Abstract
Background:
Obesity and inflammatory bowel disease (IBD) represent chronic inflammatory conditions. Bariatric surgery improves some obesity-related co-morbidities, but the effects of bariatric surgery on IBD have not been well studied.
Objectives:
To examine if bariatric surgery may attenuate colitis in an obese murine model of IBD and study the mechanisms underlying the postsurgical amelioration of intestinal inflammation.
Setting:
University of California Irvine, Department of Surgery and Microbiology laboratories.
Methods:
Obese mice were assigned to one of 2 bariatric procedures [Duodenojejunal Bypass (DJB n = 6), Sleeve Gastrectomy (SG n = 8)]. Sham-operated mice were (Sham n = 8) were used as a control. After recovering from surgery, IBD was induced by administration of 2% dextran sodium sulfate. Fecal samples were collected before and after IBD induction for microbiome analysis. Pathologic analyses and immunohistochemical staining were performed on colon.
Results:
Survival after DJB and SG was higher relative to Sham mice. Histologically, DJB mice had significantly less intestinal inflammation. The observed improvements were not related to a difference in weight among the groups. Farnesoid X receptor staining in the colon was observed quantitatively more in DJB than in SG and sham mice. A statistically significant increase in the number of Lactobacillales was observed in the stool of mice after DJB.
Conclusion:
These results suggest that bariatric surgery, in particular DJB, reduces the severity of colitis in a chemically-induced IBD murine model. The anticolitis effects of DJB may be associated with Farnesoid X receptor regulation and gut microbiome rearrangements.
Keywords: Metabolic/Bariatric surgery, Obesity, Inflammation, Colitis, Obese murine model
Inflammatory Bowel Disease (IBD) is a chronic, idiopathic, relapsing immunologically-mediated inflammatory condition that manifests as 2 major phenotypes: Crohn’s disease and ulcerative colitis (UC). Both UC and Crohn’s disease have distinct pathologic and clinical characteristics but the mechanisms underlying their pathogenesis remain poorly understood. The onset and reactivation of IBD is likely triggered by environmental factors that transiently compromise the mucosal barrier, stimulate immune responses, and alter the balance between beneficial (e.g., Lactobacillales) and pathogenic (e.g., Enterobacteriaceae) enteric gut microbiota [1].
Obesity also represents a chronic inflammatory state, and a major risk factor for other disorders including diabetes and cardiovascular diseases. The chronic inflammation observed in both obesity and IBD is underscored by the expression of pro-inflammatory markers interleukin 6, interleukin 1, and tumor necrosis factor α. Furthermore, UC and obesity are exacerbated by each other [2]. Accordingly, studies have also shown that high-fat diet (HFD)-induced obesity increases the severity of IBD in a mouse model [2,3].
Bariatric surgery results in excess weight loss and improves glucose homeostasis in morbidly obese patients [4]. Interestingly, the metabolic effects of bariatric procedures on insulin resistance and glucose metabolism are independent of weight loss [4]. The precise mechanisms behind the metabolic changes following bariatric surgery remain unclear. Recently, a new finding showed that the bile acid-activated nuclear Farnesoid X receptor (FXR) plays a key role in the metabolic improvements observed after bariatric surgery [5]. Furthermore, multiple evidences suggested that activation of FXR has beneficial effects on IBD in both animal models and humans [6,7].
A clinical study by Aminian et al. showed that bariatric surgery is feasible and safe in morbidly obese patients suffering from IBD. The authors suggested that bariatric surgery may help mitigate IBD symptoms and improve disease control [8]. In addition, a small case series suggested that weight loss caused by bariatric surgery could be useful in the pharmacologic control of IBD [9]. In this study, we sought to address whether bariatric surgery itself may impart beneficial effects on obese patients with colitis, in a manner independent of weight loss. Hence, we performed bariatric surgical procedures on HFD-induced obese mice using a dextran sodium sulfate (DSS)-induced colitis model. We hypothesized that bariatric surgery should attenuate colitis in obese mice, in part, through a rearrangement in microbiome patterns and through alterations in FXR expression.
Methods
Animals and experimental groups
Obese male C57 BL/6 J mice (Charles River, Wilmington, MA) were fed a 60% high-fat diet (Harlan Laboratories, Madison, WI) for 8 weeks starting at 6 weeks of age. Animals were allowed free access to food and water, and were maintained under 12-hour light/dark cycles. Obese mice (weight range 34–38 g) were randomly assigned into 3 groups: Duodenojejunal Bypass (DJB, n = 6), Sleeve Gastrectomy (SG, n = 8), and sham–operated (Sham, n = 8). All animal experiments were approved by the Institutional Animal Care and Use Committee (IACUC) of University of California, Irvine.
Surgical procedures
Before performing surgery, all animals were fasted 4 to 6 hours with free access to water. Based on the anatomic intestinal rearrangement, DJB and SG represent proximal intestinal bypass and restrictive procedures, respectively.
In the DJB procedure (Fig. 1 A) the duodenum is cut open distal to the pylorus, and the distal limb of the duodenum is closed [10]. Afterward, the jejunum is transected 4 cm distal to the ligament of Treitz, and anastomosed with the proximal duodenum in an end-to-end fashion. The remaining duodenojejunal limb is anastomosed to the distal jejunum 4 cm distally to the site of duodenojejunal anastomosis by end-to-side fashion. This procedure results in a bypass of the proximal intestine without any restriction of gastric volume.
Fig. 1.
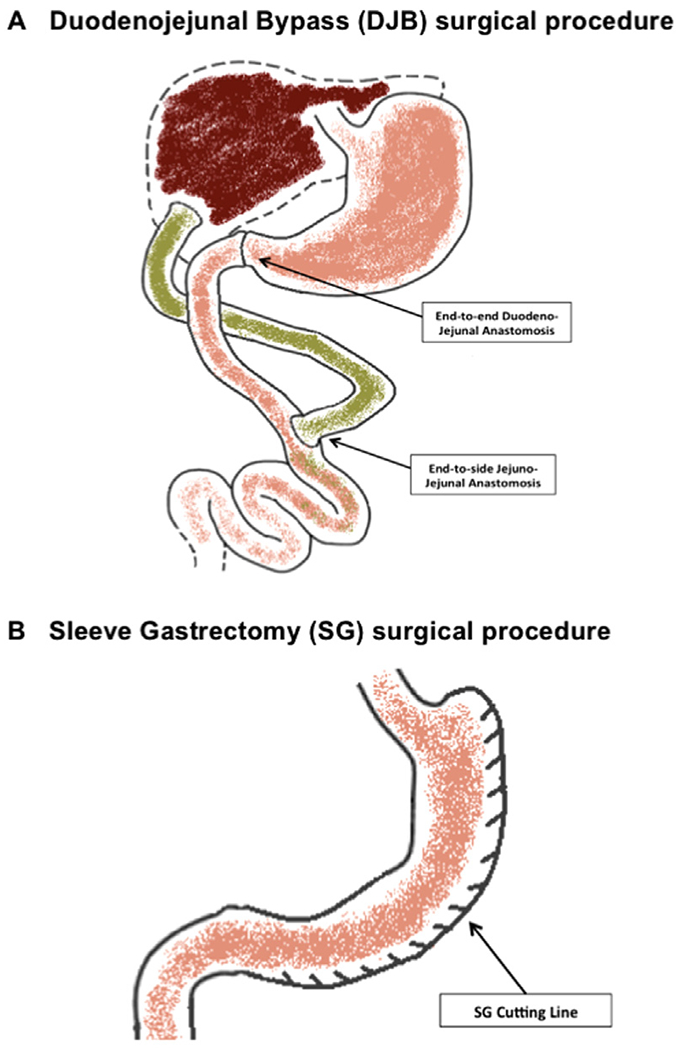
(A) The proximal duodenum is cut open and reconnected to the distal jejunal stump with an end-to-end anastomosis. The duodenojejunal limb is anastomosed to the jejunum 4 cm distally to the site of the previous anastomosis (end-to-side anastomosis). (B) Seventy percent of the stomach is divided removing the entire body and much of the gastric fundus.
Like its human counterpart, the sleeve gastrectomy procedure (Fig. 1 B) is an anatomically purely restrictive surgery. Briefly, the gastric fundus and greater curvature are freed, and 70%–80% of the stomach is divided and removed along the greater curvature over a curved hemostat. The resulting gastric sleeve is subsequently closed.
In the Sham operated mice, the stomach, duodenum and intestines are mobilized without further surgical intervention, and the mouse is kept under anesthesia for the same time period as the DJB and SG procedures (about 60 to 70 minutes).
During the operation of any procedure, about 8–10 mL of .9% saline solution were discontinuously injected on the surface of the intestine and into the mouse peritoneal cavity to maintain animal’s fluid homeostasis.
Postoperative management and IBD induction
After surgery, the animals were fasted for 16 to 18 hours with free access to water before resuming a high-fat diet. Postoperatively, the mice received 1 mL of .9% saline solution with analgesic by subcutaneous injection during the first 3 days. Animals were maintained on a high-fat diet for the remainder of the study. Our previous study showed that both DJB and SG procedures could significantly induce weight loss in HFD-induced obese mice until 2 weeks after operations. Under the HFD, the animals would start gaining weight from 10 to 14 days after operations (both DJB and SG). In this study, to minimize the influence of weight loss caused by bariatric surgery and obtain the weight-matched mice at the beginning of the DSS administration, once the animal weight reached 27–34 g (about 7 to 14 days after surgery), 2% DSS (molecular weight 36,000-50,000, MP Biochemicals, Santa Ana, CA, U.S.) was administrated in the drinking water for 7 consecutive days to induce acute colitis. At the end of DSS administration, mice were given regular drinking water. Fecal samples were collected before DSS treatment and 3 weeks after DSS administration. Fecal samples were snap-frozen in liquid nitrogen and then stored at −80°C until used for DNA extraction.
Mice were closely observed after DSS administration and were euthanized at the endpoint of the experiment (4 weeks after the start of DSS administration). The entire large bowel, from the ileo-cecal junction to the anus, was harvested. Tissue samples were prepared as spiral “Swiss rolls” and fixed in 10% formalin for 48 hours followed by the process of paraffin blocks. Paraffin sections (3μM) thick were cut, and slides with the entire length of the colon tissue were analyzed by hematoxylin and eosin (H&E) and immunohistochemical (IHC) staining.
Pathologic evaluation and IHC staining
The H&E-stained sections were evaluated in a blinded manner by 2 board-certified pathologists. The inflammation grade of tissue sections was scored as described by Dieleman et al. [11]. Briefly, the grading index was conducted according to inflammation severity, inflammation extent, crypt damage, and the percentage involvement of the ulcer or erosion in the colonic tissues. The sum of the first 3 scores was multiplied for the percentage involvement of ulcer or erosion.
To detect FXR expression by IHC staining, the paraffin-embedded colonic sections were first deparaffinized. Antigen retrieval was performed by incubating the slides in boiling 10 mM sodium citrate buffer (pH 6.0) for 20 minutes, and then at room temperature (RT) for 30 min. Afterwards, the slides were incubated in hydrogen peroxide for 10 minutes, followed by incubation in nonspecific staining blocking reagent for 30 minutes at RT. The sections were then incubated with diluted anti-FXR (rabbit polyclonal Ab, 1:200, Santa Cruz Biotechnology, CA) overnight at 4°C followed by incubation with horseradish peroxidase-labeled secondary antibody (biotinylated anti-rabbit, 1:200 for 30 minutess at RT). DAB peroxidase substrate kit (Vector Laboratories, Burlingame, CA) was used for developing visible immunocomplexes.
Microbiome analysis
The composition of the bacterial microbiota was analyzed as previously described with minor modifications [12–14]. Briefly, the DNA was extracted using the MOBIO Power-Soil DNA Isolation Kit (Carlsbad, CA, U.S.) following the manufacturer’s instruction for the PowerFecal DNA Isolation Kit. Four microliters of extracted bacterial DNA (a 1:5 dilution) were used as a template for quantitative real-time PCR reactions using the primer pairs in Table 1 A [12–14]. The 16 S gene copy numbers per μl of DNA from each sample (1 fecal pellet collected from each colon) was determined using standard curves generated with known concentrations of plasmids (Table 1 B) carrying the 16 S gene of each analyzed bacterial taxa [12,13].
Table 1A.
Primer pairs used for q-PCR on each extracted bacterial DNA
| Species | Target | Primer pairs | Reference |
|---|---|---|---|
| Eubacteria | 16 S rRNA | UniF340 5’-ACTCCTACGGGAGGCAGCAGT-3’ | Barman et al. |
| UniR514 5’-ATTACCGCGGCTGCTGGC-3’ | |||
| Firmicutes/Clostridiales | 16 S rRNA | UniF338 5’- ACTCCTACGGGAGGCAGC -3’ | Barman et al. |
| C.cocR491 5’- GCTTCTTTAGTCAGGTACCGTCAT -3’ | |||
| Firmicutes/Lactobacillales | 16 S rRNA | LabF362 5’- AGCAGTAGGGAATCTTCCA -3’ | Barman et al. |
| LabF362 5’- AGCAGTAGGGAATCTTCCA -3’ | |||
| Bacteroidetes | 16 S rRNA | BactF285 5’- GGTTCTGAGAGGAGGTCCC -3’ | Barman et al. |
| UniR338 5’- GCTGCCTCCCGTAGGAGT -3’ | |||
| Enterobacteriaceae | 16 S rRNA | Uni515 F 5’- GTGCCAGCMGCCGGCGGTAA -3’ | Barman et al. |
| Ent826 R 5’- GCCTCAAGGGCACAACCTCCAAG -3’ | |||
| Segmented Filamentous Bacteria (SFB) | 16 S rRNA | SFBF 5’- GACGCTGAGGCATGAGAGCAT -3’ | Barman et al. |
| SFBR 5’- GACGGCACGGATTGTTATTCA -3’ |
Table 1B.
Plasmids employed for investigating the microbiome on each colon sample
| Designation | Genotype | Reference |
|---|---|---|
| pSW191 | pCR2.1:: Eubacteria 16 S rRNA | Winter et al. |
| pSW192 | pCR2.1:: Clostridiales 16 S rRNA | Winter et al. |
| pSW193 | pCR:: Lactobacillales 16 S rRNA | Winter et al. |
| pSW194 | pCR2.1:: Bacteroidetes 16 S rRNA | Winter et al. |
| pSW196 | pCR2.1:: Enterobacteriaceae 16 S rRNA | Winter et al. |
| SFB | pCR2.1:: Segmented Filamentous | Barman et al. |
| Bacteria 16 S rRNA |
Statistical analysis
Statistical analyses were performed using SPSS 20.0 and GraphPad Prism Version 6. One-way ANOVA was used for comparison among the 3 groups. The 2-tailed unpaired Student’s t test was used for comparison across time points in the groups. Log-rank test was employed to determine the significance of survival rate. Data were expressed as mean ± standard error of the mean (SEM). Significance was considered for values of P < .05.
Results
Post-DSS survival rate
The observation period was set at 21 days after initiating DSS treatment. As shown in Fig. 2 A and Table 2, improved survival rate was observed in mice that had undergone DJB compared to Sham (P = .052). All 6 mice survived the 21-day period in DJB group. Seventy-five percent of SG mice and 50% of Sham mice survived until day 21.
Fig. 2.
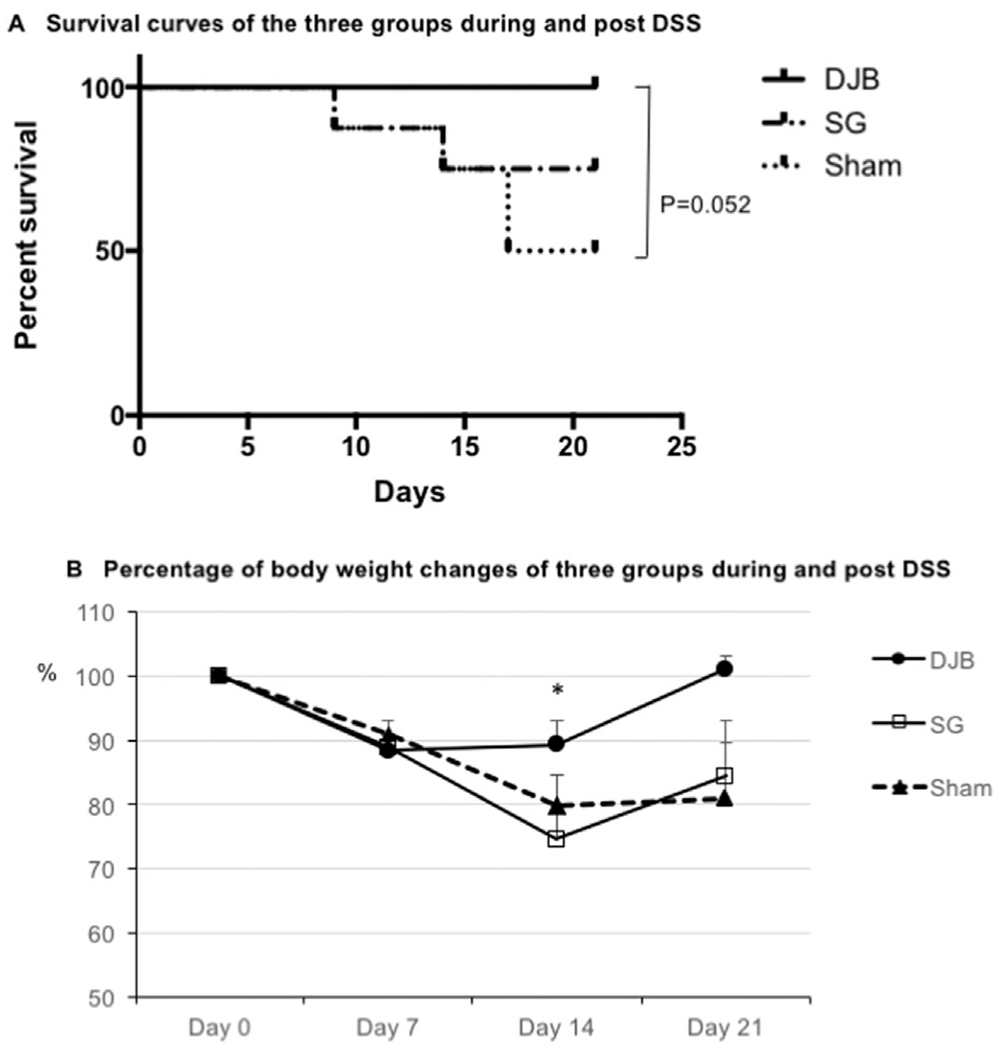
(A) Survival curves show the mice survival rate (SR) percentage after DJB, SG, and Sham surgery over the 21-day period. All DJB mice survived (n = 6, SR = 100%). Six out of 8 SG mice (SR = 75%) survived and 4 out of 8 Sham mice (SR = 50%) reached the experiment endpoint. (B) Curves show the percentage of mice weight changes after DJB, SG, and Sham surgery over the 21-day period. At the last day of DSS treatment (day 7), all 3 groups showed a similar weight loss. At day 14 DJB mice maintained a significantly higher weight compared with SG and Sham animals. At day 21 mice in DJB group gained back their original weight that is higher than SG and Sham.
*P < .05 versus SG and Sham groups.
Table 2.
Comprehensive table of the study results
| Group | Bacterial Load (Log 10, Mean ± SEM) |
BWC (% of initial weight, Mean ± SEM) |
Survival Rate |
Histologic Scores |
||||
|---|---|---|---|---|---|---|---|---|
| Pre-DSS | Post-DSS | D7 | D14 | D21 | (%) | (Mean ± SEM) | ||
| DJB | Lactobacillaesa | 6.6 ± .33 | 7.5 ± .16 | 88.4 ± 2.3 | 89.3 ± 3.7b | 101.1 ± 2.1 | 100 (n = 6) | 15.5 ± 2.9c (n = 6) |
| Enterobacteriacee | 6.7 ± .37 | 7.3 ± .17 | ||||||
| SFB | 5.2 ± .13 | 2.8 ± 1.01 | (n = 6) | (n = 6) | (n = 6) | |||
| SG | Lactobacillales | 7.0 ± .33 | 7.8 ± .02 | 88.9 ± 1.8 | 74.6 ± 2.7 | 84.5 ± 7.9 | 75 (n = 8) | 31.2 ± 1.8 (n = 6) |
| Enterobacteriacee | 6.5 ± .37 | 7.5 ± .37 | ||||||
| SFB | 5.2 ± .31 | 1.7 ± 1.35 | (n = 6) | (n = 6) | (n = 6) | |||
| Sham | Lactobacillaes | 6.7 ± .46 | 6.8 ± .09 | 91 ± 2.0 | 80 ± 4.5 | 80.1 ± 8.8 | 50 (n = 8) | 26.3 ± 2.3 (n = 4) |
| Enterobateriacee | 5.6 ± .02 | 7.7 ± .10 | ||||||
| SFB | 5.7 ± .08 | 0.1 ± .00 | (n = 6) | (n = 6) | (n = 4) | |||
BWC = weight change; DJB = duodenojejunal bypass; DSS = dextran sodium sulfate, SG = sleeve gastrectomy, SEM = standard error of the mean
Lactobacillaes bacterial load showed a statistically significant difference (Student t test) before and after DSS administration.
DJB mice showed significantly less weight loss compared with both SG and Sham mice.
DJB mice showed statistically significant lower histologic score compared with both SG and Sham mice.
Weight changes post-DSS
At the beginning of DSS administration all animals in the 3 groups were weight-matched (DJB: 28.5 ± .5 g, SG: 30.1 ± 1.0 g and Sham: 30.2 ± .5 g, P = .26, respectively). As shown in Fig. 2 B and Table 2, at day 7 of DSS administration, mice in all 3 groups lost approximately 10% of initial weight. The percentage weight change among 3 groups were similar (DJB, 88.4 ± 2.3%, n = 6; SG, 88.9 ± 1.8%, n = 6; and Sham, 91 ± 2.0%, n = 6; P = .65). At day 14, DJB mice had the same weight, while mice in the SG and sham-operated groups showed significant weight change compared to DJB group (DJB, 89.3 ± 3.7%, n = 6; SG, 74.6 ± 2.7%, n = 6; and Sham, 80 ± 4.5%, n = 6; P < .05 versus SG and Sham, respectively). At day 21, mice in the DJB group had gained more percentage weight back to pre-DSS level than in the SG and Sham groups (DJB, 101.1 ± 2.1%, n = 6; SG, 84.5 ± 7.9%, n = 6 and Sham, 80.1 ± 8.8%, n = 4; P = .10).
Pathologic changes
Of the DJB mice, 3 out of 6 exhibited moderate colonic inflammation with transmural infiltration of lymphocytes and rare crypt damage; ulcers or erosions were present in < 30% of the colon tissues. Three DJB mice exhibited mild inflammation with mucosal to submucosal infiltration of inflammatory cells and partial crypt damage, with overall intact surface epithelia (ulcers and erosions were present in < 15% of tissue). In contrast, all SG (n = 6) and sham-operated mice (n = 4), showed moderate or severe inflammation with transmural infiltration of lymphocytes and pan-colonic crypt damage; ulcers or erosions were present in 16%–45% of the colon tissue (Figs. 3 A, 3 B, and Table 2).
Fig. 3.
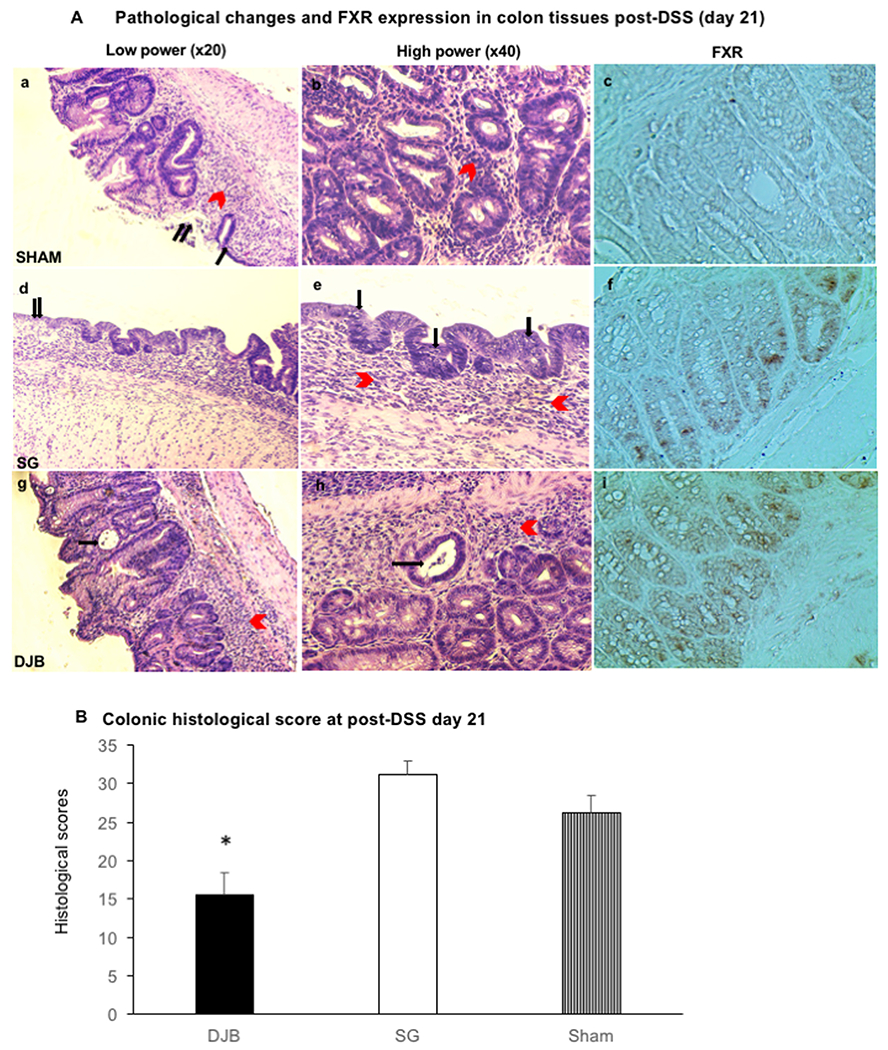
(A) H&E staining (a,b,d e,g,h) show colonic pathologic changes, while immunohistostaining (c,f,i) show FXR expression in the epithelial cells of mucosal intact glands. Overall, DJB mice (g,h) exhibited moderate colonic inflammation with lymphocytes transmural infiltration (red arrows) and isolated crypt damages (black arrows). SG mice (d,e) showed severe colonic inflammation with lymphocytes transmural infiltration (e, red arrows), mucosal erosions (d, double black arrow) and submucosal edema (d). Lymphocytes infiltration was also found in the epithelial cells as pointed by black arrows in picture e. Finally, Sham mice (a,b) showed severe lymphocytes infiltration (a, red arrow), mucosal erosion (a, single black arrow) and ulcers (a, double black arrow). Stronger specific nuclear FXR staining was detected in epithelial cells of intact glands in DJB (i) than SG (f) and Sham (c) mice. (B) The bar graph illustrates the histologic score attributed to DSS-induced colitis in DJB, SG and Sham mice. DJB showed a statistically significant lower value (15.5 ± 2.9) compared with SG (31.2 ± 1.8) and Sham (26.3 ± 2.3) mice. *P < .05 versus SG and Sham groups.
The histologic scores of DJB, SG, and sham-operated mice were 15.5 ± 2.9, 31.2 ± 1.8, and 26.3 ± 2.3, respectively (Fig. 3 B). A significantly less severe colitis in response to DSS was observed in DJB mice, compared to SG and sham-operated mice (P < .05).
A stronger nuclear expression of FXR was detected in the epithelial cells of intact glands in DJB than SG and Sham mice, as evidenced by immunohistochemical staining. FXR staining was observed quantitatively more in DJB (142 out of 899 glandular cells, about 16% were positive staining; Fig. 3A–i) than in SG mice (63 out of 787 glandular cells, about 8% were positive staining; Fig. 3A–c). These data indicate a protective role for FXR in the context of colonic inflammation.
Gut microbiota pre- and post-DSS
Given the reduced severity of colitis observed in DJB mice, we investigated whether the surgery could impart changes in the gut microbiota. Significant differences were detected in Lactobacillales, an order that includes beneficial microbes, post-DSS. In the DJB group (n = 4), Lactobacillales increased by ~9-fold post-DSS, a significant change compared to pre-DSS levels. A similar trend was observed in the SG group (n = 3), where the number of Lactobacillales increased on average 6-fold post-DSS. Importantly, no changes were observed among the microbiota analyzed in sham-operated mice (n = 2).
Discussion
In this study we report that bariatric surgery, in particular DJB, reduces the severity of DSS-induced colitis in a HFD-induced obesity mouse model. The DSS colitis model utilized in this study was employed because it recapitulates the intestinal damage frequently observed in human colitis. It should be noted that HFD and obesity are both associated with increased systemic and colonic inflammation, and HFD-induced obesity significantly increases the severity of IBD in a mouse models [3]. HFD also increases the susceptibility of mice to experimental DSS-induced colitis [2]. The high systemic and colonic inflammation, a consequence of obesity and DSS administration, likely explains the high mortality rate observed in the Sham-operated group after DSS administration.
As extensively reported in previous studies, the weight loss following bariatric surgery may be responsible for the decrease in systemic inflammation. To minimize the confounding effects weight loss could impose on our results, all animals were matched by body weight at the onset of DSS administration. As shown in Fig. 1B, obese mice initially lost weight in the first 7 days, while receiving DSS treatment. In the second week, SG and Sham-operated mice continued losing weight, whereas DJB mice maintained a relatively constant weight over the same time period. These data suggest that the protective role of proximal intestinal bypass, as exemplified by the DJB procedure, may be independent of weight loss. We also measured the quantity of water consumed during the DSS-therapy period. Notably, no significant differences among the 3 groups were observed (data not shown); hence surgery per se, did not alter water intake and DSS consumption.
It has been well-established that the gut microbiome plays an extremely important role in IBD, and in particular, several studies have shown that increased numbers of Lactobacillus prevent relapses of ulcerative colitis [15–17]. Following DSS administration, our microbiome data indicate that levels of Lactobacillales were increased in the DJB and SG, but not in the Sham-operated group, thus making this variation directly related to the specific surgical procedure and not to the DSS administration. These data suggest that the increased levels of Lactobacillales after bariatric surgical procedures may contribute to ameliorating colitis.
The bile acid nuclear receptor Farnesoid-X-Receptor (FXR) is activated by bile salts and regulates the transcription of genes involved in bile salt synthesis, transport, and metabolism in the liver and intestine [15]. It is well known that FXR plays an important role in gastrointestinal functions. A recent report proposes that FXR mediates the metabolic improvements seen after bariatric surgery, and that FXR-mediated metabolic changes eventually alter the intestinal microbiome [5]. These protective effects of FXR are supported by additional findings where activation of FXR protects against intestinal inflammation and IBD in mice [6]. Moreover, it has been reported that ileal FXR activation decreases in IBD patients [18]. Collectively, these reports, and our data suggest that activation of FXR in the intestinal tract may represent a novel therapeutic target to treat IBD in humans [7]. Interestingly, we found that FXR-specific nuclear staining was detected in colon epithelial cells of intact glands in DJB and SG mice but not in Sham-operated mice. Moreover, FXR expression was higher in the DJB group, compared with SG mice. These results suggest that bariatric surgery likely increases the expression of FXR in the colonic mucosa, thereby improving the composition and/or physiologic contribution of the gut microbiome. Furthermore, the DJB procedure that bypasses the proximal small intestine resulted in improved effectiveness in protecting against DSS-induced colonic inflammation.
It has been previously shown that antiobesity surgical procedures promote a rise in circulating levels of adiponectin with profound anti-inflammatory and insulin-sensitizing effects [19–22]. In particular, Tamao et al. reported that adiponectin exerts protective effects against DSS-induced colitis via suppressing chemokine and cytokine production in intestinal epithelial cells in a mouse model [23]. Further studies are necessary to investigate the mechanisms of the effect of bariatric surgery on systemic and colonic inflammation, and correlation of those changes to the levels of circulating chemokines and cytokines.
In the clinical setting, the relationship between IBD and obesity remains unclear, but several clinical studies showed the safety and feasibility of bariatric surgery in obese IBD patients [8,9,24,25]. On the other hand, the association between bariatric surgery and IBD is controversial [26,27]. Limitations of our study include the small sample size and the fact that we have only analyzed the effects of bariatric procedures in one model of colonic inflammation. Further research, including studies in nonobese rodent models and with spontaneous and transgenic murine IBD models, is needed to elucidate the possible specific mechanisms behind the anticolitis effect of various bariatric surgeries.
Conclusion
To the best of our knowledge, our study is the first to report that bariatric surgery reduces the severity of DSS-induced colitis in a murine model. Although further research is needed to elucidate these effects of bariatric surgery, our data suggests that bariatric procedures, especially the DJB that bypasses the proximal small intestine, reduce chemically-induced colitis via a weight loss independent modulation of FXR expression in the colon and rearrangement in gut microbiome.
Acknowledgments
This study was in part supported by Chao Family Comprehensive Cancer Center seed grant, UCI.
Footnotes
Disclosures
The authors have no commercial associations that might be a conflict of interest in relation to this article.
References
- [1].Sartor RB. Mechanisms of disease: pathogenesis of Crohn’s disease and ulcerative colitis. Nat Clin Pract Gastroenterol Hepatol 2006;3(7):390–407. [DOI] [PubMed] [Google Scholar]
- [2].Teixeira LG, Leonel AJ, Aguilar EC, et al. The combination of high-fat diet-induced obesity and chronic ulcerative colitis reciprocally exacerbates adipose tissue and colon inflammation. Lipids Health Dis 2011;10:204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Balzola F, Cullen G, Ho GT, Russell R. High-fat diet-induced obesity exacerbates inflammatory bowel disease in genetically susceptible Mdr1 a−/− male mice: Commentary. Inflamm Bowel Dis 2014;14:59–60. [Google Scholar]
- [4].Kohli R, Stefater MA, Inge TH. Molecular insights from bariatric surgery. Rev Endocr Metab Disord 2011;12(3):211–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Ryan KK, Tremaroli V, Clemmensen C, et al. FXR is a molecular target for the effects of vertical sleeve gastrectomy. Nature 2014;509(7499):183–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Gadaleta RM, van Erpecum KJ, Oldenburg B, et al. Farnesoid X receptor activation inhibits inflammation and preserves the intestinal barrier in inflammatory bowel disease. Gut 2011;60(4):463–72. [DOI] [PubMed] [Google Scholar]
- [7].Stojancevic M, Stankov K, Mikov M. The impact of farnesoid X receptor activation on intestinal permeability in inflammatory bowel disease. Can J Gastroenterol 2012;26(9):631–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Aminian A, Andalib A, Ver MR, Corcelles R, Schauer PR, Brethauer SA. Outcomes of bariatric surgery in patients with inflammatory bowel disease. Obes Surg 2016;26(6):1186–90. [DOI] [PubMed] [Google Scholar]
- [9].Colombo F, Rizzi A, Ferrari C, et al. Bariatric surgery in patients with inflammatory bowel disease: an accessible path? Report of a case series and review of the literature. J Crohns Colitis 2015;9(2):185–90. [DOI] [PubMed] [Google Scholar]
- [10].Liu W, Zassoko R, Mele T, et al. Establishment of duodenojejunal bypass surgery in mice: a model designed for diabetic research. Microsurgery 2008;28(3):197–202. [DOI] [PubMed] [Google Scholar]
- [11].Funakoshi T, Yamashita K, Ichikawa N, et al. A novel NF-kappaB inhibitor, dehydroxymethylepoxyquinomicin, ameliorates inflammatory colonic injury in mice. J Crohns Colitis 2012;6(2):215–25. [DOI] [PubMed] [Google Scholar]
- [12].Liu JZ, Jellbauer S, Poe AJ, et al. Zinc sequestration by the neutrophil protein calprotectin enhances Salmonella growth in the inflamed gut. Cell Host Microbe 2012;11(3):227–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Winter SE, Thiennimitr P, Winter MG, et al. Gut inflammation provides a respiratory electron acceptor for Salmonella. Nature 2010;467(7314):426–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Barman M, Unold D, Shifley K, et al. Enteric salmonellosis disrupts the microbial ecology of the murine gastrointestinal tract. Infect Immun 2008;76(3):907–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Herías MV, Koninkx JF, Vos JG, Huis in’t Veld JH, van Dijk JE. Probiotic effects of Lactobacillus casei on DSS-induced ulcerative colitis in mice. Int J Food Microbiol 2005;103(2):143–55. [DOI] [PubMed] [Google Scholar]
- [16].Ma Zocco, dal Verme LZ, Cremonini F, et al. Efficacy of Lactobacillus GG in maintaining remission of ulcerative colitis. Aliment Pharmacol Ther 2006;23(11):1567–74. [DOI] [PubMed] [Google Scholar]
- [17].Tang C, Kamiya T, Liu Y, et al. Inhibition of dectin-1 signaling ameliorates colitis by inducing lactobacillus-mediated regulatory t cell expansion in the intestine. Cell Host Microbe 2015;18(2):183–97. [DOI] [PubMed] [Google Scholar]
- [18].Nijmeijer RM, Gadaleta RM, van Mil SW, et al. Farnesoid X receptor (FXR) activation and FXR genetic variation in inflammatory bowel disease. PloS One 2011;6(8):e23745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Kadowaki T, Yamauchi T, Kubota N, Hara K, Ueki K, Tobe K. Adiponectin and adiponectin receptors in insulin resistance, diabetes, and the metabolic syndrome. J Clin Invest 2006;116(7):1784–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Nakatani H, Kasama K, Oshiro T, Watanabe M, Hirose H, Itoh H. Serum bile acid along with plasma incretins and serum high-molecular weight adiponectin levels are increased after bariatric surgery. Metabolism 2009;58(10):1400–7. [DOI] [PubMed] [Google Scholar]
- [21].Herder C, Peltonen M, Svensson PA, et al. Adiponectin and bariatric surgery: associations with diabetes and cardiovascular disease in the Swedish Obese Subjects Study. Diabetes Care 2014;37(5):1401–9. [DOI] [PubMed] [Google Scholar]
- [22].Kelly AS, Ryder JR, Marlatt KL, Rudser KD, Jenkins T, Inge TH. Changes in inflammation, oxidative stress and adipokines following bariatric surgery among adolescents with severe obesity. Int J Obes (Lond) 2016;40(2):275–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Nishihara T, Matsuda M, Araki H, et al. Effect of adiponectin on murine colitis induced by dextran sulfate sodium. Gastroenterology 2006;131(3):853–61. [DOI] [PubMed] [Google Scholar]
- [24].Lascano CA, Soto F, Carrodeguas L, Szomstein S, Rosenthal RJ, Wexner SD. Management of ulcerative colitis in the morbidly obese patient: is bariatric surgery indicated? Obes Surg 2006;16(6):783–6. [DOI] [PubMed] [Google Scholar]
- [25].Keidar A, Hazan D, Sadot E, Kashtan H, Wasserberg N. The role of bariatric surgery in morbidly obese patients with inflammatory bowel disease. Surg Obes Relat Dis 2015;11(1):132–6. [DOI] [PubMed] [Google Scholar]
- [26].Janczewska I, Nekzada Q, Kapraali M. Crohn’s disease after gastric bypass surgery. BMJ Case Rep 2011;2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Kotze PG, Bremer-Nones R, Kotze LM. Is there any relation between gastric bypass for morbid obesity and the development of Crohn’s disease? J Crohns Colitis 2014;8(7):712–3. [DOI] [PubMed] [Google Scholar]


