Abstract
Background
Despite the growing evidence on COVID-19, there are still many gaps in the understanding of this disease, especially in individuals in advanced age. We describe the study protocol of GeroCovid Observational, a multi-purpose, multi-setting and multicenter initiative that aims at investigating: risk factors, clinical presentation and outcomes of individuals affected by COVID-19 in acute and residential care settings; best strategies to prevent infection in long-term care facilities; and, impact of the pandemic on neuropsychologic, functional and physical health, and on medical management in outpatients and home care patients at risk of COVID-19, with a special focus on individuals with dementia.
Methods
GeroCovid involves individuals aged ≥60 years, at risk of or affected by COVID-19, prospectively or retrospectively observed since March 1st, 2020. Data are collected in multiple investigational sites across Italy, Spain and Norway, and recorded in a de-identified clinical e-Registry. A common framework was adapted to different care settings: acute wards, long-term care facilities, geriatric outpatient and home care, and outpatient memory clinics.
Results
At September 16th, 2020, 66 investigational sites obtained their Ethical Committee approval and 1618 cases (mean age 80.6 [SD=9.0] years; 45% men) have been recorded in the e-Registry. The average inclusion rate since the study start on April 25th, 2020, is 11.2 patients/day. New cases enrollment will ended on December 31st , 2020, and the clinical follow-up will end on June 30th, 2021.
Conclusion
GeroCovid will explore relevant aspects of COVID-19 in adults aged ≥60 years with high-quality and comprehensive data, which will help to optimize COVID-19 prevention and management, with practical implications for ongoing and possible future pandemics.
Trial registration
NCT04379440 (clinicaltrial.gov).
Keywords: COVID-19, Inpatients, Outpatients, Nursing Homes, Health Services for the Aged, Observational Study
Introduction
Older adults have been the part of the population most burdened by COVID-19 pandemic. Indeed, the disease mortality rate was reported to rise, in men and women, from 4.6% and 2.5% among those aged 30-39 years to 61% and 48% in individuals older than 80 years, respectively [1]. The age-related trend in mortality and the greater vulnerability of the male gender were confirmed worldwide [2], [3], [4].
Multimorbidity and frailty, whose prevalence increases with age [5,6], were supposed to partly explain the worse COVID-19 prognosis in advanced age [2,[7], [8], [9]]. “Inflammaging”, which is characterized by a complex, abnormal pattern of immune alterations promoting inflammation over a worthwhile immunologic response, is another possible cause of the age-related increased vulnerability to COVID-19 [10,11]. The cytokine storm, an ominous hallmark of the response to COVID-19, may also be more likely present and exacerbated in older people [10,12]. Apart from pathophysiologic aspects, it cannot be excluded that shortage of resources sometimes prompted to prioritize young and adult patients care, relatively limiting the access of older individuals to more intensive settings of care. In addition, it is possible that atypical presentations of COVID-19, deferring recognition and appropriate care, may have further complicated the clinical management of older patients [13].
It is also important to recognize that a considerable fraction of the excess mortality in older people has been related to poor logistic plans and organization of long-term care facilities, which made the residents at very high risk of infection and inadequate care [14,15]. Insufficient attention to appropriate disinfection practices and the shortage of protective equipment for the staff further contributed to make the nursing home an “at risk” setting for older individuals [16].
Finally, the COVID-19 pandemic affected the quality of care also of non-COVID-19 geriatric patients, especially those with dementia and psychiatric disorders, who were more likely to be burdened by the adverse effects of the loss of social contacts and continuous medical monitoring [17,18]. Even if difficult to quantify, the influence of pandemic on older multimorbid non-COVID-19 individuals determined relevant consequences, as shown by the dramatic increase of mortality after acute myocardial infarction and by the sharp worsening of psychiatric disorders observed after the beginning of the lockdown phase [19,20].
The Italian Society of Gerontology and Geriatrics, in collaboration with the Norwegian Geriatric Society, planned a multi-setting, multi-national and multi-scope registry, the Geriatric Population COVID-19 (GeroCovid) Observational Study, aimed at pursuing the following main objectives:
- to assess age-related changes in risk profile, clinical presentation, needs of care, and short- and medium-term outcomes of COVID-19 patients aged ≥60 years, in acute and residential settings;
- to explore the impact of the pandemic on the functional ability, cognitive, psychological and behavioral status of non-COVID-19 individuals aged ≥60 years, with special attention to patients with dementia;
- to identify the adaptive strategies used by outpatient and home care services to compensate for the limitation of contacts imposed by the COVID-19, with a focus on telemonitoring;
- to investigate the healthcare measures taken in long-term care facilities to prevent and contrast the COVID-19 pandemic.
This article describes the GeroCovid study protocol and provides a comprehensive overview of the methods, metrics and expected results of the project.
Methods
Study design and coordination
GeroCovid is an observational retrospective-prospective study involving adults aged ≥60 years, evaluated during the COVID-19 pandemic. The choice of this age limit will allow us to explore the study outcomes both in adults in working-age approaching retirement and those in more advanced age, capturing possible differences in risk factors, clinical course, prognosis, and burden of COVID-19. Moreover, such cut-off is currently used in several demographic statistics that report health-related data in the general population divided by 10-year age groups [21].
The study has been promoted by the Italian Society of Gerontology and Geriatrics and involves multiple investigational sites across Italy and Norway. GeroCovid includes cases observed since March 1st, 2020. Enrollment ended on December 31st, 2020 and the clinical follow-up of prospective cases will end on June 30th, 2021. The study is registered in clinicaltrial.gov (Trial Registration: NCT04379440).
GeroCovid was designed by the Italian Society of Gerontology and Geriatrics, in collaboration with Bluecompanion, which has developed and adapted a dedicated electronic registry to collect and harmonize clinical data from the different care settings. Based on the setting, the study has been structured into six main research cohorts: GeroCovid acute wards, GeroCovid home and outpatients’ care, GeroCovid dementia – drug monitoring, GeroCovid dementia – psychological health, GeroCovid long-term care facilities (LTCFs), and GeroCovid outcomes. The GeroCovid coordinating group involves clinicians specialized in geriatric medicine, experts in epidemiology, and ICT experts. This multidisciplinary group includes the coordinators of the different project cohorts. Under the supervision of the GeroCovid Principal Investigator (RAI) and the support of the methodological team (SDS, GZ), the cohorts’ coordinators closely monitor the research activities of the involved sites, including the enrollment of the study participants and the data collection. Since March 2020, the coordinating group is meeting through weekly videoconferences to discuss together the development and optimization of GeroCovid research activities, and to find solutions to possible concerns related to the study.
Study setting and objectives
GeroCovid is a multicentre and multi-setting study whose primary endpoint is represented by changes in health status, defined according to the World Health Organization (WHO) classification [22] and based on the incidence of hospitalizations, Serious Adverse Events (SAE) and death. The settings and the specific primary and secondary objectives for each GeroCovid cohort are reported in Table 1 .
Table 1.
Setting, inclusion criteria and objectives of the GeroCovid cohorts
| Cohort | Setting | Participantsinclusion criteria | Primary objective | Secondary objectives |
| GeroCovid acute wards | COVID-19 acute and post-acute wards | Patients hospitalized for SARS-CoV-2 infection | To evaluate the frequency of typical and atypical clinical presentations of COVID-19 in different age classes | - To investigate the association between clinical presentation and biochemical parameters at admission with the type and intensity of administered care, and with patients’ functional and clinical prognosis - To assess the influence of chronic diseases, polypharmacy, clinical presentation, and biochemical parameters at admission on the type and intensity of received care, and on patients’ prognosis |
| GeroCovid outcomes | Outpatient clinics involved in the care of patients recently hospitalized for COVID-19 | Individuals recently hospitalized for COVID-19, evaluated within 90 days from hospital discharge | To assess the clinical and functional outcomes of people recently hospitalized for COVID-19 | To evaluate whether clinical (e.g., hospital readmission, mortality) and functional outcomes are associated with patients’ comorbidities, the type of care setting and therapy, in-hospital COVID-19 severity, the overall length of hospital stay and social isolation |
| GeroCovid home and outpatients’ care | Geriatric outpatient and home care services | Individuals accessing geriatric outpatient or home care services, observed until 90 days from the implementation of physical distancing and remote monitoring with phone and video-call systems | To evaluate the clinical, social, functional, and psychological impact of the pandemics (related to social distancing and remote monitoring) in patients accessing geriatric outpatient or home care services | To assess the impact of the pandemics on individuals’ quality of life and dietary habits |
| GeroCovid dementia - drug monitoring | Outpatient memory clinics | Outpatients with dementia [29] on therapy with cholinesterase inhibitors, memantine and/or antipsychotics | To assess the ability of a telemonitoring approach in detecting adverse events related to anti-dementia and antipsychotic treatments | - To evaluate the change of behavioral symptoms at telemonitoring in comparison with pre-COVID-19 assessment- To explore the impact of social distancing on cognitive and functional status, depressive symptoms, and adherence to anti-dementia and antipsychotic treatments post-lock-down. |
| GeroCovid dementia – psychological health | Outpatient memory clinics | Outpatients with mild cognitive impairment or dementia | To evaluate the impact of social distancing due to COVID-19 pandemic on psychological health (anxiety, depression, perceived distress, coping responses) | To assess the effect of social distancing due to COVID-19 pandemic on the cognitive and functional status of older patients with cognitive deficits |
| GeroCovid long-term care facilities | Long-term care facilities (assisted living homes, nursing homes, retirement homes and rehabilitation centers) | Residents with suspected or confirmed SARS-CoV-2 infection | To evaluate the effectiveness of preventive measures implemented in long-term care facilities to control COVID-19 spread | - To identify the clinical and biochemical presentation of Covid-19 in residents - To assess the influence of comorbidities, ongoing treatments, previous vaccinations, and functional, cognitive and psychological status, on COVID-19 onset and course |
Study population
The GeroCovid study is consecutively enrolling individuals aged ≥60 years with or at risk of Covid-19, either retrospectively or prospectively observed. The "risk" of getting SARS-CoV-2 infection or of experiencing the negative effects of the pandemic, was specified in each setting. Of the six GeroCovid cohorts, two consider only Covid-19 patients during the hospital stay (GeroCovid acute wards) and after hospital discharge (GeroCovid outcomes). The GeroCovid LTCFs cohort involves both residents affected by Covid-19 and those at risk of getting SARS-CoV-2 infection according to suspected symptoms or contacts with Covid-19 confirmed cases. The risk of experiencing the negative effects of the pandemic was applied to the GeroCovid home and outpatients’ care, GeroCovid dementia – drug monitoring, and GeroCovid dementia – psychological health cohorts, which include, respectively, home-dwelling patients on geriatric home care and outpatients with dementia referred to memory clinic. Further details on the inclusion criteria for each GeroCovid cohort are reported in Table 1. Instead, exclusion criteria are: lack of signed informed consent to participate in the study; and, in case of impossibility to inform the patient due to her/his state of consciousness and/or awareness of disease condition, lack of a signed declaration by the investigator attesting that no explicit opt-out advanced directives by the subject existed at the inclusion in the registry.
Data collection
Trained physicians with expertise in geriatric medicine are collecting GeroCovid participants’ data in a specifically designed e-Registry, accessible through a dedicated online platform (details on the e-Registry can be found below, under “Data management and quality assurance”). Data collection in the GeroCovid e-Registry is organized in five main sections, to describe both the healthcare infrastructures (specialized COVID-19 hospitals, long-term care facilities, etc.) and the features of single cases observed across different care settings.
Characteristics of the participating centre. In the first step, the local coordinator of each site registers and describes his/her centre in the online platform. In this phase, information is given about the type (e.g., acute/post-acute ward for COVID-19 patients, outpatient clinic, memory clinic, long-term care facility) and the characteristics (e.g., number of beds, type and number of healthcare personnel, etc.) of the structure, the date in which the observation period started, the preventive measures implemented during the COVID-19 pandemics (e.g., adoption of strict limitations to family visits to patients, reduction of non-urgent specialist consultations, body temperature monitoring, isolation of suspected and confirmed COVID-19 cases).
Anamnestic information. For each GeroCovid participant, investigators collect information on his/her demographic characteristics, household setting, pre-Covid-19 lifestyle (smoking and drinking habits, mobility function and physical activity level), chronic diseases, nutritional status, frailty (using adapted criteria from Pedone et al., 2016 [23], and Fried et al., 2001 [5]), regularly used pharmacological treatments (coded through the ATC classification), ongoing or previous (until less than three months) hormonal replacement therapy, and previous influenza, anti-pneumococcal and anti-herpes zoster vaccinations. The main patient's diagnosis (and the initial hospital admission diagnosis, only for the GeroCovid acute wards patients who were originally hospitalized due to non-COVID-19 diseases), observation start and end dates, and type of outcome (classified as no major change, clinical improvement, serious adverse event, death, transfer to a different hospital, withdrawal) are also collected. Finally, recorded data also concern the impact of COVID-19 pandemics and physical distancing on social interactions, assistance in daily activities, care provision, psychological reaction and changes in care setting.
Observational phases. For each participant, investigators fill two or more observation modules corresponding to different disease- or evaluation-phases. Each observational module includes the following information: evaluation date and patient health status according to WHO classification [22], vital signs (blood pressure and heart rate), physical examination (general conditions and system-specific evaluation), anthropometry and nutritional status, diagnosis tests for SARS-CoV-2 infection (date and result of nasopharyngeal swab and/or serological tests), arterial blood gas test results, main findings at x-ray and/or computerized tomography scans, blood/urine analyses (including hematology, lipids, biochemistry, inflammatory and cardiac biomarkers, coagulation, thyroid hormones, hepatitis C screening, urine analysis), electrocardiographic test results, COVID-19-like symptoms and date of onset, procedures related to suspected COVID-19 clinical pattern, and updates of the pharmacological therapy.
Adverse events. Onset date, type (coded according to MedDRA classification), severity and outcomes of possible adverse event occurred during the observation period are recorded for each participant.
Specific evaluation scales. Specific information and evaluation scales for each GeroCovid cohort are also collected in the e-Registry. Details in this regard are reported in Table 2 .
Table 2.
Sample size and specific evaluation scales performed in each GeroCovid cohort
| Cohort | Sample size | Specific evaluation scales |
| GeroCovid acute wards | Primary outcome: frequency of typical and atypical clinical presentation of Covid-19 at older ages. Literature data: the maximum frequency of atypical COVID-19 symptoms reported by the literature is of 12% [3,24]. Sample size computation: under the hypothesis of a frequency of atypical symptoms >20% and considering a power of 80% and alpha = 0.05, the needed sample size was estimated to be at least of 128 patients (precision=7%). | ADL and IADL [30,31] |
| GeroCovid outcomes | Purposive sample of at least 100 outpatients recently hospitalized for COVID-19. | ADL and IADL [30,31]- CIRS [32] - MMSE [35]- mMRC [33] - 15-item GDS [34] - SF-36 [36] - STAI-Y [37] |
| GeroCovid home and outpatients’ care | Purposive sample of at least 100 outpatients at risk of COVID-19. | - ADL and IADL [30,31] - CIRS [32] - MMSE [35] - 5-/15-item GDS [34] - EUROQOL-5 [38] |
| GeroCovid dementia – drug monitoring | Primary outcome: incidence of adverse events related to chronic treatment with cholinesterase inhibitors and/or antipsychotic. Sample size computation: under the hypothesis of an incidence of adverse events related to chronic treatment with cholinesterase inhibitors and/or antipsychotic of about 10%, and considering an alpha=0.05, the needed sample size was estimated at least of 138 consecutive dementia outpatients (precision=5%). | - Checklist for therapeutic plan renewal of anti-cholinesterase drugs, memantine and antipsychotics - ADL and IADL [30,31] - MMSE [35] - 15-item GDS [34] - Adherence to prescribed treatment (semiquantitative assessment post-lock-down) |
| GeroCovid dementia – psychological health | Primary outcome: change in depressive and anxiety symptoms. Literature data: estimates on the prevalence of depression and anxiety in the Italian population are of 5.1% and 5.0%, respectively [39]. Chinese data showed a prevalence of depression and anxiety during COVID-19 pandemic of 16.5% and 28.8% [40]. Sample size computation: hypothesizing that the prevalence of depression and anxiety disorders in older people could double after the pandemics, and assuming a power of 80% and an alpha=0.05, the estimated sample size includes 240 individuals. Considering a drop-out of 20%, the final sample size should be of at least 290 patients. | - ADL and IADL [30,31] - CIRS [32] - MMSE [35] - MNA-SF [41] - 15-item GDS [34] - UCLA 3-items Loneliness Scale [42] - SIS [43] - COPE [44];- DASS-21 [45]; - CBI [46]; - PSS [47] - CRIq [48] - NPI [49] |
| GeroCovid long-term care facilities | Purposive sample of at least 100 institutionalized individuals with suspected or confirmed SARS-CoV-2 infection. | - ADL and IADL [30,31] - CIRS [32] - MMSE [35] - 4AT [50] - 5-/15-item GDS [34] - Cornell Scale for Depression in Dementia [51] |
Abbreviations: ADL, Activities of Daily Living; CBI, Caregiver Burden Inventory; CIRS, Cumulative Illness Rating Scale; CRIq, Cognitive Reserve Index questionnaire; DASS-21, Depression Anxiety Stress Scales-21; EUROQOL-5, European Quality of Life scale; GDS, Geriatric Depression Scale; IADL, Instrumental ADL; mMRC, modified Medical Research Council scale; MMSE, Mini-Mental State Examination; MNA-SF, Mini Nutritional Assessment Short Form; NPI, Neuropsychiatric Inventory; PSS, Perceived Stress Scale; SIS, Social Isolation Scale; STAI-Y, State-Trait Anxiety Inventory; UCLA, University of California, Los Angeles, Loneliness Scale; 4AT, 4 A's Test for delirium
Data management and quality assurance
The data collection of GeroCovid is performed in a European de-identified clinical data electronic registry. The GeroCovid e-Registry was adapted from an existing electronic platform that Bluecompanion developed in 2018 for a project called e-Trajectories. In March 2020, in conjunction with the COVID-19 pandemic, Bluecompanion made their health data collection system available to the GeroCovid initiative. E-Trajectories and its Gerocovid adaptation are based on the CleanWeb engine produced by Telemedicine Technologies (Boulogne-Billancourt, France), embedded in a dedicated web platform designed for integrating data from different sources. All data are recorded on web servers located in the European Union. ICT operations are compliant with the European General Data Protection Regulation (GDPR) and with the relevant international standards for clinical trials (ISO 9001 certification and FDA CFR 21 part 11). The platform has been developed thanks to the cooperation of the technical-scientific team of Bluecompanion and the GeroCovid cohorts’ coordinators with the goal of capturing the complexity of the geriatric patient. Moreover, to support appropriate use of the platform, investigators of each investigational sites underwent specific training sessions and dummy data entry on a “training” environment before being allowed to data entry into the production environment.
Statistical analysis
Sample size. For each GeroCovid cohort, the sample size was estimated through formal computation or following a purposive sampling strategy, depending on the specific primary outcomes and on the availability of literature data to suppose the expected effect size. Details for the estimated sample size in each GeroCovid cohort are summarized in Table 2.
Data analysis. Continuous variables will be described as mean ± standard deviation or median and interquartile range in the case of a non--normal distribution. Categorical variables will be reported as frequency values and percentages. Quantitative variables will be compared with Student's t-test and analysis of variance, or the related non-parametric tests (Mann-Whitney or Kruskal-Wallis test) after having shown a non-normal distribution. The Chi-squared or Fisher's test will be used for the categorical variables. Based on the study hypothesis, the association between exposures and outcome of interest will be tested, as appropriate, using linear or logistic regression models, Kaplan-Meier analysis or Cox regressions, and linear mixed models in the case of repeated measures over time. Possible differences by age class (60-64; 65-74, 75-84, ≥85 years) and sex in the study outcomes will be evaluated through interaction tests and stratified analyses.
Ethical aspects
The Gerocovid Observational study overarching protocol was reviewed and approved by the Campus Bio-Medico University Ethical Committee in April 2020. All participating investigational sites further submitted relevant sub-protocols to their competent local Ethical Committee and institutional review boards, as applicable, according to the Italian legislation. All investigators and the ICT team accepted to work according to the GCP (ICH E6-R2). Written or dematerialized informed consent was obtained by each patient. Alternatively, a written declaration was kept on file by the local investigator, which responded to applicable derogations during the pandemic.
All individual clinical data were anonymized before data entry. Collected data are protected and stored on private cloud hosted in an ISO 27001 DataCenter located in the European Union and cannot be lawfully accessed or read by unauthorized users. All recorded clinical data are intended for medical and scientific use for the benefit of the patients, the general and the scientific community and health authorities.
Results
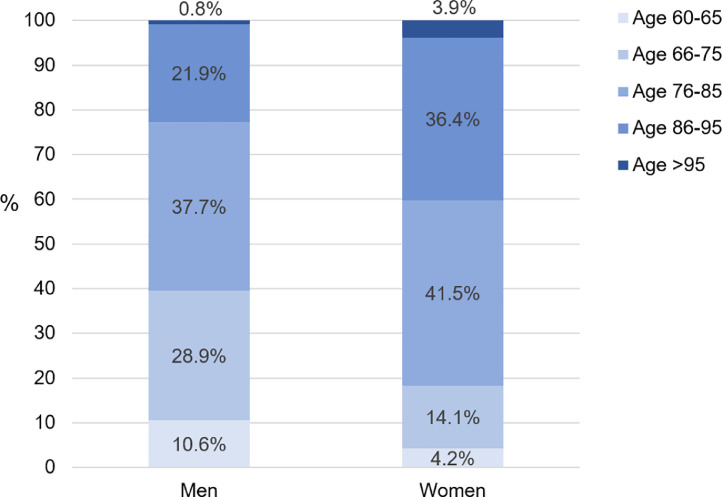
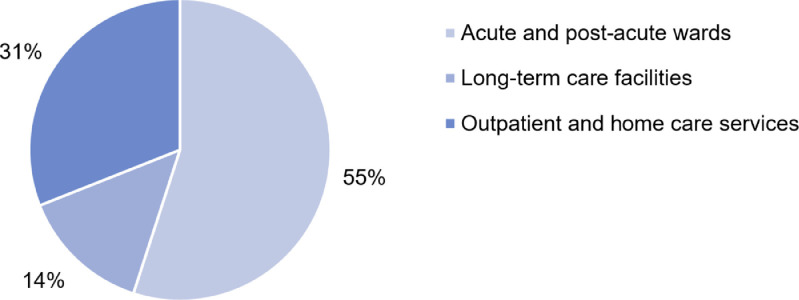
The GeroCovid data collection started on April 25th, 2020. As of September 16th, 2020, 66 investigational sites have obtained their local Ethical Committee and institutional board approval, while 24 sites are waiting for final approval. A total of 1618 observed cases (mean age 80.6±9.0 years; 45% men) to date have been recorded in the e-Registry. The age distribution of male and female participants recorded in the GeroCovid e-Registry is illustrated in Figure 1 . Of the total cases, 883 (55%) individuals were assessed in the hospital setting (GeroCovid acute wards and outcomes cohorts), 229 (14%) in long-term care facilities (GeroCovid LTCFs cohort), and 506 (31%) in outpatient or home care services (GeroCovid home and outpatients’ care, GeroCovid dementia – drug monitoring, and GeroCovid dementia – psychological health) (Figure 2 ). The current average inclusion rate is 11.2 patients/day, and the expected final sample should include more than 2000 observed cases. Preliminary results describing the finally included population will be available in November 2020, while results over the complete study duration will be available in January 2021.
Figure 1.
Age distribution of the 734 men and the 883 women enrolled in the GeroCovid initiative (data updated to the September 16th, 2020; n=1 participant had missing information on sex)
Figure 2.
Frequency of GeroCovid cases by setting of care (data updated to the September 16th, 2020)
Discussion
The GeroCovid study will contribute to increase knowledge on COVID-19 effects on individuals aged ≥60 years, the most vulnerable to the disease, by providing concrete and useful information to face the ongoing and the future pandemics. The strength of the project is the involvement of multiple centers and settings of care, which will allow exploring the multifaceted impact of COVID-19 pandemic on health status in representative subsets of the geriatric (and pre-geriatric) population. In particular, using high-quality data, the GeroCovid framework will investigate risk factors, clinical presentation and outcomes in COVID-19 inpatients; best strategies to prevent infection in long-term care facilities; impact of Covid-19 and social isolation on emotional, neuropsychologic, functional and physical health; and, possibility of a remote monitoring of drug treatment in dementia.
GeroCovid will provide original information on three main aspects of COVID-19 pandemic, i.e. SARS-CoV-2 infection onset, clinical course of Covid-19, and effects on health status of people in advanced age, including individuals at risk not affected by COVID-19.
As regards the first aspect, i.e. disease onset, the study will help to identify the factors associated with SARS-CoV-2 infection and its heterogeneous presentation. So far, inconsistent data have emerged about symptoms variability in older adults at disease onset [3,24]. GeroCovid will focus on potentially atypical COVID-19 presentations and on their associated prognostic value. An interesting contribution of GeroCovid will be provided by the involvement of long-term care facilities, which are among the major reservoirs of the frailest older population. The GeroCovid LTCFs cohort will, therefore, give useful insights on the disease onset in such individuals, as well as on the most effective preventive measures to be implemented.
With regard to clinical course, GeroCovid will use multicenter information from two European countries to recognize factors heralding a faster and more severe disease progression. The extraction of data from different geographical contexts, even at the national level, will provide a picture of the various therapeutic approaches adopted in the past months based on local guidelines and resources’ availability. Special attention will also be paid to investigate factors influencing the management of the disease, and to evaluate which therapeutic approaches may lead to better outcomes based on individual characteristics.
Finally, GeroCovid assesses the effects of the pandemic on the health of both COVID-19 patients and older people at risk of Covid-19 in specific care settings, considering physical, mental and social well-being, in accordance with the WHO Constitution [25]. This step of the project will provide important insights not only for physicians but also for healthcare systems and societies, which have to address the emerging needs of individuals in the post-acute phase of the disease [26,27]. Moreover, GeroCovid will focus on the impact of the pandemic on non-Covid-19 geriatric patients. Indeed, there is evidence that the pandemic could have affected care pathways, including cognitive status, functional abilities and psychological health of frail older people, with a special focus on dementia. Recently published data suggest that during lock-down a rapid increase of behavioral symptoms and of stress-related symptoms were observed in more than half of dementia patients and caregivers [28]. As social distancing measures are likely to last for several months, due to persistence of pandemic, it is important to quantify these emerging needs and to assess the ability of health services, including remote telemonitoring, to address them. These aspects will be investigated primarily in the GeroCovid home and outpatients’ care, GeroCovid dementia – drug treatment, and GeroCovid dementia – psychological health cohorts.
The multi-setting results of GeroCovid will contribute to developing new evidence-based recommendations promoting the prevention and management of Covid-19, and the optimization of care provision for older patients even in such emergency situations. Therefore, the potential impact of the study is not only addressed to the health of individuals, but also to the healthcare system. Indeed, evidence emerged from GeroCovid will improve the clinical management of COVID-19, possibly optimizing resource allocation and increasing readiness of healthcare and public health systems to front possible future pandemics. This will improve the resilience of healthcare systems that, in many cases, demonstrated an insufficient capacity to maintain overall efficiency in response to the current outbreak [27]. In addition, GeroCovid will inform on the burden of the disease that, even in its post-acute phase, may raise new care and assistance needs associated to non-negligible costs at the familiar, societal, and healthcare system levels. Older adults may be especially vulnerable to the consequences of COVID-19 as a disease that can alter the labile balance between multiple chronic conditions and treatments, with a negative effect on physical, mental, and functional well-being. In this sense, COVID-19 pandemic can be considered as a prototype of a stressful scenario for the frailest individuals, for our societies and healthcare systems. Consequently, information and possible solutions derived from GeroCovid will go beyond the ongoing pandemic and might be applied to future crisis of similar or different nature.
One obstacle that may influence the achievement of the expected goals of GeroCovid concerns the potential heterogeneity of data coming from different settings of care. At this regard, the GeroCovid coordinating group agreed on minimum core information shared by all study cohorts. Another possible limitation of the project is the recruitment limited to people aged 60 years or older, which will not allow GeroCovid to get a complete picture on the risk/protective factors, clinical course and outcomes of COVID-19 across all age groups, from young to older individuals. However, focusing on advanced age, the study will explore a broad set of key aspects of COVID-19 just in the part of the population that has been most burdened by the pandemic.
Conclusion
The multi-setting, multi-purpose and multicentric GeroCovid initiative is a unique opportunity to explore relevant aspects of COVID-19 with high-quality and comprehensive data on the health of individuals aged 60 years or older. This project will help to optimize COVID-19 prevention and management, with practical implications for ongoing and possible future pandemics.
Funding sources
This research did not receive any funding from agencies in the public, commercial, or not-for-profit sectors.
Declaration of Competing Interest
None.
Acknowledgements
We are thankful to our colleagues who are collaborating in data collection for their valuable contribution, and to all the study participants. We thank Gilda Borselli for her precious support for the organization of the GeroCovid initiative.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.ejim.2021.01.017.
Appendix. Supplementary materials
References
- 1.Richardson S, Hirsch JS, Narasimhan M, Crawford JM, McGinn T, Davidson KW. Presenting Characteristics, Comorbidities, and Outcomes among 5700 Patients Hospitalized with COVID-19 in the New York City Area. JAMA - J Am Med Assoc. 2020;323:2052–2059. doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Onder G, Rezza G, Brusaferro S. Case-Fatality Rate and Characteristics of Patients Dying in Relation to COVID-19 in Italy. JAMA. 2020 doi: 10.1001/jama.2020.4683. [DOI] [PubMed] [Google Scholar]
- 3.Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J. Clinical Characteristics of 138 Hospitalized Patients with 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA - J Am Med Assoc. 2020 doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Garg S, Kim L, Whitaker M, O’Halloran A, Cummings C, Holstein R. Hospitalization rates and characteristics of patients hospitalized with laboratory-confirmed coronavirus disease 2019 — COVID-net, 14 states. Morb Mortal Wkly Rep. 2020;69:458–464. doi: 10.15585/MMWR.MM6915E3. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146–M156. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 6.Vetrano DL, Palmer K, Marengoni A, Marzetti E, Lattanzio F, Roller-Wirnsberger R. Frailty and Multimorbidity: A Systematic Review and Meta-analysis. J Gerontol A Biol Sci Med Sci. 2019;74:659–666. doi: 10.1093/gerona/gly110. [DOI] [PubMed] [Google Scholar]
- 7.Hewitt J, Carter B, Vilches-Moraga A, Quinn TJ, Braude P, Verduri A. The effect of frailty on survival in patients with COVID-19 (COPE): a multicentre, European, observational cohort study. Lancet Public Heal. 2020:e444–e451. doi: 10.1016/S2468-2667(20)30146-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guo W, Li M, Dong Y, Zhou H, Zhang Z, Tian C. Diabetes is a risk factor for the progression and prognosis of COVID-19. Diabetes Metab Res Rev. 2020 doi: 10.1002/dmrr.3319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhao X, Zhang B, Li P, Ma C, Gu J, Hou P, et al. Incidence, clinical characteristics and prognostic factor of patients with COVID-19: a systematic review and meta-analysis Running title: Predictors of clinical prognosis of COVID-19. MedrxivOrg 2020:2020.03.17.20037572. https://doi.org/10.1101/2020.03.17.20037572.
- 10.Mueller AL, Mcnamara MS, Sinclair DA. Why does COVID-19 disproportionately affect the elderly? Preprints 2020:1–32. https://doi.org/10.20944/preprints202004.0548.v1.
- 11.Franceschi C, Campisi J. Chronic Inflammation (Inflammaging) and Its Potential Contribution to Age-Associated Diseases. Journals Gerontol Ser A Biol Sci Med Sci. 2014;69:S4–S9. doi: 10.1093/gerona/glu057. [DOI] [PubMed] [Google Scholar]
- 12.Meftahi GH, Jangravi Z, Sahraei H, Bahari Z. The possible pathophysiology mechanism of cytokine storm in elderly adults with COVID-19 infection: the contribution of “inflame-aging. Inflamm Res. 2020;1:3. doi: 10.1007/s00011-020-01372-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guo T, Shen Q, Guo W, He W, Li J, Zhang Y. Clinical Characteristics of Elderly Patients with COVID-19 in Hunan Province, China: A Multicenter, Retrospective Study. Gerontology. 2020:1–9. doi: 10.1159/000508734. [DOI] [PubMed] [Google Scholar]
- 14.McMichael TM, Currie DW, Clark S, Pogosjans S, Kay M, Schwartz NG. Epidemiology of COVID-19 in a Long-Term Care Facility in King County, Washington. N Engl J Med. 2020;382:2005–2011. doi: 10.1056/NEJMoa2005412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kemenesi G, Kornya L, Tóth GE, Kurucz K, Zeghbib S, Somogyi BA, et al. Nursing homes and the elderly regarding the COVID-19 pandemic: situation report from Hungary. GeroScience 2020:1. https://doi.org/10.1007/s11357-020-00195-z. [DOI] [PMC free article] [PubMed]
- 16.Rada AG. COVID-19: The precarious position of Spain’s nursing homes. BMJ. 2020;369 doi: 10.1136/bmj.m1554. [DOI] [PubMed] [Google Scholar]
- 17.Galea S, Merchant RM, Lurie N. The Mental Health Consequences of COVID-19 and Physical Distancing: The Need for Prevention and Early Intervention. JAMA Intern Med. 2020;180:817–818. doi: 10.1001/jamainternmed.2020.1562. [DOI] [PubMed] [Google Scholar]
- 18.Cuffaro L, Di Lorenzo F, Bonavita S, Tedeschi G, Leocani L, Lavorgna L. Dementia care and COVID-19 pandemic: a necessary digital revolution. Neurol Sci. 2020;41:1977–1979. doi: 10.1007/s10072-020-04512-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.De Filippo O, D’Ascenzo F, Angelini F, Bocchino PP, Conrotto F, Saglietto A. Reduced Rate of Hospital Admissions for ACS during COVID-19 Outbreak in Northern Italy. N Engl J Med. 2020 doi: 10.1056/nejmc2009166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vindegaard N, Eriksen Benros M. COVID-19 pandemic and mental health consequences: Systematic review of the current evidence. Brain Behav Immun. 2020 doi: 10.1016/j.bbi.2020.05.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Characteristics of SARS-CoV-2 patients dying in Italy Report based on available data on September 7 th, 2020. n.d.
- 22.WHO R&D Blueprint. novel Coronavirus COVID-19 - Therapeutic Trial Synopsis. Geneva, Switzerland: 2020.
- 23.Pedone C, Costanzo L, Cesari M, Bandinelli S, Ferrucci L, Antonelli Incalzi R. Are Performance Measures Necessary to Predict Loss of Independence in Elderly People? - PubMed. J Gerontol A Biol Sci Med Sci. 2016;71:84–89. doi: 10.1093/gerona/glv096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.World Health Organization. Report of the WHO-China Joint Mission on Coronavirus Disease 2019 (COVID-19). 2020.
- 25.Constitution n.d. https://www.who.int/about/who-we-are/constitution (accessed June 10, 2020).
- 26.Grabowski DC, Joynt Maddox KE. Postacute Care Preparedness for COVID-19: Thinking Ahead. JAMA - J Am Med Assoc. 2020;323:2007–2008. doi: 10.1001/jama.2020.4686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Volpato S, Landi F, Incalzi RA. A Frail Health Care System for an Old Population: Lesson form the COVID-19 Outbreak in Italy. J Gerontol A Biol Sci Med Sci. 2020;XX:1–2. doi: 10.1093/gerona/glaa087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cagnin A, Di Lorenzo R, Marra C, Bonanni L, Cupidi C, Laganà V. Behavioral and psychological effects of coronavirus disease-19 quarantine in patients with dementia. Front Psychiatry. 2020;11:1–15. doi: 10.3389/fpsyt.2020.578015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR, Kawas CH. The diagnosis of dementia due to Alzheimer's disease: Recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimer's. Dement. 2011;7:263–269. doi: 10.1016/j.jalz.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Katz S. Assessing Self-maintenance: Activities of Daily Living, Mobility, and Instrumental Activities of Daily Living. J Am Geriatr Soc. 1983;31:721–727. doi: 10.1111/j.1532-5415.1983.tb03391.x. [DOI] [PubMed] [Google Scholar]
- 31.Lawton MP, Brody EM. Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist. 1969;9:179–186. [PubMed] [Google Scholar]
- 32.Linn BS, Linn MW, Gurel L. Cumulative illness rating scale. J Am Geriatr Soc. 1968;16:622–626. doi: 10.1111/j.1532-5415.1968.tb02103.x. [DOI] [PubMed] [Google Scholar]
- 33.Mahler DA, Wells CK. Evaluation of clinical methods for rating dyspnea. Chest. 1988;93:580–586. doi: 10.1378/chest.93.3.580. [DOI] [PubMed] [Google Scholar]
- 34.Yesavage JA, Brink TL, Rose TL, Lum O, Huang V, Adey M. Development and validation of a geriatric depression screening scale: a preliminary report. J Psychiatr Res. 1982;17:37–49. doi: 10.1016/0022-3956(82)90033-4. [DOI] [PubMed] [Google Scholar]
- 35.Tombaugh TN, McIntyre NJ. The mini-mental state examination: a comprehensive review. J Am Geriatr Soc. 1992;40:922–935. doi: 10.1111/j.1532-5415.1992.tb01992.x. [DOI] [PubMed] [Google Scholar]
- 36.Ware JE, Sherbourne CD. The MOS 36-item short-form health survey (Sf-36): I. conceptual framework and item selection. Med Care. 1992;30:473–483. doi: 10.1097/00005650-199206000-00002. [DOI] [PubMed] [Google Scholar]
- 37.Spielberger C. State-Trait Anxiety Inventory for Adults - Manual, Instrument and Scoring Guide. 1983 Consult Psychol Press Inc Mind Gard Inc 2010:0–78. https://doi.org/10.1037/t06496-000.
- 38.Group TE. EuroQol - a new facility for the measurement of health-related quality of life. Health Policy (New York) 1990;16:199–208. https://doi.org/10.1016/0168-8510(90)90421-9. [DOI] [PubMed]
- 39.Depression and Other Common Mental Disorders n.d. https://www.who.int/publications/i/item/depression-global-health-estimates (accessed July 31, 2020).
- 40.Wang C, Pan R, Wan X, Tan Y, Xu L, Ho CS. Immediate psychological responses and associated factors during the initial stage of the 2019 coronavirus disease (COVID-19) epidemic among the general population in China. Int J Environ Res Public Health. 2020;17 doi: 10.3390/ijerph17051729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tsai AC, Mini Lai M-Y. Nutritional Assessment and short-form Mini Nutritional Assessment can predict the future risk of falling in older adults – Results of a national cohort study. Clin Nutr. 2014;33:844–849. doi: 10.1016/j.clnu.2013.10.010. [DOI] [PubMed] [Google Scholar]
- 42.Hughes ME, Waite LJ, Hawkley LC, Cacioppo JT. A short scale for measuring loneliness in large surveys: Results from two population-based studies. Res Aging. 2004;26:655–672. doi: 10.1177/0164027504268574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shankar A, Hamer M, McMunn A, Steptoe A. Social isolation and loneliness: Relationships with cognitive function during 4 years of follow-up in the English longitudinal study of ageing. Psychosom Med. 2013;75:161–170. doi: 10.1097/PSY.0b013e31827f09cd. [DOI] [PubMed] [Google Scholar]
- 44.Carver CS. You want to measure coping but your protocol's too long: Consider the brief COPE. Int J Behav Med. 1997;4:92–100. doi: 10.1207/s15327558ijbm0401_6. [DOI] [PubMed] [Google Scholar]
- 45.Bottesi G, Ghisi M, Altoè G, Conforti E, Melli G, Sica C. The Italian version of the Depression Anxiety Stress Scales-21: Factor structure and psychometric properties on community and clinical samples. Compr Psychiatry. 2015;60:170–181. doi: 10.1016/j.comppsych.2015.04.005. [DOI] [PubMed] [Google Scholar]
- 46.Caserta MS, Lund DA, Wright SD. Exploring the caregiver burden inventory (CBI): Further evidence for a multidimensional view of burden. Int J Aging Hum Dev. 1996;43:21–34. doi: 10.2190/2DKF-292P-A53W-W0A8. [DOI] [PubMed] [Google Scholar]
- 47.Cohen S. Perceived Stress Scale. 1994.
- 48.Nucci M, Mapelli D, Mondini S. Cognitive Reserve Index questionnaire (CRIq): a new instrument for measuring cognitive reserve. Aging Clin Exp Res. 2012;24:218–226. doi: 10.3275/7800. [DOI] [PubMed] [Google Scholar]
- 49.Cummings JL, Mega M, Gray K, Rosenberg-Thompson S, Carusi DA, Gornbein J. The Neuropsychiatric Inventory: Comprehensive assessment of psychopathology in dementia. Neurology. 1994;44 doi: 10.1212/WNL.44.12.2308. 2308–2308. [DOI] [PubMed] [Google Scholar]
- 50.Bellelli G, Morandi A, Davis DHJ, Mazzola P, Turco R, Gentile S. Validation of the 4AT, a new instrument for rapid delirium screening: A study in 234 hospitalised older people. Age Ageing. 2014;43:496–502. doi: 10.1093/ageing/afu021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Alexopoulos GS, Abrams RC, Young RC, Shamoian CA. Cornell scale for depression in dementia. Biol Psychiatry. 1988;23:271–284. doi: 10.1016/0006-3223(88)90038-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.