Abstract
In thoracolumbar vertebral tumors, reconstruction of complex multi-segment thoracolumbar vertebrae after total en-bloc spondylectomy (TES) is still challenging. In recent years, with the development of 3D printing technology, individualized 3D printed artificial vertebrae have been attempted to reconstruct complex multi-segment thoracolumbar spine. Compared with traditional titanium mesh or bone transplantation, it helps reduce long-term complications, bringing a new dawn for reconstructing multi-segment thoracolumbar spine. A 69-year-old female complained of low back pain with limited motion for 1 month. More than 2 months ago, she underwent radical mastectomy due to breast cancer (Luminal A subtype). Imageology examination revealed an osteolytic lesion involving the T11-L1 vertebra. She was performed one-stage 3-segment (T11-L1) en-bloc spondylectomy via posterior approach, and then an artificial vertebrae produced by a novel individualized 3D printing technology was used for reconstruction. The patient was follow-up for 2 years, and she recovered well, with no tumor recurrence, and no complications after spinal reconstruction. The application of individualized 3D printed artificial vertebrae in multi-segment thoracolumbar spine reconstruction can not only reconstruct the bone defect more accurately through the individualized design, but the porous design is able to achieve biomechanical performance comparable to that of cancellous bone, and it is conducive to inducing bone growth, all of which help reduce long-term mechanical complications. Furthermore, the application of artificial vertebrae in surgery can significantly shorten the operation time, reduce intraoperative blood loss and reduce the risk of perioperative complications. Therefore, individualized 3D printed artificial vertebrae is a good choice for complex multi-segment thoracolumbar spine reconstruction.
Keywords: En-bloc, pondylectomy, 3D printed, artificial vertebrae
Introduction
Breast cancer (BC) is the most common cancer among females worldwide [1], and the bone is the most common site of its distant metastasis [1,2]. In bone-metastatic events from BC, multi-segment thoracolumbar metastases can destroy the vertebral body and lead to loss of spinal stability, and even further damage the spinal cord and nerves. Usually, it is necessary to remove the entire metastasis and reconstruct the stability of the spine. With the accumulation of clinical experience and the improvement of surgical techniques, one-stage en-bloc spondylectomy of multi-segment vertebrae via posterior approach has become feasible [3-5]. However, reconstruction after resection of multi-segment thoracolumbar spine is still challenging. There is still a lack of standardized method despite decades of efforts.
In recent years, with the development of 3D printing technology, individualized 3D printed artificial vertebrae have been attempted for spine reconstruction after en-bloc spondylectomy of multi-segment vertebrae [6]. Compared with traditional titanium mesh and bone transplantation, 3D printing technology can not only reconstruct bone defects more accurately through individualized design, and achieve comparable biomechanical performance as cancellous bone with its porous structure, but also is conducive to the induction of bone growth, and reduce implant-related complications, such as loosening, subsidence and fracture, etc [7-11].
Herein, we reported a case of reconstruction of the thoracolumbar spine using individualized 3D printed artificial vertebrae after one-stage en-bloc spondylectomy of multi-segment thoracolumbar metastasis via posterior approach, and reviewed the relevant literature. Our purpose is to describe and discuss the key points and clinical efficacy of this novel and emerging technology.
Case report
Case description
A female patient, 69-year-old, complained of back pain with limited motion for 1 month without obvious cause. Visual analysis scores (VAS) were used to evaluate the degree of back pain, and the result was 7 points. More than 2 months ago, she underwent radical mastectomy due to BC (Luminal A subtype). Physical examination showed positive local tenderness and percussion pain in thoracolumbar segments, with normal muscle strength and sensation of the limbs, without neurological impairment. Imaging examination of the thoracolumbar spine by computed tomography (CT) and magnetic resonance imaging (MRI) revealed a lytic lesion involving T11 and L1 vertebral bodies (Figures 1, 2). Emission computed tomography (ECT) showed there was nuclide concentration in the T11, T12, and L1 vertebral bodies, and there was no abnormality elsewhere (Figure 3). CT guided bone biopsy confirmed that T11-L1 lesions were metastatic tumor. We assumed that the thoracolumbar vertebral lesion was a metastasis caused by BC, which was Tomita VII type, WBB staging: T11: 1-7/BD, L1: 2-7/BD, spinal instability neoplastic score (SINS) of 9 points, Tomita score for spinal metastases of 3 points, modified Tokuhashi score for spinal metastases of 12 points. Since the patient had a long life expectancy, it was appropriate for her to undergo extensive or marginal tumor resection. We planned to perform one-stage en-bloc spondylectomy of 3-segment tumor lesions via the posterior approach, and then use an individualized 3D printed artificial vertebral body for reconstruction.
Figure 1.
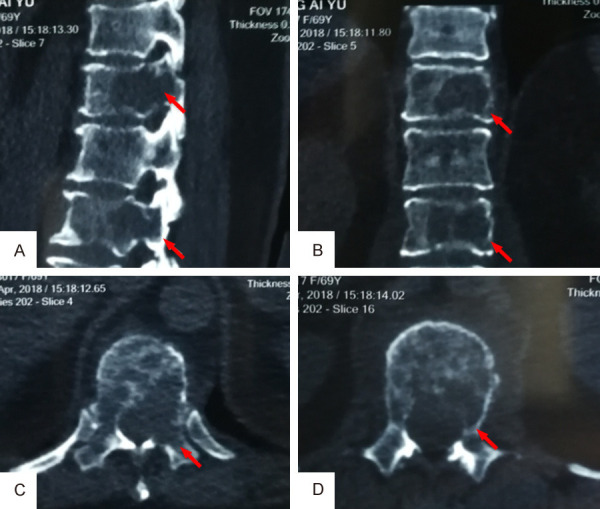
CT scanning of thoracolumbar spine showed a lytic lesion involving T11 and L1 vertebral bodies. A. Sagittal view; B. Coronal view; C. Cross section view of T11; D. Cross section view of L1.
Figure 2.
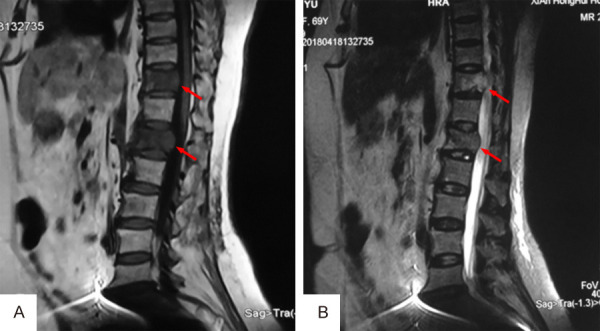
MRI examination revealed a lesion involving T11 and L1 vertebral bodies. A. Low signal on T1-weighted image; B. Mildly high signal on T2-weighted image.
Figure 3.
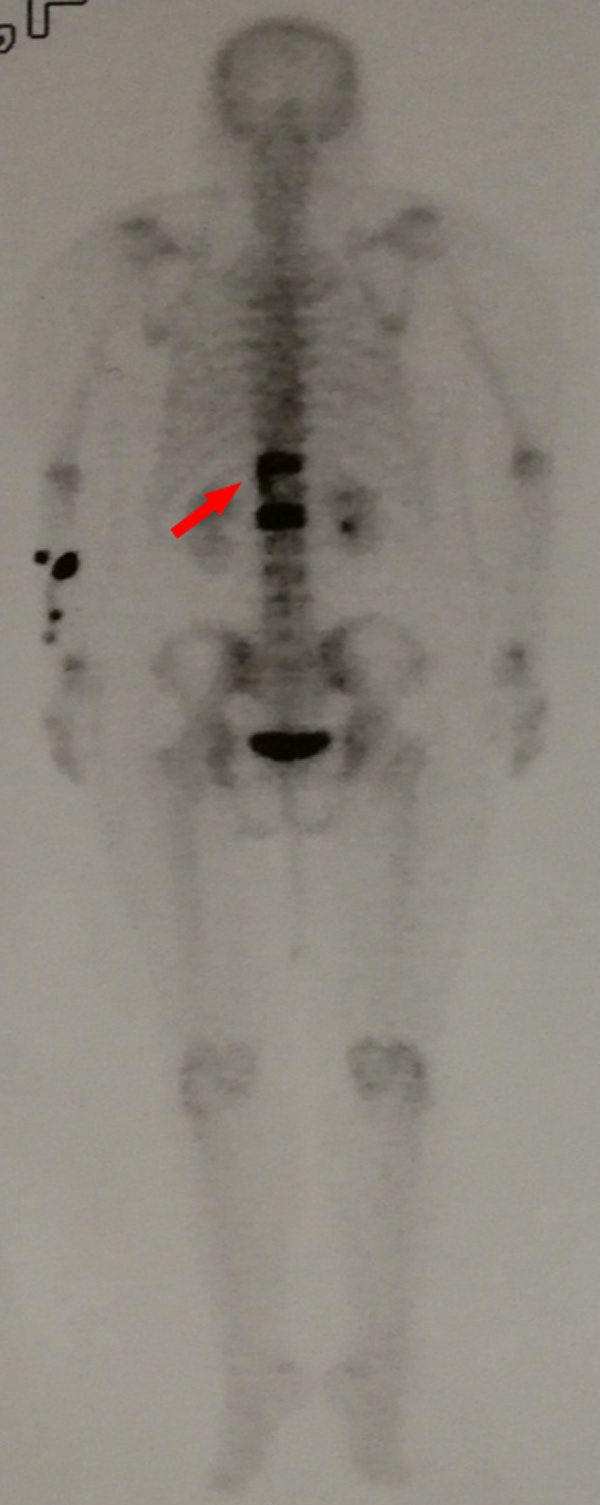
ECT showed there was nuclide concentration in the T11, L1 and even T12 vertebral bodies, no abnormality found in other parts.
Design and manufacture of individualized 3D printed artificial vertebrae
The scope of surgical resection was planned according to the imaging results, which included the osteotomy position and resected segments. MIMICS15.0 (Materialise NV, Leuven, Belgium) was used to design the shape of the personalized 3D printed artificial vertebrae. Then, according to the computer-aided design (CAD) model, the titanium alloy powder (Ti-6Al-4V) was continuously printed in 3D layer by layer using electron beam melting (EBM) technology to obtain individualized artificial vertebrae. This 3D printed titanium alloy artificial vertebrae had a porous mesh structure, the diameters of the holes and wires were 800 μm and 550 μm, respectively, and the average porosity was 50-80%. This porous structure not only has biomechanical properties comparable to cancellous bone, but also helps to induce bone growth. The individualized 3D printed artificial vertebrae was columnar. It was designed based on the patient’s thoracolumbar physiological curvature and osteotomy position of the upper and lower vertebral bodies. The contact surfaces at the upper and lower ends perfectly fitted the patient’s end plates of upper and lower vertebrae, and the spinal physiological curvature was recovered to the maximum extent. This design can increase stability and reduce the possibility of sagittal spinal misalignment, and reduce the risk of displacement and subsidence of the artificial vertebrae. Four screw holes for pedicle screws are reserved on both sides, through which pedicle screws can be placed to connect with the titanium rod to further enhance the stability of the artificial vertebrae. At the same time, in order to adapt to the actual size of the bone defect and avoid mismatch of the artificial vertebrae and normal vertebrae due to stretching or contraction of the spinal posterior column during the operation, we made 3 artificial vertebral bodies of different sizes, one of which had the same size as that measured preoperatively, and the remaining 2 vertebral bodies had a vertical axis that was increased or decreased by 3 mm respectively based on the measured value (Figure 4).
Figure 4.

Three individualized 3D printed artificial vertebral bodies of different sizes.
Surgery
Before operation, the patient underwent selective arterial embolization (blood vessels at T11-L1 segments) to reduce intraoperative bleeding. During the operation, the nerve function was monitored using somatosensory evoked potential. After general anesthesia, the patient was placed in the prone position. An incision about 25 cm in length was made at middle thoracolumbar, centered at T12 spinous process, then the skin, subcutaneous tissue and fascia were successively incised, and the subperiosteal paraspinal muscle was peeled to expose the T8-L4 spinous process, lamina and facet joint. Screws were implanted on both sides of T8-T10 and L2-L4 using manual pedicle screw technique. Then the costovertebral joints and part of the ribs on both sides of the T11-T12 vertebral body were further exposed, the ribs were cut at 3 cm from the lateral costovertebral joints. Because the left pedicles of T11 and L1 were involved, according to the preoperative image data and the 1:1 3D printed model, an ultrasonic bone scalpel was used to osteotomize the lower 1/2 spinous process and lower 1/3 lamina of T10, the superior facet joints of L2, the left side normal lamina adjacent to pedicles of T11-L1, and the right side pedicles of T11-L1. Subsequently, the entire posterior appendices of T11-L1 were removed to expose the spinal cord and nerve roots, the bilateral T11 and T12 nerve roots were cut off, while the nerve roots of L1 was retained.
During the operation, bone wax was used to seal the surfaces of pedicle osteotomy and laminectomy, and bipolar electrocoagulation and gelatin sponge compression were used to strictly stop bleeding. Afterwards, the anterior part of the vertebral body was dissected with fingers, the anterior longitudinal ligament was peeled from the vertebral body, and the malleable protective barrier was inserted between the anterior longitudinal ligament and the vertebral body, so as to protect the ventral large vessels. Then the right temporary rod was installed, and the spinal cord protector was installed on the temporary rod. After that, a wire saw was inserted, which was clinged to the lower endplate of T10 vertebrae and upper endplate of L2 vertebra to further dissect T11-L1 vertebral bodies. After we ensured that the dissected vertebral bodies were not adhered to the dura mater, the dissected diseased vertebrae were removed around the spinal cord from the left side. After measurement, the 3D printed artificial vertebrae that had the same size as measured preoperatively were finally selected for reconstruction of T11-L1. After the artificial vertebrae were placed in position, 4 pedicle screws were placed on it, and titanium rods were installed alternately, cross links were installed, and posterolateral bone graft fusion was performed to complete surgery. The operation time was 280 min, and bleeding amount was 1500 ml (Figures 5, 6).
Figure 5.
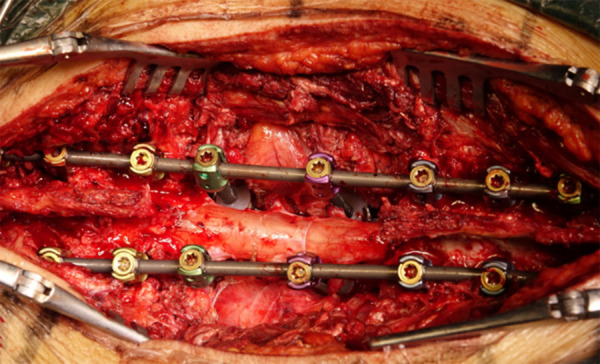
Intraoperative picture after the installation of individualized 3D printed artificial vertebrae.
Figure 6.
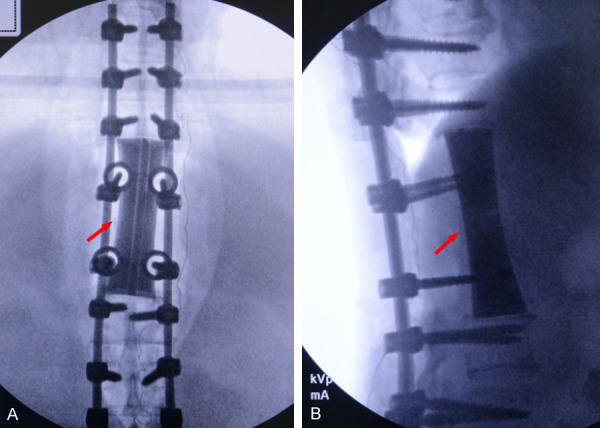
Postoperative X-ray examination showed that the internal fixation and artificial vertebral body were ideal, and the prosthesis had good contact with the upper and lower vertebral endplates.
Postoperative follow-up
After the operation, the patient was transferred to ICU for further treatment. After 3 days, the patient’s condition was stable and was transferred to a general ward. Six days after surgery, the patient’s back pain was significantly alleviated (VAS score: 2 points), and she could walk normally independently with a thoracolumbar brace.
Postoperative pathology showed a negative margin, which proved that the tumor tissue was completely removed, and a tumor-free margin was obtained. X-ray film and CT at 6 months and 2 years after the operation showed that the thoracolumbar vertebrae were in good order and the artificial vertebral body was in a good position (no loosening, displacement and subsidence) (Figures 7, 8). During a follow up of 2 years, there was no tumor recurrence, no other discomfort and the patient lived well independently.
Figure 7.
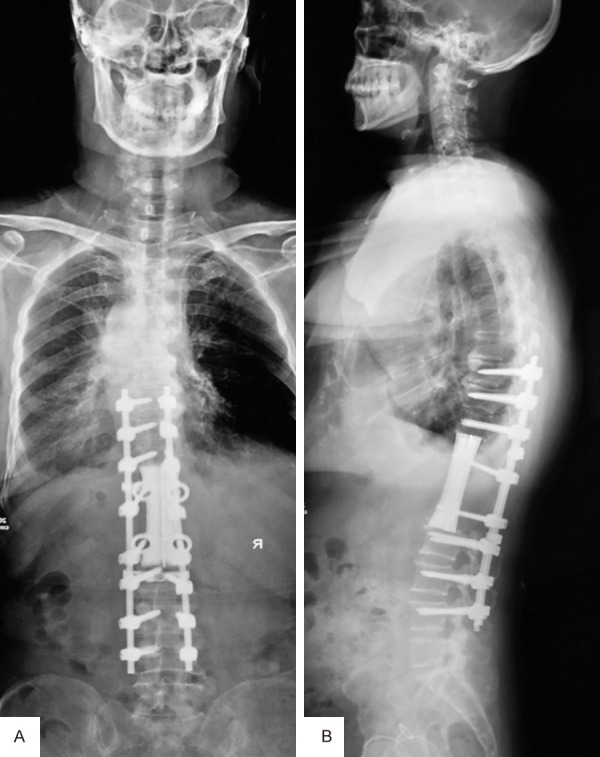
X-ray examination at 6 months after operation showed that the physiological curvature of thoracolumbar spine was well maintained and the artificial vertebral body was in a good position.
Figure 8.
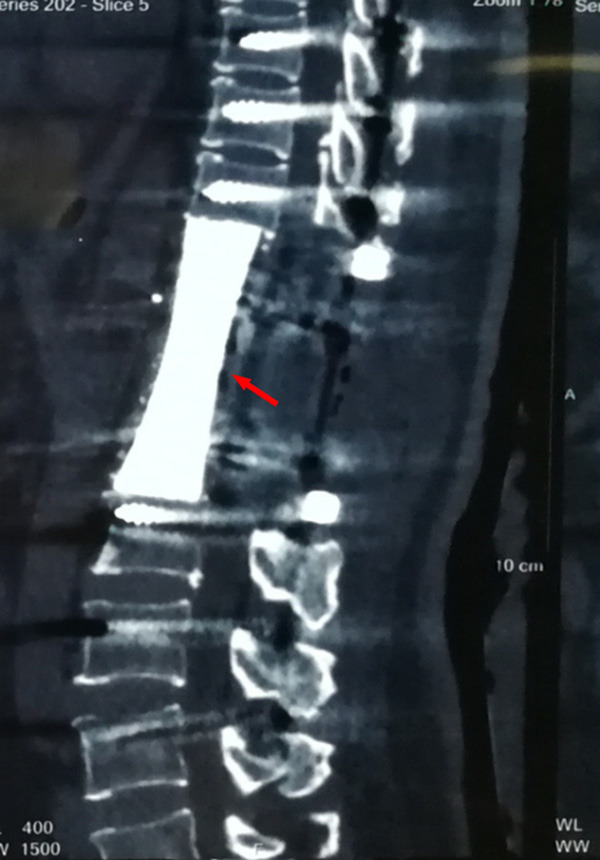
CT scanning at 2 years after operation showed that the artificial vertebral body was in a good position without loosening, fracture and subsidence.
Discussion
In spinal tumors, the purpose of total en-bloc spondylectomy (TES) is to obtain a safe margin, thereby improving survival and reducing recurrence rate, which has become the gold standard for the treatment of resectable spinal tumors [3-5]. However, one-stage posterior multi-segment TES for spinal tumors and multi-segment spine reconstruction remain challenging.
Compared with single-segment TES, multi-segment TES technology is more difficult and riskier, and has the following characteristics: (a) Wide range of resection, large amount of blood loss, high risk of perioperative complications. (b) It requires to ligate a number of segmental vessels, which easily affects the blood supply of the spinal cord, and even causes paralysis. (c) Many segments are involved, which leads to serious mechanical instability and difficult reconstruction. However, Murakami et al. [12] showed that ligating the segmental vessels of 3 adjacent segments on both sides during TES was unlikely to significantly affect the postoperative nerve function. In order to reduce intraoperative bleeding, the patient underwent selective arterial embolization (T11-L1 segmental vessels) before surgery, and there was no obvious effect on the patient’s nerve function after surgery.
Another difficulty in this case was the tumor invaded the left pedicles of T11 and L1, and osteotomy might cause tumor cells to spread. Tomita et al. [13] reported a group of 20 patients who underwent TES with osteotomy via the involved pedicles, and there was no recurrent cases. They believed that the following steps could reduce the local recurrence rate: (a) Using an electric knife to cauterize tumors in pedicles before osteotomy. (b) Using a 0.5 mm wire saw for osteotomy. (c) Using bone wax to seal osteotomy surface. (d) Using distilled water and high concentration of cisplatin (0.5 mg/ml) to soak the surgical field to further kill tumor cells which might contaminate the surgical field. However, Abe et al. [14] reported 7 cases undergoing osteotomy through the pedicle with tumor invasion, of which 2 cases with renal cancer occurred local recurrence. Therefore, how to reduce the recurrence caused by osteotomy via the pedicles with tumor invasion requires further research. Fortunately, in our patient, only the left pedicles were involved. Instead of routinely performing osteotomy from the pedicle, we used an ultrasonic bone scalpel to osteotomize the normal lamina adjacent to the pedicle on the left side of T11-L1 based on the preoperative imaging positioning and a 1:1 3D printed model, thereby avoiding intratumoral operation. In addition, during the spondylectomy, we carefully dissected the soft tissues around the vertebral bodies with our fingers to avoid damage to the large blood vessels and the pleura, and protected the large blood vessels and spinal cord with a malleable protective barrier. The patient had no adverse events such as rupture of large blood vessels during the perioperative period, and had no complications such as infection, cardiopulmonary failure, and neurological deficit after surgery. There was no tumor recurrence during the 2 years follow-up, which further confirms that one-stage posterior multi-segment TES is feasible for the treatment of thoracolumbar spine tumors.
However, after a multi-segment TES, the stability of the spine is lost, and the reconstruction of multi-segment thoracolumbar spine is still challenging. In the past, titanium mesh and bone transplantation were widely used. Nonetheless, titanium mesh and bone transplantation have problems such as insufficient fixation, poor fit with complex bone contact surfaces, uneven stress distribution, and delayed bone union, which results in a risk of implant displacement and subsidence [15]. Furthermore, long bone transplantation and long titanium mesh filling with autogenous bone can also cause additional trauma and complications [15].
In recent years, with the development of 3D printing technology, the application of individualized 3D printed artificial vertebral bodies has provided a new method for complex construction of multi-segment spine. Many studies have shown that compared with traditional titanium mesh and bone transplantation, titanium alloy artificial prostheses made by 3D printing technology can not only reconstruct bone defects more accurately through individualized design, but also achieve biomechanical properties comparable to those of cancellous bone, and at the same time, it is beneficial to induce bone growth and helps reduce long-term complications, such as loosening, subsidence and fracture, etc [7-11].
Reviewing the literature, individualized 3D printed artificial vertebral bodies have been attempted for reconstruction of the thoracolumbar spine after TES of multi-segment spinal tumors (Table 1). Chin et al. [6] reported a case of 3D printed artificial vertebral body for revision surgery after recurrence of a 3-segment lumbar tumor. This is the only report on 3D printed prosthesis used for spinal reconstruction after TES for 3-segment (L1-L3) lumbar spine tumors. In the present study, we first reported to use 3D printed titanium alloy prosthesis to reconstruct spine after TES of 3-segment thoracolumbar (T11-L1) tumor. Comparing with the 3D printed titanium alloy artificial vertebrae reported by Chin et al., we shared similar design concepts. Firstly, the artificial vertebra is designed according to the osteotomy position and bone defect morphology and is continuously 3D printed layer-bylayer using titanium alloy powder (Ti-6Al-4V) by electron beam melting (EBM) technology. The ends of the individualized 3D printed artificial vertebral body can accurately match the osteotomy surface of the upper and lower vertebral endplates. Secondly, the porous mesh design can not only achieve biomechanical properties equivalent to cancellous bone, but also is conducive to inducing bone growth, and thus enabling to achieve more stable integration between prostheses and vertebral bodies. In addition, pedicle screw holes are reserved on both sides of the 3D printed vertebrae, so that the prosthesis can be fixed on the posterior spinal instrument using the pedicle screws, which enhances the stability of the artificial vertebrae. The difference is that Chin et al. added screws to the upper and lower bone contact surfaces of the prosthesis. Because in their study, the patient with recurrence of L1-L3 giant cell tumor of bone (GCTB) have large mass, which are located in anterior-lateral vertebral bodies, they performed a first-stage posterior laminectomy internal fixation and a second-stage anterior vertebrectomy via anterolateral approach, and used spinal prosthesis reconstruction, in which the anterolateral approach allowed them to screw on the bone contact surface. In the present study, the patient was old, and underwent radical mastectomy 2 months ago. In order to avoid serious complications during the perioperative period, anterior-posterior operation was not advisable. Furthermore, the breast metastatic cancer lesions were limited to the thoracolumbar T11-L1 vertebral bodies, and T11 and T12 nerve roots could be cut off according to the needs of surgery. Hence, we performed only one-stage posterior TES resection of 3-segment (T11-L1) tumor lesions, and then used individualized 3D printed artificial vertebral body for reconstruction. This posterior only surgery saves time and reduces the patient’s extra trauma but makes it inconvenient for screw fixation on the bone contact surface. Despite this, good biomechanical stability was achieved. The patient was able to walk independently for a short distance while wearing a thoracolumbar brace 6 days after surgery. During 2 years of follow-up, the artificial vertebrae was in a good position, without displacement. However, this study is only a clinical observation of one case. Is it necessary to add screws to the upper and lower bone contact surfaces of the prosthesis in the reconstruction of multi-segment using the 3D printed vertebrae requires comparative studies and biomechanics analysis with large samples.
Table 1.
Summary of previously reported cases and present case of the application of individualized 3D printed artificial vertebrae in the reconstruction of the thoracolumbar spine after TES of multi-segment spinal tumors
| Author(s), Year Reported | Patient Age, Sex | Diagnosis | Reconstructed Segment | Outcome |
|---|---|---|---|---|
| Chin et al. [6] (2019) | 51-year-old, male | Giant Cell Tumor | L1-L3 | Healed |
| Present Case | 69-year-old, female | Breast Cancer | T11-L1 | Healed |
3D, Three dimensional; TES, Total en-bloc spondylectomy; L, lumbar vertebra; T, Thoracic vertebra.
In reviewing the report by Cai et al. [15], we learned that Liu et al. conducted a prospective study on the reconstruction of spine structure stability, in which they applied 3D printed artificial vertebrae in 4 cases with primary thoracolumbar spine tumors after multiple consecutive segment vertebrectomy. The study revealed that the application of 3D printed personalized artificial vertebrae in the reconstruction of multi-segment thoracolumbar spine could reduce the risk of displacement, subsidence and internal fixation failure due to excessively long traditional titanium mesh cage, and at the same time could avoid extra trauma and complications caused by implanting long bones. In addition, they used pedicle screws to connect the implant to the posterior internal fixation system to further enhance the stability of the implant and provide the possibility of early motion of the patient, which was similar to our design, and achieved remarkable clinical effect. However, the study is ongoing and its detailed clinical efficacy needs to be further summarized during follow-up. Nevertheless, it also shows that this technique can be effectively used in spine reconstruction after TES of thoracolumbar spine tumors.
Furthermore, with the development of 3D printing technology, individualized 3D printed artificial vertebral bodies are also used for multi-segment spine reconstruction for cervical tumors, tuberculous spondylitis, and infectious spondylitis, all of which have achieved good results [16-21]. Our case and the cases reported in literature indicate that the individualized 3D printed artificial vertebrae can significantly shorten the operation time, reduce the intraoperative blood loss and reduce the risk of perioperative complications during spinal reconstruction surgery.
In summary, with the development of 3D printing technology, the use of individualized 3D printed artificial vertebral reconstruction after multi-segment thoracolumbar TES is an effective option. However, our research and past studies are limited to case reports with short-term follow-up, and lack of long-term clinical observation data for large sample cases. Therefore, the long-term efficacy of individualized 3D printed artificial vertebral body in reconstruction of multi-segment thoracolumbar spines needs further study. In addition, according to the research results in recent years, some scholars pointed out that in the future, the research of new 3D printed biomaterials and metal microporous implants is worth expecting [15]. It is believed that with the continuous deepening of these studies, the clinical application of individualized 3D printed artificial vertebral bodies in multi-segment thoracolumbar spine reconstruction will be further expanded.
Acknowledgements
We thank the patient and her family for allowing us to describe her case in detail. This work was supported by the National Natural Science Foundation of China (No.81802167), Natural Science Research Plan in Shaanxi Province of China (No.2019JM197), and Sanitation and Health Research Fund of Shaanxi Province (2018E001).
Disclosure of conflict of interest
None.
References
- 1.Sun C, Wang B, Li J, Shangguan J, Figini M, Zhou K, Pan L, Ma Q, Zhang Z. Quantitative measurement of breast carcinoma fibrosis for the prediction in the risk of bone metastasis. Am J Transl Res. 2018;10:1852–1859. [PMC free article] [PubMed] [Google Scholar]
- 2.Khotskaya YB, Goverdhan A, Shen J, Ponz-Sarvise M, Chang SS, Hsu MC, Wei Y, Xia W, Yu D, Hung MC. S6K1 promotes invasiveness of breast cancer cells in a model of metastasis of triple-negative breast cancer. Am J Transl Res. 2014;6:361–376. [PMC free article] [PubMed] [Google Scholar]
- 3.Yoshioka K, Murakami H, Demura S, Kato S, Kawahara N, Tomita K, Tsuchiya H. Clinical outcome of spinal reconstruction after total en bloc spondylectomy at 3 or more levels. Spine (Phila Pa 1976) 2013;38:E1511–E1516. doi: 10.1097/BRS.0b013e3182a6427a. [DOI] [PubMed] [Google Scholar]
- 4.Ji X, Wang S, Oner FC, Bird JE, Lu N. Surgical management of enneking stage 3 aggressive vertebral hemangiomas with neurological deficit by one-stage posterior total en bloc spondylectomy: a review of 23 cases. Spine (Phila Pa 1976) 2020;45:E67–E75. doi: 10.1097/BRS.0000000000003192. [DOI] [PubMed] [Google Scholar]
- 5.Luzzati AD, Shah S, Gagliano F, Perrucchini G, Scotto G, Alloisio M. Multilevel en bloc spondylectomy for tumors of the thoracic and lumbar spine is challenging but rewarding. Clin Orthop Relat Res. 2015;473:858–867. doi: 10.1007/s11999-014-3578-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chin BZ, Ji T, Tang X, Yang R, Guo W. Three-level lumbar en bloc spondylectomy with three-dimensional-printed vertebrae reconstruction for recurrent giant cell tumor. World Neurosurg. 2019;129:531–537. e1. doi: 10.1016/j.wneu.2019.06.056. [DOI] [PubMed] [Google Scholar]
- 7.Shah FA, Snis A, Matic A, Thomsen P, Palmquist A. 3D printed Ti6Al4V implant surface promotes bone maturation and retains a higher density of less aged osteocytes at the bone-implant interface. Acta Biomater. 2016;30:357–367. doi: 10.1016/j.actbio.2015.11.013. [DOI] [PubMed] [Google Scholar]
- 8.Yang J, Cai H, Lv J, Zhang K, Leng H, Sun C, Wang Z, Liu Z. In vivo study of a self-stabilizing artificial vertebral body fabricated by electron beam melting. Spine (Phila Pa 1976) 2014;39:E486–E492. doi: 10.1097/BRS.0000000000000211. [DOI] [PubMed] [Google Scholar]
- 9.Yang J, Cai H, Lv J, Zhang K, Leng H, Wang Z, Liu Z. Biomechanical and histological evaluation of roughened surface titanium screws fabricated by electron beam melting. PLoS One. 2014;9:e96179. doi: 10.1371/journal.pone.0096179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xue W, Krishna BV, Bandyopadhyay A, Bose S. Processing and biocompatibility evaluation of laser processed porous titanium. Acta Biomater. 2007;3:1007–1018. doi: 10.1016/j.actbio.2007.05.009. [DOI] [PubMed] [Google Scholar]
- 11.Palmquist A, Snis A, Emanuelsson L, Browne M, Thomsen P. Long-term biocompatibility and osseointegration of electron beam melted, free-form-fabricated solid and porous titanium alloy: experimental studies in sheep. J Biomater Appl. 2013;27:1003–1016. doi: 10.1177/0885328211431857. [DOI] [PubMed] [Google Scholar]
- 12.Murakami H, Kawahara N, Tomita K, Demura S, Kato S, Yoshioka K. Does interruption of the artery of Adamkiewicz during total en bloc spondylectomy affect neurologic function? Spine (Phila Pa 1976) 2010;35:E1187–E1192. doi: 10.1097/BRS.0b013e3181e215e5. [DOI] [PubMed] [Google Scholar]
- 13.Tomita K, Kawahara N, Baba H, Tsuchiya H, Nagata S, Toribatake Y. Total en bloc spondylectomy for solitary spinal metastases. Int Orthop. 1994;18:291–298. doi: 10.1007/BF00180229. [DOI] [PubMed] [Google Scholar]
- 14.Abe E, Sato K, Murai H, Tazawa H, Chiba M, Okuyama K. Total spondylectomy for solitary spinal metastasis of the thoracolumbar spine: a preliminary report. Tohoku J Exp Med. 2000;190:33–49. doi: 10.1620/tjem.190.33. [DOI] [PubMed] [Google Scholar]
- 15.Cai H, Liu Z, Wei F, Yu M, Xu N, Li Z. 3D printing in spine surgery. Adv Exp Med Biol. 2018;1093:345–359. doi: 10.1007/978-981-13-1396-7_27. [DOI] [PubMed] [Google Scholar]
- 16.He S, Yang X, Yang J, Ye C, Liu W, Wei H, Xiao J. Customized “whole-cervical-vertebral-body” reconstruction after modified subtotal spondylectomy of c2-c7 spinal tumor via piezoelectric surgery. Oper Neurosurg (Hagerstown) 2019;17:580–587. doi: 10.1093/ons/opz077. [DOI] [PubMed] [Google Scholar]
- 17.Li X, Wang Y, Zhao Y, Liu J, Xiao S, Mao K. Multilevel 3D printing implant for reconstructing cervical spine with metastatic papillary thyroid carcinoma. Spine (Phila Pa 1976) 2017;42:E1326–E1330. doi: 10.1097/BRS.0000000000002229. [DOI] [PubMed] [Google Scholar]
- 18.Xu N, Wei F, Liu X, Jiang L, Cai H, Li Z, Yu M, Wu F, Liu Z. Reconstruction of the upper cervical spine using a personalized 3D-printed vertebral body in an adolescent with ewing sarcoma. Spine (Phila Pa 1976) 2016;41:E50–E54. doi: 10.1097/BRS.0000000000001179. [DOI] [PubMed] [Google Scholar]
- 19.Mobbs RJ, Coughlan M, Thompson R, Sutterlin CE, Phan K. The utility of 3D printing for surgical planning and patient-specific implant design for complex spinal pathologies: case report. J Neurosurg Spine. 2017;26:513–518. doi: 10.3171/2016.9.SPINE16371. [DOI] [PubMed] [Google Scholar]
- 20.Zhang YW, Deng L, Zhang XX, Yu XL, Ai ZZ, Mei YX, He F, Yu H, Zhang L, Xiao X, Xiao Y, Chen X, Zhang SL, Ge HY, Dong XP. Three-dimensional printing-assisted cervical anterior bilateral pedicle screw fixation of artificial vertebral body for cervical tuberculosis. World Neurosurg. 2019;127:25–30. doi: 10.1016/j.wneu.2019.03.238. [DOI] [PubMed] [Google Scholar]
- 21.Chung KS, Shin DA, Kim KN, Ha Y, Yoon DH, Yi S. Vertebral reconstruction with customized 3-dimensional-printed spine implant replacing large vertebral defect with 3-year follow-up. World Neurosurg. 2019;126:90–95. doi: 10.1016/j.wneu.2019.02.020. [DOI] [PubMed] [Google Scholar]


