Abstract
Mitochondria, independent double-membrane organelles, are intracellular power plants that feed most eukaryotic cells with the ATP produced via the oxidative phosphorylation (OXPHOS). Consistently, cytochrome c oxidase (COX) catalyzes the electron transfer chain’s final step. Electrons are transferred from reduced cytochrome c to molecular oxygen and play an indispensable role in oxidative phosphorylation of cells. Cytochrome c oxidase subunit 6c (COX6C) is encoded by the nuclear genome in the ribosome after translation and is transported to mitochondria via different pathways, and eventually forms the COX complex. In recent years, many studies have shown the abnormal level of COX6C in familial hypercholesterolemia, chronic kidney disease, diabetes, breast cancer, prostate cancer, uterine leiomyoma, follicular thyroid cancer, melanoma tissues, and other conditions. Its underlying mechanism may be related to the cellular oxidative phosphorylation pathway in tissue injury disease. Here reviews the varied function of COX6C in non-tumor and tumor diseases.
Keywords: Cytochrome c oxidase subunit 6c, tumor oxidative phosphorylation, mitochondria, tissue damage, cancer
Introduction
Normal mammalian cells metabolize glucose by oxidative phosphorylation of mitochondria when oxygen is sufficient, and provide abundant ATP. In the absence of oxygen, the pyruvate formed by the decomposition of glucose through glycolysis is directly reduced to lactic acid, in which only a small amount of ATP is generated. Even with sufficient oxygen, malignant tumor cells can consume plentiful glucose and produce vast lactic acid, which is named by the Warburg effect. Cancer cells rewire their metabolism to promote growth, survival, proliferation, and long-term maintenance [1]. In mammals, the energy needed for diverse vital activities is mainly provided by mitochondria. Nonetheless, Cytochrome c oxidase (COX, complex IV) plays a unique role in this process.
Structure of COX6C
COX is located in the mitochondria’s inner membrane and catalyzes the electron transfer from reduced cytochrome c to oxygen, which plays a critical regulatory role in the OXPHOS of cells and is also involved in the process of apoptosis [2]. Mammalian COX comprises the coordinated assembly of 13 subunits, in which 3 subunits encoded by mitochondrial genomes and 10 subunits by the nuclear genomes [3], and their expression varies in different organisms or different organs of the same species [4]. COX6C is encoded by the nucleus genome, followed by translated in the ribosome. After that, it is transported to the mitochondria via different pathways and ultimately integrates into the COX complex. Consistently, COX6C subunit assembled into sub-complexes that may hint rate-limiting intermediates, which plays a significant role in the function of COX and is a key regulator in the oxidative phosphorylation process of tissue cells [4,5].
COX is well known as a large complex comprised of at least 13 subunits. In detail, subunits I-III compose the catalytic core of the enzyme, which are all synthesized from mitochondrial DNA. By contrast, the remaining subunits (IV-VIII) are synthesized from cellular nuclear DNA (Figure 1) [6]. Detailedly, subunit I is located in heme A and the oxygen-binding site composed of CuB/heme A3. The voltage-dependent anion channel binds to subunit I; Besides, COX activity is inhibited at high ATP-to-ADP ratios by binding to subunit I in the matrix. However, subunit II can bind site CuA and subunit III has a role in proton pumping. Subunit IV is divided into IV-1 and IV-2, and the former shows allosteric ATP inhibition at high ATP-to-ADP ratio. It’s unclear that IV-2 is induced under hypoxia (O2)-dependent allosteric ATP inhibition. Subunit V can bind zinc. Subunit VI decreases of H+/e- from 1 to 0.5. The functions of others subunits are not definite [7].
Figure 1.
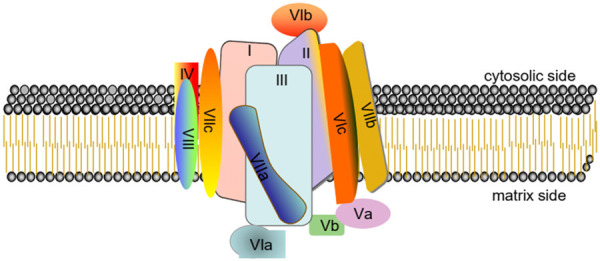
The structure of mammalian complex IV. Mammalian complex IV comprises 13 different subunits. In detail, subunits I-III compose the catalytic core of the enzyme, which are all synthesized from mitochondrial DNA. By contrast, the remaining subunits (IV, Va, Vb, VIa, VIb, VIc, VIIa, VIIb, VIIc, VIII) are synthesized from cellular nuclear DNA.
Functions of COX6C
The gene, mapped to the chromosome region 8q22.2, has 5 exons and encodes approximately 9-kDa protein consists of 75 amino acids. It is dominating expressing in mitochondria and lesser in the nucleus. Besides, this gene encodes a potential transmembrane protein that may function in vesicle-mediated transport and sorting of proteins within cells. The protein may play a role in the development and the function of the eye, hematological system, and central nervous system. Mutations of the gene could be associated with Cohen Syndrome, an inherited disease. Moreover, it has 77% amino acid sequence identity with mouse subunit VIc. Meanwhile, a pseudogene has been found on chromosomes 16p12.
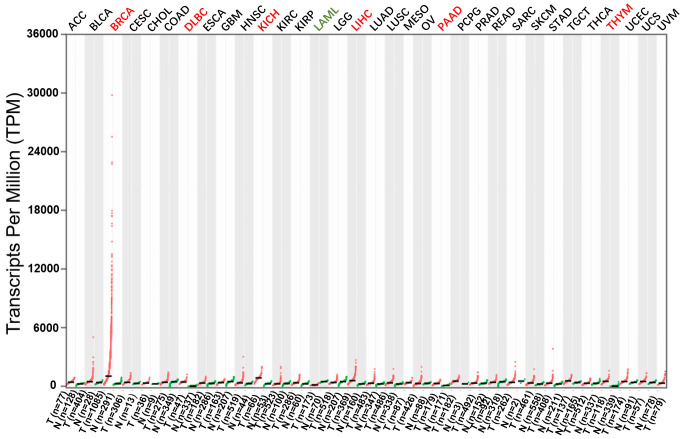
In addition, to better understand the role of COX6C in disease, we consult the Gene Expression Profiling Interactive Analysis (GEPIA) database and discover that COX6C is differentially expressed in various cancers in the GEPIA database (Figure 2). Previous studies indicated COX6C is relevant to the development of familial hypercholesteremia (FH), chronic kidney disease (CKD), diabetes, breast cancer, uterine leiomyoma, follicular thyroid cancer, melanoma tissues and other diseases. The mechanism may be correlated with the oxidative phosphorylation pathway of tissue cells in tissue injury diseases. As follows, we summarize COX6C-related diseases (Table 1) and the functions of COX6C in specific diseases (Figures 3, 4).
Figure 2.
Differential expression of COX6C in cancers in the GEPIA database. COX6C is highly expressed in BRCA, DLBC, KICH, LIHC, PAAD and THYM (Red), but it has a lower expression level in LAML (Green).
Table 1.
Current research on COX6C in non-neoplastic diseases and cancers
| Types | Quantity | references | |
|---|---|---|---|
| non-neoplastic diseases | muscle tissue damage | 3 | [8-10] |
| familial hypercholesteremia | 4 | [11-14] | |
| chronic kidney disease | 3 | [15-17] | |
| diabetes | 5 | [18-22] | |
| infectious diseases | 1 | [23] | |
| other diseases | 6 | [18,24-28] | |
| cancers | breast cancer | 3 | [29-31] |
| gastric cancer | 1 | [32] | |
| prostate cancer | 1 | [33] | |
| uterine leiomyoma | 2 | [34,35] | |
| melanoma tissues | 1 | [36] | |
| penile squamous cell carcinoma | 1 | [37] | |
| retroperitoneal lipoma | 1 | [38] | |
| follicular thyroid cancer | 1 | [39] | |
| esophageal cancer | 1 | [40] | |
| chronic lymphocytic leukemia | 1 | [41] |
Figure 3.
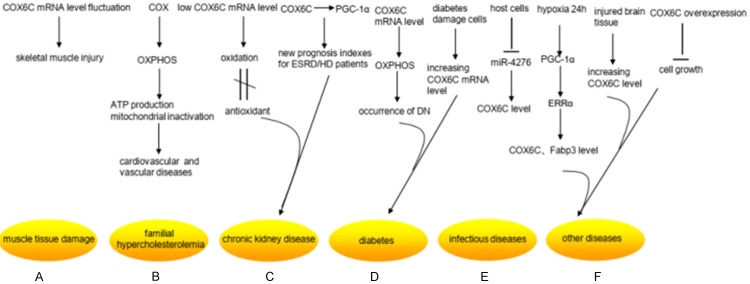
Research status of COX6C differential expression in non-neoplastic diseases. A. COX6C mRNA was much higher than that of the control group within 6 hours after contusion, but it was going down dramatically at 6-36 hours after injury in the rat muscle injury model. B. COX can regulate oxidative phosphorylation (OXPHOS) in eukaryotic enzymes, which can lead to reduced ATP production and mitochondrial inactivation. They contribute to the progression of vascular diseases. C. The expression level of the COX6C mRNA appeared lower in chronic kidney disease (CKD), which revealed the mechanism caused by the imbalance between oxidation and antioxidant defense. COX6C and peroxisome proliferator-activated receptor-gamma coactivator 1 alpha (PGC-1α), an upstream gene of COX6C, can be considered as new prognosis evaluation indexes for end-stage renal disease (ESRD)/HD patients. D. The expression level of COX6C mRNA was remarkably up-regulated in glomerular cells of patients with diabetic nephropathy (DN), manifesting a potential mechanism for the occurrence of DN. The expression of COX6C could increase in diabetes damage cells in the low-intensity laser irradiation. E. Host cells can up-regulate the expression of COX6C by silencing miR-4276 in the early stage of infection with the influenza virus when they first exposed to the influenza virus. F. PGC-1α expression increased in cardiomyocytes after 24 hours under hypoxia condition, which led to an increase in estrogen-related receptor alpha (ERRα), its downstream gene, consequently increased the mRNA expression of COX6C and Fabp3, which was two target genes of ERRα. Moreover, the level mRNA of COX6C was also differentially expressed in the damaged skin cells. And the overexpression of COX6C inhibited cell growth.
Figure 4.
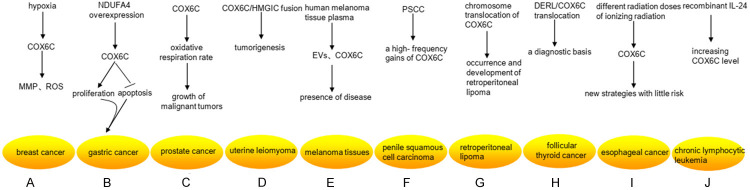
Research status of COX6C differential expression in cancers. A. Under the hypoxic conditions, the higher level of COX6C promoted the stability of mitochondrial membrane potential (MMP) and reactive oxygen species (ROS) in MCF-7/MX. B. Overexpression of NDUFA4 remarkably upregulated the expression levels of COX6C, and then promoted the proliferation and decreased the apoptosis of gastric cancer cells. C. The high level of COX6C was necessary for providing a strong oxidative respiration rate to the growth of malignant tumors. D. The COX6C gene was the fused spouse of HMGIC, which was one of the key regulators in tumorigenesis of uterine leiomyoma. E. Mitochondrial protein-enriched extracellular vesicles (EVs) and COX6C could be detected in human melanoma tissue plasma, which reflect the presence of disease. F. COX6C had a high-frequency gains in five penile squamous cell carcinoma (PSCC) cell lines. G. COX6C translocated in ETO8q chromosome breakpoint (8q22) and COX6C (8q22-q23) in retroperitoneal lipoma tissue cells, which was closely related to the occurrence and development of retroperitoneal lipoma. H. DERL/COX6C translocation may provide a diagnostic basis for follicular thyroid cancer. I. The expression of COX6C was significantly up-regulated at the early stage of different radiation doses of ionizing radiation, which may establish new strategies of alternative adjuvant therapy with little risk. J. Recombinant IL-24 stimulated the expression of COX6C after 36 hours, whereas the transcription of COX6C involved in DNA replication and metabolism was hampered within 6 hours.
COX6C and non-neoplastic diseases
The increased expression of COX6C has been reported in various invasive organelles, which always could be observed in the damaged-tissue cells. A series of biochemical processes, including OXPHOS, are usually employed. It has also been proposed that the expression level of COX6C may be considered as a marker of the degree of tissue and organ damage to guide the diagnosis and treatment of clinical diseases.
COX6C is associated with muscle tissue damage caused by disease
By real-time PCR, COX6C mRNA was regularly found to express in the rat muscle injury model, which was much higher than that of the control group within 6 hours after contusion. However, which was going down dramatically at 6-36 hours after injury. It may be valuable for estimating wound stages in combination with the results of pathological features [8]. Du and colleagues also provided similar evidence to show the expression of COX6C was positively correlated with the time of skeletal muscle injury [9]. The role of COX6C in different disease models is not consistent. Crul group reported that the expression level of COX6C was decreased in the muscle tissue of patients with acute exacerbation of chronic obstructive pulmonary disease (COPD). It indicated that various ways, such as mitochondrial dysfunction, would cause a series of pathophysiological changes under this condition. These studies may provide a strategy for treating muscle loss in acute exacerbations of some diseases [10].
COX6C and familial hypercholesterolemia
COX6C and other COXs had gene enrichment in atherosclerotic vessels of FH patients through bioinformatics analysis, and they identified a ribosomal protein as an essential regulator of atherosclerosis in FH patients depending on module analysis and gene ontology (GO) enrichment analysis. It suggested that the ribosome and oxidative phosphorylation pathway may be closely related to atherosclerosis development [11]. Moreover, OXPHOS is a metabolic pathway, through which energy is released from ATP molecules in mitochondrial cells. Meanwhile, the accumulation of reactive oxygen species (ROS) caused an increase in oxidative stress in the cells [12]. Similarly, oxidative stress has been demonstrated to play an essential role in the progression of vascular diseases, for instance, hypercholesterolemia and atherosclerosis [13]. COX has been reported to regulate OXPHOS in eukaryotic enzymes, leading to reduced ATP production and mitochondrial inactivation. Furthermore, mitochondrial dysfunction contributes to cardiovascular diseases by inducing changes in mitochondrial morphology and apoptosis [14].
COX6C and chronic kidney disease
Furthermore, Zaza et al. reported that COX6C mRNA expression was conspicuously down-regulated in peripheral blood of patients with peritoneal dialysis [15]. According to another study, including the application of clinical therapy and renal replacement therapy dirty (RRT), the expression level of the COX6C mRNA appeared noteworthy lower in patients with CKD, which revealed the mechanism for patients with CKD caused by the imbalance between oxidation and antioxidant defense. Renal tissue damage in stage 5 of CKD is associated with oxidative stress [16]. Moreover, COX6C was strongly related to the occurrence and death of hemodialysis (HD) cardiovascular disease. COX6C and peroxisome proliferator-activated receptor-gamma coactivator 1 alpha (PGC-1α), an upstream gene of COX6C, have been considered as new prognosis evaluation indexes for end-stage renal disease (ESRD)/HD patients [17].
COX6C and diabetes
It has been reported that low-intensity laser irradiation (LILI) could increase the expression of COX6C in diabetes damage cells, which proved that LILI had an impact on the mitochondrial electron transport chain (ETC, complex I-IV) and oxidative phosphorylation (ATP synthase) genes [18]. The mRNA level of PGC-1α and its downstream gene COX6C increased after exercise in insulin-sensitive people’s muscles. However, the mRNA expression of PGC-1α and COX6C was blocked and decreased in the insulin-resistant group [19]. Recently, based on the combination of transcriptomic and proteomic analyses, it has been found that avicularin could play a hypoglycemic effect to alleviate type 2 diabetes mellitus (T2DM) by regulating the expression of COX6C [20].
Besides, the expression level of COX6C mRNA was remarkably up-regulated in glomerular cells of patients with diabetic nephropathy (DN) [21]. Also, subsequent studies described that the mRNA level of COX6C was prominently increased in rats treated with telmisartan compared with their negative controls, manifesting that DN’s occurrence may be associated with mitochondrial oxidative phosphorylation pathway [22].
COX6C and infectious diseases
Host cells could upregulate the expression of COX6C by silencing miR-4276 in the early stage of infection with the influenza virus when they first came into contact with the influenza virus. It was also propounded that the influenza virus infected the host and caused changes in the miRNA profile, cell death, and tissue damage. It was speculated that COX6C was a vital player in the apoptosis pathway, which may be an essential regulator of the early stage of influenza fluidity and can be changed during the virus infection [23].
COX6C is associated with other diseases
Several literatures demonstrated that COX6C expression was increased in the injured brain tissue compared with normal control by using MALDI imaging-mass-spectrometry. COX6C would become a potential diagnostic biomarker or target for nerve recovery, and even a therapeutic target for ischemic stroke [24]. The level mRNA of COX6C was also differentially expressed in the damaged skin cells through the culture of damaged human cells in vitro [18]. Other studies indicated that PGC-1α expression increased in myocardial cells after 24 hours under hypoxia condition, which led to an increase in its downstream gene, estrogen-related receptor alpha (ERRα), consequently increased the mRNA expression of COX6C and Fabp3, which was two target genes of ERRα [25]. The estradiol/ERα signaling axis up-regulated the mitochondrial biological energy system to improve osteoblast maturation, which promoted bone formation [26].
In another paper, the researchers found that DAZAP1, a conserved RNA-binding protein, bound to COX6C pre-mRNA, but not to COX6C mRNA from intron-free expression vectors. Meanwhile, the overexpression of DAZAP1 led to the accumulation of all introns of COX6C pre-mRNA, manifesting that DAZAP1 reduced the pre-mRNA’s splicing efficiency. They also confirmed DAZAP1 silence and COX6C overexpression inhibited cell growth through cell proliferation curves in vitro. These evidences indicate that DAZAP1 is a negative regulator of pre-mRNA splicing, which may control mitochondrial energy generation by regulating COX6C expression [27].
Otherwise, COX6C may be a candidate biomarker associated with different genetic advantages of boar taint (BT) depending on differential expression analysis with co-expression analysis [28].
COX6C and cancers
Accumulating evidence illustrates that COX6C has a special relationship with breast cancer, thyroid tumors, uterine cancer, prostate cancer, esophageal cancer, and melanoma in recent years. And they put forward that the differential expression of COX6C is associated with the prognosis of some tumors and is expected to become one of the diagnostic markers of specific tumors.
COX6C and breast cancer
The expression of COX6C was up-regulated in breast cancer, confirmed through tissue microarray and other methods [29]. Several academics analyzed the ERRα expression in tumor tissues from 58 breast cancer patients via a cDNA microarray. The COX6C expression in estrogen receptor + (ER+) patients was much higher than that in non-hormone-responsive breast cancer (ER-) controls, which may play a critical role in the differentiation between ER-positive and ER-negative subtypes [30]. In addition, ATP-binding cassette superfamily G member 2 (ABCG2) and ATP synthase were increased in breast cancer cell line MCF-7/MX, which was resistant to mitoxantrone (MX). Under the hypoxic conditions, the higher level of COX6C promoted the stability of mitochondrial membrane potential (MMP) and ROS in MCF-7/MX, which provided a certain idea for targeted drug therapy of breast cancer [31].
COX6C and gastric cancer
The expression level of COX6C was remarkably up-regulated in gastric adenocarcinoma cell lines (AGS), as well as the overexpression of long non-coding MIF-AS1 or NDUFA4. It has been reported that overexpression of NDUFA4 could promote the proliferation and decreased the apoptosis of gastric cancer cells through activation of the oxidative phosphorylation pathway [32].
COX6C and prostate cancer
Another report [33] indicated that the mRNA expression level of COX6C was up-regulated in the clinical prostate tumor tissues through northern hybridization analysis. As the protein of the mitochondrial inner respiratory chain, the high level of COX6C was necessary for providing a strong oxidative respiration rate to the growth of malignant tumors, which had a meaningful clinical significance for the diagnosis of prostate cancer. Among many carcinogenic factors of prostate cancer, the transcriptional regulation of COX6C played an essential part in prostate cancer occurrence and development.
COX6C and uterine leiomyoma
In uterine leiomyoma, some scholars reported that the COX6C gene on chromosome 8q22-23 was the fused spouse of HMGIC, a novel heterotopic sequence of high mobility group protein isoform I-C [34]. They demonstrated that the first three exons of the HMGIC gene encoding three DNA binding domains fused with the second exon of the COX6C gene. Gene rearrangement identification showed that HMGIC was one of the key regulators in tumorigenesis of uterine leiomyoma. Consistently, Mine and colleagues also reported that HMGIC gene fusion was confirmed in uterine leiomyoma by RT-PCR, and COX6C translocated with RAD51B gene loci. Furthermore, it was recognized that the fusion of HMGIC DNA domain with spacer and the acidic carboxyl-terminal regulatory domain was a common tumorigenic mechanism in the development of uterine fibroids [35].
COX6C and melanoma
A groundbreaking study demonstrated mitochondrial protein-enriched extracellular vesicles (EVs) could be detected in human melanoma tissue plasma. And notably, COX6C was highly ubiquitous in the plasma of melanoma patients and was not markedly changed in breast cancer and ovarian cancer patients. The subpopulation of EVs can reflect the presence of disease, which could be another useful biomarker [36].
COX6C and penile squamous cell carcinoma
In addition, whole-genome sequencing (WGS) analyses and copy number variations (CNVs) revealed high-frequency gains of COX6C in five penile squamous cell carcinoma (PSCC) cell lines, which derived from Chinese PSCC patients [37].
COX6C and other cancers
Several reports have illustrated that COX6C was also closely related to the occurrence and development of retroperitoneal lipoma, follicular thyroid cancer, esophageal cancer, and other cancers. Concretely, COX6C translocated in ETO8q chromosome breakpoint (8q22) and COX6C (8q22-q23) in retroperitoneal lipoma tissue cells. A better classification of adipose tumor tissues could be obtained by karyotype analysis of retroperitoneal lipoma cases to assess chromosome 1 and 8 rearrangements [38]. So far, DERL/COX6C translocation has been found in thyroid cancer cell tissues, which may provide a diagnostic basis for follicular thyroid cancer [39]. In this study, the influence of ionizing radiation on esophageal cancer was evaluated by cDNA microarray. COX6C was significantly up-regulated at the early stage of different radiation doses of ionizing radiation, which may improve our understanding of radiotherapy’s molecular basis to establish new strategies of alternative adjuvant therapy [40]. Some researchers have also explored chronic lymphocytic leukemia (CLL) by utilizing a B-cell differentiation model and mRNA profiling. They demonstrated that recombinant IL-24 stimulated the expression of COX6C after 36 hours, whereas the transcription of COX6C involved in DNA replication and metabolism was hampered within 6 hours [41].
Summary and future perspectives
COX6C is an indispensable regulatory factor in the oxidative phosphorylation process of tissue cells, and plays an essential role in the mitochondrial electron transport chain of cells. Its expression is often closely related to the occurrence and development of tissue damages and tumors.
To date, the differential expression of COX6C has been found in increasing diseases. As a transcription regulator, COX6C can not only participate in oxidative phosphorylation of tissue cells, but also interact with a series of upstream regulatory proteins to regulate the biological behavior of cells. Therefore, COX6C can be recognized as a valuable biomarker for predicting the degree of tissue cell damage and the prognosis of tumor disease according to the different expression levels.
However, at present, there are still a few issues that need to be addressed. 1. It is clear that the expression levels of COX6C vary significantly in different diseases and tissue. Which specific subsets of cells are responsible for the difference? 2. In tissues, the regulatory patterns of COX6C and its upstream and downstream molecules are not clear. Whether the regulatory patterns are consistent in different tissues and cell subtypes is not clear. 3. The mechanism of COX6C as a somatic molecule in cells of different tissues remains unclear. 4. Much more studies should be performed to clarify how to take the expression level of COX6C as a marker of disease diagnosis or prognosis, even a novel target for treatment. The discussion of the above problems will greatly improve our understanding of the relevant functions of COX6C.
Acknowledgements
This work was supported in part by grants from the National Natural Science Foundation of China (81672810), the Natural Science Basic Research Project of Shaanxi Province (2020JM-362), the Institutional foundation of the first affiliated hospital of Xi’an Jiaotong University (2019ZYTS-19).
Disclosure of conflict of interest
None.
References
- 1.Liberti MV, Locasale JW. The Warburg effect: how does it benefit cancer cells? Trends Biochem Sci. 2016;41:211–8. doi: 10.1016/j.tibs.2015.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Belevich I, Verkhovsky MI. Molecular mechanism of proton translocation by cytochrome c oxidase. Antioxid Redox Signal. 2008;10:1–29. doi: 10.1089/ars.2007.1705. [DOI] [PubMed] [Google Scholar]
- 3.Schmidt TR, Jaradat SA, Goodman M, Lomax MI, Grossman LI. Molecular evolution of cytochrome c oxidase: rate variation among subunit VIa isoforms. Mol Biol Evol. 1997;14:595–601. doi: 10.1093/oxfordjournals.molbev.a025798. [DOI] [PubMed] [Google Scholar]
- 4.Barazzoni R, Short KR, Nair KS. Effects of aging on mitochondrial DNA copy number and cytochrome c oxidase gene expression in rat skeletal muscle, liver, and heart. J Biol Chem. 2000;275:3343–7. doi: 10.1074/jbc.275.5.3343. [DOI] [PubMed] [Google Scholar]
- 5.Lazarou M, Smith SM, Thorburn DR, Ryan MT, McKenzie M. Assembly of nuclear DNA-encoded subunits into mitochondrial complex IV, and their preferential integration into supercomplex forms in patient mitochondria. FEBS J. 2009;276:6701–13. doi: 10.1111/j.1742-4658.2009.07384.x. [DOI] [PubMed] [Google Scholar]
- 6.Herrmann PC, Gillespie JW, Charboneau L, Bichsel VE, Paweletz CP, Calvert VS, Kohn EC, Emmert-Buck MR, Liotta LA, Petricoin EF 3rd. Mitochondrial proteome: altered cytochrome c oxidase subunit levels in prostate cancer. Proteomics. 2003;3:1801–10. doi: 10.1002/pmic.200300461. [DOI] [PubMed] [Google Scholar]
- 7.Kadenbach B. Regulation of mammalian 13-subunit cytochrome c oxidase and binding of other proteins: role of NDUFA4. Trends Endocrinol Metab. 2017;28:761–70. doi: 10.1016/j.tem.2017.09.003. [DOI] [PubMed] [Google Scholar]
- 8.Sun JH, Zhang L, Wang XW, Du QX, Lu J, Wang YY, Sun JH. Relation between injury time and the expression of COX6C mRNA in skeletal muscle of rats after contusion. Fa Yi Xue Za Zhi. 2015;31:177–80. [PubMed] [Google Scholar]
- 9.Du QX, Wang XW, Zhang L, Li SQ, Gao CR, Wang YY. Relative expression of indicators for wound age estimation in forensic pathology. Fa Yi Xue Za Zhi. 2015;31:81–4. [PubMed] [Google Scholar]
- 10.Crul T, Testelmans D, Spruit MA, Troosters T, Gosselink R, Geeraerts I, Decramer M, Gayan-Ramirez G. Gene expression profiling in vastus lateralis muscle during an acute exacerbation of COPD. Cell Physiol Biochem. 2010;25:491–500. doi: 10.1159/000303054. [DOI] [PubMed] [Google Scholar]
- 11.Wang HX, Zhao YX. Prediction of genetic risk factors of atherosclerosis using various bioinformatic tools. Genet Mol Res. 2016;15 doi: 10.4238/gmr.15027347. [DOI] [PubMed] [Google Scholar]
- 12.Hatefi Y. The mitochondrial electron transport and oxidative phosphorylation system. Annu Rev Biochem. 1985;54:1015–69. doi: 10.1146/annurev.bi.54.070185.005055. [DOI] [PubMed] [Google Scholar]
- 13.Scherz-Shouval R, Elazar Z. ROS, mitochondria and the regulation of autophagy. Trends Cell Biol. 2007;17:422–7. doi: 10.1016/j.tcb.2007.07.009. [DOI] [PubMed] [Google Scholar]
- 14.Peng WX, Cai GD, Xia YP, Chen JN, Wu P, Wang Z, Liu GH, Wei DH. Mitochondrial dysfunction in atherosclerosis. DNA Cell Biol. 2019;38:597–606. doi: 10.1089/dna.2018.4552. [DOI] [PubMed] [Google Scholar]
- 15.Zaza G, Granata S, Masola V, Rugiu C, Fantin F, Gesualdo L, Schena FP, Lupo A. Downregulation of nuclear-encoded genes of oxidative metabolism in dialyzed chronic kidney disease patients. PLoS One. 2013;8:e77847. doi: 10.1371/journal.pone.0077847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hashad D, Elgohry I, Dwedar F. Nuclear respiratory factor-1 (NRF-1) gene expression in chronic kidney disease patients undergoing hemodialysis and mitochondrial oxidative dysregulation. Clin Lab. 2016;62:2149–54. doi: 10.7754/Clin.Lab.2016.160329. [DOI] [PubMed] [Google Scholar]
- 17.Elsayed ET, Nassra RA, Naga YS. Peroxisome proliferator-activated receptor-γ-coactivator 1α (PGC-1α) gene expression in chronic kidney disease patients on hemodialysis: relation to hemodialysis-related cardiovascular morbidity and mortality. Int Urol Nephrol. 2017;49:1835–44. doi: 10.1007/s11255-017-1628-5. [DOI] [PubMed] [Google Scholar]
- 18.Masha RT, Houreld NN, Abrahamse H. Low-intensity laser irradiation at 660 nm stimulates transcription of genes involved in the electron transport chain. Photomed Laser Surg. 2013;31:47–53. doi: 10.1089/pho.2012.3369. [DOI] [PubMed] [Google Scholar]
- 19.De Filippis E, Alvarez G, Berria R, Cusi K, Everman S, Meyer C, Lawrence JM. Insulin-resistant muscle is exercise resistant: evidence for reduced response of nuclear-encoded mitochondrial genes to exercise. Am J Physiol Endocrinol Metab. 2008;294:E607–14. doi: 10.1152/ajpendo.00729.2007. [DOI] [PubMed] [Google Scholar]
- 20.Zhu XA, Qiu ZR, Ouyang W, Miao JY, Xiong P, Mao DB, Feng KL, Li MX, Luo MN, Xiao H, Cao Y. Hepatic transcriptome and proteome analyses provide new insights into the regulator mechanism of dietary avicularin in diabetic mice. Food Res Int. 2019;125:108570. doi: 10.1016/j.foodres.2019.108570. [DOI] [PubMed] [Google Scholar]
- 21.Zhang Q, Xiao XH, Li M, Li WH, Yu M, Zhang HB, Sun XF, Mao LL, Xiang HD. Gene expression profiling in glomeruli of diabetic nephropathy rat. Exp Biol Med (Maywood) 2012;237:903–11. doi: 10.1258/ebm.2012.012032. [DOI] [PubMed] [Google Scholar]
- 22.Zhang Q, Xiao XH, Li M, Li WH, Yu M, Zhang HB, Sun XF, Mao LL, Xiang HD. Telmisartan improves kidney function through inhibition of the oxidative phosphorylation pathway in diabetic rats. J Mol Endocrinol. 2012;49:35–46. doi: 10.1530/JME-12-0020. [DOI] [PubMed] [Google Scholar]
- 23.Othumpangat S, Noti JD, Beezhold DH. Lung epithelial cells resist influenza A infection by inducing the expression of cytochrome c oxidase VIc which is modulated by miRNA 4276. Virology. 2014;468-470:256–64. doi: 10.1016/j.virol.2014.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Llombart V, Trejo SA, Bronsoms S, Morancho A, Feifei M, Faura J, García-Berrocoso T, Simats A, Rosell A, Canals F, Hernández-Guillamón M, Hernández-Guillamón J. Profiling and identification of new proteins involved in brain ischemia using MALDI-imaging-mass-spectrometry. J Proteomics. 2017;152:243–53. doi: 10.1016/j.jprot.2016.11.014. [DOI] [PubMed] [Google Scholar]
- 25.Cunningham KF, Beeson GC, Beeson CC, Baicu CF, Zile MR, McDermott PJ. Estrogen-related receptor α (ERRα) is required for adaptive increases in PGC-1 isoform expression during electrically stimulated contraction of adult cardiomyocytes in sustained hypoxic conditions. Int J Cardiol. 2015;187:393–400. doi: 10.1016/j.ijcard.2015.03.353. [DOI] [PubMed] [Google Scholar]
- 26.Lin PI, Tai YT, Chan WP, Lin YL, Liao MH, Chen RM. Estrogen/ERα signaling axis participates in osteoblast maturation via upregulating chromosomal and mitochondrial complex gene expressions. Oncotarget. 2018;9:1169–86. doi: 10.18632/oncotarget.23453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sasaki K, Ono M, Takabe K, Suzuki A, Kurihara Y. Specific intron-dependent loading of DAZAP1 onto the cox6c transcript suppresses pre-mRNA splicing efficacy and induces cell growth retardation. Gene. 2018;657:1–8. doi: 10.1016/j.gene.2018.03.005. [DOI] [PubMed] [Google Scholar]
- 28.Drag M, Skinkyte-Juskiene R, Do DN, Kogelman LJA, Kadarmideen HN. Differential expression and co-expression gene networks reveal candidate biomarkers of boar taint in non-castrated pigs. Sci Rep. 2017;7:12205. doi: 10.1038/s41598-017-11928-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Emerson JW, Dolled-Filhart M, Harris L, Rimm DL, Tuck DP. Quantitative assessment of tissue biomarkers and construction of a model to predict outcome in breast cancer using multiple imputation. Cancer Inform. 2009;7:29–40. doi: 10.4137/cin.s911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gruvberger S, Ringnér M, Chen Y, Panavally S, Saal LH, Borg A, Ferno M, Peterson C, Meltzer P. Estrogen receptor status in breast cancer is associated with remarkably distinct gene expression patterns. Cancer Res. 2001;61:5979–84. [PubMed] [Google Scholar]
- 31.Chang FW, Fan HC, Liu JM, Fan TP, Jing J, Yang CL, Hsu RJ. Estrogen enhances the expression of the multidrug transporter gene ABCG2-increasing drug resistance of breast cancer cells through estrogen receptors. Int J Mol Sci. 2017;18:163. doi: 10.3390/ijms18010163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li LH, Li YJ, Huang YG, Ouyang Y, Zhu Y, Wang YZ, Guo XD, Yuan Y, Gong KM. Long non-coding RNA MIF-AS1 promotes gastric cancer cell proliferation and reduces apoptosis to upregulate NDUFA4. Cancer Sci. 2018;109:3714–25. doi: 10.1111/cas.13801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang FL, Wang Y, Wong WK, Liu Y, Addivinola FJ, Liang P, Chen LB, Kantoff P, Pardee A. Two differentially expressed genes in normal human prostate tissue and in carcinoma. Cancer Res. 1996;56:3634–7. [PubMed] [Google Scholar]
- 34.Kurose K, Mine N, Doi D, Ota Y, Yoneyama K, Konishi H, Araki T, Emi M. Novel gene fusion of COX6C at 8q22-23 to HMGIC at 12q15 in a uterine leiomyoma. Genes Chromosomes Cancer. 2000;27:303–7. doi: 10.1002/(sici)1098-2264(200003)27:3<303::aid-gcc11>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 35.Mine N, Kurose K, Nagai H, Doi D, Ota Y, Yoneyama K, Konishi H, Araki T, Emi M. Gene fusion involving HMGIC is a frequent aberration in uterine leiomyomas. J Hum Genet. 2001;46:408–12. doi: 10.1007/s100380170059. [DOI] [PubMed] [Google Scholar]
- 36.Jang SC, Crescitelli R, Cvjetkovic A, Belgrano V, Olofsson Bagge R, Sundfeldt K, Ochiya T, Kalluri R, Lötvall J. Mitochondrial protein enriched extracellular vesicles discovered in human melanoma tissues can be detected in patient plasma. J Extracell Vesicles. 2019;8:1635420. doi: 10.1080/20013078.2019.1635420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhou QH, Deng CZ, Li ZS, Chen JP, Yao K, Huang KB, Liu TY, Liu ZW, Qin ZK, Zhou FJ, Huang WL, Han H, Liu RY. Molecular characterization and integrative genomic analysis of a panel of newly established penile cancer cell lines. Cell Death Dis. 2018;9:684. doi: 10.1038/s41419-018-0736-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Foa C, Mainguené C, Dupré F, Coindre JM, Huguet C, Kober C, Pedeutour F. Rearrangement involving chromosomes 1 and 8 in a retroperitoneal lipoma. Cancer Genet Cytogenet. 2002;133:156–9. doi: 10.1016/s0165-4608(01)00573-8. [DOI] [PubMed] [Google Scholar]
- 39.Swierniak M, Pfeifer A, Stokowy T, Rusinek D, Chekan M, Lange D, Krajewska J, Oczko-Wojciechowska M, Czarniecka A, Jarzab M, Jarzab B, Wojtas B. Somatic mutation profiling of follicular thyroid cancer by next generation sequencing. Mol Cell Endocrinol. 2016;433:130–7. doi: 10.1016/j.mce.2016.06.007. [DOI] [PubMed] [Google Scholar]
- 40.Bo H, Ghazizadeh M, Shimizu H, Kurihara Y, Egawa S, Moriyama Y, Tajiri T, Kawanami O. Effect of ionizing irradiation on human esophageal cancer cell lines by cDNA microarray gene expression analysis. J Nippon Med Sch. 2004;71:172–80. doi: 10.1272/jnms.71.172. [DOI] [PubMed] [Google Scholar]
- 41.Hadife N, Nemos C, Frippiat JP, Hamadé T, Perrot A, Dalloul A. Interleukin-24 mediates apoptosis in human B-cells through early activation of cell cycle arrest followed by late induction of the mitochondrial apoptosis pathway. Leuk Lymphoma. 2013;54:587–97. doi: 10.3109/10428194.2012.717079. [DOI] [PubMed] [Google Scholar]