Abstract
Organizing pneumonia (OP) is a sub-acute process of pulmonary tissue repair secondary to lung injury, defined histopathologically by intra-alveolar buds of granulation tissue within the lumen of distal pulmonary airspaces. It can be either cryptogenic or secondary (SOP) to different clinical conditions, namely infections. Despite being nonspecific, its diagnosis can be made by the association of clinical and imaging criteria. We report two cases of OP associated to SARS-CoV-2 pneumonia, admitted at a Portuguese tertiary hospital unit dedicated to COVID-19.
Both patients presented with severe respiratory failure with need of invasive mechanical ventilation. After initial recovery, there was worsening of dyspnea and hypoxemic respiratory failure with increase in inflammatory markers. Chest CT revealed an OP pattern. Other conditions such as superinfection, auto-immune disease and iatrogenic etiology, were excluded and corticotherapy at a dose of 1 mg/kg/day was administered. Chest CT follow up of both our patients showed complete resolution of OP pattern, with mild to moderate residual pulmonary fibrosis without honeycombing.
There is no OP to SARS-CoV-2 case series yet published describing the progress of patients after corticotherapy, although the association between systemic corticosteroids and lower all-cause mortality in patients with COVID-19 has been recently established. It is possible that, as has been described with other viruses, OP secondary to SARS-CoV-2 represents an immunological process after initial infection, presenting with elevation of inflammatory markers and cytokines storm in the bloodstream and lung tissue, which may explain the favorable response to corticosteroids.
Keywords: COVID-19, SARS-CoV-2, Coronavirus, Organizing pneumonia, Corticosteroids, Portugal
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; CRP, C-reactive protein; CT, computed tomography; LDH, lactate dehydrogenase; PCT, procalcitonin; RT-PCR, reverse transcription polymerase chain reaction
1. Introduction
Organizing pneumonia (OP) is a sub-acute process of pulmonary tissue repair secondary to lung injury. It is defined histopathologically by intra-alveolar buds of granulation tissue within the lumen of distal pulmonary airspaces. OP can be either cryptogenic or secondary to different clinical conditions, as infections, connective tissue diseases, malignancies, organ transplants, radiotherapy or certain drugs [1,2]. Despite being nonspecific, its diagnosis can be made by the association of imaging and clinical criteria [3], in particular evidence of multiple bilateral and peripheral alveolar opacities after initial improvement of fever and dyspnea [4]. The majority of patients respond to corticosteroids in addition to treatment for the underlying condition of secondary organizing pneumonia (SOP), often resulting in rapid clinical and radiological improvement. Relapses can nevertheless occur after treatment suspension [1,2].
Several case series of chest computed tomography (CT) [[5], [6], [7], [8]] and autopsy findings associating SARS-CoV-2 infection to OP have been published [9], one with histological confirmation with transbronchial biopsy [10]. However, unlike SOP associated to other coronavirus (CoV), as severe acute respiratory syndrome (SARS) and Middle East respiratory syndrome [4,11,12], and Influenza A and B [3], there is sparse information regarding the clinical course of SOP to SARS-CoV-2.
We report two cases of OP associated to SARS-CoV-2 pneumonia, admitted at a Portuguese tertiary hospital unit dedicated to COVID-19 patients in March 2020, and successfully treated with corticotherapy.
1.1. Case description 1
A 71-year-old man, with past medical history of arterial hypertension and type 2 diabetes mellitus, presented to the emergency department with fever (40 °C) for seven days. He had been diagnosed with COVID-19 two days earlier by RT-PCR testing of nasopharyngeal swab. On physical examination, the patient was febrile (39,2 °C), hypertensive (186/94 mmHg), tachycardic (107 bpm), with 96% peripheral oxygen saturation on room air and had bilateral rhonchi on lung auscultation.
Blood tests showed lymphopenia (970 lymphocytes/μL) and elevation of fibrinogen (6.78 g/L), CRP (124 mg/L), LDH (378 U/L) and AST (45 U/L); PCT and DD were at normal range. He also had respiratory and metabolic alkalemia on room air (pH 7.626, PaO2 146 mmHg, PaCO2 28.1 mmHg, HCO3− 32.6 mmol/L), with serum lactate of 2.0 mg/dL. Chest CT scan revealed multiple bilateral ground-glass opacities in all pulmonary lobes, with predominant peripheral and lower lobe distribution, with no pleural effusion or adenopathies, consistent with moderate to severe COVID-19 pneumonia. Considering the clinical symptoms and extent of pulmonary involvement on chest CT scan, the patient was admitted to COVID-19 dedicated Unit. Lopinavir/ritonavir (LOP/r) 400 g/50 mg bid and hydroxychloroquine (HCQ) 200 mg bid were started, according to institutional directives at that time, as well as prophylactic Enoxaparin.
In the first 48 hours after admission, the patient developed hypoxemic respiratory failure, with PaO2 of 60 mmHg under FiO2 of 60%. He was admitted to an Intensive Care Unit (ICU) for 17 days, with invasive mechanical ventilation for 15 days and two sessions of prone-position ventilation. Treatment with LOP/r and HCQ was maintained for a total of 10 days and the patient was medicated with ceftriaxone and azithromycin, although there was no laboratory confirmation of bacterial infection.
ICU discharge occurred at 22nd day of disease diagnosis, and the patient was readmitted at the COVID-19 dedicated Unit. He was afebrile, had no signs of respiratory distress and his blood oxygen saturation was 94% on room air. On the 30th day of disease, the patient developed dyspnea and hypoxemic respiratory failure with increase in inflammatory markers (Table 1). Chest CT scan revealed patchy linear-band opacities with curvilinear morphology and perilobular distribution, consistent with radiological pattern of OP, with >75% extension of lung parenchymal involvement (Fig. 1). A presumptive diagnosis of OP was made based on clinical and chest CT findings and prednisolone was started at a dose of 1 mg/kg/day. After 36 hours of treatment, the patient experienced significant clinical improvement, with apyrexia, dyspnea relief and progressive decrease of supplementary oxygen. Bacterial infection, autoimmune diseases and HIV infection were excluded. Nasopharyngeal swab for SARS-CoV-2 was negative and serology for SARS-CoV-2 revealed antibody response (positive IgG: 22.03 UA/mL, positive IgM: 1.03 UA/mL).
Table 1.
Comparison of clinical features and organizing pneumonia's progress between patient 1 and patient 2.
| Organizing Pneumonia | Patient 1 | Patient 2 |
|---|---|---|
| Time of presentation | 30th day of disease (8 days after ICU discharge) | 30th day of disease (10 days after ICU discharge) |
| Signs and symptoms | dyspnea and hypoxemia | worsening of hypoxemia |
| Inflammatory markers | ↑ | ↑ |
| Neutrophils (cell/μL) | 12900 | 13100 |
| Lymphocytes (cell/μL) | 1070 | 3100 |
| CRP (mg/L) | 217 | 287 |
| PCT (ng/mL) | 0,5 | 0,36 |
| Ferritin (ng/mL) | 1249 | 1574 |
| DD (mg/L) | 1,5 | 3,62 |
| Fibrinogen (g/L) | 6,3 | 6,76 |
| LDH (U/L) | 454 | 451 |
| AST (U/L) | 37 | 31 |
| ALT (U/L) | 37 | 35 |
| Treatment | prednisolone 1 mg/kg/day | methylprednisolone 1mg/kg/day |
| Ending of respiratory failure | 7 days | 6 days |
| Duration of corticotherapy | 15 weeks | 15 weeks |
| CT scan after treatment | resolution of OP pattern, mild to moderate residual pulmonary fibrosis | resolution of OP pattern, mild residual pulmonary fibrosis |
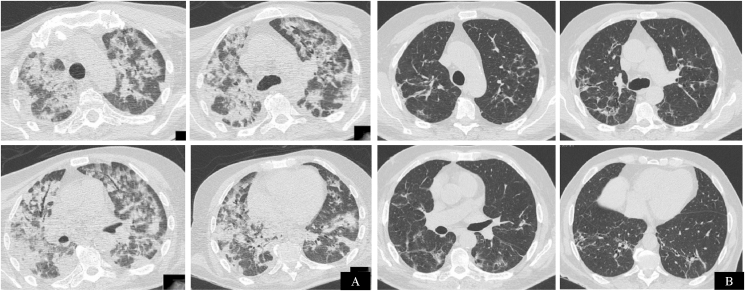
Fig. 1.
Chest CT axial images of COVID-19 pneumonia of patient 1. Diffuse patchy consolidations and ground-glass opacities in the sub-acute phase with band opacities with perilobular distribution (panel A). Chest CT performed in the last week of corticotherapy demonstrating radiological resolution of airspace disease with mild residual reticular opacities and traction bronchiectasis representing pulmonary fibrosis without honeycombing (panel B).
After 7 days of corticotherapy, the patient fully recovered from respiratory failure. He completed 16 days of full dose of corticosteroids that were tapered over 13 weeks. The patient initiated physical rehabilitation and was discharged on the 52nd day of disease. Chest CT performed in the last week of corticotherapy showed complete resolution of OP pattern, with mild to moderate residual pulmonary fibrosis.
1.2. Case description 2
An 83-year-old man was admitted with progressive dyspnea with 1-week duration. The patient denied fever, cough, orthopnea, nocturnal paroxysmal dyspnea or gastrointestinal symptoms. He had a history of arterial hypertension, type 2 diabetes mellitus and rectal cancer treated with abdominoperineal resection with permanent colostomy in 2006.
On admission at the emergency department, the patient was tachycardic (120 bpm) and hypertensive (156/77 mmHg), with 72% peripheral oxygen saturation on room air and diminished breath sound at the base of the right lung. Laboratory tests showed leukocytosis (18200/μL) with neutrophilia (16230/μL) and normal lymphocyte levels (1290/μL), and elevated inflammatory markers (CRP 354 mg/L, PCT 0.46 ng/mL, DD 3.5 mg/L, ferritin 1863 ng/mL, LDH 721 U/L, AST 67 U/L, ALT 86 U/L). Arterial blood analysis demonstrated a respiratory and metabolic alkalemia and a type I respiratory failure with 40% of FiO2 (pH 7.51, PaO2 60.9 mmHg, PaCO2 31.4 mmHg, SatO2 92.6%, HCO3− 26.9 mmol/L). Lactate was normal. Chest radiograph showed bilateral interstitial patchy airspace opacities with predominant lower lung zone distribution. The nasopharyngeal swab RT-PCR test was positive for SARS-CoV-2 infection and the patient was admitted to a COVID-19 dedicated Unit.
In the first 24 hours after admission, he developed severe acute respiratory distress syndrome (ARDS), with PaO2 of 50.4 mmHg under FiO2 of 60%, and was transferred to the ICU. He was medicated with LOP/r and HCQ (suspended 4 days later due to development of prolonged QT interval) and was discharged at the 20th day of disease, after 12 days of invasive mechanical ventilation. The patient was readmitted at our Unit on 2L/min of supplemental oxygen, but on 30th day of COVID-19 symptoms, he presented with elevated inflammatory markers (Table 1) and worsening of hypoxemia, which were not resolved by that time.
Chest CT scan revealed extensive areas of airspace disease with patchy ground-glass opacities and consolidations with peripheral and peribronchic distribution, predominantly in the mid and lower lung zones (Fig. 2). OP diagnosis was considered, and methylprednisolone was initiated at the dose of 1 mg/kg/day. Respiratory failure improved in the first 24 hours, but exacerbated again in the second day of corticotherapy, leading to readmission in the ICU on the 32nd day of disease. High flow nasal cannula oxygen (maximum of 30L/60% FiO2) and negative fluid balance strategy was initiated, maintaining the same dose of methylprednisolone. RT-PCR assay for SARS-CoV-2 was negative and serology for SARS-CoV-2 revealed antibody response (positive IgG: 20.45 UA/mL, negative IgM: 0.98 UA/mL). Blood, sputum and bronchial secretions’ cultures were negative for infectious agents and autoimmune diseases and HIV were excluded.
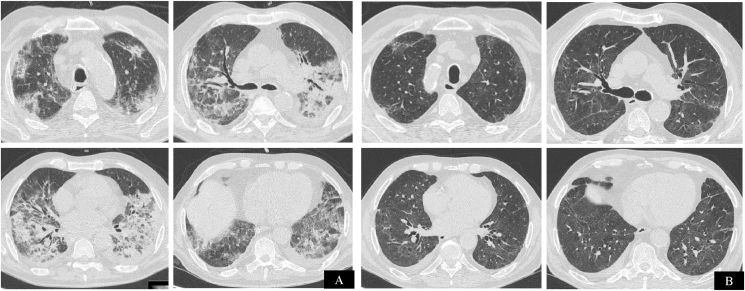
Fig. 2.
Chest CT axial images of COVID-19 pneumonia of patient 2. Patchy consolidations and ground-glass opacities with signs of organizing pneumonia in the sub-acute phase (panel A). Chest CT performed 1 month after corticosteroid suspension, demonstrating radiological resolution of airspace disease with mild residual pulmonary fibrosis without honeycombing (panel B).
The patient was discharged from ICU on the 36th day without supplemental oxygen. On the 43rd day of disease, subacute pulmonary thromboembolism was diagnosed on CT pulmonary angiography and treated with anticoagulation. Full dose of methylprednisolone was maintained for 20 days and tapered for 13 weeks. Chest CT performed 1 month after corticosteroid suspension showed resolution of pulmonary consolidations with mild residual patchy ground glass opacities, reticulation and traction bronchiectasis consistent with resolution of OP radiological pattern and mild residual pulmonary fibrosis.
2. Discussion
We describe two cases with SARS-CoV-2 infection associated with OP diagnosis based on clinical and imaging findings. The majority of OP secondary to coronaviruses’ cases are not confirmed histologically with transbronchial, percutaneous or open lung biopsies [4,10,12], although some cases are confirmed post-mortem [13]. OP secondary to viral infections are usually suspected when the patient is improving with specific treatment for pneumonia and clinical deterioration occurs, presenting with new fever, increasing inflammatory markers and worsening oxygen exchange and respiratory mechanics [14]. After excluding other conditions such as superinfection, auto-immune disease and iatrogenic etiology, the clinical hypothesis of OP can be supported with a characteristic chest CT radiological pattern that includes migratory lung parenchymal opacities, patchy consolidations and ground glass opacities with peripheral or peribronchial distribution and air bronchograms and ectatic bronchi, linear opacities with perilobular distribution, reversed halo sign and crazy paving [1]. Clinical confirmation is often presumed with a successful clinical and imaging treatment response to corticosteroids, as occurred with both our patients.
In COVID-19 pandemic, patients presently with acute respiratory symptoms and a characteristic radiological CT pattern with organizing pneumonia features are associated with high probability of SARS-CoV-2 infection. They may be used in association with RT-PCR tests to diagnose and triage patients for hospital admission and treatment [15].
There is no OP to SARS-CoV-2 case series published at current time, describing the progress of patients after corticotherapy, although the association between systemic corticosteroids and lower all-cause mortality in patients with COVID-19 has been established in the previous months by the Who REACT Group [16], RECOVERY Collaborative Group [17] and Chopra et al. [18].
OP secondary to other coronaviruses improves clinically and on imaging, but there is uncertainty about the treatment duration, time course of response and the optimal timing for corticosteroid tapering. Dosage of methylprednisolone used for OP diverges between 0,5 and 1,5 mg/kg/day and course of treatment varies from 2 months to one year, with no recurrence after corticoid suspension in most patients [1,[12], [13], [14],19].
It has been suggested that OP secondary to viruses’ infection develops after the replicative phase, since clinical presentation is subacute, occurring several weeks after the first symptoms [3] and often with undetectable viral particle in PCR assay. As with other viruses, SOP to SARS-CoV-2 may emerge as a result of an immunological process after initial infection, leading to elevation of inflammatory markers triggered by cytokines storm in the bloodstream and lung tissue, which may explain the favorable response to corticosteroids, in the absence of additional therapy such as antibiotics or therapeutic anticoagulation [13]. Different response timing may be attributable to pulmonary microthrombotic events or fluid overload [9]. In our two patients, IL-6 was not measured in the bloodstream during symptomatic disease, so we could not confirm an association with the extent of inflammation using this marker. SARS-CoV-2-IgG and IgM although detectable, cannot be used to extrapolate the magnitude of disease or response to treatment [20].
3. Conclusion
As noted previously, the use of immunomodulators in COVID-19 may be counterproductive as their use may delay eradication of the virus [21]. However, corticosteroids used during the inflammatory phase of COVID-19 improve prognosis and mortality [17].
It is not known if treatment of OP secondary to SARS-CoV-2 with corticosteroids, especially after ARDS and invasive mechanical ventilation, can prevent or decrease the development of residual pulmonary fibrosis. Large scale studies are needed to determine optimal indication, dosage and duration of corticotherapy in acute symptomatic SARS-CoV-2 infection. Clinical follow-up of these patients is required to better understand the disease course and long-term sequela.
We suspect that OP is much more common in SARS-CoV-2 despite the few reports until this day. Its timely diagnosis and treatment may lead to less ventilatory support, less redundant antibiotics and improving in overall survival.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Patient consent
Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
None.
References
- 1.Cordier J.F. Cryptogenic organising pneumonia. Eur. Respir. J. 2006;28(2):422–446. doi: 10.1183/09031936.06.00013505. [DOI] [PubMed] [Google Scholar]
- 2.Drakopanagiotakis F., Polychronopoulos V., Judson M.A. Organizing pneumonia. Am. J. Med. Sci. 2008;335(1):34–39. doi: 10.1097/MAJ.0b013e31815d829d. [DOI] [PubMed] [Google Scholar]
- 3.Asai N., Yokoi T., Nishiyama N. Secondary organizing pneumonia following viral pneumonia caused by severe influenza B: a case report and literature reviews. BMC Infect. Dis. 2017;17(1):572. doi: 10.1186/s12879-017-2677-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ajlan A.M., Ahyad R.A., Jamjoom L.G. Middle East respiratory syndrome coronavirus (MERS-CoV) infection: chest CT findings. Am. J. Roentgenol. 2014;203(4):782–787. doi: 10.2214/AJR.14.13021. [DOI] [PubMed] [Google Scholar]
- 5.Hani C., Trieu N.H., Saab I. COVID-19 pneumonia: a review of typical CT findings and differential diagnosis. Diagn Interv Imaging. 2020;101(5):263–268. doi: 10.1016/j.diii.2020.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kong W., Agarwal P.P. Chest imaging appearance of COVID-19 infection. Radiology: Cardiothoracic Imaging. 2020;2:1. doi: 10.1148/ryct.2020200028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yoon S.H., Lee K.H., Kim J.Y. Chest radiographic and ct findings of the 2019 novel coronavirus disease (Covid-19): analysis of nine patients treated in korea. Korean J. Radiol. 2020;21(4):498–504. doi: 10.3348/kjr.2020.0132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Werberich G.M., Marchiori E., Barreto M.M., Rodrigues R.S. Computed tomography findings in a Brazilian cohort of 48 patients with pneumonia due to coronavirus disease. Rev. Soc. Bras. Med. Trop. 2020;53 doi: 10.1590/0037-8682-0405-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ackermann M., Verleden S.E., Kuehnel M. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in covid-19. N. Engl. J. Med. 2020;383:120–128. doi: 10.1056/NEJMoa2015432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pogatchnik B.P., Swenson K.E., Sharifi H. Radiology-pathology correlation in recovered COVID-19, demonstrating organizing pneumonia. Am. J. Respir. Crit. Care Med. 2020;202(4):598–599. doi: 10.1164/rccm.202004-1278IM. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hwang D.M., Chamberlain D.W., Poutanen S.M. Pulmonary pathology of severe acute respiratory syndrome in Toronto. Mod. Pathol. 2005;18(1):1–10. doi: 10.1038/modpathol.3800247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim I., Lee J.E., Kim K.H. Successful treatment of suspected organizing pneumonia in a patient with Middle East respiratory syndrome coronavirus infection: a case report. J. Thorac. Dis. 2016;8(10):E1190–E1194. doi: 10.21037/jtd.2016.09.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ng W.F., To K.F., Lam W.W., Ng T.K., Lee K.C. The comparative pathology of severe acute respiratory syndrome and avian influenza A subtype H5N1—a review. Hum. Pathol. 2006;37(4):381–390. doi: 10.1016/j.humpath.2006.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cornejo R., Llanos O., Fernández C. Organizing pneumonia in patients with severe respiratory failure due to novel A (H1N1) influenza. BMJ Case Rep. 2010;2010 doi: 10.1136/bcr.02.2010.2708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Use of Chest Imaging in COVID-19: a Rapid Advice Guide. World Health Organization; Geneva: 2020. WHO/2019-nCoV/Clinical/Radiology_imaging/2020.1). Licence: CC BY-NC-SA 3.0 IGO. [Google Scholar]
- 16.The WHO rapid evidence appraisal for COVID-19 therapies (REACT) working Group. Association between administration of systemic corticosteroids and mortality among critically ill patients with COVID-19: a meta-analysis. JAMA. Published online September. 2020;2 doi: 10.1001/jama.2020.17023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.RECOVERY Collaborative Group. Horby P., Lim W.S. Dexamethasone in hospitalized patients with covid-19 - preliminary report [published online ahead of print, 2020 Jul 17] N. Engl. J. Med. 2020 doi: 10.1056/NEJMoa2021436. NEJMoa2021436. [DOI] [Google Scholar]
- 18.Chopra A., Chieng H.C., Austin A. Corticosteroid administration is associated with improved outcome in patients with severe acute respiratory syndrome coronavirus 2-related acute respiratory distress syndrome. Crit Care Explor. 2020 Jun;2(6) doi: 10.1097/CCE.0000000000000143. http://doi: 10.1097/CCE.0000000000000143 Published online 2020 Jun 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sethi G.R., Singhal K.K. Pulmonary diseases and corticosteroids. Indian J. Pediatr. 2008;75(10):1045–1056. doi: 10.1007/s12098-008-0209-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zainol Rashid Z., Othman S.N., Abdul Samat M.N., Ali U.K., Wong K.K. Diagnostic performance of COVID-19 serology assays. Malays. J. Pathol. 2020;42(1):13–21. [PubMed] [Google Scholar]
- 21.Yuen K.Y., Wong S.S. Human infection by avian influenza A H5N1. Hong Kong Med. J. 2005;11(3):189–199. [PubMed] [Google Scholar]