Abstract
Melamine adulteration in milk is a serious health concern for the consumers. A reliable and sensitive technique using silver nanoparticle (AgNPs) was developed for the detection of melamine in milk sample. The AgNPs was synthesized using culinary banana peel extract (BPE) where pH, temperature, the amount of concentration of BPE and concentration of AgNO3 was standardized. The effect of the parameters used for the synthesis of AgNPs was analyzed by observing the colour of reaction mixture and surface plasmon resonance. The AgNPs synthesized under optimum conditions were characterized by SEM-EDX, TEM and FTIR. FTIR studies reveal the effective conjugation between AgNPs and bioactive components of BPE and formation of spherical and regular shaped AgNPs were confirmed by TEM images. Presence of Ag as a dominating metal in AgNPs confirmed the formation of AgNPs. The level of melamine above 0.5 mg/L in milk could easily be detected through the interference synthesis of AgNPs.
Keywords: Banana peel, Melamine, Milk adulteration, Silver nanoparticle
Introduction
The banana fruit is generally produced by a large number of herbaceous flowering plants in the genus Musa (Merriam-Webster Online Dictionary) (Ploetz et al. 2007). In some countries, bananas used for cooking are known as plantains e.g., culinary banana are a member of the banana family. Lots of bananas are widely consumed throughout the world, but its peels are mostly wasted (Ibrahim 2015).
Silver nanoparticles (AgNPs) are nanoparticles of between 1–100 nm in size. Numerous shapes of AgNPs are produced depending on the technology used for the synthesis of AgNPs. AgNPs have distinctive photosensitive, thermal and electrical properties and is used in various products that ranging from photovoltaic to biological and chemical sensors (Ibrahim 2015). AgNPs can be synthesized by various methods; one of them is the green synthesis which involves the use of natural bio-molecules extracted from plants/microbial cells. Proteins and bioactive components in the plant extract produce the AgNPs by reducing the silver ions (Ag+). The reduction of Ag+ by different means in a solution generally gives metallic silver of nanorange (Srikar et al. 2016). Due to the presence of hydroxyl and ketonic group, the phenolic compounds have a capability to bind metals. The metal chelating property of the phenolic compounds is owed to the high nucleophilic character. The aqueous extract of bioactive components is used to reduce the Ag+ into metallic silver (Ago) and also to capping the Ago. The reducing agents reduced the Ag+ into Ago which has a tendency to form oligomeric clusters in the absence of capping/stabilizing agent (Iravani et al. 2014) therefore use of protective agents are very crucial to stabilize dispersive AgNPs. Selection of eco-friendly, harmless and stabilizing agents are the major concern in the green synthesis of AgNPs (Iravani et al. 2014).
Melamine (Mel; 1,3,5-triazine-2,4,6-triamine) adulteration in milk is a serious health concern for the consumers, which is generally used to increase the nitrogen content of the milk. Having multiple binding sites, melamine can strongly coordinate to NPs by ligand exchange with weakly surface-bound citrate ions, and cross-link with NPs (Chi et al. 2010) which interfere the synthesis of NPs formation and causes a significant shift in the characteristics spectrum and the solution color of reaction mixture changes (Elghanian et al. 1997; Park et al. 2002; Lin et al. 2006). The clearly distinguishable color change can easily be detected by the naked eye (Chi et al. 2010). There are few studies available on the melamine detection, but still a lot of research is required. In the present investigation we studied the effect of bioactive components extracted from the culinary banana peel on the synthesis of AgNPs. The effect of pH, temperature and concentration of bioactive components on the synthesis of AgNPs were studied and the rapid technique was developed to detect the melamine content in the milk sample.
Materials and methods
Materials
Kachkol variety of culinary bananas was procured from horticulture department of Tezpur University; chemicals used for the present investigation were procured from Merck India Ltd.
Methodologies
Preparation of culinary banana peel powder
Fresh, culinary banana without any patches were peeled and the peels were converted into small shards, however the pulp of banana were donated to Pobitara Madam Curie Women’s Hostel, Tezpur University. The peels were then washed with 1% acetic acid solution and then dried in a tray drier at 60 °C up to 12.5% moisture content. The dried peel powder was converted into powder using lab grain mill with mesh size of 1.0 µm.
Extraction of bioactive components from banana peel powder
Peel powder (10 g) was thoroughly mixed into 100 ml of distilled water and were heated using a heating mantle till the temperature of the mixture reached to 90 °C. The reaction mixture was cooled to 25 °C and filtered using a muslin cloth, the volume of the filtrate was made up to 20 ml using distilled water followed by addition of 20 ml of acetone. The solution was centrifuged at 1000 rpm for 5 min and supernatant was collected and stored at 4 °C for further analysis (Ibrahim 2015).
Synthesis of silver nanoparticles (AgNPs)
For synthesis of AgNPs, 50 ml of silver nitrate (1.0–2 mM) solution was prepared and then BPE) (1–2 mL) was added to it, the pH of the mixture was adjusted in between 2–6, the reaction was carried out for 5 min in a water bath at a prespecified temperature (i.e. 40–80 °C), the solution was incubated at 30 °C for 30 min in dark (Ahmed et al. 2016). The synthesis of AgNPs was confirmed by changing of the colour of the reaction mixture.
Characterization of synthesized AgNPs
UV visible spectra of synthesized AgNPs
The reaction mixture containing AgNPs was scanned from 200–800 nm using a UV visible spectrophotometer (Carry 60 UV–VIS Agilent Technologies) and the obtained spectra were recorded to obtain the characteristic AgNPs (Ahmed et al. 2016).
Transmission electron microscopy (TEM) and Energy dispersive X-ray spectroscopy (EDX)
For TEM, 2–3 µmL of aqueous AgNPs was applied to a carbon coated copper grid and dried at 60 °C, the micrographs were obtained using TEM (TECNAI G2 S-Twin, USA) operating at 80 kV. The presence of AgNPs was determined by SEM equipped with EDX.
Fourier transform infrared spectroscopy (FTIR)
The FTIR spectra were collected using FT-IR spectrophotometer (Impact 410, Nicolet USA) using KBr pellets. The pallets were scanned from 4000–400 cm−1 and spectra were obtained from 50 scans at a resolution of 4 cm−1 (Mishra et al. 2015).
Determination of total phenolic content (TPC) and DPPH free radical (DPPH*) scavenging activity of BPE
The TPC and DPPH* scavenging activity of BPE was estimated as per the method of Mishra et al. (2015).
Detection of melamine content in milk using interference synthesis of AgNPs
For interference biosynthesis based sensing experiments with milk, milk sample was pretreated with 300 g/L trichloroacetic acid and the mixture was thoroughly mixed. A white precipitate observed after 1–2 min of mixing. The mixture was filtrated using filter paper followed by the addition of 3 M NaOH to adjust the pH upto 7.0. The filtrate was re-filtered using filter paper (Zhou et al. 2011) and the pretreated milk sample was converted into five different samples, i.e. (1) without the addition of melamine (control); (2) sample containing 0.5 mg ml−1 of melamine; (3) sample with 5 mg ml−1 melamine; (4) sample with 50 mg ml−1 of melamine and (5) sample with 500 mg ml−1 of melamine. With these solutions known concentrations of AgNO3 and BPE extract was added and the synthesis reactions were carried out at specified pH and temperatures. All the reaction mixtures were then subjected to UV–Vis scan with the range of 300–800 nm to observe the effect of melamine on synthesis of AgNPs.
Statistical analysis
All samples were analyzed in triplicates and the values were reported as mean ± S.D.
Results and discussion
Synthesis and characterization of silver nanopartilces (AgNPs)
Effect of pH on synthesis of AgNPs
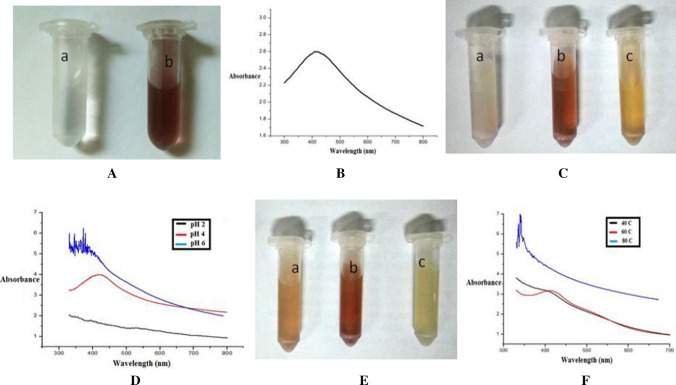
The culinary BPE used for the synthesis of AgNPs had TPC 78.2 mg 100 ml−1 GAE and 82% DPPH* scavenging activity. The synthesis of AgNPs is marked with a change in the colour of the reaction mixtures (Ibrahim 2015). The image of AgNO3 solution and AgNPs synthesized using chemicals are shown in Fig. 1Aa, Ab respectively, where a change in the color of the solution indicates the formation of AgNPs. Recorded UV spectra of the synthesized AgNPs using chemicals are shown in Fig. 1B. As the noble metals AgNPs exhibit unique optical properties that is called surface plasmon resonance (SPR) (Bindhu and Umadevi 2013). AgNPs produced characteristics SPR band due to collective oscillation of electrons on the metallic surface of silver, an intense peak at characteristics λmax indicates a high yield and narrower distribution of AgNPs (Ashraf et al. 2013).
Fig. 1.
Image of A a AgNO3 solution; b Image of AgNPs (synthesized using chemical method) containing solution, B characteristics UV–Vis spectra of AgNPs (synthesized using chemical method) containing solution; C Image of synthesized AgNPs using culinary BPE a at pH 2; b at pH 4; and at c pH 6; D UV–Vis spectra of synthesized AgNPs using culinary BPE at different pH; E Image of synthesized AgNPs using culinary BPE a at 40 °C; b at 60 °C; and c at 80 °C; and F UV–Vis spectra of synthesized AgNPs using culinary BPE at different temperature
AgNPs had a characteristics SPR band at around 400 nm in UV–Vis spectra (Darroudi et al. 2011) however, in present investigation AgNPs showed a characteristics λmax at 413 nm. NPs with a smaller number of faces and sharper vertices show resonances in a broad range of wavelengths. AgNPs usually have SPR peak in the range of 400–500 nm. The blue shift in the SPR peak is generally an indication of a decrease in particle diameter (Raza et al. 2013). In the present investigation, we tried to synthesize the AgNPs using an extract of culinary BPE through green route technology. To study the effect of pH of the solution on synthesis of AgNPs, the pH of the solution was varied from 2–8 pH, however temperature, AgNO3 and amount of BPE were kept constant at 40 °C, 1.5 mM and 1.5 ml respectively.
With the addition of BPE to the AgNO3 solution, the color of the reaction mixture changed to reddish brown color indicated the formation of AgNPs. Presence of active molecules in BPE reduced Ag+ into metallic silver Ago (Ahmed et al. 2016). Image of reaction mixture synthesized at 2, 4 and 6 pH is shown in Fig. 1Ca, Cb, Cc respectively. No change in color was observed when synthesized at pH 8 and hence not presented here, however reddish brown color was observed in reaction mixture when synthesized at pH 4. At pH 2 there was no color change and reaction mixture became very turbid which indicated that pH 2 was also not effective to reduce the silver nitrate into AgNPs. At pH 2 the functional group presents in polyphenols to reduce Ag+ were hydrolyzed or adversely affected so could not reduce the silver nitrate into AgNPs (Fig. 3) while pH 4 effectively reduced the silver ion into metallic silver which was evident from the reddish brown colour of the solution (Fig. 1Cb). Yellowish brown colour of the reaction mixture was observed when synthesized at pH 6, antioxidant property of the bioactive components may be affected at pH 6 and so reduce the capability of the antioxidants to reduce the AgNO3 into AgNPs (Mishra et al. 2015). The characteristics λmax can clearly be seen in the AgNPs solution when synthesized at pH 4 (Fig. 1D) however, there was no characteristics peak was observed in the sample synthesized at pH 2 whereas, shifting in peak from 413 nm was observed at pH 6. The UV spectra of the samples synthesized at different pH were in line with the conclusion that we drawn from the Fig. 1C. The differences in the capability to synthesize AgNPs over the pH range could be caused due to a difference in the dissociation constants (pKa) of functional groups present on the involved bioactive components (Pimprikar et al. 2009).
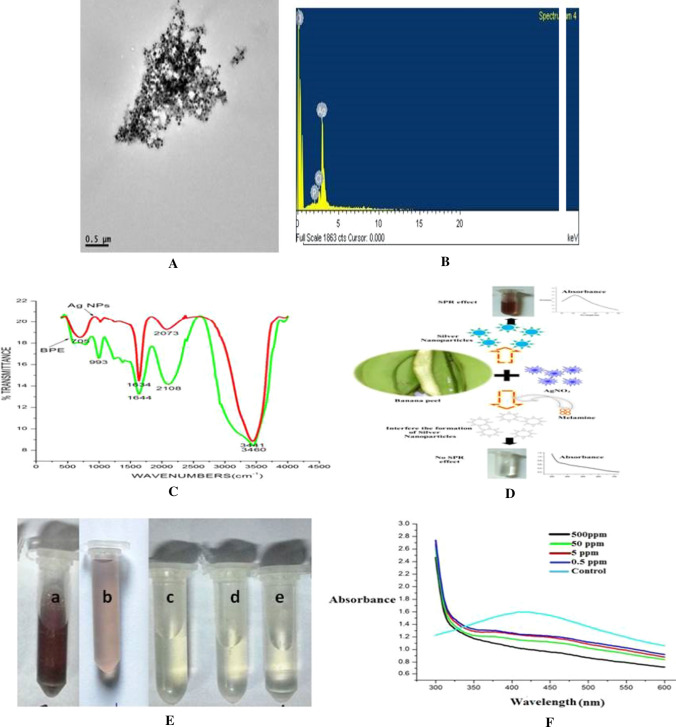
Fig. 3.
A Transmission electron microscope of synthesized AgNPs under optimum conditions; B SEM–EDX of synthesized AgNPs under optimum conditions; C FTIR spectra of BPE and synthesized AgNPs; D Mechanism of method of detection of melamine in milk; E Image of a synthesized AgNPs solution under optimum conditions containing milk sample; b AgNPs solution containing 0.5 mg/ml melamine milk sample; c AgNPs solution containing 5.0 mg/ml melamine milk sample; d AgNPs solution containing 50.0 mg/ml melamine milk sample; e AgNPs solution containing 500 mg/ml melamine milk sample; and F UV spectra of AgNPs solution of having different concentration of melamine
Effect of temperature on synthesis of AgNPs
The temperature of the mixture during the reaction were varied from 40 to 100 °C and other parameters like pH, AgNO3 and BPE were kept constant at 4, 1.5 mM and 1.5 ml respectively. Figure 1Ea, Eb, Ec presents the images of reaction mixture at 40, 60 and 80 °C respectively. No significant change in the color is observed at 100 °C synthesis temperature and so not reported here. Solution synthesized at 40 and 60 °C temperature showed a characteristic peak of SPR in the range of 410 nm, however no peak in the range of 400–500 nm was found in the reaction mixture which was synthesized at 80 °C (Fig. 1F). AgNPs synthesized at 60 °C was comparatively more effective than to 40 °C as an increase in the intensity of peak at λmax was obvious in the sample synthesized at 60 °C this may be due to the increased rate of dissociation of Ag+ from AgNO3 in the solution and hence more availability of Ag+ to react with functional group of BPE which increased the rate of formation of AgNPs in the solution. Application of exposure of high temperature like 80 °C or 100 °C adversely affected the bioactive components (responsible for reduction of Ag+) (Mishra et al. 2015) and so reduced the capability of extract to synthesize and capping of AgNPs, and thus 60 °C and pH 4 were standardized for further studies.
Effect of AgNO3 molar concentration on synthesis of AgNPs
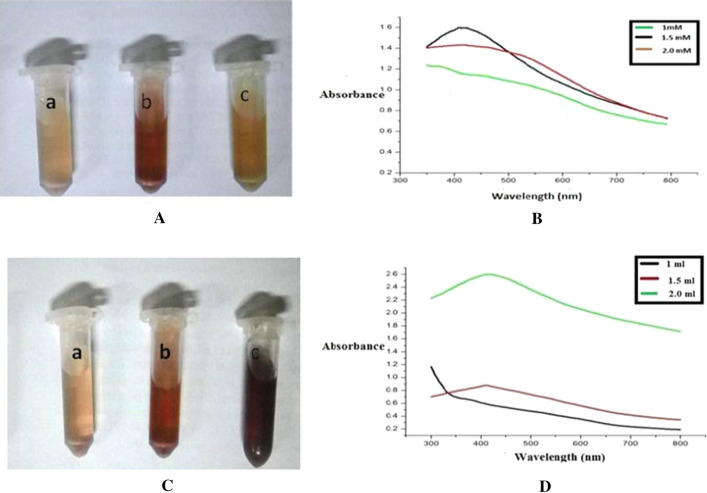
The AgNO3 level for synthesis was varied from 0.5 to 2.0 mM and other parameters like pH, temperature and BPE extract were kept constant at 4, 60 °C and 1.5 ml respectively. AgNPs produced with 0.5 mM concentration showed no significant change in the color of a solution, and not shown in the Fig. 2A. At 1 mM AgNO3 concentration the color of reaction mixtures was comparatively lighter (Fig. 2Aa) than to 1.5 mM (Fig. 2Ab). But at a concentration of 1.5 mM the colour of the reaction mixture was of reddish brown colour and at a concentration of 2 mM the colour of the mixture was orangish brown (Fig. 2Ac) in color. The SPR peak of AgNPs became distinct with increasing the concentration of AgNO3, the highest peak intensity was observed with 1.5 mM of AgNO3, but at concentration of 2 mM the SPR peak was again less pronounced (Fig. 2B) which might be due to the fact that the amount of BPE extract was not sufficiently enough for capping of all of the Ag+ present in the mixture and so 1.5 mM AgNO3 was used for further studies.
Fig. 2.
A Image of synthesized AgNPs with different concentration of AgNO3 solution: a 1.0 mM AgNO3; b 1.5 mM AgNO3; c 2.0 mM AgNO3; B UV–Vis spectra of synthesized AgNPs using culinary BPE with different concentration of AgNO3 solution; C Image of synthesized AgNPs with different concentration of BPE: a 1 ml; b 1.5 ml.; and c 2.0 ml; D UV–Vis spectra of synthesized AgNPs using culinary BPE with different concentration of BPE
Effect of BPE concentration on production of AgNPs
The BPE extract, a crucial component for synthesis of AgNPs was varied from 0.5 to 2.0 mL and the rest of the parameters were kept constant at their optimum value. The amount of BPE influenced the color of the reaction and formation of AgNPs significantly. As the amount of BPE increased in the reaction mixture from 0.5 to 2 ml the intensity of the brown color was also increased. Figure 2Da, Db, Dc show the color reaction of 1.0, 1.5 and 2 ml respectively. The dark brown color was obtained when 2 ml of BPE was used for the synthesis of AgNPs. The change in colour was almost negligible for reaction mixture containing 0.5 ml of BPE hence not shown here. Lower concentration of BPE (i.e. 0.5 and 1 ml) was not sufficient to reduce and capping of all the available Ag+ ions. When the concentration of the bioactive component used for synthesis of nanoparticle is increased, sufficient amount of biomolecules involved in the reduction of metal resulting in a more intense color. Such an effect has been observed with the bark extract of Cinnamon zeylanicum (Satishkumar et al. 2009) and with the leaves of Cinnamon camphora (Huang et al. 2007). The maximum peak intensity at λmax was observed when 2 ml of BPE was used in the reaction mixture and the 0.5 ml of BPE showed minimum intensity at λmax. A significant increase in the intensity of the peaks at characteristics λmax reflects the formation of regular, spherical and smaller particle size AgNPs. Jeeva et al. (2014) also reported that the presence of bioactive components in plant extract acts as a reducing agent and hence stabilize the AgNPs. So pH 4, temperature 60 °C, AgNO3 1.5 mM and BPE 2.0 ml were considered as optimum for the synthesis of AgNPs.
Characterization of AgNPs produced under optimum conditions
In order to characterize the synthesized AgNPs under optimum conditions, AgNPs were analyzed using TEM, SEM EDX, FTIR and spectrofluorometer. The synthesized AgNPs under standardized conditions were of circular and regular in shape (Fig. 3A). The intense and characteristic peak observed at 413 nm showed that the used technology for the production of AgNPs was sufficiently effective to produce spherical, regular, uniform and smaller sized AgNPs.
The elemental profile of AgNPs using BPE showed higher counts at 3 keV due to silver, which confirmed the production of AgNPs. Normally, metallic silver nano-crystals have characteristics, optical absorption peak approximately at 3 keV due to their SPR (Magudapathy et al. 2001). It is obvious from Fig. 3B that the silver is the only metal present in the AgNPs solution and other elements like P and chlorine are non metal by nature and may come in the solution from the BPE extract.
Synthesized AgNPs containing solutions were analyzed to recognize the major functional groups present in BPE and their probable connection in the synthesis and stabilization of AgNPs using FT-IR. The UV spectra of BPE before and after reaction with AgNO3 are shown in Fig. 3A. The bands appearing at 993, 1644, 2108 and 3460 cm−1 were due to the presence of stretching vibration in BPE, and after interaction with AgNO3 significant shift in the peaks was observed (i.e. 993–705, 1644–1634, 2108–2073, and 3460–3441 cm−1) due to the effective conjugation between functional groups of BPE and AgNPs. Bioactive components are well known to interact with metal salts via their functional groups and intervene their reduction to synthesize nanoparticles (Bar et al. 2009).
Application of synthesized AgNPs for melamine detection in milk
In our study, we developed a rapid and less expensive technique using AgNPs to detect melamine in milk sample where milk was pretreated as per the method of Zhou et al. (2011) and then varying concentration of melamine was added in the pretreated milk. The polyphenols present in the extract reduce the Ag+ ions into Ago which can be easily evident from the change in the colour of the solution. The intensity of the colour from light brown to dark brown, depends on the amount of synthesized AgNPs into the solution. When the melamine is added along with the BPE in AgNO3 solution during the synthesis of AgNPs, the melamine interferes with the biosynthesis of AgNPs. Silver can easily bind to melamine due to the presence of three amine groups (–NH2) in melamine, therefore the optical change in color can be effectively used for the detection of melamine in milk products (Daniel et al. 2017) Fig. 3C explain the interference mechanism of melamine for biosynthesis of AgNPs. The AgNO3 and BPE extract was added into the melamine containing treated milk sample to study the effect of interferences of melamine and milk on the synthesis of AgNPs. Figure 3E presents the image of reaction mixtures with having different concentrations of melamine i.e. Fig. 3Ea (control), Fig. 3Eb (0.5 mg L−1 melamine), Fig. 3Ec (5 mg L−1 melamine), Fig. 3Ed (50 mg L−1 melamine) and Fig. 3Ee (500 mg L−1 melamine). A significant decrease in the intensity of the colour of 0.5 mg L−1 melamine containing reaction mixture confirmed the interference mechanism of melamine (Fig. 3Eb). No change in colour of the solution was observed above 5 mg L−1, concentration above 5 mg L−1 completely interfered with the formation AgNPs and so no change in colour was observed. Sample without melamine (control) showed characteristic peak at λmax however sample of having 0.5 and 5 mg L−1 showed shifting of the SPR peak whereas solution with 50 and 500 mg L−1 showed no peak in the range of 400–500 nm (Fig. 3F). The concentration of melamine above 0.5 mg L−1 was effective to shift the SPR peak, however concentration of melamine above 5 mg L−1 concentration interferes with the synthesis of AgNPs and so change in colour or presence of SPR peak was not found in the sample. The limit of quantification value (LOQ) of FSSAI method for melamine detection in cow milk is 0.1 mg/kg (FSSAI Manual), therefore the further emphasis can be given in future to further develop a rapid method for detection of milk at the level of 0.1 mg/kg of milk or milk products.
Conclusion
AgNPs was synthesized effectively using bioactive components of culinary banana peel. The parameters including pH, temperature, concentration of AgNO3 solution, and amount of culinary banana extract used for synthesis significantly affected the properties of synthesized AgNPs. Characterization of AgNPs confirmed the formation of spherical and uniform sized AgNPs however FTIR study showed the effective conjugation between AgNPs and bioactive components of culinary banana extract. The synthesized AgNPs under optimized conditions was found to be effective to detect the melamine content (above 0.5 mg L−1) in milk sample.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Ahmed S, Ahmad M, Swami BL, Ikram S. Green synthesis of silver nanoparticles using Azadirachta indica aqueous leaf extract. J Radiat Res App Sci. 2016;9(1):1–7. [Google Scholar]
- Ashraf S, Abbasi AZ, Pfeiffer C, Hussain SZ, Khalid ZM, Gil PR, Parak WJ, Hussain I. Protein-mediated synthesis, pH-induced reversible agglomeration, toxicity and cellular interaction of silver nanoparticles. Colloids Surfaces B Biointerfaces. 2013;102:511–518. doi: 10.1016/j.colsurfb.2012.09.032. [DOI] [PubMed] [Google Scholar]
- Bar H, Bhui DK, Sahoo GP, Sarkar P, De SP, Misra A. Green synthesis of silver nanoparticles using latex of Jatropha curcas. Coll Surf A Physicochem Engin Asp. 2009;339(1):134–139. doi: 10.1016/j.colsurfa.2009.02.008. [DOI] [Google Scholar]
- Bindhu MR, Umadevi M. Synthesis of monodispersed silver nanoparticles using Hibiscus cannabinus leaf extract and its antimicrobial activity. Spectrochim Acta part A Mol Biomol Spectro. 2013;101:184–190. doi: 10.1016/j.saa.2012.09.031. [DOI] [PubMed] [Google Scholar]
- Chi H, Liu B, Guan G, Zhang Z, Han M-Y. A simple, reliable and sensitive colorimetric visualization of melamine in milk by unmodified gold nanoparticles. Analyst. 2010;135(5):1070. doi: 10.1039/c000285b. [DOI] [PubMed] [Google Scholar]
- Daniel SCGK, Julius LAN, Gorthi SS. Instant detection of melamine by interference biosynthesis of silver nanoparticles. Sens Acutators B Chem. 2017;238:641–650. doi: 10.1016/j.snb.2016.07.112. [DOI] [Google Scholar]
- Darroudi M, Ahmad MB, Abdullah AH, Ibrahim NA. Green synthesis and characterization of gelatin-based and sugar-reduced silver nanoparticles. Int J Nanomed. 2011;6:569–574. doi: 10.2147/IJN.S16867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elghanian R, Storhoff JJ, Mucic RC, Letsinger RL, Mirkin CA. Selective colorimetric detection of poly-nucleotides based on the distance-dependent optical properties of gold nanoparticles. Science. 1997;277:1078–1081. doi: 10.1126/science.277.5329.1078. [DOI] [PubMed] [Google Scholar]
- FSSAI Manual methods for detection of milk and milk products (2208), downloaded on 3/09/2019, pp 183–186
- Huang J, Li Q, Sun D, Lu Y, Su Y, Yang X, Hong J. Biosynthesis of silver and gold nanoparticles by novel sundried Cinnamomum camphora leaf. Nanotechnology. 2007;18(10):105104. doi: 10.1088/0957-4484/18/10/105104. [DOI] [Google Scholar]
- Ibrahim MMH. Green synthesis and characterization of silver nanoparticles using banana peel extract and their antimicrobial activity against representative microorganisms. J Rad Res App Sci. 2015;8:265–275. [Google Scholar]
- Iravani S, Korbekandi H, Mirmohammadi SV, Zolfaghari B. Synthesis of silver nanoparticles: chemical, physical and biological methods. Res Pharmaceut Sci. 2014;9(6):385. [PMC free article] [PubMed] [Google Scholar]
- Jeeva K, Thiyagarajan M, Elangovan V, Geetha N, Venkatachalam P. Caesalpinia coriaria leaf extracts mediated biosynthesis of metallic silver nanoparticles and their antibacterial activity against clinically isolated pathogens. Indus Crops Prod. 2014;52:714–720. doi: 10.1016/j.indcrop.2013.11.037. [DOI] [Google Scholar]
- Lin CX, Xie MY, Chen JJL, Liu Y, Yan H. Rolling circle amplification of a DNA nanojunction. Angew Chem Int Ed. 2006;45:7537–7539. doi: 10.1002/anie.200602113. [DOI] [PubMed] [Google Scholar]
- Magudapathy P, Gangopadhyay P, Panigrahi BK, Nair KGM, Dhara S. Electrical transport studies of Ag nanoclusters embedded in glass matrix. Phys B Condens Matter. 2001;299(1):142–146. doi: 10.1016/S0921-4526(00)00580-9. [DOI] [Google Scholar]
- Mishra P, Dutta N, Mahanta CL. Partial extraction and identification of phenolics in Amla (Emblica officinalis) seed coat powder. J Food Sci Technol. 2015;52(11):6990–7001. doi: 10.1007/s13197-015-1835-y. [DOI] [Google Scholar]
- Park SJ, Taton TA, Mirkin CA. Array-based electrical detection of DNA with nanoparticle probes. Science. 2002;295:1503–1506. doi: 10.1126/science.1066348. [DOI] [PubMed] [Google Scholar]
- Pimprikar PS, Joshi SS, Kumar AR, Zinjarde SS, Kulkarni SK. Influence of biomass and gold salt concentration on nanoparticle synthesis by the tropical marine yeast Yarrowia lipolytica NCIM 3589. Coll Surf B Biointerf. 2009;74(1):309–316. doi: 10.1016/j.colsurfb.2009.07.040. [DOI] [PubMed] [Google Scholar]
- Ploetz RC, Kepler AK, Daniells J, Nelson SC (2007) The Biology of Musa L. (banana) Banana and Plantain: an overview with emphasis on Pacific Island cultivars. In: Elevitch CR, Species profiles for Pacific Island agroforestry. Permanent agriculture resources (PAR), Hōlualoa, Hawaii. Australian Government. Retrieved 01 October 2013
- Raza S, Yan W, Stenger N, Wubs M, Mortensen NA. Blueshift of the surface plasmon resonance in silver nanoparticles: substrate effects. Opt Expr. 2013;21(22):27344–27355. doi: 10.1364/OE.21.027344. [DOI] [PubMed] [Google Scholar]
- Satishkumar M, Sneha K, Won SW, Cho CW, Kim S, Yun YS. Cinnamon zeylanicum bark extract and powder mediated green synthesis of nano-crystalline silver particles and its bactericidal activity. Coll Surf B Biointerfaces. 2009;73(2):332–338. doi: 10.1016/j.colsurfb.2009.06.005. [DOI] [PubMed] [Google Scholar]
- Srikar SK, Giri DD, Pal DB, Mishra PK, Upadhyay SN. Green synthesis of silver nanoparticles: a review. Green Sustain Chem. 2016;6(01):34. doi: 10.4236/gsc.2016.61004. [DOI] [Google Scholar]
- Zhou Q, Liu N, Qie Z, Wang Y, Ning B, Gao Z. Development of gold nanoparticle based rapid detection kit for melamine in milk product. J Agric Food Chem. 2011;59:12006–12011. doi: 10.1021/jf202919a. [DOI] [PubMed] [Google Scholar]