Abstract
Hydrolyzed collagen from the defatted Asian sea bass (Lates calcarifer) (Asbs-HC) had high hydrophobic amino acids and imino acids. When fibroblast cell was treated with Asbs-HC, there was no cytotoxicity at any concentrations (25–1000 µg/mL). Asbs-HC at 1000 µg/mL exhibited the highest cell proliferation and cell migration (p < 0.05), indicating wound healing ability. Antioxidative activities of Asbs-HC at different concentrations were determined. ABTS radical scavenging activity (ABTS-RSA) and oxygen radical absorbance capacity (ORAC) increased when Asbs-HC levels augmented up to 1 mg/mL (p < 0.05). Decreased activities in scavenging DPPH radical and chelating metal were found at higher levels of Asbs-HC (0.5 and 1 mg/mL) (p < 0.05). Molecular weight (MW) of peptides in Asbs-HC ranged from 406 to 16,120 Da. Peptide containing MW of 406 Da rendered the highest scavenging activity towards ABTS radical. Thus, Asbs-HC could be applied as antioxidant, skin nourishment and wound healing agents for food/drink fortification.
Keywords: Bioactive peptides, Defatting, Wound-healing, Fibroblast proliferation, Hydrolyzed collagen, Antioxidant
Introduction
Lipid oxidation in foods, especially those rich in polyunsatured fatty acids, takes place easily. This phenomenon induces the deterioration of food quality. Also it causes some diseases (Chotphruethipong and Benjakul 2019). Overproduction of free radicals and the decreased level of antioxidant cause oxidative stress in cells, leading to destruction of lipids, proteins and DNA. Several reports demonstrated that peptides obtained from marine protein hydrolysate possessed diverse bioactivities such as anti-inflammatory, antioxidant, immunomodulatory, anti-aging and wound-healing activities (Felician et al. 2019; Sae-leaw et al. 2016a; Venkatesan et al. 2017). Nowadays, fish-derived peptides have gained increasing interest as a potential ingredient for foods or cosmetic due to their bioactivities, especially antioxidant and tissue regeneration activities for skin nourishment (Huang et al. 2018).
Skin is a natural barrier against harmful pollutants (Pozzolini et al. 2018). Skin integrity can be damaged by several factors e.g. ultraviolet (UV) light, chemical and mechanical reactions (Pozzolini et al. 2018). Additionally, wound occurred can be infected by numerous microbes, causing infection. Wound healing could be characterized by re-epithelialization, angiogenesis, collagen deposition and granulation tissue formation (Chen et al. 2019). Recently, fish collagen has been reported to be a promoter for cell proliferation and an enhancer of various growth factors in accelerating wound healing (Huang et al. 2018).
Asian sea bass skin hydrolyzed collagen (Asbs-HC) is one of ideal natural ingredients, which was documented to have an antioxidant activity and enhance the collagen production and proliferation of fibroblast cell (Benjakul et al. 2018; Chotphruethipong et al. 2019a). Recently, Chotphruethipong et al. (2019b) produced Asbs-HC from skin defatted by porcine lipase with the aids of pulsed electric field (PEF) and vacuum impregnation, in which negligible fishy odor was detected in resulting Asbs-HC. However, no information on the antioxidative activity and wound healing property of the aforementioned Asbs-HC exists. Thus, the study aimed at examining the wound healing property and antioxidative activity of Asbs-HC. Peptide rendering antioxidant activity was also isolated and characterized.
Materials and methods
Porcine pancreas lipase (PPL) type II, phalloidin tetramethylrhodamine B isothiocyanate and other chemicals were obtained from Sigma‐Aldrich Chemical Co. (St. Louis, MO, USA). Dulbecco’s modified Eagle’s medium (DMEM), fetal bovine serum (FBS) and antibiotics were procured from Gibco BRL Life Technologies (Grand Island, NY, USA). The MRC-5 fibroblast cell line (human fetal lung) was obtained from the Cell Line Service, Heidelberg, Germany.
Enzyme assays
PPL and papain activities were measured as tailored by Chotphruethipong et al. (2019c). One unit (U) of liase activity was defined as the amount of PPL producing 1 μmol p-nitrophenol per min under the assay conditions. One unit of protease activity was defined as the amount of papain that released 0.01 µmol of tyrosine equivalent per min (µmol Tyr/min).
Asian sea bass skin preparation
Frozen Asian sea bass (Lates calcarifer) skins were procured by King-fisher Holdings Co., Ltd., Songkhla, Thailand. The skins (2 × 2 cm2) were treated with 0.1 M NaOH and subsequent washing until neutral pH was obtained (Chotphruethipong et al. 2019a).
Pretreatment of skin using pulsed electric field (PEF)-assisted process
PEF-assisted process was adopted for skin pretreatment as detailed by Chotphruethipong et al. (2019c). Alkali-treated skins (50 g) wrapped with cheese cloth were placed in a chamber (3.5 × 10 × 12 cm3). The distance of electrodes gap was 3.3 cm. Subsequently, 300 mL of water were added. PEF (electric field strength: 24 kV/cm; number of pulses: 72 ms; specific energy input: 135 kJ/kg) was employed. Pulse width and pulse repetition times were 0.1 and 20 ms, respectively.
After PEF treatment, the skins were further soaked in 10 volumes of 0.05 M citric acid for 2 h and washed using a running water as detailed by Chotphruethipong et al. (2019c).
Defatting of PEF-treated skin using PPL in combination with vacuum impregnation (VI)
Acid-treated skins (200 g) were transferred into a 5-L vacuum chamber connected with vacuum pump (VE 125 N, Zhejiang Value Mechanical and Electrical Products Co., Ltd., Zhejiang, China). The pressure during treatment was monitored using a vacuum meter (PG2, NUOVA FIMA, Novara, Italy). One liter of PPL solution (42.36 unit/g skin dry matter) was added to chamber. VI process was conducted at 4 cycles with the pressure of 100 kPa. One cycle of VI process was done for 20 min and subsequent restoration to atmosphere pressure for 10 min. Thereafter, the mixtures were further incubated at 30 ºC for 15 min and skins were washed thoroughly followed by washing the skin (Chotphruethipong et al. 2019b). The defatted skins were used for preparing hydrolyzed collagen.
Preparation of hydrolyzed collagen from defatted skin
Production of hydrolyzed collagen from defatted skin was performed using papain hydrolysis at 0.3 U/g dry matter as detailed by Chotphruethipong et al. (2019b). The reaction was conducted at 40 ºC for 90 min. Thereafter, heating (90 ºC, 15 min) was applied to inactivate papain and terminate proteolysis. The obtained hydrolysate was filtered and lyophilized as per the protocol of Chotphruethipong et al. (2019a). Asian sea bass skin hydrolyzed collagen (Asbs-HC) powder was further used for analyses.
Amino acid composition
Amino acid composition of Asbs-HC was analyzed as described by Benjakul et al. (2018).
Influence of Asbs-HC at different concentrations on MRC-5 fibroblast cells proliferation and wound healing
Cell culture
The MRC-5 fibroblast cells were cultured in complete DMEM medium containing 100 µg/mL streptomycin, 10% FBS, 100 U/mL penicillin and 2 mM L-glutamine as detailed by Singkhorn et al. (2018).
Cell proliferation assay
Asbs-HC solutions at different concentrations (0, 25, 50 100, 500 and 1000 µg/mL) were evaluated for the proliferation of cell as described by Ritto et al. (2017) and expressed as % relative to the control (without Asbs-HC). The concentrations without cytotoxicity were chosen for further studies.
Cell migration (wound healing) assay
MRC-5 cells (5 × 105 cells/well) were seeded in 6-well plates as per the method of Singkhorn et al. (2018). When cells became confluent, they were scratched using sterile P200 micropipette and subsequently washed with PBS (3 mL/well). After washing, the cells were added with the completed medium containing 1%FBS and Asbs-HC at the selected concentrations for 0 and 24 h. The wound spaces were assessed using a microscope (Olympus IX70 with DP50, Shinjuku-ku, Tokyo, Japan) as detailed by Singkhorn et al. (2018). Wound gap was calculated using an ImageJ 1.41 software (NIH, Bethesda, MD, USA), which was reported as % wound area using the equation as follows:
Lamellipodia formation
Lamellipodia formation was determined using the protocol of Singkhorn et al. (2018). MRC-5 cells (5 × 104 cells/well) were mixed with completed medium containing 1%FBS and Asbs-HC at the selected concentrations for 24 h and stained with 0.01 mg/mL of phalloidin-rhodamine (0.5 mL/well) and 0.01 mg/mL of Hoechst 33342 (0.5 mL/well) dyes for 30 min in the dark. After staining, cells were washed with PBS (1 mL/well) and cell morphology was visualized using a microscope. Lamellipodia formation was expressed as relative fluorescence intensity.
Impact of Asbs-HC at different concentrations on antioxidative activity
Asbs-HC solutions (0.1, 0.2, 0.5, 1 and 10 mg/mL) were prepared and determined for antioxidant activities as follows:
DPPH radical scavenging activity (DPPH-RSA)
DPPH-RSA was determined using the method of Chotphruethipong et al. (2017). Firstly, Asbs-HC solutions (100 μL) were mixed with 100 μL of 0.1 mM mM DPPH in 50% ethanol. The mixtures were incubated at room temperature for 30 min in the dark. The absorbance of the obtained solution was measured at 517 nm using a microplate reader (FLUOstar Omega, BMG Labtech Multi-mode microplate reader, Germany). The activity was reported as µmol Trolox equivalents (TE)/g dry sample.
ABTS radical scavenging activity (ABTS-RSA)
ABTS-RSA was measured as tailored by Chotphruethipong et al. (2017). The working solution was prepared by mixing 14.8 mM ABTS solution and 5.2 mM potassium persulphate solution at the ratio of 1:1 (v/v) and allowed them to react for 12 h at room temperature in the dark. The ABTS solution was subsequently diluted by mixing 1 mL of ABTS solution with 50 mL of distilled water to obtain an absorbance of 1.1 ± 0.02 units at 734 nm. The activity was expressed as µmol Trolox equivalents (TE)/g dry sample.
Oxygen radical absorbance capacity (ORAC)
The ORAC was determined as described by Chotphruethipong et al. (2017). The Asbs-HC at various concentrations were dissolved with 75 µM phosphate buffer (pH 7.0). Thereafter, the Asbs-HC solutions (20 μL) were loaded onto a black polystyrene, non-steriled 96-well microplate (Nunc®, Denmark), and then added with 50 μL of fluorescein (0.11 μM). The loaded microplate was equilibrated at 37 °C for 20 min in a microplate reader. Hundred microliters of AAPH (60 mM) were added to initiate reaction at 37 °C. The fluorescence intensity was measured every 5 min for 90 min with excitation and emission filters of 485 and 535 nm, respectively. The area under the fluorescence decay curve (AUC) of the samples was calculated by the normalized curves with the following equation:
where f1 and fn are the fluorescence reading at the initiation and the last measurements of the reaction, respectively. The net AUC was calculated by subtracting the AUC of the blank from that of a sample or standard. Trolox (0–200 μM) was used as standard. The ORAC was reported as µmol Trolox equivalents (TE)/g dry sample.
Metal chelating activity (MCA)
MCA was determined according to the method of Chotphruethipong et al. (2017). Asbs-HC solution (4.7 mL) was mixed with 0.1 mL of 2 mM FeCl2 and 0.2 mL of 5 mM ferrozine. The reaction mixture was allowed to stand for 20 min at room temperature. The absorbance was then read at 562 nm using a spectrophotometer (UV-1800, Shimadzu, Kyoto, Japan). The activity was expressed as µmol EDTA equivalents (EE)/g dry sample.
Molecular weight (MW) distribution
MW distribution of Asbs-HC was separated using a 2.5 × 50 cm Sephadex G-25 gel filtration column (GE Healthcare Bio-Science AB, Uppsala, Sweden) (Chotphruethipong et al. 2019a). The absorbance was recorded at 220 and 280 nm. Blue dextran (2,000,000 Da), insulin chain B (3495.89 Da), vitamin B12 (1355.4 Da), glycine-tyrosine (238.25 Da) and tyrosine (181.2 Da) were used as MW standards. MW of each fraction was estimated from the plot between available partition coefficient (Kav) and the logarithm of MW of the standards.
Statistical analysis
Experiment values were reported as mean ± standard deviation (SD). Completely randomized design (CRD) was used throughout the study. Analysis of variance (ANOVA) was carried out and mean comparisons were done using the Duncan’s multiple range test (Steel and Torrie 1980).
Results and discussion
Amino acid composition of Asbs-HC
Gly was the predominant amino acid (AA) in Asbs-HC (319 residues/1000 residues), followed by Ala (129 residues/1000 residues) and Pro (112residues/1000 residues) (Table 1). Additionally, Gln/Glu (75 residues/1000 residues) and Hyp (73 residues/1000 residues) were present. Similar AAs were found in HC prepared from Asian sea bass skin defatted using lipase without prior PEF/VI (Chotphruethipong et al. 2019a). Also, Asbs-HC had high imino acids (Hyp and Pro) content (185 residues/1000 residues). Treatment of PEF-treated skin using lipase with the aid of vacuum impregnation might increase the migration of lipase into skin, leading to augmented liberation of lipoprotein. With hydrolysis using papain, those released proteins were possibly hydrolyzed randomly. As a result, AA compositions of resulting Asbs-HC were different. Generally, Asbs-HC had high hydrophobic AAs (>65%) (Chotphruethipong et al. 2019a; Benjakul et al. 2018), which were documented to show antioxidative and biological properties, especially for skin nourishment (Benjakul et al. 2018; Chi et al. 2015).
Table 1.
Amino acid composition of hydrolyzed collagen (HC) from defatted seabass skin
| Amino acids | Content (residues/1000 residues) |
|---|---|
| Alanine (Ala) | 129 |
| Arginine (Arg) | 53 |
| Asparagine/asparatic acid (Asn/Asp) | 51 |
| Cysteine (Cys) | 1 |
| Glutamine (Gln)/glutamic acid (Gln/Glu) | 75 |
| Glycine (Gly) | 319 |
| Histidine (His) | 6 |
| Isoleucine (Ile) | 12 |
| Leucine (Leu) | 23 |
| Lysine (Lys) | 29 |
| Hydroxylysine (Hylys) | 6 |
| Methionine (Met) | 13 |
| Phenylalanine (Phe) | 14 |
| Hydroxyproline (Hyp) | 73 |
| Proline (Pro) | 112 |
| Serine (Ser) | 30 |
| Threonine (Thr) | 26 |
| Tyrosine (Tyr) | 5 |
| Valine (Val) | 23 |
| Tryptophan (Trp) | 1 |
| Total | 1000 |
| Imino acid (Hyp + Pro) | 185 |
Proliferation of MRC-5 fibroblast cell
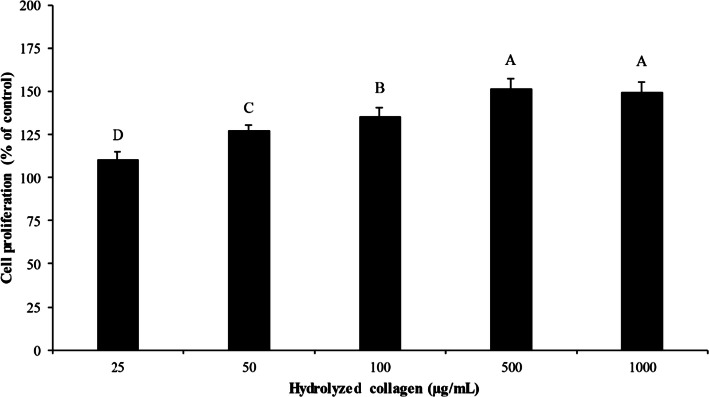
Impact of Asbs-HC at various concentrations on proliferation of fibroblast cells is depicted in Fig. 1. All concentrations had no cytotoxicity on MRC-5 cells. Cell proliferation increased as Asbs-HC concentration increased up to 500 µg/mL. Nevertheless, no difference in cell growth was obtained between 500 and 1000 µg/mL (p > 0.05). The result revealed that Asbs-HC could stimulate fibroblast proliferation in a concentration dependent manner. Effectiveness in enhancement of cell proliferation generally depends on size, composition and sequence of AAs (Sánchez and Vázquez 2017). Hydrophobic AAs were documented to promote fibroblast proliferation (Benjakul et al. 2018). Asbs-HC consisted of Gly, Ala and Pro as the predominant AAs (Table 1). Moreover, Hyp and Ser were also found (Table 1). Those aforementioned AAs could stimulate the cell proliferation of fibroblast cell (Benjakul et al. 2018; Sibilla et al. 2015). Since Asbs-HC at all concentrations tested had no cytotoxicity, they were selected for further study.
Fig. 1.
Effect of hydrolyzed collagen from defatted Asian sea bass skin at various concentrations on cell proliferation of MRC-5 fibroblast cells. Incubation time of 24 h was used. Bars represent standard deviation (n = 4). Different letters on bars indicate significant differences (p < 0.05)
Wound healing and lamellipodia formation of MRC-5 fibroblast cell
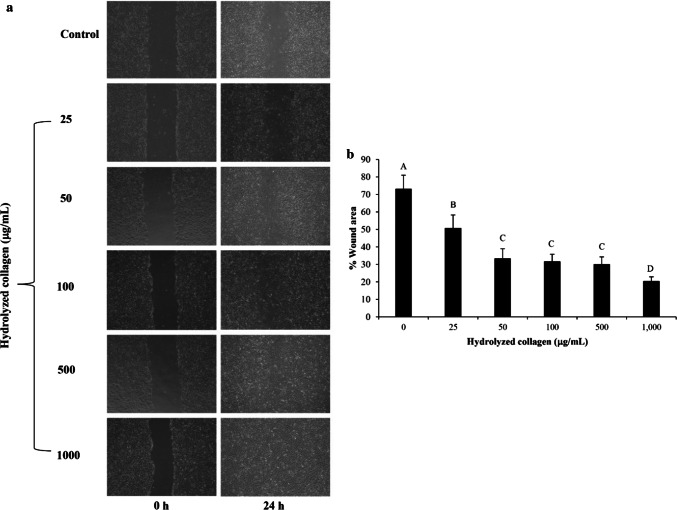
Influence of HC at various concentrations on wound healing by fibroblast cells is shown in Fig. 2. Wound healing of fibroblast was determined using monolayer scratch assay. The increase in cell migration was attained with increasing Asbs-HC concentrations after 24 h (p < 0.05). However, there was no difference (p > 0.05) in wound gap in cells treated with Asbs-HC at the levels of 50–500 µg/mL after 24 h of treatment. Those concentrations provided the effectiveness in wound healing by 65–70% as compared with the control (without Asbs-HC). The smallest wound gap was found after treatment with Asbs-HC at 1000 µg/mL for 24 h, in which the wound gap was closed by 80%. This result indicated that Asbs-HC could accelerate wound healing, especially at the high concentration. Closer gap of wound induced by Asbs-HC might be due to high content of Hyp in Asbs-HC, which was an essential AA for tissue granulation (Nayak et al. 2007). In general, collagen is a main constituent of extracellular matrix (ECM), produced by fibroblast cells (Guo and DiPietro 2010). Collagen plays a major role in wound healing by facilitating the mobility of endothelial cells to generate new blood vessels. Subsequently, the formation of granulation tissue was enhanced, leading to a reduction in wound area (Guo and DiPietro 2010). Asbs-HC was able to promote the collagen production of fibroblast cell (Benjakul et al. 2018; Chotphruethipong et al. 2019a). This might be attributed to the increased fibroblast migration as evidenced by the decreased wound gap.
Fig. 2.
Effect of hydrolyzed collagen from defatted Asian sea bass skin on the migration of MRC-5 fibroblast cells (a). The cell migration was visualized via phase contrast microscopy (scale bar = 200 μm). The percentage of the wound area was measured by comparing the change in wound area to that of the control (b). Bars represent standard deviation (n = 4). Different letters on bars indicate significant differences (p < 0.05)
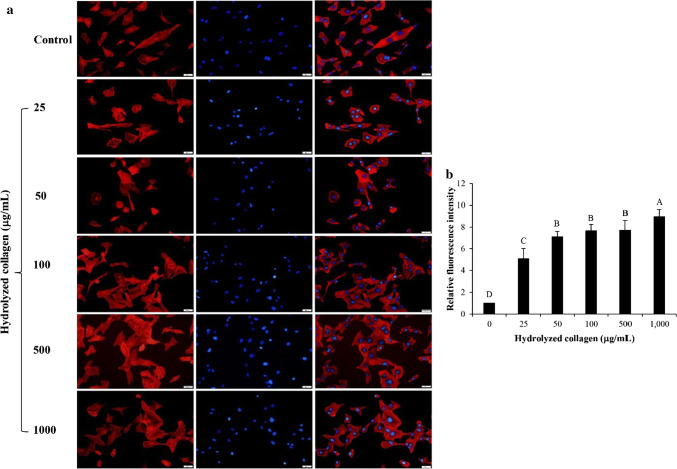
Lamellipodia formation of fibroblast cell after treatment with Asbs-HC at various concentrations for 24 h is shown in Fig. 3a. All Asbs-HC levels used could increase lamellipodia formation of fibroblast cells as shown by the increased fluorescence intensity, compared to the control (p < 0.05) (Fig. 3b). Cell migration generally relates with the protruding cell formation such as lamellipodia (Singkhorn et al. 2018). Lamellipodia is found as an actin cytoskeleton for every mobile cells, especially keratinocytes (Kopecki and Cowin 2016). The highest intensity was found in cells treated with Asbs-HC at 1000 µg/mL. Nevertheless, no difference in intensity was detected in cells treated with Asbs-HC at 25–500 µg/mL. The result was related with wound healing (Fig. 2b) as evidenced by the increased formation of lamellipodia, particularly at the higher Asbs-HC level. Thus, Asbs-HC could be applied as a supplement for skin nourishment and skin repair.
Fig. 3.
Effect of hydrolyzed collagen from defatted Asian sea bass skin on lamellipodia formation of MRC-5 fibroblast cells (a) and relative fluorescence intensity of lamellipodia after treatment with HC at various concentrations (b). Bars represent standard deviation (n = 4). Different letters on bars indicate significant differences (p < 0.05)
Antioxidative activities of Asbs-HC at various concentrations
DPPH-RSA
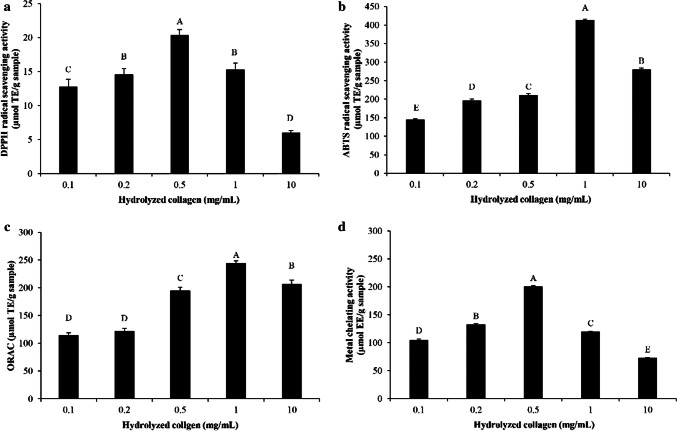
The ability in scavenging DPPH· radicals of Asbs-HC is presented in Fig. 4a. The activity was augmented as Asbs-HC levels increased up to 0.5 mg/mL (p < 0.05). DPPH-RSA depends on AA composition, in which hydrophobic AA and aromatic AA in peptide chain showed high activity (Zou et al. 2016). Asbs-HC had high amounts of hydrophobic AAs (65.1%) (Table 1). This might be related to the enhanced activity of Asbs-HC. Moreover, some AAs including His, Gly, Phe, Trp, Pro, Ala, Val and Leu can quench free radicals (Yarnpakdee et al. 2015; Zou et al. 2016). The presence of those AAs in peptide chain of Asbs-HC mostly contributed to high DPPH-RSA. Nonetheless, the activity decreased when Asbs-HC at levels of 1 and 10 mg/mL were used (p < 0.05). Lower activity was presumably owing to interaction between peptide chains at high concentration via hydrophobic-hydrophobic interaction or other bondings. Consequently, the ability in providing proton or electron was lowered. Thus, the level of Asbs-HC was an important factor determining DPPH-RSA in in vitro study.
Fig. 4.
DPPH radical scavenging activity (a), ABTS radical scavenging activity (b), ORAC (c) and metal chelating activity (d) of hydrolyzed collagen from defatted Asian sea bass skin at various concentrations. Bars represent standard deviation (n = 3). Different letters on bars indicate significant differences (p < 0.05)
ABTS-RSA
The activity in quenching ABTS radical of Asbs-HC at various concentrations is illustrated in Fig. 4b. Asbs-HC was more effective in scavenging ABTS radicals when the concentrations ranging from 0.1 to 1 mg/mL were used (p < 0.05). The result revealed that Asbs-HC could be used as hydrogen donator to ABTS·+ radical. Higher ABTS-RSA of Asbs-HC might be due to the presence of some AAs in peptide chain, especially Tyr and Phe, which was reported by Rajapakse et al. (2005). These AAs might enhance ABTS-RSA of Asbs-HC. Apart from aforementioned AAs, peptides containing Glu, Asn Gln and Asp could also increase the antioxidant activity because of excessive electrons donated to free radicals (Udenigwe and Aluko 2011). Asbs-HC had those AAs by 12.6% (Table 1). This probably contributed to the increased ability in terminating radical chain reaction. Nevertheless, lower ABTS-RSA of Asbs-HC at higher concentration (10 mg/mL) was observed, thus lowering efficiency in prevention of lipid oxidation. The excessive hydrophobic AAs of peptides possibly caused the lower ABTS-RSA (Zou et al. 2016). Thus, the ability in scavenging free radicals was not only dependent on the concentration used but also the composition of AAs in peptides.
ORAC
ORAC of Asbs-HC at different concentrations is shown in Fig. 4c. The result coincided with ABTS-RSA (Fig. 4b). The activity in inhibition of peroxyl radical increased as the level of Asbs-HC increased up to 1 mg/mL (p < 0.05). ORAC of Asbs-HC varied from 114.40 to 243.61 µmol TE/g sample. ORAC value of Asbs-HC in this study was lower than that of Asbs-HC produced by alcalase (550.25 to 623.88 μmol TE/g sample) (Sae-leaw et al. 2016). However, higher activity was obtained, compared to that of HC prepared from Pterygoplichthys disjunctivus skin using neutrase (51.43 μmol TE/g sample) (Guo et al. 2019). Different activity among various HC possibly resulted from different processes and types of enzymes as well as raw materials used. Peroxyl radical is carrier of the chain reactions and can further oxidize PUFA molecule. It initiates new chain reactions, producing lipid hydroperoxides (Nimse and Pal 2015). HC was able to quench both peroxyl and hydroxyl radicals since it contained Gly, Pro and Ala as the predominant AAs (Chotphruethipong et al. 2019a). Antioxidant peptides with these AAs were documented to have ability in inhibiting both radicals (Ambigaipalan and Shahidi 2017; Karami and Akbari-adergani 2019). The result suggested that Asbs-HC rich in hydrophobic AAs could prevent lipid oxidation induced by peroxyl radical. Additionally, the decrease in wound gap was more likely associated with antioxidative activities of Asbs-HC (Fig. 2), in which reactive oxygen species (ROS) inducing skin cell damage was scavenged by antioxidative peptides in Asbs-HC. This led to the decreased wound gap.
MCA
Asbs-HC possessed the ability in inhibiting Ferrozine–Fe2+ complex formation when the concentration was below 1 mg/mL (Fig. 4d). Decreasing MCA was observed at higher concentration (1 and 10 mg/mL). Peptides presented in Asbs-HC plausibly had different MCA, depending on sequences of AAs and chain length. Some AAs such as Gly, Asp Lys, His and Arg were involved in MCA (Saiga et al. 2003). In addition, higher amounts of polar AAs (Glu, Asp, and Ser) might contribute to higher MCA. These polar AAs contained side chains having carboxylic group (Glu and Asp) and hydroxyl group (Ser), which can chelate transition metal (Liu et al. 2010; Xiong 2010). Those AAs appeared in Asbs-HC (Table 1), which plausibly determined MCA.
Molecular weight (MW) distribution of antioxidative peptides
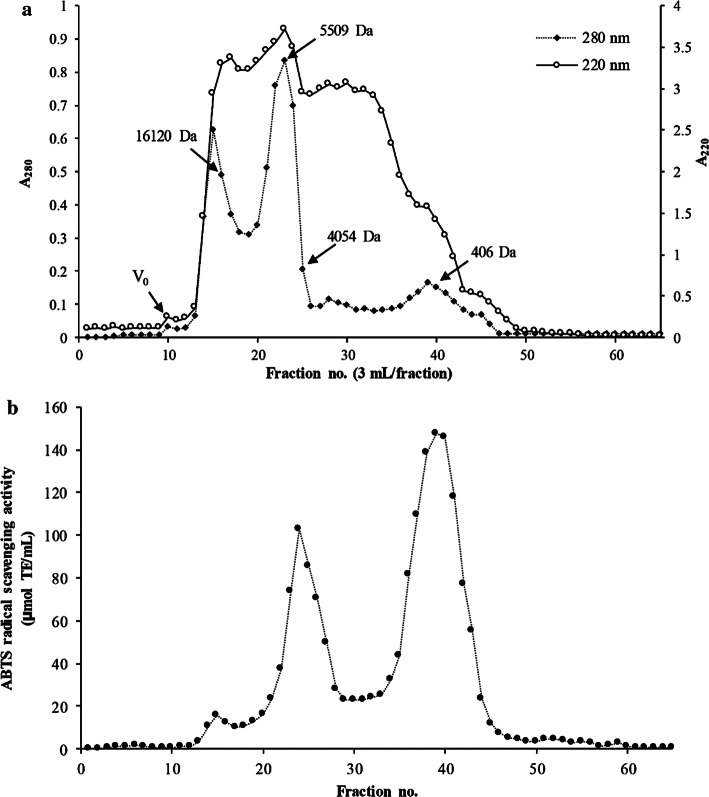
MW distribution of Asbs-HC is depicted in Fig. 5a. Asbs-HC had three major peaks of A280, representing the peptides having aromatic AAs with different MW. A220 indicates the peptides in the fractions pooled. MW of peptides in Asbs-HC ranged from 406 to 16,120 Da. When considering ABTS-RSA, the highest activity was observed in peptide with MW of 406 Da (Fig. 5b). Additionally, the peptide having MW of 4054 Da was also able to quench ABTS·+ radical at high extent. The result was in line with MW distribution in previous study, in which Asian sea bass skin peptides with MW of 500–4500 Da exhibited strong antioxidant activity (Sae-leaw et al. 2017). Different antioxidant activity of Asbs-HC was associated with MW, chain length, AA composition and sequence (Yarnpakdee et al. 2015). Kittiphattanabawon et al. (2012) noted that peptides from blacktip shark skin with MW of 644 Da had strong antioxidant activity. Antioxidant peptide from blue shark gelatin had MW < 2 kDa (Weng et al. 2014). Additionally, the peptides with MW <600 Da from cod protein hydrolysate showed high radical scavenging activity (Farvin et al. 2016). Apart from antioxidant activity, short peptides also had ability in stimulation of fibroblast proliferation (Chalisova et al. 2015). Some antioxidative peptides containing Pro-Hyp and Pro-Hyp-Gly could increase wound healing by accelerating the fibroblast mobility to the injured tissue (Iwai et al. 2005). Asbs-HC contained Gly, Hyp and Pro at high contents (Table 1). These AAs might induce the growth of fibroblast cells. Consequently, the migration of fibroblasts increased as indicated by the lower wound gap when Asbs-HC at 1000 µg/mL was implemented (Fig. 2b). Thus, Asbs-HC could be effective for skin repair.
Fig. 5.
Elution profile by Sephadex G-25 size exclusion chromatography of hydrolyzed collagen powder from defatted Asian sea bass skin (a) and ABTS radical scavenging activity (b) of different fractions
Conclusion
HC from defatted Asian sea bass skin (Asbs-HC) induced cell growth and migration of fibroblast cell, thus facilitating wound healing process. Moreover, it could scavenge both hydroxyl and peroxyl radicals as well as acted as metal chelator. Nevertheless, the appropriate concentration should be used to obtain the maximal activity. Therefore, Asbs-HC was considered as a promising natural functional ingredient or nutraceutical, especially for skin nourishment or wound healing.
Acknowledgements
This research was supported by the Thailand Research Fund under the Royal Golden Jubilee Ph.D. Program to Lalita Chotphruethipong (PHD/0183/2560). Prince of Songkla University (Grant No. AGR6302013N) and Foreign collaborative program (Grant No. AGR6302073S) were also acknowledged.
Compliance with ethical standards
Conflict of interest
The authors have declared no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Ambigaipalan P, Shahidi F. Bioactive peptides from shrimp shell processing discards: antioxidant and biological activities. J Funct Foods. 2017;34:7–17. doi: 10.1016/j.jff.2017.04.013. [DOI] [Google Scholar]
- Benjakul S, Karnjanapratum S, Visessanguan W. Hydrolysed collagen from Lates calcarifer skin: its acute toxicity and impact on cell proliferation and collagen production of fibroblasts. Int J Food Sci Technol. 2018;53:1871–1879. doi: 10.1111/ijfs.13772. [DOI] [Google Scholar]
- Chalisova N, Linkova N, Zhekalov A, Orlova A, Ryzhak G, Khavinson VK. Short peptides stimulate cell regeneration in skin during aging. Adv Gerontol. 2015;5(3):176–179. doi: 10.1134/S2079057015030054. [DOI] [PubMed] [Google Scholar]
- Chen J, Gao K, Liu S, Wang S, Elango J, Bao B, Dong J, Liu N, Wu W. Fish collagen surgical compress repairing characteristics on wound healing process in vivo. Mar Drugs. 2019;17(1):33. doi: 10.3390/md17010033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi C-F, Hu F-Y, Wang B, Li Z-R, Luo H-Y. Influence of amino acid compositions and peptide profiles on antioxidant capacities of two protein hydrolysates from skipjack tuna (Katsuwonus pelamis) dark muscle. Mar Drugs. 2015;13(5):2580–2601. doi: 10.3390/md13052580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chotphruethipong L, Aluko RE, Benjakul S. Hydrolyzed collagen from porcine lipase-defatted seabass skin: Antioxidant, fibroblast cell proliferation, and collagen production activities. J Food Biochem. 2019;43(5):1–13. doi: 10.1111/jfbc.12825. [DOI] [PubMed] [Google Scholar]
- Chotphruethipong L, Aluko RE, Benjakul S. Enhanced Asian sea bass skin defatting using porcine lipase with the aid of pulsed electric field pretreatment and vacuum impregnation. Process Biochem. 2019 doi: 10.1016/j.procbio.2019.08.012. [DOI] [Google Scholar]
- Chotphruethipong L, Aluko RE, Benjakul S. Effect of pulsed electric field-assisted process in combination with porcine lipase on defatting of seabass skin. J Food Sci. 2019;84(7):1799–1805. doi: 10.1111/1750-3841. [DOI] [PubMed] [Google Scholar]
- Chotphruethipong L, Benjakul S. Use of cashew (Anacardium occidentale L.) leaf extract for prevention of lipid oxidation in mayonnaise enriched with fish oil. Turk J Fish Aquat Sci. 2019;19(10):825–836. doi: 10.4194/1303-2712-v19_10_02. [DOI] [Google Scholar]
- Chotphruethipong L, Benjakul S, Kijroongrojana K. Optimization of extraction of antioxidative phenolic compounds from cashew (Anacardium occidentale L.) leaves using response surface methodology. J Food Biochem. 2017;41:1–10. doi: 10.1111/jfbc.12379. [DOI] [Google Scholar]
- Farvin KS, Andersen LL, Otte J, Nielsen HH, Jessen F, Jacobsen C. Antioxidant activity of cod (Gadus morhua) protein hydrolysates: fractionation and characterisation of peptide fractions. Food Chem. 2016;204:409–419. doi: 10.1016/j.foodchem.2016.02.145. [DOI] [PubMed] [Google Scholar]
- Felician FF, Yu R-H, Li M-Z, Li C-J, Chen H-Q, Jiang Y, Tang T, Qi W-Y, Xu H-M. The wound healing potential of collagen peptides derived from the jellyfish Rhopilema esculentum. Chin J Traumatol. 2019 doi: 10.1016/j.cjtee.2018.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Sa, DiPietro LA (2010) Factors affecting wound healing. J Dent Res 89(3):219–229. https://doi.org/10.1177_0022034509359125 [DOI] [PMC free article] [PubMed]
- Guo Y, Michael N, Fonseca Madrigal J, Sosa Aguirre C, Jauregi P. Protein hydrolysate from Pterygoplichthys disjunctivus, armoured catfish, with high antioxidant activity. Molecules. 2019;24(8):1628. doi: 10.3390/molecules24081628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C-Y, Wu T-C, Hong Y-H, Hsieh S-L, Guo H-R, Huang R-H. Enhancement of cell adhesion, cell growth, wound healing, and oxidative protection by gelatins extracted from extrusion-pretreated Tilapia (Oreochromis sp.) fish scale. Molecules. 2018;23(10):2406. doi: 10.3390/molecules23102406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwai K, Hasegawa T, Taguchi Y, Morimatsu F, Sato K, Nakamura Y, Higashi A, Kido Y, Nakabo Y, Ohtsuki K. Identification of food-derived collagen peptides in human blood after oral ingestion of gelatin hydrolysates. J Agric Food Chem. 2005;53(16):6531–6536. doi: 10.1021/jf050206p. [DOI] [PubMed] [Google Scholar]
- Karami Z, Akbari-adergani B. Bioactive food derived peptides: a review on correlation between structure of bioactive peptides and their functional properties. J Food Sci Technol. 2019;56(2):535–547. doi: 10.1007/s13197-018-3549-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kittiphattanabawon P, Benjakul S, Visessanguan W, Shahidi F. Gelatin hydrolysate from blacktip shark skin prepared using papaya latex enzyme: antioxidant activity and its potential in model systems. Food Chem. 2012;135(3):1118–1126. doi: 10.1016/j.foodchem.2012.05.080. [DOI] [PubMed] [Google Scholar]
- Kopecki Z, Cowin AJ (2016) The role of actin remodelling proteins in wound healing and tissue regeneration. In: Alexandrescu VA (ed) Wound healing—new insights into ancient challenges. IntechOpen, London
- Nayak B, Anderson M, Pereira LP. Evaluation of wound-healing potential of Catharanthus roseus leaf extract in rats. Fitoterapia. 2007;78(7–8):540–544. doi: 10.1016/j.fitote.2007.06.008. [DOI] [PubMed] [Google Scholar]
- Nimse SB, Pal D. Free radicals, natural antioxidants, and their reaction mechanisms. RSC Adv. 2015;5(35):27986–28006. doi: 10.1039/C4RA13315C. [DOI] [Google Scholar]
- Pozzolini M, Millo E, Oliveri C, Mirata S, Salis A, Damonte G, Arkel M, Scarfì S. Elicited ROS scavenging activity, photoprotective, and wound-healing properties of collagen-derived peptides from the marine sponge Chondrosia reniformis. Mar Drugs. 2018;16(12):465. doi: 10.3390/md16120465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajapakse N, Mendis E, Jung W-K, Je J-Y, Kim S-K. Purification of a radical scavenging peptide from fermented mussel sauce and its antioxidant properties. Food Res Int. 2005;38(2):175–182. doi: 10.1016/j.foodres.2004.10.002. [DOI] [Google Scholar]
- Ritto D, Tanasawet S, Singkhorn S, Klaypradit W, Hutamekalin P, Tipmanee V, Sukketsiri W. Astaxanthin induces migration in human skin keratinocytes via Rac1 activation and RhoA inhibition. Nutr Res Pract. 2017;11(4):275–280. doi: 10.4162/nrp.2017.11.4.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sae-leaw T, Karnjanapratum S, O'Callaghan YC, O'Keeffe MB, FitzGerald RJ, O'Brien NM, Benjakul S. Purification and identification of antioxidant peptides from gelatin hydrolysate of seabass skin. J Food Biochem. 2017;41(3):1–11. doi: 10.1111/jfbc.12350. [DOI] [Google Scholar]
- Sae-leaw T, O’callaghan YC, Benjakul S, O’brien NM. Antioxidant, immunomodulatory and antiproliferative effects of gelatin hydrolysates from seabass (Lates calcarifer) skins. Int J Food Sci Technol. 2016;51(7):1545–1551. doi: 10.1111/ijfs.13123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sae-leaw T, O’Callaghan YC, Benjakul S, O’Brien NM. Antioxidant activities and selected characteristics of gelatin hydrolysates from seabass (Lates calcarifer) skin as affected by production processes. J Food Sci Technol. 2016;53(1):197–208. doi: 10.1007/s13197-015-1989-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saiga A, Tanabe S, Nishimura T. Antioxidant activity of peptides obtained from porcine myofibrillar proteins by protease treatment. J Agric Food Chem. 2003;51(12):3661–3667. doi: 10.1021/jf021156g. [DOI] [PubMed] [Google Scholar]
- Sánchez A, Vázquez A. Bioactive peptides: a review. Food Qual Saf. 2017;1(1):29–46. doi: 10.1093/fqsafe/fyx006. [DOI] [Google Scholar]
- Sibilla S, Godfrey M, Brewer S, Budh-Raja A, Genovese L. An overview of the beneficial effects of hydrolysed collagen as a nutraceutical on skin properties: Scientific background and clinical studies. Open Nutraceuticals J. 2015 doi: 10.2174/1876396001508010029. [DOI] [Google Scholar]
- Singkhorn S, Tantisira MH, Tanasawet S, Hutamekalin P, Wongtawatchai T, Sukketsiri W. Induction of keratinocyte migration by ECa 233 is mediated through FAK/Akt, ERK, and p38 MAPK signaling. Phytother Res. 2018;32(7):1397–1403. doi: 10.1002/ptr.6075. [DOI] [PubMed] [Google Scholar]
- Udenigwe CC, Aluko RE. Chemometric analysis of the amino acid requirements of antioxidant food protein hydrolysates. Int J Mol Sci. 2011;12(5):3148–3161. doi: 10.3390/ijms12053148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatesan J, Anil S, Kim S-K, Shim M. Marine fish proteins and peptides for cosmeceuticals: a review. Mar Drugs. 2017;15(5):143. doi: 10.3390/md15050143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weng W, Tang L, Wang B, Chen J, Su W, Osako K, Tanaka M. Antioxidant properties of fractions isolated from blue shark (Prionace glauca) skin gelatin hydrolysates. J Funct Foods. 2014;11:342–351. doi: 10.1016/j.jff.2014.10.021. [DOI] [Google Scholar]
- Yarnpakdee S, Benjakul S, Kristinsson HG, Kishimura H. Antioxidant and sensory properties of protein hydrolysate derived from Nile tilapia (Oreochromis niloticus) by one-and two-step hydrolysis. J Food Sci Technol. 2015;52(6):3336–3349. doi: 10.1007/s13197-014-1394-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou T-B, He T-P, Li H-B, Tang H-W, Xia E-Q. The structure-activity relationship of the antioxidant peptides from natural proteins. Molecules. 2016;21(1):72. doi: 10.3390/molecules21010072. [DOI] [PMC free article] [PubMed] [Google Scholar]