Abstract
Background:
Sickle cell disease (SCD) is an inherited, autosomal recessive blood disorder, among the most prevalent genetic diseases, globally. While the genetic and hemolytic dynamics of SCD have been well-characterized, the etiology of SCD-related pathophysiological processes is unclear. Although limited, observational evidence suggests that environmental factors, including urban air pollution, may play a role.
Objectives:
We assessed whether daily ambient air pollution concentrations are associated with corresponding emergency department (ED) visit counts for acute SCD exacerbations in Atlanta, Georgia, during a 9-year (2005–2013) period. We also examined heterogeneity in response by age and sex.
Methods:
ED visit data were from 41 hospitals in the 20-county Atlanta, GA area. Associations between daily air pollution levels for 8 urban air pollutants and counts of SCD related ED visits were estimated using Poisson generalized linear models.
Results:
We observed positive associations between pollutants generally indicative of traffic emissions and corresponding SCD ED visits [e.g., rate ratio of 1.022 (95% CI: 1.002, 1.043) per interquartile range increase in carbon monoxide]. Age stratified analyses indicated stronger associations with traffic pollutants among children (0–18 years), as compared to older age strata. Associations involving other pollutants, including ozone and particulate matter and for models of individuals > 18 years old, were consistent a null hypothesis of no association.
Discussion:
This analysis represents the first North American study to examine acute risk among individuals with SCD to urban air pollution and provide evidence of urban air pollution, especially from traffic sources, as a trigger for acute exacerbations. These findings are consistent with a hypothesis that biological pathways, including several centrally associated with oxidative stress, may contribute towards enhanced susceptibility in individuals with SCD.
Keywords: Sickle Cell Disease, Air Pollution, Acute Health Effects, Emergency Department Visits
INTRODUCTION
Sickle cell disease (SCD) is an inherited, autosomal recessive blood disorder, among the most prevalent genetic diseases in the United States (U.S.)(Knight et al. 1999), The most common form of SCD, sickle-cell anemia (HbSS), accounts for roughly 70% of the identified disease genotypes (Rees et al. 2010), and is associated with deoxygenated-induced hemoglobin S polymerization and cellular dehydration, leading to the characteristic sickled red blood cell formation(Belhassen et al. 2001). Depending on disease severity, this process may, in turn, lead to vaso-occlusive and hemolytic manifestations (Rees et al. 2010), as well as the common clinical features, acute chest syndrome (ACS), vaso-occlusive pain episodes (VOE), systolic and diastolic hypertension, pulmonary hypertension and endothelial dysfunction (Gladwin et al. 2014; Parent et al. 2011).
While the genetic and hemolytic dynamics of SCD have been well-characterized, the clinical phenotype and spectrum of SCD complications varies widely from patient to patient despite the common genetic mutation. The etiology of SCD-related pathophysiological processes leading to this clinical variability is unclear. Unknown attribution of specific causes for pulmonary complications associated with ACS, for example, occurs in 46% of all cases (Gladwin et al. 1999). Understanding the role of environmental factors, specifically, in eliciting SCD-related response has focused, among numerous factors, on climate (Jones et al. 2005; Mekontso Dessap et al. 2014; Piel et al. 2017), including temperature, wind speed, and humidity (Tewari et al. 2015); exposure to second-hand smoke (Sadreameli et al. 2016); and dehydration (Ballas et al. 2012), as potential triggers.
It is plausible that urban air pollution is an additional potential trigger of SCD complications, given current insight into the underlying modes of action implicated in adverse air pollution-related cardiorespiratory response (Brook et al. 2009; Krishnan et al. 2012). While multiple mechanistic pathways may participate in air pollution risk, it is likely that long- and short-term exposures to specific particulate and gaseous air pollution components may contribute to acute and chronic oxidative stress, systemic inflammation, and potential adverse clinical outcomes, including endothelial dysfunction, cerebrovascular disease, atherosclerosis and myocardial infarction (Krishnan et al. 2012; Mills et al. 2005; Peretz et al. 2008). These processes may, in turn, lead to greater adhesion of the sickled erythrocytes to vascular endothelium resulting in vasculopathy (Barbosa et al. 2015). Alternatively, it is possible that chronic baseline levels of elevated systemic oxidative stress (Nur et al. 2011), another underlying SCD pathophysiologic condition, may again lead to the increased expression of circulating adhesion molecules and sensitivity to acute occlusion, pain crises, and other forms of vasculopathy (Gladwin 2006).
There have been relatively few analyses, mainly conducted in Europe and South America, that have examined associations between urban air pollution and corresponding acute complications in SCD (Braga et al. 2006; Mekontso Dessap et al. 2014; Tewari et al. 2015; Yallop et al. 2007). Findings from these studies have been equivocal regarding whether and which air pollution components may trigger adverse acute SCD responses, although a recent review paper examining a range of environmental factors concluded that numerous air pollutants appear to be predictive of a range of health responses, while noting the difficulty disentangling independent air pollution effects given their correlation other environmental factors (Tewari et al. 2015). A study in Sao Paolo, Brazil, for example, support a role for air pollution as a factor for increases in sickle cell-related emergency department visits (Braga et al. 2006). Yallop et al. (2007) also report associations between ozone (O3) levels in London and hospital admissions for ACS with pain and suggest that these events may be induced via ozone-related respiratory pathways (Yallop et al. 2007). To date, no North American studies have investigated the impact of outdoor air pollution on the heath of individuals with SCD.
To further elucidate the potential role of urban air pollution as a driver of SCD health response, we conducted the current analysis using data from the Study on Particles and Health in Atlanta (SOPHIA)(Metzger et al. 2004; Peel et al. 2005; Sarnat et al. 2013; Tolbert et al. 2007). Atlanta, as a study setting, provides an ideal location for an SCD-focused analysis, given a sizeable population of individuals with the disease living in metropolitan Atlanta, as well as it being the home to SOPHIA, which comprises one of the largest single-city timeseries assessing air pollution and health. Here, we present results from the first North American-based epidemiologic analysis examining population-level associations between air pollution and SCD-related emergency department (ED) visits, as well as differential risk by age and sex in individuals with SCD.
METHODS
We used air pollution measurements collected daily at an urban Atlanta monitoring site, Jefferson Street (JST), from January 1, 2005 to December 31, 2013. JST is located 4 km northwest of downtown Atlanta. We used this central site as an indicator of population exposures to be consistent with the assignment approach we repeatedly used in our previous epidemiologic analyses in Atlanta examining other health endpoints (Metzger et al. 2003; Peel et al. 2005; Sarnat et al. 2013; Strickland et al. 2010; Tolbert et al. 2007). JST is a former U.S Environmental Protection Agency Supersite and has also served as a primary monitoring site for the Southeastern Aerosol Research and Characterization Study (SEARCH), the Aerosol Research Inhalation Epidemiology Study (ARIES), and several ongoing epidemiologic studies as part of SOPHIA. The justification and rationale for using a single, central site as an exposure indicator for this study area as well as a discussion of the potential for measurement error from this approach, has been extensively examined previously (Sarnat et al. 2013). For this study, we elected, a priori, to use the modeling approach most consistent with other epidemiologic analyses conducted using SOPHIA data (Metzger et al. 2004; Peel et al. 2005; Sarnat et al. 2013; Tolbert et al. 2007). This analysis focused on 8 ubiquitous urban air pollutants which were measured daily and sub-daily over the analysis period: daily 1-hr maximum carbon monoxide (CO), nitrogen dioxide (NO2), sulfur dioxide (SO2); daily 8-hr maximum O3; 24-hr average fine particulate matter (PM2.5); and major PM2.5 components, sulfate (SO42-), elemental carbon (EC), and organic carbon (OC).
Patient-level ED visit data were obtained from the Georgia Hospital Association and included records from 41 of 42 acute care hospitals in the 20 county-Atlanta area. Specific data elements included admission date, hospital, and primary International Classification of Diseases 9th Revision (ICD-9) diagnosis codes, as well as age, sex, and race of the patient. The Emory University Institutional Review Board approved this study.
Sickle cell disease case groups were defined using ICD-9 codes and ED visits for each outcome were aggregated by day. We were specifically interested in ‘all SCD’ (ICD-9 code 282.6), which included visits for sickle cell anemia (HbSS) without crisis (ICD-9 code 282.61), HbSS disease with crisis (ICD-9 code 282.62), sickle-cell/Hb-C (HbSC) disease without crisis (ICD-9 code 282.63), HbSC disease with crisis (ICD-9 code 282.64), other SCD with or without crisis (ICD-9 codes 282.68 and 282.69), and unspecified SCD (ICD-9 code 282.60). As a secondary analysis, we also separately considered HbSS with crisis (ICD-9 code 282.62) as its own case group and present the results as Supplemental Material. The HbSS subset comprised approximately 80% of the ‘all SCD’ visit counts, as expected given the prevalence of HbSS as the most common form of SCD in the U.S. We hypothesized that pronounced differences in results from models of ‘all SCD’ and HbSS could indirectly indicated differences by SCD genotype. Significant results for models including HbSS, but not ‘all SCD’, for example, may provide indication of differential response to air pollution in individuals with HbSC or other SCD genotypes. Given the relative paucity of ED counts for the less prevalent SCD subtypes, we could not conduct separate, direct analyses including these outcomes.
Poisson generalized linear models were used to estimate associations of daily ambient air pollution levels and daily counts of SCD-related ED visits. The basic form of the model is:
where Yt is the count of SCD-related ED visits on day t and pollutantt is the 3-day moving average (of lag days 0, 1, and 2) air pollution concentration for time t. Given the unknown contribution of air pollution to acute SCD response, we did not define this exposure window using a disease-specific biological justification. Rather, our choice of a 3-day moving average was made, a priori, to be consistent with our previous epidemiologic models of SOPHIA data examining processes with possible inflammation-mediated pathogeneses (Krall et al. 2017; Ye et al. 2017; Strickland et al. 2010). The model also included indicator variables for day-of-week and holidays (DOW), and hospital indicator variables (hospital) to account for the entry and exit of hospitals into and from the database during the study period. Long-term trends in case presentation rates (time) were controlled using semi-parametric cubic splines, g(γ1,…,γN; x) with monthly. Cubic splines were used to control same-day maximum temperature, lag 1–2-day moving average minimum temperature, and lag 0–2-day mean dewpoint temperature (dewpoint), with knots placed at the 25th and 75th percentiles. The first and second derivatives of g(x) were continuous allowing time trends and meteorology to be modeled as smooth functions. Variance estimates were scaled to account for Poisson over-dispersion. All estimated associations were reported as rate ratios (RRs) and scaled per interquartile range (IQR) increase in pollutant concentrations. Significance is reported at p < 0.05. As noted, the model specification was selected, a priori, to be structurally similar to previous SOPHIA-based analyses (Strickland et al. 2010; Winquist et al. 2014; Krall et al. 2017; Ye et al. 2017).
Additional analyses were stratified by demographic factors, specifically by age (0–18, 19–39, 40+ years) and by sex (female/male). We also conducted a series of sensitivity analyses. To assess potential confounding by co-pollutants, sensitivity analyses included selected two-pollutant models, in order to assess the relationship between SCD and the main air pollutant of interest while controlling for a second co-pollutant. To examine the robustness of results by model specification, we conducted analyses: a) including year and month interaction terms, in place of smoothed temporal splines; b) omitting minimum temperature control variables; c) omitting season and season interaction terms; and d) replacing same-day maximum temperature and 1–2 day minimum temperature control with a 3-day moving average maximum or mean temperature cubic terms. We conducted all modeling in SAS (v9.2), using the GENMOD procedure.
RESULTS
During the time period for this analysis, there were 77,310 ‘all SCD’ ED visits (daily mean = 23.6 counts/day, standard deviation=5.4), with a substantial majority categorized as HbSS with crisis (total counts = 62,925; daily mean = 19.2 counts/day, standard deviation = 5.0). Descriptive statistics for the two outcomes, ‘all SCD’ and HbSS stratified by age, sex, and race categories, showed ED counts to be highest for the 19–39 age group, with the next highest counts for ages 0–18 (Table 1).
Table 1.
Descriptive Statistics of ‘All SCD’ and HbSS ED Visits from Jan 1, 2005 - Dec 14, 2013 stratified by Age, Sex, and Race. Differences in row totals due to missingness in emergency department record responses.
| All SCD | HbSS | |||||
|---|---|---|---|---|---|---|
| Count | Mean Visits/Day | SD | Count | Mean Visits/Day | SD | |
| Age | ||||||
| 0–18 | 17,262 | 5.3 | 2.8 | 10,226 | 3.1 | 2.0 |
| 19–39 | 46,237 | 14.1 | 3.9 | 41,115 | 12.6 | 3.8 |
| ≥ 40 | 13,811 | 4.2 | 2.1 | 11,584 | 3.5 | 2.0 |
| Sex | ||||||
| Female | 40,804 | 12.5 | 3.8 | 33,131 | 10.1 | 3.5 |
| Male | 36,503 | 11.2 | 3.5 | 29,791 | 9.1 | 3.2 |
| Race | ||||||
| Black | 45,816 | 21.1 | 5.2 | 37,323 | 17.2 | 5.0 |
| White | 121 | 0.1 | 0.2 | 96 | 0 | 0.2 |
| Hispanic | 454 | 0.2 | 0.5 | 327 | 0.2 | 0.4 |
| Other | 678 | 0.3 | 0.6 | 481 | 0.2 | 0.5 |
For the race stratification, a large majority (over 95%) of the ED visits for the time period were Black.
Broadly, pollutant concentrations during the analysis period were generally typical for Atlanta, historically (Table 2). For each of the pollutants measured, there was a general decline in pollutant levels over the time series duration, consistent with urban air pollution trends elsewhere in many cities throughout North America. During this period, there was moderate-to-strong correlation (Pearson’s correlation coefficient > 0.6) among CO, NO2, OC, and EC distributions, which we have previously attributed to shared traffic emission sources for these pollutants within the metropolitan Atlanta area (Supplemental Table 1)(Sarnat et al. 2009).
Table 2.
Descriptive statistics of three-day moving averages of urban air pollution data: Jan 1, 2005 - Dec 14, 2013.
| Pollutant (units) | N1 | Mean | SD | 25th Pctl | 50th Pctl | 75th Pctl | Maximum | IQR2 | |
|---|---|---|---|---|---|---|---|---|---|
| 1-hr max CO | (ppm) | 3284 | 0.6 | 0.4 | 0.4 | 0.5 | 0.7 | 3.7 | 0.4 |
| 1-hr max NO2 | (ppb) | 3287 | 34.4 | 11.1 | 26.3 | 33.6 | 41.7 | 77.8 | 15.3 |
| 8-hr max O3 | (ppb) | 3287 | 41.9 | 15.7 | 29.4 | 40.5 | 53.0 | 95.6 | 23.6 |
| 1-hr max SO2 | (ppb) | 3287 | 10.9 | 9.7 | 3.3 | 8.0 | 16.0 | 57.1 | 12.7 |
| 24-hr PM2.5 | (ug/m3) | 3287 | 12.5 | 5.6 | 8.6 | 11.4 | 15.3 | 45.0 | 6.7 |
| 24-hr SO42- | (ug/m3) | 3281 | 3.2 | 2.2 | 1.8 | 2.5 | 3.9 | 20.1 | 2.2 |
| 24-hr EC | (ug/m3) | 3228 | 1.0 | 0.6 | 0.6 | 0.9 | 1.2 | 5.4 | 0.6 |
| 24-hr OC | (ug/m3) | 3222 | 3.2 | 1.3 | 2.3 | 2.9 | 3.8 | 13.6 | 1.5 |
ED data for the last sixteen days of 2013 is missing
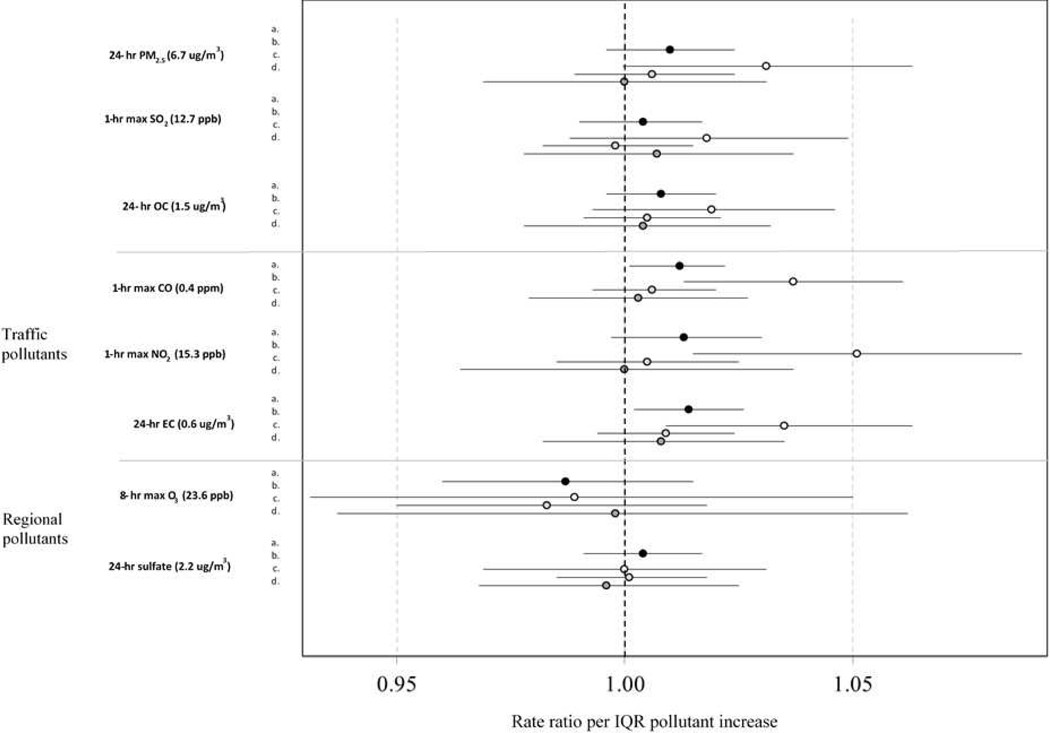
Associations between the measured pollutants and all SCD outcomes, varied by pollutant, age, and sex (Figure 1; numeric data used to generate Figure 1 shown in Supplemental Tables 2, 3). For models including the entire study population, we observed positive and significant associations between IQR increases in CO and EC and corresponding ‘all SCD’ visits (RR for CO = 1.012; 95% confidence interval [CI] = 1.001, 1.022; RR for EC= 1.014; 1.002, 1.026). In the age stratified analyses (0–18, 19–39, and ≥ 40 years), we did not observe association between any of the pollutants and ‘all SCD’ in individuals over 19 years old, although RRs from these models were predominantly ≥ 1.00 (12 of 16 models (Figure 1). In contrast, for children and adolescents (i.e., 0–18 year age strata), there were significant and positive associations between ‘all SCD’ and each of the traffic-related pollutants CO, NO2, and EC. (RR for CO = 1.037; 1.013, 1.161; NO2 = 1.051; 1015, 1.087; and EC = 1.035; 1.009, 1.063; p < 0.05), with marginal associations for PM2.5 (1.031; 0.9997, 1.063) (Figure 1; Supplement Table 3).
Figure 1.
Risk ratios and 95% CIs per inter-quartile range increase from 3-day moving average (of lag days 0, 1, and 2) models for total and age-stratified associations of emergency department visits for all SCD-related causes (ICD-9 code 282.6) with 1-hr max carbon monoxide (CO);1-hr max nitrogen dioxide (NO2);8-hr max ozone (O3);1-hr max sulfur dioxide (SO2); 24-hr fine particulate matter (PM2.5); 24-hr particulate sulfate (SO42-); 24-hr particulate elemental carbon (EC); and 24-hr particulate organic carbon (OC). ‘a’ indicates models examining the entire population; ‘b’ for age strata 0–18 years old; ‘c’ for age strata 19–39 years old; and ‘d’ for age strata 40+ years old. (Atlanta, January 1, 2005 and December 31, 2013).
Associations for each of the three, largely traffic-related pollutants with all SCD outcomes were largely driven by males (Table 3). When stratifying by sex, we observed positive associations observed for males, exclusively, with the strongest associations for models including EC (RR = 1.022; 1.005, 1.039).
Table 3.
Risk ratios and 95% CIs per inter-quartile range increase from pollution from 3-day moving average (of lag days 0, 1, and 2) for the association of emergency department visits for ‘All SCD’. Stratified by Age and Sex from January 1, 2005 - December 31, 20131
| Sex | ||
|---|---|---|
| Pollutant (units) | Female RR (95% CI) | Male RR (95% CI) |
| 24-hr PM2.5 (ug/m3) | 1.008 (0.989, 1.027) | 1.012 (0.992, 1.033) |
| 1-hr max SO2 (ppb) | 0.987 (0.969, 1.005) | 1.022 (1.003, 1.042)* |
| 24-hr OC (ug/m3) | 1.006 (0.989, 1.022) | 1.011 (0.994, 1.028) |
| Traffic-related | ||
| 1-hr max CO (ppm) | 1.006 (0.991, 1.020) | 1.018 (1.003, 1.033)* |
| 1-hr max NO2 (ppb) | 1.018 (0.996, 1.040) | 1.009 (0.986, 1.032) |
| 24-hr EC (ug/m3) | 1.006 (0.990, 1.022) | 1.022 (1.005, 1.039)* |
| Regional | ||
| 8-hr max O3 (ppb) | 1.000 (0.963, 1.038) | 0.973 (0.935, 1.012) |
| 24-hr SO42- (ug/m3) | 1.002 (0.984, 1.021) | 1.005 (0.986, 1.024) |
ED data for the last sixteen days of 2013 is missing;
IQR is 75th percentile minus 25th percentile;
= p < 0.05
Since respiratory disease and ACS are may be age-related (Strunk et al. 2008), we conducted additional sub-analyses stratifying the 0–18 year old results into 0–6, 7–10, and 11–18 age bins (data not shown). Overall ED daily sub-counts for these sub-analyses were generally very low, with low statistical power. However, in a sub-sample of models including NO2 and EC, we did not observe pronounced differences in either magnitude or strength of association among the more finely resolved age sub-strata for the children during this period.
To assess the robustness and potential confounding in the CO, NO2, and EC results, each of which was positively associated with ‘all SCD’ in single-pollutant model settings, we conducted analyses assessing changes in observed RRs results of models while including either CO, EC, or NO2, in addition to O3, SO2, PM2.5, SO42- and OC (Table 4). For EC and CO, each of the pollutants maintained positive associations with ‘all SCD’ in the co-pollutant models. For CO and EC, the addition of O3, SO2, and SO4 each individually resulted in similar confidence interval, and slightly weaker and marginally significant associations with the addition of PM2.5 and OC, individually. The results for NO2 were still positive, yet predominantly null with the individual addition of the other 5 co-pollutants. The co-pollutant models all had p-values that were equal or less than 0.10 for the main air pollutant. We did not to conduct multi-variate modeling including CO, NO2, and EC alone, given the high degree of inter-pollutant correlation, which we attribute to each acting as indicators of a shared traffic signal. This is also a practice we have used and described in previous SOPHIA-based epidemiologic analyses (Sarnat et al, 2013; Strickland et al 2010)
Table 4.
Estimated two-pollutant model risk ratios and 95% CIs per inter-quartile range increase and corresponding ED visits for ‘all SCD’ outcomes from January 1, 2005 - Dec 31, 20131
| Main Pollutant (units) | Second Pollutant (units) | SCD RR (95% CI) | Main Pollutant P-Value | Second Pollutant P-Value |
|---|---|---|---|---|
| 1-hr max CO (ppm) | 1.012 (1.001, 1.022) | 0.032 | NA | |
| 8-hr max O3 (ppb) | 1.013 (1.002, 1.024) | 0.020 | 0.201 | |
| 1-hr max SO2 (ppb) | 1.011 (1.001, 1.022) | 0.037 | 0.872 | |
| 24-hr avg PM2.5 (ug/m3) | 1.010 (0.998, 1.023) | 0.093 | 0.698 | |
| 24-hr avg SO42- (ug/m3) | 1.011 (1.001, 1.022) | 0.037 | 0.824 | |
| 24-hr avg OC (ug/m3) | 1.012 (0.999, 1.025) | 0.075 | 0.999 | |
| 1-hr max NO2 (ppb) | 1.013 (0.997, 1.030) | 0.102 | ||
| 8-hr max O3 (ppb) | 1.017 (1.000, 1.034) | 0.049 | 0.152 | |
| 1-hr max SO2 (ppb) | 1.013 (0.997, 1.030) | 0.115 | 0.770 | |
| 24-hr avg PM2.5 (ug/m3) | 1.010 (0.992, 1.029) | 0.266 | 0.481 | |
| 24-hr avg SO42- (ug/m3) | 1.013 (0.997, 1.030) | 0.120 | 0.846 | |
| 24-hr avg OC (ug/m3) | 1.010 (0.991, 1.029) | 0.318 | 0.542 | |
| 24-hr EC (ug/m3) | 1.014 (1.002, 1.026) | 0.022 | ||
| 8-hr max O3 (ppb) | 1.016 (1.004, 1.028) | 0.010 | 0.120 | |
| 1-hr max SO2 (ppb) | 1.014 (1.002, 1.026) | 0.025 | 0.989 | |
| 24-hr avg PM2.5 (ug/m3) | 1.015 (0.999, 1.031) | 0.071 | 0.854 | |
| 24-hr avg SO4 (ug/m3) | 1.015 (1.002, 1.027) | 0.022 | 0.721 | |
| 24-hr avg OC (ug/m3) | 1.018 (1.000, 1.036) | 0.054 | 0.679 | |
We conducted sensitivity analyses using the association between CO and ‘all SCD’ as an a priori test case. These models examined changes in specifications of the Poisson generalized linear models. Broadly, results were similar in direction and magnitude among the various models we assessed (results not shown), when using year and month interaction terms, in place of temporal splines, and varying meteorological and seasonal control parameters. Collectively, the sensitivity results suggest that the associations observed in the analysis were robust to model specification and potential confounding.
For all of the model results, strengths of association were generally weaker for models examining associations with HbSS as an outcome, although there were no meaningful differences in either the magnitude or direction of association as compared to models assessing ‘all SCD’ visits (Supplemental Tables 2, 3).
DISCUSSION
Using a 9-year time series of ED visits within the 20-county Atlanta area, the current findings provide evidence of increased risk from urban air pollution for acute SCD exacerbations. In this study, associations were primarily driven by excess risk in male children (individuals < 18 years old). Among the pollutants we examined, those typically associated with traffic emissions, including CO, NO2, and EC, were most strongly and positively associated with adverse response. Notably, we did not observe associations in other sex or age strata or with any of the other ubiquitous urban air pollutants we analyzed. To our knowledge, this is the first analysis examining SCD air pollution susceptibility in North America, and add to the very limited number of studies elsewhere, that have examined urban air pollution as an environmental risk factor for SCD-related morbidity. While difficult to compare across study periods and target populations, the magnitude of association we observed (with statistically significant increases in excess ED visits ranging from 1.8 to 5.1 percent per pollutant IQR) is consistent with risks reported in other Atlanta SOPHIA analyses. Strickland et al (2010), for example, report increased ED visit counts between 2.0 and 8.2 percent per pollutant IQR in a pediatric asthma population during a similar timeframe in Atlanta (Strickland et al 2010).
It is likely that several biological pathways may be involved in the observed findings and may contribute towards enhanced susceptibility. Notably, vaso-occlusion and hemolysis, primary pathophysiologic features involved in acute SCD complications, are associated with dysregulated arginine-nitric oxide metabolism (Morris et al. 2000; Morris et al. 2003; Morris et al. 2005; Tewari et al. 2015). Arginine is an α-amino acid responsible for endogenous NO production and known to be deficient in individuals with SCD (Enwonwu 1989; Morris et al. 2000; Morris et al. 2013; VanderJagt et al. 1997). An altered arginine metabolome is also found in asthma (Lara et al. 2008; Morris et al. 2004). Of interest, patients with SCD experience symptoms of asthma more frequently than the general population, and this co-morbidity is associated with adverse events such as ACS, stroke, pulmonary hypertension and early mortality (Cohen et al. 2011; Morris 2009). Relevant to this study, arginine has also been shown to be inversely associated with elevated air pollution levels (Silkoff et al. 2004) and depleted following air pollution-induced airway hyperresponsiveness, due to the augmentation of arginase (North et al. 2011). Recently, we reported findings from two independent prospective panel studies of adults with and without asthma exposed to elevated primary traffic emissions (Ladva et al. 2018; Liang et al. 2018; Liang et al. 2019). We observed associations between dysregulated metabolites in the arginine-nitric oxide pathway and elevated traffic-related air pollutants, including BC, CO, NO and PM2.5 (Liang et al. 2019), the same pollutants most associated with SCD-related ED visits in the current observational analysis.
Findings from Morris et al. (2000) lend added support for the hypothesized role of air pollution as a potential driver, in part, of SCD morbidity (Morris et al. 2000). Here, investigators observed dysregulated, reduced arginine levels during vaso-occlusive pain episodes in a panel of 36 SCD patients, reflecting a state of acute arginine substrate depletion, decreased nitric oxide production and, ultimately, increased SCD-related morbidity. It is plausible that hemolysis, vaso-occlusion, and exposures to air pollutants would have additive or synergistic effects on deregulating arginine-nitric oxide metabolism, thus leading to increased SCD morbidity manifesting in higher ED utilization. If true, the specific central role of arginine dysregulation, while anecdotal, raises the possibility that therapeutic intervention, in the form of supplementation, may help offset imbalances in metabolites participating in this paradigm.
A key finding in our analysis was that pollutants typically related to traffic emissions, including NO2, CO, and EC, were those most strongly associated with ED visits. Observational and controlled studies have provided evidence of the link between traffic-related pollution and acute adverse health (Health Effects Institute 2010), given the ability of specific pollutant components or mixtures to elicit oxidative stress. In the current analysis, we view these pollutants as indicators of a complex traffic pollution source and not necessarily reflecting true causal associations with the individual modeled pollutant itself. Similarly, it is possible that other, non-traffic sources contribute to the measured levels of the three pollutants we deemed to be traffic related. Thus, caution should be taken when inferring specific source attribution to the findings.
Analyses examining links between specific air pollutants with acute SCD-related health response have been equivocal. Most notably has been a time-series investigation, similar in design to the one we conducted, which assessed air pollution and ED visits for SCD among children in Brazil (Barbosa et al. 2015). The ED visits in this study primarily included pain manifestation as they stratified the two main causes of ED visits into pain crises and respiratory infections, and provided indication that air pollution is positively associated with vaso-occlussive events. Barbosa and colleagues focused exclusively on pediatric response (in individuals between 0 and 18 years old), and reported associations with NO2 and CO, which they too viewed as broad indicators of traffic-related pollution (Barbosa et al. 2015). In the South American analysis, the authors reported RRs for many of the pollutant models that were typically higher than those we observed (i.e., RRs for ED visits ranging from 9 to 19 percent). Another time-series analysis conducted in London reported increased hospital admissions to be positively associated with O3 levels (Yallop et al. 2007). CO was reported to be negatively associated with hospital admissions for SCD related acute pain, a finding consistent with other reported results (Mekontso Dessap et al. 2014). In their discussion of findings, Yallop and colleagues offered as an explanation that CO may serve a protective role against SCD, in promoting carboxyhemoglobin production, which is less susceptible to polymerization. A recent study of oral carbon monoxide therapy in the SCD mouse model demonstrating beneficial effects on vaso-occlusion, inflammation and anemia support this hypothesis (Belcher et al. 2006). Other studies, as well, suggest that the half-life of red blood cells is extended with the presence of CO (Beutler 1975). A similar negative association between CO and SCD-related hospital admissions was reported elsewhere (Mekontso Dessap et al. 2014). Conversely, a subsequent analysis using the London data, as well as data on pediatric hospital admissions of children with either HbSS or HbSC in London and Paris, found largely null associations with several urban air pollutants, including CO (Piel et al. 2017).
In our analysis, another main finding was that children 18 years old and younger were those most susceptible to urban air pollution. Although the biological basis for this observation is not clear, previous studies do provide some support for this conclusion. Barbosa et al. (2015) and Piel et al (2017) restricted their study to children under 18 years old (Barbosa et al. 2015; Piel et al. 2017), however, ours was the first to have analyzed stratified associations between air pollution and SCD exacerbation by age. It is probable that all children, with and without preexisting co-morbidities, may be more susceptible to air pollution given immune systems that may are not be fully developed as adults, higher minute ventilation resulting in greater per mass dosages to air pollution (Dixon 2002; Gilliland et al. 1999), and activity patterns characterized by more time spent outside than adults (Trasande 2005). It is interesting to note that children with SCD frequently experience a reversible obstructive pattern of pulmonary function (Strunk et al. 2008), while adults with SCD demonstrate a predominance of restrictive disease (Klings et al. 2006; Morris 2009), that may also differentially influence their susceptibility to air pollution.
In conducting sub-analyses of the age-stratified analyses, we were looking for some indication that RRs were higher in the younger children, specifically, where obstructive lung conditions may be more prevalent. In a sub-sample of models including NO2 and EC, we did not observe any meaningful differences among the age strata, although the power to detect statistical association given less daily ED counts in the sub-analyses was limited. These results provide some indication that the pollution-mediated ED visits for ‘all SCD’ were not driven primarily by exacerbations in asthma-like symptoms in the youngest children in the sample population. Admittedly, we cannot completely rule out this explanation for the observed differences by age. It is, however, equally unlikely that the observed elevated risk in children under 18 as compared to adults is a statistical artifact associated with differential analytical power to detect associations within the age-stratified analyses. ED visits for SCD by age were not highest in children, with 6,394 visits for ages 0–18 (children), 8,782 for ages 19–39 years, and 3,238 for ages > 40 years.
The results showing differences in observed risk by sex were also notable. We did not detect pollutant-related associations in females, both for the entire database, as well as within the pediatric subset. It is possible that different physiological processes between males and females may play a role. Although speculative, males may experience higher exposures to air pollutants than females based on their occupational choice or recreational activities and reported uncertain effect modification by sex, suggesting sex-related physiological differences, such as dermal thickness and permeability (Clougherty 2011). Divergent nitric oxide bioavailability has also been documented in men compared to women with SCD, which is another potential mechanism for sex-related differences in sickle cell morbidity (Gladwin et al. 2003). Regardless, we view the difference in findings by sex as intriguing, and deserve attention in future studies.
There were several limitations that warrant further investigation and caution when interpreting our findings. One limitation is that the air pollution data were taken from a single monitoring site in downtown Atlanta, while the ED visits were from 41 hospitals in a large, 20-county area. We have conducted numerous previous investigations of potential exposure misclassification from the use of a central site as a surrogate of population exposure in Atlanta, examining differences in observed RRs when using more proximal exposure indicators and spatially-resolved exposure modeling (Liang et al. 2018; Sarnat et al. 2008). And, while possible, we believe and have shown this form of error to be negligible within the Atlanta modeling domain. Similarly, given the limited number of daily ED counts, we did not directly examine response in individuals with other, less prevalent SCD genotypes, such HbSC or thalassemia. We did not, however, observe discernable differences in RRs from models of ‘all SCD’ visits, which includes all SCD genotypes, with those from models examining HbSS, exclusively. These results may provide some indirect indication that a pronounced differential response by SCD genotype was not present in the current study population, although it is quite possible that difference in biological response exist by SCD genotype and that a larger dataset with a greater number of non-HbSS counts would have a greater ability to detect these differences.
Together, these results comprise the largest North American epidemiological analysis examining enhanced acute risk from urban air pollution among individuals with SCD. The observed RRs, while small, were comparable to estimates we have previously reported in other sensitive populations in Atlanta, as well as those reported in other non-North American studies of air pollution and SCD. We believe these findings collectively contribute to the existing evidence that individuals with SCD may experience increased risk from urban air pollution, and traffic-related air pollution exposures, specifically. We also observed higher risks for SCD-related ED visits among male children 18 years old and younger. Broadly, these results are consistent with our prior hypothesis that individuals with SCD may be at greater risk from air pollution, given current mechanistic understandings of underlying disease pathophysiology and the ability of specific pollutant component to elicit oxidative stress-mediated acute response. While difficult to infer from a population-based observational study, it is possible that exposure to this ubiquitous pollutant source may trigger perturbations in several oxidative stress regulatory pathways. Support for this hypothesis may lie in other findings, conducted in small panels of individuals with asthma, which specifically implicate traffic pollution as a driver of changes in arginine metabolism (Ladva et al. 2018; Liang et al. 2018; Liang et al. 2019). Although speculative, it is plausible that dysregulation of this pathway for those with SCD is also involved in acute vaso-occlusion and hemolysis.
More intriguingly, the results may also point to societal and behavioral interventions for reducing exposure and mitigating adverse response in some individuals with SCD. In addition to providing support for policies aimed at lowering air pollutant emissions, these findings, along with results from mechanistic panel-based analyses, may signal a possibility of therapeutic intervention aimed at preventing specific metabolic dysregulation that potentially may be at the core of several SCD pathophysiologic responses.
Supplementary Material
Highlights.
Evidence of risk from urban air pollution for SCD exacerbations
Strongest observed associations linked to traffic related pollutants
Higher risks among male children, 18 years old and younger
ACKNOWLEDGEMENTS
This research was made possible by grants to Emory University from the US Environmental Protection Agency (USEPA; R82921301), the National Institute of Environmental Health Sciences (R01ES11294), and the Electric Power Research Institute (EP-P27723/C13172 and EP-P4353/C2124). Research reported in this publication was also supported by the National Institutes of Health-National Center for Complementary and Integrative Health grant K24AT009893 (to CRM). The content of this publication is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the USEPA. Further, USEPA does not endorse the purchase of any commercial products or services mentioned in the publication.
Footnotes
Declaration of interests
☐The authors declare the following financial interests/personal relationships which may be considered as potential competing interests:
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Ballas SK, Gupta K, Adams-Graves P. 2012. Sickle cell pain: A critical reappraisal. Blood 2012–2004-383430. [DOI] [PubMed] [Google Scholar]
- Barbosa SM, Farhat SC, Martins LC, Pereira LA, Saldiva PH, Zanobetti A, et al. 2015. Air pollution and children’s health: Sickle cell disease. Cad Saude Publica 31:265–275. [DOI] [PubMed] [Google Scholar]
- Belcher JD, Mahaseth H, Welch TE, Otterbein LE, Hebbel RP, Vercellotti GM. 2006. Heme oxygenase-1 is a modulator of inflammation and vaso-occlusion in transgenic sickle mice. The Journal of Clinical Investigation 116:808–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belhassen L, Pelle G, Sediame S, Bachir D, Carville C, Bucherer C, et al. 2001. Endothelial dysfunction in patients with sickle cell disease is related to selective impairment of shear stress-mediated vasodilation. Blood 97:1584–1589. [DOI] [PubMed] [Google Scholar]
- Beutler E 1975. The effect of carbon monoxide on red cell life span in sickle cell disease. Blood 46:253–259. [PubMed] [Google Scholar]
- Braga A, Barbosa S, Farhat S, Martins L, Pereira L, Saldiva P, et al. 2006. The expanding burden of air pollution on health: The case of sickle cell disease. Epidemiology 17(6):S223–S224. [Google Scholar]
- Brook RD, Urch B, Dvonch JT, Bard RL, Speck M, Keeler G, et al. 2009. Insights into the mechanisms and mediators of the effects of air pollution exposure on blood pressure and vascular function in healthy humans. Hypertension 54:659–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clougherty JE. 2011. A growing role for gender analysis in air pollution epidemiology. Cien Saude Colet 16:2221–2238. [DOI] [PubMed] [Google Scholar]
- Cohen RT, Madadi A, Blinder MA, DeBaun MR, Strunk RC, Field JJ. 2011. Recurrent, severe wheezing is associated with morbidity and mortality in adults with sickle cell disease. American Journal of Hematology 86:756–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon JK. 2002. Kids need clean air: Air pollution and children’s health. Fam Community Health 24:9–26. [DOI] [PubMed] [Google Scholar]
- Enwonwu C 1989. Increased metabolic demand for arginine in sickle cell anaemia. Med Sci Res 17:997–998. [Google Scholar]
- Gilliland FD, McConnell R, Peters J, Gong H Jr., 1999. A theoretical basis for investigating ambient air pollution and children’s respiratory health. Environ Health Perspect 107 Suppl 3:403–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gladwin MT, Schechter AN, Shelhamer JH, Ognibene FP. 1999. The acute chest syndrome in sickle cell disease. Possible role of nitric oxide in its pathophysiology and treatment. American Journal of Respiratory and Critical Care Medicine 159:1368–1376. [DOI] [PubMed] [Google Scholar]
- Gladwin MT, Schechter AN, Ognibene FP, Coles WA, Reiter CD, Schenke WH, et al. 2003. Divergent nitric oxide bioavailability in men and women with sickle cell disease. Circulation 107:271–278. [DOI] [PubMed] [Google Scholar]
- Gladwin MT. 2006. Deconstructing endothelial dysfunction: Soluble guanylyl cyclase oxidation and the no resistance syndrome. Journal of Clinical Investigation 116:2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gladwin MT, Barst RJ, Gibbs JS, Hildesheim M, Sachdev V, Nouraie M, et al. 2014. Risk factors for death in 632 patients with sickle cell disease in the United States and United Kingdom. PLoS One 9:e99489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassell KL. Population estimates of sickle cell disease in the US. American Journal of Preventive Medicine. 2010. April 1;38(4):S512–21. [DOI] [PubMed] [Google Scholar]
- Health Effects Institute. 2010. Traffic-related air pollution: A critical review of the literature on emissions, exposure, and health effects. Boston, MA: Health Effects Institute. [Google Scholar]
- Jones S, Duncan ER, Thomas N, Walters J, Dick MC, Height SE, et al. 2005. Windy weather and low humidity are associated with an increased number of hospital admissions for acute pain and sickle cell disease in an urban environment with a maritime temperate climate. British Journal of Haematology 131:530–533. [DOI] [PubMed] [Google Scholar]
- Klings ES, Wyszynski DF, Nolan VG, Steinberg MH. 2006. Abnormal pulmonary function in adults with sickle cell anemia. American Journal of Respiratory and Critical Care Medicine 173:1264–1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight J, Murphy TM, Browning I. 1999. The lung in sickle cell disease. Pediatr Pulmonol 28:205–216. [DOI] [PubMed] [Google Scholar]
- Krishnan RM, Adar SD, Szpiro AA, Jorgensen NW, Van Hee VC, Barr RG, et al. 2012. Vascular responses to long- and short-term exposure to fine particulate matter MESA air (Multi-Ethnic Study of Atherosclerosis and air pollution). Journal of the American College of Cardiology 60:2158–2166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladva CN, Golan R, Liang D, Greenwald R, Walker DI, Uppal K, et al. 2018. Particulate metal exposures induce plasma metabolome changes in a commuter panel study. Plos One 13:e0203468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lara A, Khatri SB, Wang Z, Comhair SA, Xu W, Dweik RA, et al. 2008. Alterations of the arginine metabolome in asthma. American Journal of Respiratory and Critical Care Medicine 178:673–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang D, Moutinho JL, Golan R, Yu T, Ladva CN, Niedzwiecki M, et al. 2018. Use of high-resolution metabolomics for the identification of metabolic signals associated with traffic-related air pollution. Environment International 120:145–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang D, Ladva CN, Golan R, Yu T, Walker DI, Sarnat SE, et al. 2019. Perturbations of the arginine metabolome following exposures to traffic-related air pollution in a panel of commuters with and without asthma. Environment International 127:503–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mekontso Dessap AM, Contou D, Dandine-Roulland C, Hemery F, Habibi A, Charles-Nelson A, Galacteros F, Brun-Buisson C, Maitre B and Katsahian S, 2014. Environmental influences on daily emergency admissions in sickle-cell disease patients. Medicine, 93(29). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metzger KB, Tolbert PE, Klein M, Peel JL, Flanders WD, Todd KH, et al. 2003. Ambient air pollution and emergency department visits for specific cardiovascular conditions, atlanta, georgia, 1993–2000. American Journal of Epidemiology 157:S27–S27. [Google Scholar]
- Metzger KB, Tolbert PE, Klein M, Peel JL, Flanders WD, Todd K, et al. 2004. Ambient air pollution and cardiovascular emergency department visits. Epidemiology 15:46–56. [DOI] [PubMed] [Google Scholar]
- Mills NL, Tornqvist H, Robinson SD, Gonzalez M, Darnley K, MacNee W, et al. 2005. Diesel exhaust inhalation causes vascular dysfunction and impaired endogenous fibrinolysis. Circulation 112:3930–3936. [DOI] [PubMed] [Google Scholar]
- Morris CR, Kuypers FA, Larkin S, Vichinsky EP, Styles LA. 2000. Patterns of arginine and nitric oxide in patients with sickle cell disease with vaso-occlusive crisis and acute chest syndrome. Journal of Pediatric Hematology/Oncology 22:515–520. [DOI] [PubMed] [Google Scholar]
- Morris CR, Morris SM Jr, Hagar W, van Warmerdam J, Claster S, Kepka-Lenhart D, et al. 2003. Arginine therapy: A new treatment for pulmonary hypertension in sickle cell disease? American Journal of Respiratory and Critical Care Medicine 168:63–69. [DOI] [PubMed] [Google Scholar]
- Morris CR, Poljakovic M, Lavrisha L, Machado L, Kuypers FA, Morris SM, Jr. 2004. Decreased arginine bioavailability and increased serum arginase activity in asthma. American Journal of Respiratory and Critical Care Medicine 170:148–153. [DOI] [PubMed] [Google Scholar]
- Morris CR, Kato GJ, Poljakovic M, Wang X, Blackwelder WC, Sachdev V, et al. 2005. Dysregulated arginine metabolism, hemolysis-associated pulmonary hypertension, and mortality in sickle cell disease. JAMA 294:81–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris CR. 2009. Asthma management: Reinventing the wheel in sickle cell disease. Am J Hematol 84:234–241. [DOI] [PubMed] [Google Scholar]
- Morris CR, Kuypers FA, Lavrisha L, Ansari M, Sweeters N, Stewart M, et al. 2013. A randomized, placebo-controlled trial of arginine therapy for the treatment of children with sickle cell disease hospitalized with vaso-occlusive pain episodes. Haematologica 98:1375–1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- North ML, Amatullah H, Khanna N, Urch B, Grasemann H, Silverman F, et al. 2011. Augmentation of arginase 1 expression by exposure to air pollution exacerbates the airways hyperresponsiveness in murine models of asthma. Respiratory Research 12:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nur E, Biemond BJ, Otten HM, Brandjes DP, Schnog JJB. 2011. Oxidative stress in sickle cell disease; pathophysiology and potential implications for disease management. American Journal of Hematology 86:484–489. [DOI] [PubMed] [Google Scholar]
- Parent F, Bachir D, Inamo J, Lionnet F, Driss F, Loko G, et al. 2011. A hemodynamic study of pulmonary hypertension in sickle cell disease. N Engl J Med 365:44–53. [DOI] [PubMed] [Google Scholar]
- Peel JL, Tolbert PE, Klein M, Metzger KB, Flanders WD, Todd K, et al. 2005. Ambient air pollution and respiratory emergency department visits. Epidemiology 16:164–174. [DOI] [PubMed] [Google Scholar]
- Peretz A, Sullivan JH, Leotta DF, Trenga CA, Sands FN, Allen J, et al. 2008. Diesel exhaust inhalation elicits acute vasoconstriction in vivo. Environmental Health Perspectives 116:937–942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piel FB, Tewari S, Brousse V, Analitis A, Font A, Menzel S, et al. 2017. Associations between environmental factors and hospital admissions for sickle cell disease. Haematologica 102:666–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao XQ, Montresor-Lopez J, Puett R, Rajagopalan S, Brook RD. 2015. Ambient air pollution: An emerging risk factor for diabetes mellitus. Current Diabetes Reports 15. [DOI] [PubMed] [Google Scholar]
- Rees DC, Williams TN, Gladwin MT. 2010. Sickle-cell disease. The Lancet 376:2018–2031. [DOI] [PubMed] [Google Scholar]
- Sadreameli SC, Eakin MN, Robinson KT, Alade RO, Strouse JJ. 2016. Secondhand smoke is associated with more frequent hospitalizations in children with sickle cell disease. Am J Hematol 91:313–317. [DOI] [PubMed] [Google Scholar]
- Sarnat JA, Brown KW, Bartell SM, Sarnat SE, Wheeler AJ, Suh HH, et al. 2009. The relationship between averaged sulfate exposures and concentrations: Results from exposure assessment panel studies in four us cities. Environmental Science & Technology 43:5028–5034. [DOI] [PubMed] [Google Scholar]
- Sarnat SE, Sarnat JA, Klein M, Goldman G, Mulholland J, Russell AG, et al. 2008. Applying alternative approaches to characterizing air pollution exposure in an epidemiologic study in Atlanta. Epidemiology 19:S38–S38. [Google Scholar]
- Sarnat SE, Sarnat JA, Mulholland J, Isakov V, Özkaynak H, Chang HH, et al. 2013. Application of alternative spatiotemporal metrics of ambient air pollution exposure in a time-series epidemiological study in Atlanta. Journal of Exposure Science and Environmental Epidemiology 23:593–605. [DOI] [PubMed] [Google Scholar]
- Silkoff PE, Bates CA, Meiser JB, Bratton DL. 2004. Single-breath exhaled nitric oxide in preschool children facilitated by a servo-controlled device maintaining constant flow. Pediatric Pulmonology 37:554–558. [DOI] [PubMed] [Google Scholar]
- Strickland MJ, Darrow LA, Klein M, Flanders WD, Sarnat JA, Waller LA, et al. 2010. Short-term associations between ambient air pollutants and pediatric asthma emergency department visits. American Journal of Respiratory and Critical Care Medicine 182:307–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strunk RC, Brown MS, Boyd JH, Bates P, Field JJ, DeBaun MR. 2008. Methacholine challenge in children with sickle cell disease: A case series. Pediatr Pulmonol 43:924–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tewari S, Brousse V, Piel FB, Menzel S, Rees DC. 2015. Environmental determinants of severity in sickle cell disease. Haematologica 100:1108–1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolbert PE, Klein M, Peel JL, Sarnat SE, Sarnat JA. 2007. Multipollutant modeling issues in a study of ambient air quality and emergency department visits in Atlanta. Journal of Exposure Science and Environmental Epidemiology 17:S29–S35. [DOI] [PubMed] [Google Scholar]
- Trasande L TG. 2005. The role of air pollution in asthma and other pediatric morbidities. J Allergy Clin Immunol 115:689–699. [DOI] [PubMed] [Google Scholar]
- VanderJagt DJ, Kanellis GJ, Isichei C, Pastuszyn A, Glew RH. 1997. Serum and urinary amino acid levels in sickle cell disease. Journal of Tropical Pediatrics 43:220–225. [DOI] [PubMed] [Google Scholar]
- Yallop D, Duncan ER, Norris E, Fuller GW, Thomas N, Walters J, et al. 2007. The associations between air quality and the number of hospital admissions for acute pain and sickle-cell disease in an urban environment. British Journal of Haematology 136:844–848. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.