Objectives
Kasai portoenterostomy(KPE) is the treatment of choice for the fatal devastating infantile type III biliary atresia (BA). The study aimed to analyze short-and long-term outcomes after this procedure and their predictors in a tertiary center. Methods: We retrospectively analyzed 410 infants who underwent KPE for type III BA in the period from February 2000 to December 2019. The overall male/female ratio was 186/224. Results: The early (>6months) complications involved 187(45.6%) of our infants with a higher incidence of early cholangitis that affected 108(26.3%) of them. The jaundice clearance at the 6th post-operative month that reached 138(33.7%) of them had an independent correlation with mild portal tracts ductal and/or ductular proliferation, using postoperative steroids therapy, and absence of early postoperative cholangitis. The early infant mortality that affected 70(17.1%) of our patients was mostly from sepsis. On the other hand, late (<6months) patients complications and mortalities affected 256(62.4%) and 240(58.5%) of patients respectively; moreover, liver failure and sepsis were the most frequent causes of late mortalities in non-transplanted and transplanted cases respectively. Lastly, the long-term (20-year) native liver survival (NLS) that reached 91(22.2%) of patients had an independent correlation with age at operation ≤ 90 days, higher preoperative mean serum alb, portal tract fibrosis grades F0 and F1, absence of intraoperative bleeding, absence of post-operative cholangitis, the occurrence of jaundice clearance at the 6th postoperative month and absence of post-operative portal hypertension (PHN). Conclusions: Sepsis had a direct effect on early and late patient mortalities after Kasai operation for type III BA; moreover, patient age at operation<90 days, higher fibrosis grades, the occurrence of postoperative cholangitis and PHN, and persistence of post-operative jaundice had negative insult on long-term postoperative outcome. So, it is crucial to modulate these factors for a better outcome.
Keywords: Biliary atresia, Short-term outcome, Long-term outcome, Survival with the native liver
Highlights
-
•
Sepsis had a direct effect on early and late patient mortalities after Kasai operation for type III BA.
-
•
Patient age at operation<90 days and higher fibrosis grades had negative insult on long-term postoperative outcome.
-
•
The occurrence of postoperative cholangitis and PHN affected long-term postoperative outcome negatively.
-
•
Persistence of post-operative jaundice had negative effect on long-term postoperative outcome.
List of abbreviations
- BA
Biliary atresia
- PHN
Portal hypertension
- ESLD
End-stage liver disease
- LFTs
Liver function tests
- US
Ultrasonography
- HIDA
Hepatobiliary iminodiacetic acid
- KPE
Kasai portoenterostomy
- NLS
Native liver survival
- LT
Liver transplantation
- INR
International normalization ratio
- UDCA
Ursodeoxycholic acid
- HPB
Hepatopancreatobiliary
- NLI
National Liver Institute
- IRB
Institutional review board
- POD
Post-operative day
- BASM
Biliary atresia splenic malformation
- TC
Triangular cord
- GO
Gastroesophageal
- IOC
Intra-operative cholangiography
- JSPS
Japanese society of pediatric surgeons
- CHD
Common hepatic duct
- CBD
Common bile duct
- PV
Portal vein
- PDS
Polydioxanone
- RUQ
Right upper quadrant
- TB
Total bilirubin
- TLS
Transplanted liver survival
- CT
Computed tomography
- GIT
Gastrointestinal tract
- VSD
Ventricular septal defect
- ASD
Atrial septal defect
- PFO
Patent foramen oval
- PDA
Patent ductus arteriosus
- DB
Direct bilirubin
- AST
Aspartate transaminase
- ALT
Alanine transaminase
- ALP
Alkaline phosphatase
- GGT
Gamma glutamate transferase
- Alb
Albumin
- CDS
Clavien Dindo system
- UTI
Urinary tract infection
- ARDS
Adult respiratory distress syndrome
- MOF
Multi-organ failure
- HPS
Hepatopulmonary syndrome
- PVT
Portal vein thrombosis
- HAT
Hepatic artery thrombosis
- TFS
Transplant free survival
- SNL
Survival of patient with native liver
- VH
Variceal hemorrhage
1. Introduction
Biliary atresia (BA) is a neonatal progressive destructive fibro-obliterative cholangiopathy of extra- and intra-hepatic biliary system with fatal outcome in the 1st 2 years of life if left untreated due to the resulting liver cirrhosis, portal hypertension (PHN), end-stage liver disease (ESLD) and liver failure [[1], [2], [3], [4], [5]]. Despite being a disease of unknown origin; viral infection, ductal plate malformations, genetic predisposition, and abnormal bile acid metabolism are possible causes [6]; while, immunologic, inflammatory, infectious, and obstructive pathways are possible theories of pathogenesis [7]. Moreover, type III BA that is characterized by biliary obstruction at the level of the porta hepatis is the most common type (<90%) with the worst prognosis [8,9].
However, early detection of this serious disease is fundamental for early surgery and better prognosis [10]. Timely Kasai portoenterostomy (KPE) by resecting the whole atretic part of the extrahepatic biliary tree and creating an anastomosis between a Roux-en-Y limb of the jejunum and the portal plate at porta hepatis remains the gold standard, first and the mainline of treatment of this devastating disease aiming to facilitate bile flow, clear jaundice and delay liver consequences leading to improved outcome [1,2].
Despite their improvement in the recent decades; jaundice clearance and long-term (20-year) native liver survival (NLS) after KPE are still in the wide ranges of 29–82% [8,[11], [12], [13], [14], [15], [16], [17]] and 14–44% [2,13,[18], [19], [20], [21]] of patients respectively, while the remaining patients with the poor outcome will need liver transplantation (LT) despite its associated limited donor availability, difficulties, morbidities, and mortalities or they will die from liver failure [16,17,[22], [23], [24], [25]]; so it is crucial to modulate factors affecting those early and/or late outcomes after KPE to improve them aiming to delay or reduce the need for LT [13].
These factors can be classified into pre-/intra-operative factors (i.e. age at surgery, surgical center and surgeon experience, liver fibrosis degree, presence and size of bile ductules at the portal plate, associated anomalies, anatomical type of BA, international normalization ratio(INR), etc) as well as postoperative parameters (i.e. using a steroid, antibiotic and/or ursodeoxycholic acid (UDCA), jaundice clearance, the occurrence of cholangitis/PHN, intrahepatic biliary cyst formation, etc) [[8], [9], [10],[26], [27], [28]]. Finally, as there is scanty literature on the long-term (20-year) outcome and its predictors after KPE for type III BA, our work aimed to analyze this important issue besides analyzing short-term outcome and its predictors in a tertiary Egyptian hepato-pancreato-biliary(HPB) center.
1.1. Patients and methods
Four hundred fifty patients underwent KPE for type III BA in the period from February 2000 to the end of 2019 in the department of HPB surgery (Higher tertiary referral center), National Liver Institute (NLI), University of Menoufia, Menoufia, Egypt. Our cohort study included 410 patients with the following inclusion criteria: infants with a sure diagnosis of BA, Type III BA, completed KPE and operation done at our Institute, however, the exclusion criteria were cases that did not complete the follow-up, cases with data loss and cases of research refusal. It is a single institutional retrospective analysis of a prospectively collected database that analyzed the short- and long-term outcomes after this operation in the period from the beginning of 2000 to mid-2020 after approval of our institutional review board (IRB); where patients were observed from POD1 until the end of June 2020 or until the death of patients with a median follow up period of 44 (range, 0.2–243) months [29,30]. The study was registered in the Chinese clinical trial registry with registration NO of ChiCTR2000038541(Web site: Chinese Clinical Trial Registry (ChiCTR))
The data were collected from our records in our HPB surgery and pediatric hepatology departments where written informed consents regarding surgeries and researches were obtained from the parents/guardians. Our work has been reported in line with the STROCSS criteria.
The recorded data included pediatric patients demographics, type of BA(Non-cystic III or cystic III), associated congenital anomalies(biliary atresia splenic malformation(BASM), cardiac or other anomalies), the onset of neonatal jaundice(since or after birth), preoperative labs(i.e. LFTs, INR, CBC, etc), preoperative abdominal US findings(hepatomegaly, splenomegaly, abnormal gallbladder(atrophic, non-contractile or absent), hepatic subcapsular flow, and the presence or absence of triangular cord(TC) sign). Preoperative PHN was identified by the presence of thrombocytopenia (platelets>150 K/μL) associated with splenomegaly on US ± gastroesophageal(GO) varices [32]. Intra-operative liver biopsy and biopsy of the excised atretic biliary tree findings, operative bleeding, blood, and plasma transfusion, operative time and postoperative hospital stay, post-operative medications, patient short- and long-term outcomes, and lastly follow up data.
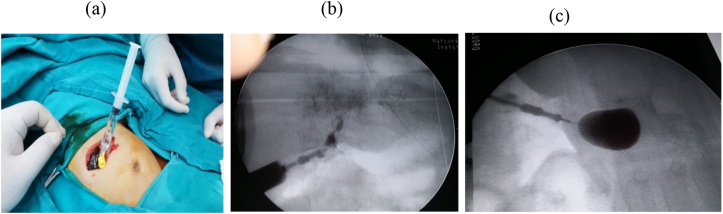
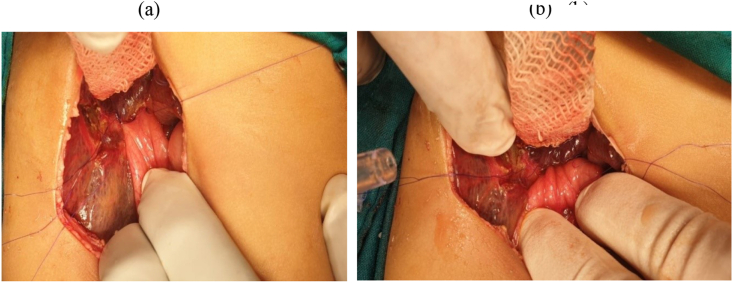
BA was diagnosed clinically (persistent jaundice, dark urine, clay stool, etc), biochemically (liver function tests (LFT), etc), by imaging (the abdominal ultrasonography (US), hepatobiliary iminodiacetic acid (HIDA) scan, etc), by duodenal intubation and measurement of intraluminal bile, pathologically (preoperative liver biopsy findings) and confirmed by operative exploration, intra-operative cholangiography(IOC) that was done whenever possible; Fig. 1(a,b,c) and by operative liver biopsy and biliary remnant pathologic findings. The macroscopic classification of BA was based on the Japanese society of pediatric surgeons (JSPS) classification where Non-cystic type III BA and cystic type III meant occlusion at the level of the porta-hepatis without and with the presence of cyst at the liver hilum respectively; Fig. 1, Fig. 2 and Fig. 1(c) respectively [8,20,33] (see Fig. 3).
Fig. 1.
a- Preparation for IOC, b- IOC shows non cystic type III BA, c- IOC shows cystic type III BA.
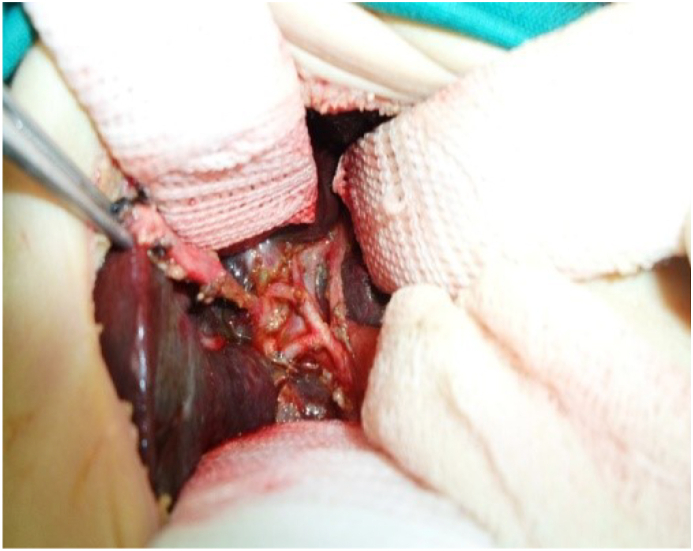
Fig. 2.
Non cystic type III BA.
Fig. 3.
Atrophic gallbladder.
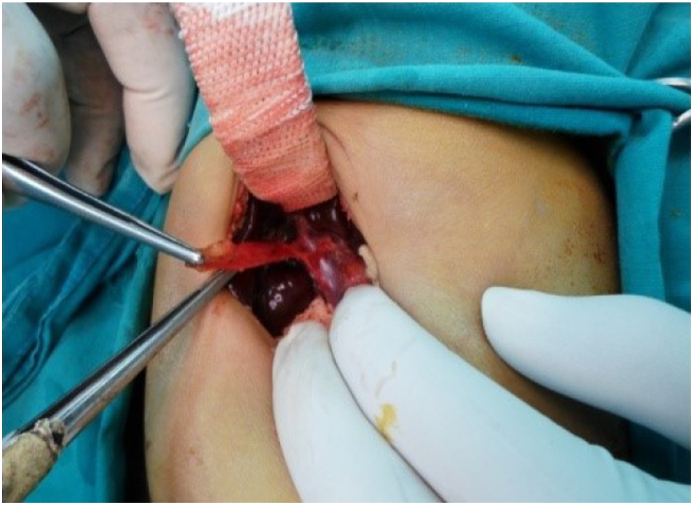
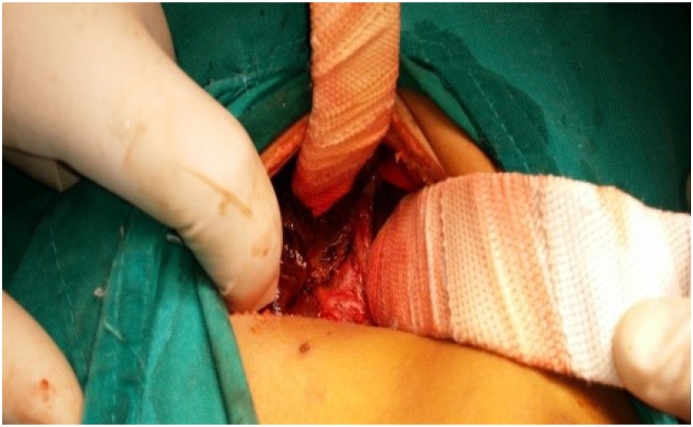
The surgical technique: Under general anesthesia by the anesthetic author of the manuscript, it was induced by inhalation of 100% oxygen and 8% sevoflurane until loss of consciousness occurred, then concentration of sevoflurane was decreased to 4%, then fentanyl 1 ug/kg and rocuronium 0.9 mg/kg were administered to facilitate oral tracheal intubation, then anesthesia was maintained by 50% oxygen/air and sevoflurane. Surgery was performed by qualified HPB surgeons who used identical techniques. In brief; a small incision in the right upper abdomen was done, then after its entry; the abdomen was grossly inspected to identify any associated anomalies. In many cases, the diagnosis of BA was confirmed by visual inspection of the liver that appeared cholestatic or fibrotic with absent, fibrotic, or atrophic gallbladder; figure [3]. However, if a normal gallbladder or hilar cyst appeared, cholangiography was performed through them to confirm the diagnosis; figure [1]. Once BA was confirmed and without liver mobilization; the gallbladder remnant was dissected from the liver bed and followed to the junction with the common hepatic duct(CHD), then the fibrous cord representing the distal common bile duct(CBD) remnant was dissected, isolated, and divided, allowing further dissection of the biliary remnant up to the portal vein (PV) bifurcation, then the dissected tissue was transected at this level of the portal plate with either a knife or sharp micro-scissor; Fig. 4, then KPE reconstruction was completed between the transected fibrous portal plate at the liver hilum and 35–50 cm Roux-en-Y jejunal limb using 5–0 or 6–0 polydioxanone(PDS) sutures, allowing drainage of bile from the small ductules located within the portal plate; Fig. 5. Lastly, a tube drain was put in the right upper quadrant (RUQ) and a wedge liver biopsy was taken before abdominal closure [2,34].
Fig. 4.
Portal plate after dissection and transaction of biliary remnant and before reconstruction.
Fig. 5.
a- KPE (Posterior layer), b- KPE (Anterior layer).
The histopathology of the liver biopsy and the biliary remnants included the followings: 1- Portal tract ductal and/or ductular proliferation that was classified according to a semi-quantitative scoring system [35] into mild (presence of 5–9 bile ducts per portal tract), moderate (≥10 bile ducts per portal tract) and marked proliferation ((≥10 bile ducts per portal tract with elongated, attenuated and angulated ducts) 2- Portal tract fibrosis that was graded according to Metaver fibrosis score [36] into F0 (no fibrosis), F1 (fibrous portal expansion), F2 (few bridges or septa) F3 (numerous bridges or septa) and F4 (cirrhosis) 3- Presence of macrophages or giant cells in portal tracts 4- The presence of remnant ducts at the porta-hepatis and their size(<or >150 μm).
Our protocol of postoperative medical and nutritional management of all patients was nearly the same during the study period and included: 1- Prophylactic antibiotics in the form of I.V. 3rd generation cephalosporin + metronidazole till the 6th postoperative day then oral cotrimoxazole for 6 months 2- Ursodeoxycholic acid (10–15 mg/kg divided into 3 doses); a choleretic drug that was given with the beginning of oral intake for 3months 3- Lipid, and lipid-soluble vitamins given for 3months 0.4- According to the treating physician's preference, some patients were given post operative steroids(oral prednisolone 2 mg/kg/day from day 5 to day 21, then 1 mg/kg/day from day 22 to day 28) [37]. Liver biopsy result, The post-KPE outcome of patients: It was classified into 1- Short-term (<6months) morbidities and mortalities (N.B cholangitis was known by fever accompanied by elevated serum bilirubin (<2.5 mg/dL), leukocytosis, and stool color change [38], however, PHN was identified as mentioned before [32], 2-Jaundice clearance at the 6th postoperative month (when serum total bilirubin(TB)≤2 mg/dl) and its predictors, 3- The early (6 months) NLS and overall survival(NLS ± transplanted liver survival (TLS)), 4- Long-term(<6months) morbidities and mortalities, 5- Long-term NLS and its predictors, 6- LT after KPE including its time and post LT survival,7- Long-term overall survival (NLS ± TLS). Moreover, for detection of the previous parameters of the outcome, patients were followed-up monthly for the 1st postoperative 6 months then 6 monthly until the end of the follow-up period by clinical evaluation, laboratory values, US, and others if needed (i.e. computed tomography (CT), upper gastrointestinal tract (GIT) endoscopy, etc).
Statistical Techniques: The data were processed with SPSS software (Statistical Product and Service Solutions, version 21, SSPS Inc, Chicago, IL, USA). Non-numerical data were expressed in frequency and % and were analyzed with the Qui square or Fisher exact tests. Numerical data were expressed as the mean and standard deviation or median and range and were compared with the T or Mann whitteny test. Univariate analysis and then multivariate analysis (by Binary logistic regression method or Cox regression method) were done to detect the relationship between the different pre-, intra-, and post-operative variables and jaundice clearance at the 6th-month post-operatively as well as the relation between these variables and long-term(20-year) NLS. The Kaplan–Meier method was used for survival analysis to assess the long-term NLS and its predictors as well as the overall survival, a P-value of <0.05 was significant [29,30].
2. Results
2.1. The characteristics of infants
They were categorized into 186(45.4%) males, and 224(54.6%) females; their median age at operation was 65 (range, 30–135) days, moreover, ages <60 days (A), 60–90 days (B) and >90 days(C) were 90(22%), 225(54.9%), and 95 (23.2%) of our infants respectively. Their median weight and height reached 4.5(range, 3–7.5) Kg, and 56 (range,49–66) cm respectively. Type III and type III cystic BA affected 390(95.1%) and 20(4.9%) of cases respectively. BA splenic malformation (BASM), cardiac anomalies (i.e. Ventricular septal defect (VSD), atrial septal defect(ASD), patent foramen oval(PFO), and patent ductus arteriosus(PDA)) and other anomalies (i.e. Situs inversus totalis, preduodenal portal vein, etc) were present in13(3.2%), 7(1.7%), and 9(2.2%) of them respectively. The jaundice was observed since and during the 1st week after birth in 268(65.4%) and 142(34.6%) of patients respectively. Preoperative laboratory values are shown in Table 1; moreover, preoperative PHN reached 7.3% of patients. According to preoperative US findings; hepatomegaly, splenomegaly, abnormal gallbladder, hepatic subcapsular flow, and positive TC sign were seen in 190(46.3%), 164(40%), 336(82%), 391(95.4%), and 142(34.6%) of our patients respectively. Table 1.
Table 1.
The characteristics of infants.
| Category | No (%) 410 (100%) Or Median(range) |
|---|---|
| Age at operation(days) (Median(range)) A (<60 days) B (60–90 days) C (>90 days) |
65(30–135) 90 (22%) 225 (54.9%) 95 (23.2%) |
| Gender Males Females |
186 (45.4%) 224 (54.6%) |
| Weight(KG) (Median(range)) | 4.5(3–7.5) |
| Height(Cm) (Median(range)) | 56(49–66) |
| Type of BA Non-cystic III Cystic III |
390 (95.1%) 20 (4.9%) |
| Associated anomalies BASM Cardiac Others |
13 (3.2%) 7 (1.7%) 9 (2.2%) |
| Onset of jaundice Since birth After birth |
268 (65.4%) 142 (34.6%) |
| Preoperative labs (Median(range)) TB(mg/dL) DB(mg/dL) AST(U/L) ALT(U/L) ALP(U/L) GGT(U/L) Alb(g/dL) INR Platelets (K/uL) |
11.9(4.3–32) 8.5(3–23) 170(51–955) 130(19–4940) 576(131–2175) 828.5(104–3310) 3.6(2.4–4.6) 1(0.8–1.3) 387 (121–1123) |
| Preoperative PHN | 30 (7.3%) |
| Findings in the preoperative US Hepatomegaly Splenomegaly Abnormal gallbladder Hepatic subcapsular flow Positive TC sign |
190 (46.3%) 164 (40%) 336 (82%) 391 (95.4%) 142 (34.6%) |
| Liver portal tracts biopsy findings Ductal and/or ductular proliferation Mild Moderate Marked Fibrosis F0 F1 F2 F3 F4 Presence of macrophages Presence of giant cells Porta hepatis remnant duct size >150 μm <150 μm No ducts |
212 (51.7%) 136 (33.2%) 62 (15.1%) 17 (4.1%) 190 (46.3%) 144 (35.1%) 40 (9.8%) 19 (4.6%) 230 (56.1%) 75 (18.3%) 210 (51.2%) 108 (26.3%) 92 (22.4%) |
| Operative bleeding | 24 (5.9%) |
| Blood transfusion | 6 (1.5%) |
| Plasma transfusion | 23 (5.6%) |
| Operative time(hours) | 4(2.5–7.5) |
| Postoperative hospital stay(days) (Median(range)) | 8(5–70) |
| Postoperative steroid use | 116 (28.3%) |
BA: Biliary atresia, BASM: Biliary atresia splenic malformation, TB: Total bilirubin, DB: Direct bilirubin, AST: Aspartate transaminase, ALT: Alanine transaminase, ALP: Alkaline phosphatase, GGT: Gamma glutamate transferase, Alb: Albumin, INR: International normalization ratio, PHN: Portal hypertension, TC: Triangular cord.
The histopathology of the intra-operative liver biopsy and the excised extrahepatic biliary tree showed the followings: 1- Mild, moderate, and marked portal tract ductal and/or ductular proliferation in 212(51.7%), 136(33.2%), and 62(15.1%) of patients respectively,2- Portal tracts fibrosis grades F0, F1, F2, F3, and F4 in17(4.1%), 190(46.3%), 144(35.1%), 40(9.8%), and 19(4.6%) of them respectively, 3- Portal tracts macrophages and giant cells in 230(56.1%), and 75(18.3%) of them respectively, 4- Porta hepatis remnant duct size >150μm,<150μm and no ducts in 210(51.2%), 108(26.3%), and 92(22.4%) of our cases respectively. Operative bleeding affected 24(5.9%) of patients where blood and plasma were transfused to 6(1.5%) and 23(5.6%) of them respectively. The median operative time and postoperative hospital stay were 4 (range, 2.5–7.5) hours and 8 (range, 5–70) days respectively. Lastly, postoperative steroid therapy was used in 116(28.3%) of patients according to the treating physician preference. Table 1.
2.2. Short-term outcome
The early (<6 months post-Kasai) complications involved 187 (45.6%) of our pediatric patients where the single patient was affected by single or multiple complications; they were classified regarding Clavien Dindo system (CDS) as 112 (27.3%), 7(1.7%), and 68(16.6%) grades II, III, and V respectively (we recorded the highest CDS in patients with multiple complications). Table 2.
Table 2.
Short-term outcome.
| Character | NO (%) 410 (100%) Or Median(range) |
|---|---|
| Early (≤6 months post Kasai) complications Infection Cholangitis UTI Chest infection Wound infection Abdominal collection Ascites Intestinal leak Bile leak Intestinal obstruction PHN Bleeding GO varices Encephalopathy |
187 (45.6%) 172 (42%) 108 (26.3%) 26 (6.3%) 85 (20.7%) 54 (13.2%) 64 (15.6%) 49 (12%) 8 (2%) 7 (1.7%) 5 (1.2%) 54 (13.2%) 8 (2%) 30 (7.3%) |
| CDS of early complications II III V |
112 (27.3%) 7 (1.7%) 68 (16.6%) |
| Post operative bilirubin level TB at 1 month (mg/dL) (Median(range)) DB at 1 month (mg/dL) (Median(range)) TB at 3 months (mg/dL) (Median(range)) DB at 3 month(mg/dL) (Median(range)) TB at 6 month (mg/dL) (Median(range)) DB at 6 month (mg/dL) (Median(range)) Jaundice clearance at 6 months |
8(0.6–19) 5.5(0.3–16) 7(0.4–19.1) 5.05(0.2–15) 6 (0.3–17.7) 4.5(0.1–16) 138 (33.7%) |
| Early patient(≤6 months) mortality The main cause of death Sepsis Liver failure GIT bleeding Aspiration pneumonia ARDS MOF |
70 (17.1%) 34 (8.3%) 25 (6.1%) 8 (2%) 1 (0.2%) 1 (0.2%) 1 (0.2%) |
| LT before or at 6months post-Kasai | 3 (0.7%) |
| Early (6 months) NLS Early (6 months) NLS (months) (Median(range)) Early (6 months) overall survival(NLS ± TLS) Early (6 months) overall survival (months) (Median(range)) |
339 (82.7%) 6(0.2–6) 340 (82.9%) 6(0.2–6) |
UTI: Urinary tract infection, PHN: Portal hypertension, GO: Gastro-esophageal, CDS: Calvien Dindo system, TB: Total bilirubin, DB: Direct bilirubin, GIT: Gastrointestinal system, ARDS: Adult respiratory distress syndrome, MOF: Multi-organ failure, LT: Liver transplantation, NLS: Native liver survival, TLS: Transplanted liver survival.
The early infection affected 172(42%) of infants in the form of cholangitis, urinary tract infection (UTI), chest infection, and wound infection that affected 108(26.3%), 26(6.3%), 85(20.7%), and 54(13.2%) of patients respectively. Cholangitis occurred in single or multiple episodes that were managed conservatively by hydration, steroids, and antibiotics with improvement in some patients and mortality from sepsis in the others (Clavien II, V). However, all cases with UTI improved after antibiotic therapy (Clavien II). On the other hand, some cases with chest infection improved after medical treatment; however, the other cases progressed to pneumonia and died from sepsis (Clavien II, V). Lastly, wound infection improved after treatment with antibiotics, wound care, and/or 2ry sutures for burst abdomen (Clavien II, III). Table 2.
Sixty-four (15.6%) of our infants were complicated with early abdominal collection in the form of ascites, intestinal and biliary leaks that affected 49 (12%), 8(2%), and 7(1.7%) of them respectively. Regarding ascites; it improved after diuretic therapy except for some cases that died from liver failure and others that underwent LT (Clavien II, III, V). However, the intestinal leak was managed surgically with success in some cases and mortality from sepsis in the others (Clavien III, V). Lastly, bile leak was treated conservatively or surgically with success in some patients and failure in others (Clavien II, III, V). Table 2.
The early intestinal obstruction that involved 5(1.2%) of infants was managed conservatively or by surgical exploration with improvement in some of them and death from sepsis in the others (Clavien II, V). On the other hand, 54 (13.2%) of our cases had early PHN with bleeding GO varices in 8 of them; these bleeding varices were managed by endoscopic injection sclerotherapy after resuscitation with unfortunate failure and mortality in all cases from massive GIT bleeding (Clavien V). Lastly, encephalopathy affected 30(7.3%) of patients who were given anti-coma measures with success in some patients and LT or mortality from liver failure in the others (Clavien II, III, V). Table 2.
The median total and direct bilirubin at1,3, and 6 post operative months were 8 mg/dL (0.6–19) and 5.5 mg/dL (0.3–16), 7 mg/dL (0.4–19.1) and 5.05 mg/dL (0.2–15), and 6 mg/dl(range, 0.3–17.7), and 4.5 mg/dL (range, 0.1–16), respectively. However, jaundice clearance at the 6th post operative month reached 138(33.7%) of our infants. Table 2.
The early patient mortality that affected 70(17.1%) of our patients was due to sepsis, liver failure, GIT bleeding, aspiration pneumonia, adult respiratory distress syndrome (ARDS), and multi-organ failure (MOF) that involved 34(8.3%), 25 (6.1%), 8(2%),1(0.2%), 1(0.2%), and 1(0.2%) of them respectively. Lastly, the early (6 months) NLS and overall survival were 339(82.7%), and 340(82.9%) respectively. Table 2.
Predictors of jaundice clearance at the 6th post-operative month:
On univariate analysis; all studied variables except patient gender, and mean preoperative (alanine transaminase (ALT), alkaline phosphatase(ALP), platelets, and gamma glutamate transferase(GGT)) were significant predictors of jaundice clearance at the 6th postoperative month, however, ALP and platelets had a trend towards significance. On the other hand, on multivariate analysis, mean preoperative direct bilirubin(DB) of 7.8 ± 2.6 mg/dl, mean Aspartate transaminase(AST) of 160.6 ± 68.3 U/L, mild portal tracts ductal and/or ductular proliferation, using postoperative steroids therapy and absence of early cholangitis post-operatively were the independent predictors of it, moreover, cystic type III BA and shorter operative time had a trend towards independent correlation with it. Table 3.
Table 3.
Predictors of jaundice clearance at the 6th postoperative month.
| Character | Jaundice clearance No = 138 |
No clearance No = 272 |
P-value Univariate analysis |
P-value Multivariate analysis |
|---|---|---|---|---|
| Age(days) (Mean ± SD) | 63.1 ± 14.2 | 73.5 ± 18.1 | 0.000 | >0.05 |
| Age category A (<60 days) B (60–90 days) C (>90 days) |
47 (34.1%) 84 (60.9%) 7 (5.1%) |
43 (15.8%) 141 (51.8%) 88 (32.4%) |
0.000 | >0.05 |
| Gender Males Females |
63 (45.7%) 75 (54.3%) |
123 (45.2%) 149 (54.8%) |
<0.05 | |
| Type of BA III III, cystic |
123 (89.1%) 15 (10.9%) |
267 (98.2%) 5 (1.8%) |
0.000 | 0.1 |
| Associated anomalies | 2 (1.4%) | 27 (9.9%) | 0.001 | >0.05 |
| BASM | 1 (0.7%) | 12 (4.4%) | 0.034 | >0.05 |
| Preoperative labs (Mean ± SD) TB(mg/dL) DB(mg/dL) AST(U/L) ALT(U/L) Alka phos(U/L) GGT(U/L) Alb(g/dL) INR Platelets (K/uL) |
10.8 ± 3.5 7.8 ± 2.6 160.6 ± 68.3 137.8 ± 74.6 588.9 ± 288.7 1078.8 ± 799.1 3.8 ± 0.5 1 ± 0.06 436.7 ± 162.5 |
12.9 ± 3.6 9.9 ± 3.3 236.2 ± 142.4 163.2 ± 305.1 635.9 ± 273.2 960.7 ± 731.8 3.4 ± 0.6 1.05 ± 0.1 398.3 ± 199.5 |
0.000 0.000 0.000 <0.05 0.1 <0.05 0.000 0.000 0.05 |
>0.05 0.003 0.001 >0.05 >0.05 >0.05 >0.05 |
| Portal tracts ductal and/or ductular proliferation Mild Moderate Marked |
119 (86.2%) 17 (12.3%) 2 (1.4%) |
93 (34.2%) 119 (43.8%) 60 (22.1%) |
0.000 | 0.03 |
| Portal tract fibrosis F0 F1 F2 F3 F4 |
11 (8%) 103 (74.6%) 19 (13.8%) 5 (3.6%) 0 |
6 (2.2%) 87 (32%) 125 (46%) 35 (12.9%) 19 (7%) |
0.000 | >0.05 |
| Presence of portal tracts macrophages | 44 (31.9%) | 186 (68.4%) | 0.000 | >0.05 |
| Presence of portal tracts giant cells | 7 (5.1%) | 68 (25%) | 0.000 | >0.05 |
| Remnant duct size at porta hepatis >150 μm <150 μm No ducts |
110 (79.7%) 19 (13.8%) 9 (6.5%) |
100 (36.8%) 89 (32.7%) 83 (30.5%) |
0.000 | >0.05 |
| Operative bleeding | 0 | 24 (8.8%) | 0.000 | >0.05 |
| Operative time(hours) (Mean ± SD) | 3.8 ± 1 | 4.4 ± 1.1 | 0.000 | 0.1 |
| Postoperative steroids | 94 (68.1%) | 22 (8.1%) | 0.000 | 0.000 |
| Early cholangitis | 10 (7.2%) | 98 (36%) | 0.000 | 0.001 |
| Early PHN | 1 (0.7%) | 53 (19.5) | 0.000 | >0.05 |
BA: Biliary atresia, BASM: Biliary atresia splenic malformation, TB: Total bilirubin, DB: Direct bilirubin, AST: Aspartate transaminase, Alb: Albumin, INR: International normalization ratio, PHN: Portal hypertension.
2.3. Long-term outcome
Two hundred fifty sexes (62.4%) of our patients had late (>6months) complications; moreover, CDS grades II, III, and V involved 7(1.7%), 19 (4.6%), and 230(56.1%) of them respectively.
The post-Kasai cholangitis (early and/or late), Late cholangitis, and cholangitis within 2 years of operation affected 175(42.7%), 99(24.1%), and 161 (39.3%) of patients respectively(i.e. most of the cholangitis episodes started in the 1st 2 years after KPE); they occurred in single or multiple attacks and were treated conservatively or by intervention radiologic drainage for the cases complicated with biliary lakes; however, the management was effective in some patients and unsuccessful with death from sepsis in the others (Clavien II, III, V). On the other hand, 15(3.7%) of cases had a late chest infection that was treated medically with success in some patients and mortality from sepsis in the others (Clavien II, V). Table 4.
Table 4.
Long-term outcome.
| Character | NO (%) 410 (100%) Or Median(range) |
|---|---|
| Late (>6 months post-Kasai) complications Cholangitis(Early and/or late) Early cholangitis Late cholangitis Cholangitis within 2 years Chest infection PHN(early and/or late) Late PHN GO varices ± GIT bleeding HPS Incisional hernia Intestinal obstruction Encephalopathy Ascites |
256 (62.4%) 175 (42.7%) 108 (26.3%) 99 (24.1%) 161 (39.3%) 15 (3.7%) 239 (58.3%) 193 (47.1%) 163 (39.8%) 34 (8.3%) 3 (0.7%) 7 (1.7%) 43 (10.5%) 186 (45.4%) |
| CDS of late complications II III V |
7 (1.7%) 19 (4.6%) 230 (56.1%) |
| LT after Kasai Onset of LT(months) (Median(range)) |
19 (4.6%) 21.5(5–118) |
| Late patient(>6 months) mortality Mortality in non transplanted cases Main causes Liver failure Sepsis GIT bleeding HPS Post transplant mortality Main causes Post transplant Sepsis Chronic rejection PVT HAT |
240 (58.5%) 230 (56.1%) 149 (36.3%) 49 (12%) 18 (4.4%) 14 (3.4%) 10 (2.4%) 5 (1.2%) 3 (0.7%) 1 (0.2%) 1 (0.2%) |
| Long-term NLS NLS (months) (Median(range)) 1-year NLS 3-year NLS 5-year NLS 10-year NLS 15-year NLS 20-year NLS Long-term overall survival Overall survival (months) (Median(range)) 1-year overall survival 3-year overall survival 5-year overall survival 10-year overall survival 15-year overall survival 20-year overall survival |
91 (22.2%) 40(0.2–243) 315 (76.8%) 236 (57.6%) 204 (49.8%) 152 (37.1%) 101 (24.6%) 91 (22.2%) 100 (24.4%) 44(0.2–243) 318 (77.6%) 244 (59.5%) 211 (51.5%) 161 (39.3%) 110 (26.8%) 100 (24.4%) |
PHN: Portal hypertension, GO: Gastro-esophageal, GIT: Gastrointestinal system, HPS: Hepatopulmonary syndrome, CDS: Calvien Dindo system, LT: Liver transplantation, PVT: Portal vein thrombosis, HAT: Hepatic artery thrombosis, NLs: Native liver survival.
PHN (early and/or late) and late PHN affected 239(58.3%) and 193(47.1%) of our patients respectively with late GO varices ± GIT bleeding in 163 of them; those varices were managed by endoscopic injection sclerotherapy and/or by band ligation in elder children with success in most cases and failure in the remaining patients that died from massive GIT bleeding despite adequate resuscitation and management (Clavien II, III, V). On the other hand, hepatopulmonary syndrome (HPS) was diagnosed in 34(8.3%) of patients with mortality in 14 of them despite aggressive support and management. (Clavien II, V) Table 4.
The postoperative late incisional hernia and intestinal obstruction complicated 3 (0.7%), and 7(1.7%) of our studied patients respectively; they were managed surgically with successful treatment in all hernia cases and failure of treatment with mortality from sepsis in some of the intestinal obstruction cases (Clavien III, V). Table 4.
Lastly, late encephalopathy and ascites affected 43(10.5%), and 186(45.4%) of patients respectively; they were managed with liver supportive treatment, anti-coma measures for encephalopathy, and diuretics for ascites with success in some patients and LT or mortality from liver failure in the others (Clavien II, III, V). Table 4.
Nineteen (4.6%) of our patients underwent LT at a median of 21.5 (range, 5–118) months where post-transplant survival reached 9/19(47.4%) of cases. Table 4.
The late (>6 months) patient mortality reached 240(58.5%) of our patients where mortality in non-transplanted cases affected 230(56.1%) of them due to liver failure, sepsis, GIT bleeding, and HPS that involved 149(36.3%), 49(12%), 18(4.4%), and 14(3.4%) of them respectively. On the other hand, post-transplant mortality was 10/19(52.6%) and 10/410(2.4%) of transplanted and all patients respectively due to post-transplant sepsis, chronic rejection, portal vein thrombosis(PVT) and hepatic artery thrombosis(HAT) in 5(1.2%), 3(0.7%), 1(0.2%), and 1(0.2%) of them respectively. Table 4.
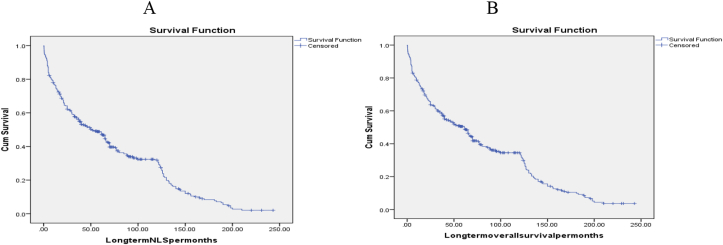
Lastly, the 1-year, 3-year, 5-year, 10-year, 15-year, and 20-year NLS reached 315(76.8%), 236(57.6%), 204(49.8%), 152(37.1%), 101(24.6%), and 91(22.2%) of them respectively. However, the 1-year, 3-year, 5-year, 10-year, 15-year, and 20-year overall survival was 318(77.6%), 244(59.5%), 211(51.5%), 161(39.3%), 110(26.8%), and 100(24.4%) of them respectively. Table 4, Fig. 6.
Fig. 6.
A-KM long-term NLS curve B-KM long-term overall survival curve.
2.4. Predictors of long-term (20-year) NLS
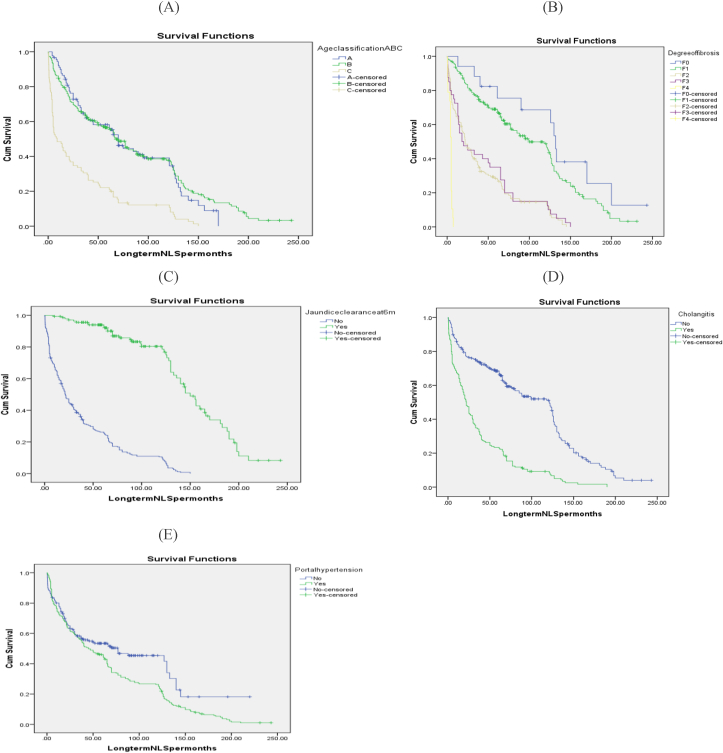
On univariate analysis; all studied variables were significant predictors of long-term NLS except patient gender, BASM anomaly, and mean preoperative (ALT, GGT, and platelets). However, on multivariate analysis; patient age ≤90 days, higher preoperative mean serum alb, portal tract fibrosis grades F0 and F1, absence of intraoperative bleeding, the occurrence of jaundice clearance at the 6th postoperative month, absence of post-operative cholangitis, and PHN were the independent predictors of it, furthermore, lower mean INR and operative time, as well as mild portal tracts ductal and/or ductular proliferation and using postoperative steroids therapy, had a trend towards independent correlation with it. Table 5, Fig. 7.
Table 5.
Predictors of long-term NLS.
| Category | NLS No (%) 91 (100%) or (Mean ± SD) |
No NLS No (%) 319 (100%) or (Mean ± SD) |
P-value Univariate analysis |
P-value Multivariate analysis |
|---|---|---|---|---|
| Age(days) (Mean ± SD) | 64 ± 12.7 | 71.8 ± 18.4 | 0.000 | >0.05 |
| Age category A (<60 days) B (60–90 days) C (>90 days) |
29 (31.9%) 59 (64.8%) 3 (3.3%) |
61 (19.1%) 166 (52%) 92 (28.8%) |
0.000 | 0.000 |
| Gender Males Females |
38 (41.8%) 53 (58.2%) |
148 (46.4%) 171 (53.6%) |
>0.05 | |
| Type of BA III III, cystic |
80 (87.9%) 11 (12.1%) |
310 (97.2%) 9 (2.8%) |
0.001 | >0.05 |
| Associated anomalies | 2 (2.2%) | 27 (8.5%) | 0.038 | >0.05 |
| BASM | 1 (1.1%) | 12 (3.8%) | >0.05 | |
| Preoperative labs (Mean ± SD) TB(mg/dL) DB(mg/dL) AST(U/L) ALT(U/L) Alka phos(U/L) GGT(U/L) Alb(g/dL) INR Platelets (K/uL) |
11.6 ± 3.3 8.4 ± 2.7 171 ± 83.3 137.5 ± 86 543.6 ± 282.8 1104.8 ± 733.2 3.9 ± 0.5 1 ± 0.04 438.5 ± 158.7 |
12.4 ± 3.7 9.4 ± 3.4 222.1 ± 135.6 159.6 ± 282.4 641.9 ± 274.5 970.7 ± 761.1 3.5 ± 0.6 1.04 ± 0.1 403.4 ± 195.8 |
0.047 0.012 0.001 >0.05 0.004 >0.05 0.000 0.000 >0.05 |
>0.05 >0.05 >0.05 >0.05 0.02 0.09 |
| Portal tracts ductal and/or ductular proliferation Mild Moderate Marked |
75 (82.4%) 16 (17.6%) 0 |
137 (42.9%) 120 (37.6%) 62 (19.4%) |
0.000 | 0.1 |
| Portal tract fibrosis F0 F1 F2 F3 F4 |
6 (6.6%) 68 (74.7%) 17 (18.7%) 0 0 |
11 (3.4%) 122 (38.2%) 127 (39.8%) 40 (12.5%) 19 (6%) |
0.000 | 0.000 |
| Presence of portal tracts macrophages | 28 (30.8%) | 202 (63.3%) | 0.000 | >0.05 |
| Presence of portal tracts giant cells | 5 (5.5%) | 70 (21.9%) | 0.000 | >0.05 |
| Remnant duct size at porta hepatis >150 μm <150 μm No ducts |
73 (80.2%) 16 (17.6%) 2 (2.2%) |
137 (42.9%) 92 (28.8%) 90 (28.2%) |
0.000 | >0.05 |
| Operative bleeding | 0 | 24 (7.5%) | 0.004 | 0.000 |
| Operative time(hours) (Mean ± SD) | 3.6 ± 1 | 4.4 ± 1.1 | 0.000 | 0.08 |
| Postoperative steroids | 63 (69.2%) | 53 (16.6%) | 0.000 | 0.1 |
| Jaundice clearance at 6 months | 84 (92.3%) | 54 (16.9%) | 0.000 | 0.000 |
| Cholangitis | 6 (6.6%) | 169 (53%) | 0.000 | 0.000 |
| PHN | 11 (12.1%) | 228 (71.5%) | 0.000 | 0.000 |
NLS: Native liver survival, BA: Biliary atresia, BASM: Biliary atresia splenic malformation, TB: Total bilirubin, DB: Direct bilirubin, AST: Aspartate transaminase, ALT: Alanine transaminase, Alka phos: Alkaline phosphatase, GGT: Gamma glutamate transferase, Alb: Albumin, INR: International normalization ratio, PHN: Portal hypertension.
Fig. 7.
KM analysis long-term NLS curves: A: Age at operation and long-term NLS (Log rank = 0.000) B- Liver fibrosis and long-term NLS (Log rank = 0.000) C- Jaundice clearance at the 6th post-operative month and long-term NLS (Log rank = 0.000) D- Post-operative cholangitis and long-term NLS (Log rank = 0.000) E− Post-operative PHN and long-term NLS (Log rank = 0.006).
3. Discussion
KPE for type III BA aims to form an internal fistula with the intrahepatic biliary tree through porta hepatis residual ductules [16] resulting in improving bile flow, clearing jaundice, restoring synthetic and excretory liver functions, and providing healthy growth [2,8] however, its better outcome occurs when performed early at the experienced center by experienced surgeons in the presence of appropriate support facilities with good postoperative care and medication [5]. Similarly, we did our best for the early detection and management of BA cases throughout the whole study period; moreover, our pediatric department designed and validated a scoring system in 2014 for achieving this goal [39].
Jaundice clearance at the 6th-month post KPE occurred in 33.7% of our patients that was comparable to the previous retrospective literature studies of different sample sizes (range; 29–82%) [8,[11], [12], [13], [14], [15], [16], [17]]; this wide variation of jaundice clearance in the literature came from different definitions of it, different periods of its occurrence as well as different postoperative medications (i.e. steroids) in those literature studies. On the other hand, it was only 27.1% in our previous study due to the difference in jaundice resolution time (3 months), patient NO, and study period [40].
Cholangitis is the most catastrophic complication after KPE occurring in 30–93% of patients [11,22,[41], [42], [43]] and is mostly ascending in nature due to the bilioenteric reconstruction; moreover, most of its attacks start within the 1st 2 years after the operation [44,45]. Similarly, and despite doing our best to prevent their occurrence by prophylactic AB for 6 months after the operation; Cholangitis (early and/or late), early cholangitis, and cholangitis within 2 years after KPE affected 42.7%, 26.3%, and 39.3% of our patients respectively, also, early cholangitis ranged from 13.2 to 48% in Suzuki et al., 2010 [16], Nightingale et al., 2017 [46] and Nio et al., 2015 [47] retrospective studies. However, late-onset cholangitis develops due to several causes (i.e. the presence of intrahepatic biliary stones, dilatations, and/or lakes) [21,44,48]; it was 24.1% and 22% in our, and Nio et al., 2004 [45] retrospective studies respectively.
PHN affects 30–70% of patients after KPE [11,49,50]; moreover, it results from the progression of liver fibrosis stimulating portosystemic shunting with fatal varices formation [44]. Similarly, PHN (early and/or late), early and late PHN affected 58.3%, 13.2%, and 47.1% of our patients respectively, however, early PHN was found in 23% of Chung et al., 2015 [51] infants, and late PHN affected 70% and 37% of patients in Lykavieris et al., 2005 [52] and Nio et al., 1997 [53] long-term follow up retrospective studies respectively.
HPS is a pulmonary complication of advanced liver cirrhosis and PHN; characterized by intrapulmonary vascular dilatations, arteriovenous shunts, poor arterial oxygenation, cyanosis, and dyspnea [54,55]; it is an independent cause of death, regardless of the stage of liver cirrhosis [56]. Similarly, it was the direct cause of deaths in 3.4% of our patients.
In our work, post KPE early and late mortalities were 17.1%, and 58.5% of patients respectively; moreover, sepsis and liver failure were the main causes of these mortalities. In similar, post KPE early (>1-year) mortality affected 21.3% of infants in Santos et al., 2009 [57] retrospective study mainly due to liver failure and sepsis, while liver failure was one of the mortality causes in Ramos-Gonzalez et al., 2019 [58] and Parolini et al., 2019 [22] retrospective studies. However, sepsis was a cause of death in Sookpotarom et al., 2006 [59] retrospective study.
Our 20-year NLS was 22.2% that lied within the literature range (14–44%) [2,13,[18], [19], [20], [21]]. However, the 20-year overall survival after KPE may reach 77.6–90% [13,33] in comparison to ours that was only 24.4% and this came from the following reasons: 1- Very low NO of our transplanted cases (4.6%) due to the absence of cadaveric LT in Egypt, low availability of living donor grafts, refusal of LT by most parents/guardians and low No of pediatric LT centers in Egypt, 2- High post-transplant mortality that reached 52.6% of transplanted cases. So, it is fundamental to do our best in the future to improve NLS post-KPE in Egypt to avoid or delay LT; moreover, our discussion aimed mainly to study the factors affecting jaundice clearance and 20-year NLS after KPE to modulate them for getting the best long-term NLS. (NB. Transplant free survival (TFS) means survival without LT and it includes the survival of a patient with his native liver (SNL) and survival of the native liver itself (NLS), so, in our discussion; we will consider SNL and NLS in the literature as TFS, also, short-, mid-and long-term TFS will mean ≤3-year, 4-year, and ≥5-year TFS respectively).
The effect of age at KPE that is affected by many factors (i.e. access to medical care, diagnosis efficiency, surgery scheduling, etc) on post-operative jaundice resolution, short-, mid-and long-term both NLS and overall survivals has been a matter of debate for years in the literature as some reports have been against its benefit, while most others have ensured its importance [60]; those ensuring studies have documented that the operation has had better outcomes when has been performed before 90 days of life due to the presence of an association between younger age at KPE and both increased porta-hepatis remnant ducts NO and decreased fibrosis grade [8,61,62]; therefore, performing it before 90 days has been the goal of most centers. Similarly, and despite the need for more improvement, only 23% of our infants underwent the operation after 90 days of life as we did our best for early detection of the disease, moreover, age<90 days at KPE was a significant predictor of postoperative jaundice resolution and independent predictor of long-term NLS in our study, also, younger age at KPE was a significant predictor of jaundice resolution in Chardot et al., 2013 [13], Pakarinen et al., 2018 [20], Parolini et al., 2019 [22], Hanalioğlu et al., 2019 [27], and Ihn et al., 2018 [63] retrospective studies. Moreover, it was an independent predictor of 4- and 5-year TFS in de Vries et al., 2012 [15] and Qiao et al., 2015 [64] retrospective studies respectively. Furthermore, it had a significant association with short- and long-term TFS outcomes in Zhen et al., 2015 [9], Chardot et al., 2013 [13], Pakarinen et al., 2018 [20], Hanalioğlu et al., 2019 [27], and Nightingale et al., 2017 [46] studies. On the other hand, it was not a predictor of jaundice resolution in Webb et al., 2017 [11], Sookpotarom et al., 2006 [59], or Yassin et al., 2020 [65] retrospective studies, also, it was not associated with short- or long-term TFS outcomes in Webb et al., 2017 [11], Jain et al., 2019 [21], Koga et al., 2013 [38], Chung et al., 2015 [51], Ramos-Gonzalez et al., 2019 [58], Ihn et al., 2018 [63], Nio et al., 2010 [66], Sasaki et al., 2016 [67], Chiang et al., 2017 [68], Nio et al., 2018 [69] or Wildhaber et al., 2003 [70] retrospective or prospective studies.
Cystic BA that is seen in 5–10% of cases has a good prognosis after KPE [71]. Similarly, cystic type III BA that affected 5% of our infants had a trend towards being an independent predictor of post-KPE jaundice resolution; moreover, it had a significant correlation with 20-year NLS, also, cystic BA was a significant predictor of good NLS in Davenport et al., 2011 [17] study and Toyosaka et al., 1993 [72] documented good NLS after successful KPE that was done for elder infant with type III cystic BA.
BA associated anomalies were a significant predictor of less both jaundice clearance and NLS in our study, similarly, it was a significant predictor of less jaundice resolution and independent predictor of poor short-term SNL in Davenport et al., 2011 [17] and Chung et al., 2015 [51] studies respectively, however it was not associated with jaundice resolution in Pakarinen et al., 2018 [20] retrospective multicenter study, moreover, it was not correlated with long-term TFS outcomes in Pakarinen et al., 2018 [20], Ramos-Gonzalez et al., 2019 [58] or Chiang et al., 2017 [68] retrospective studies. Despite being a predictor of less jaundice clearance; BASM anomaly was not associated with NLS in our work; similarly, it was not a predictor of TFS outcomes in de Vries et al., 2012 [15], Pakarinen et al., 2018 [20], or Nio et al., 2018 [69] studies. Conversely, it was a predictor of poor SNL in Shneider et al., 2006 [12] and Chardot et al., 2013 [13] studies.
Preoperative lower mean DB and AST were independent predictors of Post KPE jaundice clearance and significant predictors of good NLS in our work; similarly, lower preoperative median AST had a significant correlation with jaundice resolution in Yassin et al., 2020 [65] retrospective study, also, Preoperative lower mean DB and AST had a trend towards significant correlation with SNL in Goda et al., 2013 [73] retrospective study. The preoperative INR value reflects the secretory/synthetic liver function affecting the long-term postoperative outcomes [27]. Similarly, preoperative INR was a predictor of both jaundice clearance and long-term NLS in our work. Conversely, it was not a predictor of jaundice clearance in Yassin et al., 2020 [65] retrospective study.
The Portal tracts ductal and/or ductular proliferation seen in BA occurs as a response to chronic cholestasis and/or comes from the ductular transformation of the periportal hepatocytes [74]. In our series; the mild form of portal tracts ductal and/or ductular proliferation was an independent predictor of jaundice resolution besides having a trend towards being an independent predictor of good NLS, similarly, Santos et al., 2009 [57] and Muthukanagarajan et al., 2016 [75] found better short-term NLS with a lesser degree of biliary proliferation.
Advanced fibrosis was a significant predictor of jaundice persistence and independent predictor of poor NLS in our series, in similar, it was an independent predictor of poor long-term TFS in Hanalioğlu et al., 2019 [27] and Wildhaber et al., 2003 [70] studies. Moreover, it was a significant predictor of poor short- and long-term NLS in Webb et al., 2017 [11] and Nightingale et al., 2017 [46] retrospective studies. However, the presence of liver fibrosis or its increased grade at surgery was not associated with postoperative jaundice resolution in Hanalioğlu et al., 2019 [27], Chen G et al., 2015 [62], or Yassin et al., 2020 [65] retrospective studies. Also, advanced fibrosis at KPE was not associated with short-or long-term SNL in Zhen et al., 2015 [9], or Czubkowski et al., 2015 [74] studies.
The remnant duct size at porta-hepatis of more than 150 μm was a significant predictor of both jaundice resolution and NLS in our study; similarly, larger duct size at porta hepatis was an independent predictor of long-term TFS in Wildhaber et al., 2003 [70] study, conversely, it was not a predictor of jaundice clearance or long-term NLS outcomes in Webb et al., 2017 [11] or Ihn et al., 2018 [63] retrospective studies, also, it was not associated with long-term SNL in Koga et al., 2013 [38] prospective study.
Steroids after KPE promote postoperative biliary secretion by augmenting Cl−/HCO− exchange, increasing Na+/K+ ATPase activity, and increasing biliary acid-independent bile level; moreover, they have choleretic, anti-inflammatory, anti-edematous, and liver-protective effects [16]. In similar, and besides being significant predictors of jaundice clearance; steroids had a trend towards being independent predictors of NLS in our work; also, they improved jaundice resolution in Escobar et al., 2006 [7], Davenport et al., 2013 [37] and Chen Y et al., 2015 [76] studies; and were associated with short-term SNL in Chung et al., 2015 [51] retrospective study. On the other hand, they did not affect short- or long-term TFS outcomes in de Vries et al., 2012 [15], Ramos-Gonzalez et al., 2019 [58], or Sarkhy et al., 2011 [77] studies.
Post-KPE Jaundice resolution was an independent predictor of long-term NLS in our study; also, it was an independent predictor of short-, mid-and long-term TFS outcomes in de Vries et al., 2012 [15], Hanalioğlu et al., 2019 [27], Nightingale et al., 2017 [46], Ihn et al., 2018 [63] and Chiang et al., 2017 [68] studies, and was a significant predictor of short- and long-term TFS outcomes in Shneider et al., 2006 [12], Pakarinen et al., 2018 [20], van Heurn et al., 2004 [41], Qiao et al., 2015 [64], Czubkowski et al., 2015 [74] and Shneider et al., 2016 [78] studies. On the other hand, it was not a predictor of long-term NLS in Webb et al., 2017 [11] study.
Cholangitis affects the early postoperative biliary drainage leading to poor outcomes [45]. Moreover, it is an important variant affecting long-term survival after a successful KPE [64]. Similarly and despite our appropriate management of early and late cholangitis by hydration, aggressive AB, steroids, and/or percutaneous drainage of biliary lakes; sepsis from them was the direct cause of mortalities in 38(9.3%) of our patients, moreover, early cholangitis was an independent predictor of jaundice persistence, and cholangitis (early and/or late) was an independent predictor of poor long-term NLS outcome in our work. Also, cholangitis was an independent predictor of poor short- and long-term TFS in Chung et al., 2015 [51], Qiao et al., 2015 [64], and Wildhaber et al., 2003 [70] studies, moreover, early cholangitis was an independent predictor of poor short- and long-term SNL in Zhen et al., 2015 [9] and Hung et al., 2006 [79] studies; also, it was a significant predictor of poor mid-and long-term SNL in Koga et al., 2013 [38] and Nio et al., 2018 [69] studies; furthermore, late cholangitis was a predictor of poor long-term NLS in Jain et al., 2019 [21] study. On the other hand, cholangitis was not a predictor of jaundice resolution in Webb et al., 2017 [11] or Yassin et al., 2020 [65] studies, furthermore, it was not correlated with short-, mid- or long-term TFS outcomes in Webb et al., 2017 [11], Shneider et al., 2006 [12], de Vries et al., 2012 [15], Ihn et al., 2018 [63], Sasaki et al., 2016 [67] or Chiang et al., 2017 [68] studies.
Variceal hemorrhage (VH) is a disaster in BA patients with significant morbidity and mortality [80]. Similarly, massive GIT bleeding from early and late GO varices was the direct cause of mortalities in 26(6.3%) of our patients despite appropriate resuscitation, support, and endoscopic therapy, moreover, Post KPE early PHN was a significant predictor of jaundice persistence, while, PHN (early and/or late) was an independent predictor of poor long-term NLS outcome in our work. Also, post KPE late PHN was a significant predictor of poor long-term NLS in Jain et al., 2019 [21] study, and GO varices that required endoscopic injection sclerotherapy(EIS) was an independent predictor of poor long-term NLS in Sasaki et al., 2016 [67] study. On the other hand, post-KPE PHN was not a predictor of short- or long-term TFS in Webb et al., 2017 [11] or Chung et al., 2015 [51] studies.
The annual center volume/caseload, as well as the surgeon and center experiences, improve post-KPE outcome [8,81], similarly, our center is one of the biggest centers in Egypt performing such procedures with a mean annual caseload of 20.5 ± 10.1 cases (median 17 (range; 5–53) cases) where there was a significant correlation between annual caseload>15 cases and NLS (P = 0.008); also, mid-and long-term TFS was correlated with the higher annual caseload in Serinet et al., 2006 [14], Pakarinen et al., 2018 [20] and Davenport et al., 2004(363) [82] studies; in the same line, the center experience was a predictor of short- and long term TFS in Nightingale et al., 2017 [46], Lampela et al., 2012 [83] and Kvist and Davenport, 2011 [84] studies. Conversely, annual caseload was not a predictor of TFS outcomes in de Vries et al., 2012 [15] or Schreiber et al., 2010 [85] studies.
To the best of our knowledge; this is one of the unique studies documenting the followings: 1- The significant correlation between postoperative sepsis from different causes (i.e. cholangitis, pneumonia, bile leak, intestinal leak, and intestinal obstruction) and mortalities (P = 0.000); so it is fundamental to prevent and manage sepsis post KPE aggressively to improve outcome, 2- The significant correlation between intra-operative bleeding (N.B associated significantly with preoperative PHN (P < 0.05) and postoperative jaundice persistence, as well as its independent correlation with less long-term NLS and we think this came from the significant correlation between bleeding and both older age(P < 0.05) and higher fibrosis grade(P < 0.05) that were related to poor outcome; so, it is crucial to decrease and properly manage intra-operative bleeding for better outcome, 3- The significant association between higher preoperative mean serum alb and postoperative jaundice resolution, as well as its independent correlation with long-term NLS; so it is a must to improve the preoperative nutritional status of the infant for getting better outcome.
In conclusion: Sepsis had a direct effect on early and late patient mortalities after KPE; moreover, patient age at operation>90 days, higher fibrosis grades, the occurrence of postoperative cholangitis and PHN, and persistence of post-operative jaundice had a negative insult on the long-term postoperative outcome. So, it is crucial to modulate these factors for a better outcome.
- Forms of support received by each author for this study included good selection of cases, instructive supervision, continuous guidance, valuable suggestions and good instructions. Furthermore, the authors of the manuscript shared in its data collection, writing, and publication; moreover, the corresponding author did statistical analysis as well.
- No grant or other financial support was received for this study.
- No conflict of interest to declare.
Declaration of competing interest
No conflict of interest to declare.
Acknowledgment
Thanks to the authors of the manuscript for their efforts.
The main limitation of the study is being retrospective, done at a single center where multiple factors were studied, so, it is advisable to do further randomized multicenter prospective studies of the effect of a single factor(i.e Sepsis, age, fibrosis, cholangitis, PHN, etc) on the outcome of KPE for type III BA.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.amsu.2021.01.052.
Funding
No received a grant or other financial support for this study.
Provenance and peer review: Not commissioned, externally peer-reviewed.
Author disclosure form
The following additional information is required for submission. Please note that failure to respond to these questions/statements will mean your submission will be returned. If you have nothing to declare in any of these categories then this should be stated.
Please state whether ethical approval was given, by whom and the relevant Judgement's reference number
The approval by National liver institute (IRB), Menoufia university that was done retrospectively.
Please state any sources of funding for your research
No source of funding for this research.
Author contribution
Emad Hamdy Gad: Surgical procedures, study design, data collection, writing, statistical analysis and publication.
Yasmin Kamel: Data collection, writing and statistical analysis.
Tahany Abdel-Hameed Salem: Data collection, analysis and writing.
Mohammed Abdel-Hafez Ali: Data collection, analysis and writing.
Ahmed Nabil Sallam: Surgical procedures, data collection, analysis and writing.
Please state any conflicts of interest
No conflict of interest to declare.
Research registration unique identifying number (UIN)
Chinese clinical trial registry with registration NO of ChiCTR2000038541.
Guarantor
All the authors of this paper accept full responsibility for the work and/or the conduct of the study, had access to the data, and controlled the decision to publish.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Zhang D., Yang H.-Y., Jia J., Zhao G., Yue M., Wang J-x. Postoperative steroids after Kasai portoenterostomy for biliary atresia: a meta-analysis. Int. J. Surg. 2014;12:1203–1209. doi: 10.1016/j.ijsu.2014.08.407. [DOI] [PubMed] [Google Scholar]
- 2.Park C.J., Armenia S.J., Keung C.H., Compton J.T., Cowles R.A. Surgical modifications of the Kasai hepatoportoenterostomy minimize invasiveness without compromising short- and medium-term outcomes. J. Pediatr. Surg. 2019;54:537–542. doi: 10.1016/j.jpedsurg.2018.06.028. [DOI] [PubMed] [Google Scholar]
- 3.Andrade W.C., Silva M.M., Tannuri A.C.A., Santos M.M., Gibelli N.E.M., Tannuri U. Current management of biliary atresia based on 35 years of experience at a single center. Clinics. 2018;73:e289. doi: 10.6061/clinics/2018/e289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chardot C., Carton M., Spire-Bendelac N., Pommelet C.L., Golmard J.-L., Reding R. Is the Kasai operation still indicated in children older than 3 months diagnosed with biliary atresia? J. Pediatr. 2001;138:224–228. doi: 10.1067/mpd.2001.111276. [DOI] [PubMed] [Google Scholar]
- 5.Davenport M., Puricelli V., Farrant P., Hadzic N., Mieli-Vergani G., Portmann B. The outcome of the older (>100 Days) infant with biliary atresia. J. Pediatr. Surg. 2004;39:575–581. doi: 10.1016/j.jpedsurg.2003.12.014. [DOI] [PubMed] [Google Scholar]
- 6.Vejchapipata P., Passakonnirina R., Sookpotaroma P., Chittmittrapapa S., Poovorawan Y. High-dose steroids do not improve early outcome in biliary atresia. J. Pediatr. Surg. 2007;42:2102–2105. doi: 10.1016/j.jpedsurg.2007.08.038. [DOI] [PubMed] [Google Scholar]
- 7.Escobar M.A., Jay C.L., Brooks R.M., West K.W., Rescorla F.J., Molleston J.P. Effect of corticosteroid therapy on outcomes in biliary atresia after Kasai portoenterostomy. J. Pediatr. Surg. 2006;41:99–103. doi: 10.1016/j.jpedsurg.2005.10.072. [DOI] [PubMed] [Google Scholar]
- 8.Sanchez-Valle A., Kassira N., Varela V.C., Radu S.C., Paidas C., Kirby R.S. Biliary atresia: epidemiology, genetics, clinical update, and public health perspective. Adv. Pediatr. 2017;64:285–305. doi: 10.1016/j.yapd.2017.03.012. [DOI] [PubMed] [Google Scholar]
- 9.Zhen C., Guoliang Q., Lishuang M., Zhen Z., Chen W., Jun Z. Design and validation of an early scoring system for predicting early outcomes of type III biliary atresia after Kasai's operation. Pediatr. Surg. Int. 2015;31(6):535–542. doi: 10.1007/s00383-015-3710-3. [DOI] [PubMed] [Google Scholar]
- 10.Makin E., Davenport M. Biliary atresia. Curr. Paediatr. 2006;16:59–63. [Google Scholar]
- 11.Webb N.L., Jiwane A., Ooi C.Y., Nightinghale S., Adams S.E., Krishnan U. Clinical significance of liver histology on outcomes in biliary atresia. J. Paediatr. Child Health. 2017;53:252–256. doi: 10.1111/jpc.13371. [DOI] [PubMed] [Google Scholar]
- 12.Shneider B.L., Brown M.B., Haber B., Whitington P.F., Schwarz K., Squires R., Biliary Atresia Research Consortium A multicenter study of the outcome of biliary atresia in the United States, 1997 to 2000. J. Pediatr. 2006;148(4):467–474. doi: 10.1016/j.jpeds.2005.12.054. [DOI] [PubMed] [Google Scholar]
- 13.Chardot C., Buet C., Serinet M.-O., Golmard J.-L., Lachaux A., Roquelaure B. Improving outcomes of biliary atresia: French national series 1986–2009. J. Hepatol. 2013;58:1209–1217. doi: 10.1016/j.jhep.2013.01.040. [DOI] [PubMed] [Google Scholar]
- 14.Serinet M.O., Broué P., Jacquemin E., Lachaux A., Sarles J., Gottrand F. Management of patients with biliary atresia in France: results of a decentralized policy 1986-2002. Hepatology. 2006;44(1):75–84. doi: 10.1002/hep.21219. [DOI] [PubMed] [Google Scholar]
- 15.de Vries W., de Langen Z.J., Groen H., Scheenstra R., Peeters P.M., Hulscher J.B. Netherlands study group of biliary atresia and registry (NeSBAR). Biliary atresia in The Netherlands: outcome of patients diagnosed between 1987 and 2008. J. Pediatr. 2012;160(4):638–644. doi: 10.1016/j.jpeds.2011.09.061. [DOI] [PubMed] [Google Scholar]
- 16.Suzuki T., Hashimoto T., Kondo S., Sato Y., Hussein M.H. Evaluating patients' outcome post-Kasai operation: a 19-year experience with modification of the hepatic portoenterostomy and applying a novel steroid therapy regimen. Pediatr. Surg. Int. 2010;26:825–830. doi: 10.1007/s00383-010-2637-y. [DOI] [PubMed] [Google Scholar]
- 17.Davenport M., Ong E., Sharif K., Alizai N., McClean P., Hadzic N. Biliary atresia in England and Wales: results of centralization and new benchmark. J. Pediatr. Surg. 2011;46(9):1689–1694. doi: 10.1016/j.jpedsurg.2011.04.013. [DOI] [PubMed] [Google Scholar]
- 18.Bijl E.J., Bharwani K.D., Houwen R.H.J., de Man R.A. The long-term outcome of the Kasai operation in patients with biliary atresia: a systematic review. Neth. J. Med. 2013;71(4):170–173. [PubMed] [Google Scholar]
- 19.Nio M., Wada M., Sasaki H., Tanaka H., Okamura A. Risk factors affecting late-presenting liver failure in adult patients with biliary atresia. J. Pediatr. Surg. 2012;47:2179–2183. doi: 10.1016/j.jpedsurg.2012.09.003. [DOI] [PubMed] [Google Scholar]
- 20.Pakarinen M.P., Johansen L.S., Svensson J.F., Bjørnland K., Gatzinsky V., Stenström P. Outcomes of biliary atresia in the Nordic countries – a multicenter study of 158 patients during 2005–2016. J. Pediatr. Surg. 2018;53:1509–1515. doi: 10.1016/j.jpedsurg.2017.08.048. [DOI] [PubMed] [Google Scholar]
- 21.Jain V., Burford C., Alexander E.C., Sutton H., Dhawan A., Joshi D. Prognostic markers at adolescence in patients requiring liver transplantation for biliary atresia in adulthood. J. Hepatol. 2019;71:71–77. doi: 10.1016/j.jhep.2019.03.005. [DOI] [PubMed] [Google Scholar]
- 22.Parolini F., Boroni G., Milianti S., Tonegatti L., Armellini A., Garcia Magne M. Biliary atresia: 20-40-year follow-up with native liver in an Italian centre. J. Pediatr. Surg. 2019;54(7):1440–1444. doi: 10.1016/j.jpedsurg.2018.10.060. [DOI] [PubMed] [Google Scholar]
- 23.Shinkai M., Ohhama Y., Take H., Kitagawa N., Kudo H., Mochizuki K. Long-term outcome of children with biliary atresia who were not transplanted after the Kasai operation: >20-year experience at a children's hospital. JPGN. 2009;48:443–450. doi: 10.1097/mpg.0b013e318189f2d5. [DOI] [PubMed] [Google Scholar]
- 24.Boskovic A., Kitic I., Prokic D., Stankovic I., Grujic B. Predictive value of hepatic ultrasound, liver biopsy, and duodenal tube test in the diagnosis of extrahepatic biliary atresia in Serbian infants. Turk. J. Gastroenterol. 2014;25:170–174. doi: 10.5152/tjg.2014.5603. [DOI] [PubMed] [Google Scholar]
- 25.Hukkinen M., Kerola A., Lohi J., Heikkila P., Merras-Salmio L., Jahnukainen T. Treatment policy and liver histopathology predict biliary atresia outcomes: results after national centralization and protocol biopsies. J. Am. Coll. Surg. 2018;226:46–57. doi: 10.1016/j.jamcollsurg.2017.09.009. [DOI] [PubMed] [Google Scholar]
- 26.Wildhaber B.E. Biliary atresia: 50 years after the first Kasai. ISRN Surg. 2013;15:132089. doi: 10.5402/2012/132089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hanalioğlu D., Özen H., Karhan A., Gümüş E., Demir H., Saltık-Temizel İ.N. Revisiting long-term prognostic factors of biliary atresia: a 20-year experience with 81 patients from a single center. Turk. J. Gastroenterol. 2019;30(5):467–474. doi: 10.5152/tjg.2019.18590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chung P.H.Y., Wong K.K.Y., Lan L.C.L., Tam P.K.H. Evaluation of a standardized protocol in the use of steroids after Kasai operation. Pediatr. Surg. Int. 2008;24:1001–1004. doi: 10.1007/s00383-008-2200-2. [DOI] [PubMed] [Google Scholar]
- 29.Gad E.H., Ayoup E., Kamel Y., Zakareya T., Abbasy M., Nada A. Surgical management of laparoscopic cholecystectomy (LC) related major bile duct injuries; predictors of short-and long-term outcomes in a tertiary Egyptian center- a retrospective cohort study. Ann Med Surg (Lond). 2018;36:219–230. doi: 10.1016/j.amsu.2018.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gad E.H., Kamel Y., Alsebaey A., Mohammed A., Abdelsamee M.A. Laparoscopic cholecystectomy in patients with liver cirrhosis: 8 years experience in a tertiary center. A retrospective cohort study. Ann Med Surg (Lond). 2020;51:1–10. doi: 10.1016/j.amsu.2020.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hukkinen M., Kerola A., Lohi J., Jahnukainen T., Heikkilä P., Pakarinen M.P. Very low bilirubin after portoenterostomy improves survival of the native liver in patients with biliary atresia by deferring liver fibrogenesis. Surgery. 2019;165:843–850. doi: 10.1016/j.surg.2018.10.032. [DOI] [PubMed] [Google Scholar]
- 33.Davenport M. Biliary atresia: clinical aspects. Semin. Pediatr. Surg. 2012;21:175–184. doi: 10.1053/j.sempedsurg.2012.05.010. [DOI] [PubMed] [Google Scholar]
- 34.de Carvalho N.M.N., Torres S.M., Cavalcante J.C.B., Ximenes A.C.M., Junior J.A.L., Moreira S.O.S. Hepatoportoenterostomy surgery technique. J. Pediatr. Surg. 2019;54:1715–1718. doi: 10.1016/j.jpedsurg.2018.10.041. [DOI] [PubMed] [Google Scholar]
- 35.Lee W.S., Looi L.M. Usefulness of a scoring system in the interpretation of histology in neonatal cholestasis. World J. Gastroenterol. 2009;15:5326–5333. doi: 10.3748/wjg.15.5326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bedossa P., Poynard T. The French METAVIR cooperative study group. An algorithm for grading activity in chronic hepatitis C. Hepatology. 1996;24:289–293. doi: 10.1002/hep.510240201. [DOI] [PubMed] [Google Scholar]
- 37.Davenport M., Parsons C., Tizzard S., Hadzic N. Steroids in biliary atresia: single surgeon, single centre, prospective study. J. Hepatol. 2013;59:1054–1058. doi: 10.1016/j.jhep.2013.06.012. [DOI] [PubMed] [Google Scholar]
- 38.Koga H., Wada M., Nakamura H., Miyano G., Okawada M., Lane G.J. Factors influencing jaundice-free survival with the native liver in post-portoenterostomy biliary atresia patients: results from a single institution. J. Pediatr. Surg. 2013;48:2368–2372. doi: 10.1016/j.jpedsurg.2013.08.007. [DOI] [PubMed] [Google Scholar]
- 39.El-Guindi M.A.-S., Sira M.M., Sira A.M., Salem T.A.-H., El-Abd O.L., Konsowa H.A.-S. Design and validation of a diagnostic score for biliary atresia. J. Hepatol. 2014;61:116–123. doi: 10.1016/j.jhep.2014.03.016. [DOI] [PubMed] [Google Scholar]
- 40.Abdel-Aziz S.A.-W., Sira M.M., Gad E.H., Ayoub I., Soltan M. Preoperative alkaline phosphatase is a potential predictor of short-term outcome of surgery in infants with biliary atresia. Clin. Exp. Hepatol. 2019;5(2):155–160. doi: 10.5114/ceh.2019.85072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.van Heurn L.W.E., Saing H., Tam P.K.H. Portoenterostomy for biliary atresia: long-term survival and prognosis after esophageal variceal bleeding. J. Pediatr. Surg. 2004;39:6–9. doi: 10.1016/j.jpedsurg.2003.09.019. [DOI] [PubMed] [Google Scholar]
- 42.Decharun K., Leys C.M., West K.W., Finnell S.M.E. Prophylactic antibiotics for prevention of cholangitis in patients with biliary atresia status post-Kasai portoenterostomy: a systematic review. Clin. Pediatr. 2016;55(1):66–72. doi: 10.1177/0009922815594760. [DOI] [PubMed] [Google Scholar]
- 43.Ng V.L., Haber B.H., Magee J.C., Miethke A., Murray K.F., Michail S. Medical status of 219 children with biliary atresia surviving long-term with their native livers: results from a North American multicenter consortium. J. Pediatr. 2014;165:539–546. doi: 10.1016/j.jpeds.2014.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kelay A., Davenport M. Long-term out-look in biliary atresia. Semin. Pediatr. Surg. 2017;26:295–300. doi: 10.1053/j.sempedsurg.2017.09.003. [DOI] [PubMed] [Google Scholar]
- 45.Nio M., Sano N., Ishii T., Sasaki H., Hayashi Y., Ohi R. Cholangitis as a late complication in long-term survivors after surgery for biliary atresia. J. Pediatr. Surg. 2004;39(12):1797–1799. doi: 10.1016/j.jpedsurg.2004.08.021. [DOI] [PubMed] [Google Scholar]
- 46.Nightingale S., Stormon M.O., O'Loughlin E.V., Shun A., Thomas G., Benchimol E.I. Early post-hepatoportoenterostomy predictors of native liver survival in biliary atresia. JPGN. 2017;64:203–209. doi: 10.1097/MPG.0000000000001289. [DOI] [PubMed] [Google Scholar]
- 47.Nio M., Wada M., Sasaki H., Tanaka H., Watanabe T. Long-term outcomes of biliary atresia with splenic malformation. J. Pediatr. Surg. 2015;50:2124–2127. doi: 10.1016/j.jpedsurg.2015.08.040. [DOI] [PubMed] [Google Scholar]
- 48.Ginström D.A., Hukkinen M., Kivisaari R., Pakarinen M.P. Biliary tresia–associated cholangitis: the central role and effective management of bile lakes. JPGN. 2019;68:488–494. doi: 10.1097/MPG.0000000000002243. [DOI] [PubMed] [Google Scholar]
- 49.Shneider B.L., Abel B., Haber B., Karpen S.J., Magee J.C., Romero R. Portal hypertension in children and young adults with biliary atresia. J. Pediatr. Gastroenterol. Nutr. 2012;55:567–573. doi: 10.1097/MPG.0b013e31826eb0cf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lee S., Park H., Moon S.B., Jung S.M., Kim J.M., Kwon C.H. Long-term results of biliary atresia in the era of liver transplantation. Pediatr. Surg. Int. 2013;29(12):1297–1301. doi: 10.1007/s00383-013-3366-9. [DOI] [PubMed] [Google Scholar]
- 51.Chung P.H.Y., Wong K.K.Y., Tam P.K.H. Predictors for failure after Kasai operation. J. Pediatr. Surg. 2015;50:293–296. doi: 10.1016/j.jpedsurg.2014.11.015. [DOI] [PubMed] [Google Scholar]
- 52.Lykavieris P., Chardot C., Sokhn M., Gauthier F., Valayer J., Bernard O. Outcome in adulthood of biliary atresia: a study of 63 patients who survived for over 20 years with their native liver. Hepatology. 2005;41(2):366–371. doi: 10.1002/hep.20547. [DOI] [PubMed] [Google Scholar]
- 53.Nio M., Ohi R., Shimaoka S., Iwami D., Sano N. The outcome of surgery for biliary atresia and the current status of long-term survivors. Tohoku J. Exp. Med. 1997;181(1):235–244. doi: 10.1620/tjem.181.235. [DOI] [PubMed] [Google Scholar]
- 54.Soulaidopoulos S., Cholongitas E., Giannakoulas G., Vlachou M., Goulis I. Review article: update on current and emergent data on hepatopulmonary syndrome. World J. Gastroenterol. 2018;24(12):1285–1298. doi: 10.3748/wjg.v24.i12.1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pandey S., Sood V., Khanna R., Lal B.B., Sood A.K., Kabra S.K. Natural history, risk factors, and outcome of hepatopulmonary syndrome in pediatric liver diseases. Indian J. Gastroenterol. 2020;39(1):66–74. doi: 10.1007/s12664-020-01015-0. [DOI] [PubMed] [Google Scholar]
- 56.Iyer V.N., Swanson K.L., Cartin-Ceba R., Dierkhising R.A., Rosen C.B., Heimbach J.K. Hepatopulmonary syndrome: favorable outcomes in the MELD exception era. Hepatology. 2013;57(6):2427–2435. doi: 10.1002/hep.26070. [DOI] [PubMed] [Google Scholar]
- 57.Santos J.L., Kieling C.O., Meurer L., Vieira S., Ferreira C.T., Lorentz A. The extent of biliary proliferation in liver biopsies from patients with biliary atresia at portoenterostomy is associated with the postoperative prognosis. J. Pediatr. Surg. 2009;44:695–701. doi: 10.1016/j.jpedsurg.2008.09.013. [DOI] [PubMed] [Google Scholar]
- 58.Ramos-Gonzalez G., Elisofon S., Dee E.C., Staffa S.J., Medford S., Lillehei C. Predictors of need for liver transplantation in children undergoing hepato-portoenterostomy for biliary atresia. J. Pediatr. Surg. 2019;54:1127–1131. doi: 10.1016/j.jpedsurg.2019.02.051. [DOI] [PubMed] [Google Scholar]
- 59.Sookpotarom P., Vejchapipat P., Chittmittrapap S., Sookpotarom P., Vejchapipat P., Chittmittrapap S. Short-term results of Kasai operation for biliary atresia: experience from one institution. Asian J. Surg. 2006;29(3):188–192. doi: 10.1016/s1015-9584(09)60085-3. [DOI] [PubMed] [Google Scholar]
- 60.Rava M.V., Dzakovic A., Bentrem D.J., Reynolds M., Superina R. Trends in age for hepatoportoenterostomy in the United States. Surgery. 2010;148:785–792. doi: 10.1016/j.surg.2010.07.028. [DOI] [PubMed] [Google Scholar]
- 61.Mukhopadhyay S.G., Roy P., Chatterjee U., Datta C., Banerjee M., Banerjee S. A histopathological study of liver and biliary remnants in the long-term survivors (>10 years) of cases of biliary atresia. Indian J. Pathol. Microbiol. 2014;57(3):380–385. doi: 10.4103/0377-4929.138722. [DOI] [PubMed] [Google Scholar]
- 62.Chen G., Xue P., Zheng S., Chen L., Ma Y. A pathological scoring system in the diagnosis and judgment of prognosis of biliary atresia. J. Pediatr. Surg. 2015;50:2119–2123. doi: 10.1016/j.jpedsurg.2015.08.041. [DOI] [PubMed] [Google Scholar]
- 63.Ihn K., Ho I.G., Chang E.Y., Han S.J. Correlation between gamma-glutamyl transpeptidase activity and outcomes after Kasai portoenterostomy for biliary atresia. J. Pediatr. Surg. 2018;53(3):461–467. doi: 10.1016/j.jpedsurg.2017.10.001. [DOI] [PubMed] [Google Scholar]
- 64.Qiao G., Li L., Cheng W., Zhang Z., Ge J., Wang C. Conditional probability of survival in patients with biliary atresia after Kasai portoenterostomy: a Chinese population-based study. J. Pediatr. Surg. 2015;50:1310–1315. doi: 10.1016/j.jpedsurg.2015.03.062. [DOI] [PubMed] [Google Scholar]
- 65.Yassin N.A., El-Tagy G., Abdelhakeem O.N., Asem N., El-Karaksy H. Predictors of short-term outcome of Kasai portoenterostomy for biliary atresia in infants: a single-center study. Pediatr Gastroenterol Hepatol Nutr. 2020;23(3):266–275. doi: 10.5223/pghn.2020.23.3.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Nio M., Sasaki H., Wada M., Kazama T., Nishi K., Tanaka H. Impact of age at Kasai operation on short- and long-term outcomes of type III biliary atresia at a single institution. J. Pediatr. Surg. 2010;45(12):2361–2363. doi: 10.1016/j.jpedsurg.2010.08.032. [DOI] [PubMed] [Google Scholar]
- 67.Sasaki H., Tanaka H., Wada M., Kazama T., Nakamura M., Kudo H. Analysis of the prognostic factors of long-term native liver survival in survivors of biliary atresia. Pediatr. Surg. Int. 2016;32(9):839–843. doi: 10.1007/s00383-016-3934-x. [DOI] [PubMed] [Google Scholar]
- 68.Chiang L.W., Lee C.Y., Krishnaswamy G., Nah S.A., Kader A., Ong C. Seventeen years of Kasai portoenterostomy for biliary atresia in a single Southeast Asian paediatric centre. J. Paediatr. Child Health. 2017;53:412–415. doi: 10.1111/jpc.13379. [DOI] [PubMed] [Google Scholar]
- 69.Nio M., Wada M., Sasaki H., Tanaka H., Nakamura M., Kudo H. Using 99 mTc-DTPA galactosyl human serum albumin liver scintigraphy as a prognostic indicator in jaundice-free patients with biliary atresia. J. Pediatr. Surg. 2018;53(12):2412–2415. doi: 10.1016/j.jpedsurg.2018.08.032. [DOI] [PubMed] [Google Scholar]
- 70.Wildhaber B.E., Coran A.G., Drongowski R.A., Hirschl R.B., Geiger J.D., Lelli J.L. The Kasai portoenterostomy for biliary atresia: a review of a 27-year experience with 81 patients. J. Pediatr. Surg. 2003;38(10):1480–1485. doi: 10.1016/s0022-3468(03)00499-8. [DOI] [PubMed] [Google Scholar]
- 71.Lakshminarayanan B., Davenport M. Biliary atresia: a comprehensive review. J. Autoimmun. 2016;73:1–9. doi: 10.1016/j.jaut.2016.06.005. [DOI] [PubMed] [Google Scholar]
- 72.Toyosaka A., Okamoto E., Kawamura E., Okasora T., Nose K., Tomimoto Y. Successful Kasai operation for biliary atresia in a 9 month old. J. Pediatr. Surg. 1993;28(12):1557–1560. doi: 10.1016/0022-3468(93)90095-3. [DOI] [PubMed] [Google Scholar]
- 73.Goda T., Kawahara H., Kubota A., Hirano K., Umedaa S., Tani G. The most reliable early predictors of outcome in patients with biliary atresia after Kasai's operation. J. Pediatr. Surg. 2013;48:2373–2377. doi: 10.1016/j.jpedsurg.2013.08.009. [DOI] [PubMed] [Google Scholar]
- 74.Czubkowski P., Cielecka-Kuszyk J., Rurarz M., Kaminska D., Markiewicz-Kijewska M., Pawlowska J. The limited prognostic value of liver histology in children with biliary atresia. Ann. Hepatol. 2015;14(6):902–909. doi: 10.5604/16652681.1171781. [DOI] [PubMed] [Google Scholar]
- 75.Muthukanagarajan S.J., Karnan I., Srinivasan P., Sadagopan P., Manickam S. Diagnostic and prognostic significance of various histopathological features in extrahepatic biliary atresia. J. Clin. Diagn. Res. 2016;10(6):EC23–E27. doi: 10.7860/JCDR/2016/19252.8035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Chen Y., Nah S.A., Chiang L., Krishnaswamy G., Low Y. Postoperative steroid therapy for biliary atresia: systematic review and meta-analysis. J. Pediatr. Surg. 2015;50(9):1590–1594. doi: 10.1016/j.jpedsurg.2015.05.016. [DOI] [PubMed] [Google Scholar]
- 77.Sarkhy A., Schreiber R.A., Milner R.A., Barker C.C. Does adjuvant steroid therapy post-Kasai portoenterostomy improve outcome of biliary atresia?Systematic review and meta-analysis. Can. J. Gastroenterol. 2011;25(8):440–444. doi: 10.1155/2011/125610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Shneider B.L., Magee J.C., Karpen S.J., Rand E.B., Narkewicz M.R., Bass L.M. Total serum bilirubin within 3 months of hepatoportoenterostomy predicts short-term outcomes in biliary atresia. J. Pediatr. 2016;170:211–217. doi: 10.1016/j.jpeds.2015.11.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hung P.Y., Chen C.C., Chen W.J., Lai H.S., Hsu W.M., Lee P.H. Long-term prognosis of patients with biliary atresia: a 25 year summary. J. Pediatr. Gastroenterol. Nutr. 2006;42(2):190–195. doi: 10.1097/01.mpg.0000189339.92891.64. [DOI] [PubMed] [Google Scholar]
- 80.Chiou F.K., Ong C., Low Y., Chiang L.W., Phua K.B. Non-invasive predictors for the first variceal hemorrhage in children with biliary atresia after Kasai portoenterostomy. J Clin Exp Hepatol. 2019;9(5):581–587. doi: 10.1016/j.jceh.2019.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Stringer M.D. Biliary atresia: service delivery and outcomes. Semin. Pediatr. Surg. 2008;17:116–122. doi: 10.1053/j.sempedsurg.2008.02.007. [DOI] [PubMed] [Google Scholar]
- 82.Davenport M., De Ville de Goyet J., Stringer M.D., Mieli-Vergani G., Kelly D.A., McClean P. Seamless management of biliary atresia in England and Wales (1999-2002) Lancet. 2004;363(9418):1354–1357. doi: 10.1016/S0140-6736(04)16045-5. [DOI] [PubMed] [Google Scholar]
- 83.Lampela H., Ritvanen A., Kosola S., Koivusalo A., Rintala R., Jalanko H. National centralization of biliary atresia care to an assigned multidisciplinary team provides high-quality outcomes. Scand. J. Gastroenterol. 2012;47(1):99–107. doi: 10.3109/00365521.2011.627446. [DOI] [PubMed] [Google Scholar]
- 84.Kvist N., Davenport M. Thirty-four years' experience with biliary atresia in Denmark: a single center study. Eur. J. Pediatr. Surg. 2011;21(4):224–228. doi: 10.1055/s-0031-1271709. [DOI] [PubMed] [Google Scholar]
- 85.Schreiber R.A., Barker C.C., Roberts E.A., Martin S.R. Canadian Pediatric Hepatology Research Group. Biliary atresia in Canada: the effect of centre caseload experience on outcome. J. Pediatr. Gastroenterol. Nutr. 2010;51(1):61–65. doi: 10.1097/MPG.0b013e3181d67e5e. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.