Summary
Tumors with an impaired transporter associated with antigen processing (TAP) present several endoplasmic reticulum-derived self-antigens on HLA class I (HLA-I) which are absent on healthy cells. Selection of such TAP-independent antigens for T cell-based immunotherapy should include analysis of their expression on healthy cells to prevent therapy-induced adverse toxicities. However, it is unknown how the absence of clinically relevant antigens on healthy cells needs to be validated. Here, we monitored TAP-independent antigen presentation on various healthy cells after establishing a T cell tool recognizing a TAP-independent signal sequence receptor 1-derived antigen. We found that most but not all healthy cells present this antigen under normal and inflammatory conditions, indicating that TAP-independent antigen presentation is a variable phenomenon. Our data emphasize the necessity of extensive testing of a wide variety of healthy cell types to define clinically relevant TAP-independent antigens that can be safely targeted by immunotherapy.
Subject areas: Immunology, Cancer
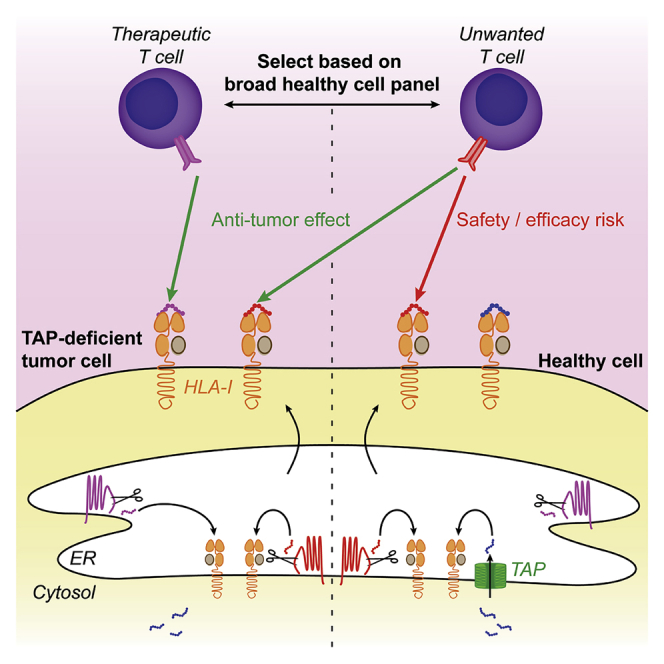
Graphical Abstract
Highlights
-
•
The ER-resident SSR1 holds an antigenic peptide that is processed independently of TAP
-
•
TAP-independent peptide presentation is functional in healthy cell types
-
•
TAP-independent SSR1-derived antigen presentation varies between healthy cells
-
•
This exposes safety and efficacy risks of clinical TAP-independent peptide targeting
Immunology; Cancer
Introduction
HLA class I (HLA-I) (neo)antigen presentation by tumor cells can induce activation of CD8+ cytotoxic T cells (Neefjes et al., 2011). This natural immunological pressure forces various tumors to downmodulate HLA-I antigen presentation through genomic or epigenetic alterations (Campoli and Ferrone, 2008; Khong and Restifo, 2002). In addition, T cell-based immunotherapies increase mutation rates in genes associated with antigen presentation such as B2M, tapasin, TAP1, and TAP2 (Gettinger et al., 2017; Jiang et al., 2010; Restifo et al., 1996; Sade-Feldman et al., 2017).
HLA-I is generated and folded in the ER, assisted by several lectin chaperones and stabilized by the beta-2 microglobulin (B2M) light chain, enabling the loading of small peptides through the collaborative effort of tapasin, calreticulin, and ERp57 (Rock et al., 2016). Conventional peptide generation is mediated by the proteasome in the cytosol. These peptides are transported into the endoplasmic reticulum (ER) by the heterodimeric transporter associated with antigen processing (TAP) complex.
Alterations in the antigen presentation machinery affect the composition of the peptide repertoire presented by HLA-I (van Hall et al., 2006). Perhaps the largest impact on the repertoire is the functional disruption of the TAP transporter (hereafter referred to as TAP-deficient), interrupting the supply of cytosolic peptides. Without TAP, HLA-I presented peptides are either derived from ER-resident proteins or enter the ER through routes other than the canonical TAP pathway (Oliveira and van Hall, 2015). Several of these so-called TAP-independent peptides are not presented on healthy TAP-proficient cells, therefore representing a potential specific immunotherapeutic target on TAP-deficient tumors (van Hall et al., 2006). Since studies targeting TAP-independent peptides in mice show low toxicity, the first proof-of-concept study targeting TAP-independent peptides in patients with non-small cell lung cancer will soon be initiated (Brolsma, 2019; Doorduijn et al., 2018). Potential target antigens for such therapy were recently identified in humans by comparing the HLA-I presented peptidome eluted from TAP-deficient and -proficient cells (Marijt et al., 2018). Because a considerable proportion of TAP-independent peptides can also be presented by at least some TAP-proficient cells (Man et al., 1992; Weinzierl et al., 2008), clinical targeting of TAP-independent antigens potentially involves significant safety and efficacy risks. Although caution is needed in designating peptides as solely being presented on TAP-deficient cells, efforts are largely lacking to validate that antigens are not at all presented by any healthy cell type with native TAP expression.
To define a validation roadmap, it would be instrumental to have a tool—such as a defined T cell clone—to monitor functional TAP-independent peptide presentation both on tumor and healthy cells. However, isolation of T cells targeting TAP-independent antigens on healthy cells may be challenging since they are likely deleted during thymic development (Takaba and Takayanagi, 2017). Therefore, human T cells that recognize a molecularly defined TAP-independent antigen on healthy cells have not been identified to date.
To bypass potential thymic deletion issues, we here investigated TAP-independent antigen recognition on healthy cells using CD8+ T cell clones derived from allogeneic repertoires (Amir et al., 2011; Van Bergen et al., 2010). We identified that the cognate antigen of one of these clones, derived from the ER-resident protein signal sequence receptor 1 (SSR1), is presented both by TAP knockout (KO) and TAP-proficient tumor cells. This ubiquitously expressed antigen is effectively presented under normal and inflammatory conditions on several but not all healthy primary cells, indicating that TAP-independent antigen presentation is a variable phenomenon. Thus, a broad healthy cell expression analysis of TAP-independent targets improves the safety profile of their immunotherapeutic application.
Results
The protein SSR1 encodes a TAP-independent peptide
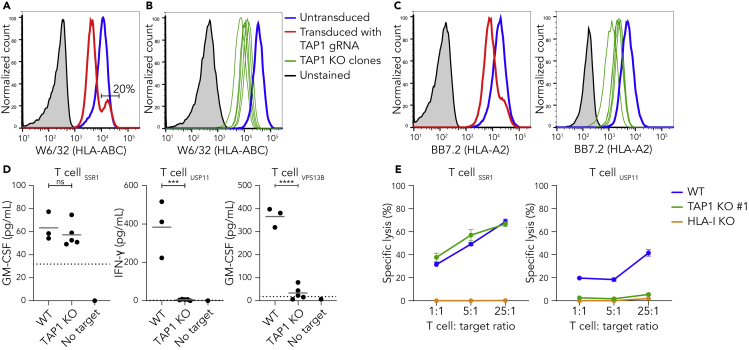
To study the effect of the TAP transporter on the functional presentation of antigens, we genetically knocked out TAP1 in human HAP1 cells. After lentiviral introduction of CRISPR/Cas9 machinery targeting the first exon of TAP1, 20% of the transduced cells still displayed surface HLA-I levels comparable to wild type cells (Figures 1A and S1). In order to prevent interference of remaining wild-type cells in T cell coculture assays, we generated five clonal TAP1 KO cell lines which showed decreased HLA-I surface expression (Figure 1B). In addition, Western blot analysis confirmed a complete lack of TAP1 expression in clone #1 (de Waard et al., 2020). Sequence analyses showed a lack of at least one amino acid in the first transmembrane domain of TAP1 in each clone (Figures S2A and S2B) (Schrodt et al., 2006).
Figure 1.
The protein SSR1 encodes a TAP-independent peptide
(A) HAP1 cells were transduced with a TAP1-specific gRNA and analyzed by flow cytometry using the pan-HLA antibody W6/32. Untransduced (blue), transduced (red) and unstained cells (gray). See Figure S1 for gating strategy.
(B) Five monoclonal cell lines were generated from the polyclonal TAP1 KO cell line in (A) and analyzed by flow cytometry. Wild-type (blue), TAP1 KO clones (green lines), unstained cells (gray). See Figure S2 for sequencing details.
(C) Same as (A) and (B) but using the HLA-A2-specific antibody BB7.2.
(D) HLA-A2-restricted SSR1-, USP11- or VPS13B-specific T cells were cocultured with three separate batches of HAP1 wild-type or each of the five clonal TAP1 KO cell lines (B) (Amir et al., 2011, Pont et al., 2019, Spaapen et al., 2007, Spaapen et al., 2008, Van Bergen et al., 2010). Culture supernatant was used for indicated cytokine ELISAs. Each datapoint represents the average of triplicate cultures. Dotted lines represent the lower detection limit of the ELISA. Student's t-test, ∗∗∗p < 0.001, ∗∗∗∗p < 0.0001, ns is not significant.
(E) The indicated T cells were cocultured with 51Cr-loaded wild-type, TAP1 KO #1 and HLA-I KO HAP1 cells (Jongsma et al., 2020) at indicated effector:target cell ratios, after which specific lysis (%) was calculated based on 51Cr release. Each datapoint represents the mean of triplicate cultures with SD.
See also Figures S1 and S2.
To analyze functional presentation of antigens in the absence of the TAP transporter, we utilized several available alloreactive CD8+ T cell clones that we previously isolated from patients following allogeneic stem cell transplantation (Amir et al., 2011; Van Bergen et al., 2010). These T cell clones recognize defined antigens, one of which is derived from the ER-localized protein SSR1 and may therefore be processed independently of TAP-mediated cytosol-to-ER transport (Pfeffer et al., 2017). Two other T cell clones recognize antigens derived from the cytosolic proteins USP11 or VPS13B (Koike and Jahn, 2019; Zhou et al., 2017). Since these antigens are presented by HLA-A2, we first confirmed that this specific allele was affected in the TAP1 KO cells (Figure 1C). Subsequent coculture of T cells with HAP1 target cells showed that USP11- and VPS13B-specific T cells recognized wild type but not TAP-deficient cells (Figure 1D, right panels). Conversely, SSR1-specific T cells were activated by the TAP-deficient cells demonstrating that the SSR1 peptide is processed independently of TAP (Figure 1D, left panel). The amount of HLA-A2 on the different TAP1 KO clones (Figure 1C) did not correlate with their capacity to stimulate SSR1-specific T cells (Figure S2C). Strikingly, HAP1 wild-type cells were capable of activating SSR1-specific T cells to a similar level as TAP-deficient cells resulting in T cell-mediated lysis of both cell lines, showing that the SSR1 peptide can be functionally presented at the cell surface even in the presence of the TAP transporter (Figures 1D and 1E). Thus, the SSR1 peptide represents a model antigen to further evaluate TAP-independent antigen presentation by healthy cells.
The SSR1 signal peptide contains the TAP-independent SSR1 antigen
SSR1 is the transmembrane spanning alpha subunit of the TRAP complex that translocates proteins cotranslationally in the ER lumen (Pfeffer et al., 2017). The epitope recognized by the SSR1-specific T cell clone is polymorphic in the population as dictated by a nonsynonymous SNP (rs10004) encoding for either a serine or leucine (SSR1-S of SSR1-L) (Figure S3) (Van Bergen et al., 2010). A 14-mer peptide containing this polymorphism (VLFRGGPRGSLAVA) was previously shown to be presented by HLA-A2 and recognized by SSR1-specific T cells, albeit with low avidity (Bijen et al., 2018). As more specific details on functionally presented SSR1 antigens may aid in defining TAP-independent peptide processing, we next investigated the HLA-I mediated presentation and recognition of additional peptides covering the SNP. In silico HLA-A2-binding predictions of SSR1 peptides covering the SNP failed to designate strong binders (Table S1). Since predictions for this SSR1 epitope can be inaccurate (Bijen et al., 2018), we biochemically determined whether SSR1 peptides other than the 14-mer are presented by HLA-A2 positive cells. Using LC-MS/MS we analyzed eluted peptides from HAP1 wild-type cells (de Waard et al., 2020), and from two HLA-A2 positive primary AML samples that genetically encode the SSR1-S peptide. In first instance, we automatically retrieved only SSR1-L peptides (13-mer and 14-mer) from the AML samples (Table 1). In addition, using the MS2 spectrum of a synthetic SSR1-S 14-mer peptide as reference, we identified this peptide in all three samples (Table 1).
Table 1.
Immunopeptidome analyses of cells genetically positive for SSR1-S
| Candidate peptide | Identified on | Genotype rs10004 | Predicted affinity for HLA-A∗02:01 (nM) |
|---|---|---|---|
| VLFRGGPRGSLAVA | HAP1, AGP2375, SWD2031 | S/S, S/L, S/L |
1403 |
| VLFRGGPRGLLAVA | AGP2375 | S/L | 649 |
| VLFRGGPRGLLAV | SWD2031 | S/L | 236 |
SSR1-S and SSR1-L candidate peptides were eluted from HAP1 cells and two primary AMLs (Hassan et al., 2013, van der Lee et al., 2019). Affinities for HLA-A∗02:01 were predicted using NetMHC4.0. See also Table S1.
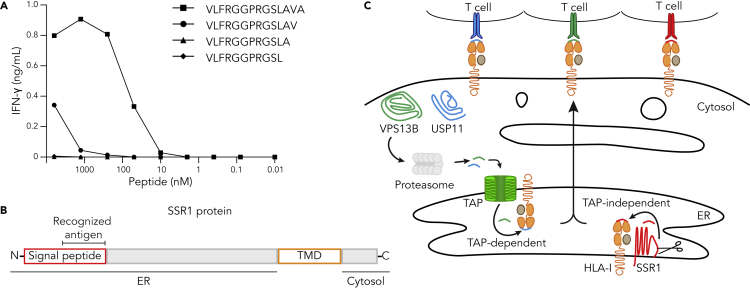
To obtain more data on presented SSR1-derived peptides, we analyzed the peptidome from additional HLA-A2 positive primary tumor samples (n = 9) and cell lines (n = 6). As both SSR1-S and SSR1-L are presented at similar levels by HLA-A2 positive cells (Bijen et al., 2018), we further focused on identifying length variants of the SSR1-L peptide. The analysis yielded four length variants of the same core peptide (VLFRGGPRGLLAVA) covering the polymorphic residue (Table 2). Coculture of SSR1-specific T cells with S-antigen-negative T2 cells loaded with decreasing amounts of synthetic candidate peptides showed superior responses against the 14-mer peptide (Figure 2A). These data establish the 14-mer peptide as the cognate epitope of the SSR1-specific T cell clone.
Table 2.
Immunopeptidome analysis reveals seven candidate peptides covering the SSR1 SNP (rs10004)
| Candidate peptide | Identified from |
Predicted affinity for HLA-A∗02:01 (nM) per variant |
|||
|---|---|---|---|---|---|
| Primary tumor | Cell line | TAP-deficient cell line | L | S | |
| VLFRGGPRGLL | – | – | T2 | 4673 | 5524 |
| VLFRGGPRGLLA | OvaL10 | UM9+ A2 | – | 5795 | 10,082 |
| VLFRGGPRGLLAV | SWD2031 | – | – | 236 | 628 |
| VLFRGGPRGLLAVA | AGP2375, CDT4512, KYE9115, OvaL10, OvaL23, GBZ10197, GPQ3361, VDL6711, ALY1143 | U266, TMD8+A2, COV413b, U2G10196, UM9+A2 | T2 | 649 | 1403 |
SSR1-L candidate peptides eluted from different primary tumors (anonymized patient numbers indicated) and cell lines. Affinities for HLA-A∗02:01 were predicted using NetMHC4.0 for both SSR1-L and SSR1-S of rs10004. See also Tables S1–S3 (Komov et al., 2018).
Figure 2.
The SSR1 signal peptide contains the TAP-independent SSR1 antigen
(A) T2 cells, loaded with different amounts of synthetic length variants of the SSR1-S peptide (peptide sequences in Table 2), were cocultured with SSR1-specific T cells after which culture supernatant was used for IFN-γ ELISA. Each datapoint represents a single measurement.
(B) Summary of the location of the antigenic peptide in the SSR1 protein. TMD, transmembrane domain (yellow).
(C) Graphic summary of subcellular localization of the three parental proteins studied here and the presumed pathway of their antigenic peptides for presentation on HLA-I to their cognate T cells.
See also Figure S3.
The location of an antigen within a transmembrane protein determines the molecular path of presentation, including the requirement for TAP transportation after proteolytic degradation (Oliveira and van Hall, 2013). SSR1 is a type-I transmembrane protein with an intra-ER domain of 189 amino acids and a cytosolic domain of 58 amino acids (Pfeffer et al., 2017). To gain insight into the presentation route of the SSR1 antigen, we analyzed the subcellular localization of the recognized peptide. The 14-mer peptide is located near the N-terminus of the SSR1 protein, spanning part of the H- and the complete C-region of the signal peptide as predicted by Phobius (Kall et al., 2007) and is likely to be C-terminally liberated in the ER by the signal peptidase complex (Figures 2B and S3) (Voldby Larsen et al., 2006; Weinzierl et al., 2008). In summary, the peptides derived from USP11 and VPS13B require TAP translocation before HLA-I binding, whereas the SSR1 peptide is readily localized in the ER and likely processed by local proteases before being loaded on HLA-I (Figure 2C).
Multiple SSR1 peptides are presented independently of TAP
To establish whether other HLA-I alleles can also present TAP-independent SSR1 peptides, we analyzed the peptidomes of TAP-deficient HAP1 cells and MCF-7 cells expressing the herpes simplex virus TAP inhibitor infected cell protein 47 (ICP47). These analyses confirmed that the antigen recognized by our SSR1-specific T cell clone was presented by TAP-deficient HAP1 cells (Table S2). In addition, we identified two SSR1-derived candidate peptides from TAP-inhibited MCF-7 cells, indicating that other SSR1 antigens may also be presented independently of TAP (Table S2 and Figure S3). Because these candidate peptides were also found on wild-type MCF-7 cells (Table S2), we questioned whether even more ER-derived SSR1 peptides can be presented by TAP-proficient cells. Examination of membrane bound and soluble HLA-I presented peptidomes from cells and sera of glioblastoma patients and non-cancerous individuals revealed multiple SSR1-derived candidate peptides (Table S3) (Shraibman et al., 2019). All candidate peptides were derived from the ER-luminal domain of SSR1 (Figure S3).
Altogether, the identification of a vast number of ER-derived SSR1 candidate peptides presented by a variety of HLA-I alleles on different cells (Tables 1 and 2, S2, and S3) indicates that multiple TAP-independent peptides derived from a single protein can be presented by TAP-proficient cells.
TAP-independent peptide presentation is functional in healthy donor cells
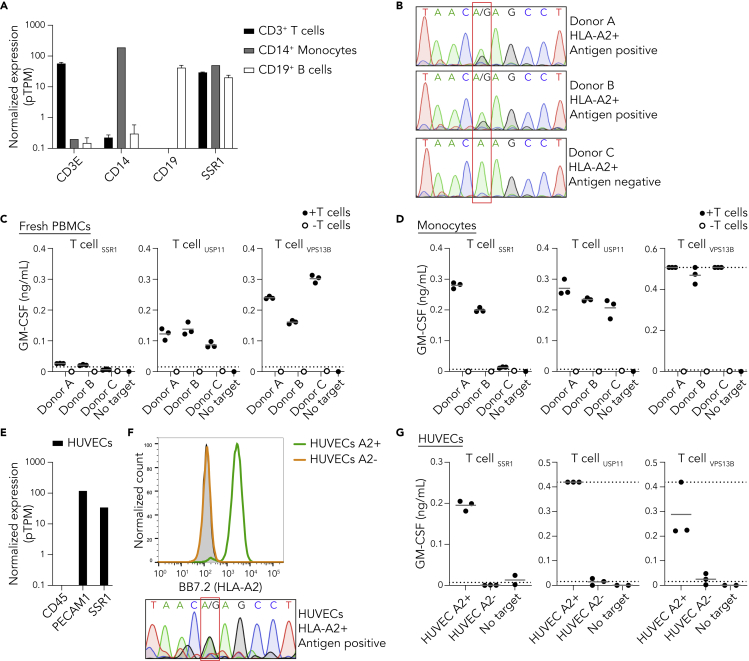
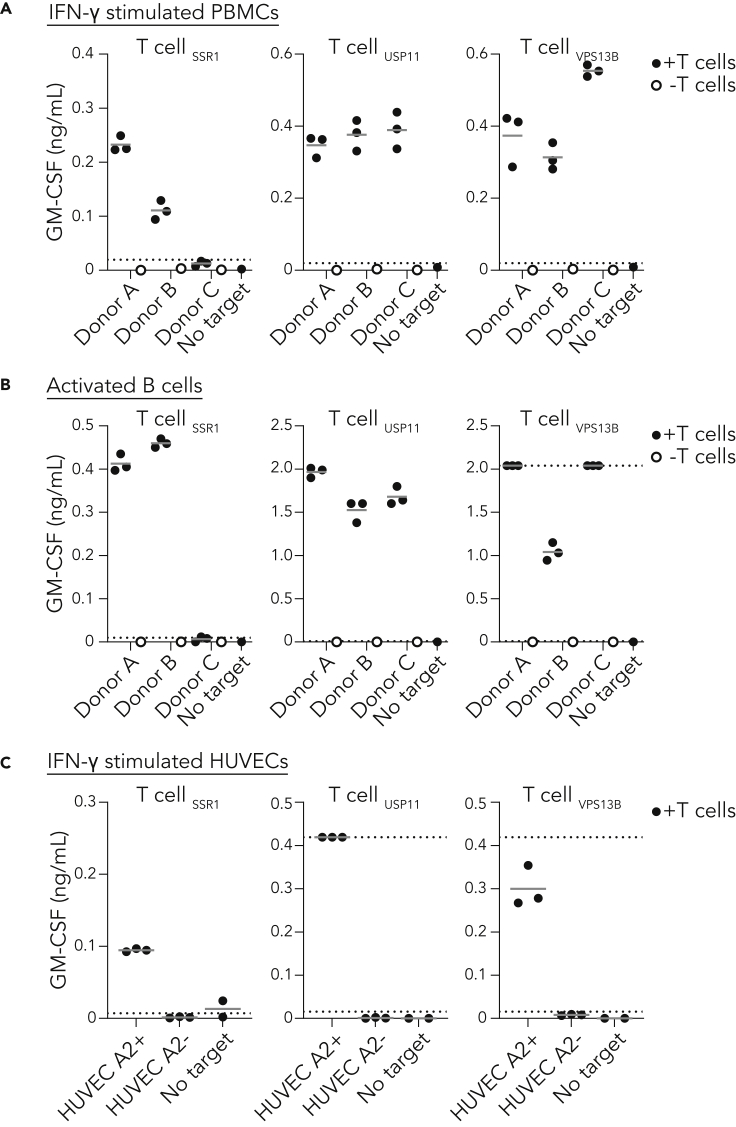
To investigate the capacity of non-transformed cells to present TAP-independent peptides, we determined the reactivity of the SSR1-specific T cell clone against healthy donor material. Monocytes, T cells and B cells, the main constituents of peripheral blood mononuclear cells (PBMCs), express SSR1 according to publicly available RNA-seq data (Figure 3A). Therefore, we isolated PBMCs from three HLA-A2 positive healthy donors and genotyped rs10004 to evaluate whether these donors were carrying the antigen recognized by the SSR1-specific T cell clone. Two donors (A and B) were heterozygous for rs10004 and thus positive for the SSR1 epitope, and one donor (C) was homozygous negative (Figure 3B). Coculture of our T cell clones with fresh PBMCs from the three donors largely failed to elicit a detectable response of SSR1-specific T cells toward antigen positive donors, while the positive control USP11- and VPS13B-specific clones were activated (Figures 3C and S4A). In contrast, monocytes isolated from the genetically positive PBMCs induced an SSR1-directed T cell response (Figures 3D and S4B). We then interrogated T cell reactivity against human umbilical cord endothelial cells (HUVECs), as a non-hematopoietic healthy cell type that expresses SSR1 (Figure 3E and S4C). SSR1-specific T cells recognized and killed selected HLA-A2 positive, SSR1-antigen positive HUVECs, but not HLA-A2 negative control HUVECs (Figures 3F, 3G, and S4D). Together, these data demonstrate that presentation of the TAP-independent SSR1-antigen is functional in multiple but not all healthy TAP-proficient cells.
Figure 3.
TAP-independent peptide presentation is functional in healthy donor cells
(A) Normalized expression of SSR1 and control CD14, CD19, and CD3E per depicted PBMC subset using the RNA HPA blood cell gene data (Uhlen et al., 2015). Data are represented as mean with SD.
(B) Sanger sequencing of the SSR1 SNP rs10004 of three HLA-A2 positive healthy donors, the position of the SNP is highlighted by the red box and consequence for antigen status is depicted.
(C) T cell coculture with PBMCs from three healthy donors (B), culture supernatant was analyzed by GM-CSF ELISA (IFN-γ data in Figure S4). Results from triplicate cultures are shown. Dotted lines represent the detection limits of the ELISA.
(D) As in (C) but using monocytes as target cells (IFN-γ data in Figure S4).
(E) Expression analysis of SSR1 and control CD45 and PECAM1 in TERT2 immortalized HUVECs using the RNA HPA cell line gene data.
(F) Flow cytometric analysis of HLA-A2 expression by HUVECs. HLA-A2 positive FACS sorted (green), HLA-A2 negative FACS sorted (orange) populations and unstained HUVECs (gray). Lower panel depicts rs10004 sequence of the HLA-A2 positive sorted HUVECs.
(G) T cell coculture with HLA-A2 positive and negative HUVECs (F). Culture supernatant was analyzed by GM-CSF ELISA (IFN-γ data in Figure S4). Each dot represents an individual measurement of triplicate cultures.
See also Figures S1 and S4.
TAP-independent peptides are presented under inflammatory conditions
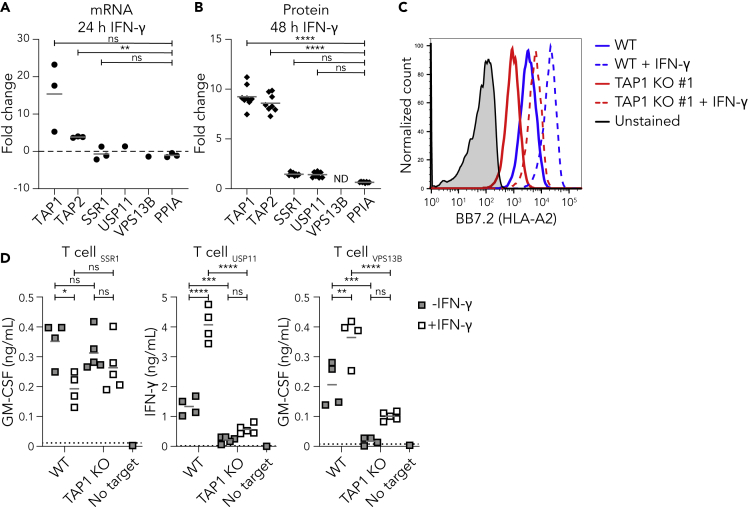
Patients receiving immunotherapy regularly experience severe inflammatory reactions and cytokine storm, which may also be the case after immunotherapeutic targeting of TAP-independent peptides (Gangadhar and Vonderheide, 2014; Hay et al., 2017; Henden and Hill, 2015). Inflammatory cytokines such as IFN-γ enhance expression of various components of the HLA-I pathway including the TAP transporter, but their influence on the expression and presentation of the antigens studied here is unknown (Jongsma et al., 2019; Ma et al., 1997). Therefore, we first analyzed the change in expression of SSR1, USP11, and VPS13B after 24 hr of IFN-γ treatment using microarray datasets from the Interferome database (Rusinova et al., 2012). In comparison to the reference gene PPIA (Riemer et al., 2012), the expression of SSR1, USP11 and VPS13B were unaffected by IFN-γ treatment, whereas TAP1 and TAP2 expression were increased (Figure 4A). Additionally, proteome analysis after 48 hr IFN-γ stimulation revealed no significant change in SSR1 and USP11 protein levels while TAP1 and TAP2 increased eight-fold (Figure 4B) (Megger et al., 2017).
Figure 4.
TAP-independent peptides are presented under inflammatory conditions
(A) TAP1, TAP2, SSR1, USP11, and VPS13B mRNA expression after 24 hr of IFN-γ stimulation was analyzed using three publicly available datasets and compared to the reference gene PPIA.
(B) TAP1, TAP2, SSR1, USP11, and VPS13B protein expression after 48 hr of IFN-γ stimulation was analyzed using publicly available MS data and compared to the reference protein PPIA (Megger et al., 2017). ND is not detected.
(C) HAP1 wild-type and TAP1 KO cells were treated with IFN-γ for 48 hr and surface HLA-A2 upregulation was measured by flow cytometry. Unstimulated (solid) or stimulated (dashed) wild type (blue) and TAP1 KO #1 (red) cells are shown, including unstimulated unstained wild type cells (gray).
(D) T cell coculture with unstimulated or IFN-γ stimulated wild-type or TAP1 KO clones (same as in Figure 1B). Culture supernatants were analyzed for the presence of cytokines using cytokine ELISA. Dotted lines represent the lower and upper detection limits of the ELISA. Repeated measures one-way ANOVA followed by Dunnett's (A), ordinary one-way ANOVA followed by Dunnett's (B) or by Sidak's (D) multiple comparisons test. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, ∗∗∗∗p < 0.0001, ns is not significant.
See also Figure S1.
To examine the effects of inflammatory conditions on TAP-independent peptide presentation, we stimulated HAP1 wild-type and TAP1 KO cells for 48 hr with IFN-γ, which resulted in increased HLA-A2 surface expression on both cell types (Figure 4C). These stimulated cells and their unstimulated controls were then exposed to the different T cell clones. The IFN-γ-induced upregulation of classical antigen presentation on wild type cells was characterized by an increased T cell response against control USP11 and VPS13B peptides (Figure 4D, right panels). These IFN-γ stimulated wild-type cells also presented significant amounts of SSR1 peptide (Figure 4D, left panel). In addition, the SSR1 peptide presentation in the absence of the TAP transporter was similar after IFN-γ stimulation (Figure 4D). These results indicate that this TAP-independent antigen presentation pathway is largely unaffected by an inflammatory IFN-γ stimulus on an immortal model cell line.
Activated healthy cells functionally present TAP-independent peptides
To evaluate whether healthy tissues present TAP-independent peptides under inflammatory conditions similar as induced by immunotherapy, we evaluated recognition of the TAP-independent SSR1 antigen on activated healthy cells. Although functional SSR1 antigen presentation by unstimulated PBMCs was negligible (Figure 3C), we observed a significant SSR1-specific T cell response against IFN-γ treated PBMCs from the SSR1 antigen-positive donors A and B but not against the antigen-negative donor C (Figure 5A). In order to test the TAP-independent antigen presentation in a broader inflammatory context, we activated B cells of the same three donors using CpG and a BCR-stimulus. The antigen-positive activated B cells were efficiently recognized and killed by SSR1-specific T cells, further substantiating that an inflammatory environment allows for TAP-independent antigen presentation in healthy cells (Figures 5B and S4E). Finally, the SSR1-specific T cells were also reactive to non-immune cells (HUVECs) pre-exposed to IFN-γ (Figure 5C). Together, our data demonstrate that healthy TAP-proficient cells can efficiently present TAP-independent peptides under steady state and inflammatory conditions.
Figure 5.
Activated healthy cells functionally present TAP-independent peptides
(A) T cell coculture with healthy donor PBMCs activated for 3 days with IFN-γ.
(B) T cell coculture with healthy donor B cells activated using CpG and B cell receptor cross-linking antibodies.
(C) T cell coculture with HLA-A2 positive and negative HUVECS activated with IFN-γ. Culture supernatants were analyzed using GM-CSF ELISA. Results from triplicate cultures are shown. Dotted lines represent lower and upper detection limit of the ELISAs.
See also Figure S4.
Discussion
Tumor-specific abrogation of the antigen presentation machinery can provide opportunities for new therapeutic approaches, such as targeting (neo)antigens that are presented only in the case of a defective TAP transporter (Marijt and van Hall, 2020; Oliveira and van Hall, 2013; van Hall et al., 2006). In our current study, we identified that an immunogenic T cell epitope originating from the ER-resident protein SSR1 is processed independently of TAP. Using an SSR1-specific T cell clone, we provide evidence that TAP-independent presentation is variable on various healthy cell types under resting and inflammatory conditions. Thus, to ensure the safety and efficacy of a therapy against TAP-independent antigens, our data imply that it is vital for every individual target to map the degree of presentation on healthy tissue.
Therapeutic targeting of non-mutated TAP-independent antigens has potential for broad clinical application, since these antigens are shared among patients with TAP-deficient tumors (Oliveira et al., 2013; van Hall et al., 2006; Voldby Larsen et al., 2006). Such immunotherapies will utilize either vaccination strategies or adoptive transfer of specific T cells. A strict prerequisite for a vaccination strategy to be effective is the lack of presentation of the targeted antigen(s) on healthy cells because otherwise specific T cells will be functionally impaired or deleted during thymic development (Doorduijn et al., 2016; Takaba and Takayanagi, 2017). Importantly, such immune tolerance inherently provides a safety margin for clinical studies in which presentation of targeted antigens would unexpectedly be not restricted to TAP-deficient cells. To increase the chance of raising an effective immune response against TAP-independent antigens, vaccination with TAP-silenced DCs could be a potential strategy (Marijt et al., 2019). Of note, there are multiple reports that in humans vaccination strategies against antigens presented on healthy tissue can break tolerance and induce severe autoimmunity, so caution is warranted (Ludewig et al., 2000; Sultan et al., 2017). The alternative for immunotherapeutic vaccination is adoptive T cell transfer, which can induce a fast anti-tumor response. A combination of adoptive transfer of T cells specific for a tumor-restricted TAP-independent antigen with repeated long peptide vaccination proved effective against murine TAP-deficient tumors (Doorduijn et al., 2016). However, presentation of a target antigen by only a subset of healthy cells can induce lethal autoimmune toxicity (Cameron et al., 2013; Morgan et al., 2013; Yee et al., 2000). Our study emphasizes that TAP-independent peptides may be presented by a subset of TAP-proficient cells. Thus, before clinical targeting of TAP-independent antigens, it is crucial to apply a thorough selection with the goal to exclude antigens that are presented on healthy cells.
To optimally select target antigens, mechanistic insights into the intracellular process of peptide selection are required. Current knowledge on how certain TAP-independent peptides fail to be selected for presentation in the presence of TAP is limited. Hypotheses are based on the peptide affinity for HLA-I, its abundance in the ER, and the distance between generation site and peptide-receptive HLA-I molecules (Durgeau et al., 2011; Oliveira et al., 2011; Oliveira and van Hall, 2013). Our human TAP-independent SSR1 antigen and its cognate T cells represent a unique toolset to further investigate existing and novel hypotheses. In support of the affinity hypothesis, the 14-mer SSR1 peptide, which has an HLA-A2 affinity well below the generally accepted threshold for presentation of 500 nM (78 nM), is selected for presentation in the presence of TAP (Bijen et al., 2018; Paul et al., 2013). However, several TAP-independent peptides that are not presented by a TAP-proficient model cell line have a strong predicted HLA-I binding, suggesting that peptide presentation decisions are not solely dictated by HLA-I affinity (Marijt et al., 2018). The second hypothesis suggests that highly abundant peptides pumped into the ER by TAP may be presented at the expense of less abundant TAP-independent peptides (Oliveira et al., 2011). In line with this hypothesis, boosting TAP-mediated competitor peptide supply into the ER by IFN-γ seemed to decrease TAP-independent SSR1 antigen presentation on wild-type cells. In contrast, a reduction in competitor peptide abundance by depleting TAP from wild-type cells failed to enhance SSR1 peptide presentation, suggesting that also other factors play a role. Because the general abundance of TAP-dependent peptides positively correlates with the expression level of TAP (Boegel et al., 2018; Durgeau et al., 2011), the presentation of TAP-independent peptides may be more efficient on cells with low TAP expression, as long as sufficient peptide-receptive HLA-I heavy chains are available. Thus, presentation of a putative TAP-independent target antigen depends at least on the expression of the antigen-containing protein, TAP and the restricted HLA-I allele, on its affinity and on the amount of competitor peptides. Therefore, safe TAP-independent clinical target definition requires assessment of a broad panel of resting and inflamed healthy cells, including cells with differential TAP and antigen-encoding gene expression. Activated B cells upregulate HLA-I presentation upon TLR9 stimulation, for example, by CpG (Jiang et al., 2011). These activated B cells prove to be efficient stimulators of CD8+ T cells, as demonstrated in Figure 5B, and can be considered for such a panel. In addition, other PBMC-derived cells and HUVECS can be included but also other healthy human cells such as but not limited to fibroblasts, keratinocytes, melanocytes, differentiated induced pluripotent stem cells or organoids from different organs.
Taken together, the TAP-independent, polymorphic SSR1 antigen provides opportunities to improve our understanding of TAP-independent peptide presentation through T cell- and MS-based experiments on healthy and TAP-deficient cells. Our data warrant testing presentation of candidate TAP-independent target antigens on a broad panel of healthy cells before clinical application.
Limitations of the study
Although we provide a direct proof of principle of TAP-independent antigen presentation by TAP-proficient cells, we have only tested a limited number of healthy donor-derived cells. Panels focusing on validating TAP-independent antigens for immunotherapy should rely on a broader panel of cell types derived from other vital tissues and organs. A technical limitation of this study is that none of the obtained TAP1 KO clones had a frameshifting mutation on both chromosomes. Although we showed absence of TAP1 by immunoblot (de Waard et al., 2020), a decrease in surface HLA-I, and a lack of presentation of cytosolic antigens, we cannot rule out residual TAP function in our model cells. The facts that the peptide is derived from a signal sequence in the ER, and that we and others eluted the SSR1 peptide from TAP-deficient T2 and 721.174 cell lines, respectively (Weinzierl et al., 2008), provide further support of the TAP-independence of the SSR1 antigen.
Resource availability
Lead contact
Further requests for resources and materials should be directed to and will be fulfilled by the lead contact, Robbert M. Spaapen (r.spaapen@sanquin.nl).
Materials availability
TAP1 KO HAP1 cells generated in this study are available from the lead contact with a completed Materials Transfer Agreement.
Data and code availability
Data of peptidome analyses generated in this manuscript are available from the lead contact on request.
Methods
All methods can be found in the accompanying Transparent methods supplemental file.
Acknowledgments
The authors thank the Sanquin Research Facility for their assistance with flow cytometry and Janine Arts and Dr. Jaap van Buul for providing HUVECs and assistance with their culture. This work was supported by the Netherlands organization for scientific research (NWO-VIDI 91719369; R.S.), KWF Alpe d’HuZes (Bas Mulder Award 2015-7982; R.S.), the Landsteiner Foundation for Blood Transfusion Research (LSBR fellowship 1842F; R.S.) and an investment Grant NWO Medium (91116004, partly financed by ZonMw) to P.A.v.V.
Authors contribution
Conceptualization and design: A.W. and R.S.; Data acquisition, analysis, and interpretation: A.W., T.V., K.H., D.S., M.J., S.B., D.M.K., A.R., R.S.; Resources and discussion: M.G., M.H., A.A., P.V., R.S.; Supervision and conceptual discussion: R.S.; Writing: A.W., R.S.; Editing: A.W., T.V. and R.S.
Declaration of interest
The authors declare no competing interests.
Published: February 19, 2021
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.isci.2021.102051.
Supplemental information
References
- Amir A.L., van der Steen D.M., Hagedoorn R.S., Kester M.G.D., van Bergen C.A.M., Drijfhout J.W., de Ru A.H., Falkenburg J.H.F., van Veelen P.A., Heemskerk M.H.M. Allo-HLA–reactive T cells inducing graft-versus-host disease are single peptide specific. Blood. 2011;118:6733–6742. doi: 10.1182/blood-2011-05-354787. [DOI] [PubMed] [Google Scholar]
- Bijen H.M., Hassan C., Kester M.G.D., Janssen G.M.C., Hombrink P., de Ru A.H., Drijfhout J.W., Meiring H.D., de Jong A.P., Falkenburg J.H.F. Specific T cell responses against minor histocompatibility antigens cannot generally Be explained by absence of their allelic counterparts on the cell surface. Proteomics. 2018;18:1700250. doi: 10.1002/pmic.201700250. [DOI] [PubMed] [Google Scholar]
- Boegel S., Löwer M., Bukur T., Sorn P., Castle J.C., Sahin U. HLA and proteasome expression body map. BMC Med. Genomics. 2018;11:36. doi: 10.1186/s12920-018-0354-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brolsma E. Oncode Inst. News.; 2019. TEIPP-targeting Immunotherapy Project Funded by Oncode Institute [WWW Document]https://www.oncode.nl/news/teipp-targeting-immunotherapy-project-funded-by-oncode-institute accessed 1.28.20. [Google Scholar]
- Cameron B.J., Gerry A.B., Dukes J., Harper J.V., Kannan V., Bianchi F.C., Grand F., Brewer J.E., Gupta M., Plesa G. Identification of a titin-derived HLA-A1-presented peptide as a cross-reactive target for engineered MAGE A3-directed T cells. Sci. Transl. Med. 2013;5:197ra103. doi: 10.1126/scitranslmed.3006034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campoli M., Ferrone S. HLA antigen changes in malignant cells: epigenetic mechanisms and biologic significance. Oncogene. 2008;27:5869–5885. doi: 10.1038/onc.2008.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Waard A.A., Verkerk T., Jongsma M.L.M., Hoefakker K., Sethumadhavan S., Gerke C., Bliss S., Kong X., Janssen G.M.C., de Ru A.H. PAKC: a novel Panel of HLA class I Antigen presentation machinery Knockout Cells from the same genetic origin. Eur. J. Immunol. 2020 doi: 10.1002/eji.202048599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doorduijn E.M., Sluijter M., Marijt K.A., Querido B.J., van der Burg S.H., van Hall T. T cells specific for a TAP-independent self-peptide remain naïve in tumor-bearing mice and are fully exploitable for therapy. Oncoimmunology. 2018;7:e1382793. doi: 10.1080/2162402X.2017.1382793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doorduijn E.M., Sluijter M., Querido B.J., Oliveira C.C., Achour A., Ossendorp F., van der Burg S.H., van Hall T. TAP-independent self-peptides enhance T cell recognition of immune-escaped tumors. J. Clin. Invest. 2016;126:784–794. doi: 10.1172/JCI83671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durgeau A., El Hage F., Vergnon I., Validire P., de Montpréville V., Besse B., Soria J.-C., van Hall T., Mami-Chouaib F. Different expression levels of the TAP peptide transporter Lead to recognition of different antigenic peptides by tumor-specific CTL. J. Immunol. 2011;187:5532–5539. doi: 10.4049/jimmunol.1102060. [DOI] [PubMed] [Google Scholar]
- Gangadhar T.C., Vonderheide R.H. Mitigating the toxic effects of anticancer immunotherapy. Nat. Rev. Clin. Oncol. 2014;11:91–99. doi: 10.1038/nrclinonc.2013.245. [DOI] [PubMed] [Google Scholar]
- Gettinger S., Choi J., Hastings K., Truini A., Datar I., Sowell R., Wurtz A., Dong W., Cai G., Melnick M.A. Impaired HLA class I antigen processing and presentation as a mechanism of acquired resistance to immune checkpoint inhibitors in lung cancer. Cancer Discov. 2017;7:1420–1435. doi: 10.1158/2159-8290.CD-17-0593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassan C., Kester M.G.D., de Ru A.H., Hombrink P., Drijfhout J.W., Nijveen H., Leunissen J.A.M., Heemskerk M.H.M., Falkenburg J.H.F., van Veelen P.A. The human leukocyte antigen–presented ligandome of B lymphocytes. Mol. Cell. Proteomics. 2013;12:1829–1843. doi: 10.1074/mcp.M112.024810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay K.A., Hanafi L.-A., Li D., Gust J., Liles W.C., Wurfel M.M., López J.A., Chen J., Chung D., Harju-Baker S. Kinetics and biomarkers of severe cytokine release syndrome after CD19 chimeric antigen receptor–modified T-cell therapy. Blood. 2017;130:2295–2306. doi: 10.1182/blood-2017-06-793141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henden A.S., Hill G.R. Cytokines in graft-versus-host disease. J. Immunol. 2015;194:4604–4612. doi: 10.4049/jimmunol.1500117. [DOI] [PubMed] [Google Scholar]
- Jiang Q., Pan H., Ye D., Zhang P., Zhong L., Zhang Z. Downregulation of tapasin expression in primary human oral squamous cell carcinoma: association with clinical outcome. Tumor Biol. 2010;31:451–459. doi: 10.1007/s13277-010-0054-4. [DOI] [PubMed] [Google Scholar]
- Jiang W., Lederman M.M., Harding C.V., Sieg S.F. Presentation of soluble antigens to CD8 + T cells by CpG oligodeoxynucleotide-primed human naive B cells. J. Immunol. 2011;186:2080–2086. doi: 10.4049/jimmunol.1001869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jongsma M.L., de Waard A.A., Raaben M., Zhang T., Cabukusta B., Platzer R., Blomen V.A., Xagara A., Verkerk T., Bliss S. The SPPL3-Defined Glycosphingolipid Repertoire Orchestrates HLA Class I-Mediated Immune Responses. Immunity. 2020;54:132–150.e9. doi: 10.1016/j.immuni.2020.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jongsma M.L.M., Guarda G., Spaapen R.M. The regulatory network behind MHC class I expression. Mol. Immunol. 2019;113:16–21. doi: 10.1016/j.molimm.2017.12.005. [DOI] [PubMed] [Google Scholar]
- Kall L., Krogh A., Sonnhammer E.L.L. Advantages of combined transmembrane topology and signal peptide prediction--the Phobius web server. Nucleic Acids Res. 2007;35:W429–W432. doi: 10.1093/nar/gkm256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khong H.T., Restifo N.P. Natural selection of tumor variants in the generation of “tumor escape” phenotypes. Nat. Immunol. 2002;3:999–1005. doi: 10.1038/ni1102-999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koike S., Jahn R. SNAREs define targeting specificity of trafficking vesicles by combinatorial interaction with tethering factors. Nat. Commun. 2019;10:1608. doi: 10.1038/s41467-019-09617-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komov L., Kadosh D.M., Barnea E., Milner E., Hendler A., Admon A. Cell surface MHC class I expression is limited by the availability of peptide-receptive “empty” molecules rather than by the supply of peptide ligands. Proteomics. 2018;18:1700248. doi: 10.1002/pmic.201700248. [DOI] [PubMed] [Google Scholar]
- Ludewig B., Ochsenbein A.F., Odermatt B., Paulin D., Hengartner H., Zinkernagel R.M. Immunotherapy with dendritic cells directed against tumor antigens shared with normal host cells results in severe autoimmune disease. J. Exp. Med. 2000;191:795–804. doi: 10.1084/jem.191.5.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma W., Lehner P.J., Cresswell P., Pober J.S., Johnson D.R. Interferon-γ rapidly increases peptide transporter (TAP) subunit expression and peptide transport capacity in endothelial cells. J. Biol. Chem. 1997;272:16585–16590. doi: 10.1074/jbc.272.26.16585. [DOI] [PubMed] [Google Scholar]
- Man S., Salter R.D., Engelhard V.H. Role of endogenous peptide in human alloreactive cytotoxic T cell responses. Int. Immunol. 1992;4:367–375. doi: 10.1093/intimm/4.3.367. [DOI] [PubMed] [Google Scholar]
- Marijt K.A., Blijleven L., Verdegaal E.M.E., Kester M.G., Kowalewski D.J., Rammensee H.-G., Stevanović S., Heemskerk M.H.M., van der Burg S.H., van Hall T. Identification of non-mutated neoantigens presented by TAP-deficient tumors. J. Exp. Med. 2018;215:2325–2337. doi: 10.1084/jem.20180577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marijt K.A., Doorduijn E.M., van Hall T. TEIPP antigens for T-cell based immunotherapy of immune-edited HLA class Ilow cancers. Mol. Immunol. 2019;113:43–49. doi: 10.1016/j.molimm.2018.03.029. [DOI] [PubMed] [Google Scholar]
- Marijt K.A., van Hall T. To TAP or not to TAP: alternative peptides for immunotherapy of cancer. Curr. Opin. Immunol. 2020;64:15–19. doi: 10.1016/j.coi.2019.12.004. [DOI] [PubMed] [Google Scholar]
- Megger D.A., Philipp J., Le-Trilling V.T.K., Sitek B., Trilling M. Deciphering of the human interferon-regulated proteome by mass spectrometry-based quantitative analysis reveals extent and dynamics of protein induction and repression. Front. Immunol. 2017;8:1139. doi: 10.3389/fimmu.2017.01139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan R.A., Chinnasamy N., Abate-Daga D., Gros A., Robbins P.F., Zheng Z., Dudley M.E., Feldman S.A., Yang J.C., Sherry R.M. Cancer regression and neurological toxicity following anti-MAGE-A3 TCR gene therapy. J. Immunother. 2013;36:133–151. doi: 10.1097/CJI.0b013e3182829903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neefjes J., Jongsma M.L.M.M., Paul P., Bakke O. Towards a systems understanding of MHC class i and MHC class II antigen presentation. Nat. Rev. Immunol. 2011;11:823–836. doi: 10.1038/nri3084. [DOI] [PubMed] [Google Scholar]
- Oliveira C.C., Querido B., Sluijter M., de Groot A.F., van der Zee R., Rabelink M.J.W.E., Hoeben R.C., Ossendorp F., van der Burg S.H., van Hall T. New role of signal peptide peptidase to liberate C-terminal peptides for MHC class I presentation. J. Immunol. 2013;191:4020–4028. doi: 10.4049/jimmunol.1301496. [DOI] [PubMed] [Google Scholar]
- Oliveira C.C., Querido B., Sluijter M., Derbinski J., van der Burg S.H., van Hall T. Peptide transporter TAP mediates between competing antigen sources generating distinct surface MHC class I peptide repertoires. Eur. J. Immunol. 2011;41:3114–3124. doi: 10.1002/eji.201141836. [DOI] [PubMed] [Google Scholar]
- Oliveira C.C., van Hall T. Alternative antigen processing for MHC class I: multiple roads Lead to rome. Front. Immunol. 2015;6:298. doi: 10.3389/fimmu.2015.00298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira C.C., van Hall T. Importance of TAP-independent processing pathways. Mol. Immunol. 2013;55:113–116. doi: 10.1016/j.molimm.2012.10.005. [DOI] [PubMed] [Google Scholar]
- Paul S., Weiskopf D., Angelo M.A., Sidney J., Peters B., Sette A. HLA class I alleles are associated with peptide-binding repertoires of different size, affinity, and immunogenicity. J. Immunol. 2013;191:5831–5839. doi: 10.4049/jimmunol.1302101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeffer S., Dudek J., Schaffer M., Ng B.G., Albert S., Plitzko J.M., Baumeister W., Zimmermann R., Freeze H.H., Engel B.D., Förster F. Dissecting the molecular organization of the translocon-associated protein complex. Nat. Commun. 2017;8:14516. doi: 10.1038/ncomms14516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pont M.J., Oostvogels R., van Bergen C.A.M., van der Meijden E.D., Honders M.W., Bliss S., Jongsma M.L.M., Lokhorst H.M., Falkenburg J.H.F., Mutis T. T cells specific for an unconventional natural antigen fail to recognize leukemic cells. Cancer Immunol. Res. 2019;7:797–804. doi: 10.1158/2326-6066.CIR-18-0137. [DOI] [PubMed] [Google Scholar]
- Restifo N.P., Marincola F.M., Kawakami Y., Taubenberger J., Yannelli J.R., Rosenberg S.A. Loss of functional beta2-microglobulin in metastatic melanomas from five patients receiving immunotherapy. J. Natl. Cancer Inst. 1996;88:100–108. doi: 10.1093/jnci/88.2.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riemer A.B., Keskin D.B., Reinherz E.L. Identification and validation of reference genes for expression studies in human keratinocyte cell lines treated with and without interferon-γ - a method for qRT-PCR reference gene determination. Exp. Dermatol. 2012;21:625–629. doi: 10.1111/j.1600-0625.2012.01537.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rock K.L., Reits E., Neefjes J. Present Yourself! By MHC class I and MHC class II molecules. Trends Immunol. 2016;37:724–737. doi: 10.1016/j.it.2016.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rusinova I., Forster S., Yu S., Kannan A., Masse M., Cumming H., Chapman R., Hertzog P.J. INTERFEROME v2.0: an updated database of annotated interferon-regulated genes. Nucleic Acids Res. 2012;41:D1040–D1046. doi: 10.1093/nar/gks1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sade-Feldman M., Jiao Y.J., Chen J.H., Rooney M.S., Barzily-Rokni M., Eliane J.-P.P., Bjorgaard S.L., Hammond M.R., Vitzthum H., Blackmon S.M. Resistance to checkpoint blockade therapy through inactivation of antigen presentation. Nat. Commun. 2017;8:1136. doi: 10.1038/s41467-017-01062-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrodt S., Koch J., Tampé R. Membrane topology of the transporter associated with antigen processing (TAP1) within an assembled functional peptide-loading complex. J. Biol. Chem. 2006;281:6455–6462. doi: 10.1074/jbc.M509784200. [DOI] [PubMed] [Google Scholar]
- Shraibman B., Barnea E., Kadosh D.M., Haimovich Y., Slobodin G., Rosner I., López-Larrea C., Hilf N., Kuttruff S., Song C. Identification of tumor antigens among the HLA peptidomes of glioblastoma tumors and plasma. Mol. Cell. Proteomics. 2019;18:1255–1268. doi: 10.1074/mcp.RA119.001524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spaapen R., van den Oudenalder K., Ivanov R., Bloem A., Lokhorst H., Mutis T. Rebuilding human leukocyte antigen class II-restricted minor histocompatibility antigen specificity in recall antigen-specific T cells by adoptive T cell receptor transfer: implications for adoptive immunotherapy. Clin. Cancer Res. 2007;13:4009–4015. doi: 10.1158/1078-0432.CCR-07-0286. [DOI] [PubMed] [Google Scholar]
- Spaapen R.M., Lokhorst H.M., van den Oudenalder K., Otterud B.E., Dolstra H., Leppert M.F., Minnema M.C., Bloem A.C., Mutis T. Toward targeting B cell cancers with CD4+ CTLs: identification of a CD19-encoded minor histocompatibility antigen using a novel genome-wide analysis. J. Exp. Med. 2008;205:2863–2872. doi: 10.1084/jem.20080713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sultan H., Trillo-Tinoco J., Rodriguez P., Celis E. Effective antitumor peptide vaccines can induce severe autoimmune pathology. Oncotarget. 2017;8:70317–70331. doi: 10.18632/oncotarget.19688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takaba H., Takayanagi H. The mechanisms of T cell selection in the thymus. Trends Immunol. 2017;38:805–816. doi: 10.1016/j.it.2017.07.010. [DOI] [PubMed] [Google Scholar]
- Uhlen M., Fagerberg L., Hallstrom B.M., Lindskog C., Oksvold P., Mardinoglu A., Sivertsson A., Kampf C., Sjostedt E., Asplund A. Tissue-based map of the human proteome. Science. 2015;347:1260419. doi: 10.1126/science.1260419. [DOI] [PubMed] [Google Scholar]
- Van Bergen C.A.M., Rutten C.E., Van Der Meijden E.D., Van Luxemburg-Heijs S.A.P., Lurvink E.G.A., Houwing-Duistermaat J.J., Kester M.G.D., Mulder A., Willemze R., Falkenburg J.H.F., Griffioen M. High-throughput characterization of 10 new minor histocompatibility antigens by whole genome association scanning. Cancer Res. 2010;70:9073–9083. doi: 10.1158/0008-5472.CAN-10-1832. [DOI] [PubMed] [Google Scholar]
- van der Lee D.I., Reijmers R.M., Honders M.W., Hagedoorn R.S., de Jong R.C., Kester M.G., van der Steen D.M., de Ru A.H., Kweekel C., Bijen H.M. Mutated nucleophosmin 1 as immunotherapy target in acute myeloid leukemia. J. Clin. Invest. 2019;129:774–785. doi: 10.1172/JCI97482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Hall T., Wolpert E.Z., van Veelen P., Laban S., van der Veer M., Roseboom M., Bres S., Grufman P., de Ru A., Meiring H. Selective cytotoxic T-lymphocyte targeting of tumor immune escape variants. Nat. Med. 2006;12:417–424. doi: 10.1038/nm1381. [DOI] [PubMed] [Google Scholar]
- Voldby Larsen M., Nielsen M., Weinzierl A., Lund O. TAP-independent MHC class I presentation. Curr. Immunol. Rev. 2006;2:233–245. [Google Scholar]
- Weinzierl A.O., Rudolf D., Hillen N., Tenzer S., van Endert P., Schild H., Rammensee H.-G., Stevanović S. Features of TAP-independent MHC class I ligands revealed by quantitative mass spectrometry. Eur. J. Immunol. 2008;38:1503–1510. doi: 10.1002/eji.200838136. [DOI] [PubMed] [Google Scholar]
- Yee C., Thompson J.A., Roche P., Byrd D.R., Lee P.P., Piepkorn M., Kenyon K., Davis M.M., Riddell S.R., Greenberg P.D. Melanocyte destruction after antigen-specific immunotherapy of melanoma. J. Exp. Med. 2000;192:1637–1644. doi: 10.1084/jem.192.11.1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Z., Luo A., Shrivastava I., He M., Huang Y., Bahar I., Liu Z., Wan Y. Regulation of XIAP turnover reveals a role for USP11 in promotion of tumorigenesis. EBioMedicine. 2017;15:48–61. doi: 10.1016/j.ebiom.2016.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data of peptidome analyses generated in this manuscript are available from the lead contact on request.