Abstract
This study was conducted to examine the effect of formulated resuscitation-promoting broths on the revival of viable but nonculturable Vibrio parahaemolyticus induced by cold and starvation stresses. Vibrio parahaemolyticus was incubated in artificial sea water at 4 °C for more than 8 months until this bacterium became undetectable, while retaining its intact cell count of more than 105 CFU/field over time. On day 250, V. parahaemolyticus was collected and enriched in tryptic soy broth supplemented with 3% NaCl, 10,000 U/mg catalase, 2% sodium pyruvate, 20 mM MgSO4, 5 mM EDTA, and a cell-free supernatant taken from V. parahaemolyticus ATCC 17802 in the stationary phase (pH 8). V. parahaemolyticus returned partially to a culturable state with a maximal cell density of 7.91 log CFU/mL in this formulated medium following 7 days of enrichment at 25 °C. In contrast, no V. parahaemolyticus was resuscitated when enriched in alkaline peptone water and tryptic soy broth.
Keywords: Food safety, Pathogen, Resuscitation, Viable but nonculturable, Vibrio parahaemolyticus
Introduction
Vibrio parahaemolyticus is a leading pathogenic bacterium of significant concern involved in food-borne diseases and illnesses globally since the consumption of seafood products contaminated with this organism can result in various clinical manifestations, such as abdominal pain, diarrhea, headache, nausea, vomiting, and septicemia (Korean Centers for Disease Control and Prevention, 2019). Generally, clam, eel, mackerel, mussel, oyster, scallop, and shrimp have been considered as the most predominant vehicles for V. parahaemolyticus as reflected in many food-borne outbreaks and recalls. Estuarine ecosystems, such as coastal area, seawater, and sediment, and even raw vegetables also have been identified to harbor large numbers of V. parahaemolyticus (Tunung et al., 2010).
Especially, V. parahaemolyticus was demonstrated to be capable of entering into a viable but nonculturable (VBNC) state when subjected to a variety of environmental stresses, including cold temperature, nutrient-deprivation, osmotic shock, and others (Dong et al., 2019; Falcioni et al., 2008; Yoon et al., 2017; Yoon et al., 2019; Yoon and Lee, 2019). V. parahaemolyticus remains viable but no longer grow on culture media that routinely support its growth and presents reduced metabolic activities, including ATP synthesis and expression of gene, RNA, and transcript, while maintaining its cellular integrity (Chaiyanan et al., 2007; Tholozan et al., 1999; Trinh et al., 2015).
Interestingly, V. parahaemolyticus would be resuscitated from a VBNC state reversibly by eliminating a causative environmental stress primarily responsible for its evolution into a VBNC state. Falcioni et al. (2008) determined that VBNC V. parahaemolyticus was capable of regaining its colony-forming ability by a temperature upshift method, thereby leading to the restoration of its cell morphology from coccal and helical forms to typical rods. Preliminarily, we observed that several V. parahaemolyticus strains had the ability to resuscitate from a VBNC state upon 2 days of enrichment in tryptic soy broth (TSB) supplemented with 3% NaCl (TSB7-3) at 25 °C, and then the resuscitated cells exhibited high levels of cytotoxicity equal to that of their normal cultures against Caco-2 and HEp-2 (data not shown). As standard bacteriological methods can neither accurately estimate the viable cell count of VBNC bacteria nor identify the formation of a VBNC state most likely due to significantly decreased culturability and metabolism (Dong et al., 2019; Oliver, 2016), pathogenic microorganisms may present a potential food safety hazard during the evolution to a VBNC state.
So far, numerous studies have been undertaken to establish an effective procedure that may be attributable to the resuscitation of microorganisms from a VBNC state. Indeed, Coutard et al. (2007) demonstrated that VBNC V. parahaemolyticus VP5 regained its colony-forming ability following a subsequent enrichment step in artificial sea water (ASW) at 25 °C for 4 days. When VBNC V. parahaemolyticus was enriched in TSB7-3 at 25 °C for 48 h, a maximum platable count of approximately 9.0 log CFU/mL was resuscitated (Wong et al., 2004). Furthermore, previous studies suggested that the presence of resuscitation-promoting stimuli, such as amino acids, antioxidants, cell-free cultures of V. vulnificus, and other biological additives, played an important role in the resuscitation of pathogenic bacteria from a VBNC state (Ayrapetyan et al., 2014; Gupte, de Rezende, and Joseph, 2003; Panutdaporn et al., 2006; Pinto et al., 2011). Despite the fact that V. parahaemolyticus can be recovered from a VBNC state in a favorable environment, which provides sufficient energy sources to encourage its biological function and growth, little is known regarding the combined effect of resuscitation-promoting stimuli on the persistence and resuscitation of VBNC V. parahaemolyticus induced under multiple environmental conditions, including acidification, high salinity, starvation, and refrigerated temperature. Importantly, there is no standardized protocol for efficient resuscitation-provoking methods involved in the restoration of V. parahaemolyticus from a VBNC state. Therefore, this study was undertaken to examine the combined effect of resuscitation-promoting stimuli, such as catalase, cell-free supernatant taken from V. parahaemolyticus ATCC 17802, sodium pyruvate, EDTA, and MgSO4, on the revival of VBNC V. parahaemolyticus induced in ASW microcosms (pH 6) supplemented with less than 30% NaCl under prolonged cold and starvation conditions. Alternatively, formulated resuscitation-promoting buffers may trigger the revival of V. parahaemolyticus persisted in cold and starvation conditions for an extended period of time, thereby contributing to the establishment of improved microbial risk surveillance programs.
Materials and methods
Preparation of microcosms
ASW microcosms were prepared according to a study conducted by Yoon et al. (2017). Considering that V. parahaemolyticus can grow or persist under osmotic environments at ≤ 7% NaCl (Cheng et al., 2004), we assumed that high levels of NaCl could be a key contributor influencing the induction of a VBNC state in V. parahaemolyticus strains. To determine the effect of different NaCl contents on the emergence of a VBNC state, ASW was modified by the addition of excessive NaCl amounts. Then, microcosms containing 5%, 10%, and 30% NaCl were designated as ASWfive, ASWten, and ASWthry, respectively, and adjusted to pH 6 using membrane-filtered 1 N lactic acid (Kanto chemical, Tokyo, Japan). Prepared ASW microcosms (500 mL) were autoclaved at 121 °C for 20 min.
Bacterial inoculums
V. parahaemolyticus ATCC 17802, V. parahaemolyticus ATCC 33844, and V. parahaemolyticus ATCC 27969 were purchased from the Korean Culture Center of Microorganisms (KCCM, Seoul-si, Republic of Korea). Each stock was maintained at − 75 °C and activated in tryptic soy broth supplemented with 3% NaCl (TSB7-3; Difco, Detroit, MI, USA) at 37 °C for 24 h. Each of V. parahaemolyticus strains was cultured in 50 mL of TSB7-3 at 37 °C for 24 h. Individually, overnight cultures of V. parahaemolyticus were harvested by centrifugation at 10,000 × g for 3 min at 4 °C, washed two times in 1 mL of ASW, and final pellets were re-suspended in 1 mL of ASW (pH 6). Each of ASW microcosms with varying concentrations of NaCl was inoculated by V. parahaemolyticus ATCC 17802, V. parahaemolyticus ATCC 33844 or V. parahaemolyticus ATCC 27969, corresponding to 107−8 log CFU/mL, and these microcosms were stored at 4 °C.
Enumeration and fluorescence microscopic assay
The ASW microcosms were withdrawn from a low temperature incubator at a regular interval. To minimize the residual NaCl effects, 1 mL of the bacterial solutions was centrifugated at 10,000 × g for 3 min at 4 °C, washed in 0.1 M PBS, and re-suspended in 0.1 M PBS. Each culture was serially diluted in alkaline peptone water (APW; Difco), and 100 μL of the diluents was spread onto tryptic soy agar (TSA; Difco) supplemented with 3% NaCl. All agar plates were incubated at 37 °C for 24–48 h.
Total and viable cell counts of V. parahaemolyticus were measured using the LIVE/DEAD BacLight® Bacterial Viability Kit (Invitrogen, Mount Waverley, VIC, Australia). The ASW microcosms were withdrawn from a low temperature incubator on day 0, 80, 100, 110 or 250. Approximately, 1 mL of the bacterial solutions was centrifugated at 10,000 × g for 3 min at 4 °C and re-suspended in 1 mL of 0.1 M PBS. Two different nucleic acid stains, SYTO9 and propidium iodide (PI) were combined at an equal volume (1:1) in a sterile microtube, and 3 μL of this mixture wad added to 1 mL of the bacterial solutions. After 15 min of incubation in the dark, 5 μL of the stained aliquots was placed onto a glass slide and covered with a coverslip. All images were obtained using an electron-fluorescence microscope (TE 2000-U, Nikon, Tokyo, Japan) at a magnification of × 40.
Resuscitation process
Resuscitation-promoting broths (RPBs) and modified APW or TSB were prepared according to a method described in Table 1. Once V. parahaemolyticus was incubated in ASW microcosms at 4 °C for 80 days (Table 2), 1 mL of the bacterial solutions was centrifuged at 13,000 × g for 3 min at 4 °C, washed twice with 0.1 M PBS, and re-suspended in 5 mL of ASW, TSB7-3 or brain heart infusion broth containing 3% NaCl (BHI; Difco). After 3 days of enrichment at 25 °C, the number of the resuscitated cells was quantified followed by direct spreading on TSA containing 3% NaCl.
Table 1.
Component of formulated resuscitation-promoting media used in this study
| Mediaa | pH | Componentb | |||||
|---|---|---|---|---|---|---|---|
| NaCl | Catalase | Pyruvate | MgSO4 | EDTA | Supernatant | ||
| APW | 8 | 1.0% | −c | − | − | − | − |
| APW7-1 | 7 | 1.0% | − | − | − | − | − |
| APW8-3 | 8 | 3.0% | − | − | − | − | − |
| APW7-3 | 7 | 3.0% | − | − | − | − | − |
| TSB | 7 | 0.5% | − | − | − | − | − |
| TSB7-3 | 7 | 3.0% | − | − | − | − | − |
| TSB7-1 | 7 | 1.0% | − | − | − | − | − |
| TSB8-3 | 8 | 3.0% | − | − | − | − | − |
| TSB8-1 | 8 | 1.0% | − | − | − | − | − |
| RPBa | 8 | 3.0% | + | + | + | + | + |
| RPBb | 8 | 3.0% | + | + | − | − | − |
| RPBc | 8 | 3.0% | + | + | + | − | − |
| RPBd | 8 | 3.0% | + | + | + | − | + |
| RPBe | 8 | 3.0% | − | − | − | − | + |
aAPW, alkaline peptone water; TSB, tryptic soy broth; RPB, resuscitation-promoting buffer. bTo formulate each of RPBs, 10,000 U/mg protein catalase, 2% sodium pyruvate, 20 mM MgSO4, 5 mM EDTA, and/or a cell-free supernatant from V. parahaemolyticus ATCC 17802 in the stationary phase were combined in this study. c + Addition; −, Non-addition
Table 2.
Reacquisition of the colony-forming ability (log CFU/mL) of VBNC V. parahaemolyticus strains persisted under cold and starvation conditions for 80 days followed by 3 days of enrichment in commercial culture broths at 25 °C
| Bacterium | Strain | Microcosm | Culture brotha | ||
|---|---|---|---|---|---|
| ASW | BHI | TSB7-3 | |||
| V. parahaemolyticus | ATCC 17802 | ASW | NGb | 6.83 | 7.43 |
| V. parahaemolyticus | ATCC 17802 | ASWfive | NG | NG | 7.87 |
| V. parahaemolyticus | ATCC 17802 | ASWten | NG | 5.63 | 3.45 |
| V. parahaemolyticus | ATCC 17802 | ASWthry | NG | 8.00 | 8.00 |
| V. parahaemolyticus | ATCC 33844 | ASW | NG | NG | 8.02 |
| V. parahaemolyticus | ATCC 33844 | ASWfive | NG | NG | 7.75 |
| V. parahaemolyticus | ATCC 33844 | ASWten | NG | NG | 8.30 |
| V. parahaemolyticus | ATCC 33844 | ASWthry | NG | NG | NG |
| V. parahaemolyticus | ATCC 27969 | ASW | NG | NG | 7.69 |
| V. parahaemolyticus | ATCC 27969 | ASWfive | NG | NG | 8.91 |
| V. parahaemolyticus | ATCC 27969 | ASWten | NG | NG | 7.93 |
| V. parahaemolyticus | ATCC 27969 | ASWthry | NG | NG | NG |
aAs described previously in the Materials and Methods, individual respective strains of V. parahaemolyticus were incubated in ASW microcosms supplemented with less than 30% NaCl at 4 °C. After 80 days, each V. parahaemolyticus strain was collected, re-suspended in ASW, BHI or TSB7-3, and enriched at 25C for 3 days. bNG, no growth
As stated above, the NaCl concentration of TSB7-3 and APW was modified, corresponding to 1–3%, and these media were adjusted to pH 7–8 by adding membrane-filtered 1 N NaOH (Kanto chemical). Upon exposure to cold and starvation stresses for 100 days (Table 3), all ASW microcosms were withdrawn, and 1 mL of the bacterial solutions was centrifugated at 10,000 × g for 3 min at 4 °C, washed in 0.1 M PBS, and re-suspended in 5 mL of the modified TSB (TSB7-1, TSB8-3 or TSB8-1) and APW (APW7-1, APW8-3 or APW7-3), respectively. Each of the cultures were enriched at 25 °C for up to 7 days.
Table 3.
Optimization of formulated culture broths containing 1–3% NaCl (pH 8–9) for resuscitating VBNC V. parahaemolyticus strains persisted under cold and starvation conditions for 100 days
| Bacterium | Strain | Microcosm | Formulate culture brotha | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| TSB7-3bhi | TSB7-1 | TSB8-3 | TSB8-1 | APW | APW7-1 | APW8-3 | APW7-3 | |||
| V. parahaemolyticus | ATCC 17802 | ASW | Growth | Growth | Growth | NG | NG | NG | NG | NG |
| V. parahaemolyticus | ATCC 17802 | ASWfive | NGb | NG | NG | NG | NG | NG | NG | NG |
| V. parahaemolyticus | ATCC 17802 | ASWten | NG | NG | NG | NG | NG | NG | NG | NG |
| V. parahaemolyticus | ATCC 17802 | ASWthry | NG | NG | NG | NG | NG | NG | NG | NG |
| V. parahaemolyticus | ATCC 33844 | ASW | Growth | Growth | Growth | Growth | NG | NG | Growth | NG |
| V. parahaemolyticus | ATCC 33844 | ASWfive | Growth | Growth | Growth | Growth | NG | NG | Growth | NG |
| V. parahaemolyticus | ATCC 33844 | ASWten | NG | Growth | Growth | Growth | NG | NG | NG | NG |
| V. parahaemolyticus | ATCC 33844 | ASWthry | NG | NG | NG | NG | NG | NG | NG | NG |
| V. parahaemolyticus | ATCC 27969 | ASW | Growth | Growth | Growth | Growth | Growth | Growth | Growth | Growth |
| V. parahaemolyticus | ATCC 27969 | ASWfive | Growth | Growth | Growth | NG | Growth | Growth | Growth | Growth |
| V. parahaemolyticus | ATCC 27969 | ASWten | Growth | NG | Growth | NG | Growth | Growth | Growth | Growth |
| V. parahaemolyticus | ATCC 27969 | ASWthry | NG | NG | NG | NG | NG | NG | NG | NG |
| Total positive samples | 6 | 6 | 7 | 4 | 3 | 5 | 3 | |||
aAs described previously in the Materials and Methods, individual respective strains of V. parahaemolyticus were incubated in ASW microcosms supplemented with less than 30% NaCl at 4 °C. After 100 days, each V. parahaemolyticus strain was collected, re-suspended in formulated culture broths and enriched at 25C for 7 days. Detail information about the formulated culture media was shown in Table 1. bNG, no growth
Individually, halophilic bacteria, such as V. parahaemolyticus ATCC 17802 and V. vulnificus ATCC 27562, were cultured in TSB7-3 at 37 °C for 24 h, whereas Escherichia coli O157:H7 ATCC 35150, Salmonella enterica serovar Typhimurium ATCC 43971, and Staphylococcus aureus ATCC 12598 were grown in TSB at 37 °C for 24 h. The overnight cultures of each bacteria were harvested by centrifugation at 13,000 × g for 3 min at 4 °C. The supernatant was collected in a sterile 50-ml conical tube and filtered using a 0.2-µm polycarbonate membrane (ADVANTEC, Tokyo, Japan) to prepare independent cell-free supernatant (CFS) fluids. TSB8-3 added with each CFS taken from V. parahaemolyticus ATCC 17802, V. vulnificus ATCC 27562, E. coli O157:H7 ATCC 35150, S. Typhimurium ATCC 43971 and Staph. aureus ATCC 12598 at a ratio of 9:1 (v/v) was designated as CFSvp, CFSvv, CFSst, CFSec, and CFSsa, respectively. Catalase (Sigma-Aldrich, St. Louis, MO, USA) or sodium pyruvate (Sigma-Aldrich) was diluted in TSB8-3, and the diluents were membrane-filtered. Both of the two antioxidants were added to TSB8-3, corresponding to 10,000 U/mg protein catalase and 2% sodium pyruvate (CSP). Then, CFSvp, CFSvv, CFSst, CFSec, CFSsa or CSP were further used to determine if these broths would stimulate the resuscitation of VBNC V. parahaemolyticus which persisted in ASW microcosms at 4 °C for 110 days (Table 4). After 3 days of enrichment at 25 °C, the number of the resuscitated cells was quantified followed by direct spreading on TSA containing 3% NaCl.
Table 4.
Optimization of the formulated culture medium TSB8-3 by adding a cell-free culture of pathogenic bacteria or a mixture of catalase and sodium pyruvate for resuscitating (log CFU/mL) VBNC V. parahaemolyticus strains persisted under cold and starvation conditions for 110 days
| Bacterium | Strain | Microcosm | TSB8-3 added with a cell-free culture of bacteria or a mixture of catalase and sodium pyruvatea | ||||||
|---|---|---|---|---|---|---|---|---|---|
| None | CFSvp | CFSvv | CFSst | CFSec | CFSsa | CSP | |||
| V. parahaemolyticus | ATCC 17802 | ASW | 7.48 | 4.60 | 3.70 | 6.78 | 7.00 | 3.48 | 5.08 |
| V. parahaemolyticus | ATCC 17802 | ASWfive | NGb | 7.75 | 4.80 | NG | 4.99 | NG | 5.76 |
| V. parahaemolyticus | ATCC 17802 | ASWten | NG | 4.08 | 4.17 | NG | NG | NG | 4.00 |
| V. parahaemolyticus | ATCC 17802 | ASWthry | NG | NG | NG | NG | NG | NG | NG |
| V. parahaemolyticus | ATCC 33844 | ASW | NG | 7.83 | 7.91 | NG | NG | NG | 8.15 |
| V. parahaemolyticus | ATCC 33844 | ASWfive | NG | NG | NG | NG | NG | NG | 7.79 |
| V. parahaemolyticus | ATCC 33844 | ASWten | NG | NG | NG | NG | NG | NG | NG |
| V. parahaemolyticus | ATCC 33844 | ASWthry | NG | NG | NG | NG | NG | NG | NG |
| V. parahaemolyticus | ATCC 27969 | ASW | 6.69 | 7.78 | 8.53 | 7.72 | 7.91 | 7.52 | 6.79 |
| V. parahaemolyticus | ATCC 27969 | ASWfive | 7.91 | 6.72 | 6.46 | 7.36 | 8.00 | 6.61 | 7.26 |
| V. parahaemolyticus | ATCC 27969 | ASWten | 6.93 | 7.45 | 6.00 | 5.93 | 6.90 | 7.36 | 5.70 |
| V. parahaemolyticus | ATCC 27969 | ASWthry | NG | NG | NG | NG | NG | NG | NG |
aAs described previously in the Materials and Methods, individual respective strains of V. parahaemolyticus were incubated in ASW microcosms supplemented with less than 30% NaCl at 4 °C. After 110 days, each V. parahaemolyticus strain was collected, re-suspended in TSB8-3 added with a cell-free culture of bacteria or a mixture of catalase and sodium pyruvate and enriched at 25C for 3 days. TSB8-3 added with a CFS taken from V. parahaemolyticus ATCC 17802, V. vulnificus ATCC 27562, E. coli O157:H7 ATCC 35150, S. Typhimurium ATCC 43971 and Staph. aureus ATCC 12598 in the stationary phase at a ratio of 9:1 (v/v) was designated as CFSvp, CFSvv, CFSst, CFSec, and CFSsa, respectively. Detail information about the formulated culture media was shown in Table 1. bNG, no growth
Individual respective resuscitation-promoting stimuli, such as 10,000 U/mg catalase, 2% sodium pyruvate, 20 mM MgSO4 (Sigma-Aldrich), 5 mM EDTA (Sigma-Aldrich), and a CFS taken from the overnight culture of V. parahaemolyticus ATCC 17802, were combined with the optimized TSB8-3 to formulate the resuscitation-promoting broths (RPBs) used in this study (Table 5). On day 250 under cold and starvation conditions, 1 mL of the bacterial solutions was centrifuged at 13,000 × g for 3 min at 4 °C, washed twice with 0.1 M PBS, and re-suspended in 5 mL of TSB8-3, RPBa RPBb RPBc RPBd or RPBe. After 7 days of enrichment at 25 °C, each solution was serially diluted in APW, and 100 μL of the diluents was spread onto TSA containing 3% NaCl. All agar plates were incubated at 37 °C for 24–48 h.
Table 5.
Resuscitation of VBNC V. parahaemolyticus strains persisted under cold and starvation conditions for 250 days followed by 7 days of enrichment in formulated resuscitation-promoting broths at 25 °C
| Bacterium | Strain | Microcosm | Formulated RPBsa | |||||
|---|---|---|---|---|---|---|---|---|
| TSB8-3 | RPBa | RPBb | RPBc | RPBd | RPBe | |||
| V. parahaemolyticus | ATCC 17802 | ASW | Growth | Growth | NG | NG | Growth | Growth |
| V. parahaemolyticus | ATCC 17802 | ASWfive | NGb | Growth | NG | NG | NG | NG |
| V. parahaemolyticus | ATCC 17802 | ASWten | NG | Growth | NG | NG | NG | NG |
| V. parahaemolyticus | ATCC 17802 | ASWthry | NG | NG | NG | NG | NG | NG |
| V. parahaemolyticus | ATCC 27969 | ASW | Growth | Growth | Growth | Growth | Growth | Growth |
| V. parahaemolyticus | ATCC 27969 | ASWfive | Growth | Growth | Growth | Growth | Growth | Growth |
| V. parahaemolyticus | ATCC 27969 | ASWten | Growth | Growth | Growth | Growth | Growth | Growth |
| V. parahaemolyticus | ATCC 27969 | ASWthry | NG | NG | NG | NG | NG | NG |
| Total positive samples | 4 | 6 | 3 | 3 | 4 | 4 | ||
aAs described previously in the Materials and Methods, individual respective strains of V. parahaemolyticus were incubated in ASW microcosms supplemented with less than 30% NaCl at 4 °C. After 250 days, each V. parahaemolyticus strain was collected, re-suspended in TSB8-3 or RPBa-e and enriched at 25C for 7 days. Detail information about the formulated culture media was shown in Table 1. bNG, no growth
Results and discussion
Induction of a VBNC state
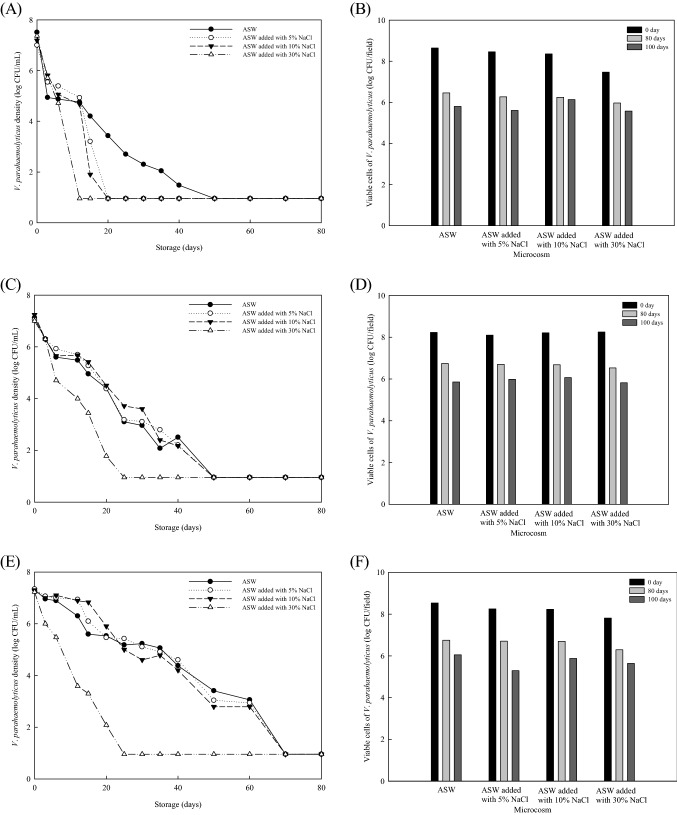
Changes in the colony-forming ability of V. parahaemolyticus ATCC 17802, V. parahaemolyticus ATCC 33844, and V. parahaemolyticus ATCC 27969 subjected to prolonged cold and starvation stresses are characterized in Fig. 1. The initial loads of V. parahaemolyticus ranged from 7.0 to 7.8 log CFU/mL. After 21 days of cold and starvation stresses, V. parahaemolyticus ATCC 17802 was reduced to approximately 3.6, ≥ 0.95, ≥ 0.95, and ≥ 0.95 log CFU/mL in ASW, ASWfive, ASWten, and ASWthry, respectively. V. parahaemolyticus ATCC 17802 survived at levels of less than 2.2 log CFU/mL in ASW on day 30. Similarly, V. parahaemolyticus ATCC 33844 was capable of surviving at levels of 2.6–3.1 log CFU/mL in ASW microcosms added with ≤ 10% NaCl at 4 °C for 40 days, while reduced by 7.2 log CFU/ml in ASWthry. V. parahaemolyticus ATCC 17802 and V. parahaemolyticus ATCC 33844 were reduced to below the detection limits (< 1.0 log CFU/ml) in all microcosms within 50 days. Approximately 21, 70, 70, and 70 days were required for V. parahaemolyticus ATCC 27969 to lose its colony-forming ability when incubated in ASW, ASWfive, ASWten, and ASWthry, respectively. Despite that each of V. parahaemolyticus strains became unculturable under cold and starvation conditions within 70 days, the viable cell number of these bacteria was between 5.5 and 6.8 log CFU/field over time as evidenced by SYTO9 and PI uptake analysis. The LIVE/DEAD BacLight assay constituting SYTO9 and PI stains has been widely used to measure the cytoplasmic membrane integrity, so called ‘viability’, of microorganisms (Dong et al., 2019; Yoon and Lee, 2019). SYTO9 is known to penetrate across intact and damaged cell membranes and interact with nucleic acid, displaying a green colored fluorescence at 480 nm excitation and 500 nm emission. In contrast, PI permeates into cytoplasm through a compromised membrane, causing a reduction of SYTO9. The loss of the permeability barrier in the stained cells presents any irreparable cellular damage, and PI exhibits a red color at 490 nm excitation and 635 nm emission. Hence, we observed the induction of a VBNC state in three V. parahaemolyticus strains persisted under cold and starvation conditions for at least 100 days, thereby indicating that an increase in the NaCl contents of the menstruum can accelerate the entry of V. parahaemolyticus into a VBNC state.
Fig. 1.
Changes in (A, C, and E) the colony-forming ability and (B, D, and F) membrane integrity of (A–B) V. parahaemolyticus ATCC 17082, (C–D) V. parahaemolyticus ATCC 33844, and (E–F) V. parahaemolyticus ATCC 27969 inoculated in ASW (pH 6) at 4 °C
The VBNC state is definitively distinct from typical starvation responses that enable microorganisms to persist in hostile environments for longer periods of time, instead of death because V. parahaemolyticus and Enterococcus faecalis in a VBNC state were found to have a specifically altered overexpression of some proteins involved in central carbohydrate metabolism, general stress response, and protein biosynthesis (Heim et al., 2002; Meng et al., 2015). A microarray-based transcriptional study also revealed that Vibrio cholerae O139, V. parahaemolyticus, and V. vulnificus continued to express their own toxin—(ctxAB, rtxA, hlyA, tl, tdh, and vvhA) and virulence-related (tcpA and TTSS) attributes after transited to a VBNC state by nutrient-deficiency coupled with cold temperature (Vora et al., 2005). Furthermore, Cappelier et al. (2007) observed that Listeria monocytogenes became avirulent in a VBNC state but regained the ability to form plaques in HT-29 monolayer cells and colonize mouse spleens after its revival to a culturable state through an embryonated egg passage. The VBNC state is a kind of survival strategy for which microorganisms adapt to withstand hostile environmental stresses, while maintaining minimal vitality requirements involved in potential infectivity or resuscitation capability.
Resuscitation from a VBNC state using conventional culture broths
Effect of conventional culture media, such as APW, ASW, BHI, and TSB, on the resuscitation of VBNC V. parahaemolyticus lasted in ASW microcosms at 4 °C for 80 days is shown in Table 2. V. parahaemolyticus failed to not only exit from a VBNC state, but also regain its colony-forming ability followed by an enrichment step in ASW (pH 7) at 25 °C. In contrast, V. parahaemolyticus returned to a culturable state with its platable count of ≥ 8.91 log CFU/mL in TSB7-3 after 3 days of temperature upshift. In part, V. parahaemolyticus ATCC 33844 and V. parahaemolyticus ATCC 27969 cells persisted in ASWthry remained nonculturable followed by an enrichment process in ASW and BHI at 25 °C for 3 days. Therefore, TSB7-3 was found to be effective in resuscitating VBNC V. parahaemolyticus more than APW.
Resuscitation may result from the re-growth of a very few undetectable cells remaining viable in stressful environments (Coutard et al., 2007; Falcioni et al., 2008). Accordingly, VBNC V. parahaemolyticus used in this study should be verified whether its conversion to a culturable state resulted from a true resuscitation, rather than underwent a reductive cell division. To avoid such a debate, V. parahaemolyticus ATCC 17802 lasted in a VBNC state for 50 days was collected, washed twice in APW, and diluted, ranging from 100 to 10−3, in APW serially. The diluents were further incubated at 25 °C for 3 days. If there are a few residual or undetected sub-culturable cells among the laboratory microcosms, the resuscitated cells continue to grow, exceeding the initial cell inoculum levels (105, 106, 107 or 108 CFU/ml) of each serial diluent during all the stages of a resuscitation process (Whitesides and Oliver, 1997). After 3 days, V. parahaemolyticus was resuscitated to 105, 106, 107 or 108 CFU/ml in the 10−3, 10−2, 10−1 or 100 diluted APW solutions (data not shown).
Formulation and optimization of RPBs
Effect of formulated culture broths containing 1–3% NaCl (pH 8–9) for resuscitating VBNC V. parahaemolyticus strains subjected to cold and starvation conditions for 100 days is shown in Table 3. Partially, 6, 6, 7 or 4 V. parahaemolyticus cultures were reverted to a culturable state after 7 days of enrichment in TSB7-3, TSB7-1, TSB8-3 or TSB8-1, irrespective of the microcosms added with varying levels of NaCl. The modified APWs were shown to be less effective for the restoration of VBNC V. parahaemolyticus. Overall, TSB8-3 exhibited the highest resuscitation capacity for VBNC V. parahaemolyticus induced by cold and starvation stresses.
The formulated culture medium TSB8-3 was further optimized by adding a cell-free culture of pathogenic bacteria or a mixture of catalase and sodium pyruvate, and its effect on the reacquisition of the colony-forming ability of VBNC V. parahaemolyticus exposed to cold and starvation stresses for 110 days is shown in Table 4. V. parahaemolyticus ATCC 17802 in ASW was resuscitated at levels of 4.60, 3.70, 6.78, 7.00, and 3.48 log CFU/mL upon enrichment in CFSvp, CFSvv, CFSst, CFSec, and CFSsa, respectively. VBNC V. parahaemolyticus ATCC 33844 induced in ASW, ASWfive, and ASWten returned to culturable at levels of 7.83–8.15 log CFU/mL in CFSvp, CFSvv, and CSP once resuscitated at 25 °C for 7 days. VBNC V. parahaemolyticus ATCC 27969 lasted in microcosms containing less than 10% NaCl at 4 °C for 110 days were revived to 6.72–7.78, 6.00–8.53, 5.93–7.36, 6.90–8.00, 6.61–7.52, and 5.70–7.26 log CFU/mL in CFSvp, CFSvv, CFSst, CFSec, CFSsa, and CSP, respectively. CFSvp exhibited the highest recovery rate (averagely 3.85 log CFU/ml) for resuscitating three V. parahaemolyticus strains in a VBNC state.
Resuscitation from a VBNC state using formulated RPBs
Table 5 shows the resuscitation of VBNC V. parahaemolyticus strains persisted under cold and starvation conditions for 250 days followed by 7 days of enrichment in formulated resuscitation-promoting broths at 25 °C. V. parahaemolyticus ATCC 17802 in ASW regained its culturability when resuscitated in TSB8-3, RPBa, RPBc, and RPBe. While VBNC V. parahaemolyticus ATCC 27969 persisted in ASW, ASWfive, and ASWten was returned to a culturable state after 7 days of enrichment in TSB8-3 and RPBa-e, V. parahaemolyticus ATCC 33844 failed to revive from a VBNC state. Overall, 6, 3, 3, 4 and 4 V. parahaemolyticus cultures were capable of reviving from a VBNC state once resuscitated in RPBa, RPBb, RPBc, RPBd, and RPBe, respectively. RPBa was the most effective in recovering V. parahaemolyticus from a VBNC state.
This study showed that the resuscitation-capability of VBNC V. parahaemolyticus was highly strain-dependent as influenced by the length of time spent under cold and starvation conditions. Indeed, when V. parahaemolyticus ATCC 17802 was induced into a VBNC state at 4 °C for 110 days, VBNC cells could be reverted to a culturable state following enrichment in TSB8-3 with or without the addition of resuscitation-promoting stimuli. In contrast, V. parahaemolyticus ATCC 17802 lasted in ASWfive, ASWten, and ASWthry at 4 °C for more than 110 days remained unculturable after 2 days of resuscitation in TSB8-3. Only V. parahaemolyticus ATCC 33844 was incapable of returning to a culturable state when incubated in microcosms at 4 °C for 250 days. As well-documented in a study of Pinto et al. (2013), VBNC cells have their own different resuscitation window periods. Behind this point, VBNC cells may lose the resuscitation capability permanently, thereby leading to death.
Previous studies showed that the phase-shift of VBNC cells to a culturable state differed significantly, depending on various physico-chemical factors, including heat shock (Chaiyanan et al., 2007), nutrient-availability (Gupte et al., 2003), and the presence of resuscitation-promoting factors (RPFs). Particularly, RPFs are characterized as muralytic enzymes, which stimulate bacterial growth and resuscitation, and known to be closely involved in cell wall remodeling (Hett et al., 2007; Kana and Mizrahi, 2009; Mukamolova et al., 2006). In the same context, it was demonstrated that amino acid, antioxidizing agent, autoinducer (AI), and CFS played an important role for the resuscitation of Vibrio spp. from a VBNC state (Ayrapetyan et al., 2014; Pinto et al., 2011). In this study, the optimized TSB8-3 supplemented with 10,000 U/mg catalase and 2% sodium pyruvate partially led to a revival of VBNC V. parahaemolyticus induced by cold and starvation stresses for more than 100 days. Interestingly, the resuscitation capacity of catalase and sodium pyruvate may be concentration-dependent. In our preliminary studies, VBNC V. parahaemolyticus still remained unculturable even after a subsequent resuscitation process with APW and TSA containing either 1000–2000 U/mg catalase or 0.1–1.0% sodium pyruvate. At the same time, VBNC cells of V. parahaemolyticus, however, was resuscitated to a culturable state, following enrichment in APW and TSA supplemented with 10,000 U/mg catalase and/or 2.0% sodium pyruvate (data not shown). In a study conducted by Mizunoe et al. (2000), dormant but viable V. parahaemolyticus VP190 was resuscitated to levels of 102−3 CFU/mL once further cultured on Luria–Bertani agar plates (LB) supplemented with either 2000 U catalase or 0.1% sodium pyruvate (in contrast, no colony was observed when VBNC V. parahaemolyticus VP190 was spread on a routine LB medium). Ramamurthy et al. (2014) determined that sodium pyruvate was responsible for the biosynthesis of some macromolecules involved in a revival of VBNC cells. Given aerobic organisms have evolved complex defense and repair systems to reduce or eliminate any cellular damage caused by reactive oxygen species (ROS) compounds, ROS might be profoundly associated with loss of culturability and induction of a VBNC state. Moreover, Morishige et al., (2013) observed that sodium pyruvate and its analogue, α-ketobutyrate presented a positive resuscitation effect on the revival of VBNC Salmonella Enteritidis induced by 0.3 mM H2O2, and there was a marked increase in the synthesis of DNA and protein when VBNC S. Enteritidis was enriched in M9 added with 30 mM sodium pyruvate at 37 °C for at least 60 min. Although α-ketobutyrate is known to be oxidized by ferredoxin and 2-oxobutyrate synthase, and finally integrated into the tricarboxylic acid (TCA) cycle, the authors demonstrated that the activation of TCA cycle may not be necessarily responsible for the resuscitation of VBNC S. Enteritidis most likely due to the failure of a cofactor, thiamine, which stimulates the production of acetyl-CoA, to repair VBNC cells in the presence of sodium pyruvate. Nevertheless, the mechanism of sodium pyruvate and α-ketobutyrate to drive the resuscitation of VBNC cells to a culturable state remains unclarified.
Interestingly, Ayrapetyan et al. (2014) showed that VBNC V. vulnificus C7184 returned to a culturable state when enriched in a formulated culture broth containing a CFS harvested from its wild counterpart. The presence of a chemically synthesized autoinducer-2 (AI-2) led to a revival of VBNC V. vulnificus, whereas some CFSs taken from AI-2 mutant V. vulnificus strains failed to do so. AIs are defined as small molecules that regulate specific gene expressions in response to fluctuation in cell population density and have a significant impact on the resuscitation of V. cholerae and E. coli from a VBNC state (Bari et al., 2013; Pinto et al., 2011). Considering that mutant strains of Mycobacterium tuberculosis with a deletion in RPF-related genes failed to revive from a VBNC state (Kana et al., 2008; Kana and Mizrahi, 2009), and VBNC bacteria, such as enterohemorrhagic E. coli, Salmonella enterica serovar Infantis, and V. parahaemolyticus, returned to a culturable state following co-culture with various eukaryotic cell lines (Senoh et al., 2014; Senoh et al., 2015), CFS may include either AI or RPF, which implies that bacteria in an inactive but viable state might be resuscitated in the presence of some resuscitation-promoting stimuli secreted by either metabolically active resident microbiota or intentionally added fermentation organisms. Reissbrodt et al. (2002) observed the resuscitation of VBNC E. coli strains to a culturable state upon exposure to the AI molecules produced by Yersinia ruckeri, strengthening this point of views.
Conclusion
To date, little is known about the effect of high NaCl contents combined with cold and starvation stresses on the entry of V. parahaemolyticus into a VBNC state or resuscitation of VBNC cells to a culturable state. In this study, we observed the induction of a VBNC state in V. parahaemolyticus exposed to ASW microcosms containing less than 30% NaCl (pH 6) at 4 °C. Formation of VBNC cells was accelerated rapidly with an increase in the NaCl concentrations. NaCl levels in a laboratory menstruum may play a critical role in the induction of a VBNC state in V. parahaemolyticus. Particularly, combined resuscitation-promoting stimuli facilitated the restoration of V. parahaemolyticus cells from a VBNC state followed by a temperature upshift. The modified TSB8-3 supplemented with different types of stimuli may lead to the resuscitation of VBNC V. parahaemolyticus strains induced in ASW microcosms at 4 °C for at least 100 days. Especially, VBNC V. parahaemolyticus returned to a culturable state following 7 days of enrichment in RPBa at 25 °C. The resuscitation capability of VBNC V. parahaemolyticus can greatly differ depending on the length of time spent under cold and starvation stresses and strain-variability. Taken together, V. parahaemolyticus can activate a preferred survival mechanism, referred to as the VBNC state, in response to unfavorable environmental conditions, concomitantly with its maintenance of minimal vitality requirements involved in the opportunistic resuscitative capability, and therefore the revival of V. parahaemolyticus from a VBNC state may pose a serious food safety hazard due to the resulting restoration of its infectivity and pathogenicity. RPBs containing various resuscitation-promoting stimuli may facilitate the revival of V. parahaemolyticus subjected to prolonged cold and starvation stresses from a VBNC state, thereby providing useful insights in establishing an effective monitoring strategy for the detection of VBNC V. parahaemolyticus.
Acknowledgements
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF; Grant No. NRF-2016R1A6A3A 11932794) and Chung-Ang University Graduate Research Scholarship in 2020.
Compliance with ethical standards
Conflict of interest
No conflict of interests was declared.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Jae-Hyun Yoon, Email: nevbge999@gmail.com.
Young-Min Bae, Email: only1617@hanmail.net.
Suyoung Jo, Email: suyoung3223@naver.com.
Sung-Kwon Moon, Email: sumoon66@cau.ac.kr.
Se-Wook Oh, Email: swoh@kookmin.ac.kr.
Sun-Young Lee, Email: nina6026@cau.ac.kr.
References
- Ayrapetyan M, Williams TC, Oliver JD. Interspecific quorum sensing mediates the resuscitation of viable but nonculturable Vibrios. Appl. Environ. Microbiol. 2014;80:2478–2483. doi: 10.1128/AEM.00080-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bari SMN, Roky MK, Mohiuddin M, Kamruzzaman M, Mekalanos JJ, Faruque SM. Quorum-sensing autoinducers resuscitate dormant Vibrio cholerae in environmental water samples. Proc. Natl. Acad. Sci. USA. 2013;110:9926–9931. doi: 10.1073/pnas.1307697110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cappelier JM, Besnard V, Roche SM, Velge P, Federighi M. Avirulent viable but nonculturable cells of Listeria monocytogenes need the presence of an embryo to be recovered in egg yolk and regain virulence after recovery. Vet. Res. 2007;38:573–583. doi: 10.1051/vetres:2007017. [DOI] [PubMed] [Google Scholar]
- Chaiyanan S, Chaiyanan SC, Grim C, Maugel T, Huq A, Colwell RR. Ultrastructure of coccoid viable but nonculturable Vibrio cholerae. Environ. Microbiol. 2007;9:393–402. doi: 10.1111/j.1462-2920.2006.01150.x. [DOI] [PubMed] [Google Scholar]
- Cheng W, Juang FM, Chen JC. The immune response of Taiwan abalone Haliotis diversicolor supertexta and its susceptibility to Vibrio parahaemolyticus at different salinity levels. Fish Shellfish Immunol. 2004;16:295–306. doi: 10.1016/S1050-4648(03)00111-6. [DOI] [PubMed] [Google Scholar]
- Coutard F, Crassous P, Droguet M, Gobin E, Colwell RR, Pommepuy M, Hervio-Heath D. Recovery in culture of viable but nonculturable Vibrio parahaemolyticus: regrowth or resuscitation. ISME J. 2007;1:111–120. doi: 10.1038/ismej.2007.1. [DOI] [PubMed] [Google Scholar]
- Dong K, Pan H, Yang D, Rao L, Zhao L, Wang Y, Liao X. Induction, detection, formation, and resuscitation of viable but non-culturable state microorganisms. Compr. Rev. Food Sci. Food Saf. 2019;19:149–183. doi: 10.1111/1541-4337.12513. [DOI] [PubMed] [Google Scholar]
- Falcioni T, Papa S, Campana R, Manti A, Battistelli M, Baffone W. State transitions of Vibrio parahaemolyticus VBNC cells evaluated by flow cytometry. Cytometry B Clin. Cytom. 2008;74:272–281. doi: 10.1002/cyto.b.20427. [DOI] [PubMed] [Google Scholar]
- Gupte AR, de Rezende CLE, Joseph SW. Induction and resuscitation of viable but nonculturable Salmonella enterica serovar Typhimurium DT104. Appl. Environ. Microbiol. 2003;69:6669–6675. doi: 10.1128/AEM.69.11.6669-6675.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hett EC, Chao MC, Steyn AJ, Fortune SM, Deng LL, Rubin EJ. A partner for the resuscitation-promoting factors of Mycobacterium tuberculosis. Mol. Microbiol. 2007;66:658–668. doi: 10.1111/j.1365-2958.2007.05945.x. [DOI] [PubMed] [Google Scholar]
- Heim S, Lleo M, Bonato B, Guzman CA, Canepari P. The viable but nonculturable state and starvation are different stress responses of Enterococcus faecalis, as determined by proteome analysis. J. Bacteriol. 2002;184:6739–6745. doi: 10.1128/JB.184.23.6739-6745.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kana BD, Mizrahi V. Resuscitation-promoting factors as lytic enzymes for bacterial growth and signaling. FEMS Immunol. Med. Microbiol. 2009;58:39–50. doi: 10.1111/j.1574-695X.2009.00606.x. [DOI] [PubMed] [Google Scholar]
- Kana BD, Gordhan BG, Downing KJ, Sung N, Vostroktunova G, Machowski EE, Tsenoval L, Young M, Kaprelyants A, Kaplan G, Mizrahi V. The resuscitation-promoting factors of Mycobacterium tuberculosis are required for virulence and resuscitation from dormancy but are collectively dispensable for growth in vitro. Mol. Microbiol. 2008;67:672–684. doi: 10.1111/j.1365-2958.2007.06078.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korean Centers for Disease Control and Prevention. Food-borne outbreaks involved in Vibrio parahaemolyticus. Available from: http://www.cdc.go.kr/CDC/cms/content/mobile/51/49751_view.html. Accessed on Dec, 10, 2019
- Meng L, Alter T, Aho T, Huehn S. Gene expression profiles of Vibrio parahaemolyticus in viable but non-culturable state. FEMS Microbiol. Ecol. 91: fiv035 (2015) [DOI] [PubMed]
- Mizunoe Y, Wai SN, Ishikawa T, Takade A, Yoshida SI. Resuscitation of viable but nonculturable cells of Vibrio parahaemolyticus induced at low temperature under starvation. FEMS Microbiol. Lett. 2000;186:115–120. doi: 10.1111/j.1574-6968.2000.tb09091.x. [DOI] [PubMed] [Google Scholar]
- Morishige Y, Fujimori K, Amano F. Differential resuscitative effect of pyruvate and its analogues on VBNC (viable but non-culturable) Salmonella. Microbes Environ. 2013;28:180–186. doi: 10.1264/jsme2.ME12174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukamolova GV, Murzin AG, Salina EG, Demina GR, Kell DB, Kaprelyants AS, Young M. Muralytic activity of Micrococcus luteus RPF and its relationship to physiological activity in promoting bacterial growth and resuscitation. Mol. Microbiol. 2006;59:84–98. doi: 10.1111/j.1365-2958.2005.04930.x. [DOI] [PubMed] [Google Scholar]
- Oliver JD. The viable but nonculturable state for bacteria: status update. Microbe. 2016;11:159–164. [Google Scholar]
- Panutdaporn N, Kawamoto K, Asakura H, Makino SI. Resuscitation of the viable but nonculturable state of Salmonella enterica serovar Oranienburg by recombinant resuscitation-promoting factor derived from Salmonella Typhimurium strain LT2. Int. J. Food Microbiol. 2006;106:241–247. doi: 10.1016/j.ijfoodmicro.2005.06.022. [DOI] [PubMed] [Google Scholar]
- Pinto D, Almeida V, Almeid-Santos M, Chambel L. Resuscitation of Escherichia coli VBNC cells depends on a variety of environmental or chemical stimuli. J. Appl. Microbiol. 2011;110:1601–1611. doi: 10.1111/j.1365-2672.2011.05016.x. [DOI] [PubMed] [Google Scholar]
- Pinto D, Santos MA, and Chambel L. Thirty years of viable but nonculturable state research: unsolved molecular mechanisms. Crit. Rev. Microbio. 41: 61-76 (2013) [DOI] [PubMed]
- Ramamurthy T, Ghosh A, Pazhani GP, Shinoda S. Current perspectives on viable but non-culturable (VBNC) pathogenic bacteria. Front. Public Health. 31: article 103 (2014) [DOI] [PMC free article] [PubMed]
- Reissbrodt R, Rienaecker I, Romanova JM, Freestone PP, Haigh RD, Lyte M, Tschäpe H, Williams PH. Resuscitation of Salmonella enterica serovar Typhimurium and enterohemorrhagic Escherichia coli from the viable but nonculturable state by heat-stable enterobacterial autoinducer. Appl. Environ. Microbiol. 2002;68:4788–4794. doi: 10.1128/AEM.68.10.4788-4794.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senoh M, Hamabata T, Takeda Y. A factor converting viable but nonculturable Vibrio cholerae to a culturable state in eukaryotic cells is a human catalase. Microbiologyopen. 2015;4:589–596. doi: 10.1002/mbo3.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senoh M, Ghosh-Banerjee J, Mizuno T, Shinoda S, Miyoshi SI, Hamabata T, Nair GB, Takeda Y. Isolation of viable but nonculturable Vibrio cholerae O1 form environmental water samples in Kolkata, India, in a culturable state. Microbiologyopen. 2014;3:239–246. doi: 10.1002/mbo3.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tholozan JL, Cappelier JM, Tissier JP, Delattre G, Federighi M. Physiological characterization of viable-but-nonculturable Campylobacter jejuni cells. Appl. Environ. Microbiol. 1999;65:1110–1116. doi: 10.1128/AEM.65.3.1110-1116.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trinh NTT, Dumas E, Le Thanh M, Degraeve P, Ben Amara C, Gharsallaoui A, Oulahal N. Effect of a Vietnamese Cinnamomum cassia essential oil and its major component trans-cinnamaldehyde on the cell viability, membrane integrity, membrane fluidity, and proton motive force of Listeria innocua. Can. J. Microbiol. 2015;61:263–271. doi: 10.1139/cjm-2014-0481. [DOI] [PubMed] [Google Scholar]
- Tunung R, Margaret S, Jeyaletchumi P, Chai LC, Tuan Zainazor TC, Ghazali FM, Nakaguchi Y, Nishibuchi M, Son R. Prevalence and quantification of Vibrio parahaemolyticus in raw salad vegetables at retail level. J. Microbiol. Biotechnol. 2010;20:391–396. doi: 10.4014/jmb.0908.08009. [DOI] [PubMed] [Google Scholar]
- Vora GJ, Meador CE, Bird MM, Bopp CA, Andreadis JD, Stenger DA. Microarray-based detection of genetic heterogeneity, antimicrobial resistance, and the viable but nonculturable state in human pathogenic Vibrio spp. Proc. Natl. Acad. Sci. USA. 2005;102:19109–19114. doi: 10.1073/pnas.0505033102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitesides MD, Oliver JD. Resuscitation of Vibrio vulnificus from the viable but nonculturable state. Appl. Environ. Microbiol. 1997;63:1002–1005. doi: 10.1128/AEM.63.3.1002-1005.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong HC, Wang P, Chen SY, Chiu SW. Resuscitation of viable but non-culturable Vibrio parahaemolyticus in a minimum salt medium. FEMS Microbiol. Lett. 2004;233:269–275. doi: 10.1111/j.1574-6968.2004.tb09491.x. [DOI] [PubMed] [Google Scholar]
- Yoon JH, Bae YM, Lee SY. Effects of varying concentrations of sodium chloride and acidic conditions on the behavior of Vibrio parahaemolyticus and Vibrio vulnificus cold-starved in artificial sea water microcosm. Food Sci. Biotechnol. 2017;26:829–839. doi: 10.1007/s10068-017-0105-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon JH, Bae YM, Moon SK, Choi C, Ryu BY, Lee SY. Detection of viable but nonculturable Vibrio parahaemolyticus induced by prolonged cold-starvation using propidium monoazide real time polymerase chain reaction. Lett Appl. Microbiol. 2019;68:537–545. doi: 10.1111/lam.13157. [DOI] [PubMed] [Google Scholar]
- Yoon JH, Lee SY. Characteristics of viable but-nonculturable Vibrio parahaemolyticus induced by nutrient-deficiency at cold temperature. Crit. Rev. Food Sci. Nutr. 2019;31:1–19. doi: 10.1080/10408398.2019.1570076. [DOI] [PubMed] [Google Scholar]