Abstract
The purpose of the study was to investigate the influence of a heteropolysacchride (HePS)-forming lactic acid bacteria (LAB) on the quality attributes of raw fermented sausages.
Therefore, salamis with the HePS-producing strain Lactobacillus plantarum TMW 1.1478 or the non-EPS-producing strain Lactobacillus sakei TMW 1.2037 (control) were manufactured using two different inoculation concentrations: more precisely, 107 CFU/g (typical starter culture concentration) or 109 CFU/g. Growth behavior, aw and pH development were recorded until a weight loss of 31% was reached and in-situ-formed EPS detected using confocal laser scanning microscopy. Moreover, the influence of the HePS formed on texture (texture profile analysis; TPA) and sensory attributes (26 panelists, ranking test) was investigated.
The final products containing L. plantarum TMW 1.1478 were found to be significantly softer (p < 0.05) than the respective control samples, an effect that was even more pronounced at the higher inoculation level of 109 CFU/g. The semi-quantitative data interpretation of the CLSM pictures revealed that the EPS were predominantly formed during the first 72 h of fermentation at 24 °C until the final pH of 4.95 ± 0.05 was reached (stationary phase). The sensory evaluation (consistency) was in accordance with the TPA results and taste was not negatively influenced by the HePS-forming strain. Results clearly indicate that EPS-producing LAB can have a negative influence on the quality of raw fermented sausages. However, these strains (in the present case L. plantarum TMW 1.1478) might be interesting for application in the field of spreadable raw sausage manufacturing.
Keywords: Starter cultures, Exopolysaccharides (EPS), Heteropolysachharides (HePS), Raw fermented sausages (salami), Texture
Introduction
In raw fermented sausage production, starter cultures are applied to guarantee that the final products have a high sensory and microbial quality (Cocolin et al. 2011; Devine and Dikeman 2014). As a substrate, sugar is usually added during processing, which is then metabolized to e.g., lactic acid during fermentation, leading to a decrease in pH (Leroy and De Vuyst 2004). However, depending on the strain and type of sugar used, lactobacilli may have the ability to also form exopolysaccharides (EPS) which, provided the amount formed is high enough, may lead to structural and, hence, textural changes of the final products (Yilmaz et al. 2015). This phenomenon is well-known in the bakery and dairy industries, where EPS-producing starter cultures are frequently used to improve the product characteristics of sourdough (Ua-Arak et al. 2015), (fat-reduced) yoghurts, and cheeses (Badel et al. 2011; Nepomuceno et al. 2016; Zhang et al. 2016) and was lately also reported for legume protein-rich foods (Xu et al. 2019). Most microbial EPS are highly soluble in water or in diluted salt solutions and can be divided into two different groups: homopolysaccharides (HoPS) and heteropolysaccharides (HePS). The latter are synthesized out of a variety of different substrates, whereas HoPS are generally synthesized out of sucrose (Wang et al. 2015). EPS are often formed under suboptimal or so-called “stress conditions”, including temperature or salt stress, in order to protect the microorganisms (Prechtl et al. 2018a; Wingender et al. 1999). The in-situ formation, however, does not only depend on the processing conditions, but also on the strain (concentration) used, growth phase, and availability of nutrients as well as their distribution (Kumar et al. 2007; Prechtl et al. 2018b). Very few studies have, as yet, focused on in-situ EPS formation in meat matrices, including the one from Hilbig et al. (2019a) who investigated EPS formation in a cooked ham model system. In their study, general requirements of EPS formation in meat matrices were also investigated with a special focus on different sugar sources and processing conditions (simulation of tumbling conditions at 2 °C and a reduced stress level at 15 °C). So far, there are only two other studies available in the current literature focusing on in-situ EPS production in raw fermented sausages. The one from Dertli et al. (2016), which examined the influence of EPS-producing lactic acid bacteria (LAB) on sucuk, a Turkish–type fermented sausage, and the work done by Hilbig et al. (2019b), which focused on the influence of in-situ EPS production on the properties of fat-reduced Teewurst. Although, in the first case, the products were found to be tougher, harder, and less adhesive compared to sucuk without an EPS-forming culture, the opposite was true for fat-reduced Teewurst (up to 50%), in which the in-situ formed EPS maintained the spreadability of the products and improved the mouthfeel. This shows that EPS-forming LAB can have very different effects, depending on the strain, processing conditions and meat matrix used. While an earlier hardening of raw fermented products could reduce the production time, a softening effect as seen for fat-reduced Teewurst would lead to a final product with undesirable properties. For this reason, the present study aimed to clarify whether a HePS-forming starter culture (Lactobacillus plantarum TMW 1.1478) is able to produce sufficient amounts of EPS in salami (typical raw fermented sausage), while reducing the pH of the product during fermentation, in order to have an influence on the product properties. In this course, the influence of different inoculation concentrations (107 and 109 CFU/g) was also investigated and all results compared to products containing a non-EPS forming culture (Lactobacillus sakei TMW 1.2037). Following the methods of Hilbig et al. (2019a), it was decided to use a qualitative (information about the location) and semi-quantitative approach for EPS detection via CLSM, since salami contains a lot more fat than cooked ham, which may influence the location and distribution of EPS in the meat matrix.
Materials
Media and chemicals
MRS agar and broth [peptone from casein 10.0 g/L, meat extract 10.0 g/L, yeast extract 4.0 g/L; d ( +)-glucose 20.0 g/L, dipotassium hydrogen phosphate 2.0 g/L, Tween® 80 1.0 g/L, di-ammonium hydrogen citrate 2.0 g/L, sodium acetate 5.0 g/L, magnesium sulfate 0.2 g/L, and manganese sulfate 0.04 g/L, ± agar–agar 14.0 g/L], as well as Anaerocult® were purchased from Merck KGaA (Darmstadt, Germany). Peptone water (pH 7.0 ± 0.2; 5 g/L) was purchased from Carl Roth GmbH + Co. KG (Karlsruhe, Germany). Plate Count Agar [agar 15.0 g/L, glucose 1.0 g/L, peptones 5.0 g/L, and yeast extract 2.5 g/L] was obtained from AppliChem GmbH (Darmstadt, Germany). Calcofluor White Stain and Concanavalin A were purchased from Sigma-Aldrich Chemie GmbH (Steinheim, Germany).
All microbiological media were prepared as specified by the respective manufacturers and autoclaved for 15 min at 121 °C.
Starter cultures
Lactobacillus plantarum TMW 1.1478 (HePS-forming strain; henceforth referred to as L. plantarum 1.1478) and L. sakei TMW 1.2037 (control strain; henceforth referred to as L. sakei 1.2037) were provided by the Technical University of Freising (Department of Technical Microbiology, Munich, Germany). The bacterial strains were stored in MRS broth containing 25% (v/v) glycerol at − 70 °C and reactivated on MRS agar prior to the experiments followed by cultivation in MRS broth at 30 °C for 48 h. Starter cultures were subsequently used in two different concentrations, more precisely at ~ 107 CFU/g and at ~ 109 CFU/g meat. In order to reach higher inoculation concentrations, the incubated MRS solution was centrifuged (Z32HK, Hermle Labortechnik GmbH, Wehingen, Germany) at 5000 rpm for 10 min and 20 °C and the resulting pellets then suspended in small aliquots of water.
Ingredients for raw sausage production
Lean pork meat (S II) and pork back fat (S VIII) were purchased at MEGA (Fachzentrum für die Metzgerei und Gastronomie eG. Stuttgart, Germany). Nitrite curing salt (NCS) was provided by Südsalz GmbH, (Heilbronn, Germany), ascorbic acid and black pepper were purchased from Gewürzmüller, (Korntal-Münchingen, Germany), and dextrose, as well as sucrose, was obtained from Südzucker AG (Mannheim, Germany).
Methods
Raw fermented sausage preparation
Four batches of raw sausages were produced using two different starter cultures as well as different initial bacterial concentrations: more precisely, the non-EPS-forming strain L. sakei 1.2037 (control; ~ 107 and 109 CFU/g) or the HePS-producing strain L. plantarum 1.1478 (~ 107 and 109 CFU/g). The meat (from different animals to account for differences in the raw material) batter was prepared in a bowl chopper (type K64DC, Seydelmann, Aalen, Germany) by chopping 20% pork back fat (S VIII, − 18 °C) and 45% lean pork meat (S II, − 10 °C). Afterwards, 3 g/kg black pepper, 5 g/kg sugar (Südzucker AG, Mannheim, Germany), the respective starter culture (either 107 or 109 CFU/g), and 0.5 g/kg ascorbate were added and mixed. In the next step, 35% ground lean pork meat (3 mm, + 2 °C) and, finally, 28 g/kg nitrate curing salt (NCS) was added and mixed to ensure a homogenous distribution of NCS. The different sausage batters were filled into cellulose casings (50 mm diameter; Nalo fasser S, Werner Niedenbeger GmbH, München, Germany) and afterwards ripened for approx. 17 days until a weight loss of 31% was reached (determined by differential weighting). In the first 7 days, the fermentation and ripening was performed at 24 °C, followed by a 10-day period at 14 °C (continuously at a relative humidity of 95%). Additionally, the sausages were smoked after 24, 48 and 72 h of fermentation in a smoking chamber (Maurer, Reichenau, Germany) at 24 °C for 15 min. For analysis purposes, duplicate samples were collected after 0, 24, 48, and 72 h, and after 9, 12, 14, and 17 days of ripening.
Microbiological analysis of raw fermented sausages
Microbiological analysis of the raw sausages was conducted at time zero (raw material ± starter culture), 24, 48, and 72 h, and after 9, 12, 14, and 17 days of ripening. To obtain viable cell counts of the different products, 10 g of sample was always taken aseptically from the core of the respective raw sausage and subsequently mixed for 1 min with 90 mL of buffered peptone water using a Masticator (Laborhomogenisator, IUL Instrument GmbH, Königswinter, Germany). Appropriate dilutions were plated on plate count agar (PCA; raw material quality) and/or MRS agar using an automated spiral plater (Don Whitley Scientific, West Yorkshire, UK) followed by incubation at 30 °C for 24–48 h under either anaerobic (MRS) or aerobic (PCA) conditions. Subsequently, the colonies were counted using an automatic colony counter (Acolyte, Synbiosis, Cambridge, UK).
pH measurement and aw determination
The pH measurement was conducted using a pH meter (WTW Microprocessor pH Meter, WTW GmbH, Weilheim, Germany) and the pH values of the different products recorded after 0, 24, 48 and 72 h, as well as after 9, 12, 14, and 17 days of ripening. Before the pH of the dried sausages was measured, the salamis were chopped using a Moulinette. The water activity (aw) was determined at the same time using the Aqua Lab device (CX-2- Decagon Devices Inc., Pullman, USA). Two independent samples were always analyzed in triplicate.
Visualization of EPS using confocal laser scanning microscopy (CLSM)
A qualitative assessment of the EPS production was performed after 0, 24, 48, 72 h, and 9, 12, 14, 17 days of ripening using the method developed by Hilbig et al. (2019a). A cylindrical metal pipe was used to take a sample (0.5 cm high, 1.5 cm wide) from the core of the respective raw fermented sausage, which was then transferred to a concave plastic slide. In order to stain in-situ-formed EPS (green), 10 µL of a diluted (1:20) Concanavalin A solution (stock: 5 mg lypolized powder in 5 mL phosphate buffer 10 mmol; pH 6) was added to the meat sample. After keeping it in the dark (12 °C) for 60 min, 10 µL of Calcoflour White Stain was added to stain the proteins (blue). The samples were analyzed using a Nikon Eclipse-Ti Inverse Microscope D- Eclipse C1 (Nikon GmbH, Düsseldorf, Germany). A 60-fold magnification lens (Nikon, Plan Flour) with immersion oil (Nikon, Plan Apo VC) was used to examine the stained meat samples. An argon laser, at 488 nm, and a red helium–neon laser, at 638 nm, were used for the excitation of EPS (green) and proteins (blue). Ten pictures of each sample were taken and further analyzed. Scales were inserted using the ImageJ software (Version 1.4.3.67, National Institutes of Health, Bethesda, MD, USA) after creating a RGB picture using the EZ-C1 3.70 Imaging Software (Nikon GmbH, Düsseldorf, Germany). The semi-quantitative analysis of the EPS was performed using the MATLAB software (The Math Works, Inc., version R2013b 8.2.0.701) following a method developed by Bosse et al. (2015). Here, the green channel of the picture was automatically transformed to a black-and-white picture and only pixels with a threshold over 0.08 were counted to remove noise. In the resulting picture, the percentage of the green area (EPS) compared to the whole area was calculated using Eq. 1:
| 1 |
Texture profile analysis
In order to determine textural changes in the raw fermented sausages, a textural profile analysis (TPA) was performed as soon as the sausages achieved 16, 23, 27, and 31% weight loss, on day 9, 12, 14, and 17, respectively. After removing the sausage casings, slicing, and storing the samples for 2 h at 12 °C (same experimental conditions), 15 samples were taken from each batch (2 cm high × 1.5 cm wide) and analyzed in a double compression test (50%; 20 s interval between cycles) at a cross-head speed of 50 mm/min using an Instron device (Model 1011, Instron Engineering Corp., Canton, MA, USA) equipped with a 100 N load cell. The hardness of the samples was determined at the first peak of compression.
Sensory analysis
The sensory evaluation of the raw fermented sausages was performed by 26 untrained panelists. Here, sausages containing either the non-EPS-forming strain L. sakei 1.2037 (control) or the HePS-forming culture L. plantarum TMW 1.1478 were cut into 1.5 cm thick slices with a diameter of about 3.5 cm and served at room temperature. For the evaluation, a ranking test was performed using a 1–4 scale for the evaluation of the following attributes: Consistency (one hardest; four softer), consistency based on preference (one more preferred; four less preferred), and taste based on preference (one favorite; four not preferred).
Statistical analysis
Each experiment was conducted twice and all measurements repeated three times. Means and standard deviations were calculated using Excel (Microsoft, Redmond, WA, USA) and SPSS (IMB SPSS Statistics 24, IBM, Germany) was used to statistically evaluate the results gained. A one—way ANOVA with a post—hoc Tukey’s test was performed to interpret TPA results, while Duncan was used for the EPS results to investigate significant differences between the samples and the control (p < 0.05).
Results and discussion
Microbial growth behavior and pH development
The viable cell counts of the raw material used (PCA) ranged between 102–103 CFU/g, indicating a good raw material quality. Typical starter cultures for raw fermented sausage production are LAB, usually used at an inoculation level of 106–107 CFU/g meat (Erkkilä et al. 2001). Among the starter cultures known for EPS-production, L. plantarum has been widely studied (Dertli et al. 2016; Fontana et al. 2015; Guidone et al. 2014; Prechtl et al. 2018b; Tallon et al. 2003) and was hence selected for the present investigation. In order to examine the influence of a typical (106–107 CFU/g) and an increased (109 CFU/g) inoculation concentration on EPS-formation, and hence on the quality parameters of raw fermented sausages, the HePS-forming strain L. plantarum 1.1478 was inoculated accordingly and its growth studied over a period of 17 days (Fig. 1). As a control, the non-EPS-forming strain L. sakei 1.2037 was used. The growth behavior of both strains was found to be very similar, with anaerobic cell counts of L. plantarum 1.1478 decreasing at the end of storage (Fig. 1). The same growth behavior could be observed in the repetition of the experiment (data not shown). LAB need fermentable sugars, which are then metabolized to organic acids leading to a decrease in the product’s pH (Leroy et al. 2006). This can also be seen in the present study, where the initial pH of 5.66 ± 0.06 decreased to a final pH (31% weight loss reached) of 4.89 ± 0.01 for L. sakei 1.2037 and of 5.035 ± 0.01 for L. plantarum 1.1478, respectively, independent of the inoculation concentration used (Fig. 2). The water activity (aw) of all sausages decreased during the 17 days of observation from 0.93 to 0.85 ± 0.03 (final product; 31% weight loss).
Fig. 1.
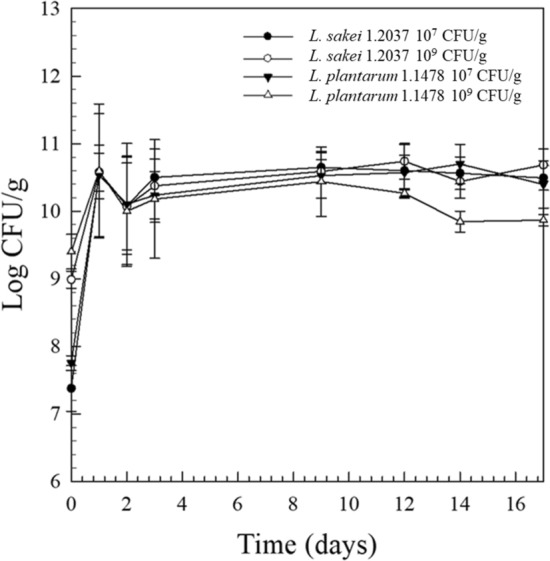
Anaerobic cell counts of raw fermented sausages produced with the non-EPS-forming strain L. sakei 1.2037 (control) or the HePS-forming strain L. plantarum 1.1478 during 17 days of fermentation and storage (7 days at 24 °C, 10 days at 14 °C). Inoculation concentrations were either 107 or 109 CFU/g. Error bars are standard deviations from two independent replicates, each examined in duplicate (n = 4)
Fig. 2.
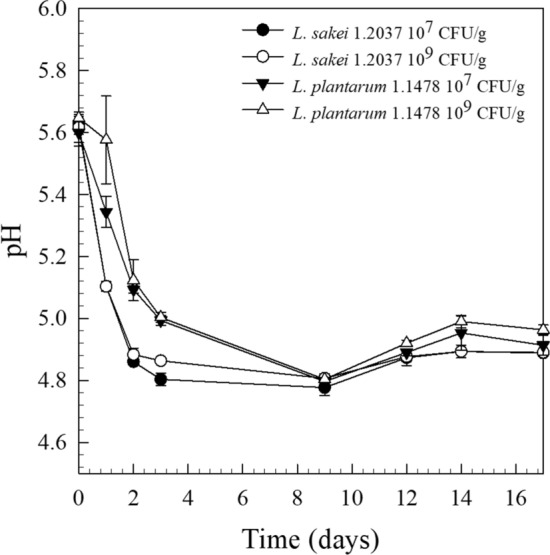
pH values of raw fermented sausages produced with the non-EPS-forming strain L. sakei 1.2037 (control) or the HePS-forming strain L. plantarum 1.1478 during 17 days of fermentation and storage (7 days at 24 °C, 10 days at 14 °C). Inoculation concentrations were either 107 or 109 CFU/g
EPS detection
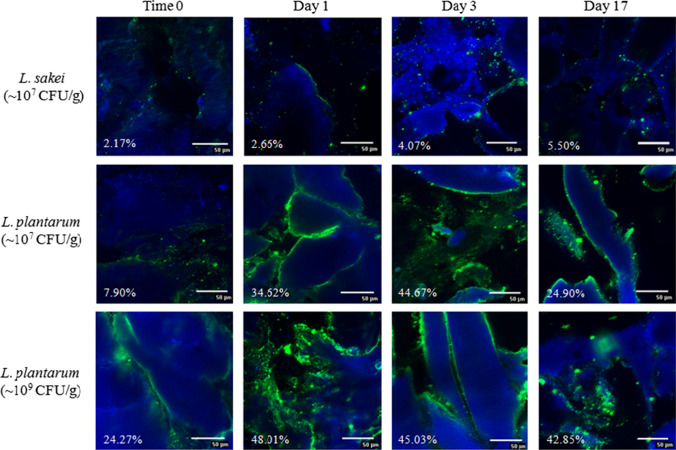
EPS formation of many mesophilic strains appears to be non-growth associated and was found to take place at both the beginning and the end of the exponential growth phase as well as during the stationary phase (Bengoa et al. 2018; Pham et al. 2000; Wang et al. 2015). The amount of HePS produced by L. plantarum ssp. was often found to be increased under suboptimal growth conditions, which was attributed to the fact that a slowing down of the cell wall synthesis results in more isoprenoid glycosyl lipid carriers available as precursor molecules for EPS formation (Sutherland 1972). The analysis of the EPS formation was done according to Hassan et al. (2002), who investigated the distribution of EPS in dairy products, including fermented milk, using Concanavalin A and Calcofluor White Stain. Figure 3 illustrates raw fermented sausage samples after production (0 days), during fermentation (1–3 days), and after reaching the final weight loss of 31% (17 days) that have either been produced with the non-EPS-forming strain L. sakei 1.2037 (107 CFU/g) or with the HePS-forming strain L. plantarum 1.1478 at an initial concentration of 107 or 109 CFU/g salami, respectively. While only very few EPS could be detected throughout the production for the control samples, which can be attributed e.g. to the autochthone meat microflora, significantly more EPS (< 0.05; after 24 h of incubation) could be found in the presence of L. plantarum 1.1478. This is in accordance with the results of the semi-quantitative data interpretation (Table 1), which followed a modified method from Bosse et al. (2015), who analyzed the distribution of staphylococci in raw ham. The EPS-formation in the present study primarily took place during the exponential, and at the beginning of the stationary growth phase, which is also reflected in the pH drop because of the sugar metabolism (Fig. 2). Since the biosynthesis of HePS is linked with the primary carbohydrate metabolism, EPS synthesis is expected to take place during the time of fermentation. This is in accordance with our findings, showing an increase in the quantities of HePS formed in the salami within the first 24–72 h of storage at 24 °C (Table 1). EPS were only located at the outer parts of the proteins, which is why the fat was not subsequently stained in the present study. However, the HePS formed were found to not be homogenously distributed in the raw sausage matrix (Fig. 3). The decrease in the overall EPS content at the later stage of the ripening or incubation phase, was already observed in other studies and was attributed to an enzymatic degradation as a result of cell lysis at the end of the stationary phase. There, intracellular glycohydrolases might be released from the cells, initiating EPS degradation (Cerning 1990; Vaningelgem et al. 2004).
Fig. 3.
Example of a qualitative analysis of the formed EPS (stained green; proteins stained blue) in raw fermented sausages produced with the non-EPS-forming strain L. sakei 1.2037 (control) or the HePS-forming strain L. plantarum 1.1478 after production (0 days), during fermentation (1 and 3 days; 24 °C) and in the final product (31% weight loss; 17 days). Inoculation concentrations were either 107 or 109 CFU/g. Presented values give the relation between the detected EPS and the total area of the picture; n = 10
Table 1.
Percentage of EPS formed (mean value) in raw fermented sausages that have been either inoculated with the non-EPS-forming strain L. sakei 1.2037 (~ 107 CFU/g and ~ 109 CFU/g), or with the HePS-forming strain L. plantarum 1.1478 (~ 107 CFU/g and ~ 109 CFU/g)
| Storage time (days) | L. sakei 1.2037 ~ 107 CFU/g | L. sakei 1.2037 ~ 109 CFU/g | L. plantarum 1.1478 ~ 107 CFU/g | L. plantarum 1.1478 ~ 109 CFU/g |
|---|---|---|---|---|
| EPS [%] | EPS [%] | EPS [%] | EPS [%] | |
| A | ||||
| 0 | 1.67 ± 1.71a | 11.28 ± 6.34b | 11.17 ± 2.96b | 4.01 ± 2.01a |
| 1 | 13.97 ± 10.47a | 10.25 ± 4.90a | 50.70 ± 16.55b | 47.14 ± 13.75b |
| 2 | 5.45 ± 2.68a | 15.33 ± 12.92a | 33.88 ± 7.81b | 55.06 ± 19.16c |
| 3 | 18.93 ± 9.11a | 18.91 ± 7.51a | 49.49 ± 18.36b | 48.74 ± 20.66b |
| 9 | 5.45 ± 3.87a | 5.78 ± 3.93a | 19.76 ± 10.42b | 18.85 ± 9.55b |
| 12 | 5.94 ± 3.94a | 8.20 ± 4.39a | 24.03 ± 11.49b | 30.68 ± 10.40b |
| 14 | 4.58 ± 1.22a | 9.32 ± 3.27a | 23.49 ± 12.60b | 27.74 ± 11.15b |
| 17 | 5.93 ± 2.33a | 4.34 ± 1,69a | 24.93 ± 9.58b | 28.06 ± 18.97b |
| B | ||||
| 0 | 13.68 ± 4.95a | 11.64 ± 4.88a | 13.68 ± 6.30a | 13.52 ± 7.69a |
| 1 | 5.01 ± 3.24a | 11.50 ± 6.28a | 30.68 ± 5.55b | 32.24 ± 16.79b |
| 2 | 15.16 ± 5.14a | 7.73 ± 4.15a | 32.48 ± 14.01b | 39.92 ± 13.05b |
| 3 | 8.26 ± 5.93a | 16.27 ± 9.58a | 37.17 ± 7.68b | 40.24 ± 4.14b |
| 9 | 8.46 ± 8.43a | 12.16 ± 7.69a | 42.09 ± 13.33c | 29.82 ± 8.80b |
| 12 | 3.75 ± 2.97a | 6.10 ± 4.06a | 30.16 ± 16.56b | 35.14 ± 16.27b |
| 14 | 16.56 ± 4.68a | 10.54 ± 5.44a | 28.61 ± 12.60b | 34.87 ± 10.61b |
| 17 | 4.81 ± 1.87a | 7.30 ± 2.46a | 22.52 ± 10.44b | 23.88 ± 13.44b |
Values with different letters show significant differences (p < 0.05) in between the line
A: First experiment; B: Repetition
Texture profile- and sensory analysis
In-situ produced HePS were found to increase gel strength, water-binding capacity and viscosity of dairy products including fat-reduced cheese and yoghurts (Cerning 1990; Hassan and Awad 2005) while Dertli et al. (2016) found HePS to increase the hardness of the sucuk. With one exception (23% weight loss, experiment A with L. plantarum 1.1478 109 CFU/g), the samples containing the HePS-forming strain were always found to be significantly softer (p < 0.05) than the respective control samples (Table 2). For instance, products (31% weight loss; final product) containing the control strain L. sakei 1.2037 (107 CFU/g) had hardness values of 110.13 ± 6.09 and 116.89 ± 8.58, respectively, while those for products containing the EPS-forming strain L. plantarum 1.1478 (107 CFU/g) were found to have significantly lower values (80.54 ± 5.98 and 84.00 ± 3.11). This effect was, in most of the cases, even more pronounced when the initial bacterial concentration for L. plantarum 1.1478 was increased from 107 to 109 CFU/g, although no significant differences could be observed with regard to HePS formation. This leads to the conclusion that the difference with regard to the HePS amount formed is too small to be covered by the semi-quantitative approach used. This is also indicated by the low amount of HePS detected by Hilbig et al. (2019b) in spreadable raw fermented sausages (teewurst containing 30–40% fat; meat particles are covered by a fat layer) that have been produced with L. plantarum 1.1478. Detected HePS-concentrations (HPLC) ranged between 0.08 ± 0.01 and 0.30 ± 0.04 g/kg dry matter while products containing the HoPS-producing strain L. sakei 1.411 were found to contain significantly higher amounts of EPS (0.46 ± 0.01–0.80 ± 0.02 g/kg dry matter). In the present study L. sakei 1.2037 has been used as a non-EPS forming control strain and L. plantarum 1.1478 as a strain able to form heteropolysaccharides under fermentation conditions. Although these strains do not belong to the same species, several studies showed that LAB contribute to textural changes by acidifying the meat batter leading to a coagulation of muscle proteins, and by forming exopolysaccharides in-situ during processing (Laranjo et al. 2017). In addition, changing the environment to acidic conditions can result in an increased activity of cathepsin D (at low pH), which is involved in muscle proteolysis (Molly et al. 1997). Changing processing and hence fermentation conditions thus has a remarkable impact on textural properties. In the present study, same fermentation conditions have been used for both strains that showed similar fermentation kinetics leading to products with pH values in the same range after storage for 9 days at 24 °C (Fig. 2). Since LAB do not display a pronounced lipolytic or proteolytic activity under the processing conditions used (lipases from lactobacilli usually display little or no activity during sausage fermentation) (Leroy et al. 2006), the observed differences in texture can be attributed to the formation of EPS, which was also shown in other studies (Hilbig et al. 2019b). This is also the reason why most commercial meat starter cultures are combined cultures of LAB and coagulase-negative staphylococci that are known for their ability to stabilize color but also for their strain-dependent proteolytic and lipolytic activity predominantly contributing to aroma formation (Ravyts et al. 2012). Although no significant differences could be observed with regard to detected HePS and inoculation concentration used, a correlation between HePS formation and influence on the quality parameters of the salami could still be made, not only with regard to the results gained from the TPA, but also from a sensory point of view: there, products containing the HePS-forming strain L. plantarum 1.1478 were rated higher in terms of consistency, which is directly associated with a softer texture (Fig. 4). However, the taste of the products containing the HePS-forming strain was not negatively affected.
Table 2.
Hardness of raw fermented sausages produced with the non-EPS-forming strain L. sakei 1.2037 (~ 107 CFU/g and ~ 109 CFU/g), or with the HePS-forming strain L. plantarum 1.1478 (~ 107 CFU/g and ~ 109 CFU/g)
| Weight loss [%] | L. sakei 1.2037 ~ 107 CFU/g | L. sakei 1.2037 ~ 109 CFU/g | L. plantarum 1.1478 ~ 107 CFU/g | L. plantarum 1.1478 ~ 109 CFU/g |
|---|---|---|---|---|
| *Hardness (N) | ||||
| A | ||||
| 16 | 56.63 ± 5.07a | 55.42 ± 2.82a | 46.48 ± 2.94b | 42.59 ± 2.76c |
| 23 | 71.90 ± 4.76a | 82.09 ± 5.63b | 56.21 ± 2.68c | 78.64 ± 3.92b |
| 27 | 87.25 ± 5.35a | 77.17 ± 4.88b | 60.67 ± 2.77c | 57.39 ± 3.88c |
| 31 | 110.13 ± 6.09a | 116.35 ± 10.35a | 80.54 ± 5.98b | 81.62 ± 4.79b |
| B | ||||
| 16 | 82.35 ± 5.05a | 74.26 ± 5.37b | 64.78 ± 8.07c | 56.00 ± 4.52d |
| 23 | 80.92 ± 3.18a | 78.61 ± 3.39a | 73.35 ± 3.38b | 55.69 ± 3.81c |
| 27 | 92.30 ± 6.41a | 93.54 ± 7.14a | 74.71 ± 5.79b | 66.37 ± 1.56c |
| 31 | 116.89 ± 8.58a | 103.32 ± 4.68b | 84.00 ± 3.11c | 69.91 ± 4.66d |
*Numbers are means ± standard deviation from 15 measurements
Values with different letters show significant differences (p < 0.05) between the lines. A: First experiment; B: Repetition
Fig. 4.
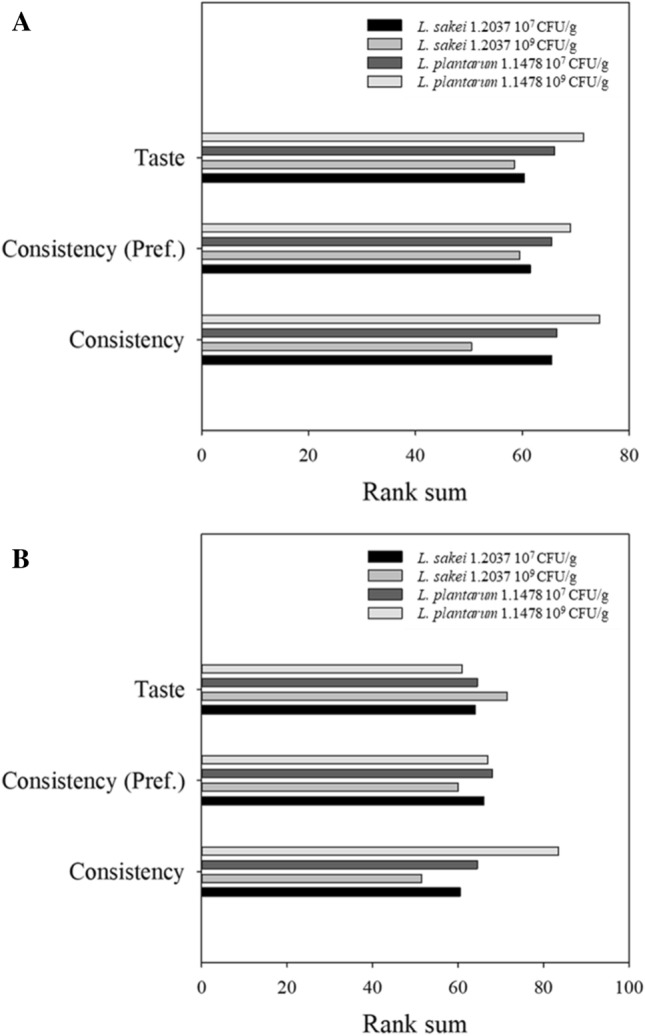
Results of the sensory evaluation (26 panelists) of raw fermented sausages (31% weight loss) produced with the non-EPS-forming strain L. sakei 1.2037 (control) or the HePS-forming strain L. plantarum 1.1478 of the first experiment (a) and the repetition (b)
With regard to the quality attributes of raw fermented sausages, the results clearly indicate that using an EPS-forming culture in raw fermented sausage manufacturing might be a real issue, since the texture of the products gets softer, which is often associated with a poorer product quality. However, the effect observed could make a positive contribution in the field of highly-perishable spreadable raw fermented sausage products, including onion mettwurst (meat particles are not covered by a fat layer), having a high product pH of 5.5–5.6 (Feiner 2006). Using EPS-forming LAB in onion mettwurst production may allow for a decrease in pH to 5.1 (close to, but above the IEP of meat proteins) while maintaining the product’s spreadability, which could, in turn, improve the shelf life of the product. This assumption is supported by the data results, since the softening effect was seen in raw fermented sausages with a pH of around 5.0 (Fig. 2). Moreover, the effects observed could even be more pronounced when using HoPS-forming LAB since higher amounts of EPS are usually produced, which may then interact with meat proteins as indicated in Fig. 3.
Conclusion
While in the food industry in-situ HePS-forming starter cultures are generally associated with a positive influence on product quality, the results of the present study showed the opposite. These starter cultures might, however, be promising in the field of spreadable raw fermented sausage manufacturing (e.g., onion mettwurst), because the softening effect observed in the present study could lead to a better spreadability. This would have the advantage that the EPS-forming culture could be used as an alternative to typically-applied hydrocolloids that have to be declared on the package. The observed softening effect of in-situ-formed EPS could even be more pronounced when a HoPS-producing starter culture is used during raw fermented sausage production, since more HoPS are usually formed due to the HePS-synthesis being much more complex and energy demanding. In future investigations one should consider using EPS-detection methods with a high sensitivity, such as high performance liquid chromatography.
Acknowledgements
This research project was supported by the German Ministry of Economics and Energy (via AiF) and the FEI (Forschungskreis der Ernährungsindustrie e.V., Bonn), Project AiF 18357 N. We would also like to thank our Master butcher Kurt Herrmann (meat pilot plant, University of Hohenheim) for his outstanding support during production.
Compliance with ethical standards
Conflict of interest
There are no conflicts of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Badel S, Bernardi T, Michaud P. New perspectives for lactobacilli exopolysaccharides. Biotechnol Adv. 2011;29:54–66. doi: 10.1016/j.biotechadv.2010.08.011. [DOI] [PubMed] [Google Scholar]
- Bengoa AA, Llamas MG, Iraporda C, Dueñas MT, Abraham AG, Garrote GL. Impact of growth temperature on exopolysaccharide production and probiotic properties of Lactobacillus paracasei strains isolated from kefir grains. Food Microbiol. 2018;69:212–218. doi: 10.1016/j.fm.2017.08.012. [DOI] [PubMed] [Google Scholar]
- Bosse R, Gibis M, Schmidt H, Weiss J. Kinetics of migration of colloidal particles in meat muscles in the absence and presence of a proteolytic enzyme to simulate non-motile bacteria penetration. Food Res Int. 2015;75:79–88. doi: 10.1016/j.foodres.2015.05.054. [DOI] [PubMed] [Google Scholar]
- Cerning J. Exocellular polysaccharides produced by lactic acid bacteria. FEMS Microbiol Lett. 1990;87:113–130. doi: 10.1111/j.1574-6968.1990.tb04883.x. [DOI] [PubMed] [Google Scholar]
- Cocolin L, Dolci P, Rantsiou K. Biodiversity and dynamics of meat fermentations: the contribution of molecular methods for a better comprehension of a complex ecosystem. Meat Sci. 2011;89:296–302. doi: 10.1016/j.meatsci.2011.04.011. [DOI] [PubMed] [Google Scholar]
- Dertli E, Yilmaz MT, Tatlisu NB, Toker OS, Cankurt H, Sagdic O. Effects of in-situ exopolysaccharide production and fermentation conditions on physicochemical, microbiological, textural and microstructural properties of Turkish-type fermented sausage (sucuk) Meat Sci. 2016;121:156–165. doi: 10.1016/j.meatsci.2016.06.008. [DOI] [PubMed] [Google Scholar]
- Devine C, Dikeman M (2014) Encyclopedia of meat sciences, 2nd edn. Elsevier, ISBN 0123847346, 9780123847348
- Erkkilä S, Petäjä E, Eerola S, Lilleberg L, Mattila-Sandholm T, Suihko M. Flavour profiles of dry sausages fermented by selected novel meat starter cultures. Meat Sci. 2001;58:111–116. doi: 10.1016/S0309-1740(00)00135-2. [DOI] [PubMed] [Google Scholar]
- Feiner G (2006) Meat products handbook: practical science and technology. In: Woodhead Publishing in food science, technology and nutrition, 1st edn. Woodhead food series (130). CRC Press, ISBN 1845690508
- Fontana C, Li S, Yang Z, Widmalm G. Structural studies of the exopolysaccharide from Lactobacillus plantarum C88 using NMR spectroscopy and the program CASPER. Carbohydr Res. 2015;402:87–94. doi: 10.1016/j.carres.2014.09.003. [DOI] [PubMed] [Google Scholar]
- Guidone A, Zotta T, Ross RP, Stanton C, Rea MC, Parente E, Ricciardi A. Functional properties of Lactobacillus plantarum strains: a multivariate screening study. LWT—Food Sci Technol. 2014;56:69–76. doi: 10.1016/j.lwt.2013.10.036. [DOI] [Google Scholar]
- Hassan AN, Awad S. Application of exopolysaccharide-producing cultures in reduced-fat cheddar cheese: cryo-scanning electron microscopy observations. J Dairy Sci. 2005;88:4214–4220. doi: 10.3168/jds.S0022-0302(05)73107-6. [DOI] [PubMed] [Google Scholar]
- Hassan AN, Frank JF, Qvist KB. Direct observation of bacterial exopolysaccharides in dairy products using confocal scanning laser microscopy. J Dairy Sci. 2002;85:1705–1708. doi: 10.3168/jds.S0022-0302(02)74243-4. [DOI] [PubMed] [Google Scholar]
- Hilbig J, Gisder J, Prechtl RM, Herrmann K, Weiss J, Loeffler M. Influence of exopolysaccharide-producing lactic acid bacteria on the spreadability of fat-reduced raw fermented sausages (Teewurst) Food Hydrocoll in press. 2019 doi: 10.1016/j.foodhyd.2019.01.056. [DOI] [Google Scholar]
- Hilbig J, Loeffler M, Herrmann K, Weiss J. Application of exopolysaccharide-forming lactic acid bacteria in cooked ham model systems. Food Res Int in press. 2019 doi: 10.1016/j.foodres.2018.10.058. [DOI] [PubMed] [Google Scholar]
- Kumar AS, Mody K, Jha B. Bacterial exopolysaccharides—a perception. J Basic Microbiol. 2007;47:103–117. doi: 10.1002/jobm.200610203. [DOI] [PubMed] [Google Scholar]
- Laranjo M, Elias M, Fraqueza MJ. The use of starter cultures in traditional meat products. J Food Qual. 2017 doi: 10.1155/2017/9546026. [DOI] [Google Scholar]
- Leroy F, De Vuyst L. Lactic acid bacteria as functional starter cultures for the food fermentation industry. Trends Food Sci Technol. 2004;15:67–78. doi: 10.1016/j.tifs.2003.09.004. [DOI] [Google Scholar]
- Leroy F, Verluyten J, De Vuyst L. Functional meat starter cultures for improved sausage fermentation. Int J Food Microbiol. 2006;106:270–285. doi: 10.1016/j.ijfoodmicro.2005.06.027. [DOI] [PubMed] [Google Scholar]
- Molly K, Demeyer D, Johansson G, Raemaekers M, Ghistelinck M, Geenen I. The importance of meat enzymes in ripening and flavour generation in dry fermented sausages. First results of a European project. Food Chem. 1997;59:539–545. doi: 10.1016/S0308-8146(97)00004-6. [DOI] [Google Scholar]
- Nepomuceno RSAC, Costa Junior LCG, Costa RGB. Exopolysaccharide-producing culture in the manufacture of Prato cheese. LWT—Food Sci Technol. 2016;72:383–389. doi: 10.1016/j.lwt.2016.04.053. [DOI] [Google Scholar]
- Pham PL, Dupont I, Roy D, Lapointe G, Cerning J. Production of exopolysaccharide by Lactobacillus rhamnosus R and analysis of its enzymatic degradation during prolonged fermentation. Appl Environ Microbiol. 2000;66:2302–2310. doi: 10.1128/AEM.66.6.2302-2310.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prechtl RM, Wefers D, Jakob F, Vogel RF. Cold and salt stress modulate amount, molecular and macromolecular structure of a Lactobacillus sakei dextran. Food Hydrocoll. 2018;82:73–81. doi: 10.1016/j.foodhyd.2018.04.003. [DOI] [Google Scholar]
- Prechtl RM, Wefers D, Jakob F, Vogel RF. Structural characterization of the surface-associated heteropolysaccharide of Lactobacillus plantarum TMW 1.1478 and genetic analysis of its putative biosynthesis cluster. Carbohydr Polym. 2018;202:236–245. doi: 10.1016/j.carbpol.2018.08.115. [DOI] [PubMed] [Google Scholar]
- Ravyts F, Vuyst LD, Leroy F. Bacterial diversity and functionalities in food fermentations. Eng Life Sci. 2012;12:356–367. doi: 10.1002/elsc.201100119. [DOI] [Google Scholar]
- Sutherland IW. Bacterial exopolysaccharides. Adv Microb Physiol. 1972 doi: 10.1016/s0065-2911(08)60190-3. [DOI] [PubMed] [Google Scholar]
- Tallon R, Bressollier P, Urdaci MC. Isolation and characterization of two exopolysaccharides produced by Lactobacillus plantarum EP56. Res Microbio. 2003;154:705–712. doi: 10.1016/j.resmic.2003.09.006. [DOI] [PubMed] [Google Scholar]
- Ua-Arak T, Jakob F, Vogel RF. Characterization of growth and exopolysaccharide production of selected acetic acid bacteria in buckwheat sourdoughs. Int J Food Microbiol. 2015;239:103–112. doi: 10.1016/j.ijfoodmicro.2016.04.009. [DOI] [PubMed] [Google Scholar]
- Vaningelgem F, Zamfir M, Adriany T, De Vuyst L. Fermentation conditions affecting the bacterial growth and exopolysaccharide production by Streptococcus thermophilus ST 111 in milk-based medium. J Appl Environ Microbiol. 2004;97:1257–1273. doi: 10.1111/j.1365-2672.2004.02418.x. [DOI] [PubMed] [Google Scholar]
- Wang J, Zhao X, Tian Z, Yang Y, Yang Z. Characterization of an exopolysaccharide produced by Lactobacillus plantarum YW11 isolated from Tibet Kefir. Carbohydr Polym. 2015;125:16–25. doi: 10.1016/j.carbpol.2015.03.003. [DOI] [PubMed] [Google Scholar]
- Wingender J, Neu TR, Flemming H-C. Microbial extracellular polymeric substances: characterization, structure and function. 1. Berlin, Heidelberg: Springer; 1999. [Google Scholar]
- Xu Y, Coda R, Holopainen-Mantila U, Laitila A, Katina K, Tenkanen M. Impact of in-situ produced exopolysaccharides on rheology and texture of fava bean protein concentrate. Food Res Int. 2019;115:191–199. doi: 10.1016/j.foodres.2018.08.054. [DOI] [PubMed] [Google Scholar]
- Yilmaz MT, Dertli E, Toker OS, Tatlisu NB, Sagdic O, Arici M. Effect of in-situ exopolysaccharide production on physicochemical, rheological, sensory, and microstructural properties of the yogurt drink ayran: an optimization study based on fermentation kinetics. J Dairy Sci. 2015;98:1604–1624. doi: 10.3168/jds.2014-8936. [DOI] [PubMed] [Google Scholar]
- Zhang L, Folkenberg DM, Amigo JM, Ipsen R. Effect of exopolysaccharide-producing starter cultures and post-fermentation mechanical treatment on textural properties and microstructure of low fat yoghurt. Int Dairy J. 2016;53:10–19. doi: 10.1016/j.idairyj.2015.09.008. [DOI] [Google Scholar]