Abstract
The asparagus of Triarrhena lutarioriparia (TL) is a popular vegetable with abundant chemical compounds in China. This study aims to optimize the ultrasound-assisted extraction (UAE) method for its content of total flavonoid and antioxidant activities by response surface methodology (RSM). Box-Behnken design was adopted to evaluate the influences of ethanol concentration, extraction time and solvent-to-sample ratio on the extraction yield of total flavonoid and the antioxidant activity. Considering the maximum content of extracted total flavonoids and antioxidant activity, the optimal extraction conditions were acquired with 70% (v/v) ethanol by UAE for 60 min at a solvent-to-sample ratio of 40 mL/g. The proportion of the extraction of total flavonoid was 15.88 mg/g and antioxidant activity reached 79.53%. The RSM would be recommended as an appropriate model for simultaneous optimization of the UAE conditions for the content of total flavonoid and the antioxidant activity of asparagus of TL.
Keywords: Asparagus of Triarrhena lutarioriparia, Ultrasound-assisted extraction, Total flavonoid, Antioxidant activity, Response surface methodology
Introduction
Triarrhena lutarioriparia (named Nandi in China) is a kind of wild plant growing naturally in the middle and lower reaches of Yangtze River marshland, widely used in ecological environment, papermaking and bioenergy field (Dang et al., 2012; Liu, 1990). The young stem is similar to bamboo shoots and also called “asparagus” or “asparagus of Triarrhena lutarioriparia” in China. The asparagus of Triarrhena lutarioriparia is with rich nutritional and edible value and medicinal value, known as “Dongting Cordyceps” (Jiang et al., 2019). In Traditional Chinese Medicine, asparagus of Triarrhena lutarioriparia can remove heat to promote salivation and increase diuresis for stranguria (Nanjing University of Chinese Medicine, 2014). Modern studies have shown that it is abundant in flavonoids, polysaccharides, sugar, protein, dietary fiber, 15 kinds of amino acids and selenium and other trace elements with good anticancer and antioxidant activities (Liao et al., 2017a; Liao et al., 2017b; Liu, 1990; Xiao et al., 2015). And the selenium content is 10 times higher than that in other vegetables, specially (Xiao et al., 2015). In China, it is not only a popular vegetable, but also a raw material for agricultural products processing such as noodle, yogurt, tablet, pastry and so on.
The studies about Triarrhena lutarioriparia researches mainly focused on cultivation, genetic breeding, storage methods and so on (He et al., 1996; He et al., 1997; Liao et al., 2017a; 2017b). The asparagus of Triarrhena lutarioriparia is becoming a popular vegetable with specific functional food adaptability and productive development potential in China. As far as we know, little is known about extraction process of active substances combined with biological activities from asparagus of Triarrhena lutarioriparia. Establishing a method of simultaneous optimization of the extraction process for active substances and biological activities from asparagus of Triarrhena lutarioriparia would be of great importance for product development in general.
Ethanol refluxing method and water decocting are the common extraction techniques for phytochemical compounds, but these extraction methods possess the shortcomings such as time-consuming and low efficiency (Xiao et al., 2014). Novel extraction techniques containing ultrasound-assisted extraction (UAE), supercritical fluid extraction (SFE) and microwave-assisted extraction (MAE), and so on, have been employed efficiently in the extraction of phytochemicals (Babamoradi et al., 2018; Chen et al., 2017; Chen et al., 2018; Kazemi et al., 2019; Li et al., 2008; Liang et al., 2018; Wu et al., 2015). In particular, UAE technology is confirmed to be a simple, green, cheap, and high efficient process. Up to now, there is no study on the extraction of flavonoid compounds from asparagus of Triarrhena lutarioriparia by UAE using response surface methodology (RSM), which is an available tool possessing mathematical model and statistical analysis function. RSM plays an important role in optimizing conditions and evaluating the influences of several different independent variables and their interplays. In RSM, though the number of experimental runs could be reduced, the acceptable results could be obtained owing to the experimental data providing sufficient information for statistics (Hwang et al., 2002; Kim et al., 2002). And Box-Behnken design (BBD) is a typical RSM with advantages of simplicity, rapidity and reliability, and so on (Ferreira et al., 2007). It was widely used in food engineering, bioprocessing, agrochemicals, pharmaceuticals and other industries to optimize extracting biological active compounds including phenolic compounds, flavonoid compounds and polysaccharides from various sources (Liu et al., 2017; Pan et al., 2017; Riciputi et al., 2018; Righi Pessoa da Silva et al., 2018).
The aim of the present study is to establish a simple, convenient and reliable method applied to simultaneously optimize the UAE conditions for the content of total flavonoid from asparagus of Triarrhena lutarioriparia and the antioxidant activity using response surface methodology. Three conventional extraction parameters including solvent-to-sample ratio, ethanol concentration and extraction time were optimized by single-factor design and RSM. The study will lay the foundation for the further applications of asparagus of Triarrhena lutarioriparia in drug or food field.
Materials and methods
Materials
Asparagus of Triarrhena lutarioriparia samples (Fig. 1) were hand-picked in Yuanjiang City China, and were authenticated by Pro. Tasi Liu, a professor of the School of Pharmacy, Hunan University of Chinese Medicine. The fresh asparagus of Triarrhena lutarioriparia were dried at 40 °C and grounded into powder. Then the powder was sifted through a 60-mesh sieve. Both 1, 1-diphenyl-2-picrylhydrazyl (DPPH) and rutin (HPLC grade) were provided by Hefei Bomei Biotechnology Co., Ltd. (Hefei, China).
Fig. 1.
Samples of asparagus of Triarrhena lutarioriparia: tender shoot of Triarrhena lutarioriparia (A), tender shoot of Triarrhena lutarioriparia without wraps (B) and tender stem of Triarrhena lutarioriparia (C)
Ultrasound extraction
Single-factor experiment
The powdered sample (1.0 g) of asparagus of Triarrhena lutarioriparia was placed into a 100 mL flask, after which a specified concentration (10%, 30%, 50%, 70%, 90%) ethanol was added according to solvent-to-sample at the ratio of 10:1, 25:1, 40:1, 55:1, 70:1. The flavonoids were extracted for a specific time (30 min, 45 min, 60 min, 75 min, 90 min) at the frequency of 40 kHz and power equals to 300 W in an ultrasonic cleaner (SB-5200DT, Ningbo Xinzhi Biotechnology Co. Ltd, Ningbo, China). The temperature was set at 40 °C by adding moderate ice into the ultrasonic cleaner at intervals to maintain the temperature. Then qualitative filter papers were used to filter the supernatant solutions and the filtrate was fixed to 100 mL. One factor varied while the others remained constant in each experiment accompanying with the dependent variables of the content of total flavonoid and DPPH inhibition.
Experimental design
BBD was applied to optimize UAE conditions of total flavonoid and the antioxidant activities of acquired extracts from asparagus of Triarrhena lutarioriparia. On the basis of the single-factor experimental results, the current experiment design comprised 17 experimental runs and experiments were implemented at random in the light of the run number designed by the Design Expert 8.0.6 software. Three independent factors including ethanol concentration (A), solvent-to-sample ratio (B), and extraction time (C) were found to affect the extraction amount of total flavonoid and the antioxidant activities of obtained extracts. BBD of three-level-three-factor was presented in Table 1 in detail.
Table 1.
Coded and uncoded levels of the three independent variables
| Independent variables | Low | Center | High |
|---|---|---|---|
| X1 (ethanol concentration) | − 1 (50%) | 0 (70%) | + 1 (90%) |
| X2 (solvent to sample ratio) | − 1 (10:1) | 0 (25:1) | + 1 (40:1) |
| X3 (extraction time) | − 1 (30 min) | 0 (45 min) | + 1 (60 min) |
The second-order polynomial Eq. (1) was adopted to fit regression analysis based on the experimental data.
| 1 |
where Y is the dependent variable (the content of total flavonoid or DPPH inhibition); Xi and Xj are the independent variables affecting the response; and β0, βi, βii and βij represent the regression coefficients (the intercept, linear, quadratic, and interaction terms, respectively) (Chen et al., 2017; Riciputi et al., 2018).
Analytical methods
Total flavonoid analysis
The concentration of total flavonoid was measured by Ultra Violet (UV) method. Stock solutions were prepared in 80% ethanol at concentration 0.42 mg/mL for rutin and stored at 4 °C. The yields of flavonoids in the extracts of asparagus of Triarrhena lutarioriparia were measured with a chromogenic system of NaNO2-Al(NO3)3-NaOH according to previous study with modifications (Hao et al., 2018). Briefly, 0.3 mL 5% NaNO2 was mixed with a known volume of extract (1.0 mL) by shaking. Then 0.3 mL 10% Al(NO3)3 was added into the mixture after reacting for 6 min. At last, 4% NaOH (4 mL) was put into the reaction mixture and the distilled water was joined into it until the total volume reached to 10 mL. Then the value of UV absorbance was determined at 510 nm after reaction for 10 min at room temperature. The content of total flavonoid from asparagus of Triarrhena lutarioriparia was measured by a standard curve with reference substance of rutin (0–0.042 mg/mL).
Antioxidant activity
According to the previous literature (Chen et al., 2017) with some appropriate modifications, DPPH scavenging activity of extraction of asparagus of Triarrhena lutarioriparia was determined using the following method. DPPH solution was dissolved and prepared in ethanol at concentrations 0.1 mM. In short, 3.0 mL DPPH solution with the concentration of 0.1 mM in ethanol was blended with 1.0 mL diluted sample solution. After the mixed solution was shaken with vortex and preserved in the dark at 25 °C for half an hour, the value of UV absorbance was detected at a wavelength of 517 nm. The percent of DPPH scavenging activity of the extraction was calculated according to the following Eq. (2):
| 2 |
where A is the absorbance of the reaction mixture containing DPPH and sample, and A0 is the absorbance of the control reaction without sample (Chen et al., 2017).
Data analyses
One-way ANOVA and Tukey test were used to examine the difference between samples in single-factor experiments. The statistical significance of the equation was analysed and evaluated by analysis of variance (ANOVA) using Design Expert 8.0.6 trial version (Chen et al., 2017; 2018). Some statistical parameters including p value of the model, coefficient of determination (R2) and p value of the lack of fit (LOF) were adopted to estimate the fitness of the polynomial equation.
Results and discussion
Single-factor test
Extraction efficiency of UAE depends on extraction time, solvent’s selectivity, solvent-to -sample ratio and the composition of the sample (Ahmad et al., 2015). Single factor tests were chosen to assess the influences of each single factor on the extraction yields of total flavonoids from asparagus of Triarrhena lutarioriparia and the antioxidant activities of the obtained extracts. As shown in Fig. 2A, the extraction amount of total flavonoid and the antioxidant activities reached the highest values when the ethanol concentration was 70%. They increased rapidly with ethanol concentration increasing from 10% to 70%, and decreased above 70%. Remarkable significant differences (p < 0.001) existed in different ethanol concentration samples. According to the theory of “like dissolves like”, the solubility of flavonoids from asparagus of Triarrhena lutarioriparia increased when the polarity of solution was gradually near to them. The solubility of flavonoids from asparagus of Triarrhena lutarioriparia ascended with the increase of ethanol concentration within 70%, and then descended over 70%. Similar results appeared in the extraction of total isoflavones in Chinese Soybean Cheese (Wang et al., 2013). As shown in Fig. 2B, the yields of the total flavonoids and the antioxidant activities went up with the solvent to sample ratio increasing until the ratio value reached 25:1 and then went down from 25:1 to 70:1. With higher ratio, the two indexes tended to become more stable. Statistical differences (p < 0.05) were observed in the samples with different solvent to sample ratios. During a certain range, the contact area of the solvent extracts could increase with an increase of the volume of extract, and the compounds could be better dissolved. In addition, dissolution of total flavonoid would tend to saturation and the content would become more and more stable when the volume of extract remains rising (Chen et al., 2017). Therefore, 25:1 was considered as the center point of the solvent to sample ratio for the next experiment. As evident from Fig. 2C, when the extraction time varied from 30 min to 90 min, the extraction amount of total flavonoid and antioxidant activities of the obtained extracts increased at first and reached to the maximum at 45 min, then decreased. There were statistical differences (p < 0.05) in the samples with different extraction time. The reason could be explained that a dynamic balance exists between extraction time and extracts with prolonging the ultrasound time (Meng, et al., 2018). And long extraction time brought about the thermal instability and degradation of the flavonoids. This phenomenon existed in the ultrasonic extraction process of flavonoids (Nacu-Briciu et al., 2011; Zheng et al., 2016).
Fig. 2.
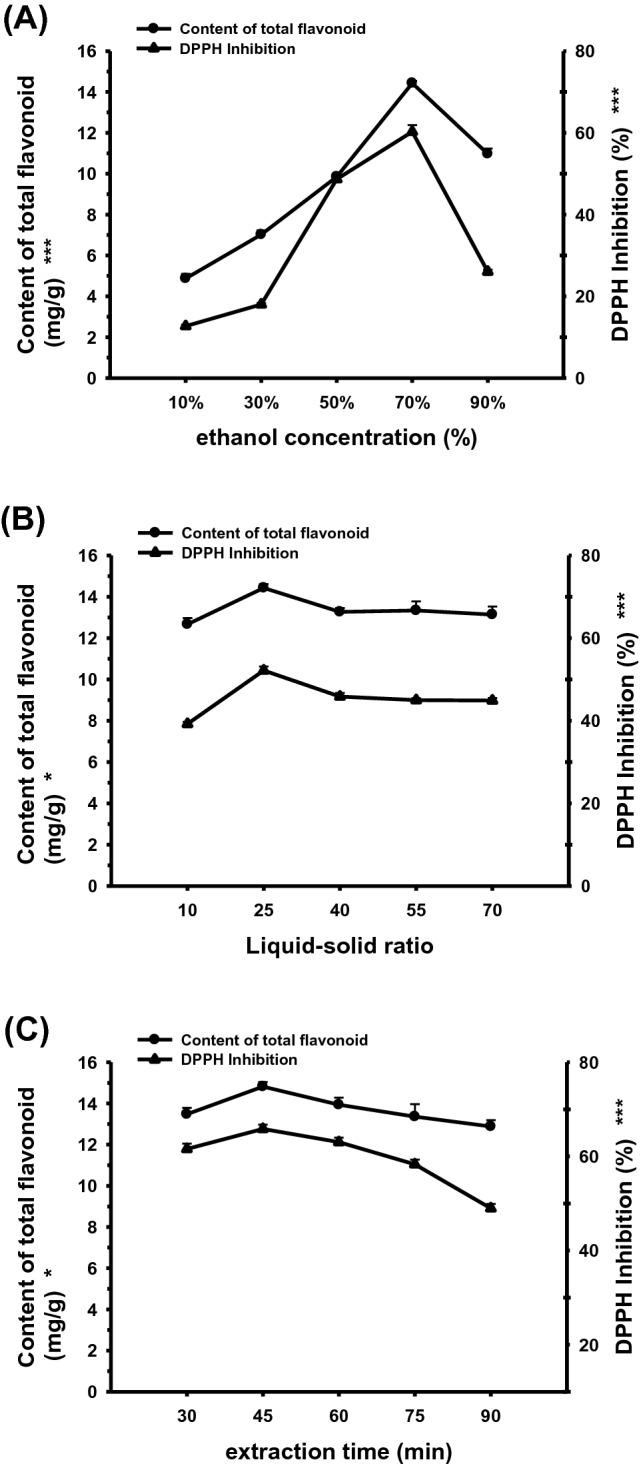
Effect of ethanol concentrations (A), solvent-to-sample ratio (B) and extraction time (C) on the content of total flavonoids and DPPH Inhibition (significant at *P < 0.05 and ***P < 0.001)
Fitting the model
After single factor tests, optimization of ethanol concentrations, solvent-to-sample ratio, and extraction time was carried out by RSM. This methodology is practical and can contribute to optimizing the extraction processes of flavonoids from various plant materials (Prakash Maran et al., 2017). Three factors and three levels were designed and seventeen experiments were performed by BBD, which was used to optimize the ultrasonic process parameters. The results of 17 experimental runs and ANOVA are listed in Tables 2 and 3, respectively. The significance of each coefficient was checked by the p values. Specifically, significant, highly significant and remarkably significant of the model terms are reflected when the p values are less than 0.05, 0.01 and 0.001, respectively (Chen et al., 2018). And the model terms present non-significant with the p values greater than 0.05.
Table 2.
The Box-Behnken experimental design and corresponding response values
| Run | A-ethanol concentration (%) | B-solvent to sample ratio (mL:g) | C-extraction time (min) | Total flavonoid content (mg/g) | DPPH inhibition (%) | ||
|---|---|---|---|---|---|---|---|
| Actual | Predicted | Actual | Predicted | ||||
| 1 | 90% | 10:1 | 45 | 14.32 | 14.42 | 43.19 | 43.22 |
| 2 | 70% | 40:1 | 30 | 14.92 | 15.04 | 79.79 | 80.86 |
| 3 | 70% | 10:1 | 30 | 15.29 | 15.26 | 62.98 | 61.86 |
| 4 | 70% | 25:1 | 45 | 16.57 | 15.99 | 70.21 | 73.49 |
| 5 | 50% | 25:1 | 60 | 11.40 | 11.47 | 77.23 | 76.14 |
| 6 | 70% | 10:1 | 60 | 15.86 | 15.74 | 76.17 | 75.11 |
| 7 | 90% | 40:1 | 45 | 14.67 | 14.61 | 51.91 | 49.76 |
| 8 | 70% | 25:1 | 45 | 15.78 | 15.99 | 71.91 | 73.49 |
| 9 | 50% | 10:1 | 45 | 10.42 | 10.47 | 57.87 | 60.02 |
| 10 | 50% | 40:1 | 45 | 11.00 | 10.90 | 77.45 | 77.42 |
| 11 | 70% | 40:1 | 60 | 16.56 | 16.59 | 78.94 | 80.06 |
| 12 | 90% | 25:1 | 60 | 14.80 | 14.83 | 53.40 | 54.44 |
| 13 | 90% | 25:1 | 30 | 14.35 | 14.28 | 46.6 | 47.69 |
| 14 | 70% | 25:1 | 45 | 15.99 | 15.99 | 74.26 | 73.49 |
| 15 | 70% | 25:1 | 45 | 15.91 | 15.99 | 72.34 | 73.49 |
| 16 | 70% | 25:1 | 45 | 15.71 | 15.99 | 78.72 | 73.49 |
| 17 | 50% | 25:1 | 30 | 10.02 | 9.99 | 71.49 | 70.45 |
Table 3.
ANOVA of quadratic models given by the BBD
| Dependent variables | Source | Sum of squares | Mean squares | F-value | P-value | Significance |
|---|---|---|---|---|---|---|
| Total flavonoid content | Model | 76.20 | 8.47 | 111.51 | < 0.0001 | *** |
| X1 | 29.26 | 29.26 | 385.36 | < 0.0001 | *** | |
| X2 | 0.20 | 0.20 | 2.61 | 0.1500 | ||
| X3 | 2.04 | 2.04 | 26.87 | 0.0013 | ** | |
| X1 X2 | 0.013 | 0.013 | 0.17 | 0.6889 | ||
| X1 X3 | 0.22 | 0.22 | 2.85 | 0.1354 | ||
| X2 X3 | 0.29 | 0.29 | 3.77 | 0.0933 | ||
| X21 | 43.18 | 43.18 | 568.61 | < 0.0001 | *** | |
| X22 | 0.15 | 0.15 | 1.94 | 0.2059 | ||
| X23 | 0.091 | 0.091 | 1.20 | 0.3091 | ||
| Residual | 0.53 | 0.076 | ||||
| Lack of Fit | 0.066 | 0.022 | 0.19 | 0.8982 | NS | |
| Pure Error | 0.47 | 0.12 | ||||
| Cor Total | 76.73 | |||||
| DPPH inhibition | Model | 2309.69 | 256.63 | 29.42 | < 0.0001 | *** |
| X1 | 988.79 | 988.79 | 113.35 | < 0.0001 | *** | |
| X2 | 286.56 | 286.56 | 32.85 | 0.0007 | *** | |
| X3 | 77.38 | 77.38 | 8.87 | 0.0206 | * | |
| X1 X2 | 29.48 | 29.48 | 3.38 | 0.1086 | ||
| X1 X3 | 0.28 | 0.28 | 0.032 | 0.8627 | ||
| X2 X3 | 49.28 | 49.28 | 5.65 | 0.0491 | * | |
| X21 | 835.49 | 835.49 | 95.78 | < 0.0001 | *** | |
| X22 | 13.59 | 13.59 | 1.56 | 0.2521 | ||
| X23 | 32.51 | 32.51 | 3.73 | 0.0949 | ||
| Residual | 61.06 | 8.72 | ||||
| Lack of Fit | 18.54 | 6.18 | 0.58 | 0.6579 | NS | |
| Pure Error | 42.52 | 10.63 | ||||
| Cor Total | 2370.75 |
***Significant at p < .001.; **Significant at p < .01.; *Significant at p < .05.; NS = Not Significant
The results of ANOVA (shown in Table 3) revealed that the models were remarkably significant (p < 0.001) for yields of total flavonoids and antioxidant activities of obtained extracts. The p-values of lack of fits of each model were 0.8982 and 0.6579 (p > 0.05), which implied that the relationship between the responses and independent variables could be explicated adequately by the predicted models, and the models could be adopted to analyze and calculate the contents of total flavonoids and antioxidant activities of the obtained extracts.
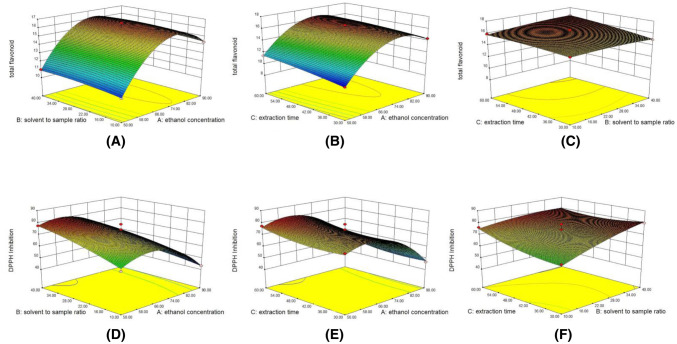
Joglejar and May (1987) suggested that the model was fitted well with R2 value at least 0.80. The values of R2 and R2-adjusted of yields of total flavonoids and the antioxidant activities were close to 1 (0.9931 and 0.9842, 0.8468 and 0.9411, respectively), which demonstrated that the consistencies between the predicted results and the experimental results were very perfect. The interactions of the variables are exhibited in the 3D graphs of response surface (Fig. 3).
Fig. 3.
Response surface plots showing the effects of investigated variables on total flavonoids content and DPPH Inhibition: (A) effects of solvent to sample ratio and ethanol concentration on total flavonoid; (B) effects of extraction time and ethanol concentration on total flavonoid; (C) effects of extraction time and solvent to sample ratio on total flavonoid; (D) effects of solvent to sample ratio and ethanol concentration on DPPH Inhibition; (E) effects of extraction time and ethanol concentration on DPPH Inhibition; (F) effects of extraction time and solvent to sample ratio on DPPH Inhibition
Effect of extraction parameters on the total flavonoids content
Experimental results of the contents of total flavonoids obtained under the designed UAE conditions are listed in Table 2. It is obvious that the total flavonoids contents ranged from 10.02 to 16.57 mg/g. The highest value of total flavonoid content (16.57 mg/g) was gained using 70% ethanol, 25:1, and 45 min. However, when the conditions were 50% ethanol, 25:1, and 30 min extraction, the content of total flavonoid was observed the lowest value (10.02 mg/g).
As listed in Table 3, the coefficients X1, X3, and X21 of total flavonoid were significant because the p values were less than 0.05, while the others had not significant influences on the extraction efficiency (p-values > 0.05). As far as the total flavonoids yield was concerned, the significances of factors were ranked as the orders: X1 > X3 > X2. The efficiency of extraction could be described by Eq. (3) in the light of regression coefficients of the model equation for the different response variables:
| 3 |
In Eq. (3), Y represents the content of total flavonoid in asparagus of Triarrhena lutarioriparia, X1, X2 and X3 are the coded variables (the ethanol concentration, solvent to sample ratio and the extraction time, respectively).
The linear effects of the ethanol concentration (X1) and extraction time (X3) showed highly significant positive effect on the extraction of flavonoid compounds (p < 0.01), while quadratic of ethanol concentration (X21) had a highly negative effect on it significantly (p < 0.01).
Figure 3B showed that extraction rates of total flavonoids in the test samples increased with prolonging extraction time. It was improved with rising the ethanol concentration up to about 80%. But if the ethanol concentration was further increased, the extraction rate of total flavonoid would decline.
Effect of extraction parameters on antioxidant activity
Experimental results of the content of DPPH inhibition obtained in the condition of designed UAE are listed in Table 2. The percentage of DPPH inhibition varied from 43.19 to 79.79%. The highest value of DPPH inhibition (79.79%) was obtained under the following condition: 70% ethanol, 25:1, and 30 min. However, the lowest DPPH inhibition value (43.19%) was observed using 90% ethanol, 10:1, and 45 min extraction.
As shown in Table 3, the coefficients X1, X2, X3, X2X3 and X21 had significant influences on DPPH inhibition (p < 0.05), and the others displayed not-significant (p > 0.05). The orders of the significances of the coefficients X1, X2, X3 were as follows: X1 > X2 > X3. According to regression coefficients, the DPPH inhibition could be explicated by Eq. (4) with Y (DPPH inhibition), and the coded variables for the ethanol concentration (X1), solvent-to-sample ratio (X2) and extraction time (X3):
| 4 |
The linear effects of solvent to sample ratio (X2) and extraction time (X3) had significant positive influences on DPPH inhibition (p-values < 0.05), while the ethanol concentration (X1), quadratic of ethanol concentration (X21), and the interaction between the solvent-to- sample ratio and extraction time (X2X3) contributed significant negatively to it (p-values < 0.05). As shown in Fig. 3D, E, DPPH inhibition in the samples ascended with the solvent-to-sample ratio increasing and extraction time extended. And it was enhanced with the ethanol concentration rising up to about 65%. And further improvement in ethanol concentration led to a decrease in DPPH inhibition.
Model validation
According to the regression analysis and 3D surface plots of each variable, the optimum conditions for the maximum content of total flavonoid and antioxidant activity (DPPH inhibition) were obtained at the following conditions: 70% (v/v) ethanol, solvent to sample ratio of 40 mL/g and extraction time for 60 min. The content of total flavonoid and DPPH inhibition were determined under the optimized conditions. As a result, the content of total flavonoid and DPPH inhibition were 15.88 mg/g and 79.53%, respectively, which matched the predicted values of 16.63 mg/g and 80.05%. The predicted values were high consistent with the model validation experimental results in our study. The model was proved to be very good and it was feasible to establish a method of optimizing UAE conditions for improving the combination of the content of total flavonoid and DPPH inhibition from asparagus of Triarrhena lutarioriparia.
In conclusion, a convenient, simple, reliable UAE extraction for increasing the content of total flavonoid and DPPH inhibition from asparagus of Triarrhena lutarioriparia was established effectively. The method had been validated and successfully applied to extraction process. The process of UAE was optimized and the influences of different variables and the interactions among the designed variables were analyzed by BBD. The optimized extraction method was helpful for the extraction and antioxidant active analysis of total flavonoid, which contributed to the further study of asparagus of Triarrhena lutarioriparia in food, medicine, and health care products.
Acknowledgements
This study was funded by Construct program of the key discipline of Chinese Pharmacy in Hunan Province (No. 2014001); Hunan Provincial Science and Technology Department Foundation (No. 2017SK2123); Hunan Provincial Natural Science Foundation of China (No. 2018JJ3876); Changsha Science and Technology Department Foundation (No. kq1701070); Cooperation fund project of Hunan University of Chinese Medicine and Yuanjiang municipal government (No. 201606).
Compliance with ethical standards
Conflict of interest
We declare that we have no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Qingming Cao, Email: cqm2000cn@163.com.
Jianye Yan, Email: yanjianye201@126.com.
Zhicheng Sun, Email: 965822816@qq.com.
Limin Gong, Email: 5065451@qq.com.
Hongnian Wu, Email: 970265604@qq.com.
Shihan Tan, Email: 349852768@qq.com.
Yating Lei, Email: 396995890@qq.com.
Bo Jiang, Email: jiangbo151230@126.com.
Yuanqing Wang, Email: wangyuanqing201@126.com, Email: wangyuanqing201@csuft.edu.cn.
References
- Ahmad A, Alkharfy KM, Wani TA, Raish M. Application of Box-Behnken design for ultrasonic-assisted extractionof polysaccharides from Paeonia emodi. Int. J. Biol. Macromol. 2015;72:990–997. doi: 10.1016/j.ijbiomac.2014.10.011. [DOI] [PubMed] [Google Scholar]
- Babamoradi N, Yousefi S, Ziarati P. Optimization of ultrasound-assisted extraction of functional polysaccharides from common mullein (Verbascum thapsus L.) flowers. J. Food Process Eng. 2018;41:e12851. doi: 10.1111/jfpe.12851. [DOI] [Google Scholar]
- Chen Y, Yin LZ, Zhao L, Shu G, Yuan ZX, Fu HL, Lv C, Lin JC. Optimization of the ultrasound-assisted extraction of antioxidant phloridzin from Lithocarpus polystachyus Rehd using response surface methodology. J. Sep. Sci. 2017;40:4329–4337. doi: 10.1002/jssc.201700686. [DOI] [PubMed] [Google Scholar]
- Chen S, Zeng Z, Hu N, Bai B, Wang H, Suo Y. Simultaneous optimization of the ultrasound-assisted extraction for phenolic compounds content and antioxidant activity of Lycium ruthenicum Murr fruit using response surface methodology. Food Chem. 2018;242:1–8. doi: 10.1016/j.foodchem.2017.08.105. [DOI] [PubMed] [Google Scholar]
- Dang N, Huang Z, Li H. Advances in biology and applications of fiber plant Triarrhena lutarioriparia. J. Trop. Subtrop. Bot. 2012;20:418–424. [Google Scholar]
- Ferreira SL, Bruns RE, Ferreira HS, Matos GD, David JM, Brandaoão GC, da Silva EG, Portugal LA, dos Reis PS, Souza AS, dos Santos WN. Box-Behnken design: an alternative for the optimization of analytical methods. Anal. Chim. Acta. 2007;597:179–186. doi: 10.1016/j.aca.2007.07.011. [DOI] [PubMed] [Google Scholar]
- Hao JY, Wan Y, Yao XH, Zhao WG, Hu RZ, Chen C, Li L, Zhang DY, Wu GH. Effect of different planting areas on the chemical compositions and hypoglycemic and antioxidant activities of mulberry leaf extracts in Southern China. PloS One. 2018;13:e0198072. doi: 10.1371/journal.pone.0198072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He L, Li A, Liu X, Zhou P. The nuclear type pattern of Chromosome of Triarrhena lutarioriparia L. Liu, sp nov. J. Hunan Agric. Univ. 1996;22:461–465. [Google Scholar]
- He L, Zhou P, Liu X. Studies on the autotetraploid of Triarrhena lutarioriparia L Liu sp nov. Acta Genet. Sin. 1997;24:544–549. [Google Scholar]
- Hwang KT, Jung ST, Lee GD, Chinnan MS, Park YS, Park HJ. Controlling molecular weight and degree of deacetylation of chitosan by response surface methodology. J. Agric. Food Chem. 2002;50:1876–1882. doi: 10.1021/jf011167u. [DOI] [PubMed] [Google Scholar]
- Jiang B, Liu S, Wang L, Shi Y, Tong Q, Yan J. Optimization of enzymatic extraction process of polysaccharides from asparagus based on antioxidant activities. Hunan J. Tradit. Chin. Med. 2019;35:157–160. [Google Scholar]
- Joglekar AM, May AT. Product excellence through design of experiments. Cereal Foods World. 1987;32:857–868. [Google Scholar]
- Kazemi M, Khodaiyan F, Labbafi M, Saeid Hosseini S, Hojjati M. Pistachio green hull pectin: Optimization of microwave-assisted extraction and evaluation of its physicochemical, structural and functional properties. Food Chem. 2019;271:663–672. doi: 10.1016/j.foodchem.2018.07.212. [DOI] [PubMed] [Google Scholar]
- Kim JT, Cha DS, Lee GD, Park TW, Kwon DK, Park HJ. Process optimization of sweet potato pulp-based biodegradable plastics using response surface methodology. J. Appl. Polym. Sci. 2002;83:423–434. doi: 10.1002/app.10070. [DOI] [Google Scholar]
- Li H, Li Y, Zhang Y, Shi H, Hu W, Zhang Z. Comparison of refluxing, ultrasonic-and microwave-assisted extraction of dihydromyricetin from Ampelopsis grossedentata. J. AOAC Int. 2008;91:1278–1283. doi: 10.1093/jaoac/91.6.1278. [DOI] [PubMed] [Google Scholar]
- Liang Y, Liu J, Zhong Q, Shen L, Yao J, Huang T, Zhou T. Determination of major aromatic constituents in vanilla using an on-line supercritical fluid extraction coupled with supercritical fluid chromatography. J. Sep. Sci. 2018;41:1600–1609. doi: 10.1002/jssc.201701097. [DOI] [PubMed] [Google Scholar]
- Liao Y, Tan Y, Fang Y, Li R. Determination of microelements in Triarrhena lutarioriparia by atomic absorption spectrometry. Centr. South Pharm. 2017;15:654–657. [Google Scholar]
- Liao Y, Tan Y, Fang Y, Li R. Effects of different storage methods on total amino acid content in tender stem of Triarrhena lutarioriparia from Yuanjiang. J. Anhui Agric. Sci. 45: 120-121, 128 (2017)
- Liu L. Unique paper-making raw materials in China: Triarrhena sacchariflorus. Plants. 1990;17:6–7. [Google Scholar]
- Liu Y, Chen F, Guo H. Optimization of bayberry juice spray drying process using response surface methodology. Food Sci. Biotechnol. 2017;26:1235–1244. doi: 10.1007/s10068-017-0169-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng Z, Zhao J, Duan H, Guan Y, Zhao L. Green and efficient extraction of four bioactive flavonoids from Pollen Typhae by ultrasound-assisted deep eutectic solvents extraction. J. Pharm. Biomed. Anal. 2018;161:246–253. doi: 10.1016/j.jpba.2018.08.048. [DOI] [PubMed] [Google Scholar]
- Nacu-Briciu RD, Cobzac SC, Baciu S. Optimum ultrasound assisted extraction conditions of some flavonoids from green tea leaves. Control quality of green tea product by TLC fingerprinting. Anal. Lett. 44: 2865-2875 (2011)
- Nanjing University of Chinese Medicine. Dictionary of Chinese Medicine. 2nd ed. Shanghai Science & Technology Press, China. pp. 1301 (2014)
- Pan H, Zhang Q, Cui K, Chen G, Liu X, Wang L. Optimization of extraction of linarin from Flos chrysanthemi indici by response surface methodology and artificial neural network. J. Sep. Sci. 2017;40:2062–2070. doi: 10.1002/jssc.201601259. [DOI] [PubMed] [Google Scholar]
- Prakash Maran J, Manikandan S, Vigna Nivetha C, Dinesh R. Ultrasound assisted extraction of bioactive compounds from Nephelium lappaceum L. fruit peel using central composite face centered response surface design. Arab. J. Chem. 10: s1145-s1157 (2017)
- Riciputi Y, Diaz-de-Cerio E, Akyol H, Capanoglu E, Cerretani L, Caboni MF, Verardo V. Establishment of ultrasound-assisted extraction of phenolic compounds from industrial potato by-products using response surface methodology. Food Chem. 2018;269:258–263. doi: 10.1016/j.foodchem.2018.06.154. [DOI] [PubMed] [Google Scholar]
- Righi Pessoa da Silva H, da Silva C, Bolanho BC. Ultrasonic-assisted extraction of betalains from red beet (Beta vulgaris L.). J. Food Process Eng. 41: e12833. (2018)
- Wang J, Geng Y, Li P, Hu F, Li L. Optimization of ultrasound-assisted extraction procedure to determine total isoflavones in Chinese soybean cheese by Box-Behnken design. Food Anal. Method. 2013;6:221–226. doi: 10.1007/s12161-012-9434-2. [DOI] [Google Scholar]
- Wu C, Wang F, Liu J, Zou Y, Chen X. A comparison of volatile fractions obtained from Lonicera macranthoides via different extraction processes: ultrasound, microwave, Soxhlet extraction, hydrodistillation, and cold maceration. Integr. Med. Res. 2015;4:171–177. doi: 10.1016/j.imr.2015.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao J, Zhao L, Shi X, Nie C, Deng F. Analysis of nutrients and heavy metals in tender stem of Triarrhena lutarioriparia from the Dongting Lake Area. Food Sci. 2015;36:104–107. [Google Scholar]
- Xiao KF, Liao XF, Cui YJ, Ren CS. Study on the new extraction methods of the flavonoids in Lithocarpus polysachyus Rehd. Food Sci. 2014;25:112–115. [Google Scholar]
- Zheng LL, Wen G, Yuan MY, Gao F. Ultrasound-assisted extraction of total flavonoids from corn silk and their antioxidant activity. Sci. Biotechnol. 2016;21:1–5. [Google Scholar]