Abstract
Attachment security is formed through interactions with a main caregiver during the first three years of life and reflects inter-individual differences in mental representations for the relationship. The striatum is known to be a key structure to initiate attachment behaviours and maintain attachment relationships as well as to modulate reward-related processing as part of the approach module in current neurobiological models of human attachment. Although findings have suggested critical roles of the striatum in inter-individual differences in attachment, most studies were based on a wide variety of tasks and very few have investigated these associations in intrinsic brain connectivity in typically developing children. In the present study, using resting-state functional magnetic resonance imaging, we examined the striatal functional connectivity according to children’s attachment security in 68 nine-year-olds (Secure attachment = 39, Insecure attachment = 29, mean age/SD = 9.62/0.69). Children with secure attachment demonstrated increased functional connectivity in the tempro-limbic region, compared to children with insecure attachment. In addition, the child-reported attachment security scores were negatively associated with the caudate-prefrontal connectivity, but positively with the putamen-visual area connectivity. These data demonstrate that inter-individual differences in attachment can be captured in striatal functional connectivity organization in the typical brain.
Keywords: Attachment security, Striatal connectivity, Resting-state fMRI, Children
1. Introduction
Attachment is based on an innate biological system which regulates proximity-seeking behaviours in infants with the goal to feel safe with a caregiver, which include separation distress, greeting reactions upon reunion and the tendency to turn to a specific caregiver for reassurance when distressed (Bowlby, 1980, 1962). With a consistent pattern of engagement of attachment processes occurring in dyadic interactions, these behaviours are gradually modulated by caregivers’ responses, and progressively form mental representations for the relationship, which Bowlby termed as “internal working models” (Bowlby, 1973). Internal Working Models operate for members of an attachment dyad to anticipate, interpret and guide interactions with partners, and lead to individual differences in the expression and organization of attachment behaviours. In attachment theory, these individual differences represent the security or quality of the relationship and can be classified into several behavioural patterns: secure and insecure (avoidant or ambivalent/resistant) types of attachment (Ainsworth et al., 1978). Initial attachment theory contended that these individual differences are maintained in a relatively consistent way over the years (McConnell and Moss, 2011; Meins et al., 2018), although more recent studies demonstrate that attachment can also be malleable under certain circumstances over time (Chopik and Grimm, 2019; for a review see Fraley, 2019). Little is known, however, about the neurobiological basis of individual differences in attachment types, particularly in typically developing children, and such knowledge is important for understanding its development as well as the possibilities for change (McConnell and Moss, 2011).
Neurobiological models of human attachment have been developed based on broader social neuroscience, such as approach vs. avoidance, or automatic vs. controlled systems in the social information processing perspectives (Gillath et al., 2016; Mikulincer and Shaver, 2003). A recent effort to provide integrative and comprehensive models of attachment synthesizing brain structures, functions, genes and neurotransmitters, has been advanced in an extended functional neuro-anatomical model of human attachment (NAMA), focussing on how attachment security is established and how the inter-individual differences in attachment operate in these models (see the review for more details: Long et al., 2020): In brief, the human attachment system is organized into two systems (affective evaluation and cognitive control) inclusive of four modules (aversion, approach, emotion regulation and mental state representation). The inter-individual differences in attachment can be explained by possible determinants of “switch point” shifts in the “push and pull” dynamics between the systems or modules.
The striatum supports a wide range of functions, including motor, cognitive, motivational, reward and emotional processes with significant projections from other cortical regions (Di Martino et al., 2008). The striatum can be embedded within a larger network of “approach” in NAMA in that the approach module promotes social interactions to seek a sense of safety, which are primarily supported by dopaminergic reward and motivation systems which include the ventral striatum, ventral tegmental area, substantia nigra and ventromedial prefrontal/orbitofrontal cortices. Furthermore, the dorsal striatum is composed of the caudate nucleus (dorsomedial part) and the putamen (dorsolateral part), regions which are involved in cognitive and motor control, respectively (Lipton et al., 2019; Yin and Knowlton, 2006). Previous literature suggested putamen network engagement in habitual behaviours and caudate network engagement for goal-directed behaviours, but these networks more likely develop in parallel and with complementary roles (Kupferschmidt et al., 2017; Robbins and Costa, 2017). Proximity-seeking in attachment is one of the strongest goal-directed behaviours emerging at an early stage of life just after the infants recognize their primary caregiver. Attachment security reflects the past and current interactions with an attachment figure, and includes specific patterns of behaviours which become habitual and automatic, and therefore operate largely at an unconscious level (Mikulincer and Shaver, 2007; Petters and Waters, 2010). This implies extensive involvement of the striatum in attachment, while initiating attachment behaviours and forming behavioural patterns in the attachment dyad.
Several neuroimaging studies have demonstrated that the striatum has important roles in explaining individual differences in adult attachment, with connections to other cortical regions known to modulate reward, emotional regulation and mentalizing processes (Antonucci et al., 2018; Vrtička et al., 2008; Vrtička and Vuilleumier, 2012). In addition, distinct neural influences have also been implicated in avoidant and anxious types of adult attachment, such that attachment avoidance is linked to blunted social reward processing and altered negative emotion perception and regulation (Strathearn et al., 2009). In contrast, attachment anxiety is linked to over-sensitivity to emotional cues and less cognitive control (Donges et al., 2012; Krause et al., 2018, 2016; Rigon et al., 2016; Schneider-Hassloff et al., 2015; Zhang et al., 2018b). An fMRI study also demonstrated a link between attachment anxiety and the activity in the ventral striatum and ventromedial orbitofrontal cortex in prediction-error response to a social reward (Poore et al., 2012). These effects can be sustained life-long, as the insecure attachment measured at 18-months predicted greater activation in prefrontal areas with reduced co-activation of nucleus accumbens in processing positive emotions 20 years later (Moutsiana et al., 2014). Further, this can also relate to psychopathology, as children and adolescents with reactive attachment disorders showed reduced activity in the caudate and nucleus accumbens in reward processing (Takiguchi et al., 2015). These reports suggest that early attachment experiences impact striatal functions, but only a few studies have examined these associations in children.
In a previous task-evoked fMRI study, children with secure attachment showed greater functional activations in the striatum, amygdalae and cingulate cortex when they were processing attachment-related cues (Choi et al., 2018). In adolescents, self-representations of attachment relationships also modulated functional activations in the amygdalae, temporal poles and dorsolateral prefrontal cortices (Debbané et al., 2017), as well as reward-related activation in the caudate (Schneider et al., 2012). A longitudinal study demonstrated that greater levels of emotional neglect were associated with blunted ventral striatum activity, which was also related to depression in adolescence (Hanson et al., 2015). Although not revealing any significant associations with attachment in the striatum, a recent study found that heightened activity in the anterior temporal pole while processing unfamiliar faces other than self and mother was related to attachment anxiety in children 8–12 years of ages (Miller et al., 2020). Although these studies have inferred that early experience in attachment relationships modulates functional organization in the brain, studies were based on a wide variety of tasks and very few reports have investigated these associations in spontaneous fluctuations of brain activity.
Resting-state functional connectivity measures the temporal correlations of spontaneous blood-oxygen-level-dependent (BOLD) signals among spatially distributed brain regions. It has provided considerable understanding of functional organization which underlies a range of sensory and cognitive processes. Corticostriatal connections are complex and interactions between functional territories are extensive (Haber, 2016). However, little is known about the association between inter-individual differences in attachment and intrinsic striatal connectivity at a whole-brain level in typically developing children. A few studies have implicated early adversity, such as neglect, maltreatment and institutional care, with functional organization of striatal connectivity. A study, which compared previously institutionalized youth to youth raised in their biological families, revealed increased ventral striatum and medial prefrontal cortex coupling and its association with parent reports of social problems (Fareri et al., 2017). Another study, although not revealing an association with striatal connectivity, reported amygdala functional connectivity using resting-state fMRI in typically developing six-to-ten-year old children, in whom parental sensitivity had been assessed at four years of age. Only in children with less sensitive parents was age positively related to stronger amygdala-medial prefrontal connectivity, suggesting that children with a lack of supportive parenting may develop their own self-regulation system instead of relying on their parents (Thijssen et al., 2017).
In the present study, we used fMRI to examine the intrinsic striatal functional connectivity of the caudate and putamen in typically developing children with secure and insecure attachment to determine how different attachment experiences contribute to the development of functional connections between the striatal structures and other cortical regions at the whole-brain level. We chose the striatum not only because of the findings from adult attachment studies but because of its critical role in a wide range of socio-emotional learning early in life, and knowing that attachment behaviours build upon those systems. We hypothesized that inter-individual differences in attachment would be observed in the prefrontal region in the caudate network related to its role in cognitive control, and the sensorimotor regions in the putamen network related their role in motor control. In addition, based on the substantial projections from multiple cortical regions to striatum and its critical role for reward and positive emotion processing as a key structure of the approach module, we speculated the inter-individual differences in attachment may also be found in limbic areas.
2. Methods
2.1. Participants
Eighty-five boys were recruited from elementary schools (mean age = 9.62 years, SD = 0.69). Those who had a history of psychiatric, neurological illness or contraindications to MRI were not included. Children’s attachment security was assessed by trained examiners using the Separation Anxiety Test (SAT), standardised in Korean (Choi, 2014; Hansburg, 1972; Resnick, 1993). The SAT is a semi-projective interview system using separation pictures to assess the quality of attachment from preschool-aged children to adolescents. All of the pictures and questions were modified according to the children’s age, sex and cultural background and were verified by a group of experts in child development research and another group of children of the same age, sex and cultural background (Choi et al., 2018). All interviews were audiotaped, transcribed verbatim and coded according to the SAT manual (Resnick, 1993), which includes nine subscales (e.g., emotional openness, devaluing of attachment, self-blame, resistance/withholding, preoccupied anger, displacement of feelings, anxiety, coherence of transcript, solutions) and assigns each child to one of the attachment classifications based on the profile in each subscale (e.g., secure, insecure avoidant, insecure preoccupied). As a result, forty-eight boys (56.5 %) were classified as secure attachment and thirty-seven boys (43.5 %) were classified as insecure attachment (27 avoidant, 10 preoccupied). The intercoder agreements for the cases were 89.4 % and the cases which showed disagreement between the coders were discussed until agreement was reached. This study was approved by the institutional review board for human subjects at the Seoul National University. All children and their parents provided written informed consent prior to study entry.
2.2. Neuropsychological assessments
Neuropsychological assessments were conducted to more fully characterise the sample by creating overall behavioural profiles. Two self-report questionnaires for children were used to assess their perception of attachment security (The Attachment Security Scale: ASS) (Kerns et al., 2001) and self-concept (the Self-Concept Inventory: SCI) (Lee and Ko, 2006). The Child Behavior Checklist (CBCL) was also completed by a caregiver to identify any internalizing and externalizing problems in the children (Achenbach and Rescorla, 2001). IQ was measured using the Korean version of Wechsler Intelligence Scale for Children Fourth Edition (K-WISC-IV) (Gwak et al., 2011), which included Verbal Comprehension Index (VCI), Perceptual Reasoning Index (PRI), Working Memory Index (WMI), Processing Speed Index (PSI) and full-scale IQ (FSIQ).
2.3. Data acquisition
MRI data were acquired on a Siemens Tim Trio 3.0 T scanner with a 32 channel head coil. Sponge padding was placed around the head to minimize head movement and the lower jaw was fixed with tape. A high resolution T1-weighted anatomical image was acquired (TR/TE = 1900/2.36 ms; FA = 9°; FOV = 192 × 240 × 256 mm; 1 mm isotropic voxels; 4.26 min acquisition time). Resting state functional images were obtained with a contrast gradient echo planar imaging pulse sequence (TR/TE = 2700/30 ms; FA 90°; FOV, matrix size = 64 × 64 × 40; 3.0 × 3.0 × 3.2 mm voxels; 150 volumes; 5.17 min acquisition time). Children watched the monitor through a mirror on the head coil, which presented landscape pictures during the structural images and was replaced with a fixation cross centred on the screen for the resting state scan.
2.4. Data preprocessing
Data preprocessing was performed using AFNI [https://afni.nimh.nih.gov], FMRIB Software Library [https://www.fmrib.ox.ac.uk/fsl], and locally developed tools. All resting-state data were slice-time and motion corrected, smoothed (6 mm FWHM), normalized to a mean intensity of 10,000, bandpass filtered between 0.01 and 0.2 Hz, and cleaned of signal contributions from the white matter, CSF, whole brain and six rigid body parameters via regression. Framewise displacement (FD) was calculated from the six rigid body parameters, and volumes with FD > 0.5 mm were censored; participants with >1/3 of their volumes censored were excluded from the analyses. The original sample included 85 children (Secure attachment = 48, Insecure attachment = 37), but 17 were excluded due to reaching clinical cut-off scores in CBCL or excessive head motion, leaving the final sample of 68 (Secure attachment = 39, Insecure attachment = 29) for analyses.
2.5. Data analyses
We used a standard seed-voxel correlation approach to compare the striatal functional connectivity between the two groups of children using FSL FEAT (Jenkinsson et al., 2012). Bilateral caudate and putamen coordinates from the Automated Anatomical Labeling (AAL; Tzourio-Mazoyer et al., 2002) atlas in MNI space were used as seeds to create striatum ROIs: left caudate (−11, 11, 9), right caudate (15, 12, 9), left putamen (−24, 4, 2), and right putamen (28, 5, 2). To account for inter-subject variability, the seed coordinates were dilated within a 10 mm spherical ROI until the minimum mean inter-voxel cross-correlation dropped below 0.8 or the number of voxels exceeded 300. First-level analysis was performed using FSL’s FEAT by correlating the mean time series of the ROIs with the time series of all voxels in the brain in each child. Second-level analysis was performed to investigate group differences (Secure vs. Insecure attachment categorized by the SAT) in functional connectivity from each seed, with and without controlling for IQ. All statistical analyses in functional connectivity differences were thresholded using clusters determined by Z>2.3 and a corrected cluster significance threshold of FWE p < .05. If significant differences were found, the mean contrast of parameter estimate (COPE) values over the significant voxels were pulled across all participants, and the brain-behaviour associations were explored by correlating the COPE values with neuropsychological characteristics (child-report attachment security scores and behavioural scores) while controlling for IQ. For behavioural correlations, Pearson R values were calculated and the resulting p-values were Bonferroni corrected and significance was held at pcorr< 0.003. In addition, the association between the whole brain striatal functional connectivity and the continuous measure of attachment security scale (ASS) were examined while controlling for IQ to determine additional clusters relevant to child-report attachment security.
3. Results
3.1. Demographic information and neuropsychological characteristics
Demographic information is presented in Table 1. Parents’ educational levels and social economic status were not different across the secure and insecure participants. The majority of parents reported that the main caregiver of the child during the first three years was the mother. Some parents reported significant maternal stress during pregnancy but the portion reporting maternal stress between the two groups did not differ. As reported in Table 2, children who were classified as insecurely attached showed significantly lower scores than children classified as secure attachment in child-reported attachment security (t(66)=-4.29, p = 0.001). The children with insecure attachment demonstrated higher internalizing and externalizing problems from the CBCL, although they are not statistically significant (Internalizing, t=0.26, p=0.80; Externalizing, t=1.21, p=0.23), but revealed significantly negative self-concept (t(65)=-2.80, p = 0.004). In CBCL symptom scales, the children in the clinical range were 7 (10.3 %) in anxious/depressed, 2 (2.9 %) in withdrawn/depressed, 6 (8.8 %) in somatic complaints, 3 (4.4 %) in social problems, 4 (5.9 %) in attention problems, 2 (2.9 %) in rule-breaking and 3 (4.4 %) in aggressive behaviour. Although the mean scores of all IQ indices were in the normal range, children with insecure attachment showed overall lower IQ scores, except on the processing speed index, (VCI, t(65)=-2.22, p = 0.03; PRI, t(65)=-2.89, p = 0.005; WMI, t(65)=-2.74, p = 0.008; FSIQ, t(65)=-3.49, p = 0.001).
Table 1.
Demographics of securely attached and insecurely attached children.
| Securely attached (N = 39) | insecurely attached (N = 29) | Statistics | |
|---|---|---|---|
| Age (years) | 9.73 ± 0.60 | 9.64 ± 0.75 | t = −0.55, p = 0.58 |
| Father’s education level | |||
| College graduation or above | 33 (86.8 %) | 22 (75.9 %) | χ2 = 2.12, p = 0.35 |
| High school graduation | 5 (13.2 %) | 6 (20.7 %) | |
| Middle school graduation | 0 (0 %) | 1 (3.4 %) | |
| Mother’s education level | |||
| College graduation or above | 32 (86.5 %) | 21 (72.4 %) | χ2 = 2.69, p = 0.26 |
| High school graduation | 5 (13.5 %) | 7 (24.1 %) | |
| Middle school graduation | 0 (0 %) | 1 (2.9 %) | |
| Primary caregiver of the child during the first three years | |||
| Mother | 36 (92.3 %) | 26 (89.7 %) | χ2 = 3.55, p = 0.47 |
| Othersa | 3 (7.7 %) | 3 (10.3 %) | |
Including father, grandparents, daycare centre or nanny.
Table 2.
Neuropsychological characteristics.
| Securely attached (N = 46) | Insecurely attached (N = 34) | Statistics | |
|---|---|---|---|
| Quality of Attachment (continuous measures) | |||
| Attachment security scale | 51.95 (4.38) | 46.14 (6.24) | t(66) = 4.29, p<0.001 |
| Child Behavior Checklist (CBCL) | |||
| Internalizing problems | 4.97 ± 5.03 | 5.28 ± 4.32 | t(65)=-0.26, p = 0.79 |
| Externalizing problems | 4.71 ± 4.63 | 6.97 ± 9.20 | t(65)=-1.21, p = 0.23 |
| Self-Concept Inventory (SCI) | |||
| General self | 59.87 (9.44) | 52.48 (12.18) | t(65) = 2.95, p = 0.004 |
| Wechsler Intelligence Scale for Children Fourth Edition (WISC-IV) | |||
| Verbal comprehension index | 116.61 (15.45) | 108.41 (14.35) | t(65)=-2.22, p = 0.03 |
| Perceptual reasoning index | 122.39 (15.76) | 111.69 (14.05) | t(65)=-2.89, p = 0.005 |
| Working memory index | 114.61 (16.39) | 104.48 (12.96) | t(65)=-2.74, p = 0.008 |
| Processing speed index | 104.11 (10.33) | 100.90 (14.30) | t(65)=-1.07, p = 0.29 |
| Full-scale IQ | 120.68 (13.93) | 109.10 (12.77) | t(65)=-3.49, p = 0.001 |
The correlations between the behavioural measures were examined without controlling for IQ (Supplementary Table 1) as well as with controlling for IQ (Supplementary Table 2). Two child-report measures for attachment security and self-concept were significantly associated with each other, independent of IQ (r = 0.55, p < 0.001). In addition, two sub-scores in the CBLC, which internalizing and externalizing problems, were also significantly correlated, independent of IQ (r = 0.056, p < 0.001).
3.2. Connectivity differences and behavioural correlations in striatal functional connectivity without controlling for IQ
Examination of resting-state functional connectivity in children with secure and insecure attachment revealed different patterns of striatal connectivity mostly in medial temporal regions. In the caudate network, children with secure attachment demonstrated increased functional connectivity between the parahippocampal gyrus and the left caudate compared to children with insecure attachment (Supplementary Table 3).
The putamen network showed more robust group differences. Children with secure attachment showed increased functional connectivity in the medial temporal cortex with the left putamen, with peak voxels in the temporal pole, hippocampus, superior temporal gyrus and parahippocampal gyrus. Increased connectivity was also found in the visual network, with peak voxels in superior occipital cortex, lingual gyrus, cuneus and calcarine sulcus (Supplementary Table 3). On other hand, children in the insecure attachment group demonstrated increased connectivity between the right supramarginal and angular gyri and the left putamen, compared to children with secure attachment (Supplementary Table 4).
Brain-behaviour associations were examined in these four clusters, which identified group differences (Supplementary Table 5). Among the four behavioural measures, the child-reported attachment security scores (ASS) was correlated with the connectivity strength to the visual cortices (R=0.40, p = 0.001) in the putamen network. In an exploratory analysis, we examined the association between this connectivity feature and children’s attention problems, as the pattern of increased connectivity in children with insecure attachment was presented in a focalised region, the anterior aspect of the TPJ, which is known to be associated with attention. Interestingly, the connectivity strength to the right supramarginal gyrus in the putamen network was positively correlated with CBCL attentional problem scores (R = 0.317, p = 0.009).
3.3. Connectivity differences and behavioural correlations in striatal functional connectivity while controlling for IQ
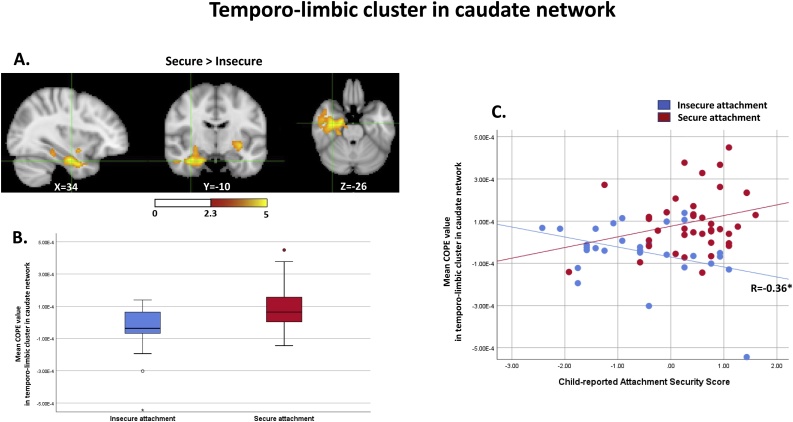
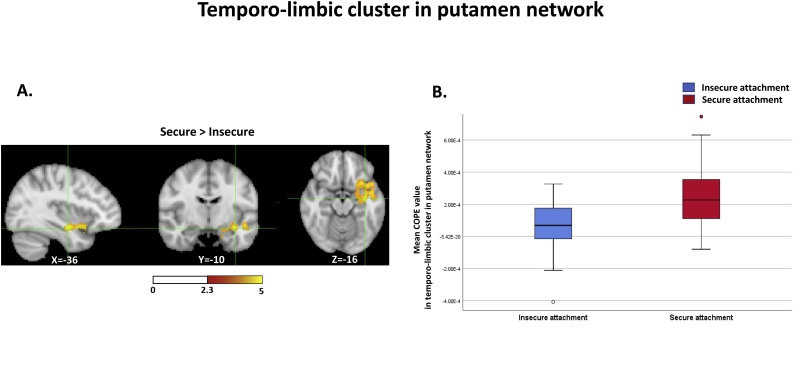
The same analyses for group comparisons were employed again, while controlling for IQ, as children in the secure and insecure attachment groups revealed significant differences in IQ; the medial temporal region connectivity remained significant. In the caudate network, the connectivity differences observed in the medial temporal regions were more pronounced after controlling for IQ (Table 3). Securely attached children demonstrated increased connectivity in bilateral parahippocampal gyri, the right hippocampus, and the left putamen, compared to children with insecure attachment (Fig. 1A and B). In the putamen network, the increased right medial temporal and temporal pole connectivity in securely attached children also remained significant after controlling for IQ, and extended to the orbital part of inferior frontal gyrus (Fig. 2A and B) (Table 4).
Table 3.
Increased functional connectivity in striatal network while controlling for IQ in securely attached children.
| Cluster | Nvoxels | Cluster p-value | Brain regions | L/R | BA | MNI coordinates (mm) |
Z | ||
|---|---|---|---|---|---|---|---|---|---|
| x | y | z | |||||||
| Caudate network | |||||||||
| 1 | 1547 | 0.000787 | Hippocampus | R | 54 | 34 | −10 | −26 | 4.23 |
| Parahippocampal gyrus | R | 36 | 20 | −8 | −26 | 4.09 | |||
| Inferior temporal gyrus | R | 20 | 42 | −24 | −22 | 3.64 | |||
| Parahippocampal gyrus | R | 36 | 38 | −32 | −18 | 3.22 | |||
| 2 | 855 | 0.0266 | Putamen | L | 49 | −32 | −12 | −6 | 3.84 |
| Parahippocampal gyrus | L | 36 | −20 | −30 | −12 | 2.63 | |||
| Putamen network | |||||||||
| 1 | 1272 | 0.0021 | Inferior frontal gyrus (Orbital) | L | 47 | −36 | −10 | −16 | 3.65 |
| Superior temporal gyrus | L | 22 | −46 | −2 | −14 | 3.56 | |||
| −48 | −8 | −12 | 3.41 | ||||||
| Temporal pole | −48 | 14 | −18 | 3.32 | |||||
| Parahippocampal gyrus | L | 36 | −26 | 2 | −28 | 3.29 | |||
PFWE <.05.
Fig. 1.
Increased functional connectivity between the caudate and the temporo-limbic region in children with secure attachment (1A) and connectivity differences in children with secure and insecure attachment (1B). The caudate-temporo limbic region connectivity had a positive association with the child-reported attachment security scores in children with secure attachment, while a negative association was observed in children with insecure attachment (r=−0.36, p = 0.05) (1C).
Fig. 2.
Increased functional connectivity between the putamen and the temporo-limbic region in children with secure attachment (2A) and connectivity differences in children with secure and insecure attachment (2B).
Table 4.
Brain regions of positively or negatively associated with child-reported attachment security scores (ASS) in striatal network.
| Cluster | Nvoxels | Cluster p-value | Brain regions | L/R | BA | MNI coordinates |
Z | ||
|---|---|---|---|---|---|---|---|---|---|
| x | y | z | |||||||
| Negatively associated with the ASS in the caudate network | |||||||||
| 1 | 1418 | 0.00149 | Superior frontal gyrus | L | −20 | 8 | 72 | 3.54 | |
| R | 18 | 32 | 42 | 3.49 | |||||
| Supplementary motor area | L | 6 | −18 | 8 | 64 | 3.39 | |||
| L | −12 | 20 | 66 | 3.38 | |||||
| 0 | 24 | 48 | 3.33 | ||||||
| Superior frontal gyrus | L | 8 | −12 | 28 | 48 | 3.30 | |||
| Positively associated with the ASS in the putamen network | |||||||||
| 1 | 2336 | 1.6e-05 | Middle occipital gyrus | R | 39 | 40 | −82 | 36 | 4.27 |
| Superior occipital gyrus | L | 19 | −24 | −90 | 34 | 4.15 | |||
| Cuneus | L | 19 | −4 | −90 | 32 | 4.11 | |||
| −10 | −90 | 30 | 4.01 | ||||||
| Superior occipital gyrus | R | 7 | 32 | −84 | 40 | 4.00 | |||
| Cuneus | R | 19 | 14 | −88 | 36 | 3.82 | |||
PFWE < .05.
The connectivity strengths from the two clusters, which identified group differences while controlling for IQ, were not associated with any behavioural measures (Supplementary Table 6) at a statistically significant level. An additional exploration revealed that the child-report attachment security scores (ASS) had reverse patterns of associations with the caudate-medial temporal connectivity between the two groups. Children with secure attachment demonstrated a positive association between the caudate-medial temporal connectivity and the ASS scores, while children with insecure attachment demonstrated negativity in this association, which was significant in insecurely attached children (r=-0.36, p = 0.05) (Fig. 1C).
3.4. Association between the whole-brain striatal connectivity and child-reported attachment security scores while controlling for IQ
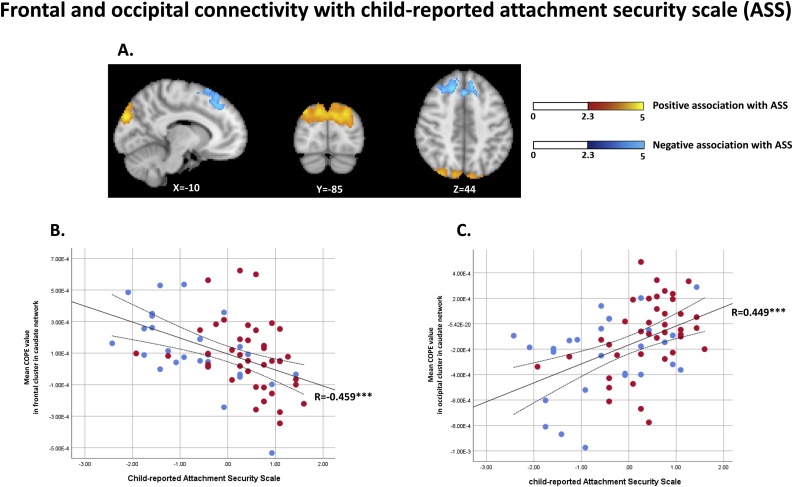
Additional clusters were identified in the association between the whole-brain striatal connectivity and the child-reported attachment security scores (ASS) (Fig. 3A). In the caudate network, the superior frontal and supplementary motor areas, which encompass dorsolateral and dorsomedial prefrontal cortices, were negatively associated with the ASS scores (Fig. 3B). In the putamen network, the visual areas, including superior and middle occipital regions and cuneus, were positively associated with the ASS scores (Fig. 3C).
Fig. 3.
The child-reported attachment security scores were negatively associated with the connectivity between the caudate and dorsolateral and dorsomedial prefrontal cortex (3A, coloured in blue, and 3B), and positively associated with the connectivity between the putamen and the superior/middle occipital visual area (3A, coloured in yellow, and 3C) (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.).
4. Discussion
In the current study, the striatal functional connectivity was examined in a large group of typically developing children according to their attachment security. Children with secure and insecure attachment demonstrated significantly different connectivity patterns in both the caudate and putamen networks, suggesting inter-individual differences in attachment can be captured in intrinsic striatal connectivity organization in the typically developing brain. More robust differences were found in the temporo-limbic regions, not sensorimotor or prefrontal regions. The prefrontal cortices are not fully developed in this young age group compared to the adult attachment studies, which have frequently reported prefrontal engagement in explaining individual differences in attachment types (Krause et al., 2018, 2016; Rigon et al., 2016; Thijssen et al., 2017). The connectivity differences between the two groups mostly represented different roles of the striatum in socio-emotional information processing. These findings correspond to adult attachment studies which showed that the fronto-striatal-limbic circuit function is modulated by attachment type during the processing of social and attachment-related cues (Perlini et al., 2019). In particular, this circuit is responsible for modulating affective evaluations and often represents an access to memory about previous experiences, thinking of negative emotions or stress responses (Coan, 2010; Vrtička and Vuilleumier, 2012; Williams et al., 2018).
Children with secure attachment demonstrated increased functional connectivity in the medial temporal cortex, including the parahippocampal gyrus and the hippocampus, and the temporal pole, with the striatum compared to children with insecure attachment. Of note, this connectivity remained significant while controlling for IQ and was even more pronounced in the caudate network. The medial temporal cortices are involved principally with memory, supporting the establishment and maintenance of long-term memory (Squire et al., 2004). The parahippocampal gyri, in particular, are anatomically and functionally closely connected with the prefrontal cortex, including the medial, dorsolateral and orbital frontal regions, and support many cognitive processes, including visuospatial processing and episodic memory (Aminoff et al., 2013). The hippocampi also play an important role in memory, learning and emotion, and reduced hippocampal volume has been implicated in maladaptive stress as well as affective psychopathology (Liu et al., 2000).
Hippocampal growth is closely linked to environmental factors, such as caregiving experiences, and abusive parenting in childhood or early life adversity causes significant reduction in hippocampal volumes (Opendak et al., 2017; Teicher et al., 2012; van Hoof et al., 2019). A recent longitudinal study demonstrated that early support enhances hippocampal development and early childhood maternal support predicted a steeper hippocampal growth trajectory (Luby et al., 2016). Interestingly, a network of regions which includes the medial temporal cortex and the striatum is integral to visuomotor associative learning. Previous studies suggested that the interaction between the medial temporal cortex and the striatum (specifically the caudate) is involved in context-dependent learning (Brown and Stern, 2014; Mattfeld and Stark, 2011) or item-set similarity learning (Stark et al., 2018), which are all very relevant to a social learning situation where stimuli that arise from other individuals have a significant role in learning. This is an important finding as individual differences in attachment security are an outcome, built upon memories and learning being fed back from children’s daily (contextual) interactions with their primary caregivers. In attachment theory (Bowlby, 1962), children develop mental representations, called “internal working models”, which operate as schematic frameworks to integrate early life experiences with their caregivers, to use those schema to understand themselves and expectations of others’ reactions to themselves, and to generalize their knowledge about the self and others to the expanded world. The internal working models are a key concept of attachment theory which shows how children develop different attachment styles and why the attachment styles may have a life-long impact on one’s social and emotional development. Our findings of different connectivity between the medial temporal cortex and the striatum contribute to the understanding of the neural mechanism underlying children’s use of their attachment experiences to learn and apply a general idea about the self and the others in the wider social context.
Furthermore, attachment studies for older populations have shown that the structural and functional connectivity within medial temporal regions including hippocampi and parahippocampal gyri reflect individual differences in attachment types. In adults, the volumes of middle temporal and parahippocampal gyri are associated with attachment insecurity (Zhang et al., 2018a). The hippocampal function is also implicated in modulating individual predisposition to react to dismissing attachment-related narratives (Krause et al., 2016), as well as in mentalizing one’s attachment figures (Laurita et al., 2019). In adolescents, a higher level of functional connectivity between the hippocampi, the middle temporal gyri and the lateral occipital cortex was associated with unresolved-disorganized attachment (van Hoof et al., 2019). These findings provide clear evidence that the individual differences in attachment types are captured by distinguishing features of the neural activation in socio-emotional processing with an engagement of temporo-limbic regions from childhood to adulthood.
Along with the medial temporal cortex, we also found increased functional connectivity between the striatum and the temporal pole in children with secure attachment. The temporal poles are activated consistently in a variety of mentalizing tasks in which participants have to think about the mental states of others (Frith and Frith, 2006; Müller-Pinzler et al., 2016; Paulus et al., 2015; Takahashi et al., 2014), as well as observed in adult attachment studies to modulate individual differences in response to attachment-related cues (Krause et al., 2016). Taken together, our results on the right medial temporal cortex and temporal pole implicate prospective links between individual differences in attachment security and the abilities to understand oneself and others. This is supported by findings in behavioural research, in which children with secure attachment exhibit better social outcomes (Bohlin et al., 2000; Groh et al., 2014; Sroufe, 2005; Veríssimo et al., 2014).
In contrast to children with secure attachment, children with insecure attachment demonstrated increased functional connectivity to the right supramarginal gyrus, although it was not significant after controlling for IQ. Parieto-striatal connectivity has structural and functional connections and is relevant to spatial attention in reinforcement learning (Jarbo and Verstynen, 2015). This region is an anterior part of temporo-parietal junction (TPJ), which is frequently associated with capacities to shift attention to unexpected stimuli (Bledowski et al., 2004; Vossel et al., 2009) as well as to understand others’ mental states (Hooker et al., 2010; Lombardo et al., 2010). A recent quantitative review on the brain activity in the TPJ across tasks reported that the supramarginal gyrus showed preferential activation for attentional reorienting in non-social tasks (Schurz et al., 2017). Another meta-analysis also showed the anterior part of TPJ was co-activated with the attentional network, whereas the posterior TPJ had more connections with typical Theory of Mind regions (Kubit and Jack, 2013). These reports suggested a potential link between early attachment experience and the maturity of the attentional system in the brain. An exploratory analysis in our study also demonstrated a possible link between these two constructs, since previous literature frequently reported attentional issues in children with insecure attachment (Fearon and Belsky, 2004; Vandevivere et al., 2014), which were linked to attention-deficit disorders (Storebø et al., 2016), even in adulthood (Atkinson et al., 2009; Dewitte et al., 2007). However, it is still unclear how attachment experiences affect the functional organization of the attentional network. One hypothesis is that different attachment expectations may influence the earliest stages of information processing, namely the attentional processing of attachment-related information (Crick and Dodge, 1994). Children with insecure attachment could start to operate a defensive mechanism, in which they try to filter out attachment-related information as it is painful, and develop a selective processing mechanism, defined as a bias, to protect themselves (Bowlby, 1980). In the short-term, an attempt to exclude attachment information could be an adaptive function, but in the long-term, this selective and biased mechanism would become an automatic process, leading them to stay insecure (Mikulincer et al., 2003), and could be generalized to their general attentional features. Our finding of increased connectivity in the anterior TPJ in children with insecure attachment might reflect their struggles to filter unexpected and unpleasant social stimuli, even at rest.
Of note, the increased supramarginal gyrus connectivity did not survive after controlling for IQ in our cohort. This suggests that the connectivity differences in the attention system is partly attributed to the differences in intellectual ability between two groups. However, there have been compelling arguments against using IQ as a covariate in cognitive studies (Dennis et al., 2009). Previous literature has shown reciprocal relations between children’s attachment representations and their cognitive ability. Children more securely attached in early childhood had better school performance and higher IQs in middle childhood (Stievenart et al., 2011; West et al., 2013). Since high IQ is associated with secure attachment, covarying for IQ would violate non-collinearity recommendations (Miller and Chapman, 2001). IQ-matched samples may help to clarify the effect of attachment security on attentional system development, but it should be considered that matching groups for IQ could create unrepresentative groups.
The early attachment experience had an impact on the functional organization in the developing brain, not only qualitatively, but also quantitatively. The connectivity differences, based on the qualitative assessment of attachment security, were not significantly associated with the child-report attachment security scores overall, but the temporo-limbic connectivity in the caudate network was negatively correlated with this quantitative measure in the insecurely attached group. In contrast, the independent analysis of the whole brain connectivity with the child-reported attachment security scores identified additional regions which were engaged in the children’s perception of attachment security. The dorsolateral and dorsomedial prefrontal cortices, which were associated with children’s negative perception of their parent-child relationship in our study, are known to be involved in cognitive control, in particular implicating less efficient or disturbed emotion regulation capacities in the adult attachment model (Vrtička and Vuilleumier, 2012). Studies have shown that individuals with unresolved or insecure attachment displayed increased activation in lateral prefrontal cortices in response to traumatic visual stimuli or while ignoring the meaning of negative emotional words (Buchheim et al., 2006; Warren et al., 2010). In children, the ability to express one’s emotions freely, even vulnerable emotions such as sadness, fear or anger, with a proper justification is one of the huge benefits of having a secure attachment with caregivers. Children with insecure attachment are unable to express vulnerable feelings openly so that they tend to shut off or restrict feelings in the avoidant type, or to be flooded or overwhelmed with disorganized feelings in the ambivalent type, resulting in emotional regulation issues. Given the majority of the insecure attachment group in our study was classified as avoidant type (69 %) and the child-reported measure included an evaluating process, the increased lateral and medial prefrontal activity may represent neurobiological mechanisms of cognitively shutting-down or restricting emotions when children with negative perception for their parents reflected on their attachment relationship.
In contrast, children’s positive perception for their attachment relationship was associated with increased activity in visual areas, including superior and middle occipital regions, in the putamen network. The visual cortex is anatomically connected to the limbic system through the inferior longitudinal fasciculus and subserves emotional functions conveyed by visual signals (Catani et al., 2003). Primary attachment figures and attachment-related scenes are visually and emotionally strong stimuli, particularly in childhood. Studies have shown that attachment types modulate visual attention processing of emotional stimuli (Dewitte, 2011; Sanscartier et al., 2016). In a recent study, the ability to utilize secure inner working models when facing self-criticism was linked to greater activity of visual system, as a neural marker of visual mental imagery (Kim et al., 2020). In line with this, our findings may provide neural evidence that children who have positive perception of their attachment relationship can easily access mental representations of their attachment figures and the relational experiences.
In conclusion, middle childhood is a relatively neglected period for attachment research and has a lack of validated measures to capture the attachment representations (Kerns et al., 2001). Here, using a semi-structured interview method with a set of separation pictures, we were able to determine how children project themselves to the child in the pictures and the vulnerabilities to the designated separation situations. Using this approach we found distinct neural underpinnings for the securely versus insecurely attached children, indicating that early attachment experiences may influence the functional organization of the typically developing brain.
Data statement
Due to the sensitive nature of the questions asked in this study, participants were assured raw data would remain confidential and would not be shared.
Declaration of Competing Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This research was supported by National Research Foundation of Korea (NRF-2013S1A5A2A01019722/NRF-2014S1A5B6038081). We thank the Department of Brain & Cognitive Sciences at Seoul National University in South Korea for support in data collection.
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.dcn.2021.100914.
Contributor Information
Eun Jung Choi, Email: eunjung.choi@sickkids.ca.
Soon-Hyung Yi, Email: ysh@snu.ac.kr.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- Achenbach T.M., Rescorla L.A. University of Vermont, Research Center for Children, Youth, & Families; Burlington, VT: 2001. Manual for the ASEBA School-Age Forms & Profiles. [Google Scholar]
- Ainsworth M., Blehar M., Waters E., Wall S. Lawrence Erlbaum; Oxford, England: 1978. Patterns of Attachment: a Psychological Study of the Strange Situation. [Google Scholar]
- Aminoff E.M., Kveraga K., Bar M. The role of the parahippocampal cortex in cognition. Trends Cogn. Sci. 2013 doi: 10.1016/j.tics.2013.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonucci L.A., Taurisano P., Coppola G., Cassibba R. Attachment style: the neurobiological substrate, interaction with genetics and role in neurodevelopmental disorders risk pathways. Neurosci. Biobehav. Rev. 2018;95:515–527. doi: 10.1016/J.NEUBIOREV.2018.11.002. [DOI] [PubMed] [Google Scholar]
- Atkinson L., Leung E., Goldberg S., Benoit D., Poulton L., Myhal N., Blokland K., Kerr S. Attachment and selective attention: disorganization and emotional Stroop reaction time. Dev. Psychopathol. 2009;21:99–126. doi: 10.1017/S0954579409000078. [DOI] [PubMed] [Google Scholar]
- Bledowski C., Prvulovic D., Goebel R., Zanella F.E., Linden D.E.J. Attentional systems in target and distractor processing: a combined ERP and fMRI study. Neuroimage. 2004;22:530–540. doi: 10.1016/j.neuroimage.2003.12.034. [DOI] [PubMed] [Google Scholar]
- Bohlin G., Hagekull B., Rydell A.M. Attachment and social functioning: a longitudinal study from infancy to middle childhood. Soc. Dev. 2000;9:24–39. doi: 10.1111/1467-9507.00109. [DOI] [Google Scholar]
- Bowlby J. Basic Books; New York: 1962. Attachment and loss: Vol. 1. Attachment. [Google Scholar]
- Bowlby J. Tavistock/Routledge; London: 1973. Attachment and Loss: Vol 2. Separation: Anxiety and Anger. [Google Scholar]
- Bowlby J. Basic Books; New York: 1980. Attachment and Loss: Vol. 3. Loss: Sadness and Depression. [Google Scholar]
- Brown T., Stern C. Contributions of medial temporal lobe and striatal memory systems to learning and retrieving overlapping spatial memories. Cereb. Cortex. 2014;24:1906–1922. doi: 10.1093/cercor/bht041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchheim A., George C., Kächele H., Erk S., Walter H. Measuring adult attachment representation in an fMRI environment: concepts and assessment. Psychopathology. 2006;39:136–143. doi: 10.1159/000091799. [DOI] [PubMed] [Google Scholar]
- Catani M., Jones D.K., Donato R., Ffytche D.H. Occipito-temporal connections in the human brain. Brain. 2003;126:2093–2107. doi: 10.1093/brain/awg203. [DOI] [PubMed] [Google Scholar]
- Choi E. Seoul National University; 2014. An fMRI Study of Children’s Neural Responses According to the Level of Attachment Security. Unpublished doctoral thesis. [Google Scholar]
- Choi E., Taylor M.J., Hong S.B., Kim C., Yi S.H. The neural correlates of attachment security in typically developing children. Brain Cogn. 2018;124:47–56. doi: 10.1016/j.bandc.2018.04.003. [DOI] [PubMed] [Google Scholar]
- Chopik W.J., Grimm K.J. Longitudinal changes and historic differences in narcissism from adolescence to older adulthood. Psychol. Aging. 2019;34:1109–1123. doi: 10.1037/pag0000379. [DOI] [PubMed] [Google Scholar]
- Coan J.A. Adult attachment and the brain. J. Soc. Pers. Relat. 2010;27:210–217. doi: 10.1177/0265407509360900. [DOI] [Google Scholar]
- Crick N., Dodge K. A review and reformulation of social information-processing mechanisms in children’s social adjustment. Psychol. Bull. 1994;115:74. [Google Scholar]
- Debbané M., Badoud D., Sander D., Eliez S., Luyten P., Vrtička P. Brain activity underlying negative self- and other-perception in adolescents: the role of attachment-derived self-representations. Cogn. Affect. Behav. Neurosci. 2017;17:554–576. doi: 10.3758/s13415-017-0497-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis M., Francis D.J., Cirino P.T., Schachar R., Barnes M.A., Fletcher J.M.J.M. Why IQ is not a covariate in cognitive studies of neurodevelopmental disorders. J. Int. Neuropsychol. Soc. 2009 doi: 10.1017/S1355617709090481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewitte M. Adult attachment and attentional inhibition of interpersonal stimuli. Cogn. Emot. 2011;25:612–625. doi: 10.1080/02699931.2010.508683. [DOI] [PubMed] [Google Scholar]
- Dewitte M., Koster E.H.W., De Houwer J., Buysse A. Attentive processing of threat and adult attachment: a dot-probe study. Behav. Res. Ther. 2007;45:1307–1317. doi: 10.1016/j.brat.2006.11.004. [DOI] [PubMed] [Google Scholar]
- Di Martino A., Scheres A., Margulies D., Kelly A., Uddin L., Shehzad Z., Biswal B., Walters J., Castellanos F., Milham M. Functional connectivity of human striatum: a resting state FMRI study. Cereb. Cortex. 2008;18:2735–2747. doi: 10.1093/cercor/bhn041. [DOI] [PubMed] [Google Scholar]
- Donges U.-S., Kugel H., Stuhrmann A., Grotegerd D., Redlich R., Lichev V., Rosenberg N., Ihme K., Suslow T., Dannlowski U. Adult attachment anxiety is associated with enhanced automatic neural response to positive facial expression. Neuroscience. 2012;220:149–157. doi: 10.1016/J.NEUROSCIENCE.2012.06.036. [DOI] [PubMed] [Google Scholar]
- Fareri D.S., Gabard-Durnam L., Goff B., Flannery J., Gee D.G., Lumian D.S., Caldera C., Tottenham N. Altered ventral striatal-medial prefrontal cortex resting-state connectivity mediates adolescent social problems after early institutional care. Dev. Psychopathol. 2017;29:1865–1876. doi: 10.1017/S0954579417001456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fearon R.M.P., Belsky J. Attachment and attention: protection in relation to gender and cumulative social-contextual adversity. Child Dev. 2004;75:1677–1693. doi: 10.1111/j.1467-8624.2004.00809.x. [DOI] [PubMed] [Google Scholar]
- Fraley R.C. Attachment in adulthood: recent developments, emerging debates, and future directions. Annu. Rev. Psychol. 2019;70:401–422. doi: 10.1146/annurev-psych-010418-102813. [DOI] [PubMed] [Google Scholar]
- Frith C.D., Frith U. The neural basis of mentalizing. Neuron. 2006 doi: 10.1016/j.neuron.2006.05.001. [DOI] [PubMed] [Google Scholar]
- Gillath O., Karantzas G.C., Fraley R.C. Adult Attachment: A Concise Introduction to Theory and Research. Elsevier; 2016. What can neuroscience, genetics, and physiology tell us about attachment? pp. 219–240. [DOI] [Google Scholar]
- Groh A.M., Fearon R.P., Bakermans-Kranenburg M.J., van IJzendoorn M.H., Steele R.D., Roisman G.I. The significance of attachment security for children’s social competence with peers: a meta-analytic study. Attach. Hum. Dev. 2014;16:103–136. doi: 10.1080/14616734.2014.883636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gwak G., Oh S., Kim C. Hakjisa; Seoul: 2011. K-WISC-IV (Korean Wechsler Intelligence Scale for Children-IV): Manual for Experts. [Google Scholar]
- Haber S.N. Corticostriatal circuitry. Dialogues Clin. Neurosci. 2016;18:7–21. doi: 10.1007/978-1-4614-6434-1_135-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansburg H.G. C.C. Thomas; Springfield: 1972. Adolescent Separation Anxiety; a Method for the Study of Adolescent Separation Problems. [Google Scholar]
- Hanson J.L., Hariri A.R., Williamson D.E. Blunted ventral striatum development in adolescence reflects emotional neglect and predicts depressive symptoms. Biol. Psychiatry. 2015;78:598–605. doi: 10.1016/j.biopsych.2015.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooker C.I., Verosky S.C., Germine L.T., Knight R.T., D’Esposito M. Neural activity during social signal perception correlates with self-reported empathy. Brain Res. 2010;1308:100–113. doi: 10.1016/j.brainres.2009.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarbo K., Verstynen T.D. Converging structural and functional connectivity of orbitofrontal, dorsolateral prefrontal, and posterior parietal cortex in the human striatum. J. Neurosci. 2015;35:3865–3878. doi: 10.1523/JNEUROSCI.2636-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinsson M., Beckmann C.F., Behrens T.E.J., Woolrich M.W., Smith S.M. FSL. Neuroimage. 2012;62(2):782–790. doi: 10.1016/j.neuroimage.2011.09.015. [DOI] [PubMed] [Google Scholar]
- Kerns K.A., Aspelmeier J.E., Gentzler A.L., Grabill C.M. Parent-child attachment and monitoring in middle childhood. J. Fam. Psychol. 2001;15:69–81. doi: 10.1037/0893-3200.15.1.69. [DOI] [PubMed] [Google Scholar]
- Kim J.J., Kent K.M., Cunnington R., Gilbert P., Kirby J.N. Attachment styles modulate neural markers of threat and imagery when engaging in self-criticism. Sci. Rep. 2020;10:1–10. doi: 10.1038/s41598-020-70772-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause A.L., Borchardt V., Li M., Van Tol M.J., Demenescu L.R., Strauss B., Kirchmann H., Buchheim A., Metzger C.D., Nolte T., Walter M. Dismissing attachment characteristics dynamically modulate brain networks subserving social aversion. Front. Hum. Neurosci. 2016;10 doi: 10.3389/fnhum.2016.00077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause A.L., Colic L., Borchardt V., Li M., Strauss B., Buchheim A., Wildgruber D., Fonagy P., Nolte T., Walter M. Functional connectivity changes following interpersonal reactivity. Hum. Brain Mapp. 2018;39:866–879. doi: 10.1002/hbm.23888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubit B., Jack A. Rethinking the role of the rTPJ in attention and social cognition in light of the opposing domains hypothesis: findings from an ALE-based meta-analysis and resting-state functional connectivity. Frontiers in Human Neuroscience. 2013;7:323. doi: 10.3389/fnhum.2013.00323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupferschmidt D., Juczewski K., Cui G., Johnson K., Lovinger D. Parallel, but dissociable, processing in discrete corticostriatal inputs encodes skill learning. Neuron. 2017;96:476–489. doi: 10.1016/j.neuron.2017.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurita A.C., Hazan C., Spreng R.N. An attachment theoretical perspective for the neural representation of close others. Soc. Cogn. Affect. Neurosci. 2019;14:237–251. doi: 10.1093/scan/nsz010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K.H., Ko J.Y. Hakjisa; Seoul: 2006. Self-Concept Inventory. [Google Scholar]
- Lipton D.M., Gonzales B.J., Citri A. Dorsal striatal circuits for habits, compulsions and addictions. Front. Syst. Neurosci. 2019;13 doi: 10.3389/fnsys.2019.00028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu D., Diorio J., Day J.C., Francis D.D., Meaney M.J. Maternal care, hippocampal synaptogenesis and cognitive development in rats. Nat. Neurosci. 2000;3:799–806. doi: 10.1038/77702. [DOI] [PubMed] [Google Scholar]
- Lombardo Michael V., Chakrabarti Bhismadev, Bullmore Edward T., Wheelwright Sally J., Sadek Susan A., Suckling J., Baron-Cohen S., Bailey A.J., Bolton P.F., Bullmore E.T., Carrington S., Chakrabarti B., Daly E.M., Deoni S.C., Ecker C., Happé F., Henty J., Jezzard P., Johnston P., Jones D.K., Lombardo M.V., Madden A., Mullins D., Murphy C., Murphy D.G., Pasco G., Sadek S.A., Spain D., Stewart R., Suckling J.S., Wheelwright S.J., Williams S.C. Shared neural circuits for mentalizing about the self and others. J. Cogn. Neurosci. 2010;22:1623–1635. doi: 10.1162/jocn.2009.21287. [DOI] [PubMed] [Google Scholar]
- Long M., Verbeke W., Ein-Dor T., Vrtička P. A functional neuro-anatomical model of human attachment (NAMA): insights from first- and second-person social neuroscience. Cortex. 2020 doi: 10.1016/j.cortex.2020.01.010. [DOI] [PubMed] [Google Scholar]
- Luby J.L., Belden A., Harms M.P., Tillman R., Barch D.M. Preschool is a sensitive period for the influence of maternal support on the trajectory of hippocampal development. Proc. Natl. Acad. Sci. U. S. A. 2016;113:5742–5747. doi: 10.1073/pnas.1601443113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattfeld A., Stark C. Striatal and medial temporal lobe functional interactions during visuomotor associative learning. Cereb. Cortex Cortex. 2011;21:647–658. doi: 10.1093/cercor/bhq144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mcconnell M., Moss E. Attachment across the life span: factors that contribute to stability and change. Aust. J. Educ. Dev. Psychol. 2011;11:60–77. [Google Scholar]
- Meins E., Bureau J.F., Fernyhough C. Mother–child attachment from infancy to the preschool years: predicting security and stability. Child Dev. 2018;89:1022–1038. doi: 10.1111/cdev.12778. [DOI] [PubMed] [Google Scholar]
- Mikulincer M., Shaver P.R. The attachment behavioral system in adulthood: activation, psychodynamics, and interpersonal processes. Adv. Exp. Soc. Psychol. 2003;35:53–152. doi: 10.1016/S0065-2601(03)01002-5. [DOI] [Google Scholar]
- Mikulincer M., Shaver P.R. Attachment in Adulthood: Structure, Dynamics, and Change. The Guilford Press; New York: 2007. The attachment behavioral system: basic concepts and principles; pp. 3–28. [Google Scholar]
- Mikulincer M., Shaver P.R., Pereg D. Attachment theory and affect regulation: the dynamics, development, and cognitive consequences of attachment-related strategies. Motiv. Emot. 2003 doi: 10.1023/A:1024515519160. [DOI] [Google Scholar]
- Miller G.A., Chapman J.P. Misunderstanding analysis of covariance. J. Abnorm. Psychol. 2001;110:40–48. doi: 10.1037/0021-843X.110.1.40. [DOI] [PubMed] [Google Scholar]
- Miller J.G., Shrestha S., Reiss A.L., Vrtička P. Neural bases of social feedback processing and self–other distinction in late childhood: the role of attachment and age. Cogn. Affect. Behav. Neurosci. 2020;20:503–520. doi: 10.3758/s13415-020-00781-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moutsiana C., Fearon P., Murray L., Cooper P., Goodyer I., Johnstone T., Halligan S. Making an effort to feel positive: insecure attachment in infancy predicts the neural underpinnings of emotion regulation in adulthood. J. Child Psychol. Psychiatry. 2014;55:999–1008. doi: 10.1111/jcpp.12198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller-Pinzler L., Rademacher L., Paulus F.M., Krach S. When your friends make you cringe: social closeness modulates vicarious embarrassment-related neural activity. Soc. Cogn. Affect. Neurosci. 2016;11:466–475. doi: 10.1093/scan/nsv130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opendak M., Gould E., Sullivan R. Early life adversity during the infant sensitive period for attachment: programming of behavioral neurobiology of threat processing and social behavior. Dev. Cogn. Neurosci. 2017 doi: 10.1016/j.dcn.2017.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulus F.M., Müller-Pinzler L., Jansen A., Gazzola V., Krach S. Mentalizing and the role of the posterior superior temporal sulcus in sharing others’ embarrassment. Cereb. Cortex. 2015;25:2065–2075. doi: 10.1093/cercor/bhu011. [DOI] [PubMed] [Google Scholar]
- Perlini C., Bellani M., Rossetti M.G., Zovetti N., Rossin G., Bressi C., Brambilla P. Disentangle the neural correlates of attachment style in healthy individuals. Epidemiol. Psychiatr. Sci. 2019;28:371–375. doi: 10.1017/S2045796019000271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petters D., Waters E. A.I., Attachment theory and simulating secure base behaviour: Dr. Bowl by meet the reverend bayes. Proceedings of the International Symposium on’ AI-Inspired Biology’, AISB Convention 2010; AISB Press, Falmer; 2010. pp. 51–58. [Google Scholar]
- Poore J.C., Pfeifer J.H., Berkman E.T., Inagaki T.K., Welborn B.L., Lieberman M.D., Forbes C.E., Crone E.A., Delgado M.R. 2012. Prediction-error in the Context of Real Social Relationships Modulates Reward System Activity. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resnick G. Westat Inc.; Rockville, MD: 1993. Measuring Attachment in Early Adolescence: a Manual for the Administration, Coding and Interpretation of the Separation Anxiety Test for 11 to 14 Year. [Google Scholar]
- Rigon A., Duff M.C., Voss M.W. Structural and functional neural correlates of self-reported attachment in healthy adults: evidence for an amygdalar involvement. Brain Imaging Behav. 2016;10:941–952. doi: 10.1007/s11682-015-9446-9. [DOI] [PubMed] [Google Scholar]
- Robbins T., Costa R. Habits. Curr. Biol. 2017;27:P1200–R1206. doi: 10.1016/j.cub.2017.09.060. [DOI] [PubMed] [Google Scholar]
- Sanscartier S., Maxwell J., Taylor E., Lockwood P. Attachment avoidance and visual attention for emotional faces over time. J. Vis. 2016;16:79. doi: 10.1167/16.12.79. [DOI] [Google Scholar]
- Schneider S., Brassen S., Bromberg U., Banaschewski T., Conrod P., Flor H., Gallinat J., Garavan H., Heinz A., Martinot J.L., Nees F., Rietschel M., Smolka M.N., Ströhle A., Struve M., Schumann G., Büchel C. Maternal interpersonal affiliation is associated with adolescents’ brain structure and reward processing. Transl. Psychiatry. 2012:2. doi: 10.1038/tp.2012.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider-Hassloff H., Straube B., Nuscheler B., Wemken G., Kircher T. Adult attachment style modulates neural responses in a mentalizing task. Neuroscience. 2015;303:462–473. doi: 10.1016/J.NEUROSCIENCE.2015.06.062. [DOI] [PubMed] [Google Scholar]
- Schurz M., Tholen M.G., Perner J., Mars R.B., Sallet J. Specifying the brain anatomy underlying temporo-parietal junction activations for theory of mind: a review using probabilistic atlases from different imaging modalities. Hum. Brain Mapp. 2017 doi: 10.1002/hbm.23675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squire L.R., Stark C.E.L., Clark R.E. The medial temporal lobe. Annu. Rev. Neurosci. 2004;27:279–306. doi: 10.1146/annurev.neuro.27.070203.144130. [DOI] [PubMed] [Google Scholar]
- Sroufe L.A. Attachment and development: a prospective, longitudinal study from birth to adulthood. Attach. Hum. Dev. 2005;7:349–367. doi: 10.1080/14616730500365928. [DOI] [PubMed] [Google Scholar]
- Stark S., Frithsen A., Mattfeld A., Stark C. Modulation of associative learning in the hippocampal-striatal circuit based on item-set similarity. Cortex. 2018;109:60–73. doi: 10.1016/j.cortex.2018.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stievenart M., Roskam I., Meunier J.C., van de Moortele G. The reciprocal relation between children’s attachment representations and their cognitive ability. Int. J. Behav. Dev. 2011;35:58–66. doi: 10.1177/0165025410370790. [DOI] [Google Scholar]
- Storebø O.J., Rasmussen P.D., Simonsen E. Association between insecure attachment and ADHD: environmental mediating factors. J. Attention Disord. 2016;20:187–196. doi: 10.1177/1087054713501079. [DOI] [PubMed] [Google Scholar]
- Strathearn L., Fonagy P., Amico J., Montague P.R. Adult attachment predicts maternal brain and oxytocin response to infant Cues. Neuropsychopharmacology. 2009;34:2655–2666. doi: 10.1038/npp.2009.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi H., Terada K., Morita T., Suzuki S., Haji T., Kozima H., Yoshikawa M., Matsumoto Y., Omori T., Asada M., Naito E. Different impressions of other agents obtained through social interaction uniquely modulate dorsal and ventral pathway activities in the social human brain. Cortex. 2014;58:289–300. doi: 10.1016/j.cortex.2014.03.011. [DOI] [PubMed] [Google Scholar]
- Takiguchi S., Fujisawa T.X., Mizushima S., Saito D.N., Okamoto Y., Shimada K., Koizumi M., Kumazaki H., Jung M., Kosaka H., Hiratani M., Ohshima Y., Teicher M.H., Tomoda A. Ventral striatum dysfunction in children and adolescents with reactive attachment disorder: functional MRI study. BJPsych Open. 2015;1:121–128. doi: 10.1192/bjpo.bp.115.001586. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Teicher M.H., Anderson C.M., Polcari A. Childhood maltreatment is associated with reduced volume in the hippocampal subfields CA3, dentate gyrus, and subiculum. Proc. Natl. Acad. Sci. U. S. A. 2012:109. doi: 10.1073/pnas.1115396109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thijssen S., Muetzel R.L., Bakermans-Kranenburg M.J., Jaddoe V.W.V., Tiemeier H., Verhulst F.C., White T., Van IJzendoorn M.H. Insensitive parenting may accelerate the development of the amygdala-medial prefrontal cortex circuit. Dev. Psychopathol. 2017;29:508–518. doi: 10.1017/S0954579417000141. [DOI] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N., Landeau B., Papathanassiou D., Crivello F., Etard O., Delcroix N., Mazoyer B., Joliot M. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15(1):273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- van Hoof M.-J., Riem M., Garrett A., Pannekoek N., van der Wee N., van IJzendoorn M., Vermeiren R. Unresolved-disorganized attachment is associated with smaller hippocampus and increased functional connectivity beyond psychopathology. J. Trauma. Stress. 2019;32:742–752. doi: 10.1002/jts.22432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandevivere E., Braet C., Bosmans G., Mueller S.C., De Raedt R. Attachment and children’s biased attentional processing: evidence for the exclusion of attachment-related information. PLoS One. 2014;9 doi: 10.1371/journal.pone.0103476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veríssimo M., Santos A.J., Fernandes C., Shin N., Vaughn B.E. Associations between attachment security and social competence in preschool children. Merrill. Palmer. Q. 2014;60:80–99. doi: 10.13110/merrpalmquar1982.60.1.0080. [DOI] [Google Scholar]
- Vossel S., Weidner R., Thiel C.M., Fink G.R. What is “Odd” in Posner’s location-cueing paradigm? Neural responses to unexpected location and feature changes compared. J. Cogn. Neurosci. 2009;21:30–41. doi: 10.1162/jocn.2009.21003. [DOI] [PubMed] [Google Scholar]
- Vrtička P., Vuilleumier P. Neuroscience of human social interactions and adult attachment style. Front. Hum. Neurosci. 2012;6:212. doi: 10.3389/fnhum.2012.00212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vrtička P., Andersson F., Grandjean D., Sander D., Vuilleumier P. Individual attachment style modulates human amygdala and striatum activation during social appraisal. PLoS One. 2008;3:e2868. doi: 10.1371/journal.pone.0002868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren S.L., Bost K.K., Roisman G.I., Silton R., Spielberg J.M., Engels A.S., Choi E., Sutton B.P., Miller G.A., Heller W. Effects of adult attachment and emotional distractors on brain mechanisms of cognitive control. Psychol. Sci. 2010;21:1818–1826. doi: 10.1177/0956797610388809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West K.K., Mathews B.L., Kerns K.A. Mother-child attachment and cognitive performance in middle childhood: an examination of mediating mechanisms. Early Child. Res. Q. 2013;28:259–270. doi: 10.1016/j.ecresq.2012.07.005. [DOI] [Google Scholar]
- Williams B., Jalilianhasanpour R., Matin N., Fricchione G.L., Sepulcre J., Keshavan M.S., LaFrance W.C., Dickerson B.C., Perez D.L. Individual differences in corticolimbic structural profiles linked to insecure attachment and coping styles in motor functional neurological disorders. J. Psychiatr. Res. 2018;102:230–237. doi: 10.1016/j.jpsychires.2018.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin H.H., Knowlton B.J. The role of the basal ganglia in habit formation. Nat. Rev. Neurosci. 2006;7:464–476. doi: 10.1038/nrn1919. [DOI] [PubMed] [Google Scholar]
- Zhang X., Deng M., Ran G., Tang Q., Xu W., Ma Y., Chen X. Brain correlates of adult attachment style: a voxel-based morphometry study. Brain Res. 2018;1699:34–43. doi: 10.1016/j.brainres.2018.06.035. [DOI] [PubMed] [Google Scholar]
- Zhang X., Ran G., Xu W., Ma Y., Chen X. Adult attachment affects neural response to preference-inferring in ambiguous scenarios: evidence from an fMRI study. Front. Psychol. 2018:9. doi: 10.3389/fpsyg.2018.00139. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.