Abstract
The aim of this study was to investigate the effects of ultraviolet-C (UV-C) irradiation on the changes in total sugars concentration and texture of ready to cook baby corn during cold storage. The baby corns were irradiated with UV-C at the dose of 0 (control), 2.2, 4.4 and 6.6 kJ m−2 and then stored at 5 ± 1 °C for 7 days. The results showed that the losses of total sugars were delayed by UV-C irradiation treatments. All the UV-C treatments significantly maintained the firmness of the treated baby corn samples and prevented the increase in electrolyte leakage, especially at 4.4 kJ m−2. Compared to control sample, the 4.4 kJ m−2 UV-C irradiated baby corn retarded the depolymerisation of pectin substances by suppressing the polygalacturonase and pectin methyl esterase activities. Therefore, the dose of 4.4 kJ m−2 could be a feasible alternative UV-C treatment maintaining texture and the total sugar concentration of ready to cook baby corn during commercial storage.
Keywords: Cell wall, Pectin, Baby corn, Texture, Ultraviolet-C
Introduction
The fresh-cut vegetables and fruit have grown recently as a result of change in consumer attitudes and rich in health promoting compounds vitamins and antioxidants. According to the World Health Organization, daily consumption of more than 400 g of fruit and vegetables was recommended. The appearance of fresh-cut fruit and vegetables is the main factor affecting consumer attributes and decision to buy. Collectively, firmness is important for consumer perceptions and withstand to stresses of postharvest handling by the commodities. The firmness loss of fruit is associated with the cell wall structure, composition and its changes during ripening (Toivonen and Brummell, 2008). In fresh-cut baby corn, fresh weight loss during transportation and high respiration rate which may lead to both of firmness and sugar content losses. Pectin can be found abundantly in the cell wall of dicotyledonous and some of monocotyledonous of non-graminaceous plants (Walter, 1991). It presents in the primary cell wall as a polysaccharide and represents different biochemical properties of the cell wall (McCann and Carpita, 2008). Pectic polysaccharides function in both, plant defense against pathogens and in plant morphogenesis as they are also a source of intercellular signaling molecules. Furthermore, it involves in cell-to-cell adhesion by calcium cross-linkage between partly de-methyl esterified homogalacturonans in the middle lamella (Jarvis et al., 2003; Vincken et al., 2003). Concerning on the human health, the qualities of fruit and vegetables are becoming an important factor. Therefore, the changes in nutritional composition of fruit and vegetables after harvest had been currently interested by the consumers. Short wavelength UV-C radiation has been found as a potentially new and environmentally friendly technology to extend the postharvest shelf-life of fruits and vegetables. UV-C treatment induced antioxidant capacity and antioxidant enzymes activities in strawberry (Erkan et al., 2008) and fresh-cut mango (González-Aguilar et al., 2007). UV-C irradiated at 7 kJ m−2 extended shelf life and reduced the increase level of total phenol, respiration rate and electrolyte leakage of red pepper (Vicente et al., 2005). Rodoni et al. (2015) found that the water-soluble pectin of UV-C treated with 10 kJ m−2 in pepper was lower than untreated due to the reduction of the disassembly of the cell wall polyuronids. UV-C retarded the accumulation of water soluble pectin and protopectin degradation and leaded to maintain firmness of tomato fruit (Lu et al., 2016). Bu et al. (2013) had also shown in cherry tomato that UV-C treated in 4.2 kJ m−2 delayed the softening of tomato by suppressing genes encoding cell wall degrading enzymes and inhibited the polygalacturonase (PG), pectin methyl esterase (PME) and cellulase activities.
A number of cell wall modifying enzymes and proteins are participated in cell wall decomposition (Brummell and Harpster, 2001). The softening processes are caused by the primary hydrolysis of polygalacturonase and pectin methyl esterase enzymes (King and O’Donoghue, 1995). A number of researches had reported that UV-C could delay these enzymes activities by targeting the genes encoding to these enzymes and resulting retarded the transcription regulation in tomato (Bu et al., 2013). UV-C treated at 3.7 kJ m−2 was induced the reduction PG and obviously inhibited PME activities by blocking the genes expression through transformation with antisense genes consequently retarded the softening of tomato. Moreover exposure to UV-C radiation maintained fruit firmness and retarded softening by reduction the activity of the cell wall degradation enzymes, enhancing the polyamine levels and consequently delays ripening and senescence of tomato (Barka et al., 2000b). Araque et al. (2019) studied UV-C irradiation on cell wall disassembly of strawberry fruit where single step at 4 kJ m−2, two step at 2 kJ m−2 (irradiated on each day 0 and 4) and multiple steps at 0.8 kJ m−2 (irradiated on each day 0, 2, 4, 6 and 8). The authors concluded that all the UV-C treatments prevented softening of strawberry by decreasing PG and PME activities and improved firmness was observed in two-step and multiple-step irradiation.
However, the main postharvest problems of baby corn are shortly shelf life due to texture and fresh weight loss. In our previous studies, we found that UV-C irradiated could maintain certain physicochemical quality of fresh-cut baby corn such as free radical scavenging activity, the loss of fresh weight, and texture during cold storage. UV-C irradiation did not affect color and visual appearance of fresh-cut baby corn. It is not known how the UV-C irradiation maintains the quality of fresh-cut baby corn. Therefore, the objective of this study was to present that pre-treatment fresh-cut baby corn by UV-C before storage could be a feasible environmentally friendly technology and which might be either improved or maintained the physicochemical parameters as well as cell wall compositions of ready to cook baby corn during commercial storage (5 ± 1 °C).
Materials and methods
Plant materials
Baby corns were obtained from a commercial farm in Thailand. They were then delivered to Food Chemistry Laboratory at Faculty of Natural Resources and Agro-Industry, Kasetsart University. The ready to cook baby corns were selected for uniformity and free from physical damages and diseases then cleaning with chlorinated water. The husks and corn silk were then removed using a sharp knife after that placed in a plastic basket under room temperature.
Ultraviolet-C (UV-C) treatments
The 120 baby corns placed in a tray which were covered with aluminum foil for UV-C treatments. The tray were placed into a box (1.32 m height, 1.85 m width and 0.8 m depth) containing two germicidal UV lamps (TUV, 30 W, Salvania, Japan) and were irradiated at distance of 70 cm for 30, 60 and 90 min to obtain dosages of 2.2, 4.4 and 6.6 kJ m−2 UV-C, respectively. To ensure the uniform treated of UV-C light, the baby corns were kept without overlapping each other and manually rotated. Non-irradiated baby corns were used as control. After treated with UV-C, 100 g of ready to cook baby corn were packed in a foam tray and then wrapped with PVC film (≈ 11 µm thickness) before storage at 5 ± 1 °C for 7 days. Total sugars concentration and texture-related factors such as firmness, tissue electrolyte leakage (EL), pectin substances and cell wall hydrolases activities were observed during storage. The experiment was designed for four treatments with three replications.
Firmness and electrolyte leakage (EL)
Firmness of ready to cook baby corn was measured using a TA-XT II texture analyzer (Stable Micro System, Surrey, England) by using flat-end probe at 40 mm depth and 30 s. The maximum force was recorded and the data were expressed as Newton (N). EL value was determined using the method described by Ergun et al. (2007). The ready to cook baby corn was sliced to 2 mm thick and 5 g of fresh-cut baby corn were soaked in deionized water. The sample was put in 30 mL of deionized water and shake for 30 min. The conductivity of the solution was measured by using a conductivity meter before and after boiling at 121 °C for 15 min. The data was calculated and expressed as the percentage of tissue EL.
Total sugars concentration assay
Total sugar content of ready to cook baby corn was assayed using the method of Dubois et al. (1956) with slight modification. A 0.5 g of baby corn was homogenized with 50 mL of distilled water and then filtered through Whatman No.1. A 2 mL of the extract was reacted with 0.05 mL of 80% phenol solution and 5 mL of concentrated sulphuric acid. The test tube allowed mixing thoroughly for a few second. The reaction were stand for 10 min and the absorbance at 490 nm wavelength was then recorded. The total sugars concentration was calculated using a linear equation of glucose standard curve. The samples were triplicated and total sugars concentration was expressed as mg glucose per kg fresh weight.
Pectin substance determination
Twenty grams of frozen fresh-cut baby corn were homogenized with 80 mL of 100% acetone and then filtered with Whatman No.1. The precipitate was washed with 200 mL of 80% acetone and then rinsed with 200 mL of 100% acetone. The acetone insoluble solid precipitate (AIS) was dried and collected for pectin substances determinations (Supapvanich and Tucker, 2013).
A 0.5 g of AIS was mixed with 20 mL of 20 mM sodium tetrahydridoborate (NaBH4) solution and shaken for 12 h at room temperature. After that the suspension was filtered using a GF/A filter paper and supernatant was retained. The pellet was then re-suspended with 20 mL of 20 mM (NaBH4) solution containing 50 mM of ethylenediaminetetraacetatic acid (EDTA), pH 8 and then shaken for 20 h in room temperature for EDTA soluble pectin. The suspension was filtered through a GF/A filter paper and supernatant was retained. The pellets was resuspended in 20 mL of 20 mM NaBH4 solution containing 50 mM sodium carbonate Na2CO3, and then shaken for 24 h in room temperature for Na2CO3 soluble pectin. All the filtrates were precipitated using acetone by making up the concentration of acetone to 80%. The precipitate was hydrolyzed with 15 mL concentrated sulphuric acid and incubated at 90 °C in water bath for 2 h and then filtered through GF/A filter paper.
The hydrolysate was used to measure pectin substances with appropriate dilution. A 2 mL of diluted solution was added with 0.05 mL of phenol and 5 mL of concentrated sulphuric acid. The sample was allowed to cold for 10 min at room temperature and the absorbance at 480 nm wavelength was then measured. The data were described as microgram D-galacturonic acid per gram AIS (µg Gal g−1 AIS).
The activities of cell wall degradation enzymes determinations
The AIS powder was extracted following the procedure described by Pressey (1983) which using extraction buffer (0.05 M sodium acetate buffer containing 1 M sodium chloride pH 6). Twenty mL of extraction buffer were added into 0.5 g of AIS and the pH was adjusted to 6 using 1 M sodium hydroxide or 1 M hydrochloric acid. The mixture was stirred continuously at 4 °C for 18 h. After that, the sample was centrifuged at 1000×g for 15 min at 4 °C. The supernatant was collected and filtered through cheesecloths. Ammonium sulphate was added to 80% of saturation and leaved at 4 °C for 1 h to precipitate protein. The precipitate was collected by centrifugation at 18,000×g for 25 min at 4 °C. The pellet was resuspended in a small amount of dialysis buffer (0.15 M sodium chloride, 0.05 M sodium acetate at pH 6). The extracted solution was dialyzed at 4 °C overnight and the buffer was changed for 8 times. The dialyzed solution was collected and then centrifuged for 5 min at 1000×g to eliminate insoluble materials. The crude extract was kept in 4 °C until used to assay PG and PME activities.
PG activity was determined using 2-cyanoacetamide as the method described by Andrew and Li (1995). A 0.5 mL of the extract was incubated in 0.5 mL of substrate solution (2.0% polygalacturonic acid, 0.15 M sodium chloride, 0.05 M sodium acetate, pH 4.0) at 37 °C for 20 h. The reaction was a mixture of 0.8 mL incubated sample and adding 4 mL of 100 mM borate buffer pH 9.2 and then 0.8 mL of 1% (w/v) 2-cyanoacetamide followed by incubation at boiling water for 10 min. The reaction was measured using a UV visible spectrophotometer at 276 nm for 5 min. The PG activity was calculated using a standard curve of D-galacturonic acid and expressed as micromole of D-galacturonic acid per gram AIS per min (µmol g−1 AIS min−1).
PME activity was measured as described by Hagerman and Austin (1986). The enzyme assay was a mixture of 200 μL of 0.5% pectin (w/v) pH 6.5, 15 μL of 0.01% bromothymol blue solution, 75 μL of distilled water and 20μL of enzyme extraction and incubated at 40 °C for 1 min and measured at 620 nm for 30 min by microplate reader. The rate of decrease between 0 and 30 min used to measure the PME activity. Calculation was carried out by against the standard curve of D-galacturonic acid and expressed as micromole of D-galacturonic acid per gram AIS per min (µmol g−1 AIS min−1).
Statistical analysis
The completely randomized design (CRD) was used. Statistical analysis was carried out using the analysis of variances (ANOVA) performed in IBM statistic SPSS version 21 software program. Mean were separated by using the Duncan’s multiple range tests (DMRT) at P ≤ 0.05 and the results were shown as mean of three replications and standard deviation (SD).
Results and discussions
Total sugars concentration
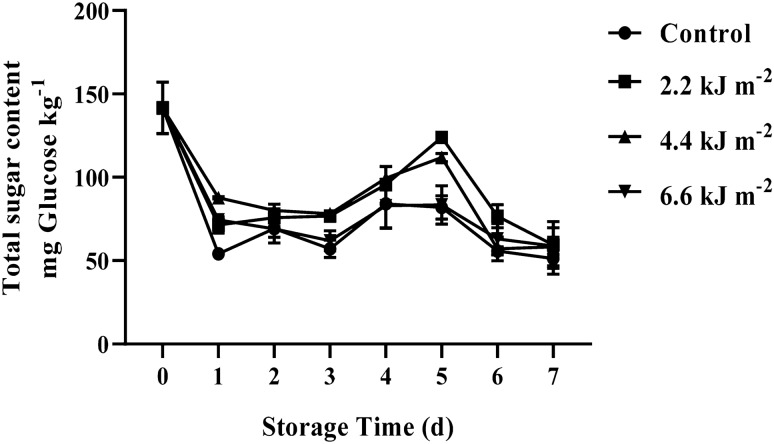
Total sugars content is one of the main qualities affecting eating quality of ready to cook baby corn as it is related to desirable taste. The total sugars concentration of all treatments was immediately decreased after storage for 1 day which that of control sample (56.16 mg kg−1) was lower than UV-C treated baby corns (64.95 to 74.46 mg kg−1) (Fig. 1). Afterwards, the total sugars concentration of 2.2 and 4.4 kJ.m−2 UV-C treated baby corns increased until day 5 of storage and then decreased while that of control and 6.6 kJ m2 UV-C treated baby corn remained constant. That of 2.2 and 4.4 kJ m−2 UV-C treated baby corns were significantly higher than that of control and 6.6 kJ m−2 UV-C treated baby corns during storage for 6 days (P < 0.05). However, at the end storage, total sugars content of all the treatments did not significantly different. These indicated that although the decreasing trend of total sugars content was found in the baby corns during storage, UV-C treatments, especially at 2.2 and 4.4 kJ m−2, could delay the loss of total sugars concentration compared to control and higher dose of UV-C treatments. Charles et al. (2016) suggested that UV-C treatment could improve taste of tomatoes due to the increase in ratio of total sugars to total organic acids. Moreover, previous work reported that the treatment of UV-C reduced respiration rate in postharvest commodities (Lemoine et al., 2007; Lu et al., 2016). It is commonly acknowledged that sugar is the main substrate of respiration mechanism thus the reduction of respiratory rate by UV-C treatment might delayed the loss of sugars content. However, UV-C treatments did not markedly affect total sugars concentration of pepper fruit (Vicente et al., 2005) and broccoli treated with UV-C was found lower the decrement of total sugar concentration during storage (Lemoine et al., 2007). The recent result revealed that UV-C treatment could retard the reduction of total sugars concentration in ready to cook baby corn rather than control treatment.
Fig. 1.
Effect of UV-C irradiation on total sugars content of ready to cook baby corn during storage at 5 ± 1 °C for 7 days. Vertical bars represent standard deviations of means, n = 3
Firmness and electrolyte leakage (EL)
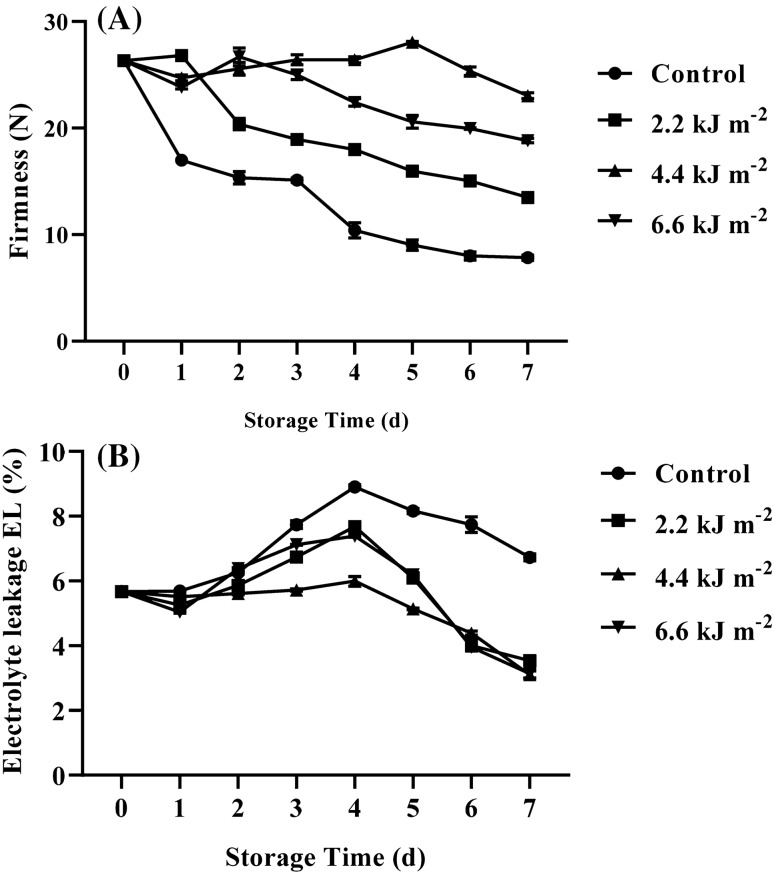
The firmness of baby corns gradually decreased in all treatments during storage (Fig. 2A). UV-C treatments could retard the reduction of firmness compared to control treatment. A marked decrease of firmness was found in control while a slight decrease was found in 2.2 and 6.6 kJ m−2. The firmness of 4.4 kJ m−2 UV-C treated baby corns remained constant throughout storage and was significantly higher than that of other samples. Therefore we suggested that UV-C irradiation effectively maintained firmness of ready to cook baby corns during commercial storage. It is widely accepted that the three main factors playing a role on texture of fresh commodities are moisture loss, cell wall modification and membrane dysfunction (Supapvanich and Tucker, 2013). Our previous study showed that UV-C treatment could retard the loss of fresh weight of fresh-cut baby corns during storage. Barka et al. (2000b) addressed that UV-C irradiation retarded reduction of cell wall depolymerization as well as cell wall hydrolases activities in tomato fruits. Promyou and Supapvanich (2020) found that the increase in tissue EL of longan pulp was retarded by UV-C treatments. Therefore, the higher firmness of UV-C treated baby corns compared to control samples might be associated to the retardation of moisture loss, cell wall components degradation and increased tissue EL.
Fig. 2.
Effect of UV-C irradiation on firmness (A) and electrolyte leakage EL percent (B) of ready to cook baby corn during storage at 5 ± 1 °C for 7 days. Vertical bars represent standard deviations of means, n = 3
The change in tissue EL of baby corns during storage as shown Fig. 2B, the increase in EL was found in all treatments during storage for 4 days and then declined until the end of storage. The EL of control sample obviously increased and was significantly higher than all UV-C treated baby corns over the storage. Among all UV-C treatments, 4.4 kJ m−2 UV-C inhibited the increase in EL being better than other UV-C treatments. The EL of 4.4 kJ m−2 UV-C treated baby corns was significantly lower than other treatment during storage for 6 days. No significant differences in EL of both 2.2 and 6.6 kJ m−2 UV-C treated baby corns were found throughout the storage. At the end of storage, EL value of all UV-C treated baby corns did not significantly different. This confirms that UV-C treatment at proper dose could inhibit tissue EL and might cause the firmness retention of baby corns during storage. Civello et al. (2006) suggested that UV-C could induce the accumulation of polyamines and Barka et al. (2000a) also addressed that UV-C treatment decreased membrane lipid peroxidation in tomato fruits. It has been accepted that the accumulation of polyamines and the inhibition of membrane lipids peroxidation strengthen membrane integrity and function. Thus these indicate why UV-C could delay the increase in tissue EL of the baby corns during storage. Moreover, the recent result was also in agreement with previous reports which UV-C irradiation reduced the increasing of EL in red pepper (Vicente et al., 2005).
Pectin substances
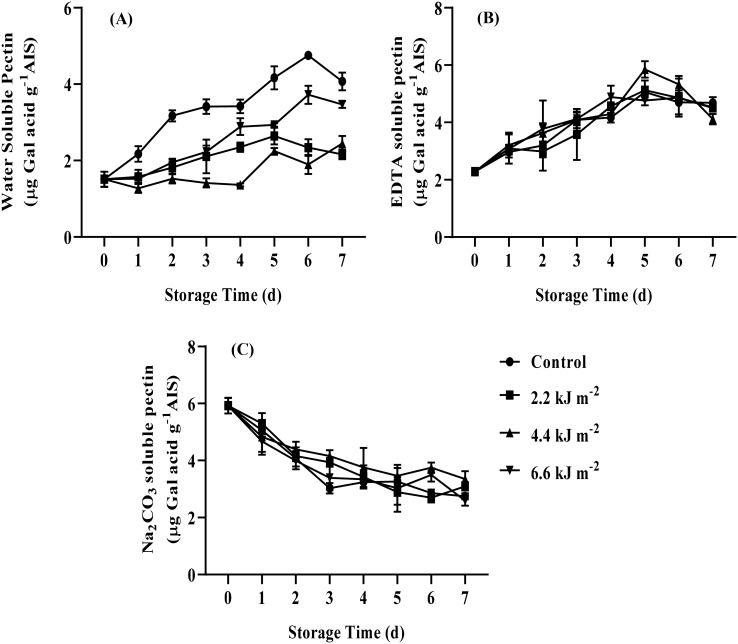
A main factor being accompanied with the loss firmness is cell wall disassembly which results in declining cell wall strength and reducing cellular adhesion (Toivonen and Brummell, 2008). Generally pectin substances are mainly found in the middle lamella and primary cell wall and play a key role on texture of fresh commodities (Prasanna et al., 2007). The effect of UV-C irradiation on pectin substances of ready to cook baby corns was shown in Fig. 3. Water soluble pectin (WSP) in ready to cook baby corns increased throughout storage period. WSP of control samples increased sharply and was significantly higher than UV-C treated baby corns (P < 0.05). The increased WSP of both 2.2 and 6.6 kJ m−2 UV-C treated baby corns was higher than that of 4.4 kJ m−2 UV-C treated baby corns which was lower than other treatments throughout storage. This was in the agreement with Rodoni et al. (2015) that increased WSP of fresh-cut pepper was delayed by UV-C irradiation during storage at 4 °C for 12 days. The EDTA soluble pectin of all treatments increased during storage for 5 days and then declined. During 4 days of storage period as well as after 6 days of storage, EDTA soluble pectin of all treatment did not significantly different. On day 5 of storage, the EDTA soluble pectin of 4.4 kJ m−2 UV-C treated baby corns was significantly higher than other treatments (Fig. 3B). It seemed to be that UV-C exposure at a proper dose could trigger an elevation of ionic interaction between pectin polymers (Araque et al., 2019). The Na2CO3 soluble pectin (NSP) of all treatments declined during storage (Fig. 3C). UV-C irradiated ready to cook baby corn showed slightly higher level of NSP when compared to control samples. The treatment of 4.4 kJ m−2 UV-C delayed the reduction of NSP rather than other treatments. The lowered WSP and EDTA soluble pectin as well as retained NSP of ready to cook baby corns treated with 4.4 kJ m−2 UV-C led to the maintenance of ready to cook baby corn texture. In the similar vein, Lu et al. (2016) found that the reduction of protopectin loss might be associated by hindering protopectin degradation and WSP progress after UV-C irradiation in tomato. Moreover, certain previous works reported that UV-C irradiation delayed pectin solubilization in cherry tomato (Bu et al., 2013) and fresh-cut pepper (Rodoni et al., 2015). In the similar vein, the recent results revealed that 4.4 kJ m−2 UV-C treatments could delay the solubilization of pectin substances as preventing the increase in WSP of baby corns during storage.
Fig. 3.
Effect of UV-C irradiation on water soluble pectin (WSP) (A), EDTA soluble pectin (B) and Na2CO3 soluble pectin (NSP) (C) of ready to cook baby corn during storage at 5 ± 1 °C for 7 days. Vertical bars represent standard deviations of means, n = 3
Cell wall degradation enzymes
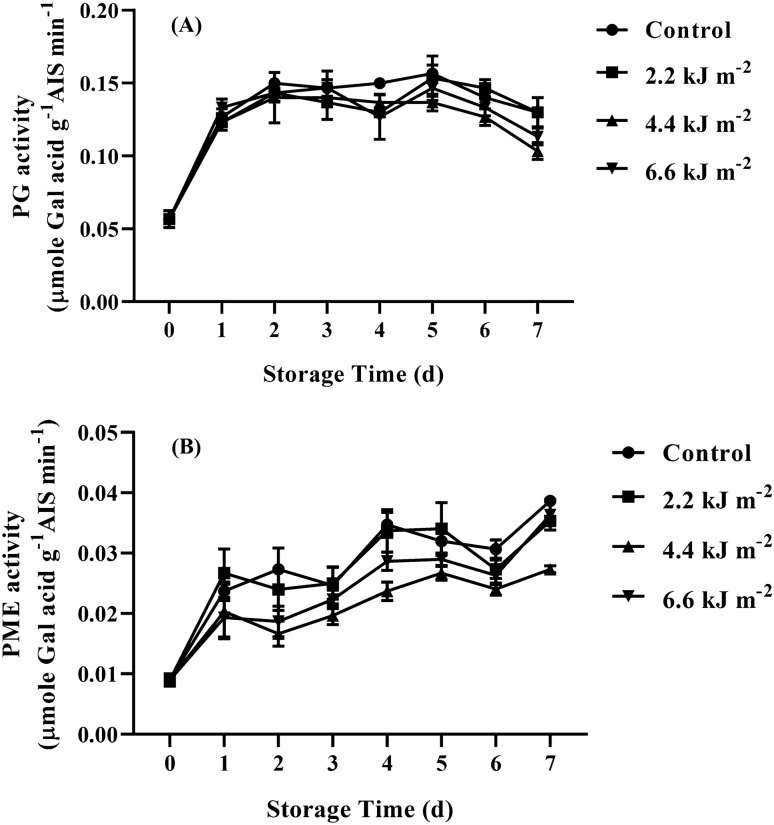
It is widely recognized that pectin solubilization is accompanied with the activities of cell wall hydrolases such as PG and PME (Brummell and Harpster, 2001). Generally, PME is acknowledged as the key enzyme involving the de-esterification of polygalacturonan chains and PG activity plays a main role hydrolyzing the de-esterified polygalacturonan causing depolymerization of pectin backbone (Wei et al., 2010). The activities of both PME and PG of the ready to cook baby corns were shown in Fig. 4. We found that the relatively low amount of PG activity was observed in ready to cook baby corns throughout storage time (Fig. 4A). The sharp increase in PG activity was observed in all the treatments during storage for 3 days and then remained constant until day 6 of storage. The decline of PG activity of all treatments was observed on day 7 of storage. After day 4 of storage, the PG activity of control sample was significantly higher than that of 4.4 kJ m−2 UV-C treated baby corns (P < 0.05). Figure 4B shows the markedly increase in PME activity of the ready to cook baby corns after storage for 1 day. Afterwards, PME activity of all the treatment seemed to be remained until day 3 of the storage and then continuously increased until the end of storage. The PME activity of 4.4 kJ m−2 UV-C treated baby corns was lower than that of other treatments and significantly lower than that of control samples over the storage (P < 0.05). No significant difference in PME activity of control and 2.2 kJ m−2 UV-C treated baby corns was found throughout the storage. The recent work indicated that UV-C treatment could retard the increments of PG and PME of ready to cook baby corns, especially the dose of 4.4 kJ m−2. As the relatively low amount of PG activity in baby corns was observed, this could be explained that PG might play a minor role in firmness loss of ready to cook baby corn. In the similar vein, Supapvanich and Tucker (2013) reported that a very low PG yield was found in netted melon fruit which it might not play the main role initiating the fruit softening. Bu et al. (2013) also reported that PG lately participated in the softening process of tomato fruit during ripening stage and UV-C treatment lowered the activities of both PG and PME. Mansourbahmani et al. (2017) also reported that UV-C irradiations significantly suppressed PG and PME activities of ‘Valouro’ tomato fruits during storage at 7 °C for 35 days. However, our results showed the total sugar of treated and control samples of baby corns are gradually decreased (Fig. 1). On the other hand PG and PME activities of baby corns increased during storage according to Fig. 4. It shows an inverse relation between sugar content and cell wall degradation enzymes activities. This suggests that the major sugars in baby corns are glucose, fructose and sucrose. The reduction of these sugars caused by high metabolic rate (respiration rate) of baby corns during storage had more affect the total sugars content rather than the increased neutral sugars by pectin depolymerisation during storage. Thus the decrease in total sugars content is related to the use of monosaccharides such as glucose and fructose in respiration of baby corns during storage. The recent results indicated that the effect of UV-C irradiation at 4.4 kJ m−2 on preventing the increments of both PG and PME activities were concomitant with the lower amount of WSP (Fig. 3A) and higher firmness (Fig. 2A) of ready to cook baby corns during storage.
Fig. 4.
Effect of UV-C irradiation on cell wall degradation enzymes polygalacturonase (PG) (A) and pectin methyl esterase (PME) (B) of fresh-cut baby corn during storage at 5 °C for 7 days. Vertical bars represent standard deviations of means, n = 3
It is concluded that UV-C irradiation at 4.4 kJ m−2 had positive effects on the maintenances of total sugars concentration and texture of ready to cook baby corn during storage. The UV irradiation maintained the high amount of total sugars concentration and firmness. The loss of firmness of ready to cook baby corns was concomitant with the increases in tissue EL and cell wall modification. The UV-C 4.4 kJ m−2 irradiation delayed the increments of tissue EL, WSP concentration and both PME and PG activities as well as the reduction of NSP concentration during storage. The reason why high level irradiation of UV-C might not be the best treatment for maintained the quality of baby corn. We found that 6.6 kJ m−2 UV-C was excessive UV-C dose that could the baby corn had superficial damage by sample tissues and caused high oxidative stress and resultant in cellular damage. This suggests that UV-C treatment at 4.4 kJ m−2 could be an alternative postharvest treatment maintaining quality attributes especially taste and texture of ready to cook baby corn during commercial storage.
Acknowledgements
This work was supported by grants from the Kasetsart University Scholarships for ASEAN for Commemoration of the 60th Birthday Anniversary of Professor Dr. Her Royal Highness Princess Chulabhorn Mahidol and Kasetsart University Research and Development Institute (KURDI) (Grant No. P3.1(D)29.61).
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Nan Theint Ngu Lwin, Email: theintngulwin@gmail.com.
Suriyan Supapvanich, Email: kusuriya@kmitl.ac.th.
Surassawadee Promyou, Email: csnsrwd@ku.ac.th.
References
- Andrews PK, Li S. Cell wall hydrolytic enzyme activity during development of nonclimacteric sweet cherry (Prunus avium L.) fruit. J. Hortic. Sci. 70: 561-567 (1995)
- Araque LCO, Ortiz CM, Darré M, Rodoni LM, Civello PM, Vicente AR. Role of UV-C irradiation scheme on cell wall disassembly and surface mechanical properties in strawberry fruit. Postharvest Biol. Technol. 2019;150:122–128. doi: 10.1016/j.postharvbio.2019.01.002. [DOI] [Google Scholar]
- Barka EA, Kalantari S, Makhlouf, J, Arul J. Effects of UV-C irradiation on lipid peroxidation markers during ripening of tomato (Lycopersicon esculentum L.) fruits. Funct. Plant Biol. 27: 147-152 (2000a)
- Barka EA, Kalantari S, Makhlouf J, Arul J. Impact of UV-C irradiation on the cell wall-degrading enzymes during ripening of tomato (Lycopersicon esculentum L.) fruit. J. Agric. Food Chem. 48: 667-671 (2000b) [DOI] [PubMed]
- Brummell DA, Harpster MH. Cell wall metabolism in fruit softening and quality and its manipulation in transgenic plants. Plant Mol. Biol. 2001;47:311–339. doi: 10.1023/A:1010656104304. [DOI] [PubMed] [Google Scholar]
- Bu J, Yu Y, Aisikaer G, Ying T. Postharvest UV-C irradiation inhibits the production of ethylene and the activity of cell wall-degrading enzymes during softening of tomato (Lycopersicon esculentum L.) fruit. Postharvest Biol. Technol. 86: 337-345 (2013)
- Civello PM, Vicente AR, Martínez GA. UV-C technology to control postharvest diseases of fruits and vegetables. Vol. 2, pp. 1-32. In: Recent advances in alternative postharvest technologies to control fungal diseases in fruits and vegetables. Troncoso-Rojas R, Tiznado-Hernández ME, González-León, A (Eds). Kerala, India (2006)
- Charles MT, Arul J, Charlebois D, Yaganza E, Rolland D, Roussel D, Merisier MJ. Postharvest UV-C treatment of tomato fruits: changes in simplesugars and organic acids contents during storage. LWT-Food Sci. Technol. 2016;65:557–564. doi: 10.1016/j.lwt.2015.08.055. [DOI] [Google Scholar]
- Dubois M, Gilles KA, Hamilton JK, Rebers P, Smith F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956;28:350–356. doi: 10.1021/ac60111a017. [DOI] [Google Scholar]
- Ergun M, Jeong J, Huber DJ, Cantliffe DJ. Physiology of fresh-cut ‘Galia’ (Cucumis melo var. reticulatus) from ripe fruit treated with 1-methylcyclopropene. Postharvest Biol. Technol. 44: 286-292 (2007)
- Erkan MS, Wang Y, Wang CY. Effect of UV treatment on antioxidant capacity, antioxidant enzyme activity and decay in strawberry fruit. Postharvest Biol. Technol. 2008;48:163–171. doi: 10.1016/j.postharvbio.2007.09.028. [DOI] [Google Scholar]
- González-Aguilar G, Villegas-Ochoa MA, Martínez-Téllez M, Gardea A, Ayala-Zavala JF. Improving antioxidant capacity of fresh-cut mangoes treated with UV-C. J. Food Sci. 2007;72:197–202. doi: 10.1111/j.1750-3841.2007.00295.x. [DOI] [PubMed] [Google Scholar]
- Hagerman AE, Austin PJ. Continuous spectrophotometric assay for plant pectin methyl esterase. J. Agric. Food Chem. 1986;34:440–444. doi: 10.1021/jf00069a015. [DOI] [Google Scholar]
- Jarvis M, Briggs S, Knox J. Intercellular adhesion and cell separation in plants. Plant Cell Environ. 2003;26:977–989. doi: 10.1046/j.1365-3040.2003.01034.x. [DOI] [Google Scholar]
- King GA, O’Donoghue EM. Unravelling senescence: new opportunities for delaying the inevitable in harvested fruit and vegetables. Trends Food Sci. Technol. 1995;6:385–389. doi: 10.1016/S0924-2244(00)89216-X. [DOI] [Google Scholar]
- Lemoine ML, Civello PM, Martínez GA, Chaves AR. Influence of postharvest UV-C treatment on refrigerated storage of minimally processed broccoli (Brassica oleracea var. Italica). J. Sci. Food Agric. 87: 1132-1139 (2007)
- Lu H, Li L, Limwachiranon J, Xie J, Luo Z. Effect of UV-C on ripening of tomato fruits in response to wound. Sci. Hortic. 2016;213:104–109. doi: 10.1016/j.scienta.2016.10.017. [DOI] [Google Scholar]
- Mansourbahmani S, Ghareyazie B, Kalatejari S, Mohammadi RS, Zarinnia V. Effect of post-harvest UV-C irradiation and calcium chloride on enzymatic activity and decay of tomato (Lycopersicon esculentum L.) fruit during storage. J. Integr. Agric. 16: 2093-2100 (2017)
- McCann MC, Carpita NC. Designing the deconstruction of plant cell walls. Curr. Opin. Plant Biol. 2008;11:314–320. doi: 10.1016/j.pbi.2008.04.001. [DOI] [PubMed] [Google Scholar]
- Prasanna V, Prabha T, Tharanathan R. Fruit ripening phenomena–an overview. Crit. Rev. Food Sci. Nutr. 2007;47:1–19. doi: 10.1080/10408390600976841. [DOI] [PubMed] [Google Scholar]
- Pressey R. β-Galactosidases in ripening tomatoes. Plant Physiol. 1983;71(1):132–135. doi: 10.1104/pp.71.1.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Promyou S, Supapvanich S. Combinative effect of salicylic acid immersion and UV-C illumination on chilling injury-related factors of longan (Dimocarpus longan Lour.) fruit. Int. J. Fruit Sci. 20: 133-148 (2020)
- Rodoni LM, Zaro MJ, Hasperué J, Concellón A, Vicente AR. UV-C treatments extend the shelf life of fresh-cut peppers by delaying pectin solubilization and inducing local accumulation of phenolics. LWT Food Sci. Technol. 2015;63:408–414. doi: 10.1016/j.lwt.2015.03.042. [DOI] [Google Scholar]
- Supapvanich S, Tucker GA. The effect if 1-methylcyclopropane (1-MCP) ib quality and cell wall hydrolases activities of fresh-cut muskmelon (Cucumis mel var reticulatus L.) during storage. Food Bioprocess Technol. 6: 2196-2201 (2013)
- Toivonen PM, Brummell DA. Biochemical bases of appearance and texture changes in fresh-cut fruit and vegetables. Postharvest Biol. Technol. 2008;48:1–14. doi: 10.1016/j.postharvbio.2007.09.004. [DOI] [Google Scholar]
- Vicente AR, Pineda C, Lemoine L, Civello PM, Martinez GA, Chaves AR. UV-C treatments reduce decay, retain quality and alleviate chilling injury in pepper. Postharvest Biol. Technol. 2005;35:69–78. doi: 10.1016/j.postharvbio.2004.06.001. [DOI] [Google Scholar]
- Vincken JP, Schols HA, Oomen RJ, McCann MC, Ulvskov P, Voragen AG, Visser RG. If homogalacturonan were a side chain of rhamnogalacturonan I. Implications for cell wall architecture. Plant Physiol. 132: 1781-1789 (2003) [DOI] [PMC free article] [PubMed]
- Walter RH. Analytical and Graphical Methods for Pectin, pp. 189-225. In: The Chemistry and Technology of Pectin. Walter RH (eds). Academic Press, San Diago, CA (1991)
- Wei J, Ma F, Shi S, Qi X, Zhu X, Yuan J. Changes and postharvest regulation of activity and gene expression of enzymes related to cell wall degradation in ripening apple fruit. Postharvest Biol. Technol. 56: 147-154 (2010)