Abstract
Our understanding of the pathogenesis of acne vulgaris is still evolving. It is known that multiple factors impact acne pathophysiology, including genetic, hormonal, inflammatory, and environmental influences. Because of its implications in many of these factors, diet has been a part of the acne discussion for decades. Several studies have evaluated the significance of the glycemic index of various foods and glycemic load in patients with acne, demonstrating individuals with acne who consume diets with a low glycemic load have reduced acne lesions compared with individuals on high glycemic load diets. Dairy has also been a focus of study regarding dietary influences on acne; whey proteins responsible for the insulinotropic effects of milk may contribute more to acne development than the actual fat or dairy content. Other studies have examined the effects of omega-3 fatty acid and γ-linoleic acid consumption in individuals with acne, showing individuals with acne benefit from diets consisting of fish and healthy oils, thereby increasing omega-3 and omega-6 fatty acid intake. Recent research into the effects of probiotic administration in individuals with acne present promising results; further study of the effects of probiotics on acne is needed to support the findings of these early studies. In this review, we discuss the current evidence regarding the diets of US patients with acne and how they may impact acne and acne treatment.
Key Points
| Despite limitations in studies investigating diet and acne, there is evidence that glycemic index, dairy content, dietary fats, and probiotics may play a role in acne and its treatment. |
| For all therapies prescribed, healthcare providers should provide patients with information on how their choice of diet could enhance their therapeutic outcomes and possibly reduce the risk of relapse upon treatment cessation. |
Introduction
Acne vulgaris is one of the most common dermatologic conditions globally [1, 2]. Unlike our knowledge regarding the heritable nature of acne and the role of hormones in acne pathogenesis, there is uncertainty regarding the role of environmental factors, including diet, in acne [3–5].
The development of acne in some populations after the adoption of a Western diet suggests that the latter plays a role in acne. Certain populations did not historically have acne: the Canadian Inuit, South African Zulus, Japanese Okinawans, Aché of Paraguay, and Kitavan islanders of Papua New Guinea. The appearance of acne in these groups has been attributed to their acceptance of Western diets, including processed foods, dairy, and refined sugars [6]. Acne is also absent in adolescents with Laron syndrome, an hereditary dwarfism disorder resulting from insensitivity to growth hormone [7]. However, some do develop acne if treatment with insulin-like growth factor (IGF-1) is undertaken. Acne resolves when IGF-1 dosing is reduced or discontinued.
Here, we examine how diet may influence acne pathogenesis, persistence, and treatment. To perform the literature search for this review, PubMed and MEDLINE were queried with combinations including but not limited to the following search terms: isotretinoin, diet and acne, glycemic index (GI) and acne, dairy and acne, and acne pathophysiology. A review of search results was performed for dates from 1977 to 2020, and relevant articles were selected. Because of the limited number of studies available, clinical studies and review articles were included if they contained information relevant to the review topic.
Pathophysiology of Acne
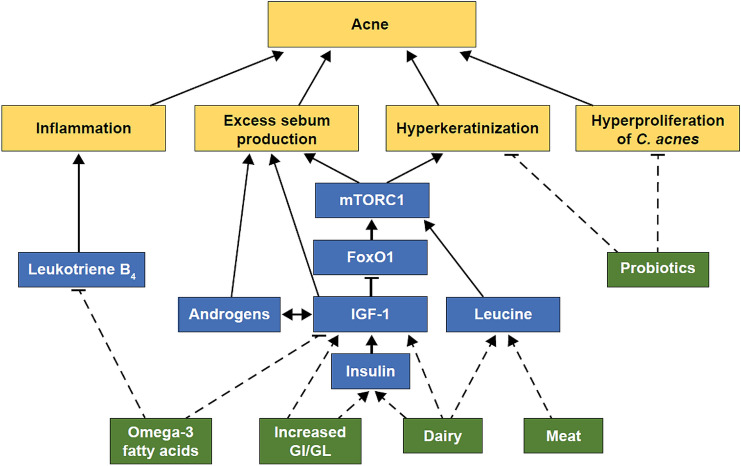
Acne pathogenesis is attributed to four key factors: excess sebum production, hyperproliferation of Cutibacterium acnes (C. acnes, formerly called Propionibacterium acnes) bacteria, hyperkeratinization of the pilosebaceous follicles, and inflammatory mechanisms [8, 9]. Excess sebum production occurs because of increased activity of androgen hormones and IGF-1 [5, 10]. Insulin-like growth factor-1 has been shown to decrease nuclear levels of the metabolic forkhead box class O transcription factor 1 (FoxO1), which leads to activation of the mammalian target of rapamycin complex 1 (mTORC1; Fig. 1) [11]. Mammalian target of rapamycin complex 1 is implicated in cell proliferation and metabolism. In acne, mTORC1 mediates sebaceous gland hyperproliferation, lipid synthesis, and hyperplasia of keratinocytes [11]. Leucine, a common amino acid in meat and dairy proteins, also activates mTORC1 [11]. Insulin-like growth factor-1 also increases androgen levels that, in turn, increase endogenous levels of IGF-1, forming a positive feedback loop that increases sebum production [10]. Hyperinsulinemia increases circulating levels of IGF-1 and insulin-growth factor-binding protein-3, directly impacting keratinocyte hyperplasia and apoptosis [10]. The expression of sterol response element-binding protein-1 is also increased by IGF-1, stimulating sebum production in sebocytes [12]. Growth hormones and inflammatory mediators are also associated with acne development [12, 13].
Fig. 1.
Dietary impact on mammalian target of rapamycin complex 1 (mTORC1) signaling. Dashed lines represent associations under discussion in this review. FoxO1 forkhead box class O transcription factor 1, GI glycemic index, GL glycemic load, IGF-1 insulin-like growth factor-1
The multimodal pathophysiology of acne affords numerous opportunities for pharmacologic intervention. The current treatment landscape for acne includes topicals (including antibiotics, benzoyl peroxide, dapsone, retinoids, or azelaic acid), orals (including antibiotics, hormonal agents, or isotretinoin), and physical interventions (including peels and laser and light therapies). Acne therapies act via a variety of mechanisms against a combination of the four key factors of acne pathogenesis [5, 9, 14–17]. Oral isotretinoin is the only therapy that directly or indirectly targets all known pathogenic aspects of acne [17]. Although the American Academy of Dermatology and clinical practice guidelines across the world recommend a variety of therapies for the treatment of each category of acne severity, isotretinoin has been the gold standard for the treatment of severe acne since its introduction in the early 1980s [9, 18].
Demographics of Patients with Acne
Acne prevalence is estimated to be 9.4% worldwide [2]. Prevalence is highest in adolescents and young adults and decreases with age beyond 30 years [19, 20]. After teenage years, acne remains more common in women than men, occurring in approximately one-half of women in their 20s, one-third in their 30s, and one-quarter in their 40s [21].
In the USA, acne is the most common condition presenting to dermatologists [22, 23]. Despite greater numbers of teenage boys than girls with acne, almost two-thirds of dermatology visits due to acne are attended by female individuals [24]. Approximately two-thirds of visits are made by patients < 25 years of age, with the mean age of those seeking treatment being 24 years [25]. Children as young as 6 years of age may be prescribed acne treatment [23, 26]. Acne medications are most often prescribed for those aged 12–14 years [24].
Diets of Patients with Acne
As patients with acne represent a broad cross-section of the US population and encompass people from all races and sexes, the diets consumed by individuals with acne represent a diverse array of food choices and nutritional regimens. Despite reducing consumption of sugar and fat over time and 77% of Americans reporting attempts to limit or avoid sugars in their diet, the majority of the US population still did not meet the American Heart Association’s 2020 Strategic Impact Goals dietary recommendations [27–29]. Multiple studies have shown that Americans are consuming too much added sugars, fat, carbohydrate, and sodium and too few whole grains [27, 30–32].
In a dietary survey of Americans in 2018, 36% reported following at least one specific eating pattern in the preceding year [29]. In total, 16% were following a form of low-carbohydrate-related diet, including the Paleo diet, a high-protein diet, and a ketogenic diet. A further 10% undertook intermittent fasting; 6% followed a gluten-free diet; 5%, a Mediterranean diet; 5%, the Whole 30 diet (an elimination diet in which participants remove certain food groups, such as sugar, grains, dairy, and alcohol, then reintroduce them over time to determine possible food sensitivities); and 4%, vegetarian or vegan diets. Many participants reported skipping at least one meal a day sometimes (27%), often (20%), or always (11%) and/or replacing at least one meal a day with snacks sometimes (30%), often (10%), or always (5%).
Vegetarian and vegan diets are increasingly popular [33]. One-third of all Americans have stopped or reduced their meat consumption. The number of vegans in the USA has grown from nearly 4 million in 2014 to 19.6 million in 2017. Plant-based milks account for 40% of all milk sales in the USA.
In the US National Health and Examination Survey between 2011 and 2014, breakfast consumption was highest in young children and older adults, with only three-quarters of adolescents and young people consuming breakfast. In individuals who consumed breakfast, this meal supplied < 20% of daily protein and total fat consumption and 20–22% of added sugar consumption [34]. Consequently, breakfast consumed by many adolescents and young adults likely contains high sugar content and provides little nutritional value.
Diets and Acne
As patients with acne represent a broad spectrum of the US population and as the latter has a variety of eating patterns with approximately one-third of the population following regimented diets, it is important to evaluate dietary aspects that may affect acne and its treatment. Current popular diets can be explored further by establishing their basic principles: Mediterranean diets including the Pioppi diet encourage low-carbohydrate, high-oil and high-vegetable, and moderate fish consumption; the ketogenic diet is defined by high-fat and low-carbohydrate consumption; and the Paleo diet typically includes high meat and very low dairy and sugar consumption [35–38]. Here, we examine the current understanding of the roles select diets have on acne (Table 1).
Table 1.
Select diets and their association with acne
| Diet | Role in acne pathophysiology | Acne findings | References |
|---|---|---|---|
| Low GL ± low GI | Reduces free androgens, increases IGFBP-3, and decreases IGF-1 levels | Evidence supportive of reduction in lesion quantity and severity of lesions | Smith et al. [39], Smith et al. [40], Burris et al. [41], Smith et al. [42], Çerman et al. [45], Burris et al. [46], Burris et al. [47], Smith et al. [51], Kwon et al. [52] |
| Dairy | Milk increases insulin and IGF-1 levels | Evidence supportive of association of milk and whey proteins in increasing acne lesions |
Rich-Edwards et al. [54], Adebamowo et al. [58], Adebamowo et al. [60], Adebamowo et al. [61], Okoro et al. [62], Grossi et al. [63], Karadag et al. [64], Duquia et al. [65] |
| Fat and fatty acids | Omega-3 fatty acids decrease IGF-1 and inhibit pro-inflammatory leukotriene B4 | Supportive of omega-3 fatty acids and γ-linoleic acid use in reduction of acne lesions | Logan [70], Li et al. [71], Simopoulos et al. [72], Zouboulis et al. [74], Jung et al. [75] |
| Vegetarian and vegan | Activation of mTORC1 decreased, leading to decreased signaling of pro-inflammatory nuclear factor-κB | No significant evidence supportive of vegan/vegetarian diets in reducing acne | Melnick [11], Young et al. [76], Stewart and Bazergy [77] |
| Probiotics | Possible production of bacteriocin-like inhibitory substances, may improve glucose metabolism and insulin levels, may increase levels of anti-inflammatory fatty acids | Mostly theoretical findings in support of acne reduction; early studies show promising results in acne improvements with probiotic supplementation | Bowe et al. [79], Kim et al. [80], Puch et al. [82], Salem et al. [83], Fabbrocini et al. [84] |
GI glycemic index, GL glycemic load, IGF-1 insulin-like growth factor-1, IGFBP-3 insulin-like growth factor-binding protein 3, mTORC mammalian target of rapamycin complex 1
Glycemic Index/Glycemic Load
The GI of a food refers to the rise in the blood glucose level, relative to pure glucose, 2 h after consumption of that food [39]. Glycemic load (GL) is a measure of a food’s ability to raise blood glucose levels, which accounts for carbohydrate in the food.
Current diets that have reduced GI or GL will have a low carbohydrate content and also typically call for a reduced intake of processed meats, bacon, added sugar, and refined grains [40]. Glycemic index and GL have been shown to affect pathways that are implicated in acne pathogenesis. For example, a low-GL diet reduces the free androgen index and increases insulin-growth factor-binding protein-3, whereas a low-GI and low-GL diet also decreases IGF-1 levels [39, 41]. In one study, a low-GL diet increased circulating levels of IGF-1-binding proteins in 12 male patients with acne (aged 15–20 years), which may suggest a reduction in the biologic activity of IGF-1 [40]. In another study involving 31 male patients with acne (aged 15–25 years) comparing a low-GL diet with a control diet for 12 weeks, there was an increased ratio of saturated-to-monounsaturated fatty acids of skin surface triglycerides and a reduction in acne lesions [42].
Ketogenic diets, which rely on a near-total reduction in carbohydrate and increased consumption of fat and protein, cause the body to source energy from ketones rather than glucose. This process is called ketosis. Ketogenic diets have been shown to reduce markers of inflammation and levels of IGF-1 [38, 43, 44].
Glycemic index and GL levels have been shown in multiple studies to be significantly higher in patients with acne than in control participants [45–47]. In particular, chocolate has been a focus of study regarding its effect on acne. In a blinded study of male individuals aged 18–35 years with acne, subjects were administered capsules with either unsweetened 100% cocoa, hydrolyzed gelatin, or a combination of the two. The study found a statistically significant increase in acne lesions after cocoa consumption, and a small-strength positive Pearson’s correlation coefficient existed between the quantity of cocoa ingested and the number of new acne lesions [48]. While the results of this study support an association between an increase in acne and cocoa consumption, limitations include utilization of only one brand of chocolate and exclusion of women. Daily consumption of chocolate and confectionaries were independently and highly associated with acne in other studies [49–51]. For example, in the recent prospective cohort NutriNet-Santé study involving 24,452 participants, the consumption of milk chocolate and sugary beverages was highly associated with current acne (odds ratio of 1.28 and 2.19, respectively) [51]. However, it is unclear whether the association of chocolate and confectionary consumption with acne could be due to the high GI of these foods or, potentially, the presence of acne increasing the intake of chocolate and sweets. Resultantly, further investigation of the effects of cocoa and chocolate on acne would be of value.
Two randomized controlled trials have shown that low-GL diets reduce acne lesion counts, body weight, and body fat compared with a carbohydrate-dense control diet in people with acne [39, 52]. Another study involved patients with mild-to-moderate acne randomized to either a low-GL diet or a high-GL diet for 10 weeks [53]. Those on a low-GL diet demonstrated significant reductions in noninflammatory and inflammatory lesion counts, smaller sebaceous glands, decreased inflammation, and reduced acne severity grading. In contrast, in university students aged 19–34 years, a study showed no association among serum glucose, insulin, leptin levels, and self-reported GI and dietary GL in patients with acne compared with those without acne (n = 49), suggesting factors other than dietary GI and serum insulin levels may contribute to acne in some adult patients with post-adolescent acne [54].
Cordain et al. [6] suggested that the absence of acne in the Kitavan Islanders of Papua New Guinea and the Aché hunter-gatherers of Paraguay was due to their low-GL diets, devoid of Western refined foods. Genetic factors were discounted as the reason for the historical lack of acne, as other South American Indians and Pacific Islanders with similar ethnic backgrounds, but more Westernized lifestyles, had considerably higher acne prevalence than the Aché and Kitavan Islanders. However, the lower acne prevalence could also be due to a higher content of omega-3 fatty acids or lower levels of milk and dairy products in the diets of these populations compared with Western diets. Accordingly, while investigation of low-GI and low-GL diets has generally produced strong evidence in support of improvements in acne with these diets, further research may be of value to explain the disparity between the results of past studies.
Dairy
Dairy remains a prominent dietary component for most Western societies. Milk consumption has been reported to increase IGF-1 levels [55]. Eighty percent of cow’s milk protein is casein, and the remaining 20% is whey proteins [56]. Whey proteins are predominantly responsible for the insulinotropic effects of milk, whereas casein stimulates IGF-1 to a greater extent than whey [56]. Hyperinsulinemia, such as that caused by the insulinotropic effects of milk whey proteins, also increases IGF-1 levels, which could provide a potential explanation for why those taking whey supplements, as has become common practice in fitness centers and weights-based athletic pursuits, present with an onset or aggravation of acne [57, 58].
The relationship between dairy and acne was first established by a questionnaire-based cohort study in 47,355 female patients with acne [59]. Adebamowo et al. [59] found a positive association with acne for total milk intake and skimmed milk intake. However, these data relied on self-reporting of diet from at least a decade prior, and dairy products were featured in only 2 of the 22 questions in the questionnaire [60].
A 3-year prospective study by the same group involving 6094 girls aged 9–15 years found a positive association between acne prevalence and consumption of full-fat, skimmed, and low-fat milk but no association with non-milk dairy foods, chocolate, and pizza [61]. A similar study in 4273 boys aged 9–15 years found only a weak association between acne and skimmed milk and no association with milks of a higher fat content [62]. Yet, these two studies were also questionnaire based and contained no blinded objective measurements. Other studies have shown a positive association between dairy consumption in some form, including ice cream, and acne without testing for a causal link [63–67].
In the NutriNet-Santé study, the consumption of milk was found to be independently associated with acne with participants with current acne consuming significantly more milk vs participants without acne (adjusted odds ratio of 1.12 and p = 0.04) [51]. However, it should be noted that 75% of this study’s participants were female, thereby, these results may not be equally representative for male individuals.
Some have postulated that the impact of dairy on acne may be due to the GI of the milk rather than the fat or dairy content [68]. However, when taking into account the GI of various dairy products, a more likely cause for the association of dairy with acne is the insulin index, defined as the elevation of insulin levels in the blood during the 2-h period after food is ingested [69]. Milk has a very high insulin index, irrespective of fat content [69, 70]. By comparison, cheese has a low insulin index, whereas ice cream has a high insulin index due to added sugar; therefore, this theory, based on hyperinsulinemia, would account for the association between ice cream and acne and the lack of association between cheese and acne.
Dietary Fats and Fatty Acids
The stereotypical Western diet contains a higher ratio of omega-6 to omega-3 fatty acids compared with the hunter-gatherer diet and similar diets rich in fish, wild game, and wild plants [71]. Certain diets, such as the Mediterranean diet, encourage consumption of fish and olive oil to increase essential fatty acid intake [37].
Omega-3 fatty acids have been shown to decrease IGF-1, which, as previously discussed, is implicated in sebum production and follicular occlusion [71, 72]. Omega-3 fatty acids also inhibit synthesis of inflammatory leukotriene B4, which in turn reduces inflammatory acne lesions [73–75]. Some studies have shown an association between lower consumption of fish and increased severity of acne [46, 65]. Increased trans-fat and saturated fat consumption was associated with increased acne severity [46].
To test the potential effects of fatty acids on acne outcomes, a randomized controlled trial compared the effects of dietary supplementation with an omega-3 fatty acid or a γ-linoleic acid with no supplementation over a 10-week period in 45 patients with mild-to-moderate acne [76]. Adding an omega-3 fatty acid supplement or a γ-linoleic acid supplement significantly reduced inflammatory and noninflammatory lesion counts. This suggests that patients with acne may benefit from increased omega-3 fatty acid or γ-linoleic acid consumption, for example through increased fish consumption.
Vegetarian and Vegan
Vegetarian and vegan diets include reduced or no intake of all types of meat (including fish) and dairy. Leucine levels are higher in meat/dairy protein-based diets compared with vegetarian or vegan diets. As leucine activates mTORC1, those consuming meat/dairy protein-based diets have increased mTORC1 activation (Fig. 1) [11]. Mammalian target of rapamycin complex 1 is a signaling protein known to activate pro-inflammatory nuclear factor κB signaling, possibly aggravating the inflammation implicated in acne [77].
Despite reduced dairy and leucine intake, Stewart and Bazergy [78] found the prevalence of vegan diets to not be significantly different between 453 patients with acne and 150 controls retrospectively screened from a family medical clinic. However, this study did not detail the contents of the vegan diets, which may have other factors, such as GL and GI, to explain the lack of difference. Because of limited studies on vegetarian and vegan diets in patients with acne, further research in this area is needed to expand our understanding of the impact of these diets on patients with acne.
Probiotics
In recent years, the microbiome has become a field of interest across the spectrum of human health and disease. In view of the role of C. acnes in acne, the balance of microbiota in the skin is of interest in acne pathogenesis and therapy. The gut microbiome is also of interest, as patients with acne have been shown to have circulating levels of endotoxins not found in controls [79].
There are theoretical effects of probiotics on acne, including the bacteriocin-like inhibitory substance produced by Streptococcus salivarius resulting in C. acnes inhibition in vitro [80] and evidence that oral Bifidobacterium lactis can improve glucose metabolism and fasting plasma insulin levels in non-insulin-dependent diabetes mellitus mouse models [81]. Bifidobacteria can be reduced by high-fat consumption [82]. Coadministration of a probiotic mix, including Lactobacillus casei, Lactobacillus bulgaricus, and Streptococcus thermophilus, with fatty acids, increases blood levels of anti-inflammatory fatty acids [83]. Although in vitro and animal studies are intriguing, they are speculative, and further in vivo evaluations are needed.
The gut microbiota have also been implicated in acne pathophysiology. Metabolites produced by gut microbiota have been shown to interact with mTOR signaling pathways, which in turn affect intestinal microbiota composition and have implications in acne pathogenesis [84]. Furthermore, in both acne vulgaris and intestinal dysbiosis, increased expression of substance P and upregulation of substance P-containing nerves have been observed [84]. Substance P can trigger an increase in proinflammatory mediators such as interleukin-6 and tumor necrosis factor-α, which have both also been implicated in acne pathogenesis [84].
In a pilot, randomized, placebo-controlled, double-blind study of 20 adult patients with acne comparing a liquid supplement containing Lactobacillus rhamnosus GG to placebo (a liquid lacking probiotics) over a 12-week period [85], those receiving Lactobacillus rhamnosus GG showed a marked improvement in acne (adjusted odds ratio of improved/markedly improved rating 28.4 vs placebo, 95% confidence interval 2.2–411.1, p < 0.05). Skin biopsies showed reduced levels of IGF-1 and increased levels of FoxO1 gene expression in the probiotic group. Although small, this study indicates that probiotics may be a beneficial and well-tolerated dietary supplement for patients with acne. More investigation into the use of probiotics in individuals with acne is necessary to support these early findings.
Effects of Diets on Acne Treatment Efficacy
For an oral medication to be effective, it must be absorbed, enter into the circulation, and be delivered to and absorbed by the target tissue [86]. This is influenced by inherent drug characteristics as well as the presence of gastrointestinal contents and their specific composition. Drug absorption characteristics of importance include solubility in the gastrointestinal tract and permeability into the enterocytes. Gastrointestinal contents can modify absorption of medications, drug solubility, stomach-emptying time, and gastrointestinal pH levels as well as form indigestible complexes and compete for enterocyte transportation [86]. All of these factors can influence adverse event profiles as well [87].
The US Center for Drug Evaluation and Research has categorized drugs into four classes based on solubility and permeability [88]. Oral acne medications such as minocycline and doxycycline are considered as Class I as both have high solubility and high permeability [89]. Spironolactone and isotretinoin, in contrast, are Class II with poor solubility in stomach contents but high permeability [89, 90]. Accordingly, it is recommended that both spironolactone and isotretinoin be taken with food to increase absorption; in particular, isotretinoin should be administered with a high-fat meal [90, 91].
Glycemic Index/Glycemic Load
A significant correlation between response to isotretinoin and GL was demonstrated in a study of 32 female patients with acne receiving isotretinoin (0.5 mg/kg) in whom a low-GL diet was a predictive marker of isotretinoin response [92]. In contrast, a randomized controlled trial compared the efficacy of topical benzoyl peroxide 2.5% gel treatment co-administered with either a low-GL diet or a control diet in 84 patients with acne [93]. Both groups showed a significant reduction in acne lesion counts after 12 weeks, with no significant difference.
Dairy
Absorption and bioavailability of tetracycline class antibiotics have been extensively studied, as the minimum inhibitory concentration must be maintained during the course of treatment in order for this class of bacteriostatic antibiotics to be effective [94]. For example, the presence of any food in the stomach can reduce absorption of doxycycline by nearly 20% [94, 95]. Moreover, for tetracycline, in which absorption is inferior to doxycycline, the bioavailability is reduced when taken with food rather than after a fast, and this reduction is greater if the food contains dairy [94]. Calcium in dairy can chelate doxycycline, reducing its absorption [95]. Minocycline absorption is less affected by stomach contents compared with doxycycline and tetracycline [95]. In a study evaluating comparative absorption of minocycline and tetracycline taken with milk and food, both drugs showed a significant reduction in absorption compared with administration with water; the effect was significantly greater for tetracycline than minocycline [96]. However, in a comparative study evaluating the absorption of an extended-release formulation of minocycline under fed (high-dairy, high-fat) and fasted states, the extended-release formulation of minocycline was shown to be unaffected by stomach contents, as measured by mean area under the curve, maximum plasma concentration, and time to maximum concentration [97]. Conversely, in a three-way crossover study comparing the delayed-release formulation of doxycycline with the standard formulation, the overall rate of absorption was increased in both formulations in a fasted state compared with an unfasted state, but there was no significant difference in the overall bioavailability between the two formulations [98].
Although there is little further evidence of a relationship between dairy intake and acne treatment efficacy, there are some data regarding supplements containing whey protein, the major protein found in milk. In a case series, five male patients aged 14–18 years with acne who experienced acne onset after starting whey protein supplements were treated with standard acne therapies [58]. All five patients had a poor response to oral antibiotics, topical retinoids, and benzoyl peroxide. When four of these patients subsequently discontinued whey protein supplementation, their acne rapidly and completely cleared. One who reinstated whey protein supplementation experienced an acne flare within 1 week. Whey protein concentrate has a high proportion of leucine (14 g/100 g of protein), which is a known activator of mTORC1 [11].
Dietary Minerals, Vitamins, and Antacids
Minerals such as calcium, magnesium, zinc, iron, and aluminum can chelate all of the tetracyclines, resulting in poor absorption and loss of efficacy [95]. Minocycline absorption is primarily reduced by dietary iron [95]. It is recommended that supplements and antacids be avoided 4–6 h before and 2 h after administration of the tetracyclines [99].
Oral and topical zinc has been a potential therapeutic of interest for acne for many years. Several studies found that treatment with oral zinc sulfate resulted in a slightly reduced number and severity of acne lesions compared with placebo, although these findings conflict with other studies that have found little clinical benefit of zinc in improving acne [100–102]. Consequently, zinc may provide a slight benefit in the reduction of acne lesions, though a consensus is needed between studies for zinc to be considered as an effective treatment for acne.
Dietary Fats and Fatty Acids
Prescribing information for isotretinoin states that coadministration with a high-fat meal enhances bioavailability. In all phase III studies evaluating the efficacy of isotretinoin, the US Food and Drug Administration mandated that dosing occur with a high-fat (50-g fat) meal [90, 103]. There has been no direct comparison of bioavailability of isotretinoin with a high-fat meal and a lower fat meal. When lidose-isotretinoin was administered to patients in the fasted state, efficacy and relapse rates were similar or lower relative to historic controls who took the drug with a 50-g fat meal [104]. The only efficacy data that exist for conventional isotretinoin are from patients following a 50-g fat meal, but the results of the pharmacokinetic study previously mentioned suggest that efficacy might suffer if isotretinoin is taken without fat. Further investigation of lidose-isotretinoin vs conventional isotretinoin would be of value in conditions of standard food intake (as opposed to a high-fat meal) and including additional supplements such as omega-3 fatty acids or olive oil. Recently in the USA, the Food and Drug Administration approved a novel formulation of lidose-isotretinoin in which the particles of the drug are micronized, increasing the surface area and resulting in improved dissolution. It is bioequivalent when taken after a high-fat meal and almost twice as bioavailable under fasted conditions despite administration at a 20% lower dose (32 mg) compared with the prior lidose-isotretinoin 40-mg dose [105, 106].
Impact of Dietary Restrictions on Drug Adherence
The importance of this information on diet is magnified by the knowledge that increased medication regimen complexity can affect consistent drug use [107]; complexity can include restriction on dietary choices and timing of meals. In addition, whereas antibiotics are more efficacious when taken without food, they are less likely to result in gastrointestinal disturbances when taken with food [95]. Barriers such as complex treatment regimens and adverse effects can lead to doses being delayed, taken in suboptimal conditions or without regard for food–drug interactions, or skipped altogether.
Conclusions
Despite limitations in studies investigating diet and acne, there is evidence that diet plays a role in acne and its treatment. There is strong support for the reduction of acne with regular consumption of omega-3 fatty acids and low-GI and low-GL diets. Similarly, several studies demonstrate milk may worsen the number and severity of acne lesions. Specifically, acne flares in individuals consuming milk may be related to whey proteins and casein via insulinotropic and IGF-1 pathways, which may explain why other dairy products such as butter or cheese have not demonstrated the same associations with acne. Low glycemic diets have generally shown favorable improvements in acne outcomes, possibly because of their effects on insulin and IGF-1; however, because of inconsistent findings in studies of patients with acne on low glycemic diets, additional treatment may be necessary in combination with changes in diet to reduce acne. Administration of probiotics shows promise in reducing acne lesions; although, further research is needed in this area to support these early findings. More investigation is necessary on the effects of vegetarian/vegan and ketogenic diets on acne, as research in this area is lacking. Efficacy of acne treatments such as tetracycline class antibiotics appears to be significantly affected by diet; in particular, there is considerable evidence that maximum absorption occurs in a fasted state with no dairy. Despite prescribing information for isotretinoin indicating that coadministration with a high-fat meal enhances bioavailability, studies have shown that lidose-isotretinoin does not require a high-fat meal for maximum bioavailability. Accordingly, new formulations of isotretinoin, such as lidose-isotretinoin, may not need coadministration with special diets. For all therapies prescribed, healthcare providers should provide patients with information on how their choice of diet could enhance their therapeutic outcomes and possibly reduce the risk of relapse upon treatment cessation.
Acknowledgments
Editorial support was provided by Tamsin Brown, MSc, of JK Associates, Inc., part of the Fishawack Group of Companies, and was funded by Sun Pharmaceutical Industries, Inc.
Declarations
Funding
The review was funded by Sun Pharmaceutical Industries, Inc., Princeton, NJ, USA.
Conflicts of Interest
Jerry Tan has been an advisor, consultant, speaker, and/or investigator for Bausch Health, Boots/Walgreens, Cipher, Galderma, and Sun Pharmaceutical Industries, Inc., and he is on the medical advisory board of the Acne and Rosacea Society of Canada. Hilary Baldwin has been an advisor, consultant, speaker, and/or investigator for Almirall, Bio-PharmX, Botanix, Dermira, EPI Health, Foamix, Galderma, La Roche-Posay, Johnson & Johnson, Mayne, Ortho-Dermatologics, Sol-Gel, and Sun Pharmaceutical Industries, Inc.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
All authors consented to publication.
Availability of data and material
Not applicable.
Code Availability
Not applicable.
Authors’ contributions
All authors contributed to the conception and development of the article, reviewed it critically for content, and approved the final version for submission.
Footnotes
The original online version of this article was revised due to retrospective open access.
Change history
12/26/2020
The original article can be found online.
References
- 1.American Academy of Dermatology. Acne. 2019. https://www.aad.org/public/diseases/acne-and-rosacea/acne. Accessed Aug 2019.
- 2.Vos T, Flaxman A, Naghavi M, Lozano R, Michaud C, Ezzati M, et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990-2010: a systemic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380(9859):2163–2196. doi: 10.1016/S0140-6736(12)61729-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bataille V, Sneider H, MacGregor A, Sasieni P, Spector T. The influence of genetics and enviromental factors in the pathogenesis of acne: a twin study of acne in women. J Invest Dermatol. 2002;119:1317–1322. doi: 10.1046/j.1523-1747.2002.19621.x. [DOI] [PubMed] [Google Scholar]
- 4.Friedman G. Twin studies of disease heritability based on medical records: application to acne vulgaris. Acta Genet Med Gemellol. 1984;33:487–495. doi: 10.1017/s0001566000005948. [DOI] [PubMed] [Google Scholar]
- 5.Arora MK, Yadav A, Saini V. Role of hormones in acne vulgaris. Clin Biochem. 2011;44(13):1035–1040. doi: 10.1016/j.clinbiochem.2011.06.984. [DOI] [PubMed] [Google Scholar]
- 6.Cordain L, Lindeberg S, Hurtado M, Hill K, Boyd Eaton S, Brand-Miller J. Acne vulgaris. Arch Dermatol. 2002;138:1584–1590. doi: 10.1001/archderm.138.12.1584. [DOI] [PubMed] [Google Scholar]
- 7.Klinger B, Anin S, Silbergeld A, Eshet R, Laron Z. Development of hyperandrogenism during treatment with insulin-like growth factor-I (IGF-I) in female patients with Laron syndrome. Clin Endocrinol (Oxf). 1998;48(1):81–87. doi: 10.1046/j.1365-2265.1998.00356.x. [DOI] [PubMed] [Google Scholar]
- 8.Kircik L. Advances in the understanding of the pathogenesis of inflammatory acne. J Drugs Dermatol. 2016;15(1 Suppl. 1):S7–S10. [PubMed] [Google Scholar]
- 9.Zaenglein A, Pathy A, Schlosser B, Alikhan A, Baldwin H, Berson D, et al. Guidelines of care for the management of acne vulgaris. J Am Acad Dermatol. 2016;74:945–973. doi: 10.1016/j.jaad.2015.12.037. [DOI] [PubMed] [Google Scholar]
- 10.Kucharska A, Szmurło A, Sińska B. Significance of diet in treated and untreated acne vulgaris. Adv Dermatol Allergol. 2016;23(2):81–86. doi: 10.5114/ada.2016.59146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Melnik B. Dietary intervention in acne: attenuation of increased mTORC1 signaling promoted by Western diet. Dermatoendocrinology. 2012;4(1):20–32. doi: 10.4161/derm.19828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Smith T, Gilliland K, Clawson G, Thiboutot D. IGF-1 induces SREBP-1 expression and lipogenesis in SEB-1 sebocytes via activation of the phosphoinositide 3-kinase (PI3-k)/Akt pathway. J Invest Dermatol. 2008;128(5):1286–1293. doi: 10.1038/sj.jid.5701155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Burris J, Rietkerk W, Woolf K. Acne: the role of medical nutrition therapy. J Acad Nutri Diet. 2013;113(3):416–430. doi: 10.1016/j.jand.2012.11.016. [DOI] [PubMed] [Google Scholar]
- 14.Leyden J, Stein Gold L, Weiss J. Why topical retinoids are mainstay of therapy for acne. Dermtol Ther (Heidelb). 2017;7(3):293–304. doi: 10.1007/s13555-017-0185-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Czernielewski J, Michel S, Bouclier M, Baker M, Hensby J. Adapalene biochemistry and the evolution of a new topical retinoid for the treatment of acne. J Eur Acad Dermatol Venereol. 2001;15(Suppl. 3):5–12. doi: 10.1046/j.0926-9959.2001.00006.x. [DOI] [PubMed] [Google Scholar]
- 16.Cotterill J. Benzoyl peroxide. Acta Derm Venereol Suppl (Stockh). 1980;1980(Suppl 89):57–63. [PubMed] [Google Scholar]
- 17.Kurokawa I, Danby F, Ju Q, Wang X, Xiang L, Xia L, et al. New developments in our understanding of acne pathogenesis and treatment. Exp Dermatol. 2009;18:821–832. doi: 10.1111/j.1600-0625.2009.00890.x. [DOI] [PubMed] [Google Scholar]
- 18.AAD. Dermatologist evaluates latest isotretinoin developments for treatment of severe acne. 2014. https://www.aad.org/media/news-releases/dermatologist-evaluates-latest-isotretinoin-developments-for-treatment-of-severe-acne. Accessed Aug 2019.
- 19.Schäfer T, Nienhaus A, Vieluf D, Berger J, Ring J. Epidemiology of acne in the general population: the risk of smoking. Br J Dermatol. 2001;145(1):100–104. doi: 10.1046/j.1365-2133.2001.04290.x. [DOI] [PubMed] [Google Scholar]
- 20.Cunliffe W, Gould D. Prevalence of facial acne vulgaris in late adolescence and in adults. BMJ. 1979;1:1109–1110. doi: 10.1136/bmj.1.6171.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Collier C, Harper J, Cantrell W, Wang W, Foster K, Elewski B. The prevalence of acne in adults 20 years and older. J Am Acad Dermatol. 2008;58(1):56–69. doi: 10.1016/j.jaad.2007.06.045. [DOI] [PubMed] [Google Scholar]
- 22.Wilmer E, Gustafson C, Ahn C, Davis S, Feldman S, Huang W. Most common dermatologic conditions encountered by dermatologists and nondermatologists. Cutis. 2014;94:285–292. [PubMed] [Google Scholar]
- 23.Davis S, Narahari S, Feldman S, Huang W, Pichardo-Geisinger R, McMichael A. Top dermatologic conditions in patients of color: an analysis of nationally representative data. J Drugs Dermatol. 2012;11(4):466–473. [PubMed] [Google Scholar]
- 24.Yentzer B, Hick J, Reese E, Uhas A, Feldman S, Balkrishnan R. Acne vulgaris in the United States: a descriptive epidemiology. Cutis. 2010;86:94–99. [PubMed] [Google Scholar]
- 25.McConnell R, Fleischer AJ, Williford P, Feldman S. Most topical tretinoin treatment is for acne vulgaris through the age of 44 years: an analysis of the National Ambulatory Medical Care Survey, 1990–1994. J Am Acad Dermatol. 1998;38:221–226. doi: 10.1016/s0190-9622(98)70598-5. [DOI] [PubMed] [Google Scholar]
- 26.Goldberg J, Dabade T, Davis S, Feldman S, Krowchuk D, Fleischer A. Changing age of acne vulgaris visits: another sign of earlier puberty. Pediatr Dermatol. 2011;28(6):645–648. doi: 10.1111/j.1525-1470.2011.01643.x. [DOI] [PubMed] [Google Scholar]
- 27.Rehm C, Peñalvo J, Afshin A, Mozaffarian D. Dietary intake among US adults, 1999–2012. JAMA. 2016;315(23):2542–2553. doi: 10.1001/jama.2016.7491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cohen E, Cragg M, deFonseka J, Hite A, Rosenberg M, Zhou B. Statistical review of US macronutrient consumption data, 1965-2011: Americans have been following dietary guidelines, coincident with the rise in obesity. Nutrition. 2015;31(5):727–732. doi: 10.1016/j.nut.2015.02.007. [DOI] [PubMed] [Google Scholar]
- 29.Food & Health Survey. 2018. https://foodinsight.org/wp-content/uploads/2018/05/2018-FHS-Report-FINAL.pdf. Accessed Aug 2019.
- 30.Wells H, Buzby J. Dietary assessment of major trends in U.S. food consumption, 1970–2005 (economic information bulletin; no. 33). United States Department of Agriculture. Washington, DC: Economic Research Service; 2008.
- 31.Krebs-Smith S, Guenther P, Subar A, Kirkpatrick S, Dodd K. Americans do not meet federal dietary recommendations. J Nutr. 2010;140:1832–1838. doi: 10.3945/jn.110.124826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Neri D, Martinez-Steele E, Monteiro C, Levy R. Consumption of ultra-processed foods and its association with added sugar content in the diets of US children, NHANES 2009–2014. Pediatr Obes. 2019;14(12):e12563. doi: 10.1111/ijpo.12563. [DOI] [PubMed] [Google Scholar]
- 33.The Vegan Society. Statistics. 2019. https://www.vegansociety.com/news/media/statistics. Accessed Aug 2019.
- 34.Drewnowski A, Rehm C, Vieux F. Breakfast in the United States: food and nutrient intakes in relation to diet quality in National Health and Examination Survey 2011–2014. A study from the International Breakfast Research Initiative. Nutrients. 2018;10(9):E1200. doi: 10.3390/nu10091200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pitt C. Cutting through the Paleo hype: the evidence for the Paleolithic diet. Aus Fam Physician. 2016;45(1–2):35–38. [PubMed] [Google Scholar]
- 36.Kirby M. What our patients are reading: the Pioppi diet. Diabetes Prim Care. 2018;20:42–45. [Google Scholar]
- 37.Simopoulos A. Omega-6/omega-3 essential fatty acid ratio and chronic diseases. Food Rev Int. 2004;20(1):77–90. [Google Scholar]
- 38.Spulber G, Spulber S, Hagenäs L, Åmark P, Dahlin M. Growth dependence on insulin-like growth-factor-I during the ketogenic diet. Epilepsia. 2009;50(2):297–303. doi: 10.1111/j.1528-1167.2008.01769.x. [DOI] [PubMed] [Google Scholar]
- 39.Smith R, Mann N, Braue A, Mäkeläinen H, Varigos G. The effect of a high-protein, low glycemic-load diet versus a conventional, high glycemic-load diet on biochemical parameters associated with acne vulgaris: a randomized, investigator-masked, controlled trial. J Am Acad Dermatol. 2007;57(2):247–256. doi: 10.1016/j.jaad.2007.01.046. [DOI] [PubMed] [Google Scholar]
- 40.Smith R, Mann N, Mäkeläinen H, Roper J, Braue A, Varigos G. A pilot study to determine the short-term effects of a low glycemic load diet on hormonal markers of acne: a nonrandomized, parallel-controlled feeding trial. Mol Nutr Food Res. 2008;52(6):718–726. doi: 10.1002/mnfr.200700307. [DOI] [PubMed] [Google Scholar]
- 41.Burris J, Shikany J, Rietkerk W, Woolf K. A low glycemic index and glycemic load diet decreases insulin-like growth factor-1 among adults with moderate and severe acne: a short-duration, 2-week randomized controlled trial. J Acad Nutri Diet. 2018;118(10):1874–1885. doi: 10.1016/j.jand.2018.02.009. [DOI] [PubMed] [Google Scholar]
- 42.Smith R, Braue A, Varigos G, Mann N. The effect of a low glycemic load diet on acne vulgaris and the fatty acid composition of skin surface triglycerides. J Dermatol Sci. 2008;50(1):41–52. doi: 10.1016/j.jdermsci.2007.11.005. [DOI] [PubMed] [Google Scholar]
- 43.Forsythe C, Phinney S, Fernandez M, Quann E, Wood R, Bibus D, et al. Comparison of low fat and low carbohydrate diets on circulating fatty acid composition and markers of inflammation. Lipids. 2008;42:65–77. doi: 10.1007/s11745-007-3132-7. [DOI] [PubMed] [Google Scholar]
- 44.Fraser D, Thoen J, Bondhus S, Haugen M, Reseland J, Djøseland O, et al. Reduction in serum leptin and IGF-1 but preserved T-lymphocyte numbers and activation after a ketogenic diet in rheumatoid arthritis patients. Clin Exp Rheumatol. 2000;18:209–214. [PubMed] [Google Scholar]
- 45.Çerman A, Aktaş E, Altunay İ, Arıcı J, Tulunay A, Ozturk F. Dietary glycemic factors, insulin resistance, and adiponectin levels in acne vulgaris. J Am Acad Dermatol. 2016;75(1):155–162. doi: 10.1016/j.jaad.2016.02.1220. [DOI] [PubMed] [Google Scholar]
- 46.Burris J, Rietkerk W, Woolf K. Relationshps of self-reported dietary factors and perceived acne severity in a cohort of New York young adults. J Acad Nutri Diet. 2014;114(3):384–392. doi: 10.1016/j.jand.2013.11.010. [DOI] [PubMed] [Google Scholar]
- 47.Burris J, Rietkerk W, Shikany J, Woolf K. Differences in dietary glycemic load and hormones in New York City adults with no and moderate/severe acne. J Acad Nutri Diet. 2017;117(9):1375–1383. doi: 10.1016/j.jand.2017.03.024. [DOI] [PubMed] [Google Scholar]
- 48.Caperton C, Block S, Viera M, Keri J, Berman B. Double-blind, placebo-controlled study assessing the effect of chocolate consumption in subjects with a history of acne vulgaris. J Clin Aesthet Dermatol. 2014;7(5):19–23. [PMC free article] [PubMed] [Google Scholar]
- 49.Wolkenstein P, Misery L, Amici J, Maghia R, Branchoux S, Cazeau C, et al. Smoking and dietary factors associated with moderate-to-severe acne in French adolescents and young adults: results of a survey using a representative sample. Dermatology. 2015;230(1):34–39. doi: 10.1159/000366195. [DOI] [PubMed] [Google Scholar]
- 50.Park S, Kwon H, Min S, Yoon J, Suh D. Epidemiology and risk factors of childhood acne in Korea: a cross-sectional community based study. Clin Exp Dermatol. 2015;40(8):844–850. doi: 10.1111/ced.12686. [DOI] [PubMed] [Google Scholar]
- 51.Penso L, Touvier M, Deschasaux M, de Edelenyi FS, Hercberg S, Ezzedine K, et al. Association between adult acne and dietary behaviors: findings from the NutriNet-Santé Prospective Cohort Study. JAMA Dermatol. 2020;2020:201602. doi: 10.1001/jamadermatol.2020.1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Smith R, Mann N, Braue A, Mäkeläinen H, Varigos G. A low-glycemic-load diet improves symptoms in acne vulgaris patients: a randomized controlled trial. Am J Clin Nutr. 2007;86(1):107–115. doi: 10.1093/ajcn/86.1.107. [DOI] [PubMed] [Google Scholar]
- 53.Kwon H, Yoon J, Hong J, Jung J, Park M, Suh D. Clinical and histological effect of a low glycaemic load diet in treatment of acne vulgaris in Korean patients: a randomized, controlled trial. Acta Derm Venereol. 2012;92:241–246. doi: 10.2340/00015555-1346. [DOI] [PubMed] [Google Scholar]
- 54.Kaymak Y, Adisen E, Ilter N, Bideci A, Gurler D, Celik B. Dietary glycemic index and glucose, insulin, insulin-like growth factor-I, insulin-like growth factor binding protein 3, and leptin levels in patients with acne. J Am Acad Dermatol. 2007;57(5):819–823. doi: 10.1016/j.jaad.2007.06.028. [DOI] [PubMed] [Google Scholar]
- 55.Rich-Edwards JW, Ganmaa D, Pollak MN, Nakamoto EK, Kleinman K, Tserendolgor U, et al. Milk consumption and the prepubertal somatotropic axis. Nutr J. 2007;27(6):28. doi: 10.1186/1475-2891-6-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kumari R, Thappa D. Role of insulin resistance and diet in acne. Ind J Dermatol Venereol Leprol. 2013;79(3):291–300. doi: 10.4103/0378-6323.110753. [DOI] [PubMed] [Google Scholar]
- 57.Pontes T, Fernandes Filho G, Trindade Ade S, Sobral Filho J. Incidence of acne vulgaris in young adult users of protein supplements in the city of João Pessoa–PB. An Bras Dermatol. 2013;88(6):907–912. doi: 10.1590/abd1806-4841.20132024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Silverberg N. Whey protein precipitating moderate to severe acne flares in 5 teenaged athletes. Cutis. 2012;90(2):70–72. [PubMed] [Google Scholar]
- 59.Adebamowo C, Spiegelman D, Danby F, Frazier A, Willett W, Holmes M. High school dietary dairy intake and teenage acne. J Am Acad Dermatol. 2005;52:207–214. doi: 10.1016/j.jaad.2004.08.007. [DOI] [PubMed] [Google Scholar]
- 60.Nurses’ Health Study. Questionnaires. 2019. https://www.nurseshealthstudy.org/participants/questionnaires. Accessed Sep 2019.
- 61.Adebamowo C, Spiegelman D, Berkey C, Danby F, Rockett H, Colditz G, et al. Milk consumption and acne in adolescent girls. Dermatol Online J. 2006;12(4):1. [PubMed] [Google Scholar]
- 62.Adebamowo C, Spiegelman D, Berkey C, Danby F, Rockett H, Colditz G, et al. Milk consumption and acne in teenaged boys. J Am Acad Dermatol. 2008;58(5):787–793. doi: 10.1016/j.jaad.2007.08.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Okoro E, Ogunbiyi A, George A, Subulade M. Association of diet with acne vulgaris among adolescents in Ibadan, southwest Nigeria. Int J Dermatol. 2016;55(9):982–988. doi: 10.1111/ijd.13166. [DOI] [PubMed] [Google Scholar]
- 64.Grossi E, Cazzaniga S, Crotti S, Naldi L, Di Landro A, Ingordo V, et al. The constellation of dietary factors in adolescent acne: a semantic connectivity map approach. J Eur Acad Dermatol Venereol. 2014;30(1):96–100. doi: 10.1111/jdv.12878. [DOI] [PubMed] [Google Scholar]
- 65.Karadag A, Balta İ, Saricaoğlu H, Kiliç S, Kelekçi K, Yildirim M, et al. The effect of personal, familial, and environmental characteristics on acne vulgaris: a prospective, multicenter, case controlled study. G Ital Dermatol Venereol. 2019;154(2):177–185. doi: 10.23736/S0392-0488.17.05532-8. [DOI] [PubMed] [Google Scholar]
- 66.Duquia R, da Silva R, dos Santos I, de Almeida Jr H, Martins Souza P, de Avelar Breunig J, Zouboulis C. Epidemiology of acne vulgaris in 18-year-old male army conscripts in a south Brazilian city. Dermatology. 2017;233:145–154. doi: 10.1159/000475775. [DOI] [PubMed] [Google Scholar]
- 67.Ismail N, Manaf Z, Azizan N. High glycemic load diet, milk and ice cream consumption are related to acne vulgaris in Malaysian young adults: a case control study. BMC Dermatol. 2012;12:13. doi: 10.1186/1471-5945-12-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bhate K, Williams H. Epidemiology of acne vulgaris. Br J Dermatol. 2012;168:474–485. doi: 10.1111/bjd.12149. [DOI] [PubMed] [Google Scholar]
- 69.Hoyt G, Hickey M, Cordain L. Dissociation of the glycaemic and insulinaemic responses to whole and skimmed milk. Br J Nutr. 2005;93:175–177. doi: 10.1079/bjn20041304. [DOI] [PubMed] [Google Scholar]
- 70.Liljeberg Elmståhl H, Björck I. Milk as a supplement to mixed meals may elevate postprandial insulinaemia. Eur J Clin Nutr. 2001;55(11):994–999. doi: 10.1038/sj.ejcn.1601259. [DOI] [PubMed] [Google Scholar]
- 71.Logan A. Omega-3 fatty acids and acne. Arch Dermatol. 2003;139(7):941–942. doi: 10.1001/archderm.139.7.941-b. [DOI] [PubMed] [Google Scholar]
- 72.Li Y, Seifert M, Ney D, Grahn M, Grant A, Allen K, et al. Dietary conjugated linoleic acids alter serum IGF-1 and IGF binding protein concentrations and reduce bone formation in rats fed (n-6) or (n-3) fatty acids. J Bone Mineral Res. 1999;14(7):1153–1162. doi: 10.1359/jbmr.1999.14.7.1153. [DOI] [PubMed] [Google Scholar]
- 73.Simopoulos A. The importance of the omega-6/omega-3 fatty acid ratio in cardiovascular disease and other chronic diseases. Exp Biol Med. 2008;233(6):674–688. doi: 10.3181/0711-MR-311. [DOI] [PubMed] [Google Scholar]
- 74.Knor T. The pathogenesis of acne. Acta Dermatovenerol Croat. 2005;13(1):44–49. [PubMed] [Google Scholar]
- 75.Zouboulis C. Zileuton, a new efficient and safe systemic anti-acne drug. Dermatoendocrinology. 2009;1(3):188–192. doi: 10.4161/derm.1.3.8368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Jung J, Kwon H, Hong J, Yoon J, Park M, Jang M, et al. Effect of dietary supplementation with omega-3 fatty acid and gamma-linolenic acid on acne vulgaris: a randomised, double-blind, controlled trial. Acta Derm Venereol. 2014;94:521–525. doi: 10.2340/00015555-1802. [DOI] [PubMed] [Google Scholar]
- 77.Young C, Koepke J, Terlecky L, Borkin M, Boyd S, Terlecky S. Reactive oxygen species in tumor necrosis factor-α-activated primary human keratinocytes: implications for psoriasis and inflammatory skin disease. J Invest Dermatol. 2008;128:2606–2614. doi: 10.1038/jid.2008.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Stewart T, Bazergy C. Hormonal and dietary factors in acne vulgaris versus controls. Dermatoendocrinol. 2018;10(1):e1442160. doi: 10.1080/19381980.2018.1442160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Juhlin L, Michaëlsson G. Fibrin microclot formation in patients with acne. Acta Derm Venereol. 1983;63(6):538–540. [PubMed] [Google Scholar]
- 80.Bowe W, Filip J, DiRienzo J, Volgina A, Margolis D. Inhibition of Propionibacterium acnes by bacteriocin-like inhibitory substances (BLIS) produced by Streptococcus salivarius. J Drugs Dermatol. 2006;5(9):868–870. [PMC free article] [PubMed] [Google Scholar]
- 81.Kim S-H, Huh C-S, Choi I-D, Jeong J-W, Ku H-K, Ra J-H, et al. The anti-diabetic activity of Bifidobacterium lactis HY8101 in vitro and in vivo. J Appl Microbiol. 2014;117:834–845. doi: 10.1111/jam.12573. [DOI] [PubMed] [Google Scholar]
- 82.Cani P, Delzenne N. Interplay between obesity and associated metabolic disorders: new insights into the gut microbiota. Curr Opin Pharmacol. 2009;9:737–743. doi: 10.1016/j.coph.2009.06.016. [DOI] [PubMed] [Google Scholar]
- 83.Puch F, Samson-Villeger S, Guyonnet D, Blachon J, Rawlings A, Lassel T. Consumption of functional fermented milk containing borage oil, green tea and vitamin E enhances skin barrier function. Exp Dermatol. 2008;17(8):668–674. doi: 10.1111/j.1600-0625.2007.00688.x. [DOI] [PubMed] [Google Scholar]
- 84.Salem I, Ramser A, Isham N, Ghannoum M. The gut microbiome as a major regulator of the gut-skin axis. Front Microbiol. 2018;9:1459. doi: 10.3389/fmicb.2018.01459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Fabbrocini G, Bertona M, Picazo Ó, Pareja-Galeano H, Monfrecola G, Emanuele E. Supplementation with Lactobacillus rhamnosus SP1 normalises skin expression of genes implicated in insulin signalling and improves adult acne. Benef Microbes. 2016;7(5):625–630. doi: 10.3920/BM2016.0089. [DOI] [PubMed] [Google Scholar]
- 86.Custodio J, Wu C-Y, Benet L. Predicting drug disposition, absorption/elimination/transporter interplay and the role of food on drug absorption. Adv Drug Deliv Rev. 2008;60(6):717–733. doi: 10.1016/j.addr.2007.08.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Porter C, Trevaskis N, Charman W. Lipids and lipid-based formulations: optimizing the oral delivery of lipophilic drugs. Nat Rev. 2007;6(3):231–248. doi: 10.1038/nrd2197. [DOI] [PubMed] [Google Scholar]
- 88.Waiver of in vivo bioavailability and bioequivalence studies for immediate-release solid oral dosage forms based on a biopharmaceutics classification system guidance for industry. Silver Springs (MD): U.S. Department of Health and Human Services; Food and Drug Administration; Center for Drug Evaluation and Research; 2017.
- 89.Wu C-Y, Benet L. Predicting drug disposition via application of BCS: transport/absorption/elimination interplay and development of a biopharmaceutics drug disposition classification system. Pharml Res. 2005;22(1):11–23. doi: 10.1007/s11095-004-9004-4. [DOI] [PubMed] [Google Scholar]
- 90.Leyden J, Del Rosso J, Baum E. The use of isotretinoin in the treatment of acne vulgaris. J Clin Aesth Dermatol. 2014;7(2 Suppl.):S3–S21. [PMC free article] [PubMed] [Google Scholar]
- 91.Spironolactone tablets 50mg: summary of product characteristics (SmPC). Devon, UK; 2019.
- 92.Preneau S, Dessinioti C, Nguyen J, Katsambas A, Dréno B. Predictive markers of response to isotretinoin in female acne. Eur J Dermatol. 2013;23(4):478–486. doi: 10.1684/ejd.2013.2033. [DOI] [PubMed] [Google Scholar]
- 93.Pavithra G, Upadya G, Rukmini M. A randomized controlled trial of topical benzoyl peroxide 2.5% gel with a low glycemic load diet versus topical benzoyl peroxide 2.5% gel with a normal diet in acne (grades 1-3) Ind J Dermatol Venereol Leprol. 2018;85:486–490. doi: 10.4103/ijdvl.IJDVL_109_17. [DOI] [PubMed] [Google Scholar]
- 94.Welling P, Koch P, Lau C, Craig W. Bioavailability of tetracycline and doxycycline in fasted and nonfasted subjects. Antimicrob Agents Chemother. 1977;11(3):462–469. doi: 10.1128/aac.11.3.462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Leyden J, Del Rosso J. Oral antibiotic therapy for acne vulgaris: pharmacokinetics and pharmacodynamic perspectives. J Clin Aesth Dermatol. 2011;4(2):40–47. [PMC free article] [PubMed] [Google Scholar]
- 96.Leyden J. Absorption of minocycline hydrochloride and tetracycline hydrochloride: effect of food, milk, and iron. J Am Acad Dermatol. 1985;12(2):308–312. doi: 10.1016/s0190-9622(85)80041-4. [DOI] [PubMed] [Google Scholar]
- 97.Plott R, Wortzman M. Key biovailability features of a new extended-release formulation of minocycline hydrochloride tablets. Cutis. 2006;78(4 Suppl.):6–10. [PubMed] [Google Scholar]
- 98.Williams DB, O’Reilly WJ, Boehm G, Story MJ. Absorption of doxycycline from a controlled release pellet formulation: the influence of food on bioavailability. Biopharm Drug Dispos. 1990;11(2):93–105. doi: 10.1002/bdd.2510110202. [DOI] [PubMed] [Google Scholar]
- 99.Medline Plus. Tetracycline. 2020. https://medlineplus.gov/druginfo/meds/a682098.html. Accessed Jan 2020.
- 100.Goransson K, Liden S, Odsell L. Oral zinc in acne vulgaris: a clinical and methodological study. Acta Derm Venereol. 1978;58(5):443–448. [PubMed] [Google Scholar]
- 101.Verma KC, Saini AS, Dhamija SK. Oral zinc sulphate therapy in acne vulgaris: a double-blind trial. Acta Derm Venereol. 1980;60(4):337–340. doi: 10.2340/0001555560337340. [DOI] [PubMed] [Google Scholar]
- 102.Weismann K, Wadskov S, Sondergaard J. Oral zinc sulphate therapy for acne vulgaris. Acta Derm Venereol. 1977;57(4):357–360. [PubMed] [Google Scholar]
- 103.Webster G, Leyden J, Gross J. Comparative pharmcokinetic profiles of a novel isotretinoin formulation (isotretinoin-Lidose) and the innovator isotretinoin formulation: a randomized, 4-treatment, crossover study. JAAD. 2013;69:762–767. doi: 10.1016/j.jaad.2013.05.036. [DOI] [PubMed] [Google Scholar]
- 104.Del Rosso JQ, Stein Gold L, Segal J, Zaenglein AL. An open-label, phase IV study evaluating lidose-isotretinoin administered without food in patients with severe recalcitrant nodular acne: low relapse rates observed over the 104-week post-treatment period. J Clin Aesthet Dermatol. 2019;12(11):13–18. [PMC free article] [PubMed] [Google Scholar]
- 105.Madan S, Kumar S, Segal J. Comparative pharmacokinetic profiles of a novel low-dose micronized-isotretinoin 32 mg formulation and lidose-isotretinoin 40 mg in fed and fasted conditions: Two open-label, randomized, crossover studies in healthy adult participants. Acta Derm Venereol. 2020;100(4):adv0049. doi: 10.2340/00015555-3381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Absorica LD™ (isotretinoin) capsules, for oral use: full prescribing information. Cranbury (NJ): Sun Pharmaceutical Industries, Inc.; 2019.
- 107.Pantuzza L, Ceccato M, Silveira M, Junqueira L, Reis A. Association between medication regimen complexity and pharmacotherapy adherence: a systematic review. Eur J Clin Pharmacol. 2017;73:1475–1489. doi: 10.1007/s00228-017-2315-2. [DOI] [PubMed] [Google Scholar]