Abstract
Pectin, found in the cell walls of fruits and vegetables, is a complex colloidal polysaccharide. In this study, pectin was extracted using ultrasound and microwave-assisted extraction methods from waste lemon, mandarin, and kiwi peel to investigate their use as alternative source of pectin. Hydrochloric acid (HCl) and nitric acid (HNO3) were used as the extracting agents. The effects of microwave power (360–600 W) and irradiation time (1, 2, 3 min) for microwave-assisted extraction (MAE) and of temperature (60 and 75 °C) and sonication time (15, 30, 45 min) on ultrasound-assisted extraction (UAE) on the yield of extracted pectin from the peels were investigated. Optimum conditions were determined for the extraction of pectin from all of the peel samples with the two extraction methods. The produced pectin yield and the degree of esterification were determined and, FT-IR and SEM analyses were performed. Kiwi peel gave the highest yield of extracted pectin using HCl as the solvent with 17.30% yield via UAE at 75 °C for 45 min and 17.97% yield via MAE at 360 W for, 3 min. It was concluded that lemon, mandarin, and kiwi peels all contained pectin and that MAE gave a better yield than UAE and could thus be used as an efficient and rapid technique for the extraction of pectin from the peels. The chemical structures of the pectin obtained using the two different extraction methods were similar and showed a high degree of methoxylation.
Keywords: Fruits, Peel, Pectin, Ultrasound, Microwave, Extraction
Introduction
Pectin is a structural heteropolysaccharide composed of α-1,4-D-galacturonic acid chains; it is found in the primary cell walls and intercellular regions of higher plants (Wang et al. 2018). It is the most complex polysaccharide in nature, making up approximately 35% of the primary cell walls in dicotyledonous and non-granular plants, 2–10% in grass, and 5% in woody tissues (Mohnen 2008). Pectin is a white, amorphous, colloidal carbohydrate found in fruit, especially apples and citrus fruits. It is an efficient, non-hazardous, easy-to-use, kinetically fast, and low-volume adsorbent that can be obtained from the internal structure of fruit peels also called agricultural waste, by various methods. It is widely used in the food, cosmetic and pharmaceutical industries owing to its thickening and emulsifying properties and also plays an important role in the biopolymer sector owing to its properties and application areas (Valdes et al. 2015; Kusrini et al. 2018). It is an additive with wide-ranging applications in the food, cosmetics, textiles and pharmaceutical industries. It is a natural polymer that can be used in many food products, such as jam, marmalade, dairy products, beverages, confectionery, sweets, yogurt, canned fish, mayonnaise, and various sauces thanks to its gelling, thickening, shine, and emulsifying properties (Kusrini et al. 2018; Sila et al. 2009). Pectin or pectic substances are effective in physiological processes, such as cell growth and cell differentiation, protecting plants against pathogens and injuries, and determining the hardness, integrity, and water-retention capacity of plants. The amount and composition of pectic molecules in fruits or vegetables affect the quality of the resulting food products (Voragen et al. 2009). Pectin is a healthy polysaccharide that us extensively used in medicine and pharmacy owing to its anti-tumor activity and the ability to -prevent the formation of cancer cells, strengthen and improve the immune system, lower cholesterol, and reduce post-prandial insulin and glucose concentrations. It has been shown to have immuno-regulatory effects in the intestine (Cho et al. 2019; Lefsih et al. 2017). Dietary pectin prevents the formation of cancer cells and is used in the treatment of colon cancer. It is used in drug delivery systems for its gelling potential, non-toxicity, and easily modifiable functional groups (Zhang et al. 2015). The carboxyl groups of pectin-forming galacturonic acid units are esterified with methanol and the degree of esterification (DE) has an effect on the gel-forming ability of pectin (Guzel and Akpinar 2017). Pectin is divided into two classes; high methoxylated (HMP) and low methoxylated (LMP) according to their degree of esterification. HMP has a degree of esterification greater than 50% whilst LMP is less than 50% (Rascon-Chu et al. 2009).
Extraction is an important technique for the isolation of bioactive products from natural sources for use in food systems (Bayar et al. 2017). Chemical extraction methods are widely used, but they can lead to serious environmental problems as they produce acidic wastewater (Yang et al. 2018). Ultrasound-assisted extraction is a clean, efficient, and eco-friendly technique that can be completed in minutes and gives a final product with higher purity (Maran et al. 2017). Microwave-assisted extraction is an innovative method that was developed as an alternative to traditional methods, benefitting from low energy consumption, easy controllability, short processing time, low solvent requirements, low cost, high efficiency, and cleanliness (Tongkham et al. 2017). UAE extract valuable components from the source using cavitation bubbles caused by ultrasonic waves. Ultrasound increases the mass transfer rate and ensures the recovery of the desired bioactive components with minimal degradation (Ponmurugan et al. 2017). With the collapse of cavitation bubbles near the surface of the plant material, an increase in pressure and temperature is observed. This causes deterioration of the plant cell walls and the components pass to the solvent (Liew et al. 2016). By using ultrasound, solvent consumption is reduced, the extraction time is decreased, repeatability is increased, the obtained product has higher purity, and less energy is consumed compared with extraction using conventional methods (Colodel and Petkowicz 2019). Microwave energy is non-ionizing radiation that can penetrate into materials. It does not change the chemical structure of the target material because it is non-ionizing. The electromagnetic radiation generated by the microwaves is transformed into molecular motion and emitted as heat. In this way, the target components are dissolved in the solvent as a result of the pressure generated by the water vapor in the cell walls in the heated sample (Ciriminna et al. 2016; Wang et al. 2016).
With the increase in the global population, waste generation is increasing and environmental pollution has become a common problem around the world. In this study, lemon, mandarin, and kiwi peels, which are rich in pectin but usually treated as waste products, were used as an alternative source for pectin extraction. The aim of this study is to obtain pectin from lemon, mandarin, and kiwi peel by UAE and MAE. The effects of extraction parameters such as temperature and sonication time for UAE and microwave power and irradiation time for MAE on the pectin yield were investigated. HCl and HNO3 were compared as extraction solvents and their effects on the pectin yield were examined. The yields from three different types of fruit peels using these two extraction methods are compared. Thus, the most efficient pectin production method and source will be determined and the product quantity and morphological structure will be investigated. The outcomes of this work will enable, food waste to be recycled to generate a natural additive, making a contribution to the national economy and environmental protection.
Materials and methods
Materials
The lemons, mandarins and kiwis used in the experiments were purchased from a local market in Istanbul, Turkey. The peels were removed from the fruit, then soaked in a hot water bath for 5 min to inactivate the enzymes. The peel samples were then washed with cold tap water followed by with distilled water. The washed peels were dried in an oven at 60 °C for 24 h to remove any moisture, then the dried peels were milled and the resulting peel powder was stored in low-density polyethylene bags at room temperature. In this study, HCl (Tekkim, Bursa, Turkey), HNO3 (Merck, Darmstadt, Germany), and ethanol (Isolab, Wertheim, Germany) were used as chemical reagents.
Ultrasound assisted extraction
Based on some preliminary experiments 2 g of powdered peel was mixed with 60 mL of 0.5 M HCl. To determine the conditions for UAE, some preliminary experiments were conducted using different ratios of dried peel-HCl and dried peel-HNO3 and the extraction solution was prepared as dried peel/solvent ratio 1:30 (g/mL). The samples were then placed in an ultrasonic water bath (Isolab, Wertheim, Germany). The temperature and reaction time were varied from 60 to 75 °C and 15 to 45 min, respectively, to determine the optimum temperature and time for the extraction. At the end of extraction, the solution was cooled to room temperature, then centrifuged for 20 min at 1500 rpm. The supernatant was removed from the centrifuge tube and coagulated by adding two volumes of ethanol (120 mL) and leaving to stand overnight at 4 °C. After the precipitation was completed, the pectin was separated by vacuum filtration and then washed with ethanol. The produced pectin was dried in an oven (MMM Ecocell 111, Munich, Germany) at 60 °C until a constant weight was reached. The same operations were carried out with the parameters determined with 0.5 M HNO3 as the solvent (Shivamathi et al. 2019; Gharibzahedia et al. 2019).
Microwave assisted extraction
Based on some preliminary experiments 2 g of dry peel powder was mixed with 0.5 M HCl (60 mL) as the extracting agent. To determine the MAE conditions, some preliminary experiments were conducted using different ratios of dried peel-HCl and dried peel-HNO3 and the extraction solution was prepared as dried peel/solvent ratio 1:30 (g/mL). To determine the optimum microwave power and irradiation time, the prepared solution was placed in the center of the microwave oven (Bosch HMT72G420, Stuttgart, Germany) and the following conditions were applied: 360 and 600 W microwave power and 1, 2, or 3 min’ irradiation time. After the irradiation, the solution was removed from the microwave and allowed to reach room temperature. All steps applied after the extractions are the same as for the UAE method. The same operations were carried out with the parameters determined with 0.5 M HNO3 used as the solvent (Gharibzahedia et al. 2019; Hosseini et al. 2016).
Determination of pectin yield and degree of esterification
Pectin yield was calculated using Eq. (1), where, A is the amount of pectin produced and, B is the initial amount of mandarin, lemon or kiwi peel powder. Yields were calculated by taking the average of three experimental sets of results for each sample (Zaid et al. 2019; Dranca et al. 2020).
| 1 |
The DE affects the gelling characteristic of pectin. It is the ratio of the methyl-esterified carboxyl groups present in a pectin sample to the total number of carboxyl groups. The chemical structure of the produced pectin was analyzed by FT-IR (IRPrestige21, Shimadzu Corporation, Kyoto, Japan) for the samples with the best yield for each extraction method and solvent. Pectin particles were mixed with KBr (1:100) and pressed into KBr pellets before FT-IR analysis. The peaks at 1745 cm−1 and 1630 cm−1 are known as the pectin’s fingerprint region. The degree of esterification was determined by FT-IR analysis according to Eq. (2). Where A1630 and A1745 represent the absorbance intensity of the non-methyl-esterified carboxyl groups at 1745 cm−1 and methyl-esterified carboxyl groups at 1630 cm−1, respectively (Liew et al. 2016; Singthong et al. 2004).
| 2 |
Preparation of pectin gels
In this study, the gel preparation was based on the method reported by the IFT (Institute of Food Technologists) Committee (1959). 13 g f sugar and 0.4 g of pectin were weighed and mixed. The mixture was then made up to 20 mL with distilled water. The volume of sugar in the solution was adjusted to 65% and the solution was then stirred magnetically with heating until it reached boiling point. After boiling 48.8% tartaric acid was added and the pH was adjusted to 2.2–2.4. The mixture was then left in a porcelain crucible for 24 h at room temperature. Subsequently, the crucible was turned upside down on a flat glass surface and left for 2 min. The gel height was then measured and the slump strength was calculated using Eq. (3). In the equation, A represents the height of the cup and B represents the height of the gel.
| 3 |
Scanning electron microscopy analysis
The morphological properties of the dried mandarin, lemon, and kiwi peels pectin were observed using a scanning electron microscope (SEM, Zeiss EVO LS10) with an accelerating voltage of 7 kV and a magnification of 10,000×.
Results and discussion
Pectin yield
Pectin was extracted from lemon, mandarin, and kiwi peels using UAE and MAE. The yield of pectin depends on the type of raw material, extraction technique, acid type, extraction time, microwave power and irradiation time for MAE, and temperature and sonication time for UAE.
Effects of UAE on pectin yield
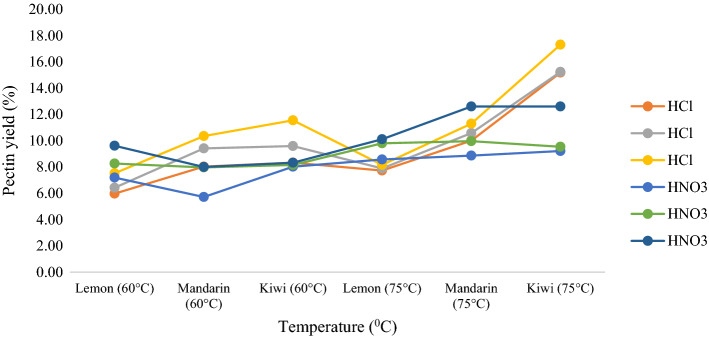
When the yield results were examined, it was seen that increasing the extraction temperature and extraction time increases the yield of the product obtained by UAE. The temperature was varied from 60 to 75 °C and the time effect was studied at 15, 30, and 45 min. The two major factors that could affect the extraction yield are cavitation and thermal effect. Ultrasound produces cavitational bubbles with a high cavitation threshold and it exploded with higher force in the medium which leads to augment the disruption of plant materials during extraction. Increasing the temperature above 75 °C reduces the surface tension and viscosity of the solvent, which decreased the extraction yield by modifying the characteristics of the ultrasonic cavitation and the mass transfer rate. The recovery of pectin was increased as the extraction time increased from 15 to 45 min and then decreased because ultrasound waves prompted the cavitational effect in the solvent medium leading to increased solvent penetration into the fruit peels and the release of pectin into the solvent. Beyond 45 min, modification and fragmentation of the pectin polysaccharide structure occurred, reducing the pectin recovery (Maran et al. 2017; Ponmurugan et al. 2017; Sengar et al. 2020). The maximum pectin yields using UAE were obtained at 45 min and 75 °C: 10.11% for lemon peel, and 11.29% for mandarin peel using HNO3 as the solvent, and 17.30% for kiwi peel using HCl as the solvent. The yields obtained for lemon and mandarin peels were higher than those reported previously from studies on waste heads of Helianthus annus (8.89%) (Ponmurugan et al. 2017), custard apple peel (8.93%) (Shivamathi et al. 2019), and apple pomace (9.18%) (Dranca et al. 2020), whilst the pectin yield obtained from the kiwi peel was higher than those reported for UAE from sisal waste (11.9%) (Yang et al. 2018), pomelo peel (14.25%) (Liew et al. 2016) and tomato waste (15.21%) (Sengar et al. 2020).
Effects of MAE on pectin yield
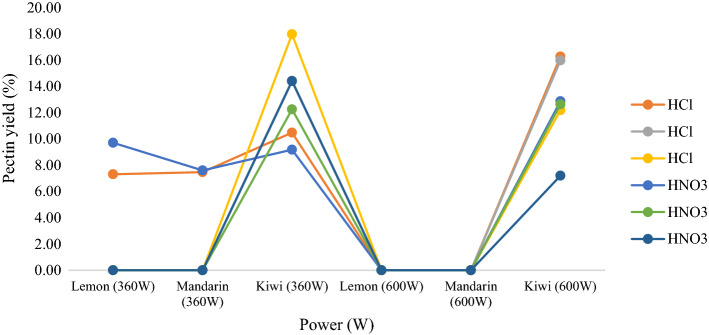
With MAE, the highest pectin yield for lemon peel was 9.71% and the highest pectin yield for mandarin peel was 7.60%, both using HNO3 as the solvent at 360 W microwave power for 1 min. The highest pectin yield for kiwi peel was 17.97% using HCl as the solvent at 360 W microwave power for 3 min. Pectin extraction from lemon and mandarin peels could not be performed at 360 W for longer than 1 min or at 600 W for any duration. This is because the resulting mixtures could not be centrifuged under the specified centrifugation conditions. After centrifugation at 1500 rpm for 20 min, the liquid phase could not be separated by Pasteur pipette because the mixture was very thick in consistency. The absorption of microwave energy in the extraction system supports thermal accumulation in the extraction solution and causes the dissolution of pectin into the solution until 1 min. However, prolonged microwave processing of pectin chain molecules causes their deterioration, whilst affecting the pectin recovery rate and decreasing efficiency. Similar outcames have been reported for pectin extraction from orange peel, waste Citrullus lanatus fruit rinds and waste Carcia papaya L. Peel, and pectic polysaccharide extraction from waste mango peel (Maran et al. 2013, 2014; Maran and Prakash 2015; Maran et al. 2015). Under the same conditions, pectin production was successfully realized by MAE from kiwi peel because an increase in the microwave power provided more irradiation energy, which enabled the penetration of the extraction solvent into the pectin source. The cell material was ruptured and the pectin release from the plant material to the solvent increased. At the same time, with increased irradiation time, there was a bigger accumulation of heat within the extraction solution and the dissolution of pectin increased. The use of HNO3 as the solvent in the extraction of pectin from lemon peel yielded more efficient results than HCl. Otherwise, the use of HCl as solvent was more effective than HNO3 in the production of pectin from kiwi and mandarin peels. The pectin yields from the lemon, mandarin, and kiwi peels were lower than those reported from studies with MAE with orange peel (29.1%) (Hosseini et al. 2016), pomelo peel (27.65%) (Liew et al. 2016), apple pomace (23.32%) (Dranca et al. 2020), and tomato waste (20.83%) (Sengar et al. 2020). However, the pectin extraction yield from kiwi peel was higher than those reported from previous studies on dragon fruit (Hylocereus polyrhizus) peel (17.2%) (Rahmati et al. 2019) and Opuntia ficus indica (12.57%) (Lefsih et al. 2017).
It can be concluded that mandarin, lemon and kiwi peels are suitable alternative sources for pectin production. It can be seen from the results presented in Table 1, and Figs. 1 and 2 that MAE is a more effective and efficient method than UAE for pectin extraction from lemon, mandarin, and kiwi peels because it has a shorter extraction time and lower; solvent and energy consumption.
Table 1.
Pectin yields (%) of ultrasound and microwave assisted extraction at different parameters
| Extraction conditions | Raw material | Pectin yield (%) | |
|---|---|---|---|
| HCl | HNO3 | ||
| UAE | |||
| 60 °C | |||
| 15 min | Lemon | 5.97 ± 0.15 | 7.19 ± 0.11 |
| Mandarin | 8.03 ± 0.05 | 5.72 ± 0.09 | |
| Kiwi | 8.33 ± 0.06 | 8.02 ± 0.05 | |
| 30 min | Lemon | 6.42 ± 0.05 | 8.26 ± 0.07 |
| Mandarin | 9.41 ± 0.06 | 7.97 ± 0.10 | |
| Kiwi | 9.59 ± 0.09 | 8.15 ± 0.05 | |
| 45 min | Lemon | 7.52 ± 0.05 | 9.61 ± 0.09 |
| Mandarin | 10.35 ± 0.05 | 8.00 ± 0.05 | |
| Kiwi | 11.55 ± 0.11 | 8.32 ± 0.14 | |
| 75 °C | |||
| 15 min | Lemon | 7.73 ± 0.12 | 8.57 ± 0.05 |
| Mandarin | 10.00 ± 0.07 | 8.86 ± 0.05 | |
| Kiwi | 15.17 ± 0.05 | 9.21 ± 0.07 | |
| 30 min | Lemon | 7.89 ± 0.10 | 9.80 ± 0.05 |
| Mandarin | 10.57 ± 0.07 | 9.96 ± 0.05 | |
| Kiwi | 15.23 ± 0.09 | 9.54 ± 0.08 | |
| 45 min | Lemon | 8.60 ± 0.06 | 10.11 ± 0.05 |
| Mandarin | 11.29 ± 0.17 | 10.33 ± 0.12 | |
| Kiwi | 17.30 ± 0.05 | 12.60 ± 0.11 | |
| MAE | |||
| 360 W | |||
| 1 min | Lemon | 7.31 ± 0.10 | 9.71 ± 0.07 |
| Mandarin | 7.47 ± 0.06 | 7.60 ± 0.05 | |
| Kiwi | 10.48 ± 0.09 | 9.18 ± 0.06 | |
| 2 min | Lemon | – | – |
| Mandarin | – | – | |
| Kiwi | 14.43 ± 0.08 | 12.25 ± 0.11 | |
| 3 min | Lemon | – | – |
| Mandarin | – | – | |
| Kiwi | 17.97 ± 0.40 | 14.40 ± 0.10 | |
| 600 W | |||
| 1 min | Lemon | – | – |
| Mandarin | – | – | |
| Kiwi | 16.27 ± 0.06 | 12.87 ± 0.05 | |
| 2 min | Lemon | – | – |
| Mandarin | – | – | |
| Kiwi | 15.98 ± 0.09 | 12.63 ± 0.07 | |
| 3 min | Lemon | – | – |
| Mandarin | – | – | |
| Kiwi | 12.18 ± 0.05 | 7.20 ± 0.07 | |
Values are mean of three replicates ± SD
Fig. 1.
Pectin yields (%) from ultrasound-assisted extraction using different extraction conditions
Fig. 2.
Pectin yields (%) from microwave-assisted extraction using different extraction conditions
Effect of pH on pectin yield
Extraction experiments were conducted at various pH (1–3) in order to examine its influence on extracted pectin yield. The results indicated that pH 2 gave the best pectin extraction yield, which is because the acidic solvent hydrolyzes insoluble pectin and converts it to a soluble state. The solubilized pectin was extracted and the pectin yield increased. Precipitation of pectin occurred above pH 2, reducing the yield. Thus, a pH of 2 gave the best pectin extraction yield. (Ponmurugan et al. 2017; Shivamathi et al. 2019; Maran et al. 2017).
Degree of esterification
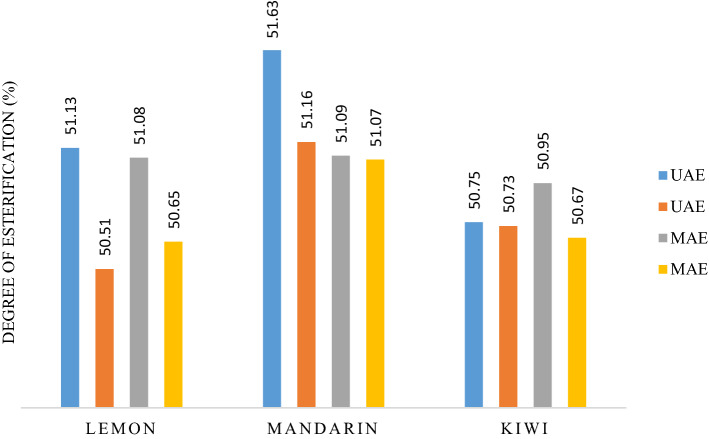
The degree of esterification is a significant parameter affecting pectin quality, its application and extraction method. Pectin with a degree of esterification above 50% is called HMP and those with less than 50% are called LMP. The degree of esterification (%) of the extracted pectin samples is presented in Table 2 and Fig. 3. The pectin extracted by UAE and MAE showed similar levels of esterification; ranging from 50.51 to 51.63%, being classed as HMP and thus suitable for use as a gelling agent and in other food applications.
Table 2.
Degree of esterification of pectin samples (%)
| Raw material | Extraction conditions | Degree of esterification (%) | ||
|---|---|---|---|---|
| HCl | HNO3 | |||
| Lemon | UAE | 75 °C and 45 min | 51.13 ± 0.19 | 50.51 ± 0.41 |
| MAE | 360 W and 1 min | 51.08 ± 0.08 | 50.65 ± 0.24 | |
| Mandarin | UAE | 75 °C and 45 min | 51.63 ± 0.58 | 51.16 ± 0.27 |
| MAE | 360 W and 1 min | 51.09 ± 0.08 | 51.07 ± 0.85 | |
| Kiwi | UAE | 75 °C and 45 min | 50.75 ± 0.34 | 50.73 ± 0.69 |
| MAE | 360 W and 3 min | 50.95 ± 0.15 | 50.67 ± 0.10 | |
Values are mean of three replicates ± SD
Fig. 3.
Degree of esterification (%) of pectin samples
Previous reports shoved that, the pectin extracted by UAE has a higher degree of esterification compared to pectin extracted by MAE; the degree of esterification of pectin extracted from tomato peel waste was 66.43% for UAE and 59.76 for MAE (Sengar et al. 2020). The degree of esterification of pectin extracted from dragon fruit by MAE ranged from 45.82 to 46.95% so it was classed as LMP (Rahmati et al. 2019). The degree of esterification of sisal pectin extracted using UAE was 44.35%; suggesting that sisal pectin is LMP (Yang et al. 2018). Hosseini et al. (2016) studied MAE of pectin from sour orange peel (SOPP). The SOPP extracted in this study showed a degree of esterification of 1.7–37.5% and thus can be classified as LMP. They concluded that pectin extracted under very severe conditions (high power, long irradiation interval) has a low degree of esterification because these conditions increase the de-esterification of polygalacturonic chains. The degree of esterification pectin obtained from lime peel by MAE was in the range of 70.81–91.58% (Rodsamran and Sothornvita 2019). Thus, lime peel pectin can be classified as HMP with a rapid-set gel formation. Dranca et al. (2020) studied the use of non-conventional techniques (MAE, UAE) for the extraction of pectin from Malus domestica ‘Fălticeni’ apple pomace. The MAE pectin showed a lower degree of esterification (73.80%) than the UAE pectin (77%).
Pectin gel formation and slump strength
When the gel formation of the pectin samples extracted from lemon, mandarin, and kiwi peels were examined, the slump strength results were the same for all extraction methods with both solvents. The slump strengths of the gels prepared using pectin extracted from lemon peel using HCl and HNO3 were calculated as 83.3% and 79.2%, respectively. The amount of pectin gel produced from lemon peel using HNO3 as the extraction solvent is relatively low and the gels formed were viscous, fluid, and they were unable to retain their structure. Thus, the gel formation capacity of the lemon peel derived pectin samples is low and the gels formed are not strong. The slump strengths of the gels made using pectin extracted from mandarin peel with both HNO3 and HCl were calculated as 83.3%. The mandarin pectin gels do not have strong gel-forming properties but have a highly fluid structure. The slump strengths of the gels made using pectin extracted from kiwi peel with both HNO3 and HCl were calculated as 87.5%. The kiwi pectin gels were more precipitated than the lemon and mandarin pectin gels so they were less potent and less effective at gel formation.
Chemical structure analysis by FT-IR
The structures of the pectin samples extracted by UAE and MAE from lemon, mandarin, and kiwi peels were determined using Fourier-transform infrared spectroscopy. The FT-IR spectra for the lemon, mandarin, and kiwi pectin samples extracted by UAE (75 °C, 45 min, HCl and 75 °C, 45 min, HNO3) and MAE method (360 W, 1 min, HCl and 360 W, 1 min, HNO3) are shown in Figs. 4, 5 and 6 respectively. The spectra for all of the extracted lemon, mandarin, and kiwi pectin samples showed the typical fingerprint of HMP.
Fig. 4.
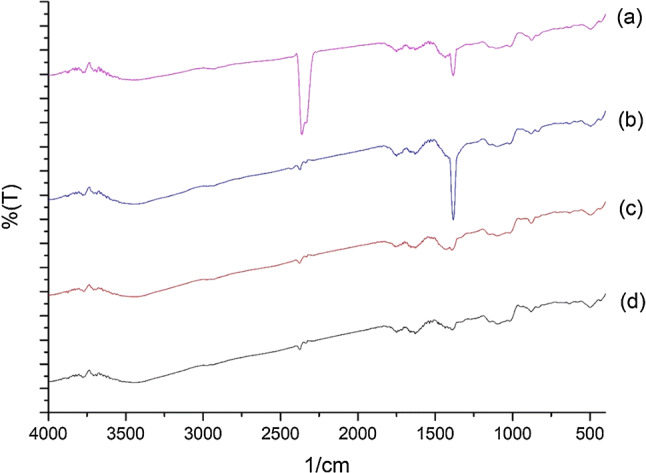
FT-IR spectra of lemon pectin samples a MAE, 360 W, 1 min, HNO3 b UAE, 75 °C, 45 min, HNO3, c MAE, 360 W, 1 min, HCl, and d UAE, 75 °C, 45 min, HCl
Fig. 5.
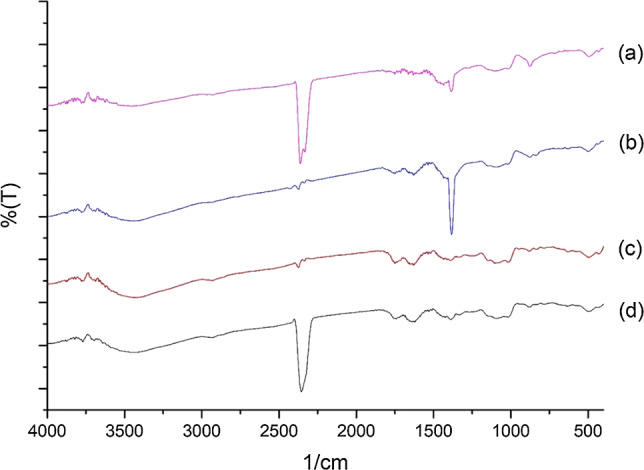
FT-IR spectra of mandarin pectin samples a MAE, 360 W, 1 min, HNO3, b UAE, 75 °C, 45 min, HNO3, c MAE, 360 W, 1 min, HCl, and d UAE, 75 °C, 45 min, HCl
Fig. 6.
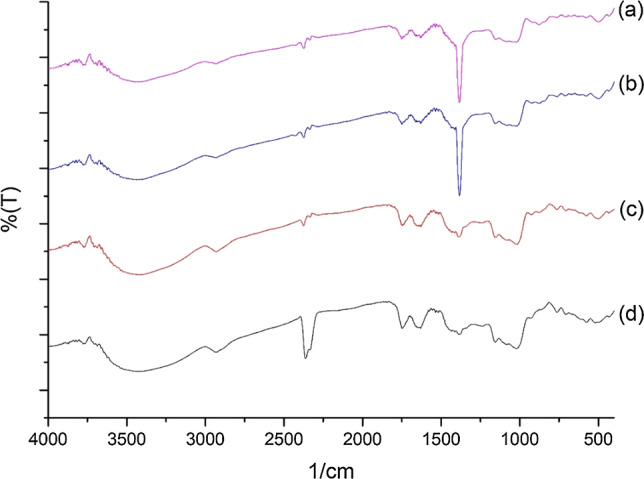
FT-IR spectra of kiwi pectin samples a MAE, 360 W, 1 min, HNO3, b UAE, 75 °C, 45 min, HNO3, c MAE, 360 W, 1 min, HCl, and d UAE, 75 °C, 45 min, HCl
The FT-IR spectra show peaks typical of the functional groups found in pectin. Absorption in the 800–1200 cm−1 wave range is defined as the fingerprint zone for carbohydrates (Tongkham et al. 2017). The peaks at around 3600–3400 cm−1, which are characteristic absorption peaks for polysaccharides, are attributed to the stretching vibrations of hydroxyl (O–H) groups and these peaks indicate that there are large numbers of OH− bonds in pectin molecules (Shivamathi et al. 2019). The absorption bands in the range of 2800–3000 cm−1 are related to the C–H of CH2 and CH3 of carbohydrates (Kazemi et al. 2019). The peak at 1740–1760 cm−1 represents the C=O stretching of esters and shows the presence of acetyl groups (COCH3) in pectin whilst the peak at 1630–1660 cm−1 is an –OH vibration band (Ponmurugan et al. 2017). The 1600–1800 cm−1 region is used for the identification and determining the quality of pectin. The absorption bands at 1380–1445 cm−1 indicate the presence of a CH3 group. The absorption bands at 1000–1200 cm−1 consist of C–O tensile vibrations and indicate the presence of methyl esters in pectin (Lefsih et al. 2017; Singthong et al. 2004; Xu et al. 2018). Thus, the FT-IR spectra for all of the pectin samples obtained from lemon, mandarin, and kiwi peels confirm the existence of polygalacturonic acid (pectin).
Results of SEM analysis
The surface morphology of the pectin samples produced using the optimized parameters for each of the fruit peels was analyzed by SEM to investigate the influence of the extraction method. Previous studies have indicated that the surface of MAE pectin appears very rough and slightly ruptured. The morphology seemed to be influenced by the rapid increase in temperature and the high internal pressure associated with this extraction method (Liew et al. 2016; Dranca et al. 2020; Kazemi et al. 2019). With UAE, the rapid increase in temperature and internal pressure, and penetration of ultrasound energy to the sample and solvent lead to the, cell wall being destroyed and ruptured, whereas no destruction was observed in samples not subjected to ultrasound treatment. The ultrasonic treatment led to a partially collapsed and relatively coarse morphology in the microstructure of the extracted pectin (Yang et al. 2018; Singthong et al. 2004; Sengar et al. 2020). SEM images of pectin obtained in this work from lemon, mandarin, and kiwi peel are presented in Fig. 7. There are some clear differences in the microstructures of the samples: the lemon and mandarin pectin shows a slight tendency to curl, while the kiwi pectin seems to be ruptured. From the results obtained in this work, it can be concluded that both ultrasonic and microwave extraction methods changed the surface morphology of pectin.
Fig. 7.
SEM image of pectin samples extracted under optimum conditions: 7 kV, 10000 magnification. a Lemon peel (UAE, 75 °C, 45 min, HNO3), b mandarin peel (UAE, 75 °C, 45 min, HCl), and c kiwi peel (MAE, 360 W, 3 min, HCl)
Conclusion
Pectin was extracted from lemon, mandarin, and kiwi peel using ultrasound and microwave assisted extraction methods. Among these three raw materials, the highest amount of pectin was obtained from kiwi peel with a yield of 17.97%. It was concluded that kiwi peel could be used as an alternative source of pectin. During the experiments, two different acids, HCl and HNO3, were investigated as extraction solvents. The pectin yield from lemon peel was higher with HNO3 whilst kiwi and mandarin peels gave higher yields with HCl. The results showed that these both acids were suitable solvents for pectin extraction. In this work, MAE was found to be more efficient that UAE since it requires less time (only 1, 2, or 3 min). The degree of esterification of the extracted pectin samples ranged from 50.51 to 51.63%, so all of the obtained pectin was HMP and could be used as gelling agents. The FT-IR spectra of extracted pectin proved that they were HMP and confirmed the presence of polygalacturonic acid. The observed differences in the surface morphology might be owing to the structural characteristics, which were influenced by the extraction processes. In conclusion, the MAE method is preferred for extraction of pectin from peel because it can be performed quickly and it is a clean, efficient, and simple technique. As a continuation of this study, the produced pectins will be used for edible film production for food packaging.
Acknowledgements
This study was supported by FYL-2018-3481 project of Yıldız Technical University Scientific Research Projects Coordinator.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Bayar N, Bouallegue T, Achour M, Kriaa M, Bougatef A, Kammoun R. Ultrasonic extraction of pectin from Opunta ficus indica cladodes after mucilage removal: optimization of experimental conditions and evaluation of chemical and functional properties. Food Chem. 2017;235:275–282. doi: 10.1016/j.foodchem.2017.05.029. [DOI] [PubMed] [Google Scholar]
- Cho E, Jung H, Lee B, Kim H, Rhee J, Yoo S. Green process development for apple-peel pectin production by organic acid extraction. Carbohyd Polym. 2019;204:97–103. doi: 10.1016/j.carbpol.2018.09.086. [DOI] [PubMed] [Google Scholar]
- Ciriminna R, Carnaroglio D, Delisi R, Arvati S, Tamburino A, Pagliaro M. Industrial feasibility of natural products extraction with microwave technology. Chem Select. 2016;3:549–555. [Google Scholar]
- Colodel C, Petkowicz C. Acid extraction and physicochemical characterization of pectin from cubiu (Solanum sessiliflorum D.) fruit peel. Food Hydrocoll. 2019;86:193–200. doi: 10.1016/j.foodhyd.2018.06.013. [DOI] [Google Scholar]
- Committee IFT. Pectin standardization. Food Technol. 1959;8:496–500. [Google Scholar]
- Dranca F, Vargas M, Oroıan M. Physicochemical properties of pectin from Malus domestica ‘Fălticeni’ apple pomace as affected by non-conventional extraction techniques. Food Hydrocoll. 2020;100:105383. doi: 10.1016/j.foodhyd.2019.105383. [DOI] [Google Scholar]
- Gharibzahedia SMT, Smith B, Guo Y. Ultrasound-microwave assisted extraction of pectin from fig (Ficus carica L.) skin: optimization, characterization and bioactivity. Carbohyd Polym. 2019;222:114992. doi: 10.1016/j.carbpol.2019.114992. [DOI] [PubMed] [Google Scholar]
- Guzel M, Akpinar Ö. Turunçgil Kabuklarından Elde Edilen Pektinlerin Karakterizasyonu ve Karşılaştırılması. Akad Gıda. 2017;15(1):17–28. doi: 10.24323/akademik-gida.304274. [DOI] [Google Scholar]
- Hosseini SS, Khodaiyan F, Yarmand MS. Optimization of microwave assisted extraction of pectin from sour orange peel and its physicochemical properties. Carbohyd Polym. 2016;140:59–65. doi: 10.1016/j.carbpol.2015.12.051. [DOI] [PubMed] [Google Scholar]
- Kazemi M, Khodaiyan F, Hosseini SS. Utilization of food processing wastes of eggplant as a high potential pectin source and characterization of extracted pectin. Food Chem. 2019;294:339–346. doi: 10.1016/j.foodchem.2019.05.063. [DOI] [PubMed] [Google Scholar]
- Kusrini E, Wicaksono W, Gınawan C, Daud NZA, Usman A. Kinetics, mechanism and thermodynamics of lanthanum adsorption on pectin extracted from durian rind. J Environ Chem Eng. 2018;6:6580–6588. doi: 10.1016/j.jece.2018.10.018. [DOI] [Google Scholar]
- Lefsih K, Giacomazza D, Dahmoune F, Mangione MR, Bulone D, San Biagio PL, Passantino R, Costa MA, Guarrasi V, Khodir M. Pectin from Opuntia ficus indica: optimization of microwave-assisted extraction and preliminary characterization. Food Chem. 2017;221:91–99. doi: 10.1016/j.foodchem.2016.10.073. [DOI] [PubMed] [Google Scholar]
- Liew SQ, Ngoh GC, Yusoff R, Teoh WH. Sequential ultrasound-microwave assisted acid extraction (UMAE) of pectin from pomelo peels. Int J Biol Macromol. 2016;93:426–435. doi: 10.1016/j.ijbiomac.2016.08.065. [DOI] [PubMed] [Google Scholar]
- Maran JP, Prakash KA. Process variables influence on microwave assisted extraction of pectin from waste Carcia papaya L. peel. Int J Biol Macromol. 2015;73:202–206. doi: 10.1016/j.ijbiomac.2014.11.008. [DOI] [PubMed] [Google Scholar]
- Maran JP, Sivakumar V, Thirugnanasambandhama K, Sridhar R. Optimization of microwave assisted extraction of pectin from orange peel. Carbohyd Polym. 2013;97(2):703–709. doi: 10.1016/j.carbpol.2013.05.052. [DOI] [PubMed] [Google Scholar]
- Maran JP, Sivakumar V, Thirugnanasambandhama K, Sridhar R. Microwave assisted extraction of pectin from waste Citrullus lanatus fruit rinds. Carbohyd Polym. 2014;101:786–791. doi: 10.1016/j.carbpol.2013.09.062. [DOI] [PubMed] [Google Scholar]
- Maran JP, Swathi K, Jeevitha P, Jayalakshmi J, Ashvini G. Microwave-assisted extraction of pectic polysaccharide from waste mango peel. Carbohyd Polym. 2015;123(5):67–71. doi: 10.1016/j.carbpol.2014.11.072. [DOI] [PubMed] [Google Scholar]
- Maran JP, Priya B, Al-Dhabi NA, Ponmurugan K, Moothy I, Sivarajasekar N. Ultrasound assisted citric acid mediated pectin extraction from industrial waste of Musa balbisiana. Ultrason Sonochem. 2017;35:204–209. doi: 10.1016/j.ultsonch.2016.09.019. [DOI] [PubMed] [Google Scholar]
- Mohnen D. Pectin structure and biosynthesis. Curr Opin Plant Biol. 2008;11:266–277. doi: 10.1016/j.pbi.2008.03.006. [DOI] [PubMed] [Google Scholar]
- Ponmurugan K, Al-Dhabi NA, Maran JP, Karthikeyan K, Moothy IG, Sivarajasekar N, Manoj JJB. Ultrasound assisted pectic polysaccharide extraction and its characterization from waste heads of Helianthus annus. Carbohyd Polym. 2017;173:707–713. doi: 10.1016/j.carbpol.2017.06.018. [DOI] [PubMed] [Google Scholar]
- Rahmati S, Abdullah A, Kang OL. Effects of different microwave intensity on the extraction yield and physicochemical properties of pectin from dragon fruit (Hylocereus polyrhizus) peels. Bioact Carbohyd Diet Fibre. 2019;18:100186. doi: 10.1016/j.bcdf.2019.100186. [DOI] [Google Scholar]
- Rascon-Chu A, Martinez-Lopez AL, Carvajal-Millan E, Leon-Renova N, Marquez-Escalante J, Romo-Chacon A. Pectin from low quality ‘golden delicious’ apples: composition and gelling capability. Food Chem. 2009;116:101–103. doi: 10.1016/j.foodchem.2009.02.016. [DOI] [Google Scholar]
- Rodsamran P, Sothornvita R. Microwave heating extraction of pectin from lime peel: characterization and properties compared with the conventional heating method. Food Chem. 2019;278:364–372. doi: 10.1016/j.foodchem.2018.11.067. [DOI] [PubMed] [Google Scholar]
- Sengar AS, Rawson A, Muthiah M, Kalakandan SK. Comparison of different ultrasound assisted extraction techniques for pectin from tomato processing waste. Ultrason Sonochem. 2020;61:104812. doi: 10.1016/j.ultsonch.2019.104812. [DOI] [PubMed] [Google Scholar]
- Shivamathi CS, Moorthy IG, Kumar RV, Soosai MR, Maran JP, Kumar RS, Varalakshmi P. Optimization of ultrasound assisted extraction of pectin from custard apple peel: potential and new source. Carbohyd Polym. 2019;225:115240. doi: 10.1016/j.carbpol.2019.115240. [DOI] [PubMed] [Google Scholar]
- Sila DN, Van Buggenhout S, Duvetter T, Fraeye I, De Roeck A, Van Loey A, Hendrickx M. Pectins in processed fruits and vegetables: part II-structure–function relationships. Compr Rev Food Sci Food Saf. 2009;8:86–104. doi: 10.1111/j.1541-4337.2009.00071.x. [DOI] [Google Scholar]
- Singthong J, Cui SW, Ningsanond S, Goff HD. Structural characterization, degree of esterification and some gelling properties of Krueo Ma Noy (Cissampelos pareira) peçtin. Carbohyd Polym. 2004;58:391–400. doi: 10.1016/j.carbpol.2004.07.018. [DOI] [Google Scholar]
- Tongkham N, Juntasalay B, Lasunon P, Sengkhamparn N. Dragon fruit peel pectin: microwave-assisted extraction and fuzzy assessment. Agric Nat Resour. 2017;51:262–267. [Google Scholar]
- Valdes A, Burgos N, Jimenez A, Garrigos MC. Natural pectin polysaccharides as edible coatings. Coatings. 2015;5:865–886. doi: 10.3390/coatings5040865. [DOI] [Google Scholar]
- Voragen AGJ, Coenen G, Verhoef RP, Schols HA. Pectin, a versatile polysaccharide present in plant cell walls. Struct Chem. 2009;20:263–275. doi: 10.1007/s11224-009-9442-z. [DOI] [Google Scholar]
- Wang H, Ding J, Ren N. Recent advances in microwave-assisted extraction of trace organic pollutants from food and environmental samples. Trends Anal Chem. 2016;75:197–208. doi: 10.1016/j.trac.2015.05.005. [DOI] [Google Scholar]
- Wang W, Chen W, Zou M, Lv R, Wang D, Hou F, Feng H, Ma X, Zhong J, Ding T, Ye X, Liu D. Applications of power ultrasound in oriented modification and degradation of pectin: a review. J Food Eng. 2018;234:98–107. doi: 10.1016/j.jfoodeng.2018.04.016. [DOI] [Google Scholar]
- Xu S-Y, Liu J-P, Huang X, Du L-P, Shi F-L, Dong R, Huang X-T, Zheng K, Liu Y, Cheong K-L. Ultrasonic-microwave assisted extraction, characterization and biological activity of pectin from jackfruit peel. LWT Food Sci Technol. 2018;90:577–582. doi: 10.1016/j.lwt.2018.01.007. [DOI] [Google Scholar]
- Yang Y, Wang Z, Hu D, Xiao K, Wu J. Efficient extraction of pectin from sisal waste by combined enzymatic and ultrasonic process. Food Hydrocoll. 2018;79:189–196. doi: 10.1016/j.foodhyd.2017.11.051. [DOI] [Google Scholar]
- Zaid RM, Mishra P, Tabassum S, Wahid ZA, Sakinah AMM. High methoxyl pectin extracts from Hylocereus polyrhizus’s peels: extraction kinetics and thermodynamic studies. Int J Biol Macromol. 2019;141:1147–1157. doi: 10.1016/j.ijbiomac.2019.09.017. [DOI] [PubMed] [Google Scholar]
- Zhang W, Xu P, Zhang H. Pectin in cancer therapy: a review. Trends Food Sci Technol. 2015;44:258–271. doi: 10.1016/j.tifs.2015.04.001. [DOI] [Google Scholar]