Abstract
This study addresses the application of native, multiple strain starter cultures for standardization of game meat sausages production. The designed starter cultures consisting of two indigenous Lactobacillus sakei and one Leuconostoc mesenteroides strains. These strains were used in both, the encapsulated and non-encapsulated form, in the game meat dough, individually or in combination, with eight treatments in total. Microbiological and physicochemical characteristics of the sausages were monitored throughout the manufacturing process, while sensory properties, biogenic amine content, and volatile compounds were evaluated in the final products. As revealed by rep-PCR, native starter cultures, encapsulated or non-encapsulated, had survived the whole sausage production process; however, to varying degrees. The application of indigenous decarboxylase negative Lb. sakei strains significantly (P < 0.05) reduced tyramine content, rapidly decreased pH and promoted the number reduction of Enterobacteriaceae and elimination of E. coli, L. monocytogenes and coliforms in ready-to-eat products. A total of 84 volatile compounds were identified by SPME–GC–MS in the eight treatment batches of game meat sausages, with only minor differences between the treatments. No significant differences in sensory traits (P > 0.05) between tested treatments were found, although treatment with the Lb. sakei strains received the highest scores for the sensory traits including cross-section, odour, hardness, aroma, and overall acceptability. Combination of multi-strain Lb. sakei starter cultures resulted in growth prevention of undesirable microbiota, reduction of tyramine content and increased the acceptability parameters of full-ripened sausages, which make them good candidates for industrial as well as artisanal application.
Electronic supplementary material
The online version of this article (10.1007/s13197-020-04587-z) contains supplementary material, which is available to authorized users.
Keywords: Game meat sausages, Native starter cultures, Encapsulation, Tyramine, Sensory properties, Volatile compounds
Introduction
The consumers demand organic food of specific taste that is minimally processed and with fewer, or preferably no preservatives (Román et al. 2017). The production of traditional, spontaneously fermented, nitrate-free game meat sausages is perceived to fulfill all of those criteria. However, such production is not standardized and may result in the oscillation of sensorial as well as microbiological quality (Žgomba Maksimović et al. 2018). Although the utilization of starter cultures is a key food safety strategy in industrial sausages production (Lücke 2000), many small-scale sausages producers still rely on traditional procedures and do not apply starter or protective cultures (Kozačinski et al. 2006; Žgomba Maksimović et al. 2018). However, in the context of microbial metabolic diversity, it must be remembered that the use of commercial starter cultures can lead to the loss of diversity and the generation of different metabolic activities which consequently directly influence the sensorial properties of sausages (García et al. 2019). Irrespectively, if the starter cultures could ensure the uniqueness of the sensorial properties of artisanal sausages and enhance microbiological safety, it is reasonable to believe that their application would be more widely distributed among the small-scale artisanal producers too.
The majority of starter cultures applied nowadays contain lactic acid bacteria (LAB), due to their recognized key role in food fermentation, positive influence on food sensorial properties, and a long history of safe use (Oliveira et al. 2018). However, not all LAB are equally efficient in all sausage types (Palavecino Prpich et al. 2015) and compared to commercial starter cultures, indigenous strains isolated from traditional fermented products often express higher metabolic activity (Frece et al. 2014; Palavecino Prpich et al. 2015).
However, to be considered as a part of the starter culture, native strains should fulfil many requirements. As such, besides proven antimicrobial and technological properties, it is essential that they show no pathogenic or toxic activities and with their application, the typical sensory properties of traditional sausages need to be preserved (Ammor and Mayo 2007). Only one strain can rarely fulfil all those requirements and therefore, multiple strains application is often much more preferable for expressing the complementary beneficial features in a food matrix. However, the capacity of starter cultures to undertake the metabolic activities in sausages is based primarily on its ability to multiply inside the meat product and to compete with the natural microbiota, despite in vitro potential and high performance under laboratory conditions (Oliveira et al. 2018).
Against this background, wild-type LAB isolated from game meat sausages were investigated for their potential use as native starter cultures, which led to the design the multiple strain starters comprising of two indigenous Lactobacillus sakei and one Leuconostoc mesenteroides strains. Although the application of other strains of Lb. sakei was reviewed (Zagorec and Champomier-Vergès 2017), the use of Leu. mesenteroides for dry fermented sausages production was not explored, even though they represent an important part of the microbiota of artisanal dry fermented sausages. For example, they were often isolated from artisanal sausages containing up to 2.5% of salt (Danilović et al. 2011; Žgomba Maksimović et al. 2018) and particular strains have contributed to the overall sensorial acceptability of sausages (Kos et al. 2019).
As known from the literature, heterofermentative LAB are often displaced in the process of microbial succession in fermented food (García et al. 2019) and additional solutions are needed to ensure their viability throughout the manufacturing process. As such, to protect the cells against the harsh conditions during fermentation and in the presence of competitive microbiota, the selected strains were encapsulated prior the application, using the ionic gelation method with alginate as a carrier (Kavitake et al. 2018).
Therefore, to investigate the feasibility of selected multiple starter cultures for the production of nitrite-free fermented game meat sausages, this study evaluated the effect of a formulation of lactic acid bacteria (LAB) on the microbiological, physicochemical, and sensory characteristics of game meat sausages. Special focus was set against the evaluation of survival rate and performance of encapsulated and non-encapsulated selected starter cultures in game meat sausages during the ripening period of 40 days at a local small-scale facility under artisanal conditions. It was hypothesized that (1) the applied native starter cultures will survive during the fermentation and ripening process, and encapsulated cultures will have a higher survival capacity, (2) there will be an inhibition of growth of undesirable microbiota and a positive effect of native starter cultures on the physicochemical properties of sausages, and (3) the typical sensory quality of traditional sausages will be preserved.
Materials and methods
Origin of LAB strains, biomass production and encapsulation
Based on previously established results from the safety and technological traits (Mrkonjic Fuka et al. 2020), two Lb. sakei and one Leu. mesenteroides strains that passed safety evaluation (were free of biogenic amine genes, sensitive to all tested antibiotics and non-hemolytic) and showed promising technological traits (acidification, proteolytic, lipolytic and antagonistic activity) were selected to be applied as native starter cultures (Table 1), alone or in combination. The association of all three strains to designed starter culture was determined using a compatibility test performed by streaking out the strains on the Man–Rogosa–Sharpe agar (MRS) 0.5 cm apart and observing any potential inhibitory effect.
Table 1.
Safety, technological and antagonistic properties of LAB strains applied as native starter cultures
| Genotype | Antibiotic resistance1 | Biogenic amine genes2 | Acidification3 | Proteolytic activity4 | Lypolitic activity5 | Antimicrobial activity6 | ||
|---|---|---|---|---|---|---|---|---|
| ΔpH after 24 h | ΔpH after 7 d | Casein | Sarcoplasmic proteins | |||||
| Lb. sakei | ||||||||
| LS0713 | – | – | 0.46 | 2.27 | – | – | + + + | Sa/W/Bc |
| LS0296 | – | – | 1.92 | 2.24 | + | – | – | Sa/St/Bc |
| Le. mesenteroides | ||||||||
| LM0532 | – | – | 2.06 | 2.43 | – | – | + + | Ec/St |
1Antibiotic resistance was tested according to EFSA where lactobacilli and leuconostocs were tested against ampicillin (2 µg—Amp), gentamicin (10 µg—Gen), erythromycin (2 µg—Ery), tetracycline (5 µg—Tet), kanamycin (30 µg—Kan), clindamycin (2 µg—Cli) and chloramphenicol (30 µg—Chl)
2Biogenic amine genes were detected by PCR assays for histamine (hdc), putrescine (odc), tyramine (tdc) and cadaverine (ldc)
3Acidification is shown as a pH change (ΔpH). pH change (ΔpH) was calculated according to the formula: ΔpH = pH1 − pH2, where pH1 is the pH of lyophilized pork meat media and pH2 is the mean pH value of lyophilized pork meat media measured 7 or 24 h after the inoculation of selected strains
4Proteolytic activity was tested on skim milk agar and media enriched with sarcoplasmic proteins. Proteolytic activity was detected as a clear zone around colonies. The diameter of the clear zone was measured and expressed as mean value in mm and compared to the proteolytic activity of Pseudomonas fluorescens WCS 417r
5Lypolitic activity was detected as a clear zone around colonies grown on tributyrin agar. The diameter of the clear zone was measured and expressed as a mean value in mm and compared to the lipolytic activity of Pseudomonas fluorescens WCS 417r
6Antimicrobial activity was tested against selected indicator bacteria: Sa—Salmonella enterica subsp. enterica (DSM 14221); L—Listeria innocua (ATCC 33090); Ec—Escherichia coli (ATCC 25922); St—Staphylococcus aureus subsp. aureus (DSM 20231); Bt—Brochotrix termospachta (LMG 17208); W—Weissella viridescens (DSM 20410); Bc—Bacillus cereus (DSM 6791)
To obtain the appropriate biomass of starter cultures, the strains were inoculated in MRS broth (100 mL) and incubated at 30 °C for 24 h. Cultures were harvested by centrifugation at 8000×g for 5 min and cell pellets were then resuspended in 100 mL of sterile skim milk solution (1.5%) and were added directly into meat batter or as encapsulated cells as described below.
Microspheres loaded with calcium ions and the selected starter cultures were prepared using the ionic gelation technique (Vinceković et al. 2016). Low viscosity sodium alginate (Sigma Aldrich, USA) and starter culture were homogenized and then dropped into a 1 mol dm−3 solution of CaCl2 (Kemika, Croatia) using a Büchi-B390 encapsulator (BÜCHI Labortechnik AG, Switzerland). The concentration of sodium alginate was 1.5% while the microsphere diameter was determined by using a nozzle size of 200 µm and the vibration frequency of 1100 Hz at a pressure of 160 mbar, respectively. Before being added to the meat dough the microcapsules were resuspended in 100 mL of sterile skim milk solution (1.5%). To determine the number of bacterial cells that survived the process of microencapsulation, encapsulated bacteria were released by adding 0.06 M sodium citrate and 0.2 M sodium bicarbonate buffer. The encapsulation yield was calculated as described previously (Khosravi Zanjani et al. 2014).
Formulation and sampling of sausages
In this study, eight treatments were prepared in three batches (n = 24), each batch with 5 kg of meat dough. All treatments were made from a mixture of domestic pig (Sus scrofa domesticus L.) meat (60%) and wild boar (Sus scrofa L.) meat (40%) and the following ingredients were added, salt (1.9%), red chilli peppers (0.50%), garlic (0.3%), red sweet peppers (0.2%), sugar (0.2%), and black peppers (0.1%). Spices were added as dried and grounded. Selected native starter cultures were applied resuspended in sterile skim milk solution either in encapsulated or non-encapsulated form as described in Table 2. Treatment B4 was used as a non-inoculated control (no starter cultures were added), and treatment B5 was used as a control with the addition of commercial starter Bitec LS-25 (Gewürzmüller, Germany) comprised of Lb. sakei and Staphylococcus carnosus. Meat dough was filled in natural casings (pig’s small intestine) with a 38 mm diameter, and the sausages were randomly stored in a drying chamber for fermentation/ripening for 40 days, with four smoking treatments under traditional conditions. Temperature and relative humidity were monitored every 30 min using data-logger LOG 32 TH (Dostmann electronic, Germany). Samples (n = 72) were taken as followed: at 0, 7, and 40 days for pH and water activity measurements as well as for complete microbiological analysis, and final products were tested for aroma volatile compounds, biogenic amine and sensory properties.
Table 2.
Formulations of starter cultures in eight treatments, with their survival (recovery) rates
| Form of application | Treatment | Identification (genotype code) | Survival rate (%) |
|---|---|---|---|
| Bacterial pellets resuspended in sterile skimmed milk solution | B1 | Lactobacillus sakei (LS0713 + LS0296) | 84.24 |
| B2 | Leuconostoc mesenteroides (LM0532) | 65.11 | |
| B3 | Lactobacillus sakei (LS0713 + LS0296) | 54.54 | |
| Leuconostoc mesenteroides (LM0532) | 44.18 | ||
| Control (no starter culture added) | B4 | n.a. | n.a. |
| Control (lyophilized commercial starter cultures resuspended in sterile distilled water) | B5 | Lactobacillus sakei, Staphylococcus carnosus | n.a. |
| Microencapsulated bacterial pellets resuspended in sterile skimmed milk solution | B6 | Lactobacillus sakei (LS0713 + LS0296) | 64.44 |
| B7 | Leuconostoc mesenteroides (LM0532) | 80.00 | |
| B8 | Lactobacillus sakei (LS0713 + LS0296) | 38.30 | |
| Leuconostoc mesenteroides (LM0532) | 44.69 |
Survival rates are expressed as a percentage of isolates recovered from each treatment with the rep-PCR patterns corresponding to the rep-PCR patterns of the inoculated starter culture(s)
n.a. not applicable
Water activity, pH and microbiological analysis
Water activity (aw) was determined using a portable analyzer, HygroPalm HP23-AW-A equipped with an HC2-AW probe (Rotronic AG, Switzerland) and pH values were measured using a portable pH-meter IQ 150 (IQ Scientific Instruments, USA).
For microbiological analysis, 25 g of sausages were taken from the central part of each sausage and were homogenized in a sterile saline solution (0.85%) using a Stomacher Lab-Blender 400 (Seward Medical, UK). The appropriate decimal dilutions were spread on different selective agar media. Enterobacteriaceae and E. coli (ISO 21528-2 2004) and coliforms (ISO 4832 2006) were determined. The presence of S. aureus was determined on Baird-Parker agar (Labo-Life Sàrl, Switzerland) supplemented with a 20% egg yolk tellurite emulsion (VWR International, Switzerland) after incubation at 37 °C for 48 h. Salmonella spp. and Listeria monocytogenes were detected according to ISO 11290-1:1996/Amd 1 (2004) and ISO 6579:2002/Amd 1 (2007), respectively. The abundance of yeasts and moulds was monitored according to ISO 21527-2 (2008) and enterococci were detected on kanamycin esculin azide agar (Biolife, Italy) after incubation at 37 °C for 48 h. Additionally, Lactobacillus spp. and Leuconostoc spp. were isolated on LamVab medium (Hartemink et al. 1997) under anaerobic conditions after 72 h at 30 °C. Approximately 15 colonies from the LamVab medium were randomly selected from one treatment and purified from each sampled time point (0, 7, and 40 days).
Fingerprinting of isolates
All collected LamVab isolates (n = 355) were screened using Gram-staining and genotyped by rep–PCR with (GTG)5 primer as described previously (Domig et al. 2014). Extraction of the template DNA for the PCR reactions was performed with the Wizard Genomic DNA Purification Kit (Promega, USA) according to the manufacturer’s instructions. The rep-PCR patterns were analyzed using the software BioNumerics version 7.6.1 (Applied Maths, Belgium) as described by Žgomba Maksimović et al. (2018) and the representatives of each cluster group were identified by 16S rRNA gene sequencing (Macrogen, Netherlands) using a previously established primer set and PCR protocol (Di Cello et al. 1997). The all obtained sequences were aligned and compared with the sequences deposited in the GenBank database using the BLAST algorithm (http://www.ncbi.nlm.nih.gov/BLAST/) and were deposited in the GenBank database under the accession numbers MH197045-197067 and MH231452-231454.
Histamine and tyramine content
The histamine and tyramine content were determined by high-performance liquid chromatography (HPLC, 1100 Agilent Technologies, USA). Extraction and derivatization of biogenic amines from the sausages were performed as described previously (Eerola et al. 1993) as too was the HPLC analysis (Žgomba Maksimović et al. 2018).
Analysis of volatile compounds
Extraction of volatile compounds was performed by headspace solid-phase microextraction (SPME), using a fused silica fiber (20 mm in length) coated with a 50/30 μm DVB/Carboxen/PDMS (Supelco, USA). Samples (5 g) were homogenized in distilled water saturated with NaCl (25 mL) using the Ultra-turrax (IKA, Germany). Following this, 10 mL of the mixture was placed in a 20 mL vial and 100 μL of 4-methyl-2-pentanol (1.2 mg/kg) was added as an internal standard. The vial was tightly capped with a PTFE septum. The vial was placed in a thermoblock at 40 °C, the SPME fiber was then exposed to the headspace for 180 min. After extraction, the SPME fiber was injected into the 6890 N gas chromatograph coupled to a 5975i mass selective detector (Agilent Technologies, USA). A capillary column DB-5 ms 30 m × 0.25 mm and a film thickness of 0.25 μm (Agilent Technologies, USA) were used, with helium as a carrier gas at a flow rate of 1.0 mL/min. The temperature program and mass spectrometry parameters were carried out as previously described (Petričević et al. 2018). An in-house mixture of C8–C20 n-alkanes was run under the same chromatographic conditions to calculate the retention indices of each of the detected compounds. The software, AMDIS 3.2 version 2.62 was used for identification of components using the NIST 2005 version 2.0 spectral library (NIST, USA), in addition to a comparison being made between the obtained retention indices and noted literature values (Adams 2007).
Sensory properties
To evaluate the influence of the native starter cultures on the final sensorial characteristics of the product but to avoid overloading of consumers, only the sausages made with non-encapsulated strains were subjected to a consumer evaluation. Sensory analysis was performed by a non-trained panel of consumers comprised of 80 participants from both genders (47.3% male and 52.7% female), with ages ranging from 18 to 64 years old. A 9-point structured scale was used where 1 meant “extremely disliked” and 9 meant “extremely liked”. Six sensory traits based on likeability were evaluated; cross-section, odour, taste, hardness, aroma, and overall. In total, five samples coded with three-digit numbers were given to each subject and presented in a completely balanced block design. Samples were cut into a thickness of 2 mm using a knife at an angle of 45°. Subjects were placed in separate booths and were instructed to use tap water and unsalted bread as palate cleansers before every sample.
Statistical analysis
The data obtained were analyzed by the SAS Studio University Edition 3.4 (SAS Institute 2015). The analysis of the viable cell counts, pH, aw, tyramine, and volatile compounds was performed using one-way ANOVA with treatment as a fixed factor and batch as a random effect. Sensory data were analyzed using mixed model repeated measures ANOVA with treatment as a fixed effect, and judge and batch as a random effect. Tukey test was used as post hoc test when the main effects/interactions analyzed were significant at P < 0.05. The CORR procedure was used for the correlation analysis and the results were expressed as a Pearson correlation coefficient (r).
Results and discussion
Biomass production and encapsulation yield
To ensure that a starter culture is effective, appropriate bacterial biomass should be produced and applied to the meat dough. The level of culture to be added depends on the product specifications, but a high viable number of cells is generally used, ranging from 5 to 9 log CFU g−1 (Oliveira et al. 2018). In this study, the viable cell counts of the non-encapsulated starters were 9.30, 9.48 and 9.63 log CFU mL−1 for B1, B3 and B2 and of encapsulated one 7.50, 8.62 and 9.38 log CFU mL−1 for B6, B8 and B7, accounting for 80.05 (B6), 90.92 (B8) and 97.48% (B7) encapsulation efficiency respectively. Furthermore, based on the compatibility test, no suppression of growth was noticed among the three selected strains when grown together (data not shown).
The survival rate of inoculated starter cultures
The ability of the starter culture to compete with the natural microbiota and to undertake the metabolic activities to improve the nutritional and microbiological quality of meat products is based on its ability to survive and multiply inside the meat product (Oliveira et al. 2018). In non-inoculated control sausages, none of three applied strains was detected. The control sausages were characterized by the highest diversity on the strain level, with a total of 27 different clusters (Supplementary Figure S1b) whereas the inoculated batches showed a reduced intraspecies diversity, especially treatment B1 (Supplementary Figure S1a) where only two clusters and domination of inoculated Lb. sakei strains were observed. Based on the rep-PCR analysis, the indigenous starter cultures had survived the whole production process, however, to varying degrees (Table 1). The highest survival rate was noted for the non-encapsulated Lb. sakei strains (B1). Despite encapsulation being superior in prolonging the viability of Leu. mesenteroides, the effect was not noticed for the treatments where Lb. sakei were used alone or in combination with Leu. mesenteroides (Table 1). However, the encapsulating biopolymers can act as a physical barrier limiting mass transfer between the bead core and the external environment (García et al. 2019; Heidebach et al. 2012; Kavitake et al. 2018). The mass transfer resistance depends on the morphology and size of the capsule, the microorganisms involved and even the fermentation medium (De Prisco et al. 2017). Moreover, the differences noticed in our study could be also related to the fact that these bacteria react differently (via the production of different metabolites) in the limited space which may lead to the mechanical stresses on the matrix and cause cell leakage from the calcium alginate microspheres into the surrounding media. To prevent, or to enable higher survivability of cells (cell leakage), lower density alginate could be used (Abd El-Salam and El-Shibiny 2015).
Microbial evolution, physicochemical properties, and biogenic amine content
At day 0, the number of LAB was between 3.78 ± 0.26 (control) and 7.10 ± 0.03 log CFU g−1 (B3) (Table 3). At day 7, the number of LAB was found to be above 8.90 log CFU g−1 for all treatments with non-encapsulated starters as well as for the treatment with the commercial starter culture (B5). However, significantly lower levels of LAB were detected in the control treatment (B4) (7.82 ± 0.01 log CFU g−1) as well as treatments with encapsulated starters (7.86 ± 0.03, 8.34 ± 0.09, and 8.09 ± 0.03 log CFU g−1, respectively) (P < 0.05). The number of LAB remained stable from day 7 till the end of production for all treatments except 1 and 2, where it was found to be reduced by up to 9.5% (P < 0.05).
Table 3.
Microbiological analysis, aw and pH values of sausages produced by the application of native starter cultures
| Treatments | Days | Selective microbiological media (log CFU g−1) | Water activity (aw) | pH | |||||
|---|---|---|---|---|---|---|---|---|---|
| LamVab (lactobacilli and Leuconostoc | KAA (enterococci) | VRBG (Enterobacte-riaceae) | DRBC (yeasts) | CCA (E. coli) | CCA (coliforms) | ||||
| B1 | 0 | 6.93 ± 0.04c | 4.33 ± 0.05 | 3.95 ± 0.08a | 3.12 ± 0.03b | 3.77 ± 0.07a | 4.15 ± 0.04a | 0.96 ± 0.00a | 5.58 ± 0.01a |
| 7 | 9.16 ± 0.16a | 4.21 ± 0.04 | 3.39 ± 0.07b | < 1.00c | 0.69 ± 0.69b | 2.19 ± 0.01b | 0.95 ± 0.00a | 5.07 ± 0.01b | |
| 40 | 8.46 ± 0.11b | 3.22 ± 0.01 | 2.07 ± 0.07c | 5.32 ± 0.00a | < 1.00b | < 1.00c | 0.88 ± 0.01b | 5.11 ± 0.02b | |
| B2 | 0 | 7.06 ± 0.02c | 4.37 ± 0.01 | 4.27 ± 0.15a | 3.70 ± 0.01b | 3.67 ± 0.04a | 4.38 ± 0.01a | 0.96 ± 0.00a | 5.56 ± 0.01a |
| 7 | 9.05 ± 0.13a | 4.16 ± 0.01 | 3.25 ± 0.01b | 3.37 ± 0.05b | 2.34 ± 0.07b | 3.05 ± 0.01b | 0.95 ± 0.00a | 5.22 ± 0.01c | |
| 40 | 8.19 ± 0.07b | 3.46 ± 0.03 | 2.48 ± 0.03c | 4.72 ± 0.09a | < 1.00c | 1.51 ± 0.76c | 0.88 ± 0.01b | 5.31 ± 0.02b | |
| B3 | 0 | 7.10 ± 0.03b | 4.25 ± 0.03a | 4.17 ± 0.01a | 4.20 ± 0.08b | 3.63 ± 0.03a | 4.14 ± 0.02a | 0.97 ± 0.00a | 5.55 ± 0.02a |
| 7 | 8.91 ± 0.12a | 4.16 ± 0.04a | 3.24 ± 0.06b | 3.24 ± 0.06c | < 1.00b | 2.41 ± 0.05b | 0.95 ± 0.00b | 5.14 ± 0.00b | |
| 40 | 8.47 ± 0.08a | 2.10 ± 1.05b | 2.15 ± 0.02c | 5.45 ± 0.01a | < 1.00b | < 1.00c | 0.88 ± 0.01c | 5.15 ± 0.02b | |
| B4 | 0 | 3.78 ± 0.26b | 4.17 ± 0.04 | 4.77 ± 0.12a | 4.22 ± 0.05b | 3.53 ± 0.22a | 3.91 ± 0.12a | 0.96 ± 0.00a | 5.59 ± 0.02a |
| 7 | 7.82 ± 0.01a | 4.26 ± 0.03 | 3.97 ± 0.02b | 3.29 ± 0.02c | 2.05 ± 0.03b | 2.91 ± 0.03b | 0.95 ± 0.00a | 5.50 ± 0.01a,b | |
| 40 | 7.75 ± 0.12a | 3.68 ± 0.02 | 2.68 ± 0.02c | 5.25 ± 0.06a | < 1.00c | 1.62 ± 0.81c | 0.87 ± 0.01b | 5.43 ± 0.02b | |
| B5 | 0 | 5.86 ± 0.05b | 4.25 ± 0.03a | 4.56 ± 0.09a | 4.42 ± 0.04b | 3.52 ± 0.08a | 4.42 ± 0.01a | 0.96 ± 0.00a | 5.58 ± 0.01a |
| 7 | 9.07 ± 0.08a | 4.19 ± 0.02a | < 1.00b | 3.03 ± 0.03c | < 1.00b | < 1.00b | 0.95 ± 0.00b | 5.00 ± 0.00b | |
| 40 | 9.12 ± 0.04a | < 1.00b | < 1.00b | 5.40 ± 0.07a | < 1.00b | < 1.00b | 0.88 ± 0.01c | 5.03 ± 0.02b | |
| B6 | 0 | 4.12 ± 0.03b | 4.28 ± 0.06 | 4.50 ± 0.15a | 4.47 ± 0.15a | 3.73 ± 0.14a | 4.18 ± 0.09a | 0.96 ± 0.00a | 5.57 ± 0.01 |
| 7 | 7.86 ± 0.03a | 4.22 ± 0.02 | 3.81 ± 0.01b | 3.31 ± 0.07b | < 1.00b | 2.82 ± 0.01b | 0.95 ± 0.00b | 5.50 ± 0.00 | |
| 40 | 7.94 ± 0.04a | 3.74 ± 0.03 | 2.74 ± 0.06c | 4.75 ± 0.03a | < 1.00b | 2.45 ± 0.05b | 0.88 ± 0.01c | 5.48 ± 0.02 | |
| B7 | 0 | 6.07 ± 0.03b | 4.19 ± 0.05 | 4.36 ± 0.11a | 4.34 ± 0.02b | 3.35 ± 0.10a | 4.34 ± 0.01a | 0.96 ± 0.00a | 5.56 ± 0.01a |
| 7 | 8.34 ± 0.09a | 4.19 ± 0.04 | 3.34 ± 0.03b | 3.25 ± 0.14c | < 1.00b | 3.04 ± 0.02b | 0.95 ± 0.00b | 5.36 ± 0.02b | |
| 40 | 8.00 ± 0.11a | 3.43 ± 0.11 | 2.66 ± 0.02c | 4.79 ± 0.01a | < 1.00b | < 1.00c | 0.89 ± 0.01c | 5.43 ± 0.02b | |
| B8 | 0 | 6.19 ± 0.07b | 4.30 ± 0.07 | 4.23 ± 0.10a | 4.16 ± 0.07b | 3.46 ± 0.04a | 4.03 ± 0.01a | 0.96 ± 0.00a | 5.57 ± 0.02a |
| 7 | 8.09 ± 0.03a | 4.16 ± 0.04 | < 1.00c | 3.14 ± 0.05c | < 1.00b | 2.39 ± 0.02b | 0.95 ± 0.00a | 5.21 ± 0.01b | |
| 40 | 8.48 ± 0.09a | 3.44 ± 0.10 | 2.52 ± 0.02b | 5.15 ± 0.08a | < 1.00b | < 1.00c | 0.89 ± 0.01b | 5.49 ± 0.03a | |
Data were expressed as mean ± SE
LamVab—Lactobacillus Anaerobic MRS (de Man, Ragosa and Sharpe Agar) with Vancomycin and Bromocresol Green (Hartemink et al. 1997). KAA = Kanamycin Aesculin Azide Agar. VRBG = Violet Red Bile Glucose Agar. DRBC = Dichloran Rose Bengal Chloramphenicol Agar. CCA = Chromogenic Coliform Agar. B1 and B6 = Lb. sakei; B2 and B7 = Le. mesenteroides; B3 and B8 = mixture of Lb. sakei and Le. mesenteroides; B4 = un-inoculated control; B5 = commercial starter cultures. B1–B3 non-encapsulated; B6–B8 encapsulated in sodium-alginate
a,b,cWithin a treatment, means without a common subscript differ (Tukey honest Test, at P ≤ 0.05)
Although the initial number of E. coli reached the value above 3.35 ± 0.10 log CFU g−1, E. coli was vanished or significantly reduced in the first week of fermentation and it was not present in all final products. Enterobacteriaceae and coliforms, initially present in amounts higher than 3.95 ± 0.08 log CFU g−1, were also affected by the acidification (r = 0.77 and r = 0.69, respectively). In the final products, coliforms were not detected in treatments B1, B3, B5, and B8 and Enterobacteriaceae were not present in B1 and B5; however, they were detected in all other sausages at a level higher than 2.00 log CFU g−1 which is a limit value that is considered satisfactory (HPA 2009).
Enterococci and yeasts were present as background microbiota throughout the whole production process. Despite the number of enterococci being positively correlated with the pH values (r = 0.48) and significantly decreasing towards the end of the production, they were still present in all final products (except B5) with values above 2.10 ± 1.05 log CFU g−1. S. aureus and Salmonella spp. were not present in any of the treatments at any time point, however, L. monocytogenes was detected in all treatments throughout the production process, except in B1 with non-encapsulated Lb. sakei where it was absent at day 7 and not detected in the final products.
No significant differences were found between treatments at any sampling point for aw (P > 0.05). At the beginning of production, aw was 0.96 ± 0.00 in all 8 treatments, which slightly declined after 7 days of production (0.95 ± 0.00) and reached between 0.87 ± 0.01 and 0.89 ± 0.01 in the final products (Table 3).
Even though the manufacturing process regarding temperature and relative humidity was not favourable or typical for fermented sausage production, there was a clear and significant pH drop, leading to the conclusion that starters used are capable of fermenting even in non-optimal artisanal production conditions. The decrease of pH was significantly correlated (r = − 0.75) to the increase of the viable LAB count (Table 3) and decrease of spoilage and pathogenic microbiota. The drop of the pH values at day 7 was found to be statistically significant for all treatments, except for the control B4 and the encapsulated B6. The lower cell counts of lactobacilli (P < 0.05) observed in these treatments at the initial stages of production could explain the slower acidification rates observed. This is supported by the opposite being seen in B1 and B5, where the highest number of LAB and the lowest pH values were detected (P < 0.05).
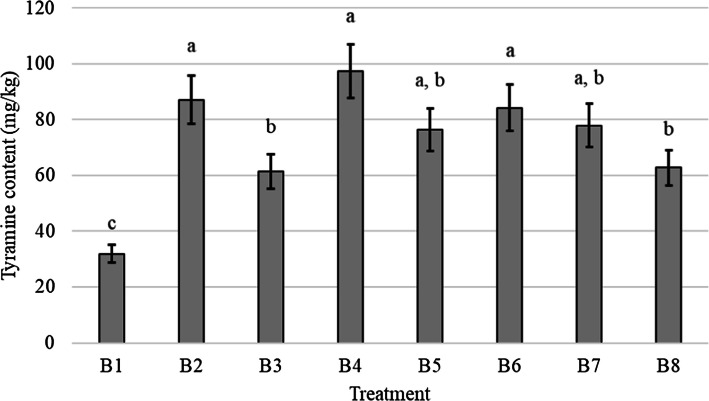
In the current study, the histamine content was found to be less than 5 mg kg−1 in all ripened sausages. However, the tyramine concentration varied between the different treatments, from 31.90 ± 4.50 (B1) to 97.20 ± 13.60 (B4). The tyramine concentration was significantly lower in batches inoculated with the indigenous decarboxylase negative, Lb. sakei strains (P < 0.05) and the highest concentration of tyramine was found in the non-inoculated control sausages (Fig. 1).
Fig. 1.
Tyramine concentration of eight game meat sausage treatments (error bars represent standard error). Treatments without a common subscript significantly differ at P ≤ 0.05. B1 and B6 = Lb. sakei; B2 and B7 = Le. mesenteroides; B3 and B8 = mixture of Lb. sakei and Le. mesenteroides; B4 = un-inoculated control; B5 = commercial starter cultures. B1-B3 non-encapsulated; B6-B8 encapsulated in sodium-alginate
Metabolite profile and sensorial quality
A total of 84 volatile compounds were identified by SPME–GC–MS (Table S1) in the 8 treatment batches of game meat sausages, with only minor differences seen between the treatments. The volatile profiles were dominated by smoke-derived volatile phenols (23.37–40.98%) which were found to be crucial compounds for the unique aroma and taste of many smoked meat products (Petričević et al. 2018). Samples with the highest content of phenols were from the non-inoculated control batches; while the samples derived from B1 and B3 had the lowest phenol content (P < 0.05). The differences in the volatile phenol profiles were mainly related to 4-methyl phenol, which was detected in most of the samples but was present in quite a large quantity in samples taken from the control treatment. Spice-derived terpenes formed the second largest group (22.15–29.13%) and they were detected in comparable amounts in all sausages (P > 0.05). Additionally, aldehydes were in a similar range in all samples (13.49–22.67%). However, larger quantities of the pentanal and hexanal forms of the above compounds were found in samples from B1 (P < 0.05). High concentrations of this volatiles usually signal flavour deterioration in meat products and consequently leads to lower consumer acceptability scores (Pham et al. 2008); however, in the current study, this was not the case.
The volatile compounds that are generated as reaction products of lipid autoxidation and microbiological metabolism (alcohols and ketones) were similar in all treatments which are possibly due to the antioxidant and antimicrobial effects of the smoke and spices (Lorenzo et al. 2013). A few sulfur compounds, aliphatic hydrocarbons, acids, and nitrogen compounds were also detected in comparable quantities in all samples. Finally, esters were found to be the least abundant of all the aroma compounds. Since esters mainly originate from bacterial metabolism, such results indicate a limited microbial impact on the volatile flavour formation in the analyzed sausages (Olivares et al. 2015). However, although, in comparison to other samples, esters were present in larger quantities in control B4, it was not relevant, due to the overall low esters level (0.10–1.03%) in all samples.
The sensory evaluation of the sausages inoculated with the non-encapsulated strains is presented in Fig. 2. No significant differences in sensory traits between tested treatments were found, although B1 with the Lb. sakei strains received the highest scores for the sensory traits including cross-section, odour, hardness, aroma, and overall acceptability. Additionally, frequency distribution was calculated as the percentage of the sum of the scores 7, 8, and 9 (the upper third), which were assigned to the highly expressed likeability of traits. The average score percentage in the upper third of all traits was the highest in B1 (66.67%), followed by B3 (63.33%), B2 (63.13%), and B5 (59.17%) while the non-inoculated control B4 had the lowest score percentage (57.50%). Due to the low presence of aromatic compounds of microbial origin in this study, the effect of starter cultures on the sensory traits and volatile compound profiles was of low importance, often also reported by others (Elias et al. 2014; Frece et al. 2014; Lorenzo et al. 2016). Despite the differences in the frequency distribution and the overall better score of B1 with the Lb. sakei starters, it appears that likeability of sausages was not significantly affected by the addition of native starter cultures and the same can be concluded for the volatile compounds profile. However, this was found beneficial, because expected sensory traits were not degraded by the addition of the native starter cultures in this study.
Fig. 2.
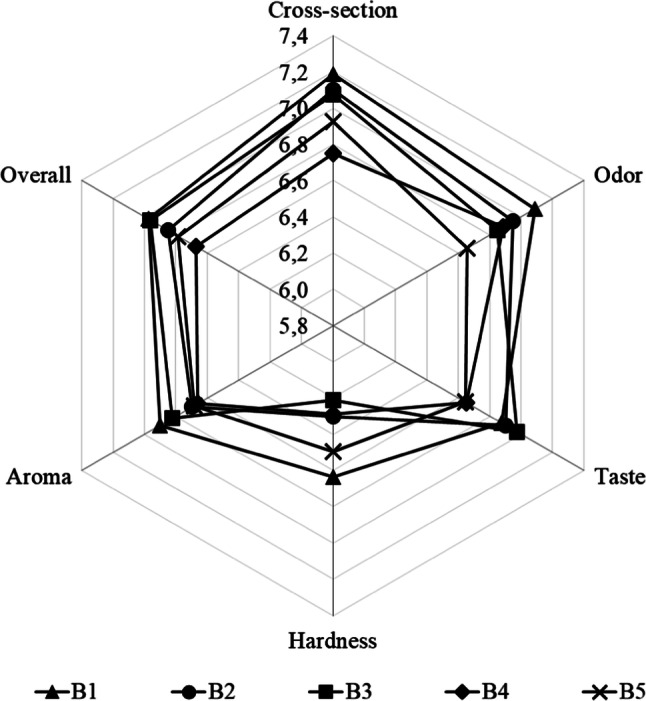
Consumer likeability scores of game meat sausages produced by the application of non-encapsulated native starter cultures. B1 = Lb. sakei; B2 = Le. mesenteroides; B3 = mixture of Lb. sakei and Le. mesenteroides; B4 = un-inoculated control; B5 = commercial starter cultures
In conclusion, two indigenous Lb. sakei strains were found to be superior in suppressing the growth of undesirable microbiota in our study, and in reducing the content of tyramine while preserving or even improving the typical sensory quality of traditional sausages. Despite the manufacturing process under artisanal conditions was not favourable for fermented sausage production, used multiple starter cultures were capable of acidification even in non-optimal production conditions which make them good candidates for industrial as well as artisanal application. The clear and positive effect of encapsulation on bacterial viability and sausage quality was not observed. Even though encapsulation prolonged the viability of Leu. mesenteroides, the effect was not noticed for the treatments where Lb. sakei were used alone or in combination with Leu. mesenteroides. Encapsulation is therefore not recommended for the strains applied in our study as neither, the viability of applied multiple starter cultures or quality of sausages was improved in comparison to non-encapsulated counterpart. However, encapsulation could still be an excellent tool to protect the cells if their survival and viability in complex food matrix are jeopardized. In particular, for application of functional starter cultures with proven probiotic potential, the combination of non-encapsulated and encapsulated bacterial cultures could be effective in order to improve the hygienic and sensory properties of fermented sausages, but also to deliver probiotics in final products.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We thank Melita Bačić and Mateja Pećina for technical assistance with samples manipulation.
Funding
This work was supported by the Croatian Science Foundation (miCROgame UIP-11-20136640).
Compliance with ethical standards
Conflict of interest
The authors declare that there is no conflict of interest regarding the publication of this paper.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Abd El-Salam MH, El-Shibiny S. Preparation and properties of milk proteins-based encapsulated probiotics: a review. Dairy Sci Techol. 2015;95:393–412. doi: 10.1007/s13594-015-0223-8. [DOI] [Google Scholar]
- Adams RP. Identification of essential oil components by gas chromatography/mass spectrometry. 4. Carol Stream: Allured Publishing Corporation; 2007. [Google Scholar]
- Ammor MS, Mayo B. Selection criteria for lactic acid bacteria to be used as functional starter cultures in dry sausage production: an update. Meat Sci. 2007;76:138–146. doi: 10.1016/j.meatsci.2006.10.022. [DOI] [PubMed] [Google Scholar]
- Danilović B, Joković N, Petrović L, Veljović K, Tolinački M, Savić D. The characterisation of lactic acid bacteria during the fermentation of an artisan Serbian sausage (Petrovská Klobása) Meat Sci. 2011;88:668–674. doi: 10.1016/j.meatsci.2011.02.026. [DOI] [PubMed] [Google Scholar]
- De Prisco A, van Valenberg HJ, Fogliano V, Mauriello G. Microencapsulated starter culture during yoghurt manufacturing, effect on technological features. Food Bioproc Technol. 2017;10:1767–1777. doi: 10.1007/s11947-017-1946-8. [DOI] [Google Scholar]
- Di Cello F, Bevivino A, Chiarini L, Fani R, Paffetti D, Tabacchioni S, Dalmastri C. Biodiversity of a Burkholderia cepacia population isolated from the maize rhizosphere at different plant growth stages. Appl Environ Microb. 1997;63:4485–4493. doi: 10.1128/AEM.63.11.4485-4493.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domig KJ, Kiss H, Petricevic L, Viernstein H, Unger F, Kneifel W. Strategies for the evaluation and selection of potential vaginal probiotics from human sources: an exemplary study. Benef Microbes. 2014;5:263–272. doi: 10.3920/BM2013.0069. [DOI] [PubMed] [Google Scholar]
- Eerola S, Hinkkanen R, Lindfors E, Hirvi T. Liquid chromatographic determination of biogenic amines in dry sausages. J AOAC Int. 1993;76:575–577. doi: 10.1093/jaoac/76.3.575. [DOI] [PubMed] [Google Scholar]
- Elias M, Potes ME, Roseiro LC, Santos C, Gomes A, Agulheiro-Santos AC. The effect of starter cultures on the portuguese traditional sausage “Paio do Alentejo” in terms of its sensory and textural characteristics and polycyclic aromatic hydrocarbons profile. J Food Res. 2014;3:45–56. doi: 10.5539/jfr.v3n3p45. [DOI] [Google Scholar]
- Frece J, Kovačević D, Kazazić S, Mrvcić J, Vahčić N, Ježek D, Hruškar M, Babić I, Markov K. Comparison of sensory properties, shelf-life and microbiological safety of industrial sausages produced with autochthonous and commercial starter cultures. Food Technol Biotechnol. 2014;52:307–316. doi: 10.17113/ftb.52.04.14.3794. [DOI] [Google Scholar]
- García C, Rendueles M, Díaz M. Liquid-phase food fermentations with microbial consortia involving lactic acid bacteria: a review. Food Res Int. 2019;119:207–220. doi: 10.1016/j.foodres.2019.01.043. [DOI] [PubMed] [Google Scholar]
- Hartemink R, Domenech VR, Rombouts FM. LAMVAB—A new selective medium for the isolation of lactobacilli from faeces. J Microbiol Methods. 1997;29:77–84. doi: 10.1016/S0167-7012(97)00025-0. [DOI] [Google Scholar]
- Heidebach T, Först P, Kulozik U. Microencapsulation of probiotic cells for food applications. Crit Rev Food Sci Nutr. 2012;52:291–311. doi: 10.1080/10408398.2010.499801. [DOI] [PubMed] [Google Scholar]
- HPA . Guidelines for assessing the microbiological safety of ready-to-eat foods placed on the market. London: Health Protection Agency; 2009. [Google Scholar]
- ISO 11290-1:1996/Amd 1 (2004) Microbiology of food and animal feeding stuffs—horizontal method for the detection and enumeration of Listeria monocytogenes—part 1: detection method—amendment 1: modification of the isolation media and the haemolysis test, and inclusion of precision data. International Organization for Standardization, Geneva
- ISO 6579:2002/Amd 1 (2007) Microbiology of food and animal feeding stuffs—horizontal method for the detection of Salmonella spp.—Amendment 1: annex D: detection of Salmonella spp. in animal faeces and in environmental samples from the primary production stage. International Organization for Standardization, Geneva
- ISO 4832 (2006) Microbiology of food and animal feeding stuffs—horizontal method for the enumeration of coliforms—colony-count technique. International Organization for Standardization, Geneva
- ISO 21527-2 (2008) Microbiology of food and animal feeding stuffs—horizontal method for the enumeration of yeasts and moulds—part 2: colony count technique in products with water activity less than or equal to 0.95. International Organization for Standardization, Geneva
- ISO 21528-2 (2004) Microbiology of food and animal feeding stuffs—horizontal methods for the detection and enumeration of Enterobacteriaceae—part 2: colony-count method. International Organization for Standardization, Geneva
- Kavitake D, Kandasamy S, Devi PB, Shetty PH. Recent developments on encapsulation of lactic acid bacteria as potential starter culture in fermented foods—a review. Food Biosci. 2018;21:34–44. doi: 10.1016/j.fbio.2017.11.003. [DOI] [Google Scholar]
- Khosravi Zanjani MA, Tarzi BG, Sharifan A, Mohammadi N. Microencapsulation of probiotics by calcium alginate-gelatinized starch with chitosan coating and evaluation of survival in simulated human gastro-intestinal condition. Iran J Pharm Res. 2014;13:843–852. [PMC free article] [PubMed] [Google Scholar]
- Kos I, Zgomba Maksimovic A, Zunabovic-Pichler M, Mayrhofer S, Domig KJ, Mrkonjic Fuka M. The influence of meat batter and sausage diameter on microbiota and sensory traits of artisanal wild boar meat sausages. Food Technol Biotechnol. 2019;57:378–387. doi: 10.17113/ftb.57.03.19.6197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozačinski L, Zdolec N, Hadžiosmanović M, Cvrtila Ž, Filipović I, Majić T. Microbial flora of the Croatian traditionally fermented sausage. Arch Lebensmittelhyg. 2006;57:141–147. [Google Scholar]
- Lorenzo JM, Carballo J, Franco D. Effect of the inclusion of chestnut in the finishing diet on volatile compounds of dry-cured ham from celta pig breed. J Integr Agric. 2013;12:2002–2012. doi: 10.1016/S2095-3119(13)60638-3. [DOI] [Google Scholar]
- Lorenzo JM, Gómez M, Purriños L, Fonseca S. Effect of commercial starter cultures on volatile compound profile and sensory characteristics of dry-cured foal sausage. J Sci Food Agric. 2016;96(4):1194–1201. doi: 10.1002/jsfa.7203. [DOI] [PubMed] [Google Scholar]
- Lücke FK. Utilization of microbes to process and preserve meat. Meat Sci. 2000;56:105–115. doi: 10.1016/S0309-1740(00)00029-2. [DOI] [PubMed] [Google Scholar]
- Mrkonjic Fuka M, Tanuwidjaja I, Zgomba Maksimovic A, Zunabovic-Pichler M, Kublik S, Hulak N, Domig KJ, Schloter M. Bacterial diversity of naturally fermented game meat sausages: sources of new starter cultures. LWT Food Sci Technol. 2020;118:108782. doi: 10.1016/j.lwt.2019.108782. [DOI] [Google Scholar]
- Olivares A, Navarro JL, Flores M. Characterization of volatile compounds responsible for the aroma in naturally fermented sausages by gas chromatography-olfactometry. Food Sci Technol Int. 2015;21:110–123. doi: 10.1177/1082013213515500. [DOI] [PubMed] [Google Scholar]
- Oliveira M, Ferreira V, Magalhães R, Teixeira P. Biocontrol strategies for Mediterranean-style fermented sausages. Food Res Int. 2018;103:438–449. doi: 10.1016/j.foodres.2017.10.048. [DOI] [PubMed] [Google Scholar]
- Palavecino Prpich NZ, Castro MP, Cayré ME, Garro OA, Vignolo GM. Autochthonous starter culture selection to keep traditions in the manufacture of dry sausages alive. Ann Microbiol. 2015;65:1709–1719. doi: 10.1007/s13213-014-1010-0. [DOI] [Google Scholar]
- Petričević S, Marušić Radovčić N, Lukić K, Listeš E, Medić H. Differentiation of dry-cured hams from different processing methods by means of volatile compounds, physico-chemical and sensory analysis. Meat Sci. 2018;137:217–227. doi: 10.1016/j.meatsci.2017.12.001. [DOI] [PubMed] [Google Scholar]
- Pham AJ, Schilling MW, Mikel WB, Williams JB, Martin JM, Coggins PC. Relationships between sensory descriptors, consumer acceptability and volatile flavor compounds of American dry-cured ham. Meat Sci. 2008;80:728–737. doi: 10.1016/j.meatsci.2008.03.015. [DOI] [PubMed] [Google Scholar]
- Román S, Sánchez-Siles LM, Siegrist M. The importance of food naturalness for consumers: results of a systematic review. Trends Food Sci Technol. 2017;67:44–57. doi: 10.1016/j.tifs.2017.06.010. [DOI] [Google Scholar]
- Vinceković M, Jalšenjak N, Topolovec-Pintarić S, Dermić E, Bujan M, Jurić S. Encapsulation of biological and chemical agents for plant nutrition and protection: chitosan/alginate microcapsules loaded with copper cations and Trichoderma viride. J Agric Food Chem. 2016;64:8073–8083. doi: 10.1021/acs.jafc.6b02879. [DOI] [PubMed] [Google Scholar]
- Zagorec M, Champomier-Vergès MC. Lactobacillus sakei: a starter for sausage fermentation, a protective culture for meat products. Microorganisms. 2017;5:56. doi: 10.3390/microorganisms5030056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Žgomba Maksimović A, Zunabovic-Pichler M, Kos I, Mayrhofer S, Hulak N, Domig KJ, Mrkonjić Fuka M. Microbiological hazards and potential of spontaneously fermented game meat sausages: a focus on lactic acid bacteria diversity. LWT Food Sci Technol. 2018;89:418–426. doi: 10.1016/j.lwt.2017.11.017. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.