Abstract
The aim of study was to optimize fermentation parameters for inulinase production from Rhizopus oryzae by a statistical approach and to carry out purification of inulinase. Five isolated fungal strains were screen out inulin degradation by using Lugol’s iodine solution. R. oryzae exhibited maximum zone of clearance around the colony and was used as an inulinase producer. The effect of carbon sources (inulin, glucose, maltose, sucrose, lactose, onion peel, stevia root, wheat bran) as medium component and fermentation parameters (temperature (25–45 °C), initial pH (4–7), time (3–7 days)) on inulinase production was investigated by Plackett–Burman Design. Wheat Bran (WB), temperature, pH, and incubation time were found to be significant for the production of inulinase (P < 0.05). Furthermore, Box–Behnken Design was employed to optimize fermentation conditions. The maximum experimental results for inulinase activity and specific activity were 348.36 EU/mL and 3621.78 EU/mg, respectively. The results were obtained at 5 days of incubation time, 35 °C of incubation temperature, initial pH of 5.5, and 2% (w/v) WB. Also, inulinase was purified by using ammonium sulfate precipitation, gel filtration chromatography with 2.19-fold and its molecular weight was found as 89.12 kDa. The optimal pH and temperature of the purified enzyme were 4.0 and 60 °C, respectively. Furthermore, the purified enzyme showed excellent stability at 60 °C. In conclusion, the present study offers cost-effective method to produce inulinase from Rhizopus oryzae. Also, it can be suggested that the purified inulinase has strong potential for usage in production of fructose syrup and other industrial areas.
Electronic supplementary material
The online version of this article (10.1007/s13197-020-04591-3) contains supplementary material, which is available to authorized users.
Keywords: Inulinase, Box–Behnken design, Purification, Submerged fermentation
Introduction
Inulin is the second most common polysaccharide found in plants after starch. It is a fructan containing β (2 → 1) fructosyl-fructose structure terminated with a sucrose unit and located in the roots and tubers of many plants such as Jerusalem artichoke, chicory, leek, garlic asparagus, dandelion, and camas (Singh and Singh 2017). Inulin rich plant has recently presented a great opportunity to produce high fructose syrup as a sweetener and a prebiotic, due to its high sweetening properties, to provide more absorption of iron and calcium. On the other hand, fructose has great importance as a sweetener because it doesn’t cause an insulin response, as the glucose-metabolic pathway is bypassed (Gong et al. 2008; El Aty et al. 2014; Ilgın et al. 2019).
Inulinases (2,1‐β‐D‐fructanohydrolase EC 3.2.1.7) are a group of important carbohydrate-degrading enzymes. They are classified into two main groups as endo‐ and exoinulinases according to their hydrolytic action. Exoinulinases produce high fructose syrups with d-fructose content over 95% by acting on the terminal fructose unit of the inulin while endoinulinases hydrolyze the internal β-2,1 glycosidic links of inulin to produce oligofructose syrups (Singh and Singh 2017). Inulinases are industrially important enzymes and have been widely used for the production of ethanol, inulin oligosaccharides, pullulan, sorbitol, single cell oil, gluconic acid, lactic acid, and citric acid besides of high fructose syrups (Ilgın et al. 2019). In particular, fructose can also be produced by acid hydrolysis of inulin or enzymatic hydrolysis of starch, but both methods are expensive and give 45% fructose yield (Singh and Singh 2017). Inulinases provide yield up to 95% pure fructose (Gill et al. 2006).
Inulinase can be produced through both submerged fermentation (SmF) and solid state fermentation (SSF) by microorganisms such as Aspergillus, Rhizopus, Penicillium, Kluyveromyces. A. niger is one of the most common microorganisms studied to obtain inulinase (Mohamed et al. 2015). However, species of Rhizopus sp. have also been reported for effective inulinase production (Ohta et al. 2002; Mohamed et al. 2015; Karam et al. 2018). Usually, SmF is the mode of inulinase production (Das et al. 2019a), although enzyme production in SSF has many advantages such as higher yield and higher product stability. Enzymes in SSF produce the surface of the solid substrate particle having low moisture content by a microorganism, but water is known to affect the whole process efficiency (Das et al. 2019b). It is difficult to control the fermentation conditions in SSF when compared to SmF (Saqib et al. 2010). SmF which has proven to be the best method for microbial inulinase production, is a widely used technique due to its advantageous properties such as ease of control and sterilization (Germec et al. 2020).
After the selection of the fermentation process, the formulation of the medium and fermentation conditions for inulinase production is a crucial issue to achieve optimal microbial growth and improved enzyme production (Singh et al. 2018). The conventional one-factor-at-a-time approach which does not guarantee to attain optimal conditions is both laborious and time-consuming to perform in the process of optimization (Trivedi et al. 2012). Statistical approaches enable the evaluation of various factors affecting enzyme production and their interactions between them the fermentation process with a minimum number of experiments (Germec et al. 2020). Response Surface Methodology (RSM) is an efficient tool for optimization of different process parameters by using experimental designs. There are two main types of RSM: central composite design (CCD) and Box–Behnken design (BBD). CCD and BBD are widely used among RSM to optimization process (Raja and Murty 2013; Shet et al. 2018; Kamble et al. 2018). BBD uses fewer design points when compared to CCD, providing the advantages of time and materials consumed due to having fewer experiments (Cordova-Villegas et al. 2019).
Production of inulinases was carried out using a wide variety of substrates. In submerged fermentation, inulin and raw materials rich in inulin, which are naturally occurring, are mostly used as a carbon source. Glucose, fructose, lactose and inulin are also pure substance options used in inulinase production (Singh and Chauhan 2017). Various low-cost substrates like Agave sisalana, and Tithonia rotundifolia extract (Kamble et al. 2019), Tithonia weed (Kamble et al. 2018), wheat bran, rice bran, banana peel and orange peel (Onilude et al. 2012) have been reported to be used for inulinase production.
Today, enzymes are utilized in more than 40 industrial sectors and to make more than 700 commercial products. The global industrial enzyme market was $ 6 billion in 2017. The market is expected to grow up to 6% in the coming years (Arnau et al. 2020). In the parallel with the increasing importance of enzymes in industries, the research on cost effect and new productive enzymes becomes important. Therefore, the objective of this study was to purify and characterize inulinase from R. oryzae as a new enzyme source. Also, this study focused on inulinase production using a low-cost medium substrate by a statistical approach.
Materials and methods
Materials
Inulin from Alfa Aesar, Sephadex (G-100) and 3, 5-dinitrosalicylic acid (DNS) from Sigma were purchased. Glucose, sucrose, maltose and lactose were purchased from Sigma-Aldrich. Other chemical reagents used in experiments were analytical grade.
Microorganisms
Rhizopus oryzae HBF351, R.oryzae HBF367, Aspergillus fumigatus HBF364, A. terreus HBF362, A. fumigatus HBF356 were isolated from soil, Turkey. The fungal strains were identified at Adnan Menderes University, Faculty of Arts and Sciences, Biology Department, Turkey. Firstly, morphological identification of the species and then molecular identification was made. ITS (The Internal Transcribed Spacer) gene regions were used for PCR. Gene sequences were obtained. Sequence analysis of microfungi was performed by using BIOEDIT program. After the sequence analysis, obtained sequence data were compared with the data in GENBANK and the molecular identification of the species was made (Supplementary Table S1 and Figure S1). Organisms showing best growth at 40 °C or 50 °C were classified as thermotolerant and thermophilic, respectively.
Determination of fungal strain producing inulinase
Fungal strains producing inulinase were screened based on their potential to form an inulolytic zone on inulin-rich medium using a rapid plate screening assay (Allais et al. 1986; Li et al. 2011). All the fungal strains were grown on potato agar plates of inulin-enriched medium (1%). The plates were incubated at 40 °C for 4 days. Thereafter, plates were flooded with Lugol’s iodine solution (1.5% potassium iodide and 1% iodine) and kept for 3–5 min. Then, plates were washed 2–3 times with distilled water and kept open for 15–20 min for the visualization of the clear hydrolytic zone. Formation of the clear hydrolytic zone around the colonies confirmed the existence of extracellular inulinase.
Inoculum and submerged state fermentation
Prior to the commencement of the study, the strains were grown and maintained on potato dextrose agar (PDA) slants at 40 °C for 4 days to obtain the inoculum. After incubation, cultures were stored at 4 °C and sub-cultured after every 1 week. Induced slant was mixed with 10 mL sterile basal medium for preparing spore suspension. The spore count in the suspension was about 1.0 × 107 spore/mL. Pre-sterilized production medium was inoculated with 2% of the spore suspension (100 mL in 250 mL Erlenmeyer’s flask).
Various carbon sources (inulin, glucose, maltose, sucrose, lactose, onion peel, stevia root, WB) as substrate were used for inulinase production. Onion peel, stevia root, WB were purchased from local market. Dried and powdered onion peel and stevia root were used. The basal medium (100 mL) composed of MgSO4·7H2O 0.6%, KH2PO4·0.5%, yeast extract 0.4%, and carbon sources (1–3%) were used for inulinase production in 250 mL Erlenmeyer flask. In order to optimize the fermenting conditions, Experiments for incubation time, initial pH, and temperature at different intervals were performed to optimize enzyme production while agitation were fixed at 120 rpm (Danial et al. 2015; Kamble et al. 2019).
Enzymes production and statistical optimization
Plackett–Burman Design for determining medium component and parameters
This experimental statistical design is used to determine the important parameters required to increase inulinase production (Kamble et al. 2019). In the light of the literature, the parameters of enzyme production were determined (Saber and El-Naggar 2009; Trivedi et al. 2012; Danial et al. 2015; Singh and Singh 2017; Singh and Chauhan 2018; Germec and Turhan 2019). The effect of carbon sources as medium component and fermentation parameters on inulinase production was investigated by PBD. Each independent variable was screened out as low (− 1) and high (+ 1) level. For this, it was used in concentrations of 1% at low (− 1) and 3% at high (+ 1) levels to test the effect of concentration amounts of various carbon sources, including inulin (A), sucrose (B), glucose (C), maltose (D), lactose (E), onion peel (F), stevia root (G), WB (H) on enzyme production. Fermentation parameters were also evaluated at low (− 1) and high (+ 1) levels; incubation temperature (25–45 °C) (J), initial pH (4–7) (K), incubation time (3–7 days) (L) (Supplementary Table S2). In 12 experimental studies, 11 variables were scanned and a smaller group of factors affecting enzyme production was obtained by ignoring the statistically insignificant factors (Table 1). All experiments were performed in duplicate and the inulinase activities were taken as response.
Table 1.
Real values of the variables in Plackett–Burman experimental design with experimental and predicted values of inulinase activity
| Media | Inulin | Sucrose | Glucose | Maltose | Lactose | Onion peel | Stevia root | Wheat bran | Temperature | pH | Time | Experimental value | Predicted value |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| % | % | % | % | % | % | % | % | °C | Day | EU/mL | EU/mL | ||
| 1 | 3.00 | 3.00 | 1.00 | 1.00 | 1.00 | 3.00 | 1.00 | 3.00 | 45 | 4 | 7 | 14.64 | 14.70 |
| 2 | 1.00 | 3.00 | 3.00 | 1.00 | 3.00 | 3.00 | 3.00 | 1.00 | 25 | 4 | 7 | 16.41 | 16.35 |
| 3 | 1.00 | 1.00 | 3.00 | 1.00 | 3.00 | 3.00 | 1.00 | 3.00 | 45 | 7 | 3 | 10.63 | 10.57 |
| 4 | 3.00 | 1.00 | 3.00 | 3.00 | 1.00 | 3.00 | 3.00 | 3.00 | 25 | 4 | 3 | 26.92 | 26.98 |
| 5 | 3.00 | 1.00 | 1.00 | 1.00 | 3.00 | 1.00 | 3.00 | 3.00 | 25 | 7 | 7 | 14.51 | 14.45 |
| 6 | 3.00 | 3.00 | 1.00 | 3.00 | 3.00 | 3.00 | 1.00 | 1.00 | 25 | 7 | 3 | 15.37 | 15.31 |
| 7 | 3.00 | 1.00 | 3.00 | 3.00 | 3.00 | 1.00 | 1.00 | 1.00 | 45 | 4 | 7 | 8.51 | 8.45 |
| 8 | 1.00 | 3.00 | 1.00 | 3.00 | 3.00 | 1.00 | 3.00 | 3.00 | 45 | 4 | 3 | 17.32 | 17.26 |
| 9 | 1.00 | 1.00 | 1.00 | 3.00 | 1.00 | 3.00 | 3.00 | 1.00 | 45 | 7 | 7 | 1.91 | 1.97 |
| 10 | 3.00 | 3.00 | 3.00 | 1.00 | 1.00 | 1.00 | 3.00 | 1.00 | 45 | 7 | 3 | 0.79 | 0.85 |
| 11 | 1.00 | 3.00 | 3.00 | 3.00 | 1.00 | 1.00 | 1.00 | 3.00 | 1.00 | 7 | 7 | 15.5 | 15.56 |
| 12 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 4 | 3 | 22.24 | 22.30 |
Optimization of parameters affecting enzyme production in fermentation medium by Box–Behnken Design
The correlation among the most significant four variables obtained from PBD for inulinase production was developed by BBD (Kamble et al. 2019). The specified range of four variables used for optimization was selected to evaluate by the BBD. The fermentation parameters, including initial pH (4–7) (A), incubation temperature (25–45 °C) (B), WB level (1–3%) (C), and incubation time (3–7 days) (D), were most significant four variables. BBD was described at three levels as high (+ 1), medium (0) and, low (− 1) (Supplementary Table S3). A total of 29 experiments were constructed in the experimental design method and the center point was repeated five times to test the reproducibility of the test results. All the experiments were carried out in duplicates and the response function coefficients were determined by regression using the experimental data and Design-Expert V7 trial version. For four variable systems, the model equation is as follows:
| 1 |
where Y is the predictable response, β0 is the model constant; A, B, C, and D are independent variables; β1, β2, β3, and β4 are linear coefficients and β11, β22, β33, and β44 are the quadratic coefficient.
After optimization, experiments were conducted to validate the predicted inulinase and specific activities under the optimum fermentation conditions predicted by the statistical model.
Protein assay
Quantitative protein concentrations were defined by using method of Bradford (Bradford 1976).
Enzyme extraction and enzyme analysis
Inulinase activity was estimated by using inulin as a substrate according to the method described by Ilgın et al. (2019) as follows: The fermented broth was centrifuged at room temperature, and 8000 rpm for 10 min. The crude enzyme (supernatant) and purified enzyme was used for enzyme activity analysis. To measure enzyme activity, the mixture of 100 μL enzyme solution and 900 μL inulin (0.5% 100 mM Na‐acetate buffer, pH 5.0) was incubated at 50 °C for 15 min. The mixture was heated at 100 °C for 10 min after 1.5 mL of dinitrosalicylic acid reagent (DNS) was added to the test tubes. The control tubes were prepared to control nonenzymatic release of sugars (100 μL sample + 1.5 mL DNS reagent + 900 μL substrate, respectively). The blank was prepared using buffer instead of enzyme solution (Ilgın et al. 2019). Absorbance measurements were performed at 540 nm. Also, invertase assay was determined similarly to inulinase assay, except that the reaction mixture consisted of sucrose. One unit of activity enzymes was defined as the amount of enzyme that catalyzes the release of 1 μmol of fructose from inulin or sucrose per minute per mL. In addition, inulinase has also a hydrolytic activity on sucrose. Therefore, the inulin/sucrose activities ratio (I/S ratio) is determined to know whether the enzyme is inulinase or invertase.
Purification of inulinase
The crude extract was precipitated using 20–80% saturated ammonium sulphate and centrifuged at 6000 rpm (10 min). After the supernatant was discarded, the resulting precipitate was resuspended in 100 mM sodium acetate buffer (pH 5.0). The precipitate was loaded onto the Sephadex G-100 column equilibrated with buffer (0.1 M sodium acetate pH 5.0). The column was drained with the same buffer at a flow rate of 1 mL/min. Fractions (1 mL) were collected. The protein amount and inulinase activity of fractions were determined (Naidoo et al. 2015).
SDS-PAGE
SDS-PAGE (Sodium dodecyl sulfate–polyacrylamide gel electrophoresis) (12% resolving gel and 5% stacking gel) method was used to estimate molecular weight of purified inulinase from Rhizopus oryzae HBF351 (Laemmli 1970).
Effect of pH and temperature on enzyme activity
The effect of pH on enzyme activity was studied by using three buffer solutions with pH values ranging from 3.0 to 9.0. The buffers used in activity assay were Na asetat (0.1 M, pH 3.0–5.0), phosphate (0.1 M, pH 6.0–7.0), and Tris–HCL (0.1 M, pH 8.0–9.0). The effect of temperature on activity and stability of enzymes was defined by measuring the residual activity of the enzyme at 25–80 °C without substrate, after incubation for 1 h. The relative activities (%) were calculated for each pH and temperature values. Also, thermostability of the enzymes at 60 °C was evaluated. Relative activities for all tests were determined by taking 100% the enzyme activity at the start of the experiment. All tests were measured by using the standard assay procedure (Jeza et al. 2018).
Statistical analysis
Design and analysis of PBD, and BBD experiments were performed using Design-Expert V7 trial version. ANOVA was used to analyze the experimental results of inulinase and specific activities.
Results and discussion
Fungus strain selection for inulinase production and identification
The most important criteria in enzyme production are the selection of microorganism and fermentation medium. Plate screening assay is widely utilized to detect extracellular hydrolytic enzymes produced by microorganisms (Li et al. 2011). To determine inulinase producer microorganisms, R. oryzae HBF351, R. oryzae HBF367, A. fumigatus HBF364, A. terreus HBF362, A. fumigatus HBF356 were screened on plate screening assay. Among the tested microorganisms, R. oryzae HBF351 strain showed a clear zone on agar plate containing inulin (Supplementary Figure S2). Thus, R. oryzae HBF351 strain was used as an inulinase producer through the study.
Determination of important variables by Plackett–Burman Design for inulinase production
Various substrates were used to carry out the production of inulinases. Especially pure inulin and raw materials rich in inulin are mostly used in submerged fermentation. Inulin for the production of inulinase acts as a single source of carbon and an inducer (Singh and Singh 2017). Inulinase are industrially important enzymes; therefore, there is a need for a new medium design to increase enzyme production with low cost substrates, as well as new sources of microorganisms able to produce inulinase. Statistical methods are increasingly seen as a stronger option for fermentation optimization because they provide clearly understanding of the relationship between variables in the medium with fewer experiments. Hence, PBD is preferred to identify the most important components in the fermentation medium to produce the highest enzyme (El Aty et al. 2014).
According to the results from the PBD, a wide variation in inulinase activity among the experiment results reflects the need for optimization of fermentation medium. The minimum and maximum inulinase activities were observed in Medium 10 and Medium 4 as 0.79 EU/mL and 26.92 EU/mL, respectively (Table 1). Germec and Turhan (2019) were screened for inulinase production using 10 different carbon sources including glucose, fructose, xylose, lactose, sucrose, inulin, sugar beet molasses, carob extract, maltrin, and whey powder and the resulting activities were found to be 10.40 EU/mL, 3.09 EU/mL, 0.44 EU/mL, 9.28 EU/mL, 21.87 EU/mL, 22.37 EU/mL, 383.73 EU/mL, 68.07 EU/mL, 11.90 EU/mL, 11.37 EU/mL, respectively. The highest inulinase was obtained by sugar beet molasses. In another study, it was reported that inulinase produce was carried out by using glucose, fructose, maltose, sucrose, rafinose, starch, inulin, artichoke tuber, dahlia tuber, chicory root, beet pulp, orange peel, cane molasses, and beet molasses. The highest activity was obtained by the usage of dahlia tuber (26.50 EU/mL) while the use of maltose resulted in the lowest activity (7.66 EU/mL) (Saber and El-Naggar 2009). These results are in line with our results, as complex carbon sources have a positive effect on the production of enzymes more than pure carbon sources.
In general, microbial inulinases also have activity on sucrose. Hence, their catalytic activity is described in terms of I/S ratio. If I/S ratio is greater than 10−2, the enzyme is inulinase. The enzyme is sucrose when I/S ratio is lower than 10−4 (Germec and Turhan 2019). To determine whether the enzyme is inulinase or invertase, the sucrase activity (77.43 EU/mL) in the 10. Medium which the highest inulinase activity (26.92 EU/mL) obtained was determined. In the present study, I/S value was 0.34. The result shows that the enzyme can be defined as inulinase. In the literature, there is a large variation in I/S ratios. The ratios in the range of 0.02–7.9 have been reported. Also, it was suggested that there seems to be a direct relationship between inulinase and invertase activities on different carbon-sources (Jain et al. 2012). Our finding also corroborates other inulinase producers such as A. tamarii (I/S: 0.76–2.42) (Saber and el-Naggar 2009), Kluyveromyces marxianus (I/S: 0.02) (Jain et al. 2012), A. tubingensis CR16 (I/S: 0.18) (Trivedi et al. 2012) and A. niger A42 (ATCC 204447) (I/S: 0.97) (Ilgın et al. 2019).
In the study, ANOVA analysis was obtained from experimental run. The P value of the model is 0.0214, which shows that the model is significant (P < 0.05). From variables, H-WB, J-incubation tempetarure, K-pH, and L-incubation time were found to be significant for the production of inulinase (P < 0.05) (Supplementary Table S4). Pareto chart of effects was plotted for identifying the important factors for inulinase production (Supplementary Fig. 3S). In the chart, it is possible to observe positive and negative values. Based on the results, maltose, WB and onion peel had positive effect on inulinase production. Inulin, sucrose, glucose, stevia root powder, initial pH, incubation temperature and time were contributed negatively. Positive effect of the variable on the response means that if the variable increase to a high level, it will mean an increase in response. On the other hand, a negative effect indicates a better response at low levels of the variable. El Aty et al. (2014) found that glucose and sucrose in medium had negative effect on inulinase production.
In the study, WB, a complex carbon source, was more effective than pure sugar sources (inulin, glucose, maltose, sucrose, lactose) as well as other complex carbon sources (onion peel, stevia root). Furthermore, it was significant only WB (P < 0.05) among carbon sources tested, for enzyme production. Hence, the work was continued only with WB as a carbon source. Previous study reported that WB was supported the highest inulinase production (Onilude et al. 2012). Trivedi et al. (2012) also observed that WB is more effective for inulinase production than sucrose, fructose, glucose, and sugar bagasse. WB is known to be good source for inulinase production because it contains 1–4% inulin. Apart from this, it has variety of soluble and thus, provides high nutritional value for microorganisms to their growth. Besides inulinase, WB has been shown to be a good source of carbon for the production of many enzymes, such as xylanase, cellulases (Trivedi et al. 2012). Also, the effect of fermentation parameters was evaluated and all parameters (pH, temperature and incubation time) were found to affect enzyme production, besides carbon sources. Therefore, for further optimization, the effect of levels of significant variables (WB, incubation temperature, pH and time) and the relationship between them on inulinase production were determined using BBD to maximize the production of inulinase.
Optimization of fermentation medium by using Box–Behnken design for maximum enzyme production
In the study, BBD was employed to define the optimum level of parameters providing maximum enzyme production. Also, relationships among the tested fermentation parameters (initial pH (A), temperature (B), WB concentration (C), and incubation time (D)) were evaluated to understand. The all experimental results from experimental design are presented in Table 2. The adequacy of the model and identification of parameters with statistically significant effects were conducted by ANOVA. Analysis of variance for response surface quadratic polynomial models of inulinase and specific activity are shown that the models are significant (P < 0.0001) (as seen in Supplementary Table S5).
Table 2.
Experimental design based Box–Behnken model for the optimization of inulinase production
| Run order | Initial pH | Incubation temperature (°C) | WB (%) | Incubation time (days) | Inulinase activity* (EU/mL) | Spesific activity* (EU/mg) |
|---|---|---|---|---|---|---|
| 1 | 5.50 | 45.00 | 2.00 | 3.00 | 291.69 | 1669.08 |
| 2 | 5.50 | 35.00 | 2.00 | 5.00 | 329.7 | 3201.78 |
| 3 | 7.00 | 35.00 | 3.00 | 5.00 | 252.3 | 726.491 |
| 4 | 4.00 | 35.00 | 1.00 | 5.00 | 247.57 | 793.287 |
| 5 | 5.50 | 25.00 | 2.00 | 3.00 | 225.63 | 980.391 |
| 6 | 5.50 | 35.00 | 3.00 | 7.00 | 264.45 | 920.512 |
| 7 | 5.50 | 35.00 | 2.00 | 5.00 | 348.36 | 3621.78 |
| 8 | 5.50 | 35.00 | 2.00 | 5.00 | 334.5 | 3241.78 |
| 9 | 5.50 | 25.00 | 1.00 | 5.00 | 267.76 | 932.034 |
| 10 | 5.50 | 35.00 | 2.00 | 5.00 | 326.24 | 3311.78 |
| 11 | 7.00 | 45.00 | 2.00 | 5.00 | 279.66 | 2384.43 |
| 12 | 5.50 | 45.00 | 3.00 | 5.00 | 344.3 | 2552.5 |
| 13 | 4.00 | 35.00 | 2.00 | 7.00 | 173.03 | 1512.12 |
| 14 | 5.50 | 45.00 | 2.00 | 7.00 | 225.33 | 1330.25 |
| 15 | 7.00 | 35.00 | 1.00 | 5.00 | 252.3 | 1008.22 |
| 16 | 5.50 | 35.00 | 2.00 | 5.00 | 330.8 | 3441.78 |
| 17 | 5.50 | 45.00 | 1.00 | 5.00 | 264.42 | 1193.38 |
| 18 | 5.50 | 25.00 | 3.00 | 5.00 | 261.9 | 1254.83 |
| 19 | 4.00 | 35.00 | 2.00 | 3.00 | 254.12 | 1900.82 |
| 20 | 4.00 | 45.00 | 2.00 | 5.00 | 262 | 2320.18 |
| 21 | 7.00 | 35.00 | 2.00 | 3.00 | 220.18 | 1051.06 |
| 22 | 5.50 | 25.00 | 2.00 | 7.00 | 177.96 | 480.787 |
| 23 | 7.00 | 35.00 | 2.00 | 7.00 | 188.06 | 648.28 |
| 24 | 4.00 | 35.00 | 3.00 | 5.00 | 313.21 | 2766.7 |
| 25 | 5.50 | 35.00 | 1.00 | 3.00 | 282.91 | 651.223 |
| 26 | 5.50 | 35.00 | 3.00 | 3.00 | 276.76 | 1326.02 |
| 27 | 7.00 | 25.00 | 2.00 | 5.00 | 205.03 | 982.348 |
| 28 | 4.00 | 25.00 | 2.00 | 5.00 | 251.57 | 2256.95 |
| 29 | 5.50 | 35.00 | 1.00 | 7.00 | 183.78 | 305.5 |
*Experimental results
For inulinase activity and specific activity responses (Y) the regression equations obtained are as follows:
| 2 |
| 3 |
The statistical significance of model terms is evaluated by their respective P value. Also, “The Lack of Fit F-value” is a special diagnostic test or adequacy of a model and shows that the lack of fit is not significant relative to the pure error. Non-significant lack of fit is desirable. For inulinase and its specific activity, the predicted R2 (0.9589 and 0.9463 respectively) and adjusted R2 (0.9798 and 0.9753, respectively) values were in reasonable agreement with the value of R2 (0.9899 and 0.9877, respectively). The values of R2 imply a correlation between the experimental results and predicted values. It shows that the experiments are very reliable.
The statistical analysis indicates that the tested variables have a significant effect on inulinase activity. In this case, A, B, C, D, AB, AC, AD, BC, CD, A2, B2, C2, D2 are significant model terms. Also, A, B, C, D, AB, AC, BC, A2, B2, C2, D2 are significant model terms for specific activity of inulinase (as shown in Supplementary Table S6).
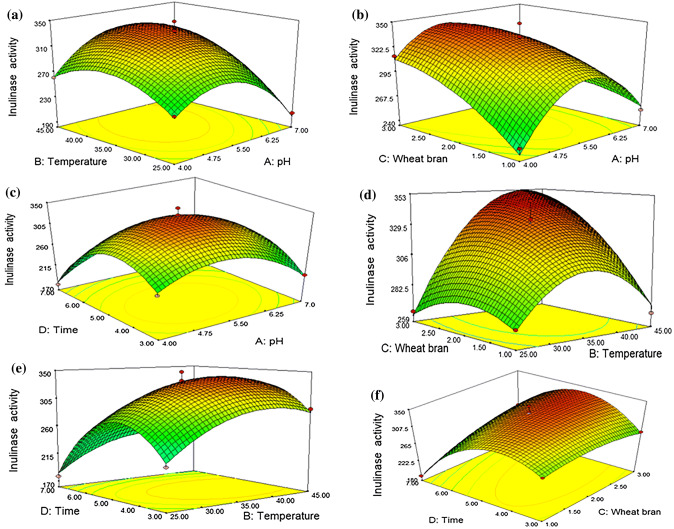
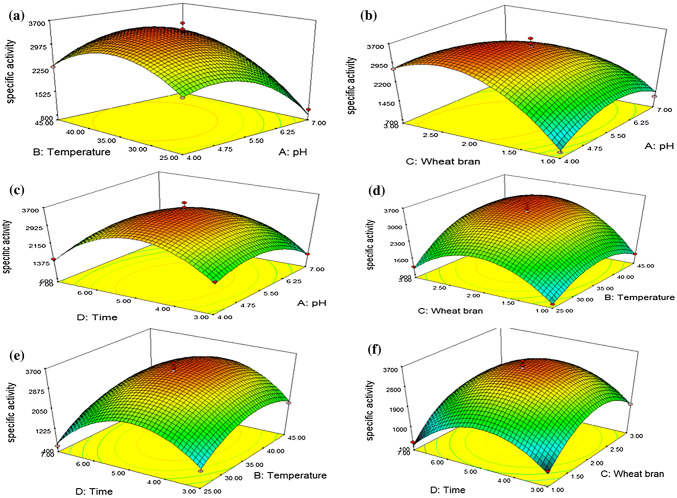
In this study, the maximum inulinase activity and specific activity were found 348.36 EU/mL and 3621.78 EU/mg, respectively (Table 2). The three-dimensional (3-D) response surface graphs were plotted to illustrate the interaction of two variables keeping other variables at their zero (central) level. The experimental results of models are shown as the 3-D plot graphical representations in Figs. 1 and 2.
Fig. 1.
3-D plots of the combined effect of two variables on inulinase activity
Fig. 2.
3-D plots of the combined effect of two variables on specific activity
Temperature and pH of the fermentation medium were reported as the most significant variables for inulinase production (Dinarvand et al. 2017). According to the Fig. 1A–C, the best enzyme production and growth rate of R. oryzae was obtained at acidic initial pH of 5.5. Furthermore, decreasing pH below 4.75 or increasing pH above 6.25 caused a decrease in inulinase activity. The finding is in accordance with the fact that most of the fungi have their optimum growth pH in an acidic range 4–6.5 (Trivedi et al. 2012; Singh and Chauhan 2017). Generally, inulinase producers from fungi are cultivated in initial fermentation medium pH between 4.5 and 7.0 (Singh and Singh 2017). Dinarvand et al. 2017 reported maximum activity for inulinase from A. niger ATCC 20611 at pH 6.5. Trivedi et al. 2012 found that increase in pH above 6.1 decreased the yield of inulinase from A. tubingensis CR16.
In the model, enzyme and specific activity were found to be affected significantly by pH (P < 0.05). Moreover, the interaction between pH and incubation time (A * D) was significant (P < 0.05) for interactive terms. However, the same relationship (A * D) was not statistically found for specific activity of inulinase (P > 0.05) (Supplementary Table S6). Specific activity of inulinase didn’t change dramatically in the range of pHs between 4.0 and 6.25 with increasing incubation time while decreased above pH 6.25. It is attributed to change of protein amount produced during fermentation. As seen in Fig. 2A–C, the highest specific activity (3621.78 EU/mg) was found at pH 5.5, similarly, to results of enzyme activity. Thus, it can be said that the amount of inulinase produced by R. oryzae was optimum with the highest activity at this pH. However, the specific activity is higher at lower pHs when compared inulinase activity; this can be explained with the low amount of produced protein at low pHs.
In the study, effect of incubation temperature was evaluated on enzyme production (Figs. 1A, E). Inulinase activity increased in the range of 30–40 °C. When the interaction between pH and temperature (A * B) for inulinase and specific activity is evaluated, it is seen that they are significant (P < 0.05) (Supplementary Table S6). Also, Fig. 2A, D, E illustrate that the highest specific activity is at 35 °C. However, the specific activity and inulinase activity began to decrease significantly at temperatures greater than 40 °C. This could be related to denaturation of enzyme. It was found at the previous report that the optimum temperature was 30 °C for inulinase production by A. niger ATCC 20611 (Dinarvand et al. 2017). The optimal fermentation temperature of most fungal species for inulinase production is 28–30 °C (Singh and Chauhan 2018).
Figure 1B, D, F show the effect of WB concentration on inulinase activity. Optimal inulinase activity was observed at 2% WB concentration, at pH 5.5 and 35 °C (348.36 EU/mL). The interaction between pH and temperature with WB concentration (A * C and B * C) for inulinase and specific activity is seen being significant (P < 0.05; Supplementary Table S6). Interestingly, an increase in WB concentration supports sustaining enzyme activity and specific activity, at a higher temperature and lower pHs (Figs. 1B–D and 2B–D). From these findings, it can be deduced that if enzyme production will be carried out at high temperatures and lower pH, the carbon source should be increased. Also, the relationship between WB concentration and incubation time was significant (C * D; P < 0.0001), but the same relationship could not be determined for specific activity (C * D; P > 0.05). This can be explained that an increase in incubation time increase other protein in the medium along with the enzyme. These results emphasize that the 2% WB concentration is enough for high yield in enzyme production. Production of inulinase from A. oryzae was carried out using WB in SMF with yield of 72.25 EU/mL (Singh and Singh 2017).
In the study, incubation time was found to have strong effect on enzyme and specific activity (P < 0.05). However, the interaction between temperature and incubation time (B * D) was insignificant for both (P > 0.05). Maximum activity was at 5 days of incubation. An increase in inulinase activity was observed with an increase in incubation period from 4th days to 5th days. Beyond fifth days, a decrease in enzyme production and specific activity may be the result of decreased nutrient sources in the medium or simultaneous production of protease. In the previous study, maximum inulinase production from Penicillium oxalicum BGPUP-4 was obtained after 5 days of incubation (Singh and Chauhan 2017).
In the study, the maximum experimental results for inulinase activity and specific activity were 348.36 EU/mL and 3621.78 EU/mg, respectively. The results were obtained at 5 days of incubation time, 35 °C of incubation temperature, initial pH of 5.5, and 2% (v/v) WB in run 7 (Table 2). Saber and El-Naggar (2009) found that maximum inulinase production from A. tamari was attained at pH 5.5 and 35 °C. Jeza et al. (2018) found that Paenibacillus sp. D9 produced a maximum of 50.9 EU/mL of inulinase activity at 30 °C, and pH 7.0 for 3 days. Danial et al. (2015) reported that the highest inulinase by Penicillium funiculosum was 163.5 EU/mL at 27 °C for 96 h. Also previous studies reported enzyme production results with different inulinase activity from various fungal source such as 72.3 U/mL from A. oryzae, 30.89 EU/mL from Penicillium oxalicum BGPUP-4 (Singh and Chauhan 2017), 3949 EU/mL from A. niger ATCC 20611 (Dinarvand et al. 2017). According to these results, the inulinase activity obtained in our study makes this fungus strain a potential candidate as an important inulinase producer.
Finally, the statistical model designed for the inulinase production was validated. To test the model validity, the predicted sets of optimized conditions which provide maximum values of both activities were obtained using the point prediction feature of the Design Expert software. The optimum condition for inulinase activity was predicted to be pH 5.20, 40.54 °C, 2.88% WB, and 4.98 days with maximal inulinase activity of 352.618 EU/mL. The optimum conditions for specific activity were predicted to be pH 5.02, 37.33 °C, 2.26% WB, and 4.87 days. The maximal specific activity was 3529.68 EU/mg. These predictions were validated by experiments in which 356.8 ± 3.45 EU/mL of inulinase activity and 3466.11 ± 4.12 EU/mg of specific activity were found. All experimental activity values were close to the predicted values. This implies confirmation of the validity of the statistical model.
Molecular mass determination and purification of inulinase
In the study, inulinase was purified from R. oryzae HBF351 with ammonium sulphate precipitation and gel filtration chromatography (Sephadex G-100). The results of purification steps of inulinase are given in Table 3. The first step for purification, the supernatant was subjected to 20–80% ammonium sulphate precipitation. Similar ammonium sulphate saturation rates were used in previous inulinase purification studies (Naidoo et al. 2015; Jeza et al. 2018). In the study, the enzyme was purified a 2.19-fold with a 20.04% yield as compared to the crude extract. The purification fold and yield obtained for inulinase purified from different sources by different methods are given in Table 4. There are also studies that are quite compatible with our results. For example, Sheng et al. (2008) purified the inulinase with a 2.44-fold increase and with a yield of about 22.4% while Mohamed et al. (2015) purified the inulinase 2 and inulinase 3 from R. oligosporus with 20 and 40-fold and with 10 and 17 yields, respectively. The results are very close to our findings from the purification fold and yield of inulinase.
Table 3.
Purification scheme for inulinase
| Purification steps | Total activity (EU) | Total protein (mg) | Specific activity (EU/mg) | Yield (%) | Purification (fold) |
|---|---|---|---|---|---|
| Culture supernatant | 20,901.6 | 5.94 | 3518.78 | 100 | 1 |
| Ammonium sulfate precipitation (20–80%) | 8178.7 | 1.35 | 6058.33 | 39.12 | 1.74 |
| Column chromatography | 4189.4 | 0.55 | 7617.09 | 20.04 | 2.19 |
Table 4.
Previous studies on the production and purification of inulinase
| Microorganism name | Bacteria/fungi | Purification method | Optimum pH | Optimum Temp. °C | Molecular weight (kDa) | Medium | Carbon source | Refs. |
|---|---|---|---|---|---|---|---|---|
| Cryptococcus aureus G7a | Yeast | Sephadex G-75 gel filtration DEAE Sepharose column chromatography | 5.0 | 50 | 60 | SMF | Inulin | Sheng et al. (2008) |
| Xanthomonas campestris pv. phaseoli KM 24 | Bacteria | Ammonium sulphat precipitation, Sephadex G-100 | 6.0 | 50 | 55 | SMF | Inulin | Naidoo et al. (2015) |
| Paenibacillus sp. D9 | Bacteria | Ammonium sulphate precipitation HiTrap QFF column and MMC column chromatography | 4.0 | 40 | 58.5 | SMF | Inulin | Jeza et al. (2018) |
| Rhizopus oligosporus NRRL 2710 | Fungus | Dialysis DEAE Sepharose Sephacryl S-200 CL-6B column chromatography | 5.0 | 50 | 76 | SMF | Artichoke leaves | Mohamed et al. (2015) |
| 60 | 30 | |||||||
| Rhizopus sp. Strain TN 96 | Fungus | Ultrafiltration DEAE-Cellulofine A-500 and Sephacryl S-200 HR column chromatography | 5.5 | 40 | 83 | SMF | Inulin Corn steep liquor | Ohta et al. (2002) |
| Aspergillus niger | Fungus | Three phase partitioning (non chromotographic process) | 4.6 | 60 | 63.8 | SMF | Inulin | Vinoth Kumar et al. (2011) |
| Rhizopus oryzae | Fungus | Ammonium sulphate precipitation, Sephadex G-100 | 4.0 | 60 | 89.12 | SMF | WB | This study |
Table 4 shows previous studies on the production and purification of inulinase from several microorganisms with chromatographic methods and three phase partitioning method. Different from those studies, inulinase was produced from R. oryzae by using WB, which is cheaper carbon source, and purified with ammonium sulphate precipitation and Sephadex G-100 column chromatography in this study with the high specific activity.
The molecular mass of inulinases from various sources ranges from 30 to 300 kDa (Singh and Singh 2017). In the study, the molecular mass of the inulinase was determined by SDS-PAGE electrophoresis (Supplementary Figure S4) and was calculated to be 89.12 kDa by comparison of standard markers proteins. The single band was observed in SDS-PAGE and was confirmed the homogeneity of the inulinase purified. The result is similar to molecular mass of inulinase from Rhizopus sp. strain TN 96 (Table 4).
Effect of pH and temperature on enzyme activity
Fungal inulinases have mostly optimal pH in a range of 4.0–7.0 (Sing and Sing 2017). In the study, the effect of pH on inulinase activity is evaluated (Supplementary Figure S5a). The purified inulinase showed optimum activity at pH 4.0 while maintenance over 50% of its activity in the ranges of pH 3–6. These findings were in correlation with several earlier reports (Jeza et al. 2018). Ohta et al. (2002) found to be 5.5 of pH optimal for inulinase from Rhizopus sp. Strain TN 96. The purified inulinase in this study can be a potential candidate for applications demanding acidic pH ranges in the industry.
The inulinases are active over a broad temperature range. The optimal temperatures of the enzymes from different microbial sources are given in Table 4. In this study, inulinase activity was measured at a ranging from 25 to 80 °C to find the optimum temperature. The inulinase activity was highest at 60 °C (Supplementary Figure S5b). Similarly, in a previous study, optimum temperatures for inulinases from R. oligosporus NRRL 2710 fungus strain were found to be 50 and 60 °C (Mohamed et al. 2015). The purified inulinase with high activity can efficiently provide fructose production in industrial applications.
In the study, thermostability of the enzyme was investigated by incubating the enzyme at 60 °C for 10 h and the remaining activity was determined. The inulinase activity retained 50.0% of its activity for 6 h, indicating that the enzyme was quite stable at 60 °C (Supplementary Figure S5c). From these results, it was seen that inulinase has a remarkable thermostability. Industrial fructose production from inulin is carried out at 60 °C, which provides a high hydrolysis rate due to the limited solubility of inulin and the possibility of microbial contamination at room temperature (Gill et al. 2006). Therefore, thermostable inulinases promise for industrial use.
Conclusion
In the study, inulinase was produced in optimum medium determined by PBD, and BBD from Rhizopus oryzae HBF351. Also, inulinase was purified with ammonium sulphate precipitation and gel filtration chromatography. The maximum inulinase activity and specific activity were 348.36 EU/mL and 3621.78 EU/mg, respectively and were obtained at 5 days of incubation time, 35 °C of incubation temperature, initial pH of 5.5, and 2% WB concentration. The high activity was obtained with WB as carbon source. It shows to be possible industrial usage of WB. Also, inulinase was purified by using ammonium sulfate precipitation, gel filtration chromatography with 2.19-fold and its molecular weight was found as 89.12 kDa. The purified inulinase was quite stable at 60 °C. This advantage makes that purified enzyme is good candidate for industrial use. As a result, this study has function to develop a more economical industrial process for the production of inulinase by cost effective carbon source. Also, R. oryzae proved a promising candidate for commercial inulinase due to high enzyme activity.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
Aydın Adnan Menderes University Scientific Research Projects (BAP-ADÜ-FEF-15011) provided financial support for the fungal isolates of this research.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Allais JJ, Kammoun S, Blanc P, Girard C, Baratti JC. Isolation and characterization of bacterial strains with inulinase activity. Appl Environ Microbiol. 1986;52:1086–1090. doi: 10.1128/AEM.52.5.1086-1090.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnau J, Yaver D, Hjort CM. Strategies and challenges for the development of industrial enzymes using fungal cell factories. In: Nevalainen H, editor. Grand challenges in fungal biotechnology. Cham: Springer; 2020. pp. 179–210. [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72(1–2):248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Cordova-Villegas LG, Cordova-Villegas AY, Taylor KE, Biswas N. Response surface methodology for optimization of enzyme-catalyzed azo dye decolorization. J Environ Eng. 2019;145(5):04019013. doi: 10.1061/(ASCE)EE.1943-7870.0001513. [DOI] [Google Scholar]
- Danial EN, Ayaz NOA, Alnahdi HSO. Production of inulinase by free and immobilized cells of Penicillium funiculosum p. 36. Braz Arc Biol Technol. 2015;58(4):636–642. doi: 10.1590/S1516-8913201500167. [DOI] [Google Scholar]
- Das D, Selvaraj R, Bhat MR. Optimization of inulinase production by a newly isolated strain Aspergillus flavus var flavus by solid state fermentation of Saccharum arundinaceum. Biocatal Agric Biotechnol. 2019;22:101363. doi: 10.1016/j.bcab.2019.101363. [DOI] [Google Scholar]
- Das D, Bhat R, Selvaraj R. Review of inulinase production using solid-state fermentation. Ann Microbiol. 2019;69(3):201–209. doi: 10.1007/s13213-019-1436-5. [DOI] [Google Scholar]
- Dinarvand M, Rezaee M, Foroughi M. Optimizing culture conditions for production of intra and extracellular inulinase and invertase from Aspergillus niger ATCC 20611 by response surface methodology (RSM) Braz J Microbiol. 2017;48(3):427–441. doi: 10.1016/j.bjm.2016.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Aty AAA, Wehaidy HR, Mostafa FA. Optimization of inulinase production from low cost substrates using Plackett–Burman and Taguchi methods. Carbohydr Polym. 2014;102:261–268. doi: 10.1016/j.carbpol.2013.11.007. [DOI] [PubMed] [Google Scholar]
- Germec M, Turhan I. Evaluation of carbon sources for the production of inulinase by Aspergillus niger A42 and its characterization. Bioproc Biosyst Eng. 2019;42(12):1993–2005. doi: 10.1007/s00449-019-02192-9. [DOI] [PubMed] [Google Scholar]
- Germec M, Gürler HN, Ozcan A, Erkan SB, Karahalil E, Turhan I. Medium optimization and kinetic modeling for the production of Aspergillus niger inulinase. Bioproc Biosyst Eng. 2020;43(2):217–232. doi: 10.1007/s00449-019-02219-1. [DOI] [PubMed] [Google Scholar]
- Gill PK, Manhas RK, Singh P. Purification and properties of a heat-stable exoinulinase isoform from Aspergillus fumigatus. Bioresour Technol. 2006;97(7):894–902. doi: 10.1016/j.biortech.2005.04.034. [DOI] [PubMed] [Google Scholar]
- Gong F, Zhang T, Chi Z, Sheng J, Li J, Wang X. Purification and characterization of extracellular inulinase from a marine yeast Pichia guilliermondii and inulin hydrolysis by the purified inulinase. Biotechnol Bioproc E. 2008;13(5):533–539. doi: 10.1007/s12257-007-0177-7. [DOI] [Google Scholar]
- Ilgın M, Germec M, Turhan I. Inulinase production and mathematical modeling from carob extract by using Aspergillus niger. Biotechnol Prog. 2019 doi: 10.1002/btpr.2919. [DOI] [PubMed] [Google Scholar]
- Jain SC, Jain PC, Kango N. Production of inulinase from Kluyveromyces marxianus using Dahlia tuber extract. Braz J Microbiol. 2012;43(1):62–69. doi: 10.1590/S1517-83822012000100007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeza S, Maseko SB, Lin J. Purification and characterization of exo-inulinase from Paenibacillus sp. d9 strain. Protein J. 2018;37(1):70–81. doi: 10.1007/s10930-017-9752-8. [DOI] [PubMed] [Google Scholar]
- Kamble PP, Kore MV, Patil SA, Jadhav JP, Attar YC. Statistical optimization of process parameters for inulinase production from Tithonia weed by Arthrobacter mysorens strain no.1. J Microbiol Methods. 2018;149:55–66. doi: 10.1016/j.mimet.2018.04.019. [DOI] [PubMed] [Google Scholar]
- Kamble PP, Suryawanshi SS, Jadhav JP, Attar YC. Enhanced inulinase production by Fusarium solani JALPK from invasive weed using response surface methodology. J Microbiol Methods. 2019;159:99–111. doi: 10.1016/j.mimet.2019.02.021. [DOI] [PubMed] [Google Scholar]
- Karam EA, Kansoh AL, Moharam ME, Hassan ME, Kansoh AL. Immobilization of inulinase produced by Rhizopus oligosporus NRRL 2549 for continuous fructose production. J Mater Environ Sci. 2018;9(8):2315–2321. [Google Scholar]
- Laemmli DK. Clevage of structual proteins during in assembly of the head of bacteriophage T4. Nature. 1970;227:680–683. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Li AX, Guo LZ, Fu Q, Lu WD. A simple and rapid plate assay for screening of inulin degrading microorganisms using Lugol’s iodine solution. Afr J Biotechnol. 2011;10(46):9518–9521. doi: 10.5897/AJB11.1367. [DOI] [Google Scholar]
- Mohamed SA, Salah HA, Moharam ME, Foda MS, Fahmy AS. Characterization of two thermostable inulinases from Rhizopus oligosporus NRRL 2710. Genet Eng Biotechnol. 2015;13(1):65–69. doi: 10.1016/j.jgeb.2014.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naidoo K, Kumar A, Sharma V, Permaul K, Singh S. Purification and characterization of an endoinulinase from Xanthomonas campestris pv. phaseoli KM 24 Mutant. Food Technol Biotechnol. 2015;53(2):146–153. doi: 10.17113/ftb.53.02.15.3902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohta K, Suetsugu N, Nakamura T. Purification and properties of an extracellular inulinase from Rhizopus sp. strain TN-96. J Biosci Bioeng. 2002;94:78–80. doi: 10.1016/S1389-1723(02)80120-7. [DOI] [PubMed] [Google Scholar]
- Onilude AA, Fadaunsi IF, Garuba EO. Inulinase production by Saccharomyces sp. in solid state fermentation using wheat bran as substrate. Annal Microbiol. 2012;62(2):843–848. doi: 10.1007/s13213-011-0325-3. [DOI] [Google Scholar]
- Raja S, Murty VR. Optimization of aqueous two-phase systems for the recovery of soluble proteins from tannery wastewater using response surface methodology. J Eng. 2013 doi: 10.1155/2013/217483. [DOI] [Google Scholar]
- Saber W, El-Naggar NE. Optimization of fermentation conditions for the biosynthesis of inulinase by the new source; Aspergillus tamarii and hydrolysis of some inulin containing agrowastes. Biotechnology. 2009;8:425–433. doi: 10.3923/biotech.2009.425.433. [DOI] [Google Scholar]
- Saqib AA, Hassan M, Khan NF, Baig S. Thermostability of crude endoglucanase from Aspergillus fumigatus grown under solid state fermentation (SSF) and submerged fermentation (SmF) Process Biochem. 2010;45(5):641–646. doi: 10.1016/j.procbio.2009.12.011. [DOI] [Google Scholar]
- Sheng J, Chi Z, Gong F, Li J. Purification and characterization of extracellular inulinase from a marine yeast Cryptococcus aureus G7a and inulin hydrolysis by the purified inulinase. Appl Biochem Biotechnol. 2008;144(2):111–121. doi: 10.1007/s12010-007-8025-y. [DOI] [PubMed] [Google Scholar]
- Shet VB, Palan AM, Rao SU, Varun C, Aishwarya U, Raja S, Goveas LC, Rao VC, Ujwal P. Comparison of response surface methodology and artificial neural network to enhance the release of reducing sugars from non-edible seed cake by autoclave assisted HCl hydrolysis. 3 Biotech. 2018;8(2):127–135. doi: 10.1007/s13205-018-1163-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh RS, Chauhan K. Inulinase production from a new inulinase producer, Penicillium oxalicum BGPUP-4. Biocatal Agric Biotechnol. 2017;9:1–10. doi: 10.1016/j.bcab.2016.10.012. [DOI] [Google Scholar]
- Singh RS, Chauhan K. Production, purification, characterization and applications of fungal inulinases. Curr Biotechnol. 2018;7(3):242–260. doi: 10.2174/2211550105666160512142330. [DOI] [Google Scholar]
- Singh R, Singh R. Inulinases. In: Ashok P, Negi S, Soccol CR, editors. Current developments in biotechnology and bioengineering: production, isolation and purification of industrial products. Amsterdam: Elsevier; 2017. pp. 423–446. [Google Scholar]
- Singh RS, Chauhan K, Kaur R, Kaur R. Inulinase production in shake-flask fermentations from Mucor circinelloides BGPUP-9. J Appl Biol Biotechnol. 2018;6(6):018–025. doi: 10.7324/JABB.2018.60603. [DOI] [Google Scholar]
- Trivedi S, Divecha J, Shah A. Optimization of inulinase production by a newly isolated Aspergillus tubingensis CR16 using low cost substrates. Carbohydr Polym. 2012;90(1):483–490. doi: 10.1016/j.carbpol.2012.05.068. [DOI] [PubMed] [Google Scholar]
- Vinoth Kumar V, Premkumar MP, Sathyaselvabala VK, Dineshkirupha S, Nandagopal J, Sivanesan S. Aspergillus niger exo-inulinase purification by three phase partitioning. Eng Life Sci. 2011;11(6):607–614. doi: 10.1002/elsc.201000180. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.