Abstract
Background
The spectrum of Coronavirus Disease 2019 (COVID-19) is broad and thus early appropriate risk stratification can be helpful. Our objectives were to define the frequency of myocardial injury using high-sensitivity cardiac troponin I (hs-cTnI) and to understand how to use its prognostic abilities.
Methods
Retrospective study of patients with COVID-19 presenting to an Emergency Department (ED) in Italy in 2020. Hs-cTnI was sampled based on clinical judgment. Myocardial injury was defined as values above the sex-specific 99th percentile upper reference limits (URLs). Most data is from the initial hospital value.
Results
426 unique patients were included. Hs-cTnI was measured in 313 (73.5%) patients; 85 (27.2%) had myocardial injury at baseline. Patients with myocardial injury had higher mortality during hospitalization (hazard ratio = 9 [95% confidence interval (CI) 4.55–17.79], p < 0.0001). Multivariable analysis including clinical and laboratory variables demonstrated an AUC of 0.942 with modest additional value of hs-cTnI. Myocardial injury was associated with mortality in patients with low APACHE II scores (<13) [OR (95% CI): 4.15 (1.40, 14.22), p = 0.014] but not in those with scores > 13 [OR (95% CI): 0.48 (0.08, 2.65), p = 0.40]. Initial hs-cTnI < 5 ng/L identified 33% of patients that were at low risk with 97.8% sensitivity (95% CI 88.7, 99.6) and 99.2% negative predictive value. Type 1 myocardial infarction (MI) and type 2 MI were infrequent.
Conclusions
hs-cTnI at baseline is a significant predictor of mortality in COVID-19 patients. A value < 5 ng/L identified patients at low risk.
Keywords: High-sensitivity cardiac troponin I, Myocardial injury, SARS-CoV-2, COVID-19, Outcomes, Risk stratification
1. Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), causing Coronavirus disease 2019 (COVID-19), has led to substantial morbidity and mortality [1]. In February 2020, Italy became the epicenter of the European outbreak [2].
The clinical spectrum of COVID-19 ranges from asymptomatic infection to severe respiratory failure and death [3], [4]. Comorbidities, such as hypertension, diabetes, and coronary artery disease, are present in nearly half of those affected [3] and are related to disease severity [4]. Similar to what has been previously reported in critically ill patients including in those with acute respiratory illness [5], [6], increases in cardiac troponin (cTn) are common in COVID-19 patients [7], [8], [9]. These increases are associated with the severity of the disease and prognosis [10], [11], [12]. The mechanisms for such elevations include increases due to underlying cardiac comorbidities, the severe acute respiratory illness that can ensue and complications associated with the disease process [13], [14]. Most studies rely on initial cTn results, using non-high sensitivity cTn assays, and a variety of thresholds, mostly non sex-specific [13]. The present study evaluated myocardial injury and its relationship to mortality in patients with COVID-19 in Padova, Italy. Using a high sensitivity cTn I (hs-cTnI) assay, we investigated the prognostic power of cTn values while correcting for clinical features of the patients. Our focus was to develop a simple method to identify high and low risk patients to facilitate triage and clinical care.
2. Methods
This was a retrospective study of patients presenting to Azienda Ospedaliera - University of Padova Emergency Department (ED), Italy, between February 21st and May 31st 2020. Patients were included if ≥18 years old with confirmed SARS-CoV-2 infection. COVID-19 due to SARS-CoV-2 was diagnosed by real-time reverse transcriptase-polymerase chain reaction (rPCR) from nasopharyngeal swabs. cTn sampling was done based on clinic need. The study was approved by the local Ethical Committee for Clinical Experimentation; informed consent was waived.
Electronic charts were reviewed by trained physicians. Variables (Table 1, Table 2, Table 3, Table 4 ) were collected and analyzed. Severity of illness scores including the Acute Physiology and Chronic Health Evaluation II (APACHE II) score and Charlson comorbidity index were calculated. Laboratory data at presentation refers to blood samples collected in the ED or within 6 h of hospitalization. cTn and D-dimer values were collected for the entire hospitalization. The first value was defined as the baseline value. All 12-lead electrocardiograms (ECG) findings were reviewed. Hs-cTnI concentrations were measured using the Abbott Architect Stat High Sensitive Troponin I assay (Abbott, Abbott Park, Illinois). This assay has a 99th percentile upper reference limit (%URL) of 16 ng/L for women and 34 ng/L for men and a limit of detection (LoD) of 2 ng/L. Myocardial injury refers to any increase above sex-specific cutoffs [15]. The use of a “rule out” cut off of 5 ng/L as proposed by others was probed [16]. Acute kidney injury (AKI) was defined according to Guidelines [17]. Acute respiratory distress syndrome (ARDS) was diagnosed by the Berlin definition [18]. Independent adjudications of all hs-cTnI increases used all available data and the criteria provided in the Fourth Universal Definition of Myocardial Infarction [15] for the diagnosis of type 1 myocardial infarction (MI), type 2 MI and myocardial injury. Outcome data was collected at 30 days after discharge. Data were collected into a computer database (REDCAP; i.e. Research Electronic Data CAPture) [19].
Table 1.
Demographic and clinical characteristics of the study population at admission stratified by outcomes. Age is expressed as median (Q1- Q3), the other variables as n (%). Percentages refer to the numbers assessed in each group. Abbreviations: BMI, body mass index. VTE: venous thromboembolism. COPD: chronic obstructive pulmonary disease. OSA: obstructive sleep apnea. HIV: human immunodeficiency virus. AIDS: acquired immune deficiency syndrome. ACEi: angiotensin converting enzyme inhibitors. ARB: angiotensin II receptor blockers.
| Number assessed Total (deceased, survivors) | Overall | Deceased | Survivors | P-value | |
|---|---|---|---|---|---|
| Overall population | 426 | 52 (12.2) | 374 (87.8) | ||
| Patient characteristics | |||||
| Age, years | 426 (52, 374) | 64.1(53.6–77.9) | 86 (76.4–90.7) | 61.6 (52.1–74.1) | <0.0001 |
| Men | 426 (52, 374) | 246 (57.7) | 30 (57.7) | 216 (57.8) | 1.000 |
| Italian nationality | 426 (52, 374) | 384 (90.1) | 51 (98.1) | 333 (89.0) | 0.093 |
| Systemic hypertension | 424 (51, 373) | 207 (48.8) | 38 (74.5) | 169 (45.3) | <0.0001 |
| BMI > 30 | 424 (51, 373) | 50 (11.8) | 4 (7.8) | 46 (12.3) | 0.49 |
| Tobacco use | 424 (51, 373) | 49 (11.6) | 5 (9.8) | 44 (11.8) | 0.82 |
| Diabetes | 424 (51, 373) | 80 (18.9) | 13 (25.5) | 67 (18.0) | 0.25 |
| Dyslipidemia | 424 (51, 373) | 88 (20.8) | 7 (13.7) | 81 (21.7) | 0.27 |
| Coronary artery disease | 424 (51, 373) | 37 (8.7) | 8 (15.7) | 29 (7.8) | 0.068 |
| Prior myocardial infarction | 424 (51, 373) | 29 (6.8) | 7 (13.7) | 22 (5.9) | 0.068 |
| Prior revascularization | 424 (51, 373) | 27 (6.4) | 7 (13.7) | 20 (5.4) | 0.032 |
| Atrial fibrillation | 424 (51, 373) | 49 (11.6) | 14 (27.5) | 35 (9.4) | 0.0006 |
| Heart failure | 424 (51, 373) | 21 (5.0) | 9 (17.6) | 12 (3.2) | 0.0003 |
| Cardiac valve disease | 424 (51, 373) | 12 (2.8) | 5 (9.8) | 7 (1.9) | 0.008 |
| Cerebrovascular disease | 424 (51, 373) | 53 (12.5) | 15 (29.4) | 38 (10.2) | 0.0004 |
| History of VTE | 424 (51, 373) | 20 (4.7) | 7 (13.7) | 13 (3.5) | 0.006 |
| Peripheral arterial disease | 424 (51, 373) | 21 (5.0) | 4 (7.8) | 17 (4.6) | 0.31 |
| Chronic Kidney disease | 424 (51, 373) | 23 (5.4) | 13 (25.5) | 10 (2.7) | <0.0001 |
| Asthma | 426 (52, 374) | 18 (4.2) | 0 (0.0) | 18 (4.8) | 0.15 |
| COPD | 426 (52, 374) | 25 (5.9) | 8 (15.4) | 17 (4.5) | 0.006 |
| Pulmonary fibrosis | 426 (52, 374) | 3 (0.7) | 3 (5.8) | 0 (0.0) | 0.002 |
| OSA | 426 (52, 374) | 10 (2.3) | 3 (5.8) | 7 (1.9) | 0.11 |
| Active or prior cancer | 424 (51, 373) | 60 (14.2) | 16 (31.4) | 44 (11.8) | 0.0008 |
| Immunocompromised | 424 (51, 373) | 18 (4.2) | 7 (13.7) | 11 (2.9) | 0.003 |
| Dementia | 424 (51, 373) | 44 (10.4) | 21 (41.2) | 23 (6.2) | <0.0001 |
| Hemiplegia | 424 (51, 373) | 4 (0.9) | 4 (7.8) | 0 (0.0) | 0.0002 |
| HIV/AIDS | 424 (51, 373) | 2 (0.5) | 1 (2.0) | 1 (0.3) | 0.23 |
| Prior therapy | |||||
| Anticoagulants | 424 (51, 373) | 61 (14.4) | 21 (41.2) | 40 (10.7) | <0.0001 |
| Antiplatelet agents | 424 (51, 373) | 71 (16.7) | 11 (21.6) | 60 (16.1) | 0.32 |
| Beta blockers | 424 (51, 373) | 70 (16.5) | 18 (35.3) | 52 (13.9) | 0.0004 |
| Ca channel blockers | 424 (51, 373) | 51 (12.0) | 7 (13.7) | 44 (11.8) | 0.65 |
| ACEi/ARB | 424 (51, 373) | 139 (32.8) | 23 (45.1) | 116 (31.1) | 0.056 |
| Steroid therapy | 424 (51, 373) | 32 (7.5) | 11 (21.6) | 21 (5.6) | 0.0005 |
Table 2.
Laboratory values at presentation by outcomes. Variables are expressed as median (Q1- Q3). Reference values at Our Institution are displayed in Supplemental Table 5. Abbreviations: APACHE II, Acute Physiology and Chronic Health Evaluation, ABG, arterial blood gas. WBC, white blood cells. Hb, hemoglobin. LDH, lactate dehydrogenase. CRP, C-reactive protein. PCT, procalcitonin.
| Number assessed Total (deceased, survivors) | Overall | Deceased | Survivors | P-value | |
|---|---|---|---|---|---|
| Severity of illness scores at presentation | |||||
| APACHE II score | 306 (40, 266) | 8 (5–11) | 13 (10–19.5) | 7 (5–10) | <0.0001 |
| Charlson comorbidity index | 422 (51, 371) | 2 (1–5) | 7 (5–9) | 2 (1–4) | <0.0001 |
| Relevant laboratory and biomarkers values at presentation | |||||
| pO2 at ABG, mmHg | 328 (45, 283) | 68.6 (59.4–79.4) | 60.9 (50.5–72.3) | 69.7 (60.2–80.7) | 0.002 |
| Lactate at ABG, mmol/L | 257 (30, 227) | 1.4 (1.0–1.9) | 1.9 (1.4–2.4) | 1.3 (1.0–1.8) | <0.0001 |
| WBC, x 10^3/uL | 406 (49, 357) | 6.1 (4.6–8.3) | 7.7 (5.4–12.3) | 5.9 (4.5–7.9) | 0.002 |
| Lymphocytes, x10^3/uL | 229 (25, 204) | 1.2 (0.9–1.6) | 0.8 (0.6–1.1) | 1.2 (0.9–1.7) | 0.0003 |
| Hb, g/L | 406 (49, 357) | 141 (129–152) | 128 (121–148) | 142 (131–152) | 0.0003 |
| Platelets, x10^3/uL | 406 (49, 357) | 195 (161–253) | 188 (146–242) | 196 (162–254) | 0.18 |
| D-dimer, ug/L | 345 (48, 297) | 216 (149–450) | 570.5 (248.5–1257.5) | 188 (149–329) | <0.0001 |
| Creatinine, umol/L | 403 (49, 354) | 83 (70–101) | 133 (91–175) | 82 (69–97) | <0.0001 |
| LDH, U/L | 275 (31, 244) | 323 (238–408) | 429 (324–692) | 302.5 (231.5–389.5) | <0.0001 |
| CRP, mg/L | 399 (48, 351) | 46 (13.5–99.6) | 122.3 (72.3–216.6) | 38 (11.9–87) | <0.0001 |
| PCT, ug/L | 100 (18, 82) | 0.1 (0.1–0.4) | 0.3 (0.2–0.5) | 0.1 (0.1–0.3) | 0.006 |
| Albumin, g/L | 307 (38, 269) | 31 (28–35) | 27 (23–29) | 32 (28–36) | <0.0001 |
| Ferritin, mcg/L | 294 (42, 252) | 676 (300–1230) | 1093.5 (570–1905) | 654.5 (267–1165.5) | 0.0003 |
| Cardiac biomarkers at presentation | |||||
| Hs-cTnI, ng/L | 313 (46, 267) | 9 (3–28) | 55.5 (32–197) | 7 (3–18) | <0.0001 |
| BNP, ng/L | 139 (30, 109) | 42 (12–138) | 156.5 (82–377) | 30 (10–68) | <0.0001 |
Table 3.
Clinical characteristics of patients in whom hs-cTnI was evaluated. Age is expressed as median (Q1- Q3), the other variables as n (%). Abbreviations: BMI, body mass index. VTE: venous thromboembolism. COPD: chronic obstructive pulmonary disease. OSA: obstructive sleep apnea. HIV: human immunodeficiency virus. AIDS: acquired immune deficiency syndrome. ACEi: angiotensin converting enzyme inhibitors. ARB: angiotensin II receptor blockers.
| Patients, n (%) |
||||
|---|---|---|---|---|
| Overall, n = 313 | Without myocardial injury n = 228 | With myocardial injury n = 85 | P-value | |
| Patient characteristics | ||||
| Deceased, | 46 (14.7) | 12 (5.3) | 34 (40.0) | <0.0001 |
| Age, years | 66.1 (55.1–79.4) | 61.3 (52.0–70.6) | 82.9 (74.9–87.0) | <0.0001 |
| Men | 176 (56.2) | 139 (61.0) | 37 (43.5) | 0.007 |
| Italian nationality | 283 (90.4) | 201 (88.2) | 82 (96.5) | 0.095 |
| Systemic hypertension | 163 (52.1) | 97 (42.5) | 66 (77.6) | <0.0001 |
| BMI > 30 | 42 (13.4) | 30 (13.2) | 12 (14.1) | 0.85 |
| Tobacco use | 36 (11.5) | 28 (12.3) | 8 (9.4) | 0.55 |
| Diabetes | 67 (21.4) | 42 (18.4) | 25 (29.4) | 0.044 |
| Dyslipidemia | 74 (23.6) | 49 (21.5) | 25 (29.4) | 0.18 |
| Coronary artery disease | 30 (9.6) | 17 (7.5) | 13 (15.3) | 0.050 |
| Prior myocardial infarction | 24 (7.7) | 13 (5.7) | 11 (12.9) | 0.053 |
| Prior revascularization | 22 (7.0) | 14 (6.1) | 8 (9.4) | 0.33 |
| Atrial fibrillation | 42 (13.4) | 20 (8.8) | 22 (25.9) | 0.0003 |
| Heart failure | 19 (6.1) | 3 (1.3) | 16 (18.8) | <0.0001 |
| Cardiac valve disease | 9 (2.9) | 1 (0.4) | 8 (9.4) | 0.0002 |
| Cerebrovascular disease | 47 (15.0) | 22 (9.6) | 25 (29.4) | 0.0001 |
| History of VTE | 18 (5.8) | 10 (4.4) | 8 (9.4) | 0.104 |
| Peripheral arterial disease | 20 (6.4) | 9 (3.9) | 11 (12.9) | 0.008 |
| Chronic Kidney disease | 22 (7.0) | 8 (3.5) | 14 (16.5) | 0.0002 |
| Asthma | 13 (4.2) | 12 (5.3) | 1 (1.2) | 0.20 |
| COPD | 24 (7.7) | 14 (6.1) | 10 (11.8) | 0.101 |
| Pulmonary fibrosis | 3 (1.0) | 1 (0.4) | 2 (2.4) | 0.18 |
| OSA | 9 (2.9) | 5 (2.2) | 4 (4.7) | 0.26 |
| Active or prior cancer | 51 (16.3) | 28 (12.3) | 23 (27.1) | 0.003 |
| Immunocompromised | 14 (4.5) | 8 (3.5) | 6 (7.1) | 0.22 |
| Dementia | 39 (12.5) | 11 (4.8) | 28 (32.9) | <0.0001 |
| Hemiplegia | 3 (1.0) | 1 (0.4) | 2 (2.4) | 0.18 |
| HIV/AIDS | 2 (0.6) | 0 (0.0) | 2 (2.4) | 0.073 |
| Connective tissue disease | 9 (2.9) | 3 (1.3) | 6 (7.1) | 0.014 |
| Peptic ulcer | 10 (3.2) | 4 (1.8) | 6 (7.1) | 0.027 |
| Prior therapy | ||||
| Anticoagulants | 48 (15.3) | 21 (9.2) | 27 (31.8) | <0.0001 |
| Antiplatelet agents | 59 (18.8) | 32 (14.0) | 27 (31.8) | 0.0006 |
| Beta blockers | 56 (17.9) | 27 (11.8) | 29 (34.1) | <0.0001 |
| Ca channel blockers | 43 (13.7) | 20 (8.8) | 23 (27.1) | 0.0001 |
| ACEi/ARB | 111 (35.5) | 72 (31.6) | 39 (45.9) | 0.024 |
| Steroid therapy | 26 (8.3) | 14 (6.1) | 12 (14.1) | 0.036 |
Table 4.
Univariate and multivariate propensity inverse probability analysis weighting association of clinical and laboratory variables with death. Myocardial injury at presentation is defined as hs-cTnI value above the sex-specific 99th % URL at baseline.
| Univariate propensity score inverse probability weighting for the association of myocardial injury with death | |||||
|---|---|---|---|---|---|
| Variable | OR (95% CI) | P-value | AUC | ||
| Myocardial injury at presentation | 8.94 (5.56, 15.04) | <0.0001 | 0.775 (0.707, 0.843) | ||
| Multivariable propensity score inverse probability weighted analysis for the risk of death | |||||
| Variable | OR (95% CI) | P-value | AUC | Sensitivity (95% CI) | Specificity (95% CI) |
| Cardiovascular risk factors (hypertension and/or diabetes) | 0.16 (0.07, 0.33) | <0.0001 | 0.942 (0.906, 0.977) | 88.8 (74.6, 95.5) | 88.4 (83.6, 91.9) |
| Structural heart disease (CAD, prior MI and/or heart failure) | 2.58 (1.15, 5.97) | 0.023 | |||
| Chronic kidney disease | 14.88 (5.15, 44.94) | <0.0001 | |||
| Age, per year | 1.13 (1.09, 1.18) | <0.0001 | |||
| APACHE II score, per point | 0.98 (0.91, 1.06) | 0.62 | |||
| CRP, per mg/dL | 1.02 (1.01, 1.02) | <0.0001 | |||
| Myocardial injury at presentation | 2.51 (1.13, 5.85) | 0.027 | |||
| Low cardiac troponin values at presentation (<12 ng/L in women, <3 ng/L in men) | 0.04 (0.0003, 0.44) | <0.0001 | |||
2.1. Statistical analysis
Continuous variables for first encounters were summarized as median (interquartile range, IQR, in parentheses); categorical variables were summarized as percentages. For univariate associations with either 30-day survival or presence of myocardial injury, continuous variables were assessed using the Wilcoxon rank sum test and categorical values using the Fisher exact test. Survival curves were plotted with the Kaplan-Meier method and log-rank tests were utilized to compare curves between patients with and without myocardial injury.
The probability of myocardial injury was assessed by generalized estimating equations (GEE) [20] with myocardial injury as dependent variable and hypertension, diabetes, coronary artery disease (CAD), prior MI and history of heart failure as independent variables, assuming a logistic distribution to adjust for repeated observations for the same patient. Similarly, GEE were used to assess sex-based differences in hs-cTnI in patients who survived and those who died with dependent variable hs-cTnI and independent variable sex, assuming a log gamma distribution. The relationships between age, sex and survival status were assessed using similar GEE with age as the dependent variable and both sex and survival status as the independent variables. Because of the small number of deaths, sex differences among those who died were assessed using the Fisher exact test. Since only 6% of patients had more than 1 admission and results were not different using GEE or traditional logistic regression, the simpler propensity-score-adjusted logistic regression [21] using Firth’s penalized likelihood methods was used to provide more robust estimates of the association between myocardial injury and death with dependent variable death and myocardial injury defined using independent variables of both sex-specific cut-offs and 26 ng/L for both sexes for the univariate model and covariates of hypertension/diabetes, any of CAD/prior MI/history of heart failure, chronic kidney disease, age (years), APACHE II score and CRP (mg/dL) for the multivariable model. For this multivariable model sensitivity and specificity, along with their score confidence intervals, were estimated at the cut-off of predicted probability that maximized sensitivity and specificity, along with positive (PPV) and negative predictive values (NPV). All analyses were performed using SAS 9.4. Statistical significance was defined as p < 0.05.
3. Results
3.1. Study population
Encounters of adult patients (n = 471) presenting to the ED between February 21st and May 31st 2020 were reviewed. There were 426 unique patients. The majority (4 0 2) had a single encounter. After assessment, 339 (79.8%) patients were hospitalized; 88.2% on a ward, 6.8% in a semi-intensive respiratory-care unit and 5% in an intensive care unit..
Patient characteristics by survival status are displayed in Table 1. Median (IQR) age was higher in non-survivors and they had more cardiovascular and non-cardiovascular comorbidities (all p < 0.032).
Time from symptom onset to presentation was 5 (3–8) days. Symptoms and vitals at presentation are shown in Supplemental Table 1 [22], [23]. Additional clinical and laboratory data by survival status are displayed in Table 2. Fifty-two patients (12.2%) died after a median of 7 (4–17) days; fifty in hospital.
3.2. Hs-cTnI and myocardial injury
Hs-cTnI was measured in 313/426 (73.5%) patients. Most initial samples were drawn in the ED (78%); the rest on admission to hospital a median of 1 day later. Dyspnea was present in 56.5% (Table 2). The ECG at presentation showed atrial fibrillation in 8.3% of patients, non-specific repolarization abnormalities in 14.1% and ischemia in 2.6%.
Using initial hs-cTnI results, myocardial injury was present in 85 patients (27.2%), 48 (56.5%) of whom were women. Patients with myocardial injury had more cardiovascular comorbidities (Table 3). Baseline D-dimer was higher in these patients [418 (202–943) ug/L versus 188 (149–368) ug/L, p < 0.0001]. By multivariable modeling, hypertension and a history of heart failure were associated with myocardial injury (OR [95% confidence interval (CI)] 3.61[1.95, 6.67] and 10.03 [2.69, 37.46], respectively).
Among those who survived, median admission hs-cTnI value was 8.4 (4–19) ng/L for men and 4 (1.9–17) ng/L for women (p = 0.0007). Among those who died, median hs-cTnI value was 40.5 (13–100) ng/L for men and 100.5 (43.5–317.5) for women (p = 0.019). Women who died were older than those who survived and older compared to men who died. They more frequently had dementia/cognitive impairment (Supplemental Table 2). Other comorbidities were similar between men and women.
The adjudicated diagnoses of those with increased hs-cTnI are shown in Supplemental Table 3. The majority of cases (88.2%) had myocardial injury without criteria for ischemia: only 7% were adjudicated with type 2 MI and 4.7% with type 1 MI.
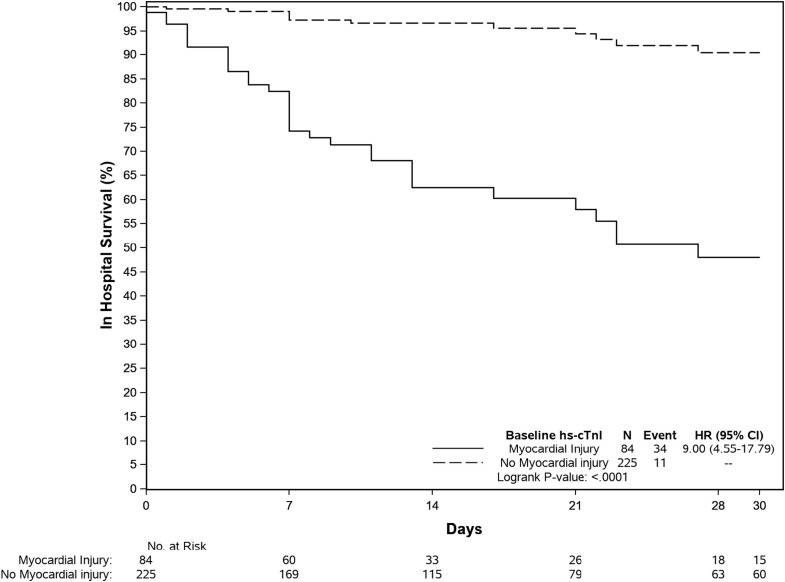
Myocardial injury was associated with an increased risk of death [34 (40%) versus 12 (5.3%); P < 0.0001]. Patients with myocardial injury had higher mortality during hospitalization (hazard ratio (HR) = 9.00 (95% CI 4.55,17.79), p < 0.0001 (Fig. 1 ).
Fig. 1.
Kaplan-Meier survival curves for mortality during index hospitalization for patients without and with myocardial injury at baseline hs-cTnI sample. Abbreviations: HR: hazard ratio; CI, confidence interval.
Myocardial injury using sex specific cut off values was univariately associated with death [OR 11.78 (95% CI 5.92, 24.86), p < 0.0001], with an area under the curve (AUC) (95% CI) of 0.775 (0.707, 0.843) (Supplemental Table 4). The association was stronger for women than for men. Using a single overall cutoff of 26 ng/L improved the AUC (95% CI) to 0.811 (0.747, 0.875). Propensity score inverse probability weighting for the association of myocardial injury with death (Table 4) provided a more robust estimate of OR (95% CI): 8.94 (5.56, 15.04). The association with all cause death remained significant when cardiac troponin was evaluated as a continuous variable. For each 10 ng/L increase in hs-cTnI the OR (95% CI) increased by 0.183 (0.128, 0.246). Peak hs-cTnI value was not more informative than the first value available. A hs-cTnI threshold of < 5 ng/L identified 103 patients (32.9%) who were at low risk of death with a sensitivity of 97.8% (95% CI 88.7, 99.6) and NPV of 99.2%. Cohort-specific cut-offs of 12 ng/L for women and 3 ng/L for men had a sensitivity of 100% (92.3, 100) and a NPV of 100%.
Using multivariable propensity score inverse probability analysis, a prior history of structural heart disease, chronic kidney disease, age, CRP and myocardial injury were all associated with an increased risk of death (Table 4). In the same model, hs-cTnI below 12 ng/L for women and 3 ng/L for men was significantly associated with favorable outcomes. After adjusting for structural heart disease and hs-cTnI, systemic hypertension and diabetes did not increase the odds of mortality.
The Apache II score at presentation was not a predictor of mortality in the multivariate model. However, in multivariable analysis, myocardial injury was a significant predictor of mortality for patients with lower APACHE II scores (<13) [OR (95% CI): 4.15 (1.40, 14.22), p = 0.014] but not in patients with scores > 13 [OR (95% CI): 0.48 (0.08, 2.65), p = 0.40].
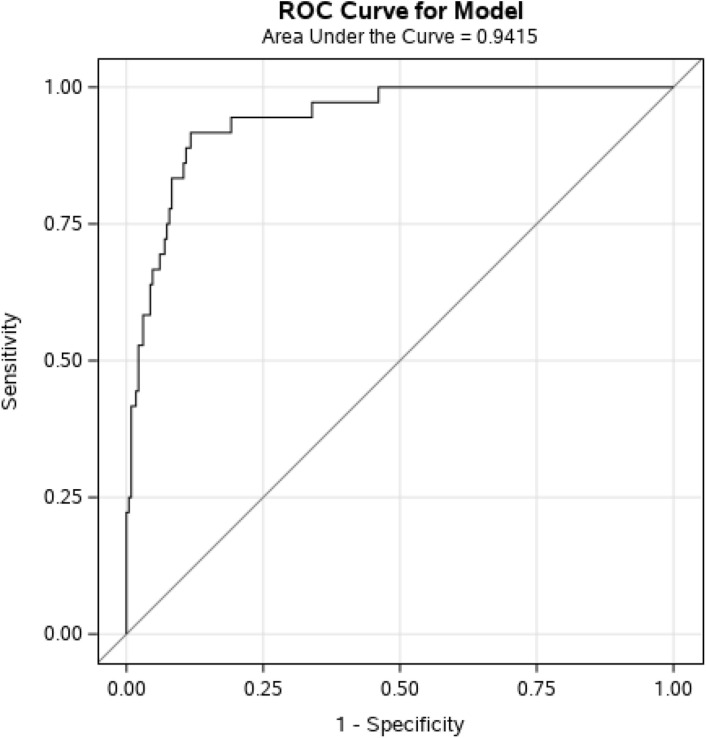
Our model for mortality demonstrated an AUC of 0.942 with a sensitivity of 88.8%, a specificity of 88.4%, a negative predictive value of 98% and a positive predictive value of 54.4% (Fig. 2 , Table 4). When analyzed without cardiac troponin, the AUC of this model was 0.924.
Fig. 2.
Receiver operating characteristic (ROC) curve for the multivariable propensity inverse probability model for mortality including hypertension and diabetes, structural heart disease, chronic kidney disease, age, APACHE II score, C-reactive protein and hs-cTnI.
4. Discussion
Our data clarify and extend previous reports and provide new insights about how to deploy cTn to evaluate patients with COVID-19. Previous studies have demonstrated that myocardial injury is frequent in critically ill patients [5], [24] and in particular in those with acute respiratory illness [6] and is associated with adverse outcomes. Similarly, myocardial injury is frequent in patients with COVID-19, especially in those with cardiovascular comorbidities, and predicts a more severe disease course [13]. Unfortunately, many studies do not describe the type of assay used, some suggest they are using a high sensitivity assay when they were not [13] and others have not used the proper 99th URLs and only a few have probed sex-specific thresholds [13]. Most studies report strong associations between myocardial injury and adverse outcomes [25]. In a recent study, hs-cTn was not a predictors of adverse events in unselected COVID-19 patients after adjustment for clinical variables [26]. Our data clarify these reports.
In our study, hs-cTnI was a simple way of anticipating an adverse clinical course or predicting a good one. Specifically, values above the 99th% URL had good predictive value for mortality especially with the use of an overall cut off value of 26 ng/L. It reached an AUC of 0.811. When integrated in a multivariable model, the AUC was as high as 0.942. However, removing hs-cTnI only reduced the model modestly to 0.925. These data are in keeping with the study from Omland et al. [26] who reported that in unselected patients hospitalized with COVID-19, cardiac biomarkers did not provide prognostic information beyond clinical characteristics and a severity of illness score.
However, that does not imply that hs-cTn values are not useful. First and importantly, similar to previous investigations [5], [6], hs-cTn values were only prognostic in those who were not severely ill. In fact, myocardial injury carried prognostic significance in patients with lower APACHE II scores but not in those with high scores. Thus, hs-cTnI helps to identify those who are less severely ill but are also at risk which might be relevant for triage of these patients. Moreover, its use may be more clinically convenient than a more complex multivariable model including clinical variables.
In addition, we identified low values in men and in women that portended better prognosis. The value of 5 ng/L, proposed as cutoff for low risk of myocardial infarction or cardiac death in previous studies [16], [27], identified 33% of patients in our cohort that were at low risk of death with a sensitivity of 97.8% and a NPV of 99.2%. Cohort specific cutoffs of 3 ng/L for men and 12 ng/L for women also predicted a favorable clinical course in the multivariable analysis. Thus, although Omland et al. [26] may be correct in one sense, their conclusion that the use of cTn values is not helpful is not in keeping with our data.
Our data are applicable to the routine clinical situation. The application of cTn testing was based on clinical need which was present in 73.5% of patients. In addition, we used a highly sensitive assay, which is infrequent in the literature [28], [29]. Other reports have used hs-cTn assays that, based on the International Federation of Clinical Chemistry (IFCC) Criteria [30], would not meet criteria for “high sensitive” assays [31]. In patients in whom samples were obtained, 27% of patients had myocardial injury. Despite a highly sensitive assay, the percentage of those with increases was lower than previously described [8], [12], [21]. This percentage, however, refers to baseline hs-cTnI values, before complications of the disease occurred. Higher prevalences in other studies may reflect sampling later during the hospital course.
In our model, cardiovascular risk factors such as hypertension and diabetes were not associated with increased mortality when adjusted for structural cardiac disease and hs-cTnI. We found that patients with hypertension and diabetes without structural heart disease had a mortality rate of 9% whereas for those who had those risk factors accompanied by the presence of structural heart disease, the mortality was 30%. These findings may reflect that clinicians were more prone to order hs-cTnI in those with risk factors which enriched them in our population. Perhaps in an “all comers” study, that would not be the case.
The mechanisms for cardiac troponin elevation are still a matter of debate. We have argued that there are phases to the disease [13]. The first related to chronic cardiovascular disease because of the propensity of the virus to infect those with cardiovascular comorbidities; the second related to critical illness with ARDS where hypoxia may play a prominent role [32], [33] and the third related to the complications of COVID-19 [34]. As in other studies, we did not find a high frequency of type 1 MI or Type 2 MI. Similarly, myocarditis was rare. This is an area of evolving knowledge [35], [36] in an environment of viral infection and pro-inflammatory cytokines [36].
In our data set, hs-cTnI values were higher in men than in women who survived [31]. However, in non-survivors, women manifested higher hs-cTnI values than men. Perhaps women presented later with more advanced stages of the disease, similar to what happens with acute coronary syndromes [37]. We were unable to find a significant difference in severity of illness parameters between the two sexes. Thus, this finding may be related to underdiagnosed comorbidities in women who died.
4.1. Limitations
This study was a retrospective, single center study. The decision to measure hs-cTnI was based on clinical judgment which can introduce selection bias. We attempted to rectify this by weighting the analysis by propensity score inverse probability weights. Furthermore cTn was not measured systematically at precise time intervals so we cannot comment on the dynamic or stable nature of the myocardial injury. In addition, other laboratory markers such as BNP were evaluated in only a limited numbers of patients. Finally, this study focused on an early characterization of patients at a time when triage and hospital placement might be relevant.
5. Conclusions
In this study of patients diagnosed with COVID-19, hs-cTnI, measured with a highly sensitive assay on admission, had the ability to provide important information about the subsequent clinical course of patients. It helped identify both those at high risk and those at low risk and alone it manifested significant predictive information. Importantly, it detected those who were less ill but at risk and those most likely to have favorable outcomes. These results can help to guide risk stratification and decisions concerning management on a prospective basis.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declaration of Competing Interest
Dr. Jaffe presently or in the past has consulted for most of the major diagnostic companies. Dr. Sandoval has participated as an advisory board/speaker for Abbott Diagnostics without personal financial compensation and on the advisory board (past) for Roche Diagnostics without personal financial compensation. All the other authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.clinbiochem.2021.01.006.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Cummings M.J., Baldwin M.R., Abrams D. Epidemiology, clinical course, and outcomes of critically ill adults with COVID-19 in New York City: a prospective cohort study. Lancet. 2020;395:1763–1770. doi: 10.1016/S0140-6736(20)31189-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.WHO Coronavirus Disease (COVID-19) Dashboard. (n.d.). Retrieved November 24, 2020, from Who.int website: http://Covid19.who.int.
- 3.Zhou F., Yu T., Du R. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guan W.J., Ni Z.Y., Hu Y. Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl. J. Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Babuin L., Vasile V.C., Rio Perez J.A. Elevated cardiac troponin is an independent risk factor for short- and long-term mortality in medical intensive care unit patients. Crit. Care Med. 2008;36:759–765. doi: 10.1097/CCM.0B013E318164E2E4. [DOI] [PubMed] [Google Scholar]
- 6.Vasile V.C., Chai H.-S., Khambatta S. Significance of elevated cardiac troponin T levels in critically ill patients with acute respiratory disease. Am. J. Med. 2010;123:1049–1058. doi: 10.1016/j.amjmed.2010.06.021. [DOI] [PubMed] [Google Scholar]
- 7.Shi S., Qin M.u., Shen B. Association of Cardiac Injury With Mortality in Hospitalized Patients With COVID-19 in Wuhan. China. JAMA Cardiol. 2020;5(7):802–810. doi: 10.1001/jamacardio.2020.0950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guo T., Fan Y., Chen M. Cardiovascular Implications of Fatal Outcomes of Patients With Coronavirus Disease 2019 (COVID-19) JAMA Cardiol. 2020;5:811–818. doi: 10.1001/jamacardio.2020.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huang C., Wang Y., Li X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan. China. The Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lala A., Johnson K.W., Januzzi J.L. Prevalence and Impact of Myocardial Injury in Patients Hospitalized With COVID-19 Infection. J Am Coll Cardiol. 2020;76:533–546. doi: 10.1016/j.jacc.2020.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Inciardi R.M., Adamo M., Lupi L. Characteristics and outcomes of patients hospitalized for COVID-19 and cardiac disease in Northern Italy. Eur Heart J. 2020;41:1821–1829. doi: 10.1093/eurheartj/ehaa388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Raad M., Dabbagh M., Gorgis S. Cardiac Injury Patterns and Inpatient Outcomes Among Patients Admitted With COVID-19. Am. J. Cardiol. 2020;133:154–161. doi: 10.1016/j.amjcard.2020.07.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sandoval Y., Januzzi J.L., Jaffe A.S. Cardiac Troponin for Assessment of Myocardial Injury in COVID-19: JACC Review Topic of the Week. J Am Coll Cardiol. 2020;76:1244–1258. doi: 10.1016/j.jacc.2020.06.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jaffe A.S., Cleland J.G.F., Katus H.A. Myocardial injury in severe COVID-19 infection. Eur. Heart J. 2020;41:2080–2082. doi: 10.1093/eurheartj/ehaa447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thygesen K., Alpert J.S., Jaffe A.S. Executive Group on behalf of the Joint European Society of Cardiology /American College of Cardiology /American Heart Association /World Heart Federation Task Force for the Universal Definition of Myocardial I. Fourth Universal Definition of Myocardial Infarction (2018) Circulation. 2018;138:e618–e651. doi: 10.1161/CIR.0000000000000617. [DOI] [PubMed] [Google Scholar]
- 16.Shah A.S.V., Anand A., Sandoval Y. High-sensitivity cardiac troponin I at presentation in patients with suspected acute coronary syndrome: a cohort study. Lancet. 2015;386:2481–2488. doi: 10.1016/S0140-6736(15)00391-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Khwaja A. KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin. Pract. 2012;120:c179–c184. doi: 10.1159/000339789. [DOI] [PubMed] [Google Scholar]
- 18.Force A.D.T., Ranieri V.M., Rubenfeld G.D. Acute respiratory distress syndrome: the Berlin Definition. JAMA. 2012;307:2526–2533. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 19.Harris P.A., Taylor R., Minor B.L. The REDCap consortium: Building an international community of software platform partners. J Biomed Inform. 2019;95 doi: 10.1016/j.jbi.2019.103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hanley J.A., Negassa A., Edwardes M.D. Statistical analysis of correlated data using generalized estimating equations: an orientation. Am. J. Epidemiol. 2003;157:364–375. doi: 10.1093/aje/kwf215. [DOI] [PubMed] [Google Scholar]
- 21.Lunceford J.K., Davidian M. Stratification and weighting via the propensity score in estimation of causal treatment effects: a comparative study. Stat. Med. 2004;23(19):2937–2960. doi: 10.1002/sim.1903. [DOI] [PubMed] [Google Scholar]
- 22.Finfer S.R., Vincent J.-L., Vincent J.-L., De Backer D. Circulatory shock. N Engl J Med. 2013;369:1726–1734. doi: 10.1056/NEJMra1208943. [DOI] [PubMed] [Google Scholar]
- 23.Six A.J., Cullen L., Backus B.E. The HEART score for the assessment of patients with chest pain in the emergency department: a multinational validation study. Crit. Pathw. Cardiol. 2013;12(3):121–126. doi: 10.1097/HPC.0b013e31828b327e. [DOI] [PubMed] [Google Scholar]
- 24.Guest T.M., Ramanathan A.V., Tuteur P.G., Schechtman K.B., Ladenson J.H., Jaffe A.S. Myocardial injury in critically ill patients. A frequently unrecognized complication. JAMA. 1995;273(24):1945–1949. [PubMed] [Google Scholar]
- 25.Lombardi C.M., Carubelli V., Iorio A. Association of Troponin Levels With Mortality in Italian Patients Hospitalized With Coronavirus Disease 2019: Results of a Multicenter Study. JAMA Cardiol. 2020;5(11):1274–1280. doi: 10.1001/jamacardio.2020.3538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Omland T., Prebensen C., Røysland R. Established Cardiovascular Biomarkers Provide Limited Prognostic Information in Unselected Patients Hospitalized With COVID-19. Circulation. 2020;142(19):1878–1880. doi: 10.1161/CIRCULATIONAHA.120.050089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chapman A.R., Lee K.K., McAllister D.A. Association of High-Sensitivity Cardiac Troponin I Concentration With Cardiac Outcomes in Patients With Suspected Acute Coronary Syndrome. JAMA. 2017;318:1913–1924. doi: 10.1001/jama.2017.17488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nie S.F., Yu M., Xie T. Cardiac Troponin I Is an Independent Predictor for Mortality in Hospitalized Patients With COVID-19. Circulation. 2020;142:608–610. doi: 10.1161/CIRCULATIONAHA.120.048789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cipriani A., Capone F., Donato F. Cardiac injury and mortality in patients with Coronavirus disease 2019 (COVID-19): insights from a mediation analysis. Intern. Emerg. Med. 2020;27:1–9. doi: 10.1007/s11739-020-02495-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Apple F.S., Jaffe A.S., Collinson P. IFCC educational materials on selected analytical and clinical applications of high sensitivity cardiac troponin assays. Clin. Biochem. 2015;48:201–203. doi: 10.1016/j.clinbiochem.2014.08.021. [DOI] [PubMed] [Google Scholar]
- 31.Apple F.S., Wu A.H.B., Sandoval Y. Sex-Specific 99th Percentile Upper Reference Limits for High Sensitivity Cardiac Troponin Assays Derived Using a Universal Sample Bank. Clin. Chem. 2020;66:434–444. doi: 10.1093/clinchem/hvz029. [DOI] [PubMed] [Google Scholar]
- 32.Nan J., Jin Y.B., Myo Y., Zhang G. Hypoxia in acute cardiac injury of coronavirus disease 2019: lesson learned from pathological studies. J Geriatr Cardiol. 2020;17:221–223. doi: 10.11909/j.issn.1671-5411.2020.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zaninotto M., Mion M.M., Padoan A. Cardiac troponin I in SARS-CoV-2-patients: The additional prognostic value of serial monitoring. Clin Chim Acta. 2020;511:75–80. doi: 10.1016/j.cca.2020.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Libby P., Luscher T. COVID-19 is, in the end, an endothelial disease. Eur Heart J. 2020;41:3038–3044. doi: 10.1093/eurheartj/ehaa623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Inciardi R.M., Lupi L., Zaccone G. Cardiac Involvement in a Patient With Coronavirus Disease 2019 (COVID-19) JAMA Cardiol. 2020;5(7):819. doi: 10.1001/jamacardio.2020.1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Basso C., Leone O., Rizzo S. Pathological features of COVID-19-associated myocardial injury: a multicentre cardiovascular pathology study. Eur. Heart J. 2020;41(39):3827–3835. doi: 10.1093/eurheartj/ehaa664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mehta L.S., Beckie T.M., DeVon H.A. Acute Myocardial Infarction in Women: A Scientific Statement From the American Heart Association. Circulation. 2016;133(9):916–947. doi: 10.1161/CIR.0000000000000351. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.