Abstract
Purpose
Lycopene has produced robust clinical effects and shows a promising chemopreventive in the oral cancer and precancerous lesions. However, much is still unknown about its mechanisms of the carotenoid in protecting against oral squamous cell carcinoma (OSCC). Insulin-like growth factor 1 (IGF1) pathway serves as a key regulatory signal pathway in the tumor microenvironment, which may be associated with the angiogenesis, tumorigenicity, and cancer proliferation. The current study was focused on elucidating the potential pathway played for lycopene to exert its function in treating with OSCC.
Materials and Methods
Firstly, we explored the dose- and time-response of CAL-27 and WSU-HN6 cells to lycopene. Both cells were incubated with various concentrations of lycopene (0.25, 0.5, 1, 2 µM). The inhibiting rate of cell proliferation was assessed using MTT assay. To observe the regulating effect of lycopene on OSCC, cell migration, apoptosis and tumor formation were detected in vitro and in vivo. The potential signaling pathways of OSCC cells treated with lycopene were analyzed by Affymetrix microarrays. And then, we investigated the changing of IGF1 signaling pathway, on the protein levels of tumor tissue after lycopene.
Results
Cell proliferation was inhibited by lycopene in a dose- and time-dependent manner. The optimum inhibition efficiencies for OSCC cells were also found. Further, the results also demonstrated that pre-treatment of OSCC with lycopene drastically induced cell apoptosis suppresses cell migration and tumor growth. Mechanistically, ingenuity pathway analysis further revealed that IGF1 pathway participate in killing effects on OSCC after treatment of lycopene. Lycopene may inhibit the pathway by regulating protein expression of IGF1, IGF binding protein (BP) 1, IGFBP3, transcription factor Jun/AP-1 (JUN), and forkhead box O1 (FOXO1).
Conclusion
These observations indicate that lycopene regulates OSCC cell growth by inhibiting IGF1 pathway, which may be a promising agent for the treatment of OSCC.
Keywords: lycopene, insulin-like growth factor 1, oral squamous cell carcinoma, cell proliferation
Introduction
Oral squamous cell carcinoma (OSCC) is not only one of the most prevalent malignancies worldwide but also can produce severe disfiguring lesions. However, evidence has been found that conventional therapies such as surgery, chemotherapy and radiation lead to the unsatisfied clinical efficacy.1 Especially for patients in early-stage OSCC, side effects of the conventional therapies should be considered seriously. Recently, the focus of tumor prevention has shifted to diet and life style.2 For cancer patients, scientific evidence recommend a diet that includes sufficient foods rich in antioxidants.3
Lycopene, an active carotenoid and potent natural source antioxidant, has proven useful in the prevention of oral precancerous lesions such as oral erythroplakia, oral submucous fibrosis and oral leukoplakia.4,5 Earlier case-control studies of serum antioxidant micronutrients and oral precancerous lesions risk observed that carotenoids may provide protection against oral cancer in Asian men.6 Such results suggest that intake of lycopene, primarily through consumption of tomatoes and red vegetables, is related to a reduced risk of OSCC, but the evidence has been inconsistent.7 Based on the previous findings, lycopene decreased intracellular and mitochondrial reactive oxygen species (ROS) level in OSCC cells, which can inhibit proliferation and induce apoptosis via PI3K/AKT/mTOR Pathway.8 Experts in increasing numbers are firmly believed that lycopene might be a promising anticancer agent in oral carcinogenesis.9
Several different mechanisms of lycopene have been found in killing tumor cells, such as induce changes in the tumor vasculature, change the cell cycle distribution and improve gap-junction communications.9–11 However, the precise mechanisms of lycopene are not well understood. Elucidation of the mechanism is very important for the development of specific nutrients. Insulin-like growth factors (IGFs) contain insulin, IGF1, and IGF2 and their receptor (insulin receptor, IGF1R, and IGF2R), and six binding proteins. After binding of IGF1 to the α subunits of IGF1R, the conformation and function of β subunit can be changed, resulting in activating tyrosine kinase mediated signalling.12 IGF1 signalling has been served as a potential tumour associated pathway, of which IGFs have many kinds of bioactivities in the tumour microenvironment. IGFs and their receptor are often overexpressed in the growth-stimulated cells, which have distinct functions in cell proliferation and differentiation. In a recent study by Liu et al13 tumor-associated macrophages (TAMs) model was induced in C57 mice, and the level of CD204 was used to identify the success of the model. And they found that TAMs may play roles in promoting tumorigenesis and metastasis by the IGF1 signalling pathway. In human colitis-associated cancer, inactivation of IGF1 receptors are directly associated with the development of cancer.14 A meta-analysis offers stronger evidence that IGF1 gene polymorphism may be responsible for the increase of susceptibility to cancer in Asian populations, which includes over 18,000 cases of various cancer.15 It is interesting that lycopene could suppress cell growth via IGF 1 in human cancer cells, according to a new study.16 Although lycopene is widely used for food and drink in Asia, there is no research study about the mechanisms of lycopene on OSCC. In this study, it is reported that IGF1 signalling is activated in OSCC cell lines after lycopene, and we examined the effects of lycopene for OSCC in vitro and in vivo.
Materials and Methods
Cell Lines and Culture
OSCC cell lines that included CAL-27 and WSU-HN6 cells were obtained from American Type Culture Collection (ATCC, USA). The cell lines were maintained in Dulbecco’s modified Eagle’s medium (Gibco, Invitrogen, Carlsbad, CA, USA) with 10% fetal bovine serum (FBS) at 37 C° under 5% CO2. Lycopene was purchased from Sigma‐Aldrich Co (L9879), which could be dissolved by tetrahydrofuran. Lycopene group was divided into four subgroups by different drug concentrations: 0.25, 0.5, 1, 2 µM, and the control group used tetrahydrofuran without lycopene. CAL-27 and WSU-HN6 cells were incubated in vitro in the above groups from 12 to 72 h. The methods for dissolving and keeping were used as described previously.8,10
Western Blotting Assay
CAL-27 and WSU-HN6 cells were lysed with RIPA buffer containing protease cocktails (Thermo Fisher Scientific, Inc.). Protein concentrations were measured with the BCA protein assay reagent kit (Beyotime, Shanghai, China). Equal amounts of total proteins were resolved on SDS-PAGE and then transferred to a PVDF membrane (Millipore, Billerica, MA, USA). The membranes were blocked with buffer (phosphate‐buffered saline containing 0.1% Tween and 5% skim milk) and incubated with primary antibodies at 4°C overnight. After washing with TBST three times, membranes were incubated for 1.5 h at room temperature with secondary antibody. After washing was repeated, the proteins were detected using electrochemiluminescence according to the manufacturer’s instructions (Thermo Fisher Scientific, Inc.). Anti-IGF1 (1:220, Atlas Antibodies Cat#HPA048946, RRID:AB_2680566), anti-IGF binding protein (BP) 1 (1:500, Santa Cruz Biotechnology Cat#sc-25257, RRID:AB_627782), anti-IGFBP3 (1:500, Santa Cruz Biotechnology Cat#sc-9028, RRID:AB_2233528), anti-transcription factor Jun/AP-1 (JUN) (1:200, Atlas Antibodies Cat# HPA059474, RRID:AB_2684030), anti-forkhead box O1 (FOXO1) (1:250, Atlas Antibodies Cat#HPA001252, RRID:AB_1078906), or anti-GAPDH (1:1000, Atlas Antibodies Cat#HPA040067, RRID:AB_10965903). GAPDH was used as an internal reference.
Cell Growth Assay
Cell viability was measured using a 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyl tetrazolium bromide (MTT) kit (Genview Cat. No. JT343, Beijing, China), according to the method described previously.13 Cells were planted into 96-well plates and incubated at 37°C. Then, 20 μL MTT solution at a concentration of 5 mg/mL was added to each well and incubated for 4 h. After removed the supernate, 10 μL of dimethyl sulfoxide was added to each well. The absorbance was detected at 490/570 nm using an automatic microplate reader (BioTek ELX808 American).
Detection of Apoptosis by Flow Cytometry
For measurement of cell apoptosis of in vitro cultured CAL-27 and WSU-HN6 cells, 1×106 cells were detected by Annexin V-FITC/PI apoptosis detection kit (eBioscience, Cat. No. 88–8007, San Diego, CA, USA). Cells were fixed, permeabilized and labeled with Annexin V-FITC/PI staining according to the manufacturer’s instructions. The apoptotic cells were assayed using a Guava EasyCyte Flow Cytometer (Millipore American). The apoptosis index (AI) of the cells of different groups was calculated using the following formula: AI (%) = (number of positive cells/total number of cells) x 100%.
Wound Healing Assay
To examine an in vitro wound healing assay, OSCC cells were seeded in 96-well plates with 100 µL culture medium supplemented with 10% FBS and then the shaped wounds ~800 µm in width were made by a WoundMaker (Essen Bioscience, Inc.). After washing three times with PBS, the cells were cultured in serum-free medium for 6 h at 37°C. The cell migration was evaluated using a light microscope (Nikon Corporation) at ×10 magnification in order to calculate the relative mobility.
Animal Experiments
The animal experiments were approved by the Institutional Animal Care and Use Committee of the Peking University Health Science Center (No. LA2018170). The experiment was performed according to Animals Usage Guideline by the Committee of Peking University Health Science Center. Athymic nude mice, 4 to 6 weeks of age, were purchased from Vital River Laboratory Animal Technology Co., Ltd (Beijing, China). All mice received humane care according to the university’s ethical guidelines. Nude mice were randomly divided into the control group and lycopene group (n=5). The control group: CAL-27 cells (1x106) were mixed in 200 μL PBS and then injected subcutaneously near the axilla of the nude mice. The lycopene group: cancer cell injection and oral administration of lycopene with a dose of 16 mg/kg twice per week. After tumor injection, gross tumor volume of all nude mice was measured using Vernier caliper (every 3 days). On day 22, the animals were euthanized, and the tumor growth curves were drawn. Gross tumor volume (cm3)= 3.14/6 x length x width2. The methods for feeding were used on the basis of previous studies.17,18
Real‐Time Quantitative PCR Analysis
Total RNA was isolated from cells by using the TRIzol Reagent (Pufei, Cat. No. 3101–100, Shanghai, China) according to the manufacturer’s protocol. Reaction mixture containing total RNA was reverse transcribed to cDNA using M-MLV Reverse Transcriptase (Promega, Cat. No. M1705, Madison, WI, USA). The data were calculated by 2−∆∆Ct method. Two groups of tissues were used to compare: primary cancer tissues and normal adjacent tissues (n=3). Significantly differentially expressed miRNAs were selected on the basis of fold change and false discovery rate (FDR) (fold change >1.5 and FDR <0.05)
DNA Microarrays and Ingenuity Pathway Analysis (IPA)
The Affymetrix GeneChip PrimeView Human Gene Expression Array was used for microarray processing to determine gene expression profiling in accordance with the manufacturer’s instructions. Pathway enrichment analysis was performed for all significant differentially expressed pr using IPA.
Statistical Analysis
Data are expressed as the mean ± standard deviation (SD) deviation and analyzed using the SPSS 17.0 Software (SPSS, Chicago, IL, USA). An unpaired Student’s t-test was performed on the difference between control group and lycopene group. The difference among groups was evaluated by one-way ANOVA. P<0.05 was deemed statistically significant difference.
Results
Lycopene Inhibits in vitro Cell Proliferation of OSCC Cell Lines
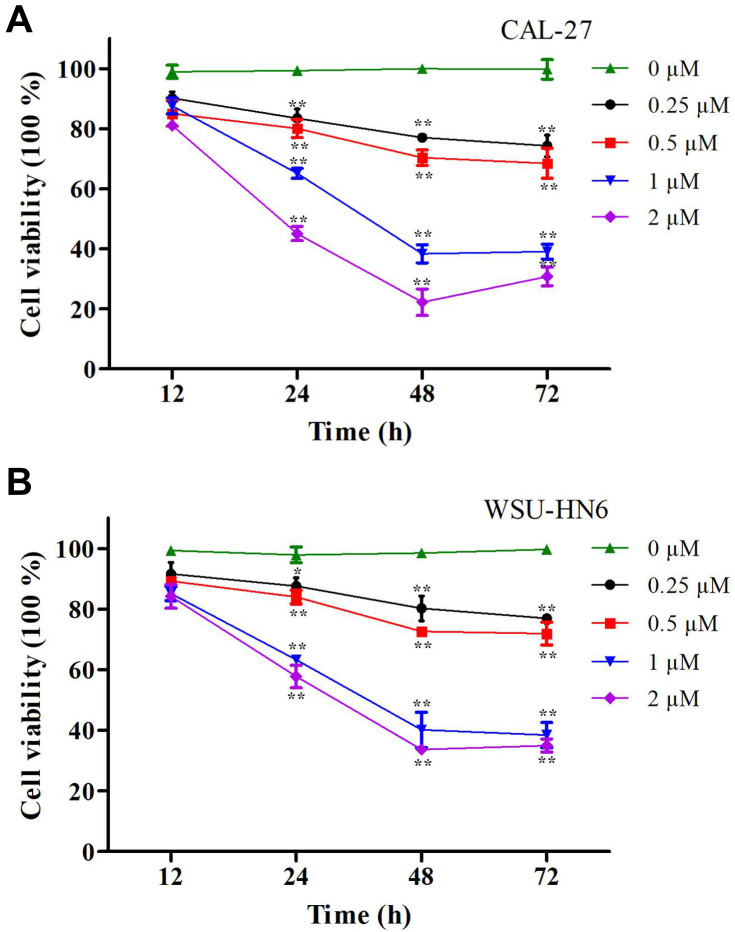
We explored the relationships between the concentrations of lycopene and cell cytotoxicity in this study. Firstly, CAL-27 and WSU-HN6 cells were incubated in different doses of lycopene (0.25, 0.5, 1, and 2 µM) at different times (12, 24, 48, and 72 h). When compared with the control group, cell viability of both cells in the lycopene group was significantly lower (P < 0.05). The results demonstrated that lycopene suppressed cell proliferation of both cells in a dose- and time-dependent manner. More importantly, we have obtained the maximum inhibition efficiencies at 2 µM lycopene after incubating 48 h later (Figure 1A and B). The IC50 of lycopene was also decreased in a time-dependent manner (12 h: 4.05 vs 2.24 mM for CAL-27 and WSU-HN6 cells, respectively; 24 h: 1.30 vs 1.26 mM for CAL-27 and WSU-HN6 cells, respectively; 48 h: 0.95 vs 0.83 mM for CAL-27 and WSU-HN6 cells, respectively; 72 h: 0.79 vs 0.61 mM for DOK and CAL-27 cells, respectively).
Figure 1.
Cell viability of OSCC cells determined by MTT assay after lycopene. (A) CAL-27 cells and (B) WSU-HN6 cells cell lines were incubated with 0.25–2 µM lycopene from 12 to 72 h. The percentage of cell viability was shown in a dose- and time-dependent manner. The data were performed independently in triplicate and presented as mean ± SD, *P<0.05, **P<0.01.
Lycopene Suppresses OSCC Cells Migration
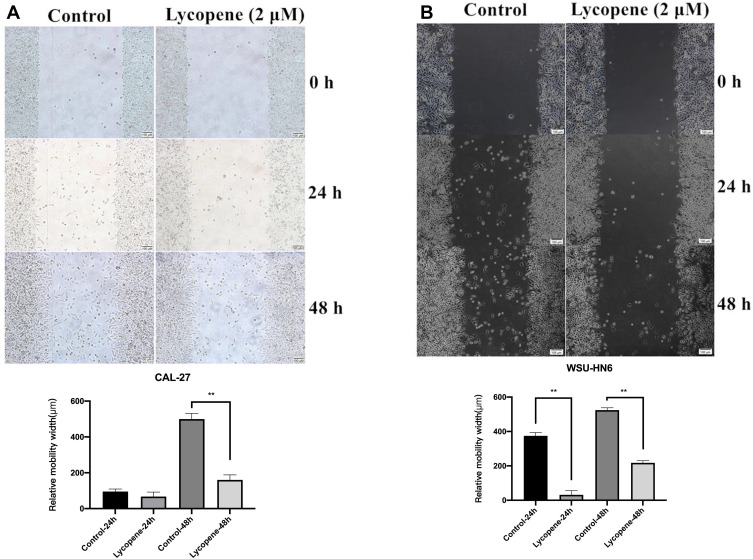
To detect the effect of 2 µM lycopene on cell migration, the wound healing assay was carried out in CAL-27 and WSU-HN6 cell lines. With 48 h of cultivation, much lower migration rates were detected in both cell lines treated with lycopene as compared with control cells (P < 0.01) (Figure 2A and B).
Figure 2.
Lycopene suppresses the cell migration of the OSCC cell lines (CAL-27, WSU-HN6). The wound-healing assay showed that the relative migration of cells incubated with lycopene in (A) CAL-27 and (B) WSU-HN6 cell lines was significantly less than the control group.
Lycopene Induces OSCC Cells Apoptosis
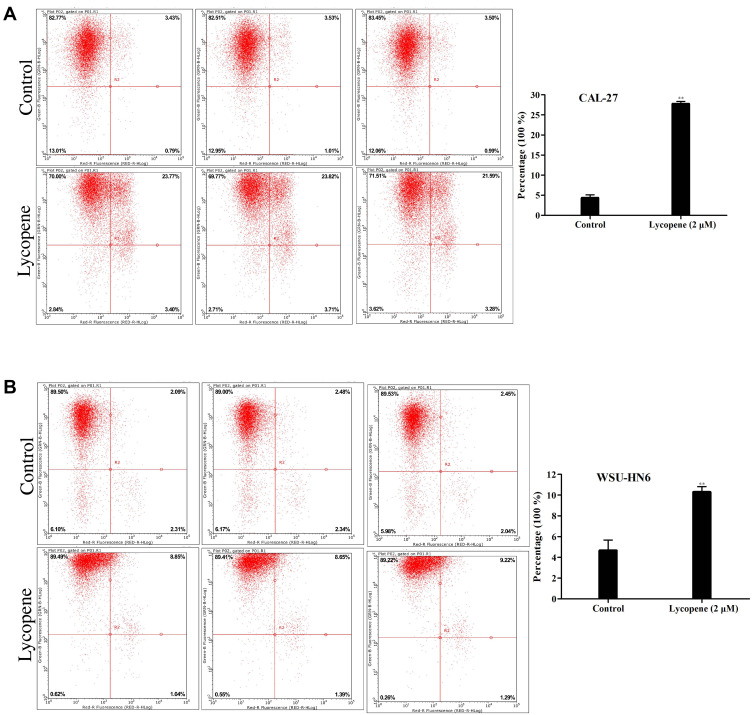
To assess the influence of lycopene on the apoptosis of OSCC cells, we used flow cytometry and AnnexinV staining to examine the apoptosis rate. It is shown clearly in Figure 3A and B which cell apoptotic rate of the two cells in the lycopene group (2 µM) increased profoundly compared with that in the control group. The percentages of apoptotic cells in the lycopene and control groups were 26.52±1.44% and 4.42± 0.17%, respectively (P<0.05) and 10.21±0.34% and 4.58±0.22%, respectively (P<0.01) in the CAL-27 cells and WSU-HN6 cells. All these results verified induction of apoptosis in OSCC cells was closely related to its effect on lycopene.
Figure 3.
Apoptosis assay using flow cytometry of (A) CAL-27 and (B) WSU-HN6 cell lines in the lycopene and control group. Representative flow cytometry figures are shown.
Lycopene Suppresses Tumor Growth in vivo
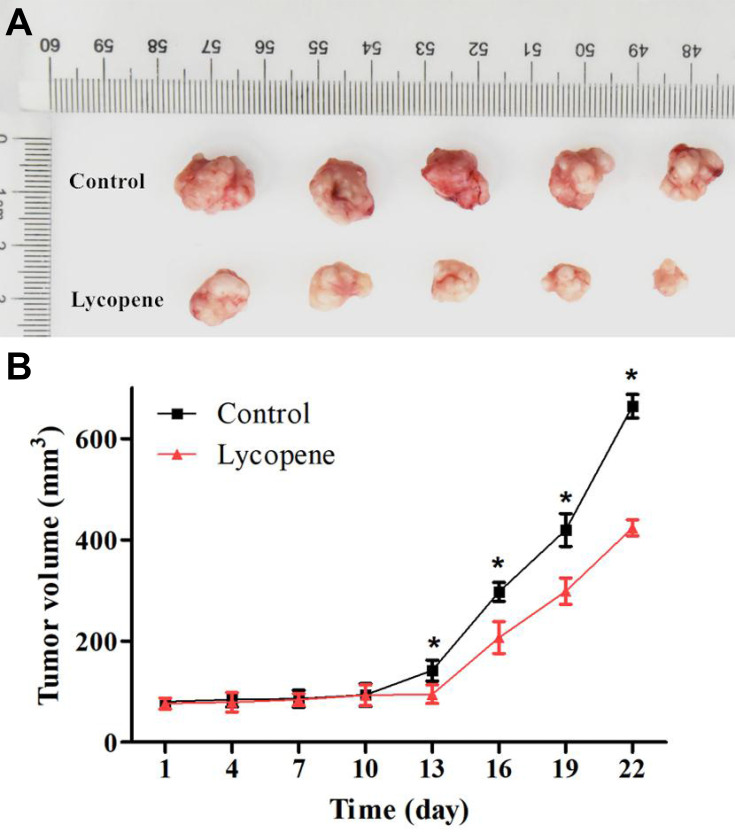
To investigate the effects of lycopene on the in vivo growth of OSCC cells, we applied the xenograft tumor model of CAL-27 cells, which were treated with cancer cell injection and/or oral administration of lycopene in nude mice (Figure 4A). And then, gross tumor volume in the lycopene group was significantly decreased after 13 days compared with those in the control group. After 22 days, the final tumor volume of the control group and lycopene group were 664.03±23.16 and 423.65±15.69 mm3, respectively (P<0.01; Figure 4B).
Figure 4.
(A) Lycopene inhibited tumor cell growth in nude mice (n = 5). (B) Tumor volume was estimated by the following equation: volume =1/2×(largest diameter)×(smallest diameter)2 and data are shown as the mean ± SD (n = 5 in each group).
Inhibition of IGF1 Signalling Pathway in OSCC Tissues After Lycopene
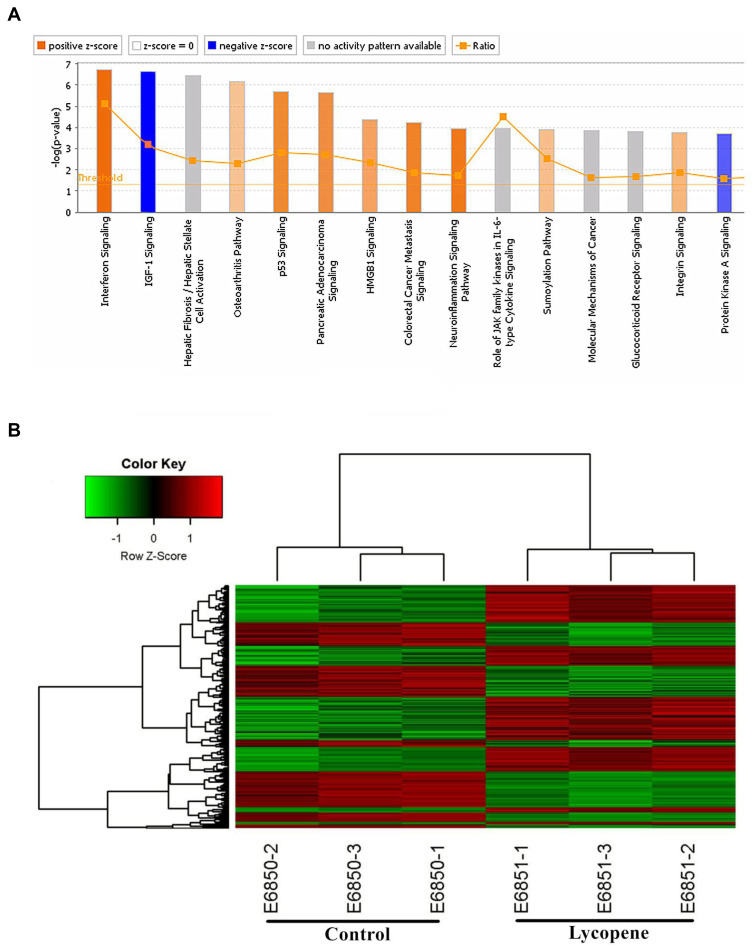
The bioinformatics analysis was performed between OSCC tissues treated with lycopene and untreated tumour tissues. In total, 997 genes were up-regulated and 572 were down-regulated. A paired Student’s t-test, applied to log2 LFQ intensity values, was used to determine the significance of gene differences between them (Figure 5A). In this study, to assess the underlying mechanisms of lycopene, we also used the Affymetrix GeneChip PrimeView Human Gene Expression Array to analyze differently expressed genes. For that, log2 values of differentially expressed proteins were normalized by Z-score. According to the result of pathway enrichment analysis, lycopene inhibited IGF1 signalling pathway significantly (Z-score<2; Figure 5B).
Figure 5.
Gene expression profiling identified an association between changes in the lycopene group. (A) Classical Pathway analysis identified genes enriched after incubating lycopene. Orange marks indicates activated (Z-score>0) and the blue marks means suppressed pathways (Z-score<0). (B) Hierarchical clustering of differentially expressed transcripts. The red dots represent upregulated relative expression, whereas green means indicates relative downregulation.
The Genes Related to the IGF1 Signalling Transduction Pathways in OSCC Tissues After Lycopene
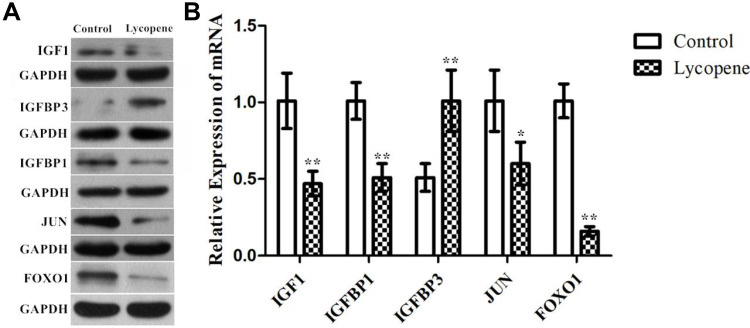
To verify the above analysis results, we observed changes in the protein expression of IGF1, IGF binding protein (BP) 1, IGFBP3, JUN and FOXO1 involved in the tumour tissues of the control and lycopene groups. We found that the levels of these proteins related to the IGF1 signalling varied obviously in the lycopene groups than in the control groups. The results showed that lycopene significantly reduced the expression levels of IGF1, IGFBP 1, JUN and FOXO1 (Figure 6A), but significantly increased in IGFBP 3 expression. Future, we also performed qRT-PCR to explore mRNA levels of them, which also supports the above data (Figure 6B). To sum up, these results suggested that lycopene has an ability to suppress IGF1 signalling transduction pathways in OSCC tissues.
Figure 6.
The protein and mRNA expression of IGF1 signalling pathway by Western blotting. Confirmation of microarray results using (A) Western blot analysis and (B) RT-qPCR of IGF1, IGFBP1, IGFBP3, JUN, and FOXO1 in tumor tissues. GAPDH was used as the internal control. In all above experiments, the data were performed independently in triplicate and presented as mean ± SD, *P<0.05, **P<0.01.
Discussion
Folk knowledge on lycopene in treating oral mucosal lesions has been focussed by the scientific community. Similar to long-held medical beliefs, lycopene intake can increase the clinical and histological response of oral leukoplakia, according to investigators at the KLES’s Institute of the Dental Sciences.19 A prospective randomized controlled trial (RCT) also revealed the advantages of lycopene in the efficacy and side effects.20 Moreover, multiple basic and clinical evidences confirm that phytochemicals may be a potential intervention means in treatment of oral cancer lesions. Among them is the target of efforts to develop anti-cancer drugs. In 2020, Wang et al8 reported that lycopene could perform the anticancer activities of oral cancer in vitro. Similar to the previous results, lycopene has suppression inhibitory effects to OSCC cell proliferation in our study, and the effect was in a time and dose-dependent manner. According to cell growth curves, we obtain the optimum inhibition efficiencies for both cells at 2 µM lycopene after incubating 48 h later. The optimum dosage of lycopene was used for further experiments. Indeed, lycopene could also remarkably induce the apoptosis in OSCC cell lines in vitro, as well as inhibit tumorigenesis in vivo.
OSCC is one of the poorest prognosis malignancies, due in part to its occult metastasis. Therefore, focusing the influence of lycopene governing OSCC migration may provide new targets for antimetastatic therapies. We sought to observe the role of lycopene function in OSCC cell migration from 0 to 48 h. Current wound healing assay data demonstrate that lycopene suppresses cell migration. Treatment of CAL-27 cells with lycopene resulted in a more pronounced inhibition of migration compared to WSU-HN6 cells. The results coincide with the discovery of previous research in other types of solid tumors. Elgass et al21 found that lycopene can inhibit matrix migration and adhesion in prostate cancer cell lines. The difference of cell migration reached statistical significance for three prostate cancer cells at 2.3 µM/l lycopene.
To improve therapy for OSCC patients, vital therapeutic targets of lycopene to explore are urgently needed. Our results demonstrated that 997 and 572 genes were up- and down-regulated in OSCC tissues treated with lycopene, respectively. The killing effect of lycopene on OSCC might be through intricate signalling pathway, which was analyzed by DNA microarray. It seemed that the IGF1 signaling pathway was potentially inhibited by lycopene in OSCC tissues (Z-score<2). It is important to note that the samples used for microarray analysis were the same as those used for the biological function tests of OSCC. IGF1 signalling is known to enhance the biological aggressiveness of HNSCC, where its impact can be amplified as a result of oxidative stress.22 Another report has noted that IGF1 signalling is also crucial for the growth of cancer cell lines without the primary oncogenic drivers.23 Karas et al24 revealed the novel mechanism of lycopene on IGF1 stimulated cell growth. With the decrease of lycopene concentration, DNA binding activity of the AP-1 transcription factor was also impaired in multiple type cancer cells. According to the result of the qRT-PCR and Western blots, several vital target genes of the IGF1 pathway was significantly up- or down-regulated in OSCC tissues after treatment of lycopene, such as IGF1, IGFBP1, IGFBP3, JUN, and FOXO1. A role for JUN phosphorylation in the regulation of cellular proliferation has been postulated. In addition, an experimental study to examine the mRNA level of JUN in tissues and its action on the critical gene in cells of OSCC.25 Lycopene was able to suppress IGF1 stimulated IR substrate 1 phosphorylation and cyclin D1 expression, to block cell cycle progression of cancer.26 These studies highlight the importance of IGF1 activity in the development of cancer. Both IGF1 and IGFBP1 expression were reduced significantly after lycopene in OSCC tissues in the present research. Notably, previous research has shown that lycopene intake increases serum total IGFBP3 concentrations in clinical trials and basic research.27,28 The presence and abnormal expression of pro-inflammatory gene, such as FOXO1 in the tumor microenvironment is consistent event within a maladjustment of chronic cellular immune response.29,30 It is identical to this study. By integrating all the results from this study, the effects of supplemental lycopene on tumor growth, apoptosis and migration in vitro and tumorigenicity in vivo might involve IGF1 pathway modulation.
Conclusion
Our present work indicates that lycopene acts as a promising treatment for human OSCC, notably, mechanistic analysis shows a critical regulatory IGF1 signaling pathway for the first time. Nevertheless, only a few in the biology function of lycopene have been well elucidated in this study. Therefore, plenty of work should focus on the function in vivo and critical mechanism, which may provide better evidence to understand the potential effects of lycopene.
Funding Statement
There is no funding to report.
Statement of Ethics
The study was approved by the Institutional Animal Care and Use Committee of the Peking University Health Science Center.
Disclosure
The authors declare that they have no financial or nonfinancial conflicts of interest for this work.
References
- 1.Mestrinho LA. Current status and future perspectives in canine oral squamous cell carcinoma. Vet Pathol. 2018;55(2):200–201. doi: 10.1177/0300985817732114 [DOI] [PubMed] [Google Scholar]
- 2.Gupta B, Bray F, Kumar N, et al. Associations between oral hygiene habits, diet, tobacco and alcohol and risk of oral cancer: a case-control study from India. Cancer Epidemiol. 2017;51:7–14. doi: 10.1016/j.canep.2017.09.003 [DOI] [PubMed] [Google Scholar]
- 3.Serna-Thomé G, Castro-Eguiluz D, Fuchs-Tarlovsky V, et al. Use of functional foods and oral supplements as adjuvants in cancer treatment. Rev Invest Clin. 2018;70(3):136–146. doi: 10.24875/RIC.18002527 [DOI] [PubMed] [Google Scholar]
- 4.Lu R, Dan H, Wu R, et al. Lycopene: features and potential significance in the oral cancer and precancerous lesions. J Oral Pathol Med. 2011;40(5):361–368. doi: 10.1111/j.1600-0714.2010.00991.x [DOI] [PubMed] [Google Scholar]
- 5.Saran G, Umapathy D, Misra N, et al. A comparative study to evaluate the efficacy of lycopene and curcumin in oral submucous fibrosis patients: a randomized clinical trial. Indian J Dent Res. 2018;29(3):303–312. doi: 10.4103/ijdr.IJDR_551_16 [DOI] [PubMed] [Google Scholar]
- 6.Nagao T, Ikeda N, Warnakulasuriya S, et al. Serum antioxidant micronutrients and the risk of oral leukoplakia among Japanese. Oral Oncol. 2000;36(5):466–470. doi: 10.1016/S1368-8375(00)00037-3 [DOI] [PubMed] [Google Scholar]
- 7.Maserejian NN, Giovannucci E, Rosner B, et al. Prospective study of vitamins C, E, and A and carotenoids and risk of oral premalignant lesions in men. Int J Cancer. 2006;120(5):970–977. doi: 10.1002/ijc.22448 [DOI] [PubMed] [Google Scholar]
- 8.Wang R, Lu X, Yu R. Lycopene inhibits epithelial-mesenchymal transition and promotes apoptosis in oral cancer via PI3K/AKT/m-TOR signal pathway. Drug Des Devel Ther. 2020;14:2461–2471. doi: 10.2147/DDDT.S251614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grabowska M, Wawrzyniak D, Rolle K, et al. Let food be your medicine: nutraceutical properties of lycopene. Food Funct. 2019;10(6):3090–3102. doi: 10.1039/C9FO00580C [DOI] [PubMed] [Google Scholar]
- 10.Kim M, Kim SH, Lim JW, et al. Lycopene induces apoptosis by inhibiting nuclear translocation of β-catenin in gastric cancer cells. J Physiol Pharmacol. 2019;70(4). doi: 10.26402/jpp.2019.4.11. [DOI] [PubMed] [Google Scholar]
- 11.Jeong Y, Lim JW, Kim H. Lycopene inhibits reactive oxygen species-mediated NF-κB signaling and induces apoptosis in pancreatic cancer cells. Nutrients. 2019;11(4):762. doi: 10.3390/nu11040762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Iams WT, Lovly CM. Molecular pathways: clinical applications and future direction of insulin-like growth factor-1 receptor pathway blockade. Clin Cancer Res. 2015;21(19):4270–4277. doi: 10.1158/1078-0432.CCR-14-2518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu L, Wang X, Li X, et al. Upregulation of IGF1 by tumor-associated macrophages promotes the proliferation and migration of epithelial ovarian cancer cells. Oncol Rep. 2018;39(2):818–826. doi: 10.3892/or.2017.6148 [DOI] [PubMed] [Google Scholar]
- 14.Ostermann AL, Wunderlich CM, Schneiders L, et al. Intestinal insulin/IGF1 signalling through FoxO1 regulates epithelial integrity and susceptibility to colon cancer. Nat Metab. 2019;1(3):371–389. doi: 10.1038/s42255-019-0037-8 [DOI] [PubMed] [Google Scholar]
- 15.Xu GP, Chen WX, Xie WY, et al. The association between IGF1 gene rs1520220 polymorphism and cancer susceptibility: a meta-analysis based on 12,884 cases and 58,304 controls. Environ Health Prev Med. 2018;23(1):38. doi: 10.1186/s12199-018-0727-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tjahjodjati SS, Umbas R, Satari M. The protective effect of lycopene on prostate growth inhibitory efficacy by decreasing insulin growth factor-1 in Indonesian human prostate cancer cells. Res Rep Urol. 2020;12:137–143. doi: 10.2147/RRU.S232745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tang FY, Pai MH, Kuo YH, et al. Concomitant consumption of lycopene and fish oil inhibits tumor growth and progression in a mouse xenograft model of colon cancer. Mol Nutr Food Res. 2012;56(10):1520–1531. doi: 10.1002/mnfr.201200098 [DOI] [PubMed] [Google Scholar]
- 18.Yang CM, Yen YT, Huang CS, et al. Growth inhibitory efficacy of lycopene and β-carotene against androgen-independent prostate tumor cells xenografted in nude mice. Mol Nutr Food Res. 2011;55(4):606–612. doi: 10.1002/mnfr.201000308 [DOI] [PubMed] [Google Scholar]
- 19.Singh M, Krishanappa R, Bagewadi A, et al. Efficacy of oral lycopene in the treatment of oral leukoplakia. Oral Oncol. 2004;40(6):591–596. doi: 10.1016/j.oraloncology.2003.12.011 [DOI] [PubMed] [Google Scholar]
- 20.Kumar A, Bagewadi A, Keluskar V, et al. Efficacy of lycopene in the management of oral submucous fibrosis. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007;103(2):207–213. doi: 10.1016/j.tripleo.2006.07.011 [DOI] [PubMed] [Google Scholar]
- 21.Elgass S, Cooper A, Chopra M. Lycopene treatment of prostate cancer cell lines inhibits adhesion and migration properties of the cells. Int J Med Sci. 2014;11(9):948–954. doi: 10.7150/ijms.9137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rajbhandari N, Lin W-C, Wehde BL, et al. Autocrine IGF1 signaling mediates pancreatic tumor cell dormancy in the absence of oncogenic drivers. Cell Rep. 2017;18(9):2243–2255. doi: 10.1016/j.celrep.2017.02.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Elferink LA, Resto VA. Receptor-tyrosine-kinase-targeted therapies for head and neck cancer. J Signal Transduct. 2011;2011:982879. doi: 10.1155/2011/982879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Karas M, Amir H, Fishman D, et al. Lycopene interferes with cell cycle progression and insulin-like growth factor I signalling in mammary cancer cells. Nutr Cancer. 2000;36(1):101–111. doi: 10.1207/S15327914NC3601_14 [DOI] [PubMed] [Google Scholar]
- 25.Xu H, Jin X, Yuan Y, et al. Prognostic value from integrative analysis of transcription factors c-Jun and Fra-1 in oral squamous cell carcinoma: a multicenter cohort study. Sci Rep. 2017;7(1):7522. doi: 10.1038/s41598-017-05106-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nahum A, Zeller L, Danilenko M, et al. Lycopene inhibition of IGF-induced cancer cell growth depends on the level of cyclin D1. Eur J Nutr. 2006;45(5):275–282. doi: 10.1007/s00394-006-0595-x [DOI] [PubMed] [Google Scholar]
- 27.Jiang B, Zhang X, Du -L-L, et al. Possible roles of insulin, IGF-1 and IGFBPs in initiation and progression of colorectal cancer. World J Gastroenterol. 2014;20(6):1608–1613. doi: 10.3748/wjg.v20.i6.1608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Valadez-Bustos N, Escamilla-Silva EM, García-Vázquez FJ, et al. Oral administration of microencapsulated B. longum BAA-999 and lycopene modulates IGF-1/IGF-1R/IGFBP3 protein expressions in a colorectal murine model. Int J Mol Sci. 2019;20(17):4275. doi: 10.3390/ijms20174275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Song H, Lai L, Liu M, et al. Investigating the role and mechanism of microRNA-196a in oral squamous cell carcinoma by targeting FOXO1. Exp Ther Med. 2020;19(6):3707–3715. doi: 10.3892/etm.2020.8614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zheng M, Cao M-X, Yu X-H, et al. STAT3 promotes invasion and aerobic glycolysis of human oral squamous cell carcinoma via inhibiting FoxO1. Front Oncol. 2019;9:1175. doi: 10.3389/fonc.2019.01175 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]