Background: A few weeks after the beginning of the outbreak of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), uncontrolled trials and in vitro studies suggested that chloroquine and hydroxychloroquine may have efficacy in treating coronavirus disease 2019 (COVID-19) (1). Although very preliminary, the results of these studies received extraordinary media coverage and several high-profile figures, including some health regulatory authorities, endorsed the use of chloroquine and hydroxychloroquine to treat COVID-19. This led to a huge demand for and substantial use of these drugs; the Centers for Disease Control and Prevention identified an 80-fold increase in new prescriptions for hydroxychloroquine, particularly by nondermatologists and nonrheumatologists, between March 2019 and March 2020 (2). However, major clinical trials have concluded that hydroxychloroquine and chloroquine are unlikely to be effective in treating or preventing COVID-19, leading the U.S. Food and Drug Administration (FDA) to suspend its emergency use authorization for these drugs on 15 June 2020 (3). Nevertheless, little is known about the impact of this massive use of chloroquine and hydroxychloroquine on the number and nature of induced adverse drug reactions (ADRs).
Objective: To use the FDA Adverse Event Reporting System (FAERS) database to quantify the change in number and type of reported ADRs associated with hydroxychloroquine and chloroquine since the beginning of the outbreak, compared with 2018 and 2019.
Methods and Findings: We extracted 21 305 reports of 152 201 suspected ADRs concerning chloroquine and hydroxychloroquine from the FAERS database from 1 January 2018 to 30 September 2020 (4). We present ADRs reported each year across several parameters: number of ADRs, patient sex, and severity and nature of ADRs.′
The number of reported ADRs for chloroquine and hydroxychloroquine more than doubled in 2020 (n = 11 493 and 89 607 ADRs) compared with the same months in 2018 (n = 4681 and 25 035 ADRs) and 2019 (n = 5131 and 37 559 ADRs). Daily and cumulative cases reported each year are presented in Figure 1, with dates of noteworthy events highlighted. The most represented countries in 2020 were Canada (n = 7276 cases), the United States (n = 1282 cases), France (n = 511 cases), Spain (n = 931 cases), and Italy (n = 379 cases). Of ADRs reported in 2020, 97.1% were considered serious (defined as fatal, life threatening, requiring hospitalization, or resulting in substantial disability or incapacity and other medically important conditions) whereas 73.4% and 84.8% were defined as serious in 2018 and 2019, respectively. Likewise, 5.1% of reported cases (n = 589) in 2020 were fatal, compared with 3.1% (n = 147) in 2018 and 1.9% (n = 97) in 2019. The types of ADRs seemed to be similar over time (Figure 2); however, a higher proportion of reported events in some categories, such as cardiac disorders, were fatal. Although the ages of patients who reported ADRs were similar in the 3 periods studied, the proportion of reported ADRs was greater among men in 2020 compared to 2018 and 2019.
Figure 1. Cumulative (.

top ) and weekly ( bottom ) ADR reports associated with chloroquine and hydroxychloroquine and related noteworthy events from January to September 2018, 2019, and 2020.
Noteworthy events that favor hydroxychloroquine or chloroquine use are shown in the green-shaded boxes, events that may potentially discourage use in the red-shaded boxes. ADR = adverse drug reaction; AZM = azithromycin; COVID-19 = coronavirus disease 2019; CQ = chloroquine; CV = cardiovascular; EUA = emergency use authorization; FDA = U.S. Food and Drug Administration; HCQ = hydroxychloroquine; RECOVERY = Randomised Evaluation of COVID-19 Therapy.
Figure 2. Distribution of the main types of reported ADRs associated with chloroquine and hydroxychloroquine from January to June 2018, 2019, and 2020.
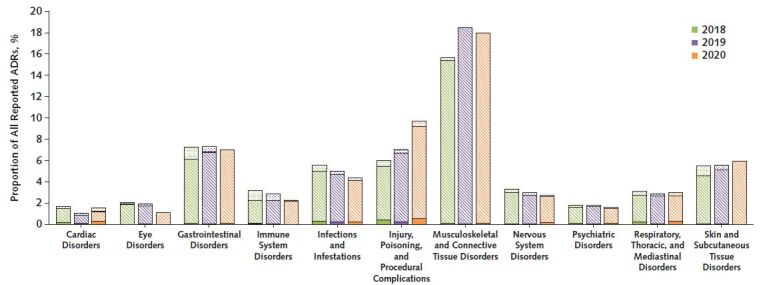
Dots represent nonserious events, stripes serious events, and solid colors fatal events. ADR = adverse drug reaction.
Discussion: Our results show an increase in reported ADRs associated with hydroxychloroquine and chloroquine use during the first months of the SARS-CoV-2 outbreak, when use of these medications was substantially higher. Reported ADRs seemed to be concomitant with positive endorsements by public leaders, media, or health regulatory authorities. The countries in 2020 with the highest reported ADRs were also those most affected by the virus during this period. Despite the FDA's revocation of its emergency use authorization, the number of reported ADRs remained high, potentially reflecting the persistent use of hydroxychloroquine across the world. The higher proportion of serious and fatal events observed in 2020 has several explanations, including changes in patient characteristics, comorbid conditions, and coprescription with other medications that may interact with these drugs, as well as higher dosages or drug pharmacokinetic modifications in patients with COVID-19. Interpretation of the results, particularly the comparison of annual reporting trends and case characteristics, is limited by underreporting and selective reporting of ADRs in pharmacovigilance databases, and some cases may have been confounded by prescription indication or patients' underlying conditions (5).
The dramatic increase in reported ADRs associated with chloroquine and hydroxychloroquine during the SARS-CoV-2 pandemic raises concerns about the potential harms of widespread use of these medications in the absence of proven benefit.
Footnotes
This article was published at Annals.org on 26 January 2021
References
- 1. Roustit M , Guilhaumou R , Molimard M , et al; French Society of Pharmacology and Therapeutics (SFPT). Chloroquine and hydroxychloroquine in the management of COVID-19: much kerfuffle but little evidence. Therapie. 2020 Jul - Aug;75:363-70. [PMID: ] doi: 10.1016/j.therap.2020.05.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kuehn BM . Shifting hydroxychloroquine patterns raise concern. JAMA. 2020;324:1600. [PMID: ] doi: 10.1001/jama.2020.20311 [DOI] [PubMed] [Google Scholar]
- 3.U.S. Food and Drug Administration. Coronavirus (COVID-19) Update: FDA Revokes Emergency Use Authorization for Chloroquine and Hydroxychloroquine. FDA news release. Accessed at www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-revokes-emergency-use-authorization-chloroquine-and on 28 December 2020.
- 4.U.S. Food and Drug Administration. Home page. Accessed at www.fda.gov/home on 4 November 2020.
- 5. Alatawi YM , Hansen RA . Empirical estimation of under-reporting in the U.S. Food and Drug Administration Adverse Event Reporting System (FAERS). Expert Opin Drug Saf. 2017;16:761-7. [PMID: ] doi: 10.1080/14740338.2017.1323867 [DOI] [PubMed] [Google Scholar]


