Abstract
Background: Radiotherapy has been widely used in the treatment of hepatocellular carcinoma (HCC). However, whether the patients should receive radiotherapy before or after surgical treatment has not been studied. The objective of the study was to compare the efficacy of the treatment in HCC patients who received pre-surgery and post-surgery radiotherapy. Methods: Data from the Surveillance, Epidemiology, and End Results (SEER) database were analyzed. Patients with surgery combined with radiotherapy were included into the study. The outcome measures were overall survival (OS) and cancer-specific survival (CSS). Propensity score matching (PSM) was used to reduce selection bias. Results: Before PSM, the median OS (mOS: 82 months) and median CSS (mCSS: NA) in the pre-surgery group were longer than in the post-surgery group (mOS: 21 months; mCSS: 20 months; P<0.001 for both). After PSM, the mOS and mCSS in the pre-surgery group were longer than in the post-surgery group (mOS: 45 vs. 26 months, P=0.011; mCSS: 60 vs. 26 months, P=0.003). The subgroup analysis documented that patients with single tumor, liver resection, and American Joint Committee on Cancer (AJCC) stage I and II had longer mOS and mCSS if they received pre-surgery rather than post-surgery radiotherapy (all P<0.05). Multivariate regression analysis showed patients with post-surgery radiotherapy had a higher risk of mortality than patients with post-surgery radiotherapy. Conclusion: HCC patients with single tumor, AJCC stage I and II, or with liver resection who received pre-surgery radiotherapy have better survival benefits than patients receiving post-surgery radiotherapy, particularly if internal radiotherapy was used.
Keywords: Hepatocellular carcinoma, surgery, radiotherapy, time, efficacy
Introduction
Liver cancer is one of the most common and deadly cancers worldwide [1]. Hepatocellular carcinoma (HCC) accounts for approximately 75%-85% of primary liver cancers [2]. The guideline recommends that patients with early HCC should receive transplantation or liver resection or radiofrequency ablation, while patients with intermediate or advanced HCC should receive transarterial chemoembolization (TACE) and molecular targeted drugs. However, transplantation is difficult to realize in most early HCC patients due to the strict selection criteria and an extremely limited supply of organs. Although liver resection and ablation are suitable choices for early HCC patients, treatments are associated with a high recurrence rate [3,4]. The recurrence is similarly high, and the tumor response rate is low in intermediate and advanced HCC patients treated with TACE and molecular targeted drugs [5,6]. Thus, palliative treatments, in particular, radiotherapy, are recommended for patients who have received standard therapy.
Radiotherapy comprises external and internal radiotherapy, and the use of both forms in HCC treatments has been expanding in recent years. HCC patients treated with a combination of radiotherapy and other treatments experience longer survival than patients subjected to a single therapy [7-15]. A large number of studies have demonstrated that patients treated with the combination of radiotherapy and surgery, including liver resection and transplantation, had survived longer than patients treated with surgery alone [11,16,17].
Radiotherapy conducted before the surgery might lower the tumor grade, which can increase the benefit of radical treatments, while radiotherapy performed after the surgery might destroy residual tumor cells that were not removed during the operation. However, currently, no available studies address addressing the question of whether HCC patients should receive radiotherapy before or after the surgery. Therefore, an analysis was performed utilizing the data from the Surveillance, Epidemiology, and End Results (SEER) database to compare the clinical outcomes of surgically treated HCC patients who received pre-surgery radiotherapy and post-surgery radiotherapy.
Materials and methods
Patient selection
The data used in the study were extracted from the SEER database which covered approximately 34.6% of the U.S population by using the SEER*stat software. The SEER database routinely collect data on patient demographics, primary tumor site, tumor morphology, stage at diagnosis, and first course of treatment, and they follow up with patients for vital status. The study was approved by the ethics committee of the hospital and the patient’s consent was waived by the committee because the study was conducted based on SEER database. But the data used in the study were approved by the SEER program (the reference ID: 12577-Nov2019). Patient diagnosed with HCC (International Classification of Diseases for Oncology, Third Edition (ICD-O-3), histology codes 8170/3-8175/3, site code C220.0), from 2004 to 2015 were included in the analysis. The inclusion criteria were: (1) age between 30 and 84 years; (2) patients receiving radiotherapy combined with surgery (patients with radiotherapy before and after surgery, and with unknown time of radiotherapy were excluded); (3) patients with a complete record of survival time (patients with survival code 0 were excluded); (4) patients with a complete record of surgical treatments (patients with surgery codes 0 and 99 were excluded) (Supplementary Figure 1). A total of 436 patients met these criteria and were included in the analysis. Additionally, 15121 patients with surgery alone were also included into analysis.
Statistical analysis
Continuous variables (age, year of diagnosis, and tumor size) were converted to categorical variables. Categorical variables between the two groups were compared by Chi-square and Fisher’s exact test. Patient survival was analyzed using the Kaplan-Meier method and compared by the log-rank test. The variables (except for tumor size) which might have affected the OS and CSS of patients were analyzed by the Cox proportional risk model and multivariate regression. In the subgroup analysis, the adjusted multivariate regression model was used to test whether post-surgery radiotherapy increased the risk of mortality and cancer-specific mortality. The adjusted multivariate regression analysis accounted for the variations in age, gender, diagnosis, size, race, marital status, and chemotherapy.
Age, gender, race, year of diagnosis, marital status, AJCC staging, tumor size, number of tumors, administration of chemotherapy, and the type and time of radiotherapy were used to perform PSM. The optimal caliper of the PSM model was set as 0.2. The 1:1 ratio matching generated 114 pairs of patients. After the PSM, the use of chemotherapy remained different between the two groups (P<0.001) (Table 1).
Table 1.
The baseline characteristics of patients Before PSM
| Characteristics | Before PSM | After PSM | ||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Before surgery (n=182, %) | After surgery (n=254, %) | P value | Before surgery (n=114, %) | After surgery (n=114, %) | P value | |
| Age (Years) | 0.001 | 0.815 | ||||
| 30-49 | 11 (6) | 20 (7.9) | 7 (6.1) | 6 (5.3) | ||
| 50-69 | 142 (78) | 155 (61) | 80 (70.2) | 77 (67.5) | ||
| 70-84 | 29 (16) | 79 (31.1) | 27 (23.7) | 31 (27.2) | ||
| Gender | 0.014 | 0.207 | ||||
| Male | 149 (81.9) | 182 (71.7) | 92 (80.7) | 84 (73.7) | ||
| Female | 33 (18.1) | 72 (28.3) | 22 (19.3) | 30 (26.3) | ||
| Race | 0.397 | 0.649 | ||||
| White | 131 (72) | 196 (77.2) | 80 (70.2) | 85 (74.6) | ||
| Black | 21 (11.5) | 27 (10.6) | 14 (12.3) | 14 (12.3) | ||
| Other | 30 (16.5) | 31 (12.2) | 20 (17.5) | 15 (13.1) | ||
| Year of diagnosis | <0.001 | 0.405 | ||||
| 2004-2007 | 23 (12.6) | 66 (26) | 19 (16.7) | 26 (22.8) | ||
| 2008-2011 | 38 (20.9) | 73 (28.7) | 28 (24.6) | 30 (26.3) | ||
| 2012-2015 | 121 (66.5) | 115 (45.3) | 67 (58.7) | 58 (50.9) | ||
| Marital status | 0.265 | 0.919 | ||||
| Married | 114 (62.6) | 145 (57.1) | 63 (55.3) | 60 (52.6) | ||
| Single | 61 (33.5) | 91 (35.8) | 45 (39.5) | 48 (42.1) | ||
| Other | 7 (3.9) | 18 (7.1) | 6 (5.2) | 6 (5.3) | ||
| AJCC staging | <0.001 | 0.710 | ||||
| I | 67 (36.8) | 79 (31.1) | 38 (33.3) | 45 (39.5) | ||
| II | 63 (34.6) | 54 (21.3) | 30 (26.3) | 33 (28.9) | ||
| III | 30 (16.5) | 67 (26.4) | 26 (22.8) | 21 (18.4) | ||
| IV | 12 (6.6) | 35 (13.7) | 12 (10.6) | 10 (8.8) | ||
| Unknown | 10 (5.5) | 19 (7.5) | 8 (7) | 5 (4.4) | ||
| Tumor size | 0.014 | 0.425 | ||||
| ≤3 cm | 56 (30.8) | 74 (29.1) | 32 (28.1) | 42 (36.8) | ||
| 3-5 cm | 55 (30.2) | 49 (19.3) | 25 (21.9) | 23 (20.2) | ||
| >5 cm | 62 (34.1) | 105 (41.3) | 49 (43) | 39 (34.2) | ||
| Unknown | 9 (4.9) | 26 (10.3) | 8 (7) | 10 (8.8) | ||
| Tumor number | 0.241 | 0.702 | ||||
| 1 | 145 (79.7) | 193 (76) | 88 (77.2) | 87 (76.3) | ||
| 2 | 34 (18.7) | 48 (18.9) | 24 (21.1) | 23 (20.2) | ||
| 3 | 3 (1.6) | 10 (3.9) | 2 (1.7) | 4 (3.5) | ||
| >3 | 0 (0) | 3 (1.2) | 0 (0) | 0 (0) | ||
| Chemotherapy | 0.965 | <0.001 | ||||
| Yes | 77 (42.3) | 108 (42.5) | 46 (40.4) | 13 (11.4) | ||
| No/Unknown | 105 (57.7) | 146 (57.5) | 68 (59.6) | 101 (88.6) | ||
| Surgery | <0.001 | 0.194 | ||||
| Transplantation | 85 (46.7) | 11 (4.3) | 19 (16.7) | 10 (8.8) | ||
| Liver resection | 45 (24.7) | 104 (40.9) | 43 (37.7) | 45 (39.5) | ||
| Non-liver resection | 52 (28.6) | 139 (54.8) | 52 (45.6) | 59 (51.7) | ||
| Radiotherapy | <0.001 | 0.244 | ||||
| Internal radiotherapy | 133 (73.1) | 104 (40.9) | 75 (65.8) | 60 (52.6) | ||
| External radiotherapy | 41 (22.5) | 136 (53.6) | 34 (29.8) | 48 (42.1) | ||
| Internal and external radiotherapy | 1 (0.5) | 3 (1.2) | 1 (0.9) | 1 (0.9) | ||
| Unknown | 7 (3.9) | 11 (4.3) | 4 (3.5) | 5 (4.4) | ||
All statistical analyses were conducted using SPSS 24.0 (IBM, Corp, NY, USA) and Stata 14.0 (StataCorp, College Station, TX, USA). P-value <0.05 was considered statistically significant.
Results
Patients
A total of 436 patients were included in the study. Among them, 182 received pre-surgery radiotherapy, and 254 received post-surgery radiotherapy. In the pre-surgery group, 130 patients had AJCC stage I and II, 145 had a single tumor, 45 received liver resection, 133 were given internal radiotherapy, and 41 were treated with external radiotherapy. In the post-surgery group, 133 patients had AJCC stage I and II, 193 had a single tumor, 104 patients received liver resection, 104 patients were given internal radiotherapy, and 136 were treated with external radiotherapy (Table 1). And the baseline characteristics of patients with surgery alone or surgery combined with radiotherapy were presented in Supplementary Table 1.
Survival outcomes
Firstly, we compared the efficacy of patients with surgery alone with patients received surgery combined with radiotherapy. The mOS and mCSS of patients with surgery alone (mOS: 53 months, 95% CI: 51.2-54.8; mCSS: 73 months, 95% CI: 68.7-77.3) were longer than patients with surgery combined radiotherapy (mOS: 31 months, 95% CI: 26.6-35.4; mCSS: 33 months, 95% CI: 26.4-39.6; both P<0.001). However, the mOS and mCSS of patients with pre-surgery radiotherapy (mOS: 82 months, 95% CI: 43.6-54.8; mCSS: not available) were longer than patients with surgery alone (mOS: 53 months, 95% CI: 51.2-54.8; mCSS: 73 months, 95% CI: 68.7-77.3; P=0.026 and P=0.050) (Supplementary Figure 2).
Then, we compared the efficacy of patients with pre-surgery radiotherapy with patients received post-surgery radiotherapy. Before PSM, compared to patients with post-surgery radiotherapy, those with pre-surgery radiotherapy had longer mOS (82 months, 95% CI: 18.3-23.7 vs. 21 months, 95% CI: 43.6-120.4; P<0.001) and mCSS (NA; vs. 20 months, 95% CI: 17.1-22.9; P<0.001) (Figure 1). The multivariate regression analysis documented that patients with post-surgery radiotherapy had higher all-cause mortality risk (HR=1.785, 95% CI: 1.295-2.461; P<0.001) and cancer-specific mortality risk (HR=1.928, 95% CI: 1.316-2.824; P=0.001) than patients with pre-surgery radiotherapy (Table 2).
Figure 1.
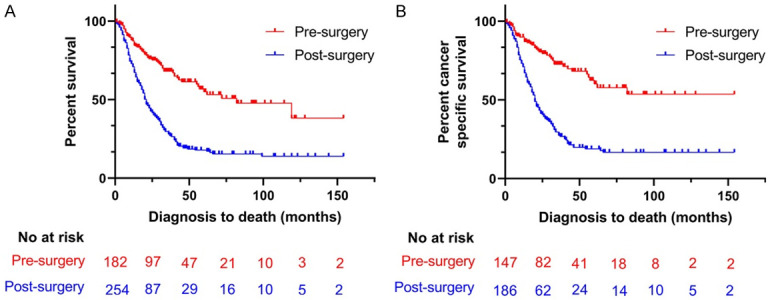
Kaplan-Meier curve of patients before PSM; A. Kaplan-Meier curve of overall survival (OS); B. Kaplan-Meier curve of cancer-specific survival (CSS).
Table 2.
Multivariate analysis for OS and CSS before PSM
| Characteristics | OS | CSS | ||
|---|---|---|---|---|
|
|
|
|||
| HR (95% CI) | P value | HR (95% CI) | P value | |
| Age (Years) | ||||
| 30-49 | Reference | Reference | ||
| 50-69 | 0.495 (0.288, 0.849) | 0.011 | 0.561 (0.302, 1.042) | 0.067 |
| 70-84 | 0.508 (0.285, 0.904) | 0.032 | 0.627 (0.273, 1.017) | 0.056 |
| Gender | ||||
| Male | Reference | Reference | ||
| Female | 0.647 (0.471, 0.889) | 0.007 | 0.672 (0.457, 0.989) | 0.044 |
| Race | ||||
| White | Reference | Reference | ||
| Black | 1.584 (1.068, 2.348) | 0.022 | 1.377 (0.824, 2.301) | 0.222 |
| Other | 0.712 (0.470, 1.079) | 0.109 | 0.654 (0.399, 1.074) | 0.093 |
| Year of diagnosis | ||||
| 2004-2007 | Reference | Reference | ||
| 2008-2011 | 0.728 (0.518, 1.023) | 0.067 | 0.679 (0.457, 1.009) | 0.055 |
| 2012-2015 | 0.744 (0.553, 1.086) | 0.138 | 0.624 (0.414, 0.939) | 0.024 |
| Marital status | ||||
| Married | Reference | Reference | ||
| Single | 0.849 (0.635, 1.134) | 0.268 | 0.880 (0.621, 1.248) | 0.474 |
| Other | 0.839 (0.635, 1.425) | 0.515 | 0.924 (0.516, 1.656) | 0.791 |
| AJCC staging | ||||
| I | Reference | Reference | ||
| II | 1.126 (0.777, 1.632) | 0.530 | 1.133 (0.726, 1.769) | 0.581 |
| III | 2.085 (1.445, 3.008) | <0.001 | 1.724 (1.113, 2.671) | 0.015 |
| IV | 5.370 (3.437, 8.390) | <0.001 | 5.046 (3.012, 8.455) | <0.001 |
| Unknown | 0.909 (0.522, 1.581) | 0.735 | 0.900 (0.448, 1.808) | 0.767 |
| Tumor number | ||||
| 1 | Reference | Reference | ||
| 2 | 1.209 (0.862, 1.377) | 0.271 | 0.786 (0.372, 1.659) | 0.527 |
| 3 | 0.882 (0.437, 1.778) | 0.725 | N | |
| >3 | 0.553 (0.129, 2.371) | 0.425 | N | |
| Chemotherapy | ||||
| Yes | Reference | Reference | ||
| No/Unknown | 1.054 (0.807, 1.377) | 0.699 | 1.024 (0.749, 1.401) | 0.880 |
| Surgery | ||||
| Transplantation | Reference | Reference | ||
| Liver resection | 2.613 (1.541, 4.431) | <0.001 | 3.589 (1.859, 6.928) | <0.001 |
| Non-liver resection | 3.711 (2.224, 6.192) | <0.001 | 5.447 (2.862, 10.367) | <0.001 |
| Radiotherapy | ||||
| Internal radiotherapy | Reference | Reference | ||
| External radiotherapy | 1.393 (1.046, 1.856) | 0.023 | 1.409 (1.000, 1.987) | 0.050 |
| Internal and external radiotherapy | 1.041 (0.318, 3.408) | 0.947 | 1.339 (0.393, 4.565) | 0.640 |
| Unknown | 0.691 (0.321, 1.487) | 0.345 | 0.600 (0.230, 1.565) | 0.297 |
| Time of radiotherapy | ||||
| Pre-surgery | Reference | Reference | ||
| Post-surgery | 1.785 (1.295, 2.461) | <0.001 | 1.928 (1.316, 2.824) | 0.001 |
After PSM, similar results were obtained. In comparison with patients in the post-surgery group, patients in the pre-surgery group had longer mOS (45 months, 95% CI: 27.1-62.9 vs. 26 months, 95% CI: 19-33; P=0.011) and mCSS (60 months, 95% CI: 39.2-80.8 vs. 26 months, 95% CI: 17.4-34.6; P=0.003) (Figure 2). After excluding potential confounding variables, multivariate regression analysis demonstrated that patients with post-surgery radiotherapy had higher all-cause mortality risk (HR=1.702, 95% CI: 1.154-2.510; P=0.007) and cancer-specific mortality (HR=2.131, 95% CI: 1.296-3.504; P=0.003) than patients with pre-surgery radiotherapy (Table 3).
Figure 2.
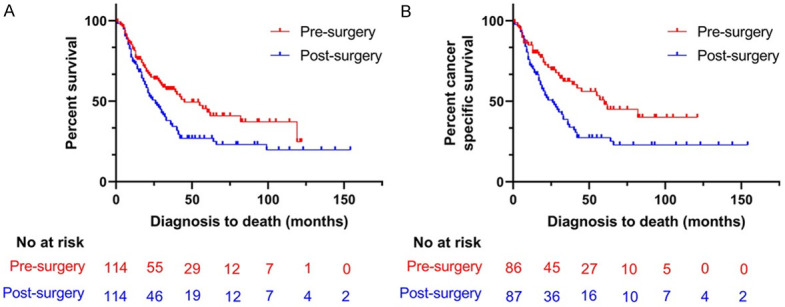
Kaplan-Meier curve of patients after PSM; A. Kaplan-Meier curve of OS; B. Kaplan-Meier curve of CSS.
Table 3.
Multivariate analysis for OS and CSS after PSM
| Characteristics | OS | CSS | ||
|---|---|---|---|---|
|
|
|
|||
| HR (95% CI) | P value | HR (95% CI) | P value | |
| Age (Years) | ||||
| 30-49 | Reference | Reference | ||
| 50-69 | 0.746 (0.300, 1.854) | 0.528 | 1.157 (0.377, 3.551) | 0.799 |
| 70-84 | 0.840 (0.323, 2.187) | 0.721 | 1.201 (0.375, 3.851) | 0.758 |
| Gender | ||||
| Male | Reference | Reference | ||
| Female | 0.558 (0.339, 0.919) | 0.022 | 0.597 (0.327, 1.088) | 0.092 |
| Race | ||||
| White | Reference | Reference | ||
| Black | 1.708 (0.999, 2.923) | 0.051 | 1.379 (0.667, 2.849) | 0.386 |
| Other | 0.570 (0.324, 1.005) | 0.052 | 0.510 (0.279, 0.933) | 0.029 |
| Year of diagnosis | ||||
| 2004-2007 | Reference | Reference | ||
| 2008-2011 | 0.744 (0.442, 1.255) | 0.268 | 0.585 (0.310, 1.102) | 0.097 |
| 2012-2015 | 0.763 (0.462, 1.259) | 0.290 | 0.510 (0.281, 1.069) | 0.029 |
| Marital status | ||||
| Married | Reference | Reference | ||
| Single | 0.818 (0.544, 1.229) | 0.333 | 0.853 (0.517, 1.408) | 0.535 |
| Other | 1.214 (0.545, 2.704) | 0.635 | 1.624 (0.695, 3.796) | 0.263 |
| AJCC staging | ||||
| I | Reference | Reference | ||
| II | 1.250 (0.768, 2.035) | 0.370 | 1.366 (0.762, 2.447) | 0.295 |
| III | 2.335 (1.402, 3.889) | 0.001 | 2.129 (1.158, 3.913) | 0.015 |
| IV | 5.475 (2.726, 10.994) | <0.001 | 5.582 (2.535, 12.291) | <0.001 |
| Unknown | 0.666 (0.268, 1.658) | 0.382 | 0.789 (0.225, 2.767) | 0.711 |
| Tumor number | ||||
| 1 | Reference | Reference | ||
| 2 | 1.282 (0.802, 2.050) | 0.299 | 1.185 (0.427, 3.291) | 0.745 |
| 3 | 0.389 (0.091, 1.658) | 0.202 | N | |
| Chemotherapy | ||||
| Yes | Reference | Reference | ||
| No/Unknown | 1.001 (0.631, 1.586) | 0.998 | 0.864 (0.496, 1.507) | 0.607 |
| Surgery | ||||
| Transplantation | Reference | Reference | ||
| Liver resection | 2.189 (1.056, 4.538) | 0.035 | 4.100 (1.567, 10.725) | 0.004 |
| Non-liver resection | 3.346 (1.635, 8.847) | 0.001 | 6.336 (2.440, 16.452) | <0.001 |
| Radiotherapy | ||||
| Internal radiotherapy | Reference | Reference | ||
| External radiotherapy | 1.706 (1.134, 2.566) | 0.010 | 1.828 (1.117, 2.990) | 0.016 |
| Internal and external radiotherapy | 0.925 (0.109, 7.824) | 0.943 | 1.388 (0.128, 15.037) | 0.787 |
| Unknown | 0.689 (0.202, 2.354) | 0.552 | 0.840 (0.233, 3.022) | 0.789 |
| Time of radiotherapy | ||||
| Pre-surgery | Reference | Reference | ||
| Post-surgery | 1.702 (1.154, 2.510) | 0.007 | 2.131 (1.296, 3.504) | 0.003 |
Subgroup analysis of outcomes
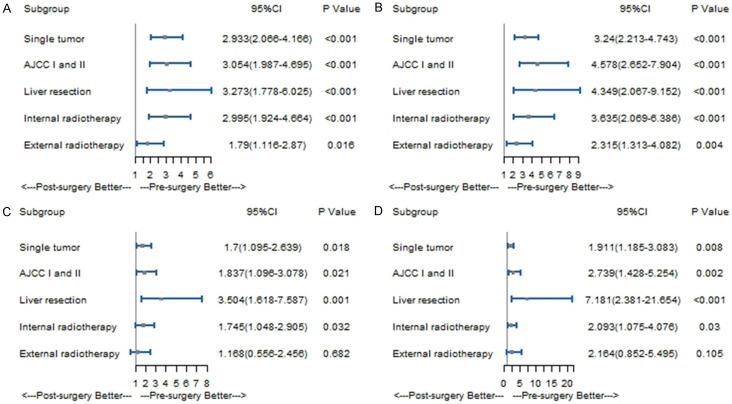
Before PSM, the results of the subgroups analysis showed that pre-surgery radiotherapy resulted in longer mOS and mCSS than post-surgery radiotherapy in patients with single tumor (mOS: 119 months, 95% CI: 14.4-223.6 vs. 20 months, 95% CI: 18-22; P<0.001; mCSS: NA vs. 20 months; 95% CI: 17.7-22.3; P<0.001), external radiotherapy (mOS: 40 months, 95% CI: 23.9-56.1 vs. 20 months, 95% CI: 17.2-22.8; P=0.002; mCSS: 42 months, 95% CI: 9-75 vs. 20 months, 95% CI: 16.1-23.9; P=0.002), internal radiotherapy (mOS: NA vs. 21 months, 95% CI: 17-25; P<0.001; mCSS: NA vs. 23 months, 95% CI: 17.1-28.9; P<0.001), AJCC stage I and II (mOS: NA vs. 29 months, 95% CI: 23.4-34.6; P<0.001; mCSS: NA vs. 27 months, 95% CI: 19.1-34.9; P<0.001), and liver resection (mOS: 62 months, 95% CI: NA vs. 21 months, 95% CI: 17.1-24.9; P<0.001; mCSS: NA vs. 21 months, 95% CI: 16.5-25.5; P<0.001) (Supplementary Figure 3). After adjustment for age, gender, year of diagnosis, tumor size, race, marital status and chemotherapy, post-surgery radiotherapy was associated with a higher all-cause mortality risk and cancer-specific mortality risk than pre-surgery radiotherapy in patients with a single tumor (all-cause mortality risk: HR=2.933, 95% CI: 2.066-4.166, P<0.001; cancer-specific mortality risk: HR=3.240, 95% CI: 2.213-4.743, P<0.001), external radiotherapy (all-cause mortality risk: HR=1.790, 95% CI: 1.116-2.870, P=0.016; cancer-specific mortality risk: HR=2.315, 95% CI: 1.313-4.081, P=0.004), internal radiotherapy (all-cause mortality risk: HR=2.995, 95% CI: 1.924-4.664, P<0.001; cancer-specific mortality risk: HR=3.635, 95% CI: 2.069-6.386, P<0.001), AJCC stage I and II (all-cause mortality risk: HR=3.054, 95% CI: 1.987-4.695, P<0.001; cancer-specific mortality risk: HR=4.578, 95% CI: 2.652-7.904, P<0.001), and liver resection (all-cause mortality risk: HR=3.273, 95% CI: 1.778-6.025, P<0.001; cancer-specific mortality risk: HR=4.349, 95% CI: 2.067-9.152; P<0.001) (Figure 3A, 3B).
Figure 3.
Forest plot of subgroup analysis; A. Predictors for OS of subgroup analysis before PSM; B. Predictors for CSS of subgroup analysis before PSM; C. Predictors for OS of subgroup analysis after PSM; D. Predictors for CSS of subgroup analysis after PSM.
After PSM, comparable results were obtained. Pre-surgery radiotherapy resulted in longer mOS and mCSS than post-surgery radiotherapy in patients with a single tumor (mOS: 57 months, 95% CI: 33.6-80.4 vs. 21 months, 95% CI: 13.3-28.7; P=0.017; mCSS: 60 months, 95% CI: 26.8-93.2 vs. 23 months, 95% CI: 14.6-31.4; P=0.008), internal radiotherapy (mOS: 55 months, 95% CI: 31.2-78.8 vs. 24 months, 95% CI: 15.8-32.2; P=0.010; mCSS: 82 months, 95% CI: 39.3-124.7 vs. 21 months, 95% CI: 10.7-31.8; P=0.007), AJCC stage I and II (mOS: 60 months, 95% CI: 15.7-104.3 vs. 31 months, 95% CI: 24.9-37.1; P=0.008; mCSS: 82 months, 95% CI: NA vs. 33 months, 95% CI: 25.2-40.8; P=0.004) and liver resection (mOS: NA vs. 22 months, 95% CI: 13.4-30.6; P<0.001; mCSS: NA vs. 21 months, 95% CI: 10.5-31.5; P<0.001) (Supplementary Figure 4). The results of the adjusted Cox proportional risk model showed that post-surgery radiotherapy was associated with higher all-cause mortality risk and cancer-specific mortality risk than pre-surgery radiotherapy in patients with single tumor (all-cause mortality risk: HR=1.700, 95% CI: 1.095-2.639, P=0.018; cancer-specific mortality risk: HR=1.911, 95% CI: 1.185=3.083, P=0.008), internal radiotherapy (all-cause mortality risk: HR=1.745, 95% CI: 1.048-2.905, P=0.032; cancer-specific mortality risk: HR=2.093, 95% CI: 1.075-4.076, P=0.030), AJCC stage I and II (all-cause mortality risk: HR=1.837; 95% CI: 1.096-3.078, P=0.021; cancer-specific mortality risk: HR=2.739, 95% CI: 1.428-5.254, P=0.002), and liver resection (all-cause mortality risk: HR=3.504, 95% CI: 1.618-7.587; P=0.001; cancer-specific mortality risk: HR=7.181, 95% CI: 2.381-21.654; P<0.001) (Figure 3C, 3D).
Discussion
Although several guidelines do not emphasize the use of radiotherapy in the treatment of HCC much due to the lack of high-quality evidence [1,18-20], some prospective and retrospective studies have indicated that patients with HCC can obtain survival benefits from radiotherapy [9,15,21]. These findings promoted a broader use of radiotherapy in the treatment of HCC patients, particularly in combination with other treatments. In this study, the results showed that patients with surgery alone had longer overall survival and cancer-specific survival than patients with surgery combined with radiotherapy. However, patients with pre-surgery radiotherapy had longer overall survival and cancer-specific survival than patients with surgery alone, which meant that HCC patients could get more survival benefits from pre-surgery radiotherapy than surgery alone. The efficacy of HCC patients with pre-surgery radiotherapy and post-surgery radiotherapy was still unclear yet. Thus, we compared the efficacy of patients received pre-surgery radiotherapy with patients received post-surgery radiotherapy based on prior results.
The major finding of the current retrospective study was that HCC patients who received radiotherapy before surgery experienced better survival benefits than those who received radiotherapy after surgery. However, the number of patients undergoing liver transplantation in the pre-surgery group was higher than in the post-surgery group, and, in the multivariate regression analysis, a liver transplant was a factor strongly favoring a longer OS and CSS of patients. To reduce selection bias, the PSM model was used. Although the factor of whether patients received chemotherapy was unbalanced between the two groups, the multivariable regression analysis showed that the factor of chemotherapy was not an independent predictor for OS and CSS of patients, which meant that chemotherapy did not influence the OS and CSS of all patients. After PSM, the only factor that remained unbalanced between the groups was the treatment with chemotherapy, and the multivariate regression analysis showed that this variable did not influence the OS and CCS of patients, and the OS and CSS of patients in the pre-surgery group were longer than in the post-surgery group. It might be because radiotherapy could lower the grade of patients with HCC, which could render the tumor more suitable for radical treatments, thus providing a good chance of complete removal. However, the patients that received post-surgery radiotherapy might be at a disadvantage due to the presence of residual tumor cells after surgery, potentially leading to recurrence. Moreover, the recurrence of the tumor after surgery would accelerate the death of patients. Previous studies have shown that patients with small or early HCC who were subjected to radiotherapy had survival benefits comparable to those of patients who received radiofrequency ablation or liver resection [22,23]. Therefore, the analysis included subgroups characterized by a single tumor, internal radiotherapy, external radiotherapy, AJCC stage I and II, and liver resection. The efficacy of pre-surgery and post-surgery radiotherapy in these patients with compared, and the results of the subgroup analysis showed that patients with a single tumor, internal radiotherapy, AJCC stage I and II, and liver resection had longer mOS and mCSS if they had received radiotherapy before rather than after the surgery. This conclusion was reached regardless of the application of PSM.
Several studies indicated that age, gender, race, AJCC staging, marital status, tumor size, number of tumors, administration of chemotherapy, and the methods of surgery might influence the survival of patients with HCC who received radiotherapy or surgery [24-30]. Therefore, this study included all factors which might influence OS and CSS of patients treated with a combination of surgery and radiotherapy, and performed multivariate regression analysis. Regardless of whether the PSM was performed, the results demonstrated that patients with post-surgery radiotherapy had a higher risk of all-cause mortality and cancer-specific mortality than patients with pre-surgery radiotherapy. In the subgroup analysis, to reduce collinearity, the factors of age, gender, year of diagnosis, tumor size, race, marital status, and treatment with chemotherapy were included in the adjusted Cox proportional risk model. This approach was used since the number of patients in the subgroups was small. This analysis confirmed that patients receiving post-surgery radiotherapy who had a single tumor, internal radiotherapy, AJCC stage I and II, and liver resection had a higher risk of all-cause mortality and cancer-specific mortality than patients subjected to radiotherapy. This finding was obtained both without and with PSM.
Some limitations are present in this investigation. First, it was designed as a retrospective study, which inevitably led to selection bias. However, the selection bias was reduced by PSM, which ensured a better balance in the baseline characteristics, making the two groups more comparable. Second, the liver function and physical condition of the patients were unknown since the SERR database does not provide this information. This deficiency might have influenced the results. Future retrospective or prospective studies should include these factors in the analysis to confirm the results of the current work. Third, the radiation dose received by the patients is unknown. The absence of these data might influence the interpretation of the results on the survival of patients because patients treated with high radiation dose could survive longer than those receiving a low dose of radiation. However, the study considered most of the factors that may affect the survival of patients and demonstrated that pre-surgery radiotherapy afforded better survival benefits than post-surgery radiotherapy. Prospective studies are necessary to unequivocally prove the validity of conclusions reached in the current analysis.
Conclusion
This study using the SEER database documented that the efficacy of HCC treatment is better in patients who received radiotherapy before surgery than in patients subjected to radiotherapy after surgery. Pre-surgery radiotherapy is most effective in patients with a single tumor, AJCC stage I and II, liver resection, and when internal radiotherapy is used.
Acknowledgements
This study was funded by the National Natural Science of foundation of China (No. 81873919, recipient: Chuansheng Zheng). We acknowledge that SEER database provided the data for this study.
Disclosure of conflict of interest
None.
Supporting Information
References
- 1.European Association for the Study of the Liver. Electronic address: easloffice@easloffice.eu; European Association for the Study of the Liver. EASL clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2018;69:182–236. doi: 10.1016/j.jhep.2018.03.019. [DOI] [PubMed] [Google Scholar]
- 2.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 3.Roayaie S, Obeidat K, Sposito C, Mariani L, Bhoori S, Pellegrinelli A, Labow D, Llovet JM, Schwartz M, Mazzaferro V. Resection of hepatocellular cancer ≤2 cm: results from two Western centers. Hepatology. 2013;57:1426–1435. doi: 10.1002/hep.25832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hwang S, Lee YJ, Kim KH, Ahn CS, Moon DB, Ha TY, Song GW, Jung DH, Lee SG. The impact of tumor size on long-term survival outcomes after resection of solitary hepatocellular carcinoma: single-institution experience with 2558 patients. J Gastrointest Surg. 2015;19:1281–1290. doi: 10.1007/s11605-015-2849-5. [DOI] [PubMed] [Google Scholar]
- 5.Cheng AL, Kang YK, Chen Z, Tsao CJ, Qin S, Kim JS, Luo R, Feng J, Ye S, Yang TS, Xu J, Sun Y, Liang H, Liu J, Wang J, Tak WY, Pan H, Burock K, Zou J, Voliotis D, Guan Z. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10:25–34. doi: 10.1016/S1470-2045(08)70285-7. [DOI] [PubMed] [Google Scholar]
- 6.Xiao EH, Guo D, Bian DJ. Effect of preoperative transcatheter arterial chemoembolization on angiogenesis of hepatocellular carcinoma cells. World J Gastroenterol. 2009;15:4582–4586. doi: 10.3748/wjg.15.4582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen L, Kan X, Sun T, Ren Y, Cao Y, Yan L, Liang B, Xiong B, Zheng C. Transarterial chemoembolization combined with iodine 125 seeds versus transarterial chemoembolization combined with radiofrequency ablation in the treatment of early- and intermediate-stage hepatocellular carcinoma. BMC Gastroenterol. 2020;20:205. doi: 10.1186/s12876-020-01355-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhao Y, Zhu X, Wang H, Dong D, Gao S, Zhu X, Wang W. Safety and efficacy of transcatheter arterial chemoembolization plus radiotherapy combined with sorafenib in hepatocellular carcinoma showing macrovascular invasion. Front Oncol. 2019;9:1065. doi: 10.3389/fonc.2019.01065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hardy-Abeloos C, Lazarev S, Ru M, Kim E, Fischman A, Moshier E, Rosenzweig K, Buckstein M. Safety and efficacy of liver stereotactic body radiation therapy for hepatocellular carcinoma after segmental transarterial radioembolization. Int J Radiat Oncol Biol Phys. 2019;105:968–976. doi: 10.1016/j.ijrobp.2019.09.006. [DOI] [PubMed] [Google Scholar]
- 10.Kim YJ, Jung J, Joo JH, Kim SY, Kim JH, Lim YS, Lee HC, Kim JH, Yoon SM. Combined transarterial chemoembolization and radiotherapy as a first-line treatment for hepatocellular carcinoma with macroscopic vascular invasion: necessity to subclassify Barcelona Clinic Liver Cancer stage C. Radiother Oncol. 2019;141:95–100. doi: 10.1016/j.radonc.2019.08.009. [DOI] [PubMed] [Google Scholar]
- 11.Wang L, Wang W, Rong W, Li Z, Wu F, Liu Y, Zheng Y, Zhang K, Siqin T, Liu M, Chen B, Wu J. Postoperative adjuvant treatment strategy for hepatocellular carcinoma with microvascular invasion: a non-randomized interventional clinical study. BMC Cancer. 2020;20:614. doi: 10.1186/s12885-020-07087-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sandroussi C, Dawson LA, Lee M, Guindi M, Fischer S, Ghanekar A, Cattral MS, McGilvray ID, Levy GA, Renner E, Greig PD, Grant DR. Radiotherapy as a bridge to liver transplantation for hepatocellular carcinoma. Transpl Int. 2010;23:299–306. doi: 10.1111/j.1432-2277.2009.00980.x. [DOI] [PubMed] [Google Scholar]
- 13.Katz AW, Chawla S, Qu Z, Kashyap R, Milano MT, Hezel AF. Stereotactic hypofractionated radiation therapy as a bridge to transplantation for hepatocellular carcinoma: clinical outcome and pathologic correlation. Int J Radiat Oncol Biol Phys. 2012;83:895–900. doi: 10.1016/j.ijrobp.2011.08.032. [DOI] [PubMed] [Google Scholar]
- 14.Li J, Xing J, Yang Y, Liu J, Wang W, Xia Y, Yan Z, Wang K, Wu D, Wu L, Wan X, Yang T, Gao C, Si A, Wang H, Wu M, Lau WY, Chen Z, Shen F. Adjuvant (131)I-metuximab for hepatocellular carcinoma after liver resection: a randomised, controlled, multicentre, open-label, phase 2 trial. Lancet Gastroenterol Hepatol. 2020;5:548–560. doi: 10.1016/S2468-1253(19)30422-4. [DOI] [PubMed] [Google Scholar]
- 15.Wei X, Jiang Y, Zhang X, Feng S, Zhou B, Ye X, Xing H, Xu Y, Shi J, Guo W, Zhou D, Zhang H, Sun H, Huang C, Lu C, Zheng Y, Meng Y, Huang B, Cong W, Lau WY, Cheng S. Neoadjuvant three-dimensional conformal radiotherapy for resectable hepatocellular carcinoma with portal vein tumor thrombus: a randomized, open-label, multicenter controlled study. J. Clin. Oncol. 2019;37:2141–2151. doi: 10.1200/JCO.18.02184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mourad M, Mabrut JY, Chellakhi M, Lesurtel M, Prevost C, Ducerf C, Rode A, Merle P, Mornex F, Mohkam K. Neoadjuvant conformal radiotherapy before liver transplantation for hepatocellular carcinoma: a propensity score matched analysis of postoperative morbidity and oncological results. Future Oncol. 2019;15:2517–2530. doi: 10.2217/fon-2019-0127. [DOI] [PubMed] [Google Scholar]
- 17.Uemura T, Kirichenko A, Bunker M, Vincent M, Machado L, Thai N. Stereotactic body radiation therapy: a new strategy for loco-regional treatment for hepatocellular carcinoma while awaiting liver transplantation. World J Surg. 2019;43:886–893. doi: 10.1007/s00268-018-4829-x. [DOI] [PubMed] [Google Scholar]
- 18.Burak KW, Sherman M. Hepatocellular carcinoma: consensus, controversies and future directions. A report from the canadian association for the study of the liver hepatocellular carcinoma meeting. Can J Gastroenterol Hepatol. 2015;29:178–184. doi: 10.1155/2015/824263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cheung TT, Kwok PC, Chan S, Cheung CC, Lee AS, Lee V, Cheng HC, Chia NH, Chong CCN, Lai TW, Law ALY, Luk MY, Tong CC, Yau TCC. Hong Kong consensus statements for the management of unresectable hepatocellular carcinoma. Liver Cancer. 2018;7:40–54. doi: 10.1159/000485984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heimbach JK, Kulik LM, Finn RS, Sirlin CB, Abecassis MM, Roberts LR, Zhu AX, Murad MH, Marrero JA. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology. 2018;67:358–380. doi: 10.1002/hep.29086. [DOI] [PubMed] [Google Scholar]
- 21.Sun J, Yang L, Shi J, Liu C, Zhang X, Chai Z, Lau WY, Meng Y, Cheng SQ. Postoperative adjuvant IMRT for patients with HCC and portal vein tumor thrombus: an open-label randomized controlled trial. Radiother Oncol. 2019;140:20–25. doi: 10.1016/j.radonc.2019.05.006. [DOI] [PubMed] [Google Scholar]
- 22.Hara K, Takeda A, Tsurugai Y, Saigusa Y, Sanuki N, Eriguchi T, Maeda S, Tanaka K, Numata K. Radiotherapy for hepatocellular carcinoma results in comparable survival to radiofrequency ablation: a propensity score analysis. Hepatology. 2019;69:2533–2545. doi: 10.1002/hep.30591. [DOI] [PubMed] [Google Scholar]
- 23.Sapisochin G, Barry A, Doherty M, Fischer S, Goldaracena N, Rosales R, Russo M, Beecroft R, Ghanekar A, Bhat M, Brierley J, Greig PD, Knox JJ, Dawson LA, Grant DR. Stereotactic body radiotherapy vs. TACE or RFA as a bridge to transplant in patients with hepatocellular carcinoma. An intention-to-treat analysis. J Hepatol. 2017;67:92–99. doi: 10.1016/j.jhep.2017.02.022. [DOI] [PubMed] [Google Scholar]
- 24.Zhang XP, Wang K, Gao YZ, Wei XB, Lu CD, Chai ZT, Zhen ZJ, Li J, Yang DH, Zhou D, Fan RF, Yan ML, Xia YJ, Liu B, Huang YQ, Zhang F, Hu YR, Zhong CQ, Lin JH, Fang KP, Cheng ZH, Wu MC, Lau WY, Cheng SQ. Prognostic model for identifying candidates for hepatectomy among patients with hepatocellular carcinoma and hepatic vein invasion. Br J Surg. 2020;107:865–877. doi: 10.1002/bjs.11524. [DOI] [PubMed] [Google Scholar]
- 25.Nathan H, Schulick RD, Choti MA, Pawlik TM. Predictors of survival after resection of early hepatocellular carcinoma. Ann Surg. 2009;249:799–805. doi: 10.1097/SLA.0b013e3181a38eb5. [DOI] [PubMed] [Google Scholar]
- 26.Olesinski J, Mithieux F, Guillaud O, Hilleret MN, Lombard-Bohas C, Henry L, Boillot O, Walter T, Partensky C, Paliard P, Valette PJ, Vuillez JP, Borson-Chazot F, Scoazec JY, Dumortier J. Survival and prognostic factors after adjuvant (131)iodine-labeled lipiodol for hepatocellular carcinoma: a retrospective analysis of 106 patients over 20 years. Ann Nucl Med. 2017;31:379–389. doi: 10.1007/s12149-017-1165-4. [DOI] [PubMed] [Google Scholar]
- 27.Zhang W, Wang X, Huang R, Jin K, Zhangyuan G, Yu W, Yin Y, Wang H, Xu Z, Sun B. Prognostic value of marital status on stage at diagnosis in hepatocellular carcinoma. Sci Rep. 2017;7:41695. doi: 10.1038/srep41695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang X, Chen M, Wei R, Wang Z. External radiation versus internal radiation for patients with advanced unresectable HCC -A SEER based study. J Cancer. 2019;10:1171–1180. doi: 10.7150/jca.28983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang L, Yan L, Niu H, Ma J, Yuan BY, Chen YH, Zhuang Y, Hu Y, Zeng ZC, Xiang ZL. A nomogram to predict prognosis of patients with unresected hepatocellular carcinoma undergoing radiotherapy: a population-based study. J Cancer. 2019;10:4564–4573. doi: 10.7150/jca.30365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mazzaferro V, Sposito C, Zhou J, Pinna AD, De Carlis L, Fan J, Cescon M, Di Sandro S, Yi-Feng H, Lauterio A, Bongini M, Cucchetti A. Metroticket 2.0 model for analysis of competing risks of death after liver transplantation for hepatocellular carcinoma. Gastroenterology. 2018;154:128–139. doi: 10.1053/j.gastro.2017.09.025. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.