Abstract
Excessive softening of fruits during the ripening process leads to rapid deterioration. N-glycan processing enzymes are reported to play important roles during fruit ripening associated softening. Efforts have been made to identify and purify β-D-N-acetyl hexosaminidase (β-Hex) from strawberry fruit and also to investigate its function during ripening. More than that, the postharvest treatment effect of alginate oligosaccharides (AOS) at a concentration of 0.1 g L−1 on fruit firmness and the activity of N-glycan processing enzymes were also investigated during the storage of strawberry. Results demonstrated that the full-length of β-Hex1 and β-Hex2 genes were 2186 and 2013 bp, including an ORF of 1598 and 1724 bp and encoding 532 and 574 amino acids with a predicted molecular weight of 60 and 71 kDa, respectively. Moreover, β-hex enzyme activity and the expression of their encoding genes increased during the ripening of strawberry. In addition, postharvest application of AOS delayed the loss of firmness and suppressed the activity of N-glycan processing enzymes (α-Man and β-Hex) along with N-glycan processing enzymes associated genes expression resulting in delayed fruit softening. Therefore, our study suggests that N-glycan processing enzymes may play roles in strawberry softening and AOS treatment suppressed enzymes activity and preserve firmness of the fruit.
Electronic supplementary material
The online version of this article (10.1007/s13197-020-04576-2) contains supplementary material, which is available to authorized users.
Keywords: β-D-N-acetyl hexosaminidase, Cloning, Identification, Characterization, Firmness, Softening, Alginate oligosaccharide
Introduction
The garden strawberry (Fragaria × ananassa Duch) belongs to the family Rosaceae, is one of the most prominent and economically important fruit in the world. It is highly appreciated by consumers due to attractive color, delicious taste, distinct flavor and high nutritional quality (Bose et al. 2019). Short shelf life and rapid softening during ripening are the important post-harvest problems of this fruit. Texture is one of the vital characters for fleshy fruit because its linked to consumer’s sense of touch and texture softening is associated with loss of firmness, accelerating postharvest spoilage, challenging fruit transportation and reducing quality and shelf life of fruit (Posé et al. 2011). Hence, to fortify the texture of strawberry and to reduce postharvest loss during storage is of great interest.
To reduce postharvest losses and enhanced shelf life, strawberries are externally treated with chitosan (Gol et al. 2013), abscisic acid (Jiang and Joyce 2003), methyl jasmonate (Saavedra et al. 2016), salicylic acid (Asghari and Babalar 2009) and calcium chloride (Langer et al. 2019) etc. It is shown that the use of different chemical compounds such as imazalil (IMZ), pyrimethanil (PYR), fludioxonil (FLU), thiabendazole (TBZ) etc. to prolong shelf life and quality, enhance the risk of adding toxic substance with fruit resulting in harmful impact on health and environment (Palou 2018). Very recent, the use of natural non-toxic compounds including chitosan (Chaiprasart et al. 2006), chitosan oligosaccharides (COS) (Yanqiu et al. 2019), alginate oligosaccharides (AOS) (Bose et al. 2019) for fruit storage and preservation, showing great preference. Several reports revealed that postharvest treatment of COS significantly delayed ripening and softening of aprium (Ma et al. 2014), cherries (Kerch et al. 2011) and strawberries (Yanqiu et al. 2019). Similar with COS, AOS also proved a nontoxic biostimulator and more useful for maintaining fruit quality (Battacharyya et al. 2015; Bose et al. 2019). AOS was prepared in our laboratory by enzymatic hydrolysis from sodium alginate which was extracted from brown algae (Bose et al. 2019; Huang et al. 2013). Recent report suggested that strawberry fruit treated with AOS, prolong shelf life by delaying ABA accumulation, and cell wall degradation (Bose et al. 2019). Accordingly, studying the molecular process and ripening associated softening of strawberry would be the main target to extend the shelf life.
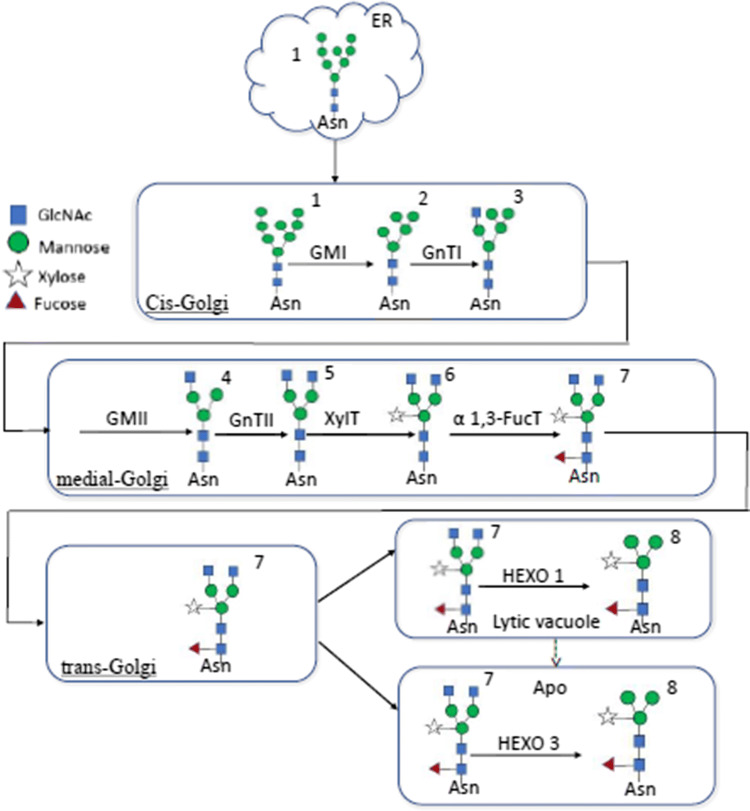
Considerable studies have reported that strawberry fruit softening during ripening is widely accompanied with enzymes mediated cell wall degradation and inhibiting the activity of these enzymes such as polygalacturonase (Quesada et al. 2009), pectin methyl esterase (Osorio et al. 2008), pectate lyase (Youssef et al. 2009) and endoglucanase (Mercado et al. 2010) are not sufficient for delaying fruit texture softening. Possibly, there are many more factors including N-glycoprotein are participating in the fruit softening process during ripening. The inhibition of N-glycosylation can delay fruit ripening suggesting that N-glycan processing may be play significant roles in the ripening process (Priem et al. 1993). N-glycoproteins are commonly found in the plant cell wall and extensively take part in activity of different physiological mechanisms including fruit ripening and softening (Bourque et al. 1999) and free N-glycans appear as the antecedent of glycosylation or proteolysis of glycoprotein. These N-glycans are responsible for the stability of several biological activities of glycoproteins and can protect the protein from proteolytic degradation (Rayon et al. 1998). In plants, N-glycan processing is initiated in the endoplasmic reticulum, the below figure showing previously proposed protein N-glycosylation pathway in plants (Bosch et al. 2013).
Recent report suggested that N-glycan processing enzymes α-Mannosidase (α-Man) and β-D-N-acetyl hexosaminidase (β-Hex) bring out into open their involvement in the ripening associated softening of different fleshy fruit (Ghosh et al. 2010; Meli et al. 2010). For this reason, among N-glycan processing enzymes, we have targeted the β-Hex which degrades the N-glycan form N-glycoprotein, that was found to play important roles in the fruit ripening process. This enzyme was recently reported to take part in the degradation of cell wall N-glycoprotein and generate free N-glycans which accelerate ripening associated softening of tomato (Irfan et al. 2014) and pepper (Ghosh et al. 2010). β-Hex, the member of GH family 20 cleaves the terminal N-acetyl-D-hexosamine residues and produce the paucimannosidic N-glycans present in most of the plant glycoproteins (Gutternigg et al. 2007; Strasser et al. 2007). Previous report also suggested that the activity of β-Hex are higher during fruit ripening and softening (Jagadeesh et al. 2004), and fruit shelf life can be extended by repressing the expression of β-Hex through RNA interference. It is also noted that blocking the activity of β-Hex enzymes, increased capsicum fruit shelf life due to the slow degradation of N-glycoprotein resulting in delayed softening (Ghosh et al. 2010). However, till to date β-Hex in strawberry has not been identified, and the role of β-Hex enzymes during ripening of strawberry remain unexplored. So, their molecular functions need to be elucidated.
Therefore, in this study, we first report the cloning, isolation, characterization and expression of β-Hex genes in strawberry. Furthermore, we also examined β-Hex enzyme activity during strawberry fruit postharvest storage, explore the effect of AOS application on fruit firmness, β-Hex, and another N-glycan processing enzyme α-Man activity and related gene expression.
Materials and methods
Gene cloning and sequence analysis
Total strawberry RNA was extracted using TaKaRa RNAiso Reagent (TakaRa, Dalian, China) and 1 mg of total RNA was used as template for the synthesis of cDNA by PrimeScript™ 1st Strand cDNA Synthesis kit (Takara) according to the manufacturer’s instruction. The nucleotide sequence was identified by sequencing (service provided by BGI (Beijing, China) and the Open Reading Frame (ORF) was mapped by using ORF finder (https://www.ncbi.nlm.nih.gov/gorf/). Gene-specific primers were designed and synthesized during this study are describe in supplementary Table 1.
Table 1.
Effect of metal ions on β-N-acetyl hexosaminidase from strawberry
| Metal ions | Relative activity (%) β-Hex1 | Relative activity (%) β-Hex2 |
|---|---|---|
| Control | 100 | 100 |
| Na+ | 76.5 ± 1.99** | 70.8 ± 0.18** |
| Mg2+ | 65.96 ± 0.66** | 78.3 ± 0.57** |
| Ca2+ | 67.0 ± 0.07** | 100.7 ± 0.72NS |
| Fe2+ | 82.7 ± 2.47** | 105.5 ± 0.26* |
| Zn2+ | 13.5 ± 0.17** | 26.4 ± 0.06** |
| Cu2+ | 13.48 ± 0.36** | 54.2 ± 1.28** |
| Mn2+ | 108.5 ± 0.11** | 116.4 ± 0.45** |
Data represent the means ± SE (Standard Error). β-Hex activity was relative to the control (100%). The * and ** represent means significantly different according to T test at P < 0.05 and P < 0.01 level, respectively and NS mean not significant
In order to subclone the gene of β-Hex1 and β-Hex2 into the expression vector pPICZαA, the recombinant expression vector was constructed by RF-cloning following the previously described method (Van and Lowe 2006). Multiple sequence alignments were performed with Basic Local Alignment Search Tool (BLAST, https://blast.ncbi.nlm.nih.gov/Blast.) on NCBI. Sequence domains were analyzed using the online Simple Modular Architecture Research Tool (SMART, https://smart.embl-heidelberg.de/). Multiple alignments of amino acid sequences were performed using the Vector NTI software program.
Expression and purification of recombinant protein
The recombinant plasmids were expressed into Pichia pastoris X-33, and performed in Buffered Glycerol-complex Medium (BMGY) and Buffered Methanol-complex Medium (BMMY) media at 28 °C (1% yeast extract, 2% peptone, 1.34% YNB, 4 × 10−5% biotin, 0.1 mol L−1 potassium phosphate, pH 6.0 and 1% glycerol or 0.5% methanol as carbon sources, respectively). After expression, the harvested culture supernatant was purified using Nickel-Nitrilo Triacetic Acid (Ni–NTA) Sepharose™ excel chromatography resin (GE Healthcare, Amersham, Sweden) and then verified by SDS-PAGE analysis. PNGase F is the most effective enzymatic method for removing almost all N-linked oligosaccharides from glycoproteins. The PNGase F enzymatic assay was performed according to manufacturer’s instruction (New England Biolab, UK).
Effects of pH, temperature and metal ions on the activity of β-Hex
The effects of pH, temperature and metal ions on the activity of β-Hex were perfomred according to previously described method (Jin et al. 2002). Briefly, the optimum pH for β-Hex1 and β-Hex2 activity was determined using 0.05 mol L−1 p-nitrophenol (pNP) in 0.2 mol L−1 NaAc-HAc buffer (pH 3.0–8.0). The optimum temperature for the β-Hex1 and β-Hex2 activity determination was performed with 0.005 mol L−1 pNP in 0.1 mol L−1 NaAc-HAc (pH 5.0) using incubation temperatures range from 20 to 70 °C for 60 min.
For determination of metal ion effect on β-Hex1 and β-Hex2 activity, a volume of 0.75 mL of β-Hex1 and β-Hex2 in 0.02 mol L−1 Tris–HCl (pH 7.5) was diluted with 0.25 g L−1 BSA and incubated for 2 h with 0.25 mL of 0.2 mol L−1 Ethylene Diamine Tetra Acetic Acid (EDTA) containing 0.5 mol L−1 NaCl (pH 7.5). An incubation control was made with 0.05 mol L−1 Tris–HCl (pH 7.5) containing 0.5 mol L−1 NaCl instead of EDTA. The EDTA-treated β-Hex-1 and β-Hex-2 was desalted using PD10 columns (GE Healthcare) previously equilibrated with 0.1 mol L−1 NaAc-HAc (pH 4.5). Elution was performed with 2 mL of equilibration buffer. The effect of metal ions was studied by incubating 100 μL of desalted sample at room temperature with 10 μL of 0.001 mol L−1 concentrations of the following salts: NaCl, CaCl2, CuSO4, MgCl2, MnCl2, FeSO4 and ZnCl2, respectively.
Plant materials and treatment
Commercially mature, uniform size and shape with 15–16 g weighted, more or less similar color without any defect cultivated strawberries (Fragaria × ananassa Duch) were collected from farmer’s field located at 39° 25′ to 40° 12′ N latitude and 122° 29′ to 123° 31′ E longitude (Zhuanghe city, Liaoning Province, China) on April 2018. A total of 180 randomly selected strawberries were divided into two groups and each group consisted of 90 strawberries and each replication contained 30 fruits.
AOS was prepared in our laboratory from sodium alginate which was extracted from brown algae (viscosity, 250 cP). It was 69 h hydrolysate of Alg2A (an alginate lyase from Flavobacterium sp. S20) and purified by a cation exchange column. The degree of polymerization is 2–7 and M/G is 7:3 (Bose et al. 2019; Huang et al. 2013). AOS powder was weighted with the help of an electric balance and dissolved in distilled water. Initially, 5 concentrations of AOS (0.05, 0.1, 0.25, 0.5 and 1.0 g L−1) were used during our previous study (Bose et al. 2019). Among these five concentrations, AOS 0.05, 0.1 g L−1 exhibited better results compared to other treatments and we noticed that AOS 0.1 g L−1 treatment most effectively preserved fruit quality and increased shelf life of strawberry. Based on our previous studied results, we used 0.1 g L−1 AOS as a treatment and one control group in this experiment. Between two groups of fruit, 1 group was treated with AOS solution and other group was steeped with distilled water for 1 min and then placed on brown paper at room temperature for drying surface liquid. Strawberries were stored at 20 ± 2 °C with 80 ± 2% relative humidity up to 6 days. From each fruit group, 15 fruits were taken at every alternate day for measuring the firmness and then cut into small pieces, group wise packed with aluminum foil, immediately dipped in liquid nitrogen and then stored at − 80 °C for enzyme activity analysis.
Determination of flesh firmness
Fruit flesh firmness was determined by “TA.XT.plus Texture Analyser” (Stable Micro Systems Ltd., Surrey UK). At every alternate day, 15 fruits from each treatment group were used for firmness determination. Fruits were longitudinally divided into two equal parts and two measurements were done on opposite sides of each fruit by penetrating the probe at a distance of 5 mm into the fruit with pre- and post-test speed 1 mm s−1. The firmness of fruits was estimated from maximum penetration force of probe while breakage of fruit tissues and average value was expressed as Newton (N) (Fig. 1).
Fig. 1.
Protein N-Glycosylation pathway in plants. ER: endoplasmic reticulum; Apo: apoplast. 1: Man8; 2: Man5; 3: Man5Gn; 4: MGn; 5: GnGn; 6: GnGnX; 7: GnGnXF3; 8: MMXF3 (paucimannosidic structure). GMI: Golgi-α-mannosidase I; GnTI: N-acetylglucosaminyltransferase I;GMII: Golgi-α-mannosidase II; GnTII: N-acetylglucosaminyltransferase II; XylT: β1,2-xylosyltransferase; α1,3-FucT: HEXO1, HEXO3: β-N-acetylhexosaminidase 1 and 3
Strawberry in-vivo enzymes (α-Man and β-Hex) activity determination
α-Man and β-Hex enzymes activity were determined according to the method described by Jagadeesh et al. (2004) with some modifications. Briefly, 5 g frozen strawberry fruits were ground in liquid nitrogen with the help of mortar and pestle. Transfer 2.0 g fruit powder in a 50 mL centrifuge tube and suspended with 10 mL of 0.1 mol L−1 sodium acetate (NaAc) buffer (pH 4.5) containing 0.5% polyvinylpyrrolidone (PVP), then kept overnight at 4 °C. The supernatant was collected by centrifugation at 10,000×g for 15 min at 4 °C and collected liquid was used as crude enzyme for enzyme activity determination. A volume of 50 μL NaAc (0.1 mol L−1) buffer (pH 4.5) and 200 μL of substrate (pNP-α-Man and pNP-GlcNAc, 1 mm L−1) was taken in a 2 mL tube, heated in a water bath at 37 °C for 5 min, then 50 μL of crude enzyme was added to the tube, and again heated for 15 min at 50 °C. The reaction was terminated by adding 1 mL of 0.5 mol L−1 sodium carbonate, after 5 min the absorbance was measured at 410 nm. P-nitrophenol was used as standard for calculation of enzyme activity. One unit was defined as 1.0 A410 unit mg−1 sample.
Real-time quantitative PCR (RT-qPCR) analysis
For RT-qPCR analysis, 5 μL Monad PCR mix (Monad Biotech Co. Ltd, Shanghai, China), 1 μL of each gene specific forward and reverse primer, 2 μL cDNA, and 1 μL Diethyl Pyrocarbonate (DEPC) water were mixed together in a final volume 10 μL. The nucleotide sequences of the primer pair used for each gene in this study are presented in supplementary Table 1. DNA amplification was conducted by using RT-qPCR machine (qTOWER 2.2, Analytik Jena AG, German) under following thermocycling conditions: 94 °C for 10 min followed by 40 cycles at 94 °C for 10 s and at 60 °C for 1 min, finally melting for 0.06 s. 26S–18S was used as reference gene to normalize small differences in the amount of the used template. The specificity of the PCR amplification was confirmed with a melt curve analysis. The relative expressions of desire genes were analyzed using the 2−ΔΔCt formula (Livak and Schmittgen 2001).
Statistical analysis
The present study was conducted by using completely randomized design with three replications. The collected data were compiled and analyzed by using SPSS 22.0 (IBM, New York, USA) software. The level of significant difference was considered at P < 0.05 and P < 0.01.
Results and discussion
Cloning of strawberry β-Hex
The function of β-Hex during ripening and softening were observed by cloning the genes from strawberry using degenerate primers and then consistently evaluated their activity. The sequencing results showed that they share 98 and 97% similarity with PREDICTED Fragaria Vesca subsp. Vesca β-Hex1 (XM004307513) and β-Hex2 base sequence (XM004301762), respectively. The full-length of β-Hex1 and β-Hex2 were further cloned. The length of β-Hex1 gene is 2186 bp (Supplementary Fig. 1 a, i.e. Fig. S1 a), including an ORF of 1598 bp (449–2047 bp), encoding 532 amino acids with a predicted molecular weight of 60 kDa (Fig.S1 b). The length of β-Hex2 gene is 2013 bp (Fig. S1 a), containing an ORF of 1724 bp (244–1968 bp), encoding 574 amino acids with a predicted molecular mass of 71 kDa (Fig. S1 b). Their C-terminal is the Glycohydro 20 domain, as shown in supplementary Fig. 2 (Fig. S2), indicating that β-Hex1 and β-Hex2 are members of the GH 20 family. Our findings also validated previously reported results that after cloning and functional characterization of β-Hex from capsicum fruit, it was noted that β-Hex is the member of GH family 20 which cleaves the terminal β-D-N-acetylglucosamine residues of N-glycans (Cao et al. 2014; Ghosh et al. 2010).
The phylogenetic analysis of β- Hex
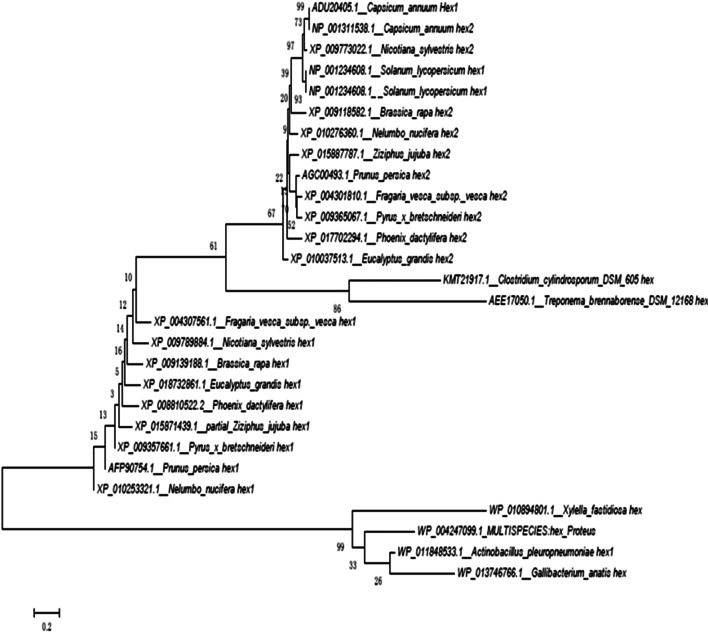
The nucleotide sequence identity analysis of the two β-Hex genes were carried out in NCBI data using blastx program, and the amino acid sequences encoded by the ORFs of Pp β-Hex1 and Pp β-Hex2 were named Fragaria vesca Hex1 (FvHex1) and Fragaria vesca Hex2 (FvHex2).
FvHex1 had high similarity to Nicotiana sylvestris Hex1 (75%) and Brassica rapa Hex1 (74%), and FvHex2 had high similarity to Prunus persica Hex2 (87%) and Pyrus bretschneideri Hex2 (80%). The sequences of all the genes mentioned above were used for multiple alignment analysis (Fig. 2). Results showed that FvHex1 and FvHex2 belongs to two different branches, and along evolutionary distance existed in different branches, with only 28.0% of sequence identity between FvHex1 and FvHex2. Similar to our research, differences in structure and function was also reported in several previous study such as PpHex1 and PpHex2 in peach fruit (Cao et al. 2014), AtHex1 and AtHex2 in Arabidopsis (Liebminger et al. 2011).
Fig. 2.
Phylogenetic tree of Fragaria vesca subsp. Vesca β-Hex1 and β-Hex2
Purification and identification of recombinant β-Hex1 and β-Hex2
Both FvHex1 and FvHex2 were expressed and purified successfully, with molecular weight between 70 and 90 kDa, which was larger than the theoretical molecular weight (69.54 kDa/71 kDa), indicating the presence of N-glycosylated sites in both enzymes (Fig. S3 a and b). For the purpose of confirmation that the recombinant β-Hex were N-glycosylated modified proteins, the purified β-Hex was digested with the glycosidase PNGase F under native and SDS-PAGE (Fig. S3 c). The molecular weight of β-Hex1 and β-Hex2 decreased to 70 kDa, which is consistent with the theoretical molecular weight (69.5 kDa), indicating that both β-Hex1 and β-Hex2 are N-glycosylated modified protein, and the N-glycan branching may not introduce steric hindrance. Under natural conditions, the glycosidase PNGase F can approach the glycosylation site and excise the glycan.
Characterization of FvHex1 and FvHex2
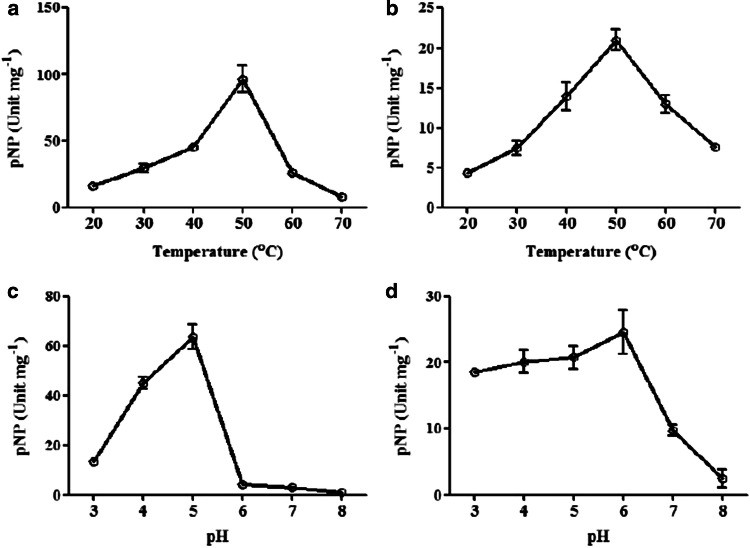
The activity of β-Hex1 and β-Hex2 increased gradually at temperature range from 20 to 50 °C and then decreased (Fig. 3a, b). From our result we noticed that the β-Hex1 and β-Hex2 both enzymes showed maximum activity at 50 °C. This result was agreement with previously described results obtained by purification and characterization of β-N-acetyl hexosaminidase from rice seeds and also noted that the purified enzyme showed the highest activity at 50 °C and was stable at 20–40 °C (Jin et al. 2002).
Fig. 3.
a, b Effect of temperature on β-Hex1, β-Hex2 activity; c, d effect of pH on β-Hex1, β-Hex2 activity. Data represent the means ± SE (Standard Error)
The effect of pH on the activities of the enzymes was examined using the corresponding p-nitrophenyl glycosides as substrates. The optimum β-Hex1 activity was noted at pH 4.0 whereas for β-Hex2, it was 5.0 (Fig. 3c, d). Our results validated previously described result and they reported that the maximum activity of pNP-GlcNAc was showed at pH 5.0 and activity of this enzyme was stable at pH 5.0–5.5 in rice seeds (Jin et al. 2002).
To specify the effect of metal ions on enzyme activity, we performed the enzyme reaction under standard condition. Enzyme activity in the absence of any metal ions was considered as 100%. The effect of various metal ions on the β-Hex enzyme activity is presented in Table 1. Among the studied metal ions, the highest enzyme activity was recorded from Mn2+ in respect of both β-Hex1 and β-Hex2 whereas the enzyme activity was strongly inhibited in the presence of Cu2+ and Zn2+ in case of β-Hex1 and β-Hex2, respectively. Metal ions play important roles in the biological function of many enzymes. Different metal ions had inhibitory and enhanced effect on β-Hex enzyme activity was reported by several researchers in many crops. For instance, β-Hex enzyme activity strongly inhibited by Ag2+, Cu2+ and Fe3+ in wheat leaf (Barber and Ride 1989) and enhanced by Mn2+, Mg2+ and Ca2+ in pea (Harley and Beevers 1987).
In order to clarify the effect of N-glycosylation on the activities of β-Hex1 and β-Hex2, the activities of β-Hex1 and β-Hex2 were detected before and after PNGaseF enzyme treatment. PNGase F is a highly active enzyme that can be used to efficiently deglycosylate N-linked glycoproteins for characterization of glycan structure and function, as well as for glycan-site identification. N-Glycans were removed by using PNGaseF (New England Biolabs, Ipswich, Massachusetts, USA) under denaturing conditions, according to the supplier’s instructions and visualized by SDS-PAGE (Fig. S3 c). The results revealed that the activities of β-Hex1 and β-Hex2 were decreased by 10.03 and 27.48% of the activity of correspondence glycoprotein, respectively indicating significant influence of glycosylation on the enzyme activity. Previous report noticed that β-Hex is a cell wall glycoprotein which is able to cleave terminal β-D-N-acetyl glucosamine residues of N-glycans (Ghosh et al. 2010) but they didn’t provided any direct evidence. In our research we, first time provided direct evidence that the β-Hex enzyme is a glycoprotein which validated previous results.
β-Hex enzyme activity and related gene expression
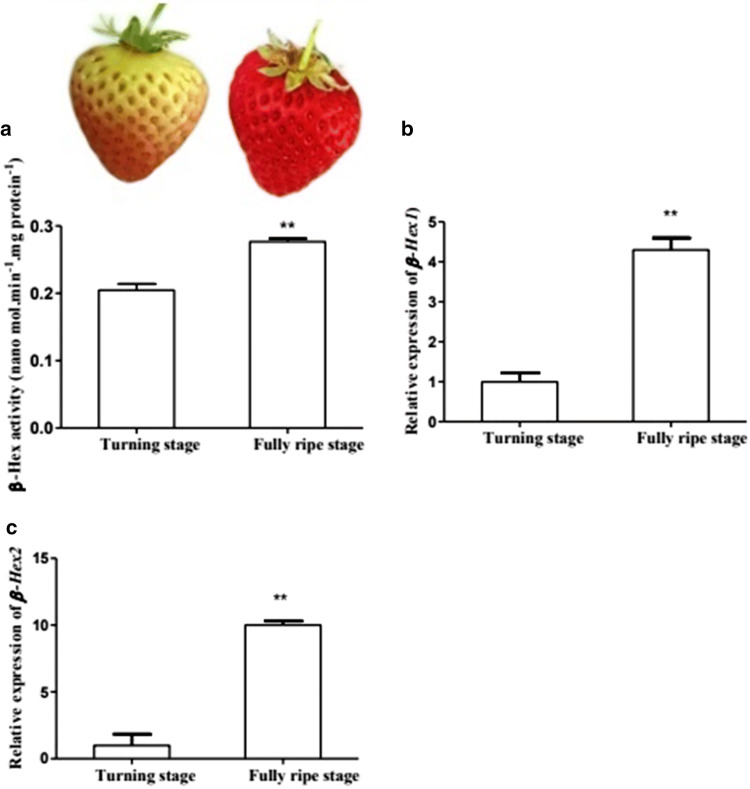
To know the probable function of β-Hex enzyme in ripening associated softening, activity and encoding gene expression was observed. The result showed that the β-Hex enzyme activity increased during ripening of strawberry fruit (Fig. 4a). Similar trend of results was also reported previously in capsicum fruits and noted that this enzyme activity was higher during ripening and silencing of this enzyme activity reduced softening resulting in enhances fruit shelf life (Ghosh et al. 2010). The expression level of β-Hex1 and β-Hex2 genes were also increased (Fig. 4b, c) during ripening of strawberry. Our results were also similar with previously reported results that β-Hex gene expression was higher during ripening of peach fruits and suppressing the expression of these genes increased shelf life of fruits (Cao et al. 2014). All these results indicated that β-Hex play crucial role in ripening and ripening associated softening of fruits during postharvest storage.
Fig. 4.
a β-Hex enzyme activity and gene expression analysis of b β-Hex1, c β-Hex2 during storage of strawberry fruits. Data represent the means ± SE. The * and ** represent means significantly different according to T test at P < 0.05 and P < 0.01 level, respectively
Effect of AOS on fruit firmness
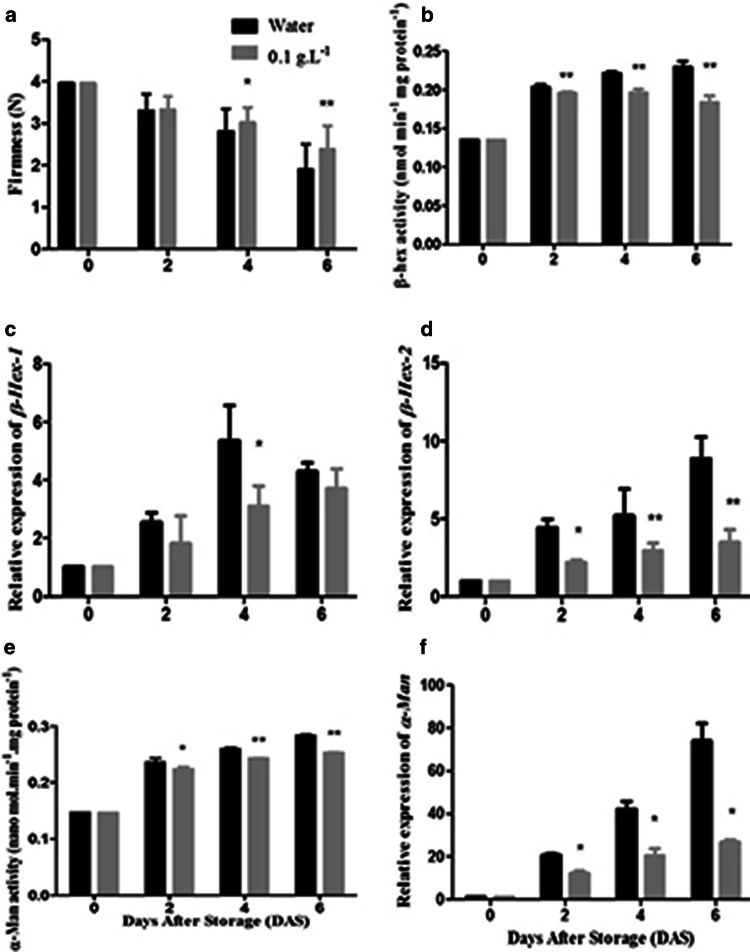
AOS has been reported to play important roles on fruit quality preservation. To understand the possible effect of AOS during strawberry fruit ripening and softening, we also studied the fruit firmness. Enhancement of desirable texture during storage and ripening of strawberry is the key to prolonging shelf life. Fruit firmness closely associated with softening, is one of the vital quality indices. Immediately after AOS treatment, both treated and untreated fruits showed same firmness and it decreased during prolong storage period. It was observed that AOS at a concentration of 0.1 g L–1 significantly (P < 0.05) delayed the loss of firmness compared to untreated fruit (Fig. 5a) and after 6 days of storage the higher firmness was noted from AOS treatment. Previous report suggested that fruit softening closely linked with cell wall degradation and modifications resulting from the activity of some cell wall degenerating enzymes (Brummell 2006). COS postharvest treatment could delay the firmness reduction of strawberry (Kerch et al. 2011; Yanqiu et al. 2019) and aprium fruit (Ma et al. 2014). In our result, we also observed that AOS postharvest treatment had a good efficiency to retain firmness of strawberry fruit.
Fig. 5.
Postharvest treatment effect of AOS on strawberry fruit (a) firmness (b) β-Hex enzyme activity (c) β-Hex1 gene expression (d) β-Hex2 gene expression (e) α-Man enzyme activity (f) α-Man gene expression during storage. Data represent the means ± SE (Standard Error). The * and ** represent means significantly different according to T test at P < 0.05 and P < 0.01 level, respectively
AOS effect on β-Hex enzyme activity and related gene expression on strawberry ripening
To know the probable function of AOS on β-Hex enzyme activity during ripening associated softening, their encoding gene expression was observed after AOS postharvest treatment. In this experiment, our result showed that β-Hex enzymes activity increased during ripening and noted maximum after 6 days of storage whereas fruit treated with AOS significantly (P < 0.05) inhibited the β-Hex enzymes activity (Fig. 5b). Previous study reported that inhibition of β-Hex enzymes activity, increase fruit shelf life and delay the softening of tomato (Meli et al. 2010) which validated our results. So, results suggested that AOS have great potential to inhibit β-Hex enzymes activity, as a result higher firmer fruit was obtained during storage compared to control.
Furthermore, we also examined the expression of β-Hex encoding gene expression during storage of strawberry and our results revealed that gene expression increased with the time of storage period which is consistent with enzymes activity. AOS at a concentration of 0.1 g L−1 had inhibiting effect on both β-Hex1 and β-Hex2 genes expression during storage. AOS treatment significantly (P < 0.05) restrained β-Hex1 gene expression during 4 days of storage (Fig. 5c), but in respect of β-Hex2, significantly (P < 0.01) suppressing effect of AOS treatment was recorded during entire storage period (Fig. 5d). Previous report suggested that, when peach fruit treated with ethylene antagonist like 2-Acetaindo-1,2-dideoxynojirimycin (2-AND), reduced the β-Hex enzyme activity as well as suppressed the β-Hex encoding gene expression resulting delayed ethylene production and softening of fruit (Cao et al. 2014). In our study, we noted that after AOS application, β-Hex enzyme activity as well as encoding genes expression were suppressed resulting in delayed fruit softening and increased shelf life. Considering the role of β-Hex enzymes in relation to fruit firmness and softening, another N-glycan processing enzyme (α-Man) activity and related gene expression were also investigated during storage after AOS postharvest treatment.
AOS effect on α-Man enzyme activity and related gene expression
α-Man is another N-glycan processing enzyme, member of the glycosyl hydrolase 38 (GH 38) family enzyme (Fig. S4) and its activity was shown to increase during ripening of several fruit including tomato, capsicum, mango, papaya (Ghosh et al. 2010; Hossain et al. 2009; Meli et al. 2010; Priya and Prabha 1997). To know the possible role of α-Man in strawberry fruit softening during storage, α-Man enzyme activity and related gene expression was observed. During storage, α-Man enzyme activity increased and noted maximum after 6 days of storage. Our results showed that AOS treatment significantly (P < 0.05) repressed the α-Man enzyme activity (Fig. 5e). Similar result was previously reported in case of tomato fruit ripening and they also noticed that suppression activity of α-Man enzyme, increase fruit shelf life and delay the softening (Meli et al. 2010). In our study, we observed that AOS postharvest treatment inhibited the activity of this enzyme, as a result higher firmer fruit was obtained during storage compared to control.
In addition, we assessed the expression of α-Man encoding gene expression during storage of strawberry and our results revealed that gene expression increased with prolong storage period which is similar with enzymes activity. AOS at a concentration of 0.1 g L−1 significantly (P < 0.05) repressed the expression of α-Man gene during storage i.e. untreated fruit exhibited higher expression compared to AOS treated fruit throughout the storage period (Fig. 5f) which implied that AOS suppressed the α-Man encoding gene expression. Our results were in line with previously reported results in capsicum fruit and noted that this enzyme encoding gene expression was higher during ripening and silencing of this gene reduced softening resulting in enhances fruit shelf life (Ghosh et al. 2010). The possible reason is that AOS postharvest treatment hinders both enzyme activities during storage of strawberry fruit. Above results suggested that α-Man and β-Hex genes play important roles in softening of strawberry fruit and AOS showed obvious inhibition effect against these enzymes and encoding genes resulting delay softening. The commercial ways to improve shelf life and delay softening of strawberry fruit and comparison of our results with previous literature are present in supplementary table 2.
Conclusion
In our study, we have cloned, purified and characterized N-glycan processing enzyme encoding gene β-Hex from strawberry fruit for the first time. In addition, we examined the activity of this enzyme along with another N-glycan processing enzyme α-Man during storage. Their increasing activity and gene expression implied that they involved in the regulation of strawberry postharvest softening. AOS postharvest treatment has a good effect on retaining strawberry firmness, it also exerts obvious inhibition effect on the expression and activity of this N-glycan processing enzymes, which further validates N-glycan processing can be targeted for the improvement of shelf life in strawberry fruit.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
Santosh Kumar Bose and Prianka Howlader were supported by CAS-TWAS president fellowship Ph.D. program and they are very grateful to CAS-TWAS.
Author contributions
WW and YH: Conceptualization, HY and SKB: methodology, HY and SKB: software, WW and YH: validation, HY and SKB: formal analysis, HY and SKB: investigation, WW and YH: resources, YH : data curation, SKB and PH: writing—original draft preparation, SKB, WW, YH and PH: writing—review and editing, YH: supervision, YH: project administration, YH: funding acquisition.
Funding
This work was supported by the National Key R&D Program of China, China [2017YFD0200900 and 2017YFD0200902], CAS Scientific Technological Service Network (STS) Project (KFJ-STS-ZDTP-048-02), the Natural Science Foundation of Liaoning Province (2019-MS-31), Heng Yin was supported by Liaoning Revitalization Talents Program, China [XLYC1807041].
Compliance with ethical standards
Conflict of interest
The authors declare no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Santosh Kumar Bose, Yanqiu He and Prianka Howlader have contributed equally.
Contributor Information
Wenxia Wang, Email: wangwx@dicp.ac.cn.
Heng Yin, Email: yinheng@dicp.ac.cn.
References
- Asghari M, Babalar M (2009) Use of salicylic acid to increase strawberry fruit total antioxidant activity. In: Paper presented at the VI international postharvest symposium, p 877
- Barber M, Ride J. Purification and properties of a wheat leaf N-acetyl-β-D-hexosaminidase. Plant Sci. 1989;60:163–172. doi: 10.1016/0168-9452(89)90162-3. [DOI] [Google Scholar]
- Battacharyya D, Babgohari MZ, Rathor P, Prithiviraj B. Seaweed extracts as biostimulants in horticulture. Sci Hortic. 2015;196:39–48. doi: 10.1016/j.scienta.2015.09.012. [DOI] [Google Scholar]
- Bosch D, Castilho A, Loos A, Schots A, Steinkellner H. N-Glycosylation of Plant-produced recombinant proteins. Curr Pharm Des. 2013;19:5503–5512. doi: 10.2174/1381612811319310006. [DOI] [PubMed] [Google Scholar]
- Bose SK, Howlader P, Jia X, Wang W, Yin H. Alginate oligosaccharide postharvest treatment preserve fruit quality and increase storage life via Abscisic acid signaling in strawberry. Food Chem. 2019;283:665–674. doi: 10.1016/j.foodchem.2019.01.060. [DOI] [PubMed] [Google Scholar]
- Bourque S, Binet MN, Ponchet M, Pugin A, Lebrun-Garcia A. Characterization of the cryptogein binding sites on plant plasma membranes. J Biol Chem. 1999;274:34699–34705. doi: 10.1074/jbc.274.49.34699. [DOI] [PubMed] [Google Scholar]
- Brummell DA. Cell wall disassembly in ripening fruit. Funct Plant Biol. 2006;33:103–119. doi: 10.1071/FP05234. [DOI] [PubMed] [Google Scholar]
- Cao L, Zhao C, Su S, Luo C, Han M. The role of β-hexosaminidase in peach (Prunus persica) fruit softening. Sci Hortic. 2014;169:226–233. doi: 10.1016/j.scienta.2014.02.015. [DOI] [Google Scholar]
- Chaiprasart P, Handsawasdi C, Pipattanawong N. Effect of chitosan coating and calcium chloride treatment on postharvest qualities of strawberry fruit (Fragaria x ananassa) Acta Hortic. 2006;708:337–342. doi: 10.17660/ActaHortic.2006.708.58. [DOI] [Google Scholar]
- Ghosh S, Meli VS, Kumar A, Thakur A, Chakraborty N, Chakraborty S, Datta A. The N-glycan processing enzymes α-mannosidase and β-D-N-acetylhexosaminidase are involved in ripening-associated softening in the non-climacteric fruits of capsicum. J Exp Bot. 2010;62:571–582. doi: 10.1093/jxb/erq289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gol NB, Patel PR, Rao TR. Improvement of quality and shelf-life of strawberries with edible coatings enriched with chitosan. Postharvest Biol Technol. 2013;85:185–195. doi: 10.1016/j.postharvbio.2013.06.008. [DOI] [Google Scholar]
- Gutternigg M, Kretschmer-Lubich D, Paschinger K, Rendić D, Hader J, Geier P, Ranftl R, Jantsch V, Lochnit G, Wilson IB. Biosynthesis of truncated N-linked oligosaccharides results from non-orthologous hexosaminidase-mediated mechanisms in nematodes, plants, and insects. J Biol Chem. 2007;282:27825–27840. doi: 10.1074/jbc.M704235200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harley SM, Beevers L. Isozymes of β-N-acetylhexosaminidase from pea seeds (Pisum sativum L.) Plant physiol. 1987;85:1118–1122. doi: 10.1104/pp.85.4.1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hossain MA, Nakamura K, Kimura Y. α-Mannosidase involved in turnover of plant complex type N-glycans in tomato (Lycopersicum esculentum) fruits. Biosci Biotechnol Biochem. 2009;73:140–146. doi: 10.1271/bbb.80561. [DOI] [PubMed] [Google Scholar]
- Huang L, Zhou J, Li X, Peng Q, Lu H, Du Y. Characterization of a new alginate lyase from newly isolated Flavobacterium sp. S20. J Ind Microbiol Biot. 2013;40:113–122. doi: 10.1007/s10295-012-1210-1. [DOI] [PubMed] [Google Scholar]
- Irfan M, Ghosh S, Kumar V, Chakraborty N, Chakraborty S, Datta A. Insights into transcriptional regulation of β-DN-acetylhexosaminidase, an N-glycan-processing enzyme involved in ripening-associated fruit softening. J Exp Bot. 2014;65:5835–5848. doi: 10.1093/jxb/eru324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jagadeesh BH, Prabha TN, Srinivasan K. Activities of β-hexosaminidase and α-mannosidase during development and ripening of bell capsicum (Capsicum annuum var. variata) Plant Sci. 2004;167:1263–1271. doi: 10.1016/j.plantsci.2004.06.031. [DOI] [Google Scholar]
- Jiang Y, Joyce DC. ABA effects on ethylene production, PAL activity, anthocyanin and phenolic contents of strawberry fruit. Plant Growth Regul. 2003;39:171–174. doi: 10.1023/A:1022539901044. [DOI] [Google Scholar]
- Jin YL, Jo YY, Kim KY, Shim JH, Kim YW, Park RD. Purification and characterization of β-N-acetylhexosaminidase from rice seeds. BMB Rep. 2002;35:313–319. doi: 10.5483/BMBRep.2002.35.3.313. [DOI] [PubMed] [Google Scholar]
- Kerch G, Sabovics M, Kruma Z, Kampuse S, Straumite E. Effect of chitosan and chitooligosaccharide on vitamin C and polyphenols contents in cherries and strawberries during refrigerated storage. Eur Food Res Technol. 2011;233:351–358. doi: 10.1007/s00217-011-1525-6. [DOI] [Google Scholar]
- Langer SE, Marina M, Burgos JL, Martínez GA, Civello PM, Villarreal NM. Calcium chloride treatment modifies cell wall metabolism and activates defense responses in strawberry fruit (Fragaria× ananassa, Duch) J Sci Agric. 2019;99:4003–4010. doi: 10.1002/jsfa.9626. [DOI] [PubMed] [Google Scholar]
- Liebminger E, Veit C, Pabst M, Batoux M, Zipfel C, Altmann F, Mach L, Strasser R. β-N-Acetylhexosaminidases HEXO1 and HEXO3 are responsible for the formation of paucimannosidic N-glycans in Arabidopsis thaliana. J Biol Chem. 2011;286:10793–10802. doi: 10.1074/jbc.M110.178020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Ma L, Cao J, Xu L, Zhang X, Wang Z, Jiang W. Effects of 1-methylcyclopropene in combination with chitosan oligosaccharides on post-harvest quality of aprium fruits. Sci Hortic. 2014;179:301–305. doi: 10.1016/j.scienta.2014.09.052. [DOI] [Google Scholar]
- Meli VS, Ghosh S, Prabha T, Chakraborty N, Chakraborty S, Datta A. Enhancement of fruit shelf life by suppressing N-glycan processing enzymes. Proc Natl Acad Sci. 2010;107:2413–2418. doi: 10.1073/pnas.0909329107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercado JA, Trainotti L, Jiménez-Bermúdez L, Santiago-Doménech N, Posé S, Donolli R, Barcelo M, Casadoro G, Alfaro FP, Quesada MA. Evaluation of the role of the endo-β-(1,4)-glucanase gene FaEG3 in strawberry fruit softening. Postharvest Biol Technol. 2010;55:8–14. doi: 10.1016/j.postharvbio.2009.08.004. [DOI] [Google Scholar]
- Osorio S, Castillejo C, Quesada M, Medina-Escobar N, Brownsey GJ, Suau R, Heredia A, Botella MA, Valpuesta V. Partial demethylation of oligogalacturonides by pectin methyl esterase 1 is required for eliciting defence responses in wild strawberry (Fragaria vesca) Plant J. 2008;54:43–55. doi: 10.1111/j.1365-313X.2007.03398.x. [DOI] [PubMed] [Google Scholar]
- Palou L. Postharvest treatments with GRAS salts to control fresh fruit decay. Hortic. 2018;4:46. doi: 10.3390/horticulturae4040046. [DOI] [Google Scholar]
- Posé S, García-Gago JA, Santiago-Doménech N, Pliego-Alfaro F, Quesada MA, Mercado JA. Strawberry fruit softening: role of cell wall disassembly and its manipulation in transgenic plants. Genes, Genomes, Genet. 2011;5:40–48. [Google Scholar]
- Priem B, Gitti R, Bush CA, Gross KC. Structure of ten free N-glycans in ripening tomato fruit (arabinose is a constituent of a plant N-glycan) Plant physiol. 1993;102:445–458. doi: 10.1104/pp.102.2.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priya Sethu K, Prabha T. Alpha-D-Mannosidase from Capsicum Annuum. Phytochemistry. 1997;44:383–387. doi: 10.1016/S0031-9422(96)00490-6. [DOI] [Google Scholar]
- Quesada MA, Blanco-Portales R, Pose S, Garcia-Gago JA, Jimenez-Bermudez S, Munoz-Serrano A, Caballero JL, Pliego-Alfaro F, Mercado JA, Munoz-Blanco J. Antisense down-regulation of the FaPG1 gene reveals an unexpected central role for polygalacturonase in strawberry fruit softening. Plant Physiol. 2009;150:1022–1032. doi: 10.1104/pp.109.138297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rayon C, Lerouge P, Faye L. The protein N-glycosylation in plants. J Exp Bot. 1998;49:1463–1472. doi: 10.1093/jxb/49.326.1463. [DOI] [Google Scholar]
- Saavedra GM, Figueroa NE, Poblete LA, Cherian S, Figueroa CR. Effects of preharvest applications of methyl jasmonate and chitosan on postharvest decay, quality and chemical attributes of Fragaria chiloensis fruit. Food Chem. 2016;190:448–453. doi: 10.1016/j.foodchem.2015.05.107. [DOI] [PubMed] [Google Scholar]
- Strasser R, Bondili JS, Schoberer J, Svoboda B, Liebminger E, Glössl J, Altmann F, Steinkellner H, Mach L. Enzymatic properties and subcellular localization of Arabidopsis β-N-acetylhexosaminidases. Plant physiol. 2007;145:5–16. doi: 10.1104/pp.107.101162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den Ent F, Lowe J. RF cloning: a restriction-free method for inserting target genes into plasmids. J Biochem Biophys Methods. 2006;67:67–74. doi: 10.1016/j.jbbm.2005.12.008. [DOI] [PubMed] [Google Scholar]
- Yanqiu H, Bose SK, Mengyu W, Tongmei L, Wenxia W, Hang L, Heng Y. Effects of chitosan oligosaccharides postharvest treatment on the quality and ripening related gene expression of cultivated strawberry fruits. J Berry Res. 2019;9(1):1–16. doi: 10.3233/JBR-180294. [DOI] [Google Scholar]
- Youssef SM, Jiménez-Bermúdez S, Bellido ML, Martín-Pizarro C, Barceló M, Abdal-Aziz SA, Caballero JL, López-Aranda JM, Pliego-Alfaro F, Muñoz J, Quesada MA. Fruit yield and quality of strawberry plants transformed with a fruit specific strawberry pectate lyase gene. Sci Hortic. 2009;119(2):120–5. doi: 10.1016/j.scienta.2008.07.011. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.