Abstract
Phenylalanine hydroxylase (PAH) deficiency is an inborn error of phenylalanine (Phe) metabolism that results in the buildup of dietary Phe to potentially toxic levels. Poorly controlled Phe levels in women of childbearing age are particularly worrisome due to the toxic effect of elevated Phe on fetal development. Pegvaliase was recently approved as an enzyme substitution therapy to reduce Phe concentrations in adult patients with PAH deficiency who have suboptimal Phe control on existing management. During the pegvaliase clinical trials pregnant patients were excluded from participation, but the approved label does not contraindicate its use during pregnancy. This case report describes the outcome of the first PAH deficient patient who elected to continue treatment with pegvaliase during pregnancy and reviews the lessons learned and future considerations.
1. Introduction
Phenylalanine hydroxylase (PAH) deficiency, also called phenylketonuria (PKU; OMIM 261660) is an autosomal recessive disorder caused by pathogenic mutations in the PAH gene that results in deficient phenylalanine hydroxylase necessary for the catabolism of the essential amino acid phenylalanine (Phe). PAH deficiency causes a spectrum of clinical phenotypes ranging from mild hyperphenylalaninemia to severe deficiency and is characterized by the inability to properly convert dietary Phe into tyrosine with subsequent elevations of Phe in the blood and brain [1]. Elevated Phe is neurotoxic and if undiagnosed and untreated, PAH deficiency can result in reduced executive functioning, behavioral and mental health problems and irreversible intellectual disability. With the advent of newborn screening, PAH deficiency is diagnosed pre-symptomatically and dietary interventions can be implemented at birth to ameliorate most neurologic consequences allowing affected individuals to lead full lives [2].
Historically, the mainstay of treatment for PAH deficiency has centered around interventions aimed at the restriction of dietary protein and supplemented with Phe free medical formula and modified low protein foods. The diet approach in theory is straight forward, but adherence is difficult due to the restrictive nature of the diet. More recently, nondietary pharmacologic approaches have been developed as adjunctive therapy. Sapropterin dihydrochloride (sapropterin; marketed as Kuvan), has been FDA approved since 2007 and is a synthetic form of the naturally occurring cofactor of the PAH enzyme tetrahydrobiopterin (BH4) [3]. In patients with BH4 responsive PAH deficiency, sapropterin can lower blood Phe levels and increase Phe tolerance. In 2018, pegvaliase (marketed as Palynziq), was approved as an injectable enzyme substitution therapy to lower blood Phe levels independent of the PAH enzyme or its BH4 cofactor in adult patients with uncontrolled Phe concentrations (>600 μmol/L) [4,5]. In some individuals, pegvaliase treatment has allowed the transition to a normal diet, dramatically improving quality of life.
Poorly controlled Phe levels in women of childbearing age are particularly worrisome due to the risk of fetal harm and raises additional challenges that are unique to the PAH deficient population. During pregnancy, Phe crosses the placenta by active transport and results in fetal levels that are higher than maternal blood levels [6,7]. Poorly controlled Phe levels during pregnancy can result in maternal PKU syndrome with known teratogenic effects to the fetus (microcephaly, congenital heart defects, low birth weight and developmental disability) [7,8]. Current guidelines recommend tight control of blood Phe levels prior to conception and during pregnancy (60–360 μmol/L) to prevent toxic elevated Phe exposure to the developing fetus and persistently low levels which may cause fetal harm [1,9]. In a French study, there was an increase proportion of IUGR found in offspring of maternal PKU patients when Phe levels were < 120 μmol/L during the 2nd and 3rd trimester [10]. Outcome from the interim report from the PKU MOMS sub-registry did not support this finding [9] and some centers advocate for stricter Phe control during pregnancy (60–240 μmol/L) to avoid complications associated with elevated levels [1,11].
Sapropterin is a treatment option for pregnant women with BH4 responsive PAH deficiency who cannot achieve recommended ranges of blood Phe during pregnancy with diet therapy alone [9]. Although listed as a class C medication, preliminary data from the PKU MOMS sub-registry found sapropterin use during pregnancy to be generally well tolerated and associated with lower mean blood Phe levels [9]. Rationale for its continued use centers around the understanding that elevated maternal Phe levels are teratogenic and the potential benefits of lower blood Phe levels outweigh the limited risk of sapropterin use in pregnancy [3,9]. During the pegvaliase clinical trials, pregnancy was an exclusion criterion [12] and treatment during pregnancy outside the animal population has not been studied.
A surveillance program is in place [5], but there are currently no well controlled studies to monitor the outcomes of pregnant women who are treated with pegvaliase. Studies with pregnant animals without PAH deficiency treated with pegvaliase raised concerns about potential fetal risks [5]. These studies were undertaken using pegvaliase at up to 7.5 times the maximum recommended dose and were associated with signs of toxicity in the parent animal [5]. Despite the lack of human data and the unknown risks, current prescribing guidelines allow for the conditional use of pegvaliase during pregnancy given the known toxic/teratogenic effects of high Phe levels on the developing fetus [5,8]. The following report describes the first case of a healthy infant born to a mother with PAH deficiency on treatment with pegvaliase. We also highlight the challenges we faced, and the changes made in our clinic based on our experience with the patient.
2. Case report
This is a 22-year-old female with PAH deficiency, identified through newborn screening (NBS) and started on a Phe-restricted diet in the neonatal period. Her natural protein tolerance was estimated at 8 g per day and Phe levels fluctuated during childhood between 400 and 700 μmol/L while treated with diet and medical foods alone. Sapropterin was introduced at 9 years of age with improved Phe levels (~130 μmol/L) while natural protein tolerance increased to 14 g. Her care was subsequently transferred to other centers due to insurance changes. She returned to our clinic for management at 21 years of age where treatment fatigue (to medical products and low protein diet) was identified as the major cause of suboptimal Phe control (>1000 μmol/L). This led to pegvaliase treatment with the goal to improve diet and plasma Phe levels.
The patient's Phe level at treatment initiation was 1332 μmol/L, and her protein intake was estimated at 45 g per day (off medical foods and formula). Our center's pegvaliase protocol included monthly clinic visits and Phe level monitoring during the titration phase, but the patient was not able to visit or submit a blood spot specimen until week 6 of treatment. At that time, she was receiving pegvaliase 10 mg weekly and her Phe level was 1269 μmol/L (similar to her pre-treatment level). She submitted the next sample at week 10 of treatment (20 mg daily) and her Phe level decreased abruptly to 2.1 μmol/L. The patient was instructed to increase natural protein by 20 g per day (goal intake = 65 g/day), the frequency of Phe level sampling was increased (as frequent as daily) and the test center was changed to improve lab result turnaround time. Eventually, the combination of increased protein intake and decreased pegvaliase frequency (20 mg, 3×/week; Tues, Thurs, Sat) resulted in improved Phe levels by week 20 of treatment.
The patient did not experience any significant adverse events during the titration or maintenance phase (arthralgia, injection site erythema/reaction, fatigue, pain, pyrexia or anaphylaxis) and the recommended manufacturer's dose/titration schedule was followed [5]. She was evaluated for migraines at week 18 of treatment with associated low Phe levels, though she had a history of migraines prior to initiation of pegvaliase.
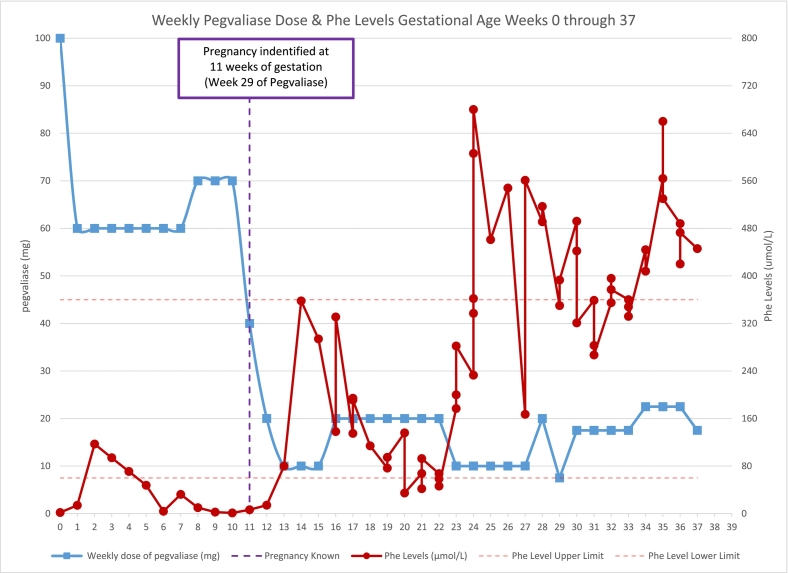
During weeks 20–28 of treatment, her pegvaliase dose was changed from 20 mg 3×/week to 10 mg daily to provide better steady state levels and our dietitians continued to work with the patient to improve her protein intake. At week 29 of treatment, her dose was titrated to 10 mg 3×/week (Tues, Thurs, Sat) based on persistently low Phe levels. The patient visited a local emergency room for acute vomiting and was found to be pregnant, leading her to self-discontinue pegvaliase treatment until her scheduled metabolic appointment one week later. After a long discussion in clinic, reviewing the potential benefits and risks of continued treatment with pegvaliase, treatment was resumed at 10 mg weekly (Fig. 1, Table 1). Phe levels improved when natural protein intake was increased to 80 g/day utilizing a standard liquid meal replacement (20 g protein per serving; recommended intake = 1 per day) and blood samples were monitored closely. Evaluation by an obstetrician at week 32 of treatment estimated the fetal gestational age to be 14 weeks, with an estimated date of conception around week 18 of treatment (8 weeks following the precipitous drop in plasma Phe levels).
Fig. 1.
Table 1.
Gestational age in weeks, weekly dose of pegvaliase and corresponding Phe level in table format.
| Gestational Age (weeks) | Weekly dose of pegvaliase (mg) | Phe Levels (μmol/L) |
|---|---|---|
| 0 | 100 | 1.8 |
| 1 | 60 | 14 |
| 2 | 60 | 117 |
| 3 | 60 | |
| 4 | 60 | |
| 5 | 60 | 47.7 |
| 6 | 60 | 3.9 |
| 7 | 60 | 32.3 |
| 8 | 70 | 10.1 |
| 9 | 70 | 2.5 |
| 10 | 70 | 1.13 |
| 11 | 40 | 6.4 |
| 12 | 20 | 14.1 |
| 13 | 10 | 79.9 |
| 14 | 10 | 358 |
| 15 | 10 | 294 |
| 16 | 20 | 138, 331 |
| 17 | 20 | 135, 194, 194, 191 |
| 18 | 20 | 114 |
| 19 | 20 | 76.5, 94.7 |
| 20 | 20 | 136, 34.7 |
| 21 | 20 | 67.5, 41.9, 92.6 |
| 22 | 20 | 67.2, 58.6, 46.3 |
| 23 | 10 | 177, 200, 282 |
| 24 | 10 | 233, 362, 337, 606, 680 |
| 25 | 10 | 461 |
| 26 | 10 | 548 |
| 27 | 10 | 167, 561 |
| 28 | 20 | 491, 517 |
| 29 | 7.5 | 350, 393 |
| 30 | 17.5 | 492, 442, 321 |
| 31 | 17.5 | 359, 283, 267 |
| 32 | 17.5 | 355, 396, 377 |
| 33 | 17.5 | 360, 348, 332 |
| 34 | 22.5 | 444, 408 |
| 35 | 22.5 | 564, 660, 530 |
| 36 | 22.5 | 488, 420, 473 |
| 37 | 17.5 | 446 |
During weeks 14–16 of pregnancy, Phe levels rose and her pegvaliase dose was increased with subsequent improved Phe levels. The patient remained on the same dose (10 mg twice a week) from week 16–19 of pregnancy with steady Phe levels (sampling 3×/week) while consuming about 60 g of natural protein per day. Phe levels dropped during week 20–22 of pregnancy without changes in pegvaliase dose or daily protein intake. To avoid maternal/fetal Phe deficiency, pegvaliase dose frequency was decreased from twice weekly to once weekly while Phe sample frequency varied between daily to twice weekly (week 23–27 of pregnancy). Phe levels improved initially, but then fluctuated depending on the time between sampling and pegvaliase dose. Request for 2.5 mg pegvaliase formulation was made during week 27 of pregnancy and her dose was increased to 10 mg twice a week until the new formulation became available. From week 29–33 of pregnancy, Phe levels improved while receiving 2.5 mg daily. For the remainder of the pregnancy, her pegvaliase dose was adjusted between 2.5 and 5 mg/day based on Phe levels as they became available.
3. Pregnancy and delivery
The patient received good prenatal care and was followed closely by her obstetrician throughout the course of her pregnancy. The initial anatomy ultrasound at 20 weeks gestation suggested club foot, but subsequent evaluation by a maternal fetal medicine specialist showed normal anatomy. Estimated fetal weight at 32 weeks was 2293 g (95th percentile). Due to gestational hypertension (first noted at week 23 of pregnancy), the patient was induced at 37 weeks and 3 days. A healthy male infant was delivered by vaginal delivery with APGAR scores of 8 and 9 at 1 and 5 min respectively. Birth weight was 3610 g (89th percentile), birth length was 53 cm (96th percentile), and head circumference was 35 cm (83rd percentile). The baby required a short course of phototherapy for mild jaundice prior to discharge. Due to the association of congenital heart disease with maternal PKU, an echocardiogram was performed with normal results. The baby passed his newborn hearing screen and was discharged home with mother at 2 days of life. NBS sample obtained between 24 and 48 h of life was not flagged for PKU or other NBS conditions.
4. Discussion
To our knowledge, our patient is the first human subject to continue with pegvaliase during pregnancy.
Our patient's unplanned pregnancy was identified at 11 weeks gestation during a period of persistently low Phe levels. When low Phe levels were first identified, the patient was instructed to increase natural protein in her diet, but she struggled to incorporate high protein foods into her daily routine. She battled with increased anxiety due to a psychological aversion to high protein foods and a historical pattern of protein avoidance. When it became clear that diet measures alone were not enough to address her low Phe levels, we began reducing her pegvaliase dose. Once we were made aware of the pregnancy, there was an increased urgency to improve the patient's Phe levels. Although we realized pregnancy was not the sole factor for the patient's low levels, we speculated that fetal needs did increase Phe utilization and played a role in the patient's Phe levels. By week 13 of pregnancy, after further pegvaliase dose adjustments and renewed efforts to increase dietary protein intake, we were able to achieve Phe levels within the target range (60–360 μmol/L). We continued with frequent lab monitoring, diet modifications and pegvaliase dose adjustments, but biochemical control fluctuated during the pregnancy with periods of low (<60 μmol/L) and starting during week 24 of pregnancy, intermittent elevated maternal Phe levels (>360 μmol/L).
Periods of suboptimal Phe fluctuations were attributed to a combination of socioeconomic, behavioral, and logistical factors that hindered our ability to adjust treatment in a timely fashion. The patient lived on a military base more than 3 h away from our center, making frequent sampling in clinic unrealistic. Barriers to consistent Phe sampling included security measures at the base, the patient's work schedule, lack of Phe monitoring at the base hospital and inconsistent compliance with home sample requests. We provided pre-paid overnight delivery FedEx envelopes, sent reminder aids in the form of phone calls, alarms, HIPPA compliant provider to patient messaging, clinical staff outreach, and secure emails with only limited improvement. The patient was consistent with monthly visits to our outreach clinic where Phe levels were obtained.
Another barrier to timely treatment adjustment was the lack of immediate access to various pegvaliase strengths (2.5 mg, 10 mg, 20 mg). When a dose adjustment was indicated, there was an approximate 3-week delay from the time of the order change to the time of delivery. Therefore, we were limited to prescribing once or twice-weekly drug administration while we waited for delivery of lower dosage strengths. In the future, a maternal PKU pegvaliase kit, containing several syringes of each strength, would allow for timely and on demand dose adjustment as needed.
Despite the challenges, our patient delivered a healthy baby boy without cardiac, anthropometric, or other anatomic anomalies. However, the infant's long-term neurocognitive outcomes are unknown and developmental monitoring is needed to ensure normal growth and development. We did not observe an association between very low Phe levels (<30 μmol/L) and fetal harm as cautioned in the pegvaliase prescribing guidelines [5]. Phe levels were higher in the later part of the pregnancy and we recognize the benefit of sustained Phe levels within the goal treatment range (60–360 μmol/L). The decision to lower the pegvaliase dose at week 23 of pregnancy, which retrospectively led to a period of Phe instability, was based on the cautionary statement from the guidelines.
After delivery, due to personal preference, our patient chose to formula feed her infant. Before this decision was made, we considered allowing breastfeeding while on treatment with pegvaliase as the benefits of breastfeeding have been well documented (protection against chronic disease, improved cognitive development, lower rates of obesity) [13,14]. Furthermore, in a recent case report, it was noted that any potential trace amounts of pegvaliase in breast milk should not alter the infant's nutrition since pegvaliase is a polypeptide and would likely be hydrolyzed by intestinal proteases in the infant's GI tract [8]. However, if breastfeeding were allowed, we would have monitored the mother's Phe levels closely to guard against Phe deficiency in the infant [8].
Prior to pegvaliase initiation, all patients in our clinic are counseled regarding the unknown effects of treatment during pregnancy and instructed to use reliable birth control. Despite these precautions and as seen in the PRISM studies, patients have become pregnant while on treatment with pegvaliase [12]. Having experienced more than one pregnancy in our patient population (n = 2, one ending in elective termination), we have added pregnancy testing prior to treatment initiation and then monthly to further reinforce the need for reliable contraception and to identify unplanned pregnancies as early as possible.
Our experience in a single patient case, found pegvaliase treatment during pregnancy did not result in anatomic anomalies associated with maternal PKU syndrome. However, we recognize the need for caution in extrapolating our findings in a single case report to a larger patient population. Additionally, central nervous system functions in the infant (cognitive, behavioral and executive functioning) cannot be adequately assessed at this time and will require long term follow up to assess developmental outcomes.
Although the current prescribing guidelines allow for conditional use during pregnancy, we recommend careful consideration given the paucity of evidence on pregnancy outcomes with pegvaliase treatment. In our patient, pre-pegvaliase Phe levels were frequently >1000 μmol/L leading us to speculate that the risks of fetal exposure to elevated maternal Phe levels exceeded the unknown risk of pegvaliase treatment. In the future, as data from the established surveillance program becomes available, confidence may increase regarding pegvaliase treatment during pregnancy. Our experience also highlights the various barriers to timely Phe monitoring and the need for a maternal PKU pegvaliase kit containing multiple dosage strengths that would permit more expedient and responsive maternal PKU management.
Contributor Information
Monica Boyer, Email: mboyer@choc.org.
Richard Chang, Email: RChang@choc.org.
References
- 1.Vockley J., Andersson H.C., Antshel K.M., Braverman N.E., Burton B.K., Frazier D.M., Mitchell J., Smith W.E., Thompson B.H., Berry S.A. Phenylalanine hydroxylase deficiency: diagnosis and management guideline, genetics in medicine official journal of the American College of Medical. Genetics. 2014;16:188–200. doi: 10.1038/gim.2013.157. [DOI] [PubMed] [Google Scholar]
- 2.Mitchell J.J., Trakadis Y.J., Scriver C.R. Phenylalanine hydroxylase deficiency, genetics in medicine official journal of the American College of Medical. Genetics. 2011;13:697–707. doi: 10.1097/gim.0b013e3182141b48. [DOI] [PubMed] [Google Scholar]
- 3.Muntau A.C., Adams D.J., Bélanger-Quintana A., Bushueva T.V., Cerone R., Chien Y.-H., Chiesa A., Coşkun T., de Las Heras J., Feillet F., Katz R., Lagler F., Piazzon F., Rohr F., van Spronsen F.J., Vargas P., Wilcox G., Bhattacharya K. International best practice for the evaluation of responsiveness to sapropterin dihydrochloride in patients with phenylketonuria. Mol. Genet. Metab. 2019;127:1–11. doi: 10.1016/j.ymgme.2019.04.004. [DOI] [PubMed] [Google Scholar]
- 4.Longo N., Dimmock D., Levy H., Viau K., Bausell H., Bilder D.A., Burton B., Gross C., Northrup H., Rohr F., Sacharow S., Sanchez-Valle A., Stuy M., Thomas J., Vockley J., Zori R., Harding C.O. Evidence- and consensus-based recommendations for the use of pegvaliase in adults with phenylketonuria. Genet. Med. 2019;21:1851–1867. doi: 10.1038/s41436-018-0403-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.US Food and Drug Administration Drugs@FDA. 2020. https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/761079s000lbl.pdf
- 6.Bouchlariotou S., Tsikouras P., Maroulis G. Undiagnosed maternal phenylketonuria: own clinical experience and literature review. J. Matern. Fetal Neonatal Med. 2009;22:943–948. doi: 10.1080/14767050902994697. [DOI] [PubMed] [Google Scholar]
- 7.Maternal Phenylketonuria . Vol. 122. 2008. Pediatrics; pp. 445–449. [DOI] [Google Scholar]
- 8.Rohr F., Kritzer A., Harding C.O., Viau K., Levy H.L. Discontinuation of Pegvaliase therapy during maternal PKU pregnancy and postnatal breastfeeding: a case report. Mol. Genet. Metab. Rep. 2020;22:100555. doi: 10.1016/j.ymgmr.2019.100555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grange D.K., Hillman R.E., Burton B.K., Yano S., Vockley J., Fong C.-T., Hunt J., Mahoney J.J., Cohen-Pfeffer J.L. Sapropterin dihydrochloride use in pregnant women with phenylketonuria: an interim report of the PKU MOMS sub-registry. Mol. Genet. Metab. 2014;112:9–16. doi: 10.1016/j.ymgme.2014.02.016. [DOI] [PubMed] [Google Scholar]
- 10.Teissier R., Nowak E., Assoun M., Mention K., Cano A., Fouilhoux A., Feillet F., Ogier H., Oger E., de Parscau L. Maternal phenylketonuria: low phenylalaninemia might increase the risk of intra uterine growth retardation. J. Inherit. Metab. Dis. 2012;35:993–999. doi: 10.1007/s10545-012-9491-0. [DOI] [PubMed] [Google Scholar]
- 11.Krishnamoorthy U., Dickson M. Maternal phenylketonuria in pregnancy. Obstet. Gynaecol. 2005;7:28–33. doi: 10.1576/toag.7.1.028.27039. [DOI] [Google Scholar]
- 12.Thomas J., Levy H., Amato S., Vockley J., Zori R., Dimmock D., Harding C.O., Bilder D.A., Weng H.H., Olbertz J., Merilainen M., Jiang J., Larimore K., Gupta S., Gu Z., Northrup H. Pegvaliase for the treatment of phenylketonuria: results of a long-term phase 3 clinical trial program (PRISM) Mol. Genet. Metab. 2018;124:27–38. doi: 10.1016/j.ymgme.2018.03.006. [DOI] [PubMed] [Google Scholar]
- 13.Stuebe A. The risks of not breastfeeding for mothers and infants, reviews in. Obstet. Gynecol. 2009;2:222–231. [PMC free article] [PubMed] [Google Scholar]
- 14.Binns C., Lee M., Low W.Y. The long-term public health benefits of breastfeeding. Asia Pac. J. Public Health. 2016;28:7–14. doi: 10.1177/1010539515624964. [DOI] [PubMed] [Google Scholar]