, being transferred to ICU, or receiving invasive mechanical ventilation.
3.2. Viral clearance
SARS-CoV-2 RNA testing turned to negative in all patients of the CM group while in one case of WM group the viral RNA was still positive by the end of observation period. The SARS-CoV-2 RNA persisted for a median time of 12 days (IQR, 6.50-14.00; 95%CI, 9–14) in the CM group, as compared with 15.5 days (IQR, 12.75-18.25; 95%CI, 13–18) in the WM group, that is, the median time of SARS-CoV-2 RNA conversion to negative in the CM group was shorter than that in the WM group.
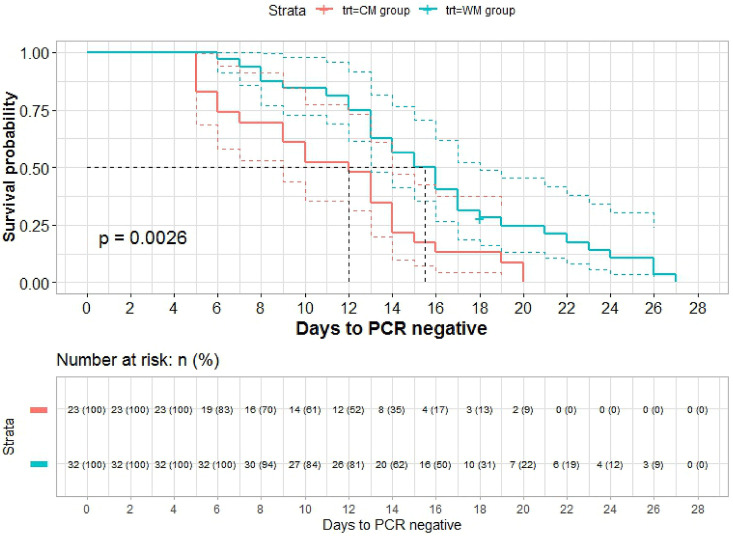
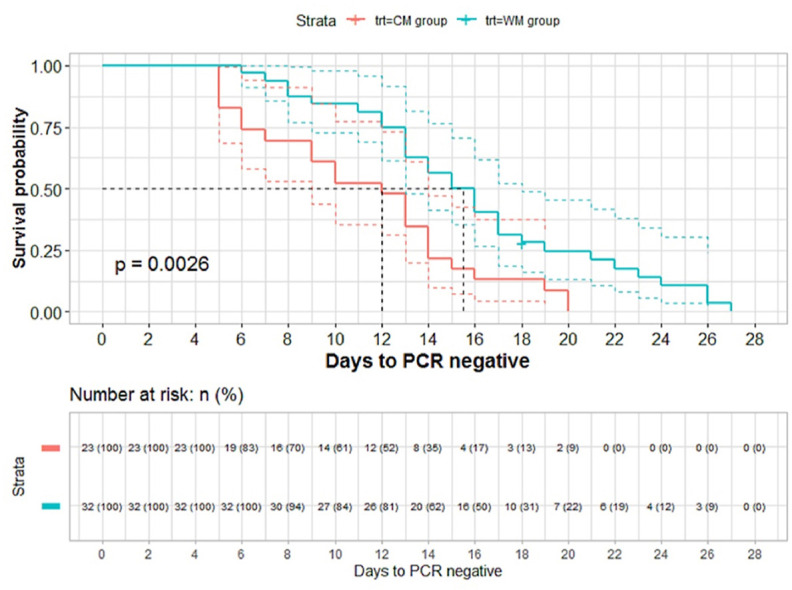
The ratio of nucleic acid negative conversion of CM group at different follow-up time points was significantly higher than that of WM group by log-rank test (P =0.0026, <0.01) (Fig. 2 ). The same results were obtained with the further calibration analysis by using the stratified log-rank test (P<0.02) and the multivariate Cox proportional hazards model (HR: 2.281, P =0.018).
Fig. 2.
Survival curve by Kaplan-Meier of CM and WM
The x-coordinate is the interval time from the diagnosed date to nucleic acid negative conversion date, and the y-coordinate is non-nucleic acid negative transformation rate (1-nucleic acid negative transformation rate) at different follow-up time point. Red line is CM group and blue line is WM group. On average, the hazard ratio of nucleic acid negative transformation after diagnosis is more than 2 in CM group compared to WM group in the survival curve. Furthermore, according to the number of patients at baseline and nucleic acid negative transformation at different follow-up time points to estimate the number of theoretical nucleic acid negative transformation. The ratio of nucleic acid negative transformation is significantly higher than that of WM by log-rank test (P = 0.0026, <0.01). (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
3.3. Discharge
During the treatment period of 16 days, 45 of the 55 patients were discharged. Among them, 20 cases (20/23, 87.0%) were discharged from the CM group, and 25 cases (25/32, 78.1%) were discharged from the WM group. The discharge rates of the two groups had no significant difference (P = 0.629). The other 10 patients remained in hospital until March 5th, 2020 were transferred to other wards for treatment and were eventually discharged.
3.4. Imaging features
In chest CT, the two groups showed typical changes of severe pneumonia, wide GGO, consolidation, Mixed GGO with consolidation mainly distributed in the middle, lower and peripheral lung areas. However, these features were not significantly different between two groups except the distribution areas.
The scores of pulmonary inflammatory lesions' feature, for example, consolidation sign in each group were significantly reduced, indicating that the inflammation was significantly absorbed after treatment (Table 2 ). After CM group with the therapy, the scores of the distribution of inflammatory lesions in both lungs (except the upper right lung) were significantly reduced (Table 3 ); however, the score for the lesion area in the WM group also decreased, and there was no significant difference between the scores obtained before and after treatment. The scores for total distribution and for the left upper lung were significantly decreased in CM group compared with WM group, which means that the inflammation absorption was in a wider range in CM group (Fig. 3 ).
Table 2.
The score of CT imaging for lesion’s feature.
| Groups | N | Baseline (n/N) |
Outcome Value (n/N) |
Inter-group P value | Extra-group P value | |
|---|---|---|---|---|---|---|
| Ground Glass Opacity (GGO) | WM | 21 | 19/21 | 18/21 | > 0.999 | 0.709 |
| CM | 18 | 17/18 | 15/18 | > 0.999 | ||
| Consolidation | WM | 21 | 17/21 | 9/21 | 0.046 | 0.738 |
| CM | 18 | 14/18 | 8/18 | 0.041 | ||
| Mixed.GGO.and.consolidation | WM | 21 | 17/21 | 11/21 | 0.131 | 0.529 |
| CM | 18 | 11/18 | 7/18 | > 0.999 | ||
| Bronchial.wall.thickening | WM | 21 | 3/21 | 1/21 | 0.480 | 0.700 |
| CM | 18 | 3/18 | 2/18 | > 0.999 | ||
| Reticulation | WM | 21 | 12/21 | 9/21 | 0.617 | 0.695 |
| CM | 18 | 12/18 | 9/18 | > 0.999 | ||
| Subpleural.bands | WM | 21 | 16/21 | 15/21 | 0.371 | 0.005 |
| CM | 18 | 6/18 | 6/18 | 0.480 | ||
| Traction.bronchiectasis | WM | 21 | 2/21 | 1/21 | > 0.999 | 0.282 |
| CM | 18 | 0 | 0 | - |
Note. CM denotes Chinese medicine; WM Western medicine. The Chinese medicine group and the western medicine group showed severe pneumonia lesion signs. Consolidation reduced in each group (P<0.05). Subpleural bands was different between two groups (P=0.005).
Table 3.
The score of CT imaging for lesion’s distribution.
| Groups | N | Baseline Median(Q1,Q3) |
Outcome Value Median(Q1,Q3) |
Inter-group P value | Inter-group difference | Extra-group P value | |
|---|---|---|---|---|---|---|---|
| Right Upper region | WM | 21 | 1.00 (0.00, 1.00) | 1.00 (1.00, 1.00) | > 0.999 | 0.00 (0.00, 0.00) | 0.274 |
| CM | 18 | 1.00 (1.00, 2.00) | 1.00 (1.00, 1.75) | 0.072 | 0.00 (0.00, 0.00) | ||
| Right Middle region | WM | 21 | 1.00 (1.00, 2.00) | 1.00 (1.00, 2.00) | 0.777 | 0.00 (0.00, 0.00) | 0.130 |
| CM | 18 | 2.00 (1.00, 2.00) | 1.00 (1.00, 2.00) | 0.020 | 0.00 (-1.00, 0.00) | ||
| Right Lower region | WM | 21 | 1.00 (1.00, 2.00) | 1.00 (1.00, 1.00) | 0.129 | 0.00 (0.00, 0.00) | 0.065 |
| CM | 18 | 2.00 (1.25, 3.00) | 1.00 (1.00, 2.00) | 0.005 | -0.50 (-1.00, 0.00) | ||
| Left Upper region | WM | 21 | 1.00 (0.00, 1.00) | 1.00 (1.00, 1.00) | 0.773 | 0.00 (0.00, 0.00) | 0.002 |
| CM | 18 | 1.00 (1.00, 1.75) | 1.00 (0.00, 1.00) | 0.008 | 0.00 (-1.00, 0.00) | ||
| Left Middle region | WM | 21 | 2.00 (1.00, 2.00) | 1.00 (1.00, 2.00) | 0.530 | 0.00 (0.00, 0.00) | 0.237 |
| CM | 18 | 1.00 (1.00, 2.00) | 1.00 (1.00, 1.75) | 0.020 | 0.00 (-1.00, 0.00) | ||
| Left Lower region | WM | 21 | 2.00 (1.00, 2.00) | 1.00 (1.00, 2.00) | 0.129 | 0.00 (0.00, 0.00) | 0.251 |
| CM | 18 | 2.00 (1.00, 2.00) | 1.00 (1.00, 2.00) | 0.011 | 0.00 (-1.00, 0.00) | ||
| Total score | WM | 21 | 9.00 (5.00, 10.00) | 7.00 (6.00, 9.00) | 0.482 | 0.00 (-2.00, 0.00) | 0.025 |
| CM | 18 | 9.00 (7.00, 13.50) | 6.00 (4.25, 10.00) | <0.001 | -2.00 (-2.75, -1.25) |
Note. CM denotes Chinese medicine; WM denotes Western medicine. The lesions distributed morein the lower and the peripheral region.The inflammation area (except in the right upper lung) significantly reduced after Chinese medicine treatment (P<0.05).The scores of the inflammation area involving in the left upper lung and the whole lung significantly decreased in the Chinese medicine group compared with the western medicine (P=0.002, 0.025).
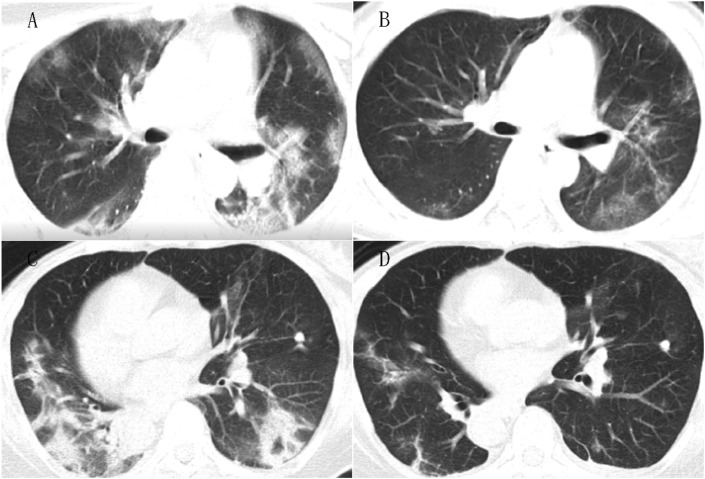
Fig. 3.
Chest CT images
3A. Chest CT of case (Wu XX) from WM group, at admission, consolidation combined with ground-glass opacities in both middle lungs. 3B. Follow up CT after 10 days WM treatment, lesions obviously reduced in scope and density. 3C. Chest CT of the case (Wang XX) from CM group, at admission the flaky consolidation with a little ground-glass opacities in both lower lobe. 3D. Follow up CT after 7 days' CM treatment, the infiltrating lesions absorbed significantly.
3.5. Laboratory examinations
On-admission lymphocytopenia was present in 52.7% (29/55) of patients, the absolute value of neutrophils/lymphocytes was abnormally increased in 87.3% (48/55) of patients. Most patients had elevated levels of erythrocyte sedimentation rate (ESR) (43/52, 82.7%), high sensitivity C-reactive protein (hs-CRP) (36/55, 65.5%), interleukin-6 (IL-6) (35/51, 68.6%), amyloid A (33/49, 67.3%) and serum ferritin (SF) (43/53, 81.1%).
After treatment, the improvement of hs-CRP and SF in the CM group was better than those of the WM group (after the correction of covariate, P < 0.05) (Table 4 ).
Table 4.
Observed values and changes from baseline for outcome.
| Observed Values |
Change from baseline |
P value |
|||
|---|---|---|---|---|---|
| WM |
CM |
WM |
CM |
||
| Body temperature (°C)—Median (IQR) | |||||
| Baseline | 36.60 (36.40, 36.82) | 36.70 (36.40, 37.05) | 0.00 (-0.40, 0.20) | 0.00 (-0.35, 0.35) | >0.05 |
| Outcome | 36.50 (36.27, 36.70) | 36.70 (36.60, 36.80) | |||
| Heart Rate—Median(IQR) | |||||
| Baseline | 84.00 (77.25, 96.00) | 96.00 (89.00, 101.00) | 2.00 (-9.25, 8.00) | -3.00 (-21.50, 1.00) | >0.05 |
| Outcome | 82.00 (80.00, 85.25) | 88.00 (80.00, 93.00) | |||
| Breathe—Median(IQR) | |||||
| Baseline | 21.00 (20.00, 22.00) | 22.00 (21.50, 23.00) | -1.00 (-2.00, 0.00) | 0.00 (-1.00, 1.00) | >0.05 |
| Outcome | 20.00 (20.00, 20.25) | 22.00 (21.50, 23.00) | |||
| Systolic blood pressure (mmHg)—Median(IQR) | |||||
| Baseline | 125.50 (119.00, 135.25) | 128.00 (122.00, 140.50) | 0.00 (-16.00, 0.00) | -7.00 (-18.50, 0.00) | >0.05 |
| Outcome | 120.50 (114.75, 129.00) | 123.00 (114.00, 131.00) | |||
| Diastolic blood pressure (mmHg)—Median(IQR) | |||||
| Baseline | 80.50 (71.50, 86.00) | 85.00 (79.50, 91.00) | 0.00 (-8.25, 0.00) | -7.00 (-15.00, 0.00) | >0.05 |
| Outcome | 76.50 (68.00, 85.00) | 78.00 (72.00, 82.50) | |||
| White-cell count (×10∧9/L) —Median(IQR) | |||||
| Baseline | 5.48 (4.54, 6.98) | 4.44 (3.81, 6.12) | -0.05 (-1.61, 0.76) | 0.68 (-0.40, 1.36) | >0.05 |
| Outcome | 5.15 (4.44, 6.00) | 5.58 (4.47, 6.74) | |||
| Lymphocyte count (10∧9/L) —Median(IQR) | |||||
| Baseline | 1.06 (0.84, 1.61) | 1.25 (0.80, 1.58) | 0.25 (-0.08, 0.54) | 0.33 (0.00, 0.71) | >0.05 |
| Outcome | 1.42 (1.08, 1.68) | 1.43 (1.25, 1.92) | |||
| Lymphocyte percentage (%)—Median(IQR) | |||||
| Baseline | 18.60 (15.33, 27.20) | 22.30 (18.90, 32.25) | 5.20 (-0.55, 10.15) | 2.00 (-2.85, 8.25) | >0.05 |
| Outcome | 25.20 (21.53, 30.72) | 27.60 (22.95, 32.80) | |||
| Neutrophil count/lymphocyte count—Median(IQR) | |||||
| Baseline | 3.82 (2.25, 5.19) | 3.16 (1.77, 4.15) | -1.15 (-3.36, 0.02) | -0.43 (-1.56, 0.24) | >0.05 |
| Outcome | 2.60 (1.84, 3.14) | 2.24 (1.76, 2.95) | |||
| Erythrocyte sedimentation (mm/h)—Median(IQR) | |||||
| Baseline | 49.00 (30.25, 60.25) | 41.50 (29.00, 60.50) | 0.00 (0.00, 0.00) | -7.50 (-16.75, 0.00) | >0.05 |
| Outcome | 36.00 (22.25, 51.98) | 32.50 (16.75, 44.00) | |||
| High sensitivity C-reactive protein (mg/L)—Median(IQR) | |||||
| Baseline | 17.55 (3.40, 38.00) | 16.50 (2.15, 54.35) | 0.00 (-5.75, 0.00) | -9.50 (-45.45, -0.15) | <0.05 |
| Outcome | 8.30 (1.42, 21.12) | 1.70 (0.80, 6.35) | |||
| Ferroprotein (ng/mL)—Median(IQR) | |||||
| Baseline | 457.30 (254.55, 663.88) | 435.20 (244.65, 764.78) | 0.00 (-57.13, 0.00) | -50.94 (-287.09, 0.00) | <0.05 |
| Outcome | 402.15 (258.20, 626.06) | 351.96 (193.22, 430.84) | |||
| D dimer (ug/mL) —Median(IQR) | |||||
| Baseline | 0.84 (0.47, 1.20) | 0.55 (0.33, 0.91) | 0.00 (0.00, 0.06) | 0.00 (0.00, 0.00) | >0.05 |
| Outcome | 0.90 (0.66, 1.21) | 0.66 (0.32, 0.90) | |||
| Interleukin-6 (pg/mL) —Median(IQR) | |||||
| Baseline | 8.17 (6.04, 10.49) | 9.46 (7.12, 12.62) | 0.00 (0.00, 0.00) | 0.00 (-3.17, 0.07) | >0.05 |
| Outcome | 8.05 (5.73, 11.01) | 8.51 (6.38, 11.58) | |||
| Aspartate aminotransferase (U/L) —Median(IQR) | |||||
| Baseline | 30.00 (22.75, 41.50) | 29.00 (23.00, 48.00) | -8.00(-16.00, -1.00) | -4.00 (-17.50, 0.00) | >0.05 |
| Outcome | 21.00 (17.00, 32.00) | 24.00 (18.00, 29.00) | |||
| Alanine aminotransferase (U/L) — Median(IQR) | |||||
| Baseline | 29.50 (22.75, 55.50) | 25.00 (16.50, 48.50) | -1.00 (-11.25, 2.50) | 0.00 (-11.50, 4.50) | >0.05 |
| Outcome | 26.50 (18.75, 52.50) | 28.00 (20.00, 47.00) | |||
| Creatine kinase (U/L)—Median(IQR) | |||||
| Baseline | 54.00 (45.00, 87.25) | 79.00 (64.50, 135.50) | -4.00 (-22.50, 0.00) | -22.00 (-90.00, -3.50) | >0.05 |
| Outcome | 48.00 (35.00, 65.25) | 54.00 (45.50, 67.50) | |||
| Hypersensitive troponin (pg/mL) — Median(IQR) | |||||
| Baseline | 2.20 (0.95, 3.75) | 4.60 (2.20, 8.40) | 0.00 (0.00, 0.00) | 0.00 (-1.55, 0.00) | >0.05 |
| Outcome | 2.20 (0.95, 3.50) | 2.80 (1.50, 5.85) | |||
| Myohemoglobin (ng/mL) — Median(IQR) | |||||
| Baseline | 38.50 (30.23, 48.15) | 41.10 (23.90, 60.90) | 0.00 (0.00, 0.00) | 0.00 (-9.85, 0.00) | >0.05 |
| Outcome | 36.45 (28.00, 47.02) | 31.20 (23.00, 41.50) | |||
| Lactate dehydrogenase (U/L) —Median(IQR) | |||||
| Baseline | 262.50 (212.75, 329.50) | 238.00 (207.00, 334.50) | -33.50 (-75.25, 0.00) | -43.00 (-94.50, 0.00) | >0.05 |
| Outcome | 202.00 (171.75, 247.75) | 203.00 (189.50, 220.50) | |||
Note. CM,Chinese medicine; WM,Western medicine; IQR,interquartile range.
3.6. Safety
After treatment, 4 patients in the WM group and 2 patients in the CM group showed mild elevation of alanine aminotransferase, and one patient in each group showed elevation of aspartate aminotransferase, and no other adverse events were recorded. Since the virus attacks the liver, kidney and other organs in the course of the disease, the correlation between the observed adverse events and the medication is not clear yet.
4. Discussion
In this study, a total of 55 patients met the study conditions. We compared the efficacy and safety of enrolled severe COVID-19 patients treated with CM and WM in the same period. The results showed that there was no case of death, being transferred to ICU, or receiving invasive mechanical ventilation in two groups during hospitalization. The median time of SARS-CoV-2 RNA clearance in CM and WM group were 12 days and 15.5 days, the ratio of nucleic acid negative conversion of CM group at different follow-up time points was significantly higher than that of WM group (HR: 2.281, P = 0.018). Further, the chest CT imaging showed more widely lung lesion opacity was absorbed in the CM group. The hs-CRP and SF decreased significantly in the CM group (P < 0.05). There was no significant difference in adverse events in terms of liver function and renal function between the two groups.
4.1. Viral clearance
In general, the duration of positive detection of viral nucleic acid in patients is an important factor in evaluating the risk of virus transmission and prognosis. Recent clinical studies found that the RNA detection of respiratory virus in dead patients with COVID-19 continues to be positive, and the average virus shedding period of discharged patients was 20 days (Zhou et al., 2020). Although there is no evidence that antiviral therapy can shorten the virus shedding time of SARS-CoV-2 (Zhou et al., 2020), the results of the present study showed that CM treatment could significantly shorten the SARS-CoV-2 RNA persistence time and cleared the virus more quickly, which is of significance to reduce the risk of disease transmission and improve the prognosis of patients.
4.2. CT score changes
Chest CT is a valuable diagnostic tool for clinical management of COVID-19-related lung diseases (Harmon et al., 2020). The semi-quantitative CT imaging analysis in this study showed that the inflammation imaging signs and the infiltrated lung area in severe patients were basically consistent with recent COVID-19 imaging results (Chung et al., 2020; Kanne, 2020). The major lung CT findings of the disease include extensive GGO, consolidation, and sub-pleural bands in the peripheral parts of the middle and lower lungs. Pulmonary consolidation is a well-known CT sign of the lung inflammation at the peak. The disperse of consolidation was observed in both groups, but more lung lesion opacity absorbed in CM group. Recent autopsy and lung replacement pathology reports have showed that SARS-CoV-2 mainly causes inflammatory reactions characteristically in deep airway and alveolar level, producing extensive inflammatory exudation and mucus filling in the alveolar cavity. It has suggested that eliminating inflammatory exudation and mucus from the small airways is an important solution (Liu et al., 2020). It has been previously verified that the anti-inflammatory and expectorant effects of CM in viral pneumonia and SARS (Zhu et al., 2020; Liang et al., 2003).
4.3. Potential mechanism of CM in improve lymphopenia and reduce inflammatory
The results in this study showed that combination therapy in CM group could improve lymphopenia and reduce inflammatory biomarkers such as hs-CRP and SF. A recent study also showed that integrated treatments could significantly improve the lymphocytes, serum amyloid-A (SAA), CRP and ESR (Xia et al., 2020), CM could improve the immune and inflammatory response induced by SARS-Cov-2 infection.
With regard to the above results in the CM group, we think that it has a potential relationship with the pharmacological function of the CM used. A large number of previous studies have shown that some CM extracts can enhance or regulate the function of the immune system, stimulate the production of endogenous interferons, and have anti-inflammatory and anti-allergic effect (Liu, 2020). Previous studies have proved that the active ingredients in Chinese herbal medicine composed of Huashi Baidu granule have the effects of anti-inflammation and regulating immunity. Polysaccharide from Ephedra sinica Stapf reduced airway and pulmonary inflammation by regulating inflammatory cytokines (Liang et al., 2018), andrographolide could reduce the pathological changes of lung tissue and the expression of inflammatory cytokines in mice induced by influenza A virus (Ding et al., 2017), patchouli alcohol could alleviate lipopolysaccharide-induced acute lung injury in mice through anti-inflammatory and antioxidant effects (Su et al., 2016), and stragaloside has good anti-inflammatory and immunostimulatory activities (Qi et al., 2017), which is of great significance to improve the immune function of the body.
Xiyanping injection is made by sulfonation process of andrographis B extracted from Andrographis paniculata, it produces antibacterial and antiviral effects, and has been widely used in the treatment of bronchitis, tonsillitis, bacillary dysentery and other infectious diseases in China (Yang et al., 2019), and some studies have shown that Xiyanping injection ameliorates lipopolysaccharide-induced acute lung injury in mice by down-regulating MAPK and NF-κB pathways (Peng et al., 2016).
The main components of Xuebijing injection are hydroxysafflor yellow A, paeoniflorin oxide, Ligusticum striatum DC., lactone I and paeoniflorin, etc. It can be used for infective systemic inflammatory response syndrome and for the treatment of organ function damage in multiple organ dysfunction syndrome (MODS) (Ma et al., 2020; Sun et al., 2010; Song et al., 2020).
It has been reported that 7.2% of COVID-19 patients have acute cardiac injury (Wang et al., 2020). Shenmai injection is extracted from Panax ginseng and Ophiopogon japonicas. Some studies have shown that Shenmai injection could protect cardiomyocytes through energy metabolism pathway (Wang et al., 2019). A randomized, double-blind, multi-center, placebo-controlled clinical study showed that integrative treatment with standard medicines plus Shenmai injection can improve the chronic heart failure (CHF) (Xian et al., 2016). In addition, animal experimental study showed that Shenmai injection can protect the lung from injury induced by intestinal Imax R injury, which may be mediated by inhibiting the activation of p38 MAPK (Zhao et al., 2019).
Dynamic immune response plays an important role in shaping the process of COVID-19 (Ong et al., 2020), cytokine storm (CS) is an important node of COVID-19 's transformation from mild to severe (Ma et al., 2020; Coperchini et al., 2020; Vaninov, 2020; Hu et al., 2020), and it is also one of the causes of severe or critical death (Ma et al., 2020). Improving the immune function of patients and reducing the storm of inflammatory factors are two key links in the treatment of severe COVID-19 patients (Ma et al., 2020). The results of CM treatment in this study suggests that CM has obvious anti-inflammatory effect, and has a good effect in preventing death and disease progression. It is worth mentioning that the treatment scheme in the CM group has been incorporated into Diagnosis and Treatment Protocol for COVID-19” released by NHC of the PRC (NHC, 2020), which is recommended for the treatment of severe cases of COVID-19.
5. Limitations
This study has all the limitations of a retrospective case series study; the sample size was small. To further evaluate the efficacy and safety of the CM treatment for COVID-19, rigorously designed prospective randomized clinical trials are warranted.
6. Conclusion
The overall results of CM treatment in this study were good in prevention of death and exacerbation of the disease at early stage of the COVID-19 epidemic in Wuhan, China. While the CM therapy shows a better effects in SARS-CoV-2 RNA clearance, lung lesion opacity absorbed and reducing inflammation in severe COVID-19 patients, which is effective and safe therapy for treating severe COVID-19 and reducing mortality.
Ethics statement
This study was approved by the Research Ethics Committee of Wuhan Jinyintan Hospital (KY-2020–43.01) and China Academy of Chinese Medical Sciences (CACM S-IRB2020-003-1). The requirement for informed consent was waived by the Ethics Committee as a retrospective study.
Funding
This study was funded by the National Key Research and Development Plan for the Emergency Management of Novel Coronavirus Pneumonia (No. 2020YFC0841500).
Declaration of competing interest
The authors declare that they have no conflict of interest.
Acknowledgements
The authors gratefully acknowledge all patients and health-care workers in the Wuhan Jinyintan Hospital. We also acknowledge Hongjun Yang, Xin Shen, Xinghua Xiang, Yunfei Xin, and all research assistants participated in collection of clinical data, and technical support.
References
- Ajlan A.M., Ahyad R.A., Jamjoom L.G., et al. Middle east respiratory syndrome coronavirus (MERS-CoV) infection: chest CT findings. AJR Am. J. Roentgenol. 2014;203:782–787. doi: 10.2214/AJR.14.13021. [DOI] [PubMed] [Google Scholar]
- Arshad Ali S., Baloch M., Ahmed N., et al. The outbreak of Coronavirus Disease 2019 (COVID-19)-An emerging global health threat. J. Infect. Publ. Health. 2020;13(4):644–646. doi: 10.1016/j.jiph.2020.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z., Nakamura T. Statistical evidence for the usefulness of Chinese medicine in the treatment of SARS. Phytother Res. 2004;18(7):592–594. doi: 10.1002/ptr.1485. [DOI] [PubMed] [Google Scholar]
- Chung M., Bernheim A., Mei X., et al. CT Imaging features of 2019 novel coronavirus (2019-nCoV) Radiology. 2020;295(1):202–207. doi: 10.1148/radiol.2020200230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coperchini F., Chiovato L., Croce L., et al. The cytokine storm in COVID-19: an overview of the involvement of the chemokine/chemokine-receptor system. Cytokine Growth Factor Rev. 2020;53:25–32. doi: 10.1016/j.cytogfr.2020.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding Y., Chen L., Wu W., et al. Andrographolide inhibits influenza A virus-induced inflammation in a murine model through NF-κB and JAK-STAT signaling pathway. Microb. Infect. 2017;19(12):605–615. doi: 10.1016/j.micinf.2017.08.009. [DOI] [PubMed] [Google Scholar]
- Harmon S.A., Sanford T.H., Xu S., et al. Artificial intelligence for the detection of COVID-19 pneumonia on chest CT using multinational datasets. Nat. Commun. 2020;11(1):4080. doi: 10.1038/s41467-020-17971-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho T.Y., Wu S.L., Chen J.C., et al. Emodin blocks the SARS coronavirus spike protein and angiotensin-converting enzyme 2 interaction. Antivir. Res. 2007;74(2):92–101. doi: 10.1016/j.antiviral.2006.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu B., Huang S., Yin L. The cytokine storm and COVID-19. J. Med. Virol. 2020 doi: 10.1002/jmv.26232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C., Wang Y., Li X., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia W., Gao W. Is traditional Chinese medicine useful in the treatment of SARS? Phytother Res. 2003;17(7):840–841. doi: 10.1002/ptr.1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanne J.P. Chest CT findings in 2019 novel coronavirus (2019-nCoV) infections from Wuhan, China: key points for the radiologist. Radiology. 2020;295(1):16–17. doi: 10.1148/radiol.2020200241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau J.T., Leung P.C., Wong E.L., et al. The use of an herbal formula by hospital care workers during the severe acute respiratory syndrome epidemic in Hong Kong to prevent severe acute respiratory syndrome transmission, relieve influenza-related symptoms, and improve quality of life: a prospective cohort study. J. Alternative Compl. Med. 2005;11:49–55. doi: 10.1089/acm.2005.11.49. [DOI] [PubMed] [Google Scholar]
- Leung P.C. The efficacy of Chinese medicine for SARS: a review of Chinese publications after the crisis. Am. J. Chin. Med. 2007;35(4):575–581. doi: 10.1142/S0192415X07005077. [DOI] [PubMed] [Google Scholar]
- Li J.H., Wang R.Q., Guo W.J., et al. Efficacy and safety of traditional Chinese medicine for the treatment of influenza A (H1N1): a meta-analysis. J. Chin. Med. Assoc. 2016;79(5):281–291. doi: 10.1016/j.jcma.2015.10.009. [DOI] [PubMed] [Google Scholar]
- Li Y., Li J., Zhong D., et al. Clinical practice guidelines and experts' consensuses of traditional Chinese herbal medicine for novel coronavirus (COVID-19): protocol of a systematic review. Syst. Rev. 2020;9(1):170. doi: 10.1186/s13643-020-01432-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Liu X., Guo L., Li J., Zhong D., Zhang Y., et al. Traditional Chinese herbal medicine for treating novel coronavirus (COVID-19) pneumonia: protocol for a systematic review and meta-analysis. Syst. Rev. 2020;9(1):75. doi: 10.1186/s13643-020-01343-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang A., Ling Y., Liu H. Application of traditional Chinese Medicine in Prevention and treatment of Severe acute respiratory syndrome (SARS) Pharm. J. Chin People’s Liberation Army. 2003;19(5):367–369. (In Chinese) [Google Scholar]
- Liang S., Meng X., Wang Z., et al. Polysaccharide from Ephedra sinica Stapf inhibits inflammation expression by regulating Factor-β1/Smad2 signaling. Int. J. Biol. Macromol. 2018;106:947–954. doi: 10.1016/j.ijbiomac.2017.08.096. [DOI] [PubMed] [Google Scholar]
- Liu J. Study on the effect of active components of traditional Chinese Medicine on the Multi-target Therapy of COVID-19. Chin. J. Integr. Tradit. West. Med. 2020:1–6. (In Chinese) [Google Scholar]
- Liu Q., Wang R., Qu G., et al. A macroscopic observation report on the systematic anatomy of COVID-19 patient’s autopsy. J. Forensic Med. 2020;36(1):1–3. (In Chinese) [Google Scholar]
- Liu X., Zhang M., He L., et al. Chinese herbs combined with Western medicine for severe acute respiratory syndrome (SARS) Cochrane Database Syst. Rev. 2012;10 doi: 10.1002/14651858.CD004882.pub3. Cd004882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Q., Qiu M., Zhou H., et al. The study on the treatment of Xuebijing injection (XBJ) in adults with severe or critical Corona Virus Disease 2019 and the inhibitory effect of XBJ against SARS-CoV-2. Pharmacol. Res. 2020;160:105073. doi: 10.1016/j.phrs.2020.105073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NHC . .; 2020. Diagnosis and Treatment Protocol for COVID-19 (Trial Version 7)https://www.who.int/docs/default-source/wpro---documents/countries/china/covid-19-briefing-nhc/1-clinical-protocols-for-the-diagnosis-and-treatment-of-covid-19-v7.pdf?sfvrsn=c6cbfba4_2 [Google Scholar]
- Ong E.Z., Chan Y.F.Z., Leong W.Y., et al. A dynamic immune response shapes COVID-19 progression. Cell Host Microbe. 2020;27(6):879–882. doi: 10.1016/j.chom.2020.03.021. e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng S., Hang N., Liu W., et al. Andrographolide sulfonate ameliorates lipopolysaccharide-induced acute lung injury in mice by down-regulating MAPK and NF-κB pathways. Acta Pharm. Sin. B. 2016;6(3):205–211. doi: 10.1016/j.apsb.2016.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi Y., Gao F., Hou L., et al. Anti-inflammatory and immunostimulatory activities of astragalosides. Am. J. Chin. Med. 2017;45(6):1157–1167. doi: 10.1142/S0192415X1750063X. [DOI] [PubMed] [Google Scholar]
- Rees E.M., Nightingale E.S., Jafari Y., et al. COVID-19 length of hospital stay: a systematic review and data synthesis. BMC Med. 2020;18(1):270. doi: 10.1186/s12916-020-01726-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Y., Yao C., Yao Y., et al. XueBiJing injection versus placebo for critically ill patients with severe community-acquired pneumonia: a randomized controlled trial. Crit. Care Med. 2020;47(9):e735–743. doi: 10.3760/cma.j.issn.2095-4352.2019.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su Z., Liao J., Liu Y., et al. Protective effects of patchouli alcohol isolated from Pogostemon cablin on lipopolysaccharide-induced acute lung injury in mice. Exp. Ther. Med. 2016;11(2):674–682. doi: 10.3892/etm.2015.2918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J., Xue Q., Guo L., et al. Xuebijing protects against lipopolysaccharide-induced lung injury in rabbits. Exp. Lung Res. 2010;36:211–218. doi: 10.3109/01902140903312123. [DOI] [PubMed] [Google Scholar]
- Vaninov N. In the eye of the COVID-19 cytokine storm. Nat. Rev. Immunol. 2020;20(5):277. doi: 10.1038/s41577-020-0305-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D., Hu B., Hu C., et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in wuhan, China. J. Am. Med. Assoc. 2020;323(11):1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S., Ye L., Wang L. Protective mechanism of shenmai on myocardial ischemia-reperfusion through the energy metabolism pathway. Am. J. Tourism Res. 2019;11:4046–4062. [PMC free article] [PubMed] [Google Scholar]
- WHO a . 2020. WHO Director-General's Remarks at the Media Briefing on 2019-nCoV on 11 February 2020.https://www.who.int/dg/speeches/detail/who-director-general-s-remarks-at-the-media-briefing-on-2019-ncov-on-11-february-2020 [Google Scholar]
- WHO b . 2020. Weekly Operational Update on COVID-19 - 30 October 2020.https://www.who.int/publications/m/item/weekly-operational-update---30-october-2020 [Google Scholar]
- WHO c . 2020. Director-General's Opening Remarks at the Media Briefing on COVID-19-11 March 2020.https://www.who.int/zh/dg/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19---11-march-2020 (2020-03-25 Retrieved March, 11, 2020. [Google Scholar]
- Wu L., Chen Y., Ma Y., et al. Clinical practice guideline on treating influenza in adult patients with Chinese patent medicines. Pharmacol. Res. 2020;105101 doi: 10.1016/j.phrs.2020.105101. [DOI] [PubMed] [Google Scholar]
- Xia W., An C., Zheng C., et al. Clinical observation on 34 patients with novel coronavirus nneumonia (COVID-19) treated with intergrated traditional Chinese and Western medicine. J. Trasdit. Chin. Med. 2020;61(5):375–382. (In Chinese) [Google Scholar]
- Xian S., Yang Z., Lee J., et al. A randomized, double-blind, multicenter, placebo-controlled clinical study on the efficacy and safety of Shenmai injection in patients with chronic heart failure. J. Ethnopharmacol. 2016;186:136–142. doi: 10.1016/j.jep.2016.03.066. [DOI] [PubMed] [Google Scholar]
- Xin S., Cheng X., Zhu B., et al. Clinical retrospective study on the efficacy of Qingfei Paidu decoction combined with Western medicine for COVID-19 treatment. Biomed. Pharmacother. 2020;129:110500. doi: 10.1016/j.biopha.2020.110500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Q.W., Li Q., Zhang J., et al. Crystal structure and anti-inflammatory and anaphylactic effects of andrographlide sulphonate E in Xiyanping, a traditional Chinese medicine injection. J. Pharm. Pharmacol. 2019;71(2):251–259. doi: 10.1111/jphp.13028. [DOI] [PubMed] [Google Scholar]
- Zhang H.T., Huang M.X., Liu X., et al. Evaluation of the adjuvant efficacy of Natural herbal medicine on COVID-19: a retrospective matched case-control study. Am. J. Chin. Med. 2020;48(4):779–792. doi: 10.1142/S0192415X20500391. [DOI] [PubMed] [Google Scholar]
- Zhao J., Jia Y., Tang Y., et al. Effects of Shenmai injection on the expression of p38MAPK and the apoptosis-related genes in lung injury induced by intestinal ischemia/reperfusion in rats. Zhongguo Ying Yong Sheng Li Xue Za Zhi. 2019;35:65–68. doi: 10.12047/j.cjap.5713.2019.016. [DOI] [PubMed] [Google Scholar]
- Zhao W., Zhong Z., Xie X.Z. CT scans of patients with 2019 novel coronavirus (COVID-19) pneumonia. Theranostics. 2020;10(10):4606–4613. doi: 10.7150/thno.45016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou F., Yu T., Du R., et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;(20) doi: 10.1016/S0140-6736(20)30566-3. pii: S0140-6736. 30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu N., Zhang D., Wang W., et al. A novel coronavirus from patients with pneumonia in China, 2019. N. Engl. J. Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]