The gut microbiota plays a crucial role in the development of the immune system and confers benefits or disease susceptibility to the host. Emerging studies have indicated the gut microbiota could affect pulmonary health and disease through cross talk between the gut microbiota and the lungs. Gut microbiota dysbiosis could lead to acute or chronic lung disease, such as asthma, tuberculosis, and lung cancer. In addition, the composition of the gut microbiota may be associated with different lung diseases, the prevalence of which also varies by age.
KEYWORDS: gut microbiota, lung, lung disease, immunity, microbiome
ABSTRACT
The gut microbiota plays a crucial role in the development of the immune system and confers benefits or disease susceptibility to the host. Emerging studies have indicated the gut microbiota could affect pulmonary health and disease through cross talk between the gut microbiota and the lungs. Gut microbiota dysbiosis could lead to acute or chronic lung disease, such as asthma, tuberculosis, and lung cancer. In addition, the composition of the gut microbiota may be associated with different lung diseases, the prevalence of which also varies by age. Modulation of the gut microbiota through short-chain fatty acids, probiotics, and micronutrients may present potential therapeutic strategies to protect against lung diseases. In this review, we will provide an overview of the cross-talk between the gut microbiota and the lungs, as well as elucidate the underlying pathogenesis and/or potential therapeutic strategies of some lung diseases from the point of view of the gut microbiota.
INTRODUCTION
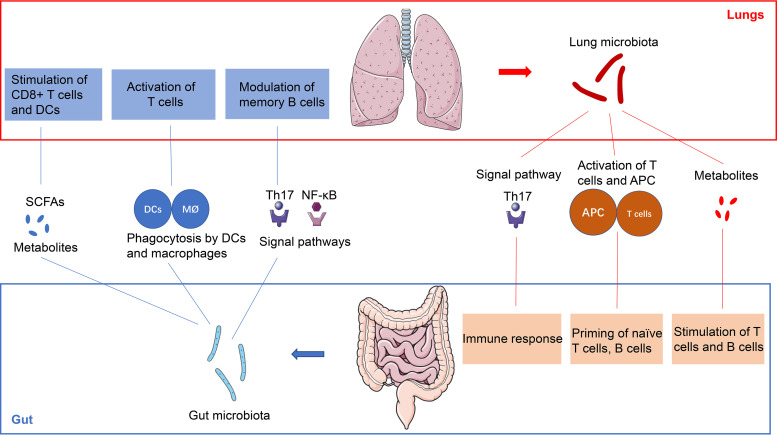
The gut microbiota, which is considered the “second genome” and “forgotten organ” of the human body, contains a metagenome that exceeds our own genome by 100 times and exerts a crucial role relevant for human health and disease (1). The composition and diversity of the gut microbiota are determined by the environment, genetics, and immunity (2). In addition, the homeostasis of the gut microbiota is essential for some physiologic functions, such as vitamin synthesis, maturation of the immune system, and pathogen infection prevention (3). Besides, emerging evidence has revealed that gut microbiota dysbiosis is related to some gastrointestinal diseases, including inflammatory bowel disease (IBD), cirrhosis, and colorectal cancer (CRC) (4). Interestingly, recent studies show that the gut microbiota is also an important moderator of immune responses, inflammation, and the development of lung disease, such as pneumonia, asthma, and lung cancer (5). The lungs are not sterile and are colonized by different communities of microbiota because of exposure to environmental stimuli, and the cross talk between the gut microbiota and lung microbiota may play an important role in some common lung diseases (6). Given this, an increasing number of studies are considering the potential mechanism of the gut microbiota in lung homeostasis and diseases, which led to the coining of the gut-lung axis concept (Fig. 1) and may potentially serve as a new direction for lung disease treatment (7). However, data on this topic are scarce, and the mechanisms by which the gut microbiota affects lung homeostasis and diseases are poorly understood and remain to be studied in detail. In this review, we provide an overview of the bidirectional effect of the gut and the lung and a recent understanding of the immunological interaction of the gut-lung axis, as well as some potential therapeutic strategies for manipulation of the gut microbiota in the treatment of lung diseases (Fig. 1; Fig. 2).
FIG 1.
The gut-lung axis. The cross talk between the gut microbiota and lungs can be mediated by the microbiota and its products as well as immune cells, and the gut-lung axis can be divided in two directions. For instance, gut segmented filamentous bacteria (SFBs), commensal gut microbiota constituents colonizing the ileum, can activate the Th17 signaling pathway, which modulates memory B cells in the lungs and the response to lung autoimmune manifestations and pulmonary fungal infections. In addition, metabolites from the gut microbiota, such as SCFAs, can stimulate and promote the differentiation of T cells, which supports anti-inflammatory and immunomodulatory actions in the lungs. In addition, the gut microbiota enters the intestinal mucosa and may be phagocytosed by antigen-presenting cells (APCs), such as DCs and macrophages. APCs transfer to the lungs, stimulate T cells, and induce the immune response in the lungs. On the other hand, the lung microbiota exhibits similar effects and influences the immune system and homeostasis of the gut through the gut-lung axis.
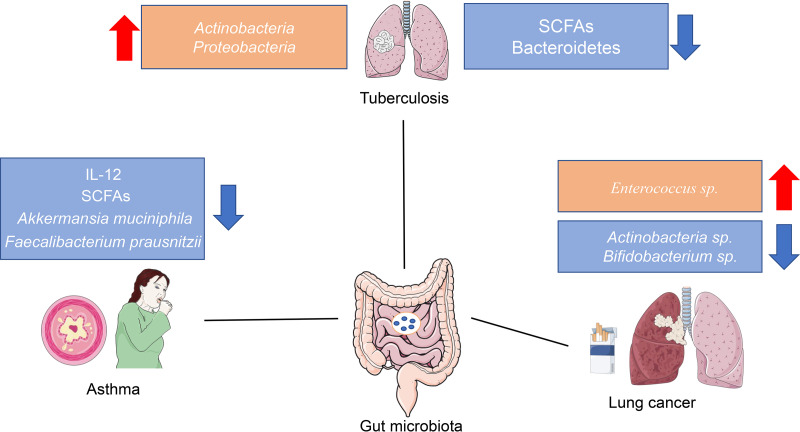
FIG 2.
The dysbiosis of the gut microbiota in some lung diseases. An increasing number of studies have suggested that the composition of the gut microbiota and its products change in different lung diseases. For example, obvious decreases in Akkermansia muciniphila, Faecalibacterium prausnitzii, and SCFA levels are observed in patients with asthma. Some studies found that the abundances of Actinobacteria spp. and Bifidobacterium spp. decreased in patients with lung cancer. However, the underlying mechanism and relationship between the gut microbiota and lung diseases are still unclear, and additional animal studies and clinical trials are required to understand the complex interactions of the gut microbiota and lung diseases.
GUT-LUNG AXIS
Recently, with the development of microbiological analysis, increasing evidence has indicated that the gut microbiota could provide essential benefits to host health by enhancing local defenses against enteral pathogens and regulating immune homeostasis (8). Changes in the constituents of the gut microbiota are related to altered immune responses and homeostasis in the respiratory system (9). An increasing number of epidemiological and experimental studies have highlighted an essential cross talk between the gut microbiota and the lungs, termed the “gut-lung axis,” though the underlying mechanisms and pathways are still unknown (6). This axis allows for the passage of gut microbiota metabolites, endotoxins, and cytokines into the bloodstream connecting the intestinal tissues with the lungs (10). Disturbances in the composition and function of the gut microbiota, termed dysbiosis, play a crucial role in alterations of immune responses and are associated with lung disorders and respiratory infections (11) (Fig. 2).
Cross talk from the gut to the lung.
Recent evidence has shown that local changes in the gut microbiota could influence immunity in distal tissues, especially the respiratory tract. For instance, clinical and experimental studies suggest that dysbiosis of gut microbiota plays a critical role in the pathogenesis of IBD (12). Some patients with IBD also present with respiratory tract changes, such as progressive airway involvement and pulmonary vasculature alterations, which indicate immunological cross talk between the gut and the lungs (13).
Emerging studies have shown that the gut microbiota plays a crucial role in the local immune system by modulating the neutrophil response and proinflammatory signals (14). In addition, fragments and metabolites of surviving gut microbiota can modulate the lung immune response by translocating across the intestinal barrier and the mesenteric lymphatic system, an essential pathway between the lungs and the gut (15). For example, gut microbiota-mediated production of various short-chain fatty acids (SCFAs), such as butyrate, exerts broad anti-inflammatory effects by modulating immune cell migration and suppressing the activation of NF-κB pathways (16). The increased levels of SCFAs enhance the generation of dendritic cell precursors, which protect against allergic inflammation in the lungs (17). And some studies have reported that SCFAs could stimulate Tregs to protect against airway inflammation by inhibiting histone deacetylases (HDACs) or activating acetate and propionate (18). Propionate could induce an enhanced generation of dendritic cell precursors and macrophages to modulate the allergic inflammatory response in lung disease via free fatty acid receptors (FFAR3) (19). In addition, epidemiological studies suggested that a diet rich in fiber could stimulate beneficial bacteria to generate SCFAs and modulate innate immunity and lung inflammation, which decreased the risk of chronic obstructive pulmonary disease (COPD) and were beneficial for lung health (20). Furthermore, an increasing amount of evidence has shown that butyrate, as an inducer of forkhead lineage-transcription factor (FoxP3), could attenuate lung inflammation by suppressing Th2 responses and inducing regulatory T cells (17). On the other hand, a recent study found a striking decrease in SCFA-producing bacteria in patients with active tuberculosis (TB), which may indicate the important role of SCFAs in the pathogenesis of tuberculosis (21).
In addition, some metabolites, such as lipoteichoic acid, peptidoglycan, and lipopolysaccharide, can initiate the Toll-like receptor (TLR) pathway and activate antigen-specific CD4 and CD8 T cells, as well as pathogen-specific antibodies that induce lung immune responses (22). Ichinohe et al. showed that a dose of lipopolysaccharide delivered to influenza-infected mice activated the immune response in the lungs (23), and Yang et al. supplemented lipopolysaccharide to mice, which induced E. coli pneumonia and indicated that the gut microbiota could enhance bacterial clearance during E. coli pneumonia through TLR4 (24). Furthermore, recent evidence suggests that gut segmented filamentous bacteria (SFBs) colonizing the ileum could activate CD4+ T cell polarization into the Th17 pathway and protect against pulmonary fungal infections, as well as induce autoimmune manifestations of lungs (25). On the other hand, gut lymphocytes can enter the systemic circulation and bind to some non-tissue-specific receptors in lung tissues during intestinal inflammation, such as inflammatory bowel disease (IBD), which might cause mishoming of these gut immune cells into the lung tissues (24). In that case, the inflammatory response in the intestinal tract can be mirrored in the lungs.
Cross talk from the lung to the gut.
With the development of culture-independent techniques of microbial identification, Hilty et al. have indicated the presence of a lung microbiota in humans (26). Recent studies suggested that the lung microbiota could promote the turnover of the lung immune system and inhibit an excessive immune response in acute infection of the lungs (27). Evolving evidence on the microbiota indicates that the gut-lung axis is bidirectional, implying the possibility of communication from the lung to the gut (17). Although the influence of the lung microbiota on intestinal immunity and the intestinal microbiota is poorly understood, some studies have demonstrated that lung inflammation could affect the intestinal microbiota and lead to some diseases, such as irritable bowel syndrome (IBS) (28).
Wang et al. found that the CCL25/CCR9 axis could induce the recruitment of lung-derived CD4+ T cells into the intestinal tract, which mediated the disturbance of the intestinal microbiota and caused damage to the intestinal immune system (29). In addition, a preclinical study indicated that influenza infection of the lung induced dysbiosis of the gut microbiota, which increased the proportion of Enterobacteriaceae as well as decreasing the abundances of lactococci and lactobacilli in the intestinal tract (30). Furthermore, some patients with chronic lung disorders, such as COPD, exhibit not only lung microbiota dysbiosis but also gut microbiota disturbance, leading to IBS (31). Vital et al. also indicated that the local pulmonary allergic response of asthma patients could affect the composition of the gut microbiota and cause intestinal immune injury (32).
According to traditional Chinese medicine, the lung and the intestine are a pair of related organ systems, named “biao-li” (32). Many studies have suggested that the trachea-lung tree and the gastrointestinal tract share an embryological origin of the primitive gut, which activates the maturity of some similar submucosal lymphoid tissues and plays an important role in both acquired and innate immunity. The disturbance of lung homeostasis could affect the homeostasis of the gut (33). For instance, some studies have shown that the inhalation of LPS into mouse lungs through the airway significantly increased the number of bacteria in the gut, and it has been shown that pneumonia could decrease gut epithelial proliferation and induce intestinal injury (34).
Overall, the gut and lung exhibit many common features, and the cross talk between the gut and lung is affected by internal and external relationships. Further studies are required to elucidate the mechanism of epithelium-mediated immune events in the lung and gut, as well as to support a better understanding of the cross talk among distant organs, which may help in exploring potential therapeutic strategies for mucosal inflammatory diseases in both the gut and lung.
ROLE OF THE GUT MICROBIOTA IN LUNG HOMEOSTASIS AT DIFFERENT AGES
An increasing number of studies have suggested that the composition of the gut microbiota varies by race, sex, and age (35). Emerging evidence indicates that people of different ages are prone to different lung diseases, which might be attributed to the composition of the gut microbiota, such as asthma in teenagers and COPD or lung cancer in elderly people (36). Understanding the effects of the gut microbiota on lung homeostasis at different ages may support potential therapeutic strategies for these complicated lung diseases.
Neonatal period.
The neonatal period is a critical developmental window of the lung immune system, and it may be modulated by genetic and environmental factors (37). Recent studies suggest that the gut microbiota plays an important role in the both the early development of lung immunity and the late establishment of a stable adult gut microbiota. Aberrations and alterations in the composition of the gut microbiota may lead to pediatric disorders and a series of diseases later in life (38).
The gut microbiota of neonates originates from the mother and is affected by the mode of delivery, medical factors, and feeding (39). Fredrik et al. suggested that, compared to that of infants delivered by Caesarean section, the gut microbiota of vaginally delivered infants showed significantly more resemblance to that of their mothers, and breastfeeding plays a critical role in the shaping and maturation of the gut microbiota during the first year of life (40). Prevotella and Lactobacillus spp. are more prevalent in infants born by vaginal delivery, while more Staphylococcus spp. are observed in those born by Caesarean section (41). In addition, a study showed the gut microbiota shaped the repertoires of immune cells and directed the postnatal ontogeny of type 3 innate lymphoid cells (ILC3s) in the lungs, which maintain homeostasis at mucosal barrier sites and promote resistance to pneumonia in newborns (42). In addition, Penders et al. suggested that Cesarean deliveries and abuse of antibiotics in early life might not only induce gut microbiota dysbiosis but also increase the risk of pneumonia and sepsis (43). A recent study showed that vancomycin exerted significant selective effects on the gut microbiota and altered the susceptibility of newborn mice to Th2- or Th1/Th17-driven lung inflammatory disease, indicating the importance of the gut microbiota to lung homeostasis (44). Furthermore, aberrant gastrointestinal colonization has been found in preterm infants with bronchopulmonary dysplasia (BPD). The anaerobic colonization of these preterm newborns has been delayed, and higher levels of Enterobacteriaceae, Enterococcus spp., and opportunistic pathogens are observed in their feces compared with those in the feces of term newborns (45).
Elderly period.
Many studies have suggested that the function of the lung immune system declines in elderly people, which increases the risk of infections and chronic inflammatory diseases and increases the mortality of elderly people (46). On the other hand, recent evidence has indicated that the structure of the gut microbiota also changes with age and may affect the immune response of the lungs (47). Marius et al. showed that a decrease in the Bacteroidetes-to-Firmicutes ratio and increasing abundances of taxa such as Ruminococcaceae, Lachnospiraceae, and Rikenellaceae occurred in old mice, which exaggerated the pulmonary inflammatory response and enhanced susceptibility to allergic airway disease (48). In addition, Chen et al. suggested that the abuse of antibiotics could disturb the composition of the gut microbiota community and increase pneumonia susceptibility in elderly people (49). Furthermore, Charlson et al. observed an increase in Proteobacteria, such as Eggerthella, Proteus, and Salmonella spp., in elderly patients with COPD compared to that in control subjects, and exposure to cigarette smoke induced dysbiosis of the gut microbiota and dysfunctional changes in the intestinal mucosal barrier, which aggravated the inflammatory responses of the lungs (50). Also, some studies have suggested that the composition of gut microbiota fluctuates with severity of COPD during an acute exacerbation of COPD or with the use of steroids (51).
In summary, the composition of the gut microbiota changes with different stages of life, which significantly influences the immune system and homeostasis of the lungs. However, the mechanisms by which the gut microbiota impacts lung homeostasis have not yet been fully identified, and additional animal studies and clinical trials are urgently required to elucidate the roles of the gut microbiota in the lungs at different ages, which may help us to understand the mechanism of some complicated lung diseases.
ROLE OF THE GUT MICROBIOTA IN LUNG DISEASES
Until now, studies of lung microbiota are poor and data on the gut-lung axis are mostly supported in only one direction: from the gut to the lung. Recent studies have suggested that alterations in the gut microbiota and metabolites are associated with changes in immune responses and inflammation of the lungs, which play an important role in lung diseases, such as asthma, pneumonia, and lung cancer (52). Some evidence indicated that dysbiosis of the gut microbiota might affect the incidence rate of lung disease, the response to drugs, and prognosis (42).
Asthma.
Asthma is the most common chronic lower respiratory tract condition in childhood, involving severe respiratory symptoms of shortness of breath, wheezing, and coughing (53), and the prevalence of asthma has increased to more than 300 million people worldwide and is anticipated to increase to 400 million by 2025 (54). The pathogenesis of asthma is still not well understood, and some studies have suggested it might be associated with hyperactivation of the T helper 2 (Th2) arm of adaptive immunity and genetic, infectious, and nutritional factors (55).
Currently, animal models and clinical trials have suggested that the gut microbiota plays a crucial role in the pathogenesis of asthma (56). Long-term stability of the gut microbiota begins at approximately 2 years of age and is affected by the mode of childbirth, breastfeeding, and antibiotic use (57). Studies suggest that one-year-old children suffer an increased risk of asthma with an immature gut microbiota composition (58). Stiemsma et al. observed dysbiosis of the gut microbiota in a population of children diagnosed with asthma, including an increase in Streptococcus pneumoniae and Haemophilus influenzae and a decrease in Veillonella, Faecalibacterium, and Rothia abundances (59). In addition, Faecalibacterium prausnitzii and Akkermansia muciniphila abundances decreased in the intestinal tract of the asthma group compared to those in the control group, where these relative abundances play a critical role in modulating secreted metabolites to suppress inflammation of the lung, such as increased interleukin 10 (IL-10) and decreased IL-12 levels (60). Furthermore, in addition to the altered composition of the gut microbiota, disturbances in metabolite levels have also been observed (43). Some evidence has shown that the bile acids generated by the gut microbiota might lead to Th2-type inflammation and mediate the development of asthma (61). A significant decrease in SCFAs (acetate, butyrate, and isoacids), which could generate an extrathymic Treg pool and thereby modulate the immune system and inhibit the development of asthma, was observed in the feces of asthma patients compared to those in the healthy group (17). On the other hand, deficiency of vitamin D in childhood may directly influence the abundance or diversity of the gut microbiota as well as antigen processing by dendritic cells, which may disrupt the mucosal barrier and promote the sensitization or abnormal tolerization of some allergens (62).
In conclusion, dysbiosis of the gut microbiota might result in chronic inflammatory respiratory disorders, such as asthma. These findings indicate an association of the gut microbiota with the host immune response and the development of asthma. However, the underlying mechanisms of how the gut microbiota modulates the lung immune response and inflammation remain complex and elusive. Further investigations are required to improve our understanding of the role of the gut microbiota in the pathogenesis of asthma. Improved understanding of these mechanisms raises the potential of therapies to improve or prevent asthma by targeting the gut microbiota.
Tuberculosis.
TB is a worldwide health concern that has been extensively studied both experimentally and clinically, and it has been reported that almost one-third of the world’s population has TB (63). The gut microbiota plays a crucial role in the development of host immunity and defense, and some studies observed alterations in the gut microbiota in TB patients and indicated that dysbiosis of the gut microbiota is essential for the pathogenesis of TB (64).
Recent studies have shown disturbances in bacterial species abundance and richness in the gastrointestinal tract of mice and patients with TB. Luo et al. found that Actinobacteria and Proteobacteria were significantly enriched, while the Bacteroidetes abundance was decreased in the intestinal tract of TB patients compared to those in healthy controls (65). In addition, the abundance of SCFA-producing bacteria was significantly decreased in TB patients, which led to the loss of SCFA producers and associated pathways and induced systemic inflammation and impairment of the lung immune system (66). On the other hand, the increase in Actinobacteria abundance was associated with enhanced T cell responses to vaccination in infants (67). Interestingly, Perry et al. showed that TB patients with Helicobacter pylori infection had more Th1-like cytokines and gamma interferon (IFN-γ) than TB patients without H. pylori and were less likely to develop active or drug-resistant TB, which indicates the bystander effects of H. pylori that modify the risk of TB and contribute to the control of TB infection (68). Furthermore, Liu et al. suggested that changes in the gut microbiota could be regarded as a biomarker to identify the difference between tuberculosis patients and healthy controls or the difference between new and recurrent tuberculosis patients (65).
However, only a few studies have demonstrated the interaction between the gut microbiota and TB, and the distinct relationship between them still needs to be determined. These studies indicate that dysbiosis of the gut microbiota may contribute to the pathogenesis of TB and the underlying beneficial role of the gut microbiota in therapeutic and immunization strategies for TB.
Lung cancer.
Lung cancer is one of the deadliest malignancies, with growing mortality as well as morbidity, and a leading cause of cancer-related death worldwide, posing a great threat to human health (69). Recently, an increasing number of studies have suggested the crucial role of the gut microbiota in various cancers, such as colorectal cancer (CRC), hepatocellular carcinoma (HCC), and lung cancer (70). The interactions between lung cancer and the gut microbiota attracted much attention in efforts to understand the complex characteristics of the gut microbiota and possible mechanisms of the gut microbiota in the prevention, carcinogenesis, and therapy of lung cancer.
A large number of studies have indicated that the composition of the gut microbiota in patients with lung cancer exhibited significant differences from that in healthy controls. Increased levels of Enterococcus spp. and decreased levels of Actinobacteria spp. and Bifidobacterium spp. are observed in the feces of patients with lung cancer (71). In addition, Zhuang et al. showed that normal controls had a significantly higher functional spectrum of their gut microbiota, while patients with lung cancer showed decreased levels of various metabolites and enhanced cancer susceptibility via multiple pathways (72). Furthermore, the normal tumor biomarkers for the early diagnosis, tumor staging, and metastasis of lung cancer include cytokeratin 19 fragment (CYFRA21-1), carcinoembryonic antigen (CEA), and neuron-specific enolase (NSE). Some studies have suggested that changes in the components and species of the gut microbiota may serve as a more convenient and efficient biomarker of the lung cancer system (73). On the other hand, dysbiosis of the gut microbiota is associated with the risk of immune-related diarrhea after treatment with anti-programmed cell death protein 1 (anti-PD-1) antibodies in lung cancer patients (74).
However, the causality between lung cancer and the gut microbiota is still underexplored, and further mechanistic insight into the interactions and pathways is expected, which might provide new insight into the pathogenesis of the lung cancer system. The gut microbiota may serve as a potential target for the prevention and treatment of lung cancer.
Other lung diseases.
In addition, emerging studies have shown the role of the gut microbiota in other lung diseases. For instance, some evidence indicated an intimate link between the gut microbiota and pathogenesis of cystic fibrosis in the lungs (75). The richness, abundance, and diversity of the gut microbiota significantly change in children with cystic fibrosis, such as the decreases in Firmicutes spp., Bifidobacterium adolescentis, and Eubacterium rectale and the increase in Streptococcus spp., Clostridium difficile, and Escherichia coli (76). In addition, dysbiosis of the gut microbiota and dysfunctional changes of the intestinal mucosal barrier were observed in patients with COPD, and a diet rich in fiber could decrease the risk of COPD by enhancing the activity of beneficial bacteria in the intestinal tract (77). Furthermore, Hanada et al. indicated that acute viral infection of the lung, such as influenza, may induce dysbiosis of the gut microbiota and induce the development of postviral bacterial pneumonia (78).
In conclusion, the vital cross talk between the gut microbiota and lung may contribute to the pathogenesis of lung diseases, and additional animal experiments and clinical trials are required to understand the underlying mechanisms of the gut microbiota in lung diseases.
POTENTIAL THERAPEUTIC STRATEGIES FOR LUNG DISEASES BY MODULATING THE GUT MICROBIOTA
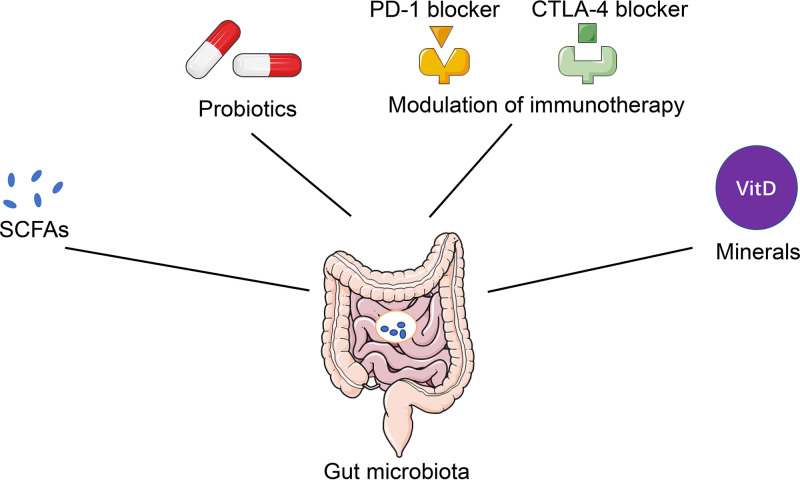
Because of the crucial role of the gut microbiota in lung health and diseases, whether modulation of the gut microbiota represents a potential therapeutic strategy for lung diseases has been investigated by an increasing number of clinical and experimental studies. Interventions, including SCFA, probiotics, and modulation of immunotherapy, have been attempted in subjects with lung diseases (Fig. 3).
FIG 3.
Potential therapeutic strategies for lung diseases by modulating the gut microbiota. Increasingly, clinical and experimental studies have validated some promising therapeutic strategies for common lung diseases, such as SCFAs, probiotics, minerals, and modulation of immunotherapy by targeting the gut microbiota, which regulate inflammation and the immune response in the lungs and may be regarded as potential therapeutic approaches for lung diseases in the future.
Probiotics.
The World Health Organization defined probiotics as “live microorganisms which, when administered in adequate amounts, confer a health benefit on the host,” including Lactobacillus, Saccharomyces, and Bifidobacterium spp., which lead to changes in the composition of the intestinal microbiota and improve microbial balance in the gut (79). Numerous studies have shown the immunomodulatory and anti-inflammatory effects of probiotics in lung diseases (80–82).
Recently, some evidence suggested that probiotics could decrease the number of pulmonary exacerbations and improve the quality of life in patients with cystic fibrosis. A randomized clinical trial indicated that Lactobacillus rhamnosus could restore the gut microbiota and reduce microbial richness and lung inflammation in children with cystic fibrosis (83). In addition, Khailova et al. found that the administration of L. rhamnosus modulated the inflammatory response and homeostasis of the lung by improving gut permeability in mouse models of Pseudomonas aeruginosa pneumonia (84). Furthermore, some probiotics have been confirmed to protect against tumor cells or elevate the efficacy of antitumor medicines. For instance, Zhu et al. indicated that Bifidobacterium infantis could increase the necrosis rate of lung cancer and prolong the survival time of C57BL/6 mice with lung cancer (85). Gui et al. suggested that Lactobacillus acidophilus could increase the antitumor effect of cisplatin and the survival rates of model mice (86). On the other hand, a randomized synbiotic trial suggested that one third of the lower respiratory tract infections in developing countries (India) could be effectively prevented using an oral synbiotic containing Lactobacillus plantarum (87). Therefore, the use of probiotics represents a novel approach for the prevention and treatment of lung diseases, and further animal experiments and clinical trials are required to elucidate the underlying mechanisms of probiotics to treat lung diseases.
Modulation of immunotherapy.
The recent clinical success of immune checkpoint inhibitors has become a turning point in the treatment of lung cancer (88). Immune checkpoint inhibitors (ICIs), including anti-PD-1/programmed cell death 1 ligand 1 (PD-L1) and anti-cytotoxic T lymphocyte-associated protein 4 (anti-CTLA-4) antibodies, have significantly expanded the horizons of therapeutic strategies in lung cancer (89).
Accumulating studies have suggested that the gut microbiota can affect the efficacy of immunotherapy on lung cancer (90). For instance, a recent clinical trial indicated that the level of gut microbiome diversity influenced the anti-PD-1 efficacy in advanced non-small-cell lung carcinoma (NSCLC) patients. An increase in memory T and NK cells was observed in the peripheral blood samples of NSCLC patients with high gut microbiota diversity, which may be attributed to the differentiation of Th1 lymphocytes and M1 macrophages, upregulation of PD-1 expression on lymphocytes, and activation of helper/cytotoxic T cells (91). In addition, some studies have shown that germfree animal models exhibit a reduction in T helper 17 (pTh17) cells and low efficacy of cyclophosphamide in patients with lung cancer, which may be attributed to the deficiency of Gram-positive bacteria and nonstimulation of the memory Th1 immune response (92). Furthermore, an increasing number of studies indicate that abuse of antibiotics could reduce the diversity of the gut microbiota, which significantly decreases the antitumor effects of ICIs and leads to a poor response to immunotherapy in mouse models of lung cancer (93). On the other hand, the immunostimulatory effects of CTLA-4 blockade by Bacteroides fragilis have been observed in NSCLC mice (94). These results indicate the critical role of the gut microbiota in immunotherapy of patients with lung cancer and highlight the potential of gut microbiota manipulation in immunotherapy of lung cancer.
Other potential therapeutic strategies.
In addition, vitamin D can maintain the homeostasis of the gut microbiota and reduce proinflammatory cytokine levels, which has been attempted as a therapeutic approach to modulate the gut microbiota in cystic fibrosis (95). Moreover, emerging studies have shown that a diet enriched with acidic oligosaccharides could enrich the species of the gut microbiota such as Sutterella wadsworthensis and Bifidobacterium species that are involved in the generation of butyrate and propionate, which limit the number and severity of pulmonary exacerbations after P. aeruginosa infection (96). A recent study of Chinese herbal medicines indicated that a pentaherbs formula could suppress a variety of immune effector cells and alleviate symptoms of allergic asthma by altering the composition of the gut microbiota and metabolites (97). On the other hand, fiber-enriched diet and exercise attribute to the releases of SCFAs (acetate, butyrate, and hexanoate) by gut microbiota and upregulation of anti-inflammatory cytokines and antioxidant enzymes, which benefits human health (98, 99).
In summary, modulation of the gut microbiota represents a promising therapeutic strategy for lung diseases, and additional clinical and experimental studies are required to understand the effects and mechanisms of these therapeutic approaches properly in the future.
Conclusion. Increasing evidence indicates an intimate relationship between the gut microbiota and lung health and disease. The gut microbiota plays a crucial role in the development of the lung immune system, and alterations in the composition of the gut microbiota may lead to immune responses and disease development in the lungs. Manipulation of the gut microbiota and metabolites may serve as potential strategies for lung diseases. However, it is not clear whether dysbiosis of the gut microbiota is a cause or a consequence of disease initiation or progression, and the underlying mechanisms and effects of the gut-lung axis are still poorly understood. With the development of culture-independent techniques and microbial science, we may understand the role of the gut microbiota in lung health and disease as well as prevent lung disease by modulating the gut microbiota.
ACKNOWLEDGMENTS
The study was supported by the National Natural Science Foundation of China (award no. 81972327).
All authors actively contributed to the conceptual development of this article.
We report no conflicts of interest.
REFERENCES
- 1.O'Hara AM, Shanahan F. 2006. The gut flora as a forgotten organ. EMBO Rep 7:688–693. doi: 10.1038/sj.embor.7400731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lynch SV, Pedersen O. 2016. The human intestinal microbiome in health and disease. N Engl J Med 375:2369–2379. doi: 10.1056/NEJMra1600266. [DOI] [PubMed] [Google Scholar]
- 3.Clemente JC, Ursell LK, Parfrey LW, Knight R. 2012. The impact of the gut microbiota on human health: an integrative view. Cell 148:1258–1270. doi: 10.1016/j.cell.2012.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fond G, Boukouaci W, Chevalier G, Regnault A, Eberl G, Hamdani N, Dickerson F, Macgregor A, Boyer L, Dargel A, Oliveira J, Tamouza R, Leboyer M. 2015. The “psychomicrobiotic”: targeting microbiota in major psychiatric disorders: a systematic review. Pathol Biol (Paris) 63:35–42. doi: 10.1016/j.patbio.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 5.Wang J, Li F, Tian Z. 2017. Role of microbiota on lung homeostasis and diseases. Sci China Life Sci 60:1407–1415. doi: 10.1007/s11427-017-9151-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Budden KF, Gellatly SL, Wood DL, Cooper MA, Morrison M, Hugenholtz P, Hansbro PM. 2017. Emerging pathogenic links between microbiota and the gut-lung axis. Nat Rev Microbiol 15:55–63. doi: 10.1038/nrmicro.2016.142. [DOI] [PubMed] [Google Scholar]
- 7.Bird L. 2012. Gut microbiota influences liver disease. Nat Rev Immunol 12:153. doi: 10.1038/nri3177. [DOI] [PubMed] [Google Scholar]
- 8.Marsland BJ, Trompette A, Gollwitzer ES. 2015. The gut-lung axis in respiratory disease. Ann Am Thorac Soc 12 Suppl 2:S150–S156. doi: 10.1513/AnnalsATS.201503-133AW. [DOI] [PubMed] [Google Scholar]
- 9.Voynow JA, Hendricks-Munoz KD. 2018. The gut-lung axis and pulmonary responses to ozone. Am J Respir Cell Mol Biol 59:281–282. doi: 10.1165/rcmb.2018-0140ED. [DOI] [PubMed] [Google Scholar]
- 10.Dang AT, Marsland BJ. 2019. Microbes, metabolites, and the gut-lung axis. Mucosal Immunol 12:843–850. doi: 10.1038/s41385-019-0160-6. [DOI] [PubMed] [Google Scholar]
- 11.Wedgwood S, Warford C, Agvatisiri SR, Thai PN, Chiamvimonvat N, Kalanetra KM, Lakshminrusimha S, Steinhorn RH, Mills DA, Underwood MA. 2020. The developing gut-lung axis: postnatal growth restriction, intestinal dysbiosis, and pulmonary hypertension in a rodent model. Pediatr Res 87:472–479. doi: 10.1038/s41390-019-0578-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zuo T, Ng SC. 2018. The gut microbiota in the pathogenesis and therapeutics of inflammatory bowel disease. Front Microbiol 9:2247. doi: 10.3389/fmicb.2018.02247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cozzi D, Moroni C, Addeo G, Danti G, Lanzetta MM, Cavigli E, Falchini M, Marra F, Piccolo CL, Brunese L, Miele V. 2018. Radiological patterns of lung involvement in inflammatory bowel disease. Gastroenterol Res Pract 2018:5697846. doi: 10.1155/2018/5697846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Enaud R, Prevel R, Ciarlo E, Beaufils F, Wieers G, Guery B, Delhaes L. 2020. The gut-lung axis in health and respiratory diseases: a place for inter-organ and inter-kingdom crosstalks. Front Cell Infect Microbiol 10:9. doi: 10.3389/fcimb.2020.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Piersigilli F, Van Grambezen B, Hocq C, Danhaive O. 2020. Nutrients and microbiota in lung diseases of prematurity: the placenta-gut-lung triangle. Nutrients 12:469. doi: 10.3390/nu12020469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Anand S, Mande SS. 2018. Diet, microbiota and gut-lung connection. Front Microbiol 9:2147. doi: 10.3389/fmicb.2018.02147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Trompette A, Gollwitzer ES, Yadava K, Sichelstiel AK, Sprenger N, Ngom-Bru C, Blanchard C, Junt T, Nicod LP, Harris NL, Marsland BJ. 2014. Gut microbiota metabolism of dietary fiber influences allergic airway disease and hematopoiesis. Nat Med 20:159–166. doi: 10.1038/nm.3444. [DOI] [PubMed] [Google Scholar]
- 18.Thorburn AN, McKenzie CI, Shen S, Stanley D, Macia L, Mason LJ, Roberts LK, Wong CH, Shim R, Robert R, Chevalier N, Tan JK, Mariño E, Moore RJ, Wong L, McConville MJ, Tull DL, Wood LG, Murphy VE, Mattes J, Gibson PG, Mackay CR. 2015. Evidence that asthma is a developmental origin disease influenced by maternal diet and bacterial metabolites. Nat Commun 6:7320. doi: 10.1038/ncomms8320. [DOI] [PubMed] [Google Scholar]
- 19.Galisteo M, Duarte J, Zarzuelo A. 2008. Effects of dietary fibers on disturbances clustered in the metabolic syndrome. J Nutr Biochem 19:71–84. doi: 10.1016/j.jnutbio.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 20.Macia L, Thorburn AN, Binge LC, Marino E, Rogers KE, Maslowski KM, Vieira AT, Kranich J, Mackay CR. 2012. Microbial influences on epithelial integrity and immune function as a basis for inflammatory diseases. Immunol Rev 245:164–176. doi: 10.1111/j.1600-065X.2011.01080.x. [DOI] [PubMed] [Google Scholar]
- 21.Saitou M, Nemoto D, Utano K, Suzuki T, Lefor AK, Togashi K, Niitsuma K. 2018. Identification of intestinal abnormalities in patients with active pulmonary tuberculosis using small bowel capsule endoscopy. Endosc Int Open 6:E1103–E1108. doi: 10.1055/a-0655-2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Round JL, Lee SM, Li J, Tran G, Jabri B, Chatila TA, Mazmanian SK. 2011. The Toll-like receptor 2 pathway establishes colonization by a commensal of the human microbiota. Science 332:974–977. doi: 10.1126/science.1206095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ichinohe T, Pang IK, Iwasaki A. 2010. Influenza virus activates inflammasomes via its intracellular M2 ion channel. Nat Immunol 11:404–410. doi: 10.1038/ni.1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tsay T-B, Yang M-C, Chen P-H, Hsu C-M, Chen L-W. 2011. Gut flora enhance bacterial clearance in lung through toll-like receptors 4. J Biomed Sci 18:68. doi: 10.1186/1423-0127-18-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McAleer JP, Nguyen NL, Chen K, Kumar P, Ricks DM, Binnie M, Armentrout RA, Pociask DA, Hein A, Yu A, Vikram A, Bibby K, Umesaki Y, Rivera A, Sheppard D, Ouyang W, Hooper LV, Kolls JK. 2016. Pulmonary Th17 antifungal immunity is regulated by the gut microbiome. J Immunol 197:97–107. doi: 10.4049/jimmunol.1502566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hilty M, Burke C, Pedro H, Cardenas P, Bush A, Bossley C, Davies J, Ervine A, Poulter L, Pachter L, Moffatt MF, Cookson WOC. 2010. Disordered microbial communities in asthmatic airways. PLoS One 5:e8578. doi: 10.1371/journal.pone.0008578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rooks MG, Garrett WS. 2016. Gut microbiota, metabolites and host immunity. Nat Rev Immunol 16:341–352. doi: 10.1038/nri.2016.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang J, Li F, Wei H, Lian ZX, Sun R, Tian Z. 2014. Respiratory influenza virus infection induces intestinal immune injury via microbiota-mediated Th17 cell-dependent inflammation. J Exp Med 211:2397–2410. doi: 10.1084/jem.20140625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang J, Tian Z. 2015. How lung infection leads to gut injury. Oncotarget 6:42394–42395. doi: 10.18632/oncotarget.6470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Looft T, Allen HK. 2012. Collateral effects of antibiotics on mammalian gut microbiomes. Gut Microbes 3:463–467. doi: 10.4161/gmic.21288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang H, Liu JS, Peng SH, Deng XY, Zhu DM, Javidiparsijani S, Wang GR, Li DQ, Li LX, Wang YC, Luo JM. 2013. Gut-lung crosstalk in pulmonary involvement with inflammatory bowel diseases. World J Gastroenterol 19:6794–6804. doi: 10.3748/wjg.v19.i40.6794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vital M, Harkema JR, Rizzo M, Tiedje J, Brandenberger C. 2015. Alterations of the murine gut microbiome with age and allergic airway disease. J Immunol Res 2015:892568. doi: 10.1155/2015/892568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ekbom A. 2004. The epidemiology of IBD: a lot of data but little knowledge. How shall we proceed? Inflamm Bowel Dis 10 Suppl 1:S32–S34. doi: 10.1097/00054725-200402001-00007. [DOI] [PubMed] [Google Scholar]
- 34.Coopersmith CM, Stromberg PE, Davis CG, Dunne WM, Amiot DM 2nd, Karl IE, Hotchkiss RS, Buchman TG. 2003. Sepsis from Pseudomonas aeruginosa pneumonia decreases intestinal proliferation and induces gut epithelial cell cycle arrest. Crit Care Med 31:1630–1637. doi: 10.1097/01.CCM.0000055385.29232.11. [DOI] [PubMed] [Google Scholar]
- 35.Wu Q, Turturice B, Wagner S, Huang Y, Gupta PK, Schott C, Metwally A, Ranjan R, Perkins D, Alegre ML, Finn P, Budinger GRS, Shilling R, Bharat A. 2019. Gut microbiota can impact chronic murine lung allograft rejection. Am J Respir Cell Mol Biol 60:131–134. doi: 10.1165/rcmb.2018-0139LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gentile CL, Weir TL. 2018. The gut microbiota at the intersection of diet and human health. Science 362:776–780. doi: 10.1126/science.aau5812. [DOI] [PubMed] [Google Scholar]
- 37.Dowling DJ, Levy O. 2014. Ontogeny of early life immunity. Trends Immunol 35:299–310. doi: 10.1016/j.it.2014.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lathrop SK, Bloom SM, Rao SM, Nutsch K, Lio CW, Santacruz N, Peterson DA, Stappenbeck TS, Hsieh CS. 2011. Peripheral education of the immune system by colonic commensal microbiota. Nature 478:250–254. doi: 10.1038/nature10434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Eggesbø M, Moen B, Peddada S, Baird D, Rugtveit J, Midtvedt T, Bushel PR, Sekelja M, Rudi K. 2011. Development of gut microbiota in infants not exposed to medical interventions. APMIS 119:17–35. doi: 10.1111/j.1600-0463.2010.02688.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bäckhed F, Roswall J, Peng Y, Feng Q, Jia H, Kovatcheva-Datchary P, Li Y, Xia Y, Xie H, Zhong H, Khan MT, Zhang J, Li J, Xiao L, Al-Aama J, Zhang D, Lee YS, Kotowska D, Colding C, Tremaroli V, Yin Y, Bergman S, Xu X, Madsen L, Kristiansen K, Dahlgren J, Wang J. 2015. Dynamics and stabilization of the human gut microbiome during the first year of life. Cell Host Microbe 17:852. doi: 10.1016/j.chom.2015.05.012. [DOI] [PubMed] [Google Scholar]
- 41.Furusawa Y, Obata Y, Fukuda S, Endo TA, Nakato G, Takahashi D, Nakanishi Y, Uetake C, Kato K, Kato T, Takahashi M, Fukuda NN, Murakami S, Miyauchi E, Hino S, Atarashi K, Onawa S, Fujimura Y, Lockett T, Clarke JM, Topping DL, Tomita M, Hori S, Ohara O, Morita T, Koseki H, Kikuchi J, Honda K, Hase K, Ohno H. 2013. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature 504:446–450. doi: 10.1038/nature12721. [DOI] [PubMed] [Google Scholar]
- 42.Dickson RP, Erb-Downward JR, Huffnagle GB. 2013. The role of the bacterial microbiome in lung disease. Expert Rev Respir Med 7:245–257. doi: 10.1586/ers.13.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Penders J, Thijs C, Vink C, Stelma FF, Snijders B, Kummeling I, van den Brandt PA, Stobberingh EE. 2006. Factors influencing the composition of the intestinal microbiota in early infancy. Pediatrics 118:511–521. doi: 10.1542/peds.2005-2824. [DOI] [PubMed] [Google Scholar]
- 44.Russell SL, Gold MJ, Reynolds LA, Willing BP, Dimitriu P, Thorson L, Redpath SA, Perona-Wright G, Blanchet MR, Mohn WW, Finlay BB, McNagny KM. 2015. Perinatal antibiotic-induced shifts in gut microbiota have differential effects on inflammatory lung diseases. J Allergy Clin Immunol 135:100–109. doi: 10.1016/j.jaci.2014.06.027. [DOI] [PubMed] [Google Scholar]
- 45.Hill CJ, Lynch DB, Murphy K, Ulaszewska M, Jeffery IB, O'Shea CA, Watkins C, Dempsey E, Mattivi F, Tuohy K, Ross RP, Ryan CA, O'Toole PW, Stanton C. 2017. Evolution of gut microbiota composition from birth to 24 weeks in the INFANTMET cohort. Microbiome 5:21. doi: 10.1186/s40168-016-0213-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Larbi A, Franceschi C, Mazzatti D, Solana R, Wikby A, Pawelec G. 2008. Aging of the immune system as a prognostic factor for human longevity. Physiology (Bethesda) 23:64–74. doi: 10.1152/physiol.00040.2007. [DOI] [PubMed] [Google Scholar]
- 47.Brandenberger C, Li N, Jackson-Humbles DN, Rockwell CE, Wagner JG, Harkema JR. 2014. Enhanced allergic airway disease in old mice is associated with a Th17 response. Clin Exp Allergy 44:1282–1292. doi: 10.1111/cea.12388. [DOI] [PubMed] [Google Scholar]
- 48.Hooper LV, Littman DR, Macpherson AJ. 2012. Interactions between the microbiota and the immune system. Science 336:1268–1273. doi: 10.1126/science.1223490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chen LW, Chen PH, Hsu CM. 2011. Commensal microflora contribute to host defense against Escherichia coli pneumonia through Toll-like receptors. Shock 36:67–75. doi: 10.1097/SHK.0b013e3182184ee7. [DOI] [PubMed] [Google Scholar]
- 50.Xin X, Dai W, Wu J, Fang L, Zhao M, Zhang P, Chen M. 2016. Mechanism of intestinal mucosal barrier dysfunction in a rat model of chronic obstructive pulmonary disease: an observational study. Exp Ther Med 12:1331–1336. doi: 10.3892/etm.2016.3493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mammen MJ, Sethi S. 2016. COPD and the microbiome. Respirology 21:590–599. doi: 10.1111/resp.12732. [DOI] [PubMed] [Google Scholar]
- 52.Gensollen T, Iyer SS, Kasper DL, Blumberg RS. 2016. How colonization by microbiota in early life shapes the immune system. Science 352:539–544. doi: 10.1126/science.aad9378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.von Mutius E. 2016. The microbial environment and its influence on asthma prevention in early life. J Allergy Clin Immunol 137:680–689. doi: 10.1016/j.jaci.2015.12.1301. [DOI] [PubMed] [Google Scholar]
- 54.Lange P, Parner J, Vestbo J, Schnohr P, Jensen G. 1998. A 15-year follow-up study of ventilatory function in adults with asthma. N Engl J Med 339:1194–1200. doi: 10.1056/NEJM199810223391703. [DOI] [PubMed] [Google Scholar]
- 55.Kang YB, Cai Y, Zhang H. 2017. Gut microbiota and allergy/asthma: from pathogenesis to new therapeutic strategies. Allergol Immunopathol (Madr) 45:305–309. doi: 10.1016/j.aller.2016.08.004. [DOI] [PubMed] [Google Scholar]
- 56.Prince BT, Mandel MJ, Nadeau K, Singh AM. 2015. Gut microbiome and the development of food allergy and allergic disease. Pediatr Clin North Am 62:1479–1492. doi: 10.1016/j.pcl.2015.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lai PS, Kolde R, Franzosa EA, Gaffin JM, Baxi SN, Sheehan WJ, Gold DR, Gevers D, Xavier RJ, Phipatanakul W. 2018. The classroom microbiome and asthma morbidity in children attending 3 inner-city schools. J Allergy Clin Immunol 141:2311–2313. doi: 10.1016/j.jaci.2018.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ardissone AN, de la Cruz DM, Davis-Richardson AG, Rechcigl KT, Li N, Drew JC, Murgas-Torrazza R, Sharma R, Hudak ML, Triplett EW, Neu J. 2014. Meconium microbiome analysis identifies bacteria correlated with premature birth. PLoS One 9:e90784. doi: 10.1371/journal.pone.0090784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Watson RL, de Koff EM, Bogaert D. 2019. Characterising the respiratory microbiome. Eur Respir J 53:1801711. doi: 10.1183/13993003.01711-2018. [DOI] [PubMed] [Google Scholar]
- 60.Demirci M, Tokman HB, Uysal HK, Demiryas S, Karakullukcu A, Saribas S, Cokugras H, Kocazeybek BS. 2019. Reduced Akkermansia muciniphila and Faecalibacterium prausnitzii levels in the gut microbiota of children with allergic asthma. Allergol Immunopathol (Madr) 47:365–371. doi: 10.1016/j.aller.2018.12.009. [DOI] [PubMed] [Google Scholar]
- 61.Belkaid Y, Hand TW. 2014. Role of the microbiota in immunity and inflammation. Cell 157:121–141. doi: 10.1016/j.cell.2014.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Litonjua AA, Weiss ST. 2007. Is vitamin D deficiency to blame for the asthma epidemic? J Allergy Clin Immunol 120:1031–1035. doi: 10.1016/j.jaci.2007.08.028. [DOI] [PubMed] [Google Scholar]
- 63.Khan N, Vidyarthi A, Nadeem S, Negi S, Nair G, Agrewala JN. 2016. Alteration in the gut microbiota provokes susceptibility to tuberculosis. Front Immunol 7:529. doi: 10.3389/fimmu.2016.00529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dubourg G, Lagier JC, Armougom F, Robert C, Hamad I, Brouqui P, Raoult D. 2013. The gut microbiota of a patient with resistant tuberculosis is more comprehensively studied by culturomics than by metagenomics. Eur J Clin Microbiol Infect Dis 32:637–645. doi: 10.1007/s10096-012-1787-3. [DOI] [PubMed] [Google Scholar]
- 65.Luo M, Liu Y, Wu P, Luo DX, Sun Q, Zheng H, Hu R, Pandol SJ, Li QF, Han YP, Zeng Y. 2017. Alternation of gut microbiota in patients with pulmonary tuberculosis. Front Physiol 8:822. doi: 10.3389/fphys.2017.00822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hu Y, Feng Y, Wu J, Liu F, Zhang Z, Hao Y, Liang S, Li B, Li J, Lv N, Xu Y, Zhu B, Sun Z. 2019. The gut microbiome signatures discriminate healthy from pulmonary tuberculosis patients. Front Cell Infect Microbiol 9:90. doi: 10.3389/fcimb.2019.00090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhang D, Li S, Wang N, Tan HY, Zhang Z, Feng Y. 2020. The cross-talk between gut microbiota and lungs in common lung diseases. Front Microbiol 11:301. doi: 10.3389/fmicb.2020.00301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Perry S, de Jong BC, Solnick JV, de la Luz Sanchez M, Yang S, Lin PL, Hansen LM, Talat N, Hill PC, Hussain R, Adegbola RA, Flynn J, Canfield D, Parsonnet J. 2010. Infection with Helicobacter pylori is associated with protection against tuberculosis. PLoS One 5:e8804. doi: 10.1371/journal.pone.0008804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Blandin Knight S, Crosbie PA, Balata H, Chudziak J, Hussell T, Dive C. 2017. Progress and prospects of early detection in lung cancer. Open Biol 7:170070. doi: 10.1098/rsob.170070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gori S, Inno A, Belluomini L, Bocus P, Bisoffi Z, Russo A, Arcaro G. 2019. Gut microbiota and cancer: how gut microbiota modulates activity, efficacy and toxicity of antitumoral therapy. Crit Rev Oncol Hematol 143:139–147. doi: 10.1016/j.critrevonc.2019.09.003. [DOI] [PubMed] [Google Scholar]
- 71.Carbone C, Piro G, Di Noia V, D'Argento E, Vita E, Ferrara MG, Pilotto S, Milella M, Cammarota G, Gasbarrini A, Tortora G, Bria E. 2019. Lung and gut microbiota as potential hidden driver of immunotherapy efficacy in lung cancer. Mediators Inflamm 2019:7652014. doi: 10.1155/2019/7652014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhuang H, Cheng L, Wang Y, Zhang YK, Zhao MF, Liang GD, Zhang MC, Li YG, Zhao JB, Gao YN, Zhou YJ, Liu SL. 2019. Dysbiosis of the gut microbiome in lung cancer. Front Cell Infect Microbiol 9:112. doi: 10.3389/fcimb.2019.00112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bai Y, Shen W, Zhu M, Zhang L, Wei Y, Tang H, Zhao J. 2019. Combined detection of estrogen and tumor markers is an important reference factor in the diagnosis and prognosis of lung cancer. J Cell Biochem 120:105–114. doi: 10.1002/jcb.27130. [DOI] [PubMed] [Google Scholar]
- 74.Liu T, Xiong Q, Li L, Hu Y. 2019. Intestinal microbiota predicts lung cancer patients at risk of immune-related diarrhea. Immunotherapy 11:385–396. doi: 10.2217/imt-2018-0144. [DOI] [PubMed] [Google Scholar]
- 75.Schippa S, Iebba V, Santangelo F, Gagliardi A, De Biase RV, Stamato A, Bertasi S, Lucarelli M, Conte MP, Quattrucci S. 2013. Cystic fibrosis transmembrane conductance regulator (CFTR) allelic variants relate to shifts in faecal microbiota of cystic fibrosis patients. PLoS One 8:e61176. doi: 10.1371/journal.pone.0061176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Burke DG, Fouhy F, Harrison MJ, Rea MC, Cotter PD, O’Sullivan O, Stanton C, Hill C, Shanahan F, Plant BJ, Ross RP. 2017. The altered gut microbiota in adults with cystic fibrosis. BMC Microbiol 17:58. doi: 10.1186/s12866-017-1006-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kanhere M, He J, Chassaing B, Ziegler TR, Alvarez JA, Ivie EA, Hao L, Hanfelt J, Gewirtz AT, Tangpricha V. 2018. Bolus weekly vitamin D3 supplementation impacts gut and airway microbiota in adults with cystic fibrosis: a double-blind, randomized, placebo-controlled clinical trial. J Clin Endocrinol Metab 103:564–574. doi: 10.1210/jc.2017-01983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hanada S, Pirzadeh M, Carver KY, Deng JC. 2018. Respiratory viral infection-induced microbiome alterations and secondary bacterial pneumonia. Front Immunol 9:2640. doi: 10.3389/fimmu.2018.02640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Williams NT. 2010. Probiotics. Am J Health Syst Pharm 67:449–458. doi: 10.2146/ajhp090168. [DOI] [PubMed] [Google Scholar]
- 80.Jafari SA, Mehdizadeh-Hakkak A, Kianifar HR, Hebrani P, Ahanchian H, Abbasnejad E. 2013. Effects of probiotics on quality of life in children with cystic fibrosis; a randomized controlled trial. Iran J Pediatr 23:669–674. [PMC free article] [PubMed] [Google Scholar]
- 81.Tsai YL, Lin TL, Chang CJ, Wu TR, Lai WF, Lu CC, Lai HC. 2019. Probiotics, prebiotics and amelioration of diseases. J Biomed Sci 26:3. doi: 10.1186/s12929-018-0493-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Forsythe P. 2011. Probiotics and lung diseases. Chest 139:901–908. doi: 10.1378/chest.10-1861. [DOI] [PubMed] [Google Scholar]
- 83.Bruzzese E, Callegari ML, Raia V, Viscovo S, Scotto R, Ferrari S, Morelli L, Buccigrossi V, Lo Vecchio A, Ruberto E, Guarino A. 2014. Disrupted intestinal microbiota and intestinal inflammation in children with cystic fibrosis and its restoration with Lactobacillus GG: a randomised clinical trial. PLoS One 9:e87796. doi: 10.1371/journal.pone.0087796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Khailova L, Baird CH, Rush AA, Barnes C, Wischmeyer PE. 2017. Lactobacillus rhamnosus GG treatment improves intestinal permeability and modulates inflammatory response and homeostasis of spleen and colon in experimental model of Pseudomonas aeruginosa pneumonia. Clin Nutr 36:1549–1557. doi: 10.1016/j.clnu.2016.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zhu H, Li Z, Mao S, Ma B, Zhou S, Deng L, Liu T, Cui D, Zhao Y, He J, Yi C, Huang Y. 2011. Antitumor effect of sFlt-1 gene therapy system mediated by Bifidobacterium Infantis on Lewis lung cancer in mice. Cancer Gene Ther 18:884–896. doi: 10.1038/cgt.2011.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Gui QF, Lu HF, Zhang CX, Xu ZR, Yang YH. 2015. Well-balanced commensal microbiota contributes to anti-cancer response in a lung cancer mouse model. Genet Mol Res 14:5642–5651. doi: 10.4238/2015.May.25.16. [DOI] [PubMed] [Google Scholar]
- 87.Panigrahi P, Parida S, Nanda NC, Satpathy R, Pradhan L, Chandel DS, Baccaglini L, Mohapatra A, Mohapatra SS, Misra PR, Chaudhry R, Chen HGH, Johnson JA, Morris JG, Paneth N, Gewolb IH. 2018. A randomized synbiotic trial to prevent sepsis among infants in rural India. Nature 553:238–238. doi: 10.1038/nature25006. [DOI] [PubMed] [Google Scholar]
- 88.Herbst RS, Baas P, Kim DW, Felip E, Pérez-Gracia JL, Han JY, Molina J, Kim JH, Arvis CD, Ahn MJ, Majem M, Fidler MJ, de Castro G Jr., Garrido M, Lubiniecki GM, Shentu Y, Im E, Dolled-Filhart M, Garon EB. 2016. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet 387:1540–1550. doi: 10.1016/S0140-6736(15)01281-7. [DOI] [PubMed] [Google Scholar]
- 89.Brahmer J, Reckamp KL, Baas P, Crinò L, Eberhardt WE, Poddubskaya E, Antonia S, Pluzanski A, Vokes EE, Holgado E, Waterhouse D, Ready N, Gainor J, Arén Frontera O, Havel L, Steins M, Garassino MC, Aerts JG, Domine M, Paz-Ares L, Reck M, Baudelet C, Harbison CT, Lestini B, Spigel DR. 2015. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med 373:123–135. doi: 10.1056/NEJMoa1504627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Shaikh FY, Gills JJ, Sears CL. 2019. Impact of the microbiome on checkpoint inhibitor treatment in patients with non-small cell lung cancer and melanoma. EBioMedicine 48:642–647. doi: 10.1016/j.ebiom.2019.08.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Jin Y, Dong H, Xia L, Yang Y, Zhu Y, Shen Y, Zheng H, Yao C, Wang Y, Lu S. 2019. The diversity of gut microbiome is associated with favorable responses to anti-programmed death 1 immunotherapy in Chinese patients with NSCLC. J Thorac Oncol 14:1378–1389. doi: 10.1016/j.jtho.2019.04.007. [DOI] [PubMed] [Google Scholar]
- 92.Viaud S, Saccheri F, Mignot G, Yamazaki T, Daillere R, Hannani D, Enot DP, Pfirschke C, Engblom C, Pittet MJ, Schlitzer A, Ginhoux F, Apetoh L, Chachaty E, Woerther PL, Eberl G, Berard M, Ecobichon C, Clermont D, Bizet C, Gaboriau-Routhiau V, Cerf-Bensussan N, Opolon P, Yessaad N, Vivier E, Ryffel B, Elson CO, Dore J, Kroemer G, Lepage P, Boneca IG, Ghiringhelli F, Zitvogel L. 2013. The intestinal microbiota modulates the anticancer immune effects of cyclophosphamide. Science 342:971–976. doi: 10.1126/science.1240537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Routy B, Le Chatelier E, Derosa L, Duong CPM, Alou MT, Daillère R, Fluckiger A, Messaoudene M, Rauber C, Roberti MP, Fidelle M, Flament C, Poirier-Colame V, Opolon P, Klein C, Iribarren K, Mondragón L, Jacquelot N, Qu B, Ferrere G, Clémenson C, Mezquita L, Masip JR, Naltet C, Brosseau S, Kaderbhai C, Richard C, Rizvi H, Levenez F, Galleron N, Quinquis B, Pons N, Ryffel B, Minard-Colin V, Gonin P, Soria JC, Deutsch E, Loriot Y, Ghiringhelli F, Zalcman G, Goldwasser F, Escudier B, Hellmann MD, Eggermont A, Raoult D, Albiges L, Kroemer G, Zitvogel L. 2018. Gut microbiome influences efficacy of PD-1-based immunotherapy against epithelial tumors. Science 359:91–97. doi: 10.1126/science.aan3706. [DOI] [PubMed] [Google Scholar]
- 94.Vétizou M, Pitt JM, Daillère R, Lepage P, Waldschmitt N, Flament C, Rusakiewicz S, Routy B, Roberti MP, Duong CP, Poirier-Colame V, Roux A, Becharef S, Formenti S, Golden E, Cording S, Eberl G, Schlitzer A, Ginhoux F, Mani S, Yamazaki T, Jacquelot N, Enot DP, Bérard M, Nigou J, Opolon P, Eggermont A, Woerther PL, Chachaty E, Chaput N, Robert C, Mateus C, Kroemer G, Raoult D, Boneca IG, Carbonnel F, Chamaillard M, Zitvogel L. 2015. Anticancer immunotherapy by CTLA-4 blockade relies on the gut microbiota. Science 350:1079–1084. doi: 10.1126/science.aad1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Vanstone MB, Egan ME, Zhang JH, Carpenter TO. 2015. Association between serum 25-hydroxyvitamin D level and pulmonary exacerbations in cystic fibrosis. Pediatr Pulmonol 50:441–446. doi: 10.1002/ppul.23161. [DOI] [PubMed] [Google Scholar]
- 96.Bernard H, Desseyn JL, Bartke N, Kleinjans L, Stahl B, Belzer C, Knol J, Gottrand F, Husson MO. 2015. Dietary pectin-derived acidic oligosaccharides improve the pulmonary bacterial clearance of Pseudomonas aeruginosa lung infection in mice by modulating intestinal microbiota and immunity. J Infect Dis 211:156–165. doi: 10.1093/infdis/jiu391. [DOI] [PubMed] [Google Scholar]
- 97.Tsang MS, Cheng SW, Zhu J, Atli K, Chan BC, Liu D, Chan HY, Sun X, Chu IM, Hon KL, Lam CW, Shaw PC, Leung PC, Wong CK. 2018. Anti-inflammatory activities of pentaherbs formula and its influence on gut microbiota in allergic asthma. Molecules 23:2776. doi: 10.3390/molecules23112776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Mailing LJ, Allen JM, Buford TW, Fields CJ, Woods JA. 2019. Exercise and the gut microbiome: a review of the evidence, potential mechanisms, and implications for human health. Exerc Sport Sci Rev 47:75–85. doi: 10.1249/JES.0000000000000183. [DOI] [PubMed] [Google Scholar]
- 99.Adak A, Khan MR. 2019. An insight into gut microbiota and its functionalities. Cell Mol Life Sci 76:473–493. doi: 10.1007/s00018-018-2943-4. [DOI] [PMC free article] [PubMed] [Google Scholar]