Abstract
Serotonin is involved in a wide range of mental capacities essential for navigating the social world, including emotion and impulse control. Much recent work on serotonin and social functioning has focused on decision-making. Here we investigated the influence of serotonin on human emotional reactions to social conflict. We used a novel computerised task that required mentally simulating social situations involving unjust harm and found that depleting the serotonin precursor tryptophan—in a double-blind randomised placebo-controlled design—enhanced emotional responses to the scenarios in a large sample of healthy volunteers (n = 73), and interacted with individual differences in trait personality to produce distinctive human emotions. Whereas guilt was preferentially elevated in highly empathic participants, annoyance was potentiated in those high in trait psychopathy, with medium to large effect sizes. Our findings show how individual differences in personality, when combined with fluctuations of serotonin, may produce diverse emotional phenotypes. This has implications for understanding vulnerability to psychopathology, determining who may be more sensitive to serotonin-modulating treatments, and casts new light on the functions of serotonin in emotional processing.
Subject terms: Human behaviour, Predictive markers
Introduction
A unified function for serotonin (5-hydroxytryptamine; 5-HT) has, perhaps unsurprisingly, proven to be elusive. It is hypothesised to have a role in many psychiatric disorders1, and is implicated in a wide range of mental functions including aversive processing, impulse control and social behaviour2. These domains can be viewed under a unified framework by considering how serotonin impacts Pavlovian (stimulus-outcome, including emotional) and instrumental (stimulus-response-outcome; operant) processes that underlie both social and nonsocial functions3. Much recent work on the relationship between serotonin, aversive processing and human social behaviour has focused on instrumental action in the context of behavioural economic games and moral dilemmas4–6. Here we examined emotional reactions to social scenarios depicting unjust harm.
Studies of healthy volunteers have primarily employed two techniques to investigate serotonin function: acute tryptophan depletion (ATD), a dietary technique that temporarily lowers brain serotonin levels by depleting its biosynthetic precursor, tryptophan7–11 and treatment with single doses of selective serotonin reuptake inhibitors (SSRIs). SSRIs are generally assumed to increase extracellular serotonin; however, it should be noted that single rather than chronic doses can paradoxically decrease serotonin in projection areas2,12. ATD studies have revealed disinhibition of retaliatory behaviour in the face of perceived injustice4, modelled using the Ultimatum Game (UG), while single-dose SSRI has had prosocial effects5.
Morally relevant social emotions, meanwhile, lie at the interface between moral standards and socially appropriate behaviour13. Moral standards prohibit behaviours that are likely to have negative consequences for the well-being of others. Emotions are in part Pavlovian in nature, and while ATD has been shown to modulate Pavlovian processes in nonsocial domains14,15, here we tested the influence of ATD on emotion in a social context. We asked whether ATD would enhance morally relevant negative emotions evoked by social scenarios in which a person was unjustly harmed: these served as Pavlovian cues. Using a novel task, we prompted participants to reflect on the situations and specifically assessed emotions involving annoyance, guilt, shame and feeling “bad”. Reporting one’s emotional reactions after mentally simulating hypothetical social scenarios implicitly calls on autobiographical memories, which should result in a diversity of subjective experiences tied to personal qualities and expectations about social behaviour. We therefore also tested the influence of three personality traits—empathy, psychopathy and impulsivity—on how serotonin would affect emotion.
Research on moral emotions has focused mostly on the self-conscious negative-emotions guilt and shame13. Guilt often relates to a negative appraisal of a specific behaviour, whereas shame tends to involve a negative evaluation of the self:13 “If only I hadn’t” as opposed to “If only I weren’t”16. While guilt is part of the diagnostic criteria for depression17, proneness to shame most consistently relates to an array of psychiatric conditions, including symptoms of depression18–20, anxiety19, post-traumatic stress disorder21–24 and eating disorders25,26, as well as more specific symptoms such as low self-esteem27, suicidal ideation28, anger29,30 and aggression30. Importantly, guilt is thought to become maladaptive primarily when it is fused with shame13.
Evidence from patients with damage to the ventromedial prefrontal cortex (vmPFC) and incarcerated individuals with psychopathy provides a plausible connection between moral emotions and social behaviour. The vmPFC is an area central to emotion regulation31, with dense serotonergic innervation32. Individuals with vmPFC damage also show increased retaliatory behaviour to unfairness on the UG33, which mirrors the ATD findings4,6. This is furthermore analogous to the UG results from psychopathic individuals34, where vmPFC dysfunction is a feature35, as is reduced guilt36. Impaired moral behaviour following damage to the vmPFC has meanwhile been conceptualised as a manifestation of diminished guilt37. The implication here is that guilt is related to inhibition of antisocial behaviour, as modelled by restraint in behavioural economic games like the UG. Studying the effects of ATD on guilt could therefore inform how moral emotion and behaviour are integrated.
Proneness to guilt consistently correlates with empathy, which refers to the ability to share the affective experiences of others13. Empathy, while not a discrete emotion, is a morally relevant emotional capacity13 and trait38. Guilt appears to foster reparative action, promote empathy13 and increase altruistic acts39. Elevated empathy, moreover, has been correlated with severity of depression, and proposed as a risk factor for its development40. Importantly, empathy is classically absent in psychopathy41.
We were especially interested in empathy because moral decision-making in individuals high in trait empathy has been shown to be particularly sensitive to manipulations of serotonin5. Furthermore, the serotonin 2 A (5-HT2A) receptor agonists lysergic acid diethylamide (LSD), psilocybin, and 3,4-methylenedioxymethamphetamine (MDMA), have all been shown to enhance empathy42–44. To extend these findings we therefore tested the hypothesis that ATD would interact with trait empathy to amplify morally relevant social emotion. Given the consistent correlations between guilt-proneness and empathy13, we predicted guilt would be the most likely emotion to be affected.
Conversely, psychopathy is characterised by emotional dysfunction reflected in reduced guilt and empathy45, and an increased risk for aggression. Aggression can be either goal-directed (e.g. a premeditated crime), or reactive: an explosive, impulsive response to frustration36. Many psychiatric conditions increase the risk for reactive aggression, however psychopathy is unique in that there is an increased risk for both reactive and goal-directed aggression36. Aggression is in turn traditionally associated with low serotonin46, including evidence from studies of violent offenders47. We explored whether psychopathic traits are likewise related to morally relevant emotions, and whether ATD modulates this relationship. We predicted that psychopathic traits might have the most pronounced effect on feeling annoyed, which invokes the notion of frustration.
While some, but not all, aggression can be impulsive, aggression and impulsivity are distinct. Indeed, discrete serotonergic circuits modulate aggressive versus impulsive behaviour in mice48. ATD can induce “waiting impulsivity” (diminished action restraint while waiting for a reward) and “impulsive choice” (accepting small immediate rewards over larger delayed ones) in healthy individuals6,49. More impulsive choice has been correlated with increased aggressive impulses to perceived injustice on the UG, which was heightened further by ATD6. We therefore asked whether trait impulsivity on the Barratt Impulsiveness Scale50 was related to increased annoyance following ATD.
To test our hypotheses, we employed ATD in a double-blind, randomised, placebo-controlled, between-groups design, in healthy volunteers. We predicted that ATD would enhance negative emotion overall, and that individual differences in empathy, psychopathy, and impulsivity would influence how ATD modulated the profile of emotion. In line with the traditional disconnection between psychopathy and empathy, we predicted that there would be dissociation between how trait empathy and psychopathy interact with neurochemical status to modulate annoyance, guilt, shame and feeling bad. Given the established connection between serotonin and impulsivity49,51,52, and retaliatory behaviour6, we also hypothesised that high-trait impulsivity would be related to increased feelings of annoyance, which would be further potentiated by ATD in these individuals.
Materials and methods
Participants
Seventy-three healthy participants (mean age 24.6, 39 males) completed the experiment. Participants were medically healthy and screened to be free from any psychiatric disorders, using the Mini-International Neuropsychiatric Interview53. Individuals who reported, during screening, having a first-degree relative (parent or sibling) with a psychiatric disorder were also excluded. Other exclusion criteria included neurological disorders, current use of any regular medication excluding contraceptive pills, and past use of neurological or psychiatric medication. Further exclusion criteria and measures to ensure a matching of the groups are reported in the Results and Supplementary Material. Participants gave informed consent before the start of the study and were paid for their participation.
General procedure
The protocol was approved by the Cambridge Central Research Ethics Committee (16/EE/0101), and the study took place at the National Institute for Health Research/Wellcome Trust Clinical Research Facility at Addenbrooke’s Hospital in Cambridge, England. Participants arrived in the morning of the study day having fasted for at least 9 h beforehand and completed a baseline 16-item visual analogue scale (VAS) to assess mood and other feelings including alertness. The VAS was also completed during the middle and end of the day. Participants then gave a baseline blood sample, and ingested either a placebo or tryptophan depletion drink. Simple randomization was employed. In the afternoon, participants gave a second blood sample, ~4.5 h after ingesting the drink. The Moral Emotions task (described below) was administered following the nadir of plasma tryptophan levels, which has been reported to occur in the sixth hour after consuming the amino-acid mixture54. Other tasks that will be reported elsewhere preceded the Moral Emotions task in the following order and examined: behavioural attributions55, probabilistic reversal learning56, theory of mind57, Pavlovian conditioning58, avoidance learning59 and deterministic reversal learning. The administration of multiple tasks within a single ATD study is a common approach taken by several studies cited within this manuscript4,6,14,49,60. Participants additionally attended a short afternoon session the day before, with no pharmacological manipulation, where they completed baseline questionnaires and three unrelated tasks, which will not be reported here. The present laboratory experiment was conducted once.
Acute tryptophan depletion
Tryptophan is the amino-acid precursor necessary to synthesize brain serotonin. Acute tryptophan depletion (ATD) is a widely used dietary manipulation, which results in a rapid decrease in the synthesis and release of serotonin7–11. Participants were randomly assigned to receive either ATD or a placebo condition, in a double-blind, between-groups design. The depletion group received a drink that contained a balance of all the essential amino acids except for tryptophan. The placebo group received the same drink except that it included tryptophan. Blood plasma samples were collected to verify depletion and analysed using high performance liquid chromatography (HPLC).
Moral emotions task
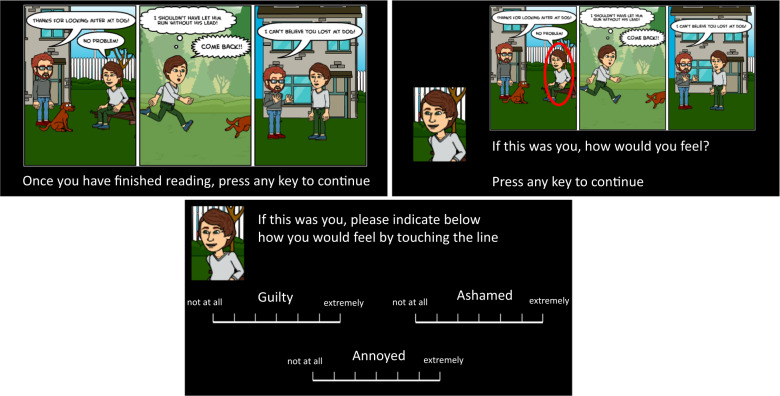
We employed a novel task, part of the EMOTICOM neuropsychological testing battery57,61, to measure feelings of guilt, shame, annoyance and feeling “bad”. Using a touchscreen computer, participants were presented with cartoons of social scenarios—Pavlovian cues—in which someone was unjustly harmed, either intentionally or unintentionally. We then interrogated emotional reactions to these scenarios by asking participants to reflect, and report how they would feel if they were the victim or agent of harm. An example of one trial is depicted in Fig. 1. There were 28 randomised trials, composed of 14 different cartoons, which were each presented twice—once where participants were prompted to identify as the victim, and once where they were asked to identify as the agent. The specific instruction was, “If this was you, please indicate below how you would feel by touching the line.” The four different emotions were measured using four unnumbered touchscreen scales, with seven rungs to choose from, where the first rung was labelled “not at all”, scored as 1, and the seventh labelled “extremely”, scored as 7. Half of the scenarios involved an intentional harm. In the other half, the harm committed was accidental. The task was self-paced.
Fig. 1. Moral emotions task schematic57.
Three example slides of a trial are shown. Feeling “bad” was assessed with a rating scale on a fourth slide.
Statistics
Sample size was chosen based on a recent analogous study that also employed the EMOTICOM neuropsychological testing battery, in healthy volunteers, and using a serotonergic manipulation62. Data were analysed using MATLAB (MathWorks) and SPSS (IBM). MATLAB code is available as supplementary material. All statistical tests were two-sided. Homogeneity of variance in t-tests was verified with Levene’s test, and degrees of freedom were adjusted when this assumption was violated. The Greenhouse–Geisser correction was used where applicable, in designs with within-subjects factors, to correct for violation of the sphericity assumption as determined by Mauchly’s test. Adjustments for multiple comparisons were not deemed necessary.
Results
Seventy-three participants completed the study: 37 underwent depletion (20 males), while the remaining 36 received placebo (19 males).
Effects of ATD on mood ratings
Mood ratings were unaffected by ATD. We collected rating data from 65 participants (n = 33 depletion) on how happy or sad they were feeling before the task, after depletion had taken effect, and these ratings did not differ from those of participants in the placebo group (t(63) = −0.727, p = 0.47). There were also no baseline differences in depressive symptoms (t(71) = −1.258, p = 0.212) using the Beck Depression Inventory-II63.
How serotonin depletion modulates emotional ratings overall
We tested whether serotonin depletion potentiated emotions overall, irrespective of individual differences. To do this we performed repeated measures analysis of variance (ANOVA) incorporating all four emotions measured. Serotonin status (ATD versus placebo) was the between-subjects factor, and the within-subjects factors were emotion (annoyance, guilt, shame, and feeling bad), agency (agent or victim of harm) and intentionality (intentional or unintentional harm). Indeed, serotonin depletion potentiated emotion overall (F(1,71) = 5.959, p = 0.017, ηp2 = 0.077), shown in Table 1 and Supplementary Fig. 1. There were highly significant main effects for emotion (F(2,155) = 171.744, p = 7.4 × 10−42, ηp2 = 0.708), agency (F(1,71) = 630.212, p = 4.9 × 10−37, ηp2 = 0.899) and intentionality (F(1,71) = 11.799, p = 0.001, ηp2 = 0.143), regardless of serotonergic status, which supports task validity and suggests individuals were attuned to the social context of the scenarios. Emotional ratings were significantly greater when imagining being the agent compared to the victim, and when the harm committed was intentional rather than unintentional. There were no interactions between serotonin status and agency or intentionality (all p > 0.05). Our core results in this study, however, came from analyses of how individual differences interacted with the serotonin-depleted state to modulate emotion, which now follow.
Table 1.
Summary of results on personality traits.
| Annoyance | Guilt | Shame | Bad | |||||
|---|---|---|---|---|---|---|---|---|
| Placebo | ATD | Placebo | ATD | Placebo | ATD | Placebo | ATD | |
| Impulsivity | − | |||||||
| Psychopathy | ↑↑ | |||||||
| Empathy | ↑↑ | + | + | |||||
Arrows (↑↑) indicate a significant enhancement of the emotion by ATD at high levels of the personality trait shown; Plus sign (+) indicates a significant positive relationship between the personality trait and the emotion that was not significantly modulated by ATD; Minus sign (−) likewise indicates a significant negative relationship. Emotions are collapsed across agency and intentionality.
Correlations between trait measures
First, we ensured that our trait measures of interest were not correlated with one another. Scores on the impulsivity and psychopathy scales were not correlated (r(73) = −0.113, p = 0.342). Empathy scores were likewise not correlated with impulsivity (r(73) = 0.094, p = 0.427) or psychopathy (r(73) = 0.015, p = 0.897).
How trait empathy modulates emotional effects of serotonin depletion
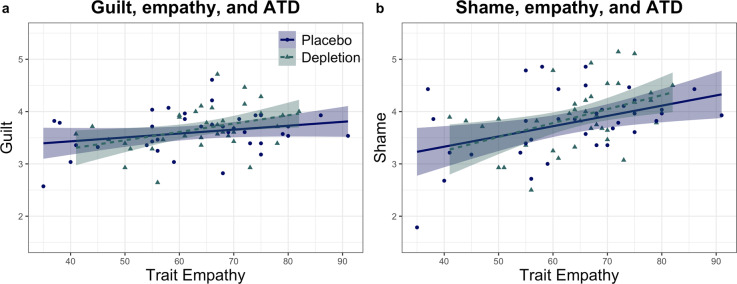
Given prior evidence that single-dose SSRI had a more pronounced effect on social behaviour in highly empathic participants5, a central question in our study was whether the serotonin-depleted state and empathic trait interacted to influence emotion. Indeed, we found that the self-conscious emotion guilt was more sensitive to serotonin depletion in individuals with high-trait empathy. We ensured self-reported empathy at baseline, using the Interpersonal Reactivity Index (IRI)38, was matched between placebo and depletion (t(63) = 0.442, p = 0.660; Levene’s test F(71) = 4.569, p = 0.036). We analysed emotional ratings via analysis of covariance (ANCOVA), using empathy and serotonin status (ATD versus placebo) as factors in a between-subjects interaction term, controlling for main effects, and agency (agent or victim) and intent (intentional or unintentional) as within-subjects factors. For guilt ratings, this revealed a significant four-way serotonin × empathy × agency × intentionality interaction (F(1,69) = 5.596, p = 0.021, ηp2 = 0.075). Guilt ratings were significantly higher in more empathic individuals following serotonin depletion (r(37) = 0.385, p = 0.019), and not under placebo (r(36) = 0.265, p = 0.118), irrespective of agency or intentionality, seen in Fig. 2a and Table 1. Follow-up tests showed that the agency and intentionality effect was driven by a highly significant relationship between empathy and guilt, when imagining inflicting (being the agent of) harm unintentionally (r(37) = 0.43, p = 0.008). There was additionally a three-way interaction between agency, intentionality and group (F(1,69) = 4.765, p = 0.032, ηp2 = 0.065). Critically, however, this three-way interaction disappeared when empathy was not included as a factor in the (otherwise identical) model (F(1,71) = 0.608, p = 0.438, ηp2 = 0.008). We were also able to reproduce this core result on guilt, using a quartiles approach whereby individuals with an empathy score in the top 25% were deemed “high empathy” and the bottom 25% “low empathy”. ANCOVA with empathy (high versus low) and serotonin status (ATD versus placebo) as factors in a between-subjects interaction term, controlling for main effects, and agency (agent or victim) and intent (intentional or unintentional) as within-subjects factors, again revealed a significant four-way serotonin × empathy × agency × intentionality interaction effect on guilt (F(1,36) = 7.028, p = 0.012, ηp2 = 0.163). While the ANCOVA approach, as above, did not yield a significant interaction of serotonin and empathy on shame (F < 0.42, p > 0.05, ηp2 < 0.007 for all terms involving serotonin status), empathy and overall shame ratings were highly correlated in both the depletion (r(37) = 0.437, p = 0.007) and placebo groups (r(36) = 0.425, p = 0.01). These data for shame are shown in Fig. 2b and Table 1. The ANCOVA on annoyance was not significant (F < 2, p > 0.05, ηp2 < 0.03 for all terms involving serotonin status), as was the case for the ANCOVA on feeling “bad” (F < 1.2, p > 0.05, ηp2 = 0.02 for all terms involving serotonin status). Guilt, therefore, was uniquely affected by the interaction between trait empathy and the serotonin-depleted state. Serotonin induced a distinct emotional profile in highly empathic individuals.
Fig. 2. Effects of trait empathy on how serotonin depletion influences emotion.
Shading indicates 1 standard error. Each point represents the average emotion ratings for each individual, collapsed across agency and intentionality. a The highly empathic reported more guilt following depletion relative to when on placebo. b Shame was significantly elevated in individuals high in trait empathy on both placebo and depletion.
How trait psychopathy modulates emotional effects of serotonin depletion
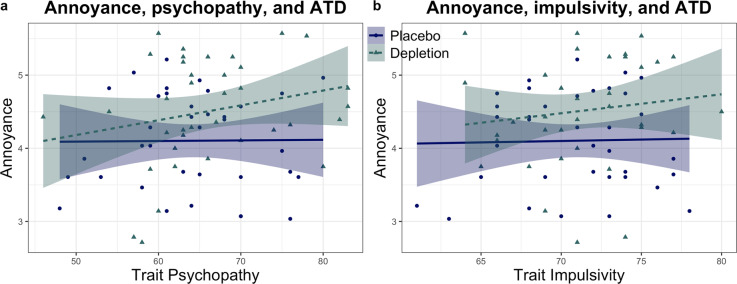
Trait psychopathy also interacted with the serotonin-depleted state to modulate emotion. We ensured psychopathic traits assessed with the Levenson Self-Report Psychopathy Scale64 at baseline were matched in the placebo and depletion groups (t(71) = 1.132, p = 0.261). ANCOVA with serotonin status (ATD versus placebo) and psychopathy as factors in a between-subjects interaction term, controlling for main effects, and agency and intentionality as within-subjects factors revealed a significant serotonin × psychopathy × intentionality three-way interaction for feelings of annoyance (F(1,69) = 7.172, p = 0.009, ηp2 = 0.094). With increasing trait psychopathy, individuals felt even more annoyed following serotonin depletion, seen in Fig. 3a and Table 1. Intentionality significantly interacted with psychopathy to influence annoyance under placebo (F(1,34) = 5.163, p = 0.03, ηp2 = 0.132) and not following depletion (F(1,34) = 2.237, p = 0.144, ηp2 = 0.06). In other words, the influence of psychopathy on annoyance depended on intentionality when on placebo, but on depletion those high in trait psychopathy were more annoyed regardless of intentionality. There was additionally a significant serotonin × intentionality interaction (F(1,69) = 7.161, p = 0.009, ηp2 = 0.094). Critically, however, this two-way interaction disappeared when psychopathy was not included as a factor in the (otherwise identical) model (F(1,71) = 0.043, p = 0.836, ηp2 = 0.001). Next, we assessed guilt using the same ANCOVA approach: there was no serotonin × psychopathy interaction (F(1,69) < 2.8, p > 0.05, ηp2 < 0.04 for all terms involving serotonin status). There was no serotonin × psychopathy interaction for shame (F(1,69) < 3.8, p > 0.05, ηp2 < 0.055 for all terms involving serotonin status) nor for feeling bad (F(1,69) < 2.1, p > 0.05, ηp2 < 0.03 for all terms involving serotonin status).
Fig. 3. Effects of trait psychopathy and impulsivity on emotional effects of serotonin depletion.
Shading indicates 1 standard error. Each point represents the average emotion ratings for each individual, collapsed across agency and intentionality. a Annoyance was potentiated by serotonin depletion in high-trait psychopathy. b Trait impulsivity did not significantly enhance the effects of ATD on annoyance.
Trait impulsivity and emotional effects of serotonin depletion
Trait impulsivity, measured at baseline with the Barratt Impulsiveness Scale50, was also matched between groups, and did not interact with the serotonin-depleted state to modulate emotion. Data on impulsivity are summarised in Table 1. First, we assessed feelings of annoyance using ANCOVA with serotonin status (ATD versus placebo) and impulsivity as factors in a between-subjects interaction term, controlling for main effects, and agency (agent or victim) and intent (intentional or unintentional) as within-subjects factors: there was no interaction between serotonin and impulsivity (F(1,69) < 1.7, p > 0.05, ηp2 < 0.025 for all terms involving serotonin status). These data are shown in Fig. 3b. The same was true for all terms involving serotonin status in the ANCOVAs on guilt (F(1,69) < 0.7, p > 0.05, ηp2 < 0.01), shame (F(1,69) < 0.4, p > 0.05, ηp2 < 0.01) and feeling bad (F(1,69) < 2.6, p > 0.05, ηp2 < 0.04).
Principal components analysis
We also explored whether there was any structure underlying the task measurements that was not detected by our prior analyses. To do this, we used principal components analysis (PCA) on the 16 outcome variables from the task (see Supplementary for methods; factor loadings are shown in Supplementary Table 1). The validity of this PCA was confirmed by comparing the number of components, and the variables that clustered together, to a PCA on the same task in the original larger dataset from a non-pharmacological study of 186 healthy participants57. We then interpreted how the task measurements from our experiment clustered into the four principal components. Component 1 centred on annoyance with others for having done harm to oneself—in other words, outward frustration. The predominant theme of component 2 was inward frustration, or annoyance with oneself for having harmed another. Components 3 and 4 centred on the self-conscious negative-emotions guilt and shame. Component 3 captured these emotions when the participant was the agent, component 4 when the participant was the victim of harm. We then used the estimated factor scores for each individual to assess how serotonin depletion modulated the constructs captured by the four components. ANOVA with serotonin status (ATD versus placebo) as a between-subjects factor, and the four components as levels of a within-subjects factor, revealed a significant serotonin × component interaction (F(3,213) = 3.165, p = 0.025, ηp2 = 0.043). There was no main effect of serotonin depletion (F(1,71) = 2.187, p = 0.144, ηp2 = 0.030). Follow-up t-tests revealed the values of component 2 (t(71) = 2.124, p = 0.037) and component 4 (t(71) = 2.290, p = 0.025) were each significantly greater following depletion relative to placebo, as seen in Supplementary Fig. 2. Inward frustration, or annoyance, for having harmed another—captured by component 2—was potentiated by serotonin depletion. When the victim of harm in the task, self-conscious negative emotion was also exacerbated by serotonin depletion (component 4).
Blood analysis
Blood results are shown in Fig. 4. The ratio of tryptophan to large neutral amino acids (TRP:LNAAs; tryptophan to valine, methionine, isoleucine, leucine, tyrosine and phenylalanine) was calculated, as this is thought to be most reflective of the extent of brain serotonin depletion65. We then performed a t-test on the change in the TRP:LNAA ratio between samples taken at baseline and approximately 4.5 h following administration of the mixture. Plasma levels were unavailable for two participants: one due to a staff processing error, and one due to difficulty with venepuncture. We achieved a robust depletion of tryptophan (t(60) = −19.163, p = 3.01 × 10−27).
Fig. 4. Blood analysis results.
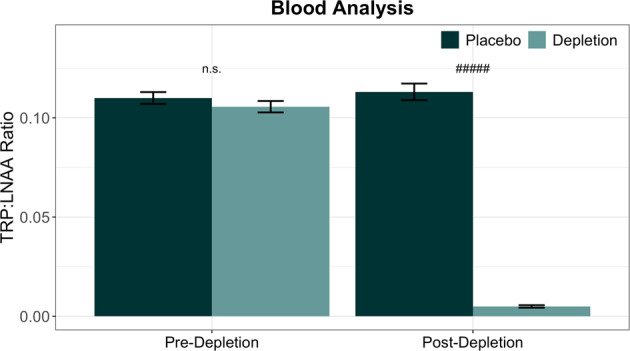
Error bars represent 1 standard error. Significance at p < 5 ×10−27 is denoted by #####. n.s. denotes not significant.
Correlation analyses
Extent of depletion was not correlated with any particular emotion, reinforcing the importance of accounting for traits to uncover effects of ATD on social emotion. Change in TRP:LNAA ratio was not correlated with annoyance (r = −0.208, p = 0.081), guilt (r = −0.121, p = 0.314), shame (r = −0.057, p = 0.636), or feeling bad (r = −0.088, p = 0.464). There were correlations between emotion ratings: collapsed across serotonergic status, annoyance, guilt, and shame were all positively correlated with one another (p < 0.05), consistent with the nonspecific enhancement of emotion in the absence of considering traits. Guilt and shame were both negatively correlated with feeling bad (p < 0.05). These correlations, with exact p-values and correlation coefficients, are presented in Supplementary Table 2.
Summary of results
Results are summarised in Table 1. Serotonin depletion enhanced emotion overall. Examining individual trait differences revealed a deeper story. Guilt was significantly enhanced by serotonin depletion in highly empathic participants, which was a distinctive emotional profile. This was driven by guilt when the agent of an unintentional harm. Trait empathy, furthermore, was highly correlated with shame ratings in both the placebo and depletion groups. Greater trait psychopathy following serotonin depletion, meanwhile, was associated with enhancement of annoyance. PCA revealed a component where guilt and shame clustered together when the victim of harm, which was potentiated by ATD.
Discussion
Using a novel test that cued autobiographical memories, we showed that serotonin depletion heightened emotional reactions when mentally simulating social scenarios involving unjust harm. While emotion was enhanced nonspecifically at the group level, harnessing baseline individual differences revealed that personality traits play a critical role in shaping which distinctive types of emotions are affected by serotonin depletion. A key result was that individuals high in trait empathy showed a distinct profile of enhanced guilt following serotonin depletion. This contrasted with how variation in trait psychopathy (classically associated with lack of empathy and guilt) influenced the relationship between serotonin depletion and emotional reactions: only annoyance was potentiated. This dissociation, in other words, mirrored the antithetical nature of empathy and psychopathy. Previous studies have shown that traits can influence the magnitude of effects of serotonin manipulations on social behaviour;5,6 we now show that traits modulated the quality, as well as the magnitude, of social emotion following serotonin depletion. Traits contribute to an individual’s model of the world, and therefore shape prior expectations about social interactions: we propose the influence of traits on how serotonin modulated emotions can be thought of as constituting biological “priors”.
Emotions prepare the body for action or inaction66. Our findings on the serotonergic modulation of social emotion converge with the literature on social decision-making, and we propose that our results represent a Pavlovian influence that can shape social behaviour3. The deployment of empathy has additionally been described as having a Pavlovian character, which can shape behaviour triggered by cues signalling harm3. While we did not measure behaviour, there are some intriguing parallels between our findings on emotional reactions to unjust harm, and studies of retaliatory behaviour to unfairness, as assessed using the Ultimatum Game4,6 which we highlight below. In the UG, individuals must split a sum of money with another, and are given the opportunity to reject unfair offers, in which case neither player gets paid4,6. Indeed, the UG has been studied under serotonin depletion and in relation to empathy and psychopathy.
Empathy was of central importance to our analysis. This was motivated by the observation that social behaviour in highly empathic participants was especially sensitive to single-dose SSRI administration: these individuals showed the greatest reduction in retaliatory behaviour to unfairness5. Critically, we found that individuals high in trait empathy had a distinct profile of enhanced emotion: guilt was amplified. Empathy and guilt are consistently correlated13, and guilt may even promote empathy13. Feelings of guilt have been associated with real-life altruistic acts39. Guilt has been proposed to restrain antisocial behaviour as modelled by laboratory tests: a diminished sense of guilt is thought to contribute to dysfunctional social behaviour following vmPFC damage37, and is also a feature of psychopathy36. The present observation that increased guilt in the highly empathic under ATD was driven by instances of unintentionally inflicting harm in a social setting is entirely consistent with these accounts.
Shame, furthermore, was highly correlated with empathy. This was true in both the placebo and depletion conditions, which could have led to a ceiling effect, leaving less room for further enhancement of shame by ATD in the highly empathic, on top of an already elevated baseline under placebo. Guilt and shame, moreover, clustered together in the PCA when the victim of harm, and this component was enhanced by serotonin depletion regardless of personality traits. Guilt and shame are distinguishable yet can overlap13, which is also true of their neural correlates67. While guilt and shame were correlated, the literature indicates that different events can be guilt-inducing or shame-inducing for different people13. That different types of emotion were enhanced in tandem at the group level, furthermore, does not imply the quality of these emotions cannot be distinguished by the person experiencing them.
Shame-free guilt is seen as possibly adaptive—for instance by promoting reparations—while proneness to shame is seen as a risk for, and is indeed associated with a wide range of psychopathology13. Importantly, guilt is thought to become maladaptive primarily when it is fused with shame13. Guilt overlaid with shame is most likely a source of rumination13. The hippocampus has been reported to be involved in the experience of shame68, and failure of hippocampal serotonin is suggested to contribute to rumination69. SSRIs, meanwhile, improve hippocampal function in depression70. Individuals with hippocampal damage, moreover, appear to show heightened reactive emotionality congruent with their behaviour in moral decision-making tasks, which is antithetical to the pattern seen with vmPFC lesions71. While the relationship with our results is merely speculative, hippocampal dysfunction is a feature of numerous psychiatric conditions, as is social dysfunction: a recent framework proposes these two well-established phenomena can be unified through the purported role of the hippocampus in organising social information (memories), via relational maps that support simulations of social outcomes72.
Indeed, there are reported links between elevated empathy and depression40. Individuals sensitive to distress in others may be more likely to experience personal distress, and this has been highlighted as a vulnerability factor for depression39,40. At the same time, there is evidence for diminished deployment of theory of mind—non-affective perspective taking—in depression73, and this combination raises the possibility that sensitivity to distress in oneself and others may become misattributed or inappropriately directed inward.
While mood was unaffected in our study, consistent with the literature on ATD in healthy individuals74, ATD can transiently reinstate low mood in depressed individuals successfully treated with an SSRI74. By using trait measurements and a task that elicited emotions, however, we were able to uncover a pattern reminiscent of depression: more guilt in the highly empathic under serotonin depletion. Indeed, this task has already been used to detect possible latent vulnerabilities in a healthy population with trait paranoia61. We propose that empathy, which produced a qualitatively unique emotional profile under ATD, may represent an important proxy for sensitivity to changes in serotonin.
Conversely, psychopathic individuals classically have impairments in guilt and empathy, and an increased risk for aggressive behaviour, especially following frustration36,45. This is consonant with our results. We found that the emotional profile following serotonin depletion in healthy individuals high in psychopathic traits dissociated from what we observed in the highly empathic: annoyance was instead amplified following unjust harm. This result is furthermore in line with existing literature on social decision-making: clinically psychopathic individuals show an analogous pattern of behaviour on the UG34 to the disinhibited aggressive impulses seen in ATD studies of healthy volunteers4, that is also quantitatively similar to how individuals with vmPFC lesions behave on the UG34. Critically, vmPFC damage is associated with impaired emotion regulation, and individuals with such lesions tend to exhibit anger and irritability particularly following frustration in their personal lives33. Diminished structural and functional connectivity between the vmPFC and amygdala in clinically psychopathic individuals35 is indeed thought to be a central mechanism underlying the condition. Interactions between these structures are furthermore sensitive to ATD in healthy individuals viewing facial signs of aggression60. That serotonin depletion made participants high in trait psychopathy more annoyed by social injustice may be relevant for understanding how serotonin affects the emotional basis of retaliatory behaviour to unfairness4,6. This view is supported by work showing that such behavioural reactions are associated with self-reported anger in healthy volunteers75. Trait anger and psychopathy in violent offenders indeed appears to reflect 5-HT1B receptor levels76, which moreover fits with the vast literature implicating serotonergic dysfunction in aggression46,51,77.
The individual differences we observed in response to a challenge of brain serotonin are likely in part related to the relative contribution of the multiple serotonin subsystems in the brain. Importantly, preferential dysfunction in the median or dorsal raphe nuclei, which innervate, among other regions, the hippocampus and prefrontal cortex, respectively, has been putatively related to phenotypes as divergent as depression and antisocial personality disorder, respectively; both nuclei project to the amygdala46. Recent data underscore the complexity of serotonin subsystems, revealing that even within the dorsal raphe there are subsystems that have distinct and at times opposing functions: both activate to reward but have opposing responses to aversion78.
A limitation of our experiment is we did not measure serotonin (5-HT) directly: we measured plasma tryptophan levels following depletion, as tryptophan is the amino-acid precursor of serotonin and ATD has been shown to produce transient reductions in central serotonin synthesis in humans11. While the validity of ATD as a method to manipulate central serotonin has been questioned79, this position has been rebutted on the basis of considerable evidence9. Consonant results between human studies employing ATD, and rodent experiments that induce profound serotonin loss using the neurotoxin 5,7-dihydroxytryptamine (5,7-DHT), bolster the case that ATD reduces central serotonin. A prime example comes from studies of waiting impulsivity, which can be induced in humans following ATD49, and in rats after serotonin depletion via 5,7-DHT80. It is also important to note that the present task did not measure positive emotions. While ATD is associated with evoking negative biases2, consistent with our results, future work will be required to clarify whether positive emotions to social scenarios would be blunted or potentiated by ATD. Another limitation is that our study was reliant on self-reported emotion. Future work could, for instance, seek to understand how self-reported guilt evoked by the present task may relate to a computational measure purported to reflect guilt, estimated based on choice in behavioural economic games37.
Our data from a novel test, that required drawing on autobiographical memories to mentally simulate cued social scenarios, demonstrate that there are important individual differences in the way serotonin influences how we react emotionally to social injustice. This should not come as a surprise given the intricacy of the serotonin systems and the complexities of human emotion and behaviour. While serotonin depletion potentiated the magnitude of emotion nonspecifically at the group level, personality traits played a critical role in shaping which distinctive types of emotions were affected. There was a qualitative dissociation in the way trait empathy, relative to psychopathy, amplified social emotion following serotonin depletion. Previous ATD studies on social cognition, by contrast, examined behavior rather than emotion and found changes in the magnitude but not the quality of effects5,6. We propose that traits in conjunction with the memories our task evoked represent biological priors, which prime individuals to have different emotional reactions in the social world. Our data indicate serotonin would affect the gain. Given emotions are a prescription for action66, it follows that our results could represent how serotonin impacts social behaviour via underlying emotional responses, positioned at the nexus of a social Pavlovian influence over action (Pavlovian action selection). When considering apparent paradoxes in the serotonin literature2,46 and designing future studies, it is critical to note that the quality and magnitude of effects of a single serotonin manipulation can depend on personality. These data additionally inform the neurochemical basis of psychopathology associated with excessive emotions such as guilt and shame. Our findings on the interaction between the serotonin-depleted state and personal attributes could help inform which individuals are particularly vulnerable to pathological emotional reactions, and who may be more amenable to serotonin-modulating treatments, with implications for psychiatric classification in frameworks such as the Research Domain Criteria81.
Supplementary information
Acknowledgements
This research was funded by a Wellcome Trust Senior Investigator Award (104631/Z/14/Z) to T.W.R. B.J.S. receives funding from the National Institute for Health Research (NIHR) Cambridge Biomedical Research Centre (Mental Health Theme); the views expressed are those of the authors and not necessarily those of the NIHR or the Department of Health and Social Care. R.N.C.’s research is supported by the UK Medical Research Council (MC_PC_17213). J.W.K. is supported by a Gates Cambridge Scholarship. We would like to thank the staff at the NIHR/Wellcome Trust Clinical Research Facility at Addenbrooke’s Hospital, where the study was conducted, and Rachel Kyd of the Cambridge University Hospital Research & Development Office for assistance with study approval.
Conflict of interest
T.W.R. discloses consultancy with Cambridge Cognition, Greenfields Bioventures and Unilever; he receives research grants from Shionogi & Co and GlaxoSmithKline and royalties for CANTAB from Cambridge Cognition and editorial honoraria from Springer Verlag and Elsevier. B.J.S. discloses consultancy with Cambridge Cognition, Greenfield BioVentures, and Cassava Sciences, and receives royalties for CANTAB from Cambridge Cognition. R.N.C. consults for Campden Instruments and receives royalties from Cambridge Enterprise, Routledge, and Cambridge University Press. J.W.K., F.E.A., R.Y., D.M.C., A.M.A-S., and A.P. declare no conflict of interest.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
accompanies this paper at (10.1038/s41398-020-00880-9).
References
- 1.Dayan P, Huys QJM. Serotonin in affective control. Annu. Rev. Neurosci. 2009;32:95–126. doi: 10.1146/annurev.neuro.051508.135607. [DOI] [PubMed] [Google Scholar]
- 2.Cools R, Roberts AC, Robbins TW. Serotoninergic regulation of emotional and behavioural control processes. Trends Cogn. Sci. 2008;12:31–40. doi: 10.1016/j.tics.2007.10.011. [DOI] [PubMed] [Google Scholar]
- 3.Gesiarz F, Crockett MJ. Goal-directed, habitual and pavlovian prosocial behavior. Front. Behav. Neurosci. 2015;9:1–16. doi: 10.3389/fnbeh.2015.00135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Crockett MJ, Clark L, Tabibnia G, Lieberman MD, Robbins TW. Serotonin modulates behavioral reactions to unfairness. Science. 2008;320:1739. doi: 10.1126/science.1155577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Crockett MJ, Clark L, Hauser MD, Robbins TW. Serotonin selectively influences moral judgment and behavior through effects on harm aversion. Proc. Natl. Acad. Sci. USA. 2010;107:17433–17438. doi: 10.1073/pnas.1009396107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Crockett MJ, Clark L, Lieberman MD, Tabibnia G, Robbins TW. Impulsive choice and altruistic punishment are correlated and increase in tandem with serotonin depletion. Emotion. 2010;10:855–862. doi: 10.1037/a0019861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bel N, Artigas F. Reduction of serotonergic function in rat brain by tryptophan depletion: effects in control and fluvoxamine-treated rats. J. Neurochem. 1996;67:669–676. doi: 10.1046/j.1471-4159.1996.67020669.x. [DOI] [PubMed] [Google Scholar]
- 8.Biggio G, Fadda F, Fanni P, Tagliamonte A, Gessa GL. Rapid depletion of serum tryptophan, brain tryptophan, serotonin and 5-hydroxyindoleacetic acid by a tryptophan-free diet. Life Sci. 1974;14:1321–1329. doi: 10.1016/0024-3205(74)90440-8. [DOI] [PubMed] [Google Scholar]
- 9.Crockett MJ, et al. Converging evidence for central 5-HT effects in acute tryptophan depletion. Mol. Psychiatry. 2012;17:121–123. doi: 10.1038/mp.2011.106. [DOI] [PubMed] [Google Scholar]
- 10.Hood SD, Bell CJ, Nutt DJ. Acute tryptophan depletion. Part I: rationale and methodology. Aust. N. Z. J. Psychiatry. 2005;39:558–564. doi: 10.1080/j.1440-1614.2005.01627.x. [DOI] [PubMed] [Google Scholar]
- 11.Nishizawa S, et al. Differences between males and females in rates of serotonin synthesis in human brain. Proc. Natl. Acad. Sci. USA. 1997;94:5308–5313. doi: 10.1073/pnas.94.10.5308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nord M, Finnema SJ, Halldin C, Farde L. Effect of a single dose of escitalopram on serotonin concentration in the non-human and human primate brain. Int. J. Neuropsychopharmacol. 2013;16:1577–1586. doi: 10.1017/S1461145712001617. [DOI] [PubMed] [Google Scholar]
- 13.Tangney JP, Stuewig J, Mashek DJ. Moral emotions and moral behavior. Annu. Rev. Psychol. 2007;58:345–372. doi: 10.1146/annurev.psych.56.091103.070145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Crockett MJ, Clark L, Apergis-Schoute AM, Morein-Zamir S, Robbins TW. Serotonin modulates the effects of Pavlovian Aversive predictions on response vigor. Neuropsychopharmacology. 2012;37:2244–2252. doi: 10.1038/npp.2012.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hindi Attar C, Finckh B, Büchel C. The influence of serotonin on fear learning. PLoS ONE. 2012;7:e42397. doi: 10.1371/journal.pone.0042397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Niedenthal PM, Tangney JP, Gavanski I. ‘If Only I Weren’t’ Versus ‘If Only I Hadn’t’: distinguishing shame and guilt in counterfactual thinking. J. Pers. Soc. Psychol. 1994;67:585–595. doi: 10.1037/0022-3514.67.4.585. [DOI] [PubMed] [Google Scholar]
- 17.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th edn. (American Psychiatric Association,: Washington, DC, 2013).
- 18.Ashby JS, Rice KG, Martin JL. Perfectionism, shame, and depressive symptoms. J. Couns. Dev. 2006;84:148–156. doi: 10.1002/j.1556-6678.2006.tb00390.x. [DOI] [Google Scholar]
- 19.Crossley D, Rockett K. The experience of shame in older psychiatric patients: a preliminary enquiry. Aging Ment. Healh. 2005;9:368–373. doi: 10.1080/13607860500131252. [DOI] [PubMed] [Google Scholar]
- 20.Stuewig J, McCloskey LA. The relation of child maltreatment to shame and guilt among adolescents: psychological routes to depression and delinquency. Child Maltreat. 2005;10:324–336. doi: 10.1177/1077559505279308. [DOI] [PubMed] [Google Scholar]
- 21.Brewin CR, Andrews B, Rose S. Fear, helpnessness, and horror in posttraumatic stress disorder: investigating DSM-IV criterion A2 in victims of violent crime. J. Trauma Stress. 2000;13:499–509. doi: 10.1023/A:1007741526169. [DOI] [PubMed] [Google Scholar]
- 22.Feiring C, Taska LS. The persistence of shame following sexual abuse: a longitudinal look at risk and recovery. Child Maltreat. 2005;10:337–349. doi: 10.1177/1077559505276686. [DOI] [PubMed] [Google Scholar]
- 23.Leskela J, Dieperink M, Thuras P. Shame and posttraumatic stress disorder. J. Trauma Stress. 2002;15:223–226. doi: 10.1023/A:1015255311837. [DOI] [PubMed] [Google Scholar]
- 24.Orsillo SM, Heimberg RG, Juster HR, Garrett J. Social phobia and PTSD in vietnam veterans. J. Trauma Stress. 1996;9:235–252. doi: 10.1002/jts.2490090207. [DOI] [PubMed] [Google Scholar]
- 25.Murray C, Waller G, Legg C. Family dysfunction and bulimic psychopathology: the mediating role of shame. Int. J. Eat. Disord. 2000;28:84–89. doi: 10.1002/(SICI)1098-108X(200007)28:1<84::AID-EAT10>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 26.Sanftner JL, Barlow DH, Marschall DE, Tangney JP. The relation of shame and guilt to eating disorder symptomatology. J. Soc. Clin. Psychol. 1995;14:315–324. doi: 10.1521/jscp.1995.14.4.315. [DOI] [Google Scholar]
- 27.Feiring C, Taska L, Lewis M. Adjustment following sexual abuse discovery: the role of shame and attributional style. Dev. Psychol. 2002;38:79–92. doi: 10.1037/0012-1649.38.1.79. [DOI] [PubMed] [Google Scholar]
- 28.Bryan CJ, Morrow CE, Etienne N, Ray-Sannerud B. Guilt, shame, and suicidal ideation in a military outpatient clinical sample. Depress Anxiety. 2013;30:55–60. doi: 10.1002/da.22002. [DOI] [PubMed] [Google Scholar]
- 29.Harper FWK, Arias I. The role of shame in predicting adult anger and depressive symptoms among victims of child psychological maltreatment. J. Fam. Violence. 2004;19:367–375. doi: 10.1007/s10896-004-0681-x. [DOI] [Google Scholar]
- 30.Tangney JP, Wagner PE, Hill-Barlow D, Marschall DE, Gramzow R. Relation of shame and guilt to constructive versus destructive responses to anger across the lifespan. J. Pers. Soc. Psychol. 1996;70:797–809. doi: 10.1037/0022-3514.70.4.797. [DOI] [PubMed] [Google Scholar]
- 31.Schiller D, Delgado MR. Overlapping neural systems mediating extinction, reversal and regulation of fear. Trends Cogn. Sci. 2010;14:268–276. doi: 10.1016/j.tics.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hornung, J. in Handbook of the Behavioral Neurobiology of Serotonin (eds. Muller C. P., Jacobs B. L.) (Elsevier, London, 2010) pp 51–64.
- 33.Koenigs M, Tranel D. Irrational economic decision-making after ventromedial prefrontal damage: evidence from the Ultimatum Game. J. Neurosci. 2007;27:951–956. doi: 10.1523/JNEUROSCI.4606-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Koenigs M, Kruepke M, Newman JP. Economic decision-making in psychopathy: a comparison with ventromedial prefrontal lesion patients. Neuropsychologia. 2010;48:2198–2204. doi: 10.1016/j.neuropsychologia.2010.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Motzkin JC, Newman JP, Kiehl KA, Koenigs M. Reduced prefrontal connectivity in psychopathy. J. Neurosci. 2011;31:17348–17357. doi: 10.1523/JNEUROSCI.4215-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Blair RJR. Psychopathy, frustration, and reactive aggression: the role of ventromedial prefrontal cortex. Br. J. Psychol. 2010;101:383–399. doi: 10.1348/000712609X418480. [DOI] [PubMed] [Google Scholar]
- 37.Krajbich I, Adolphs R, Tranel D, Denburg NL, Camerer CF. Economic games quantify diminished sense of guilt in patients with damage to the prefrontal cortex. J. Neurosci. 2009;29:2188–2192. doi: 10.1523/JNEUROSCI.5086-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Davis, M. H. A multidimensional approach to individual differences in empathy. JSAS Catalog of Selected Documents in Psychology, vol. 10, p. 85. https://scholar.google.com/scholar?hl=en&as_sdt=0%2C31&q=A+multidimensional+approach+to+individual+differences+in+empathy&btnG= (1980).
- 39.O’Connor, L. E., Berry, J. W., Lewis, T., Mulherin, K. & Crisostomo, P. S. Empathy and depression: the moral system on overdrive. Empathy Ment. Illn. 49–75. https://www.cambridge.org/core/books/empathy-in-mental-illness/C903C59E67101C5992B1196322A48D40 (2007).
- 40.O’Connor LE, Berry JW, Weiss J, Gilbert P. Guilt, fear, submission, and empathy in depression. J. Affect. Disord. 2002;71:19–27. doi: 10.1016/S0165-0327(01)00408-6. [DOI] [PubMed] [Google Scholar]
- 41.Blair J, et al. Theory of mind in the psychopath. J. Forensic Psychiatry. 1996;7:15–25. doi: 10.1080/09585189608409914. [DOI] [Google Scholar]
- 42.Dolder PC, Schmid Y, Müller F, Borgwardt S, Liechti ME. LSD acutely impairs fear recognition and enhances emotional empathy and sociality. Neuropsychopharmacology. 2016;41:2638–2646. doi: 10.1038/npp.2016.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hysek CM, et al. MDMA enhances emotional empathy and prosocial behavior. Soc. Cogn. Affect. Neurosci. 2014;9:1645–1652. doi: 10.1093/scan/nst161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pokorny T, Preller KH, Kometer M, Dziobek I, Vollenweider FX. Effect of psilocybin on empathy and moral decision-making. Int. J. Neuropsychopharmacol. 2017;20:747–757. doi: 10.1093/ijnp/pyx047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jones AP, Happé FGE, Gilbert F, Burnett S, Viding E. Feeling, caring, knowing: different types of empathy deficit in boys with psychopathic tendencies and autism spectrum disorder. J. Child Psychol. Psychiatry. 2010;51:1188–1197. doi: 10.1111/j.1469-7610.2010.02280.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Deakin, J. F. W. Depression and antisocial personality disorder: two contrasting disorders of 5HT function. In Neuropsychopharmacology. In Neuropsychopharmacology. Journal of Neural Transmission. Supplementa, vol 64. 10.1007/978-3-7091-6020-6_5 (Springer, Vienna, 2003). [DOI] [PubMed]
- 47.Linnoila M, et al. Low cerebrospinal fluid 5-hydroxyindoleacetic acid concentration differentiates impulsive from nonimpulsive violent behavior. Life Sci. 1983;33:2609–2614. doi: 10.1016/0024-3205(83)90344-2. [DOI] [PubMed] [Google Scholar]
- 48.Nautiyal KM, et al. Distinct circuits underlie the effects of 5-HT1B receptors on aggression and impulsivity. Neuron. 2015;86:813–826. doi: 10.1016/j.neuron.2015.03.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Worbe Y, Savulich G, Voon V, Fernandez-Egea E, Robbins TW. Serotonin depletion induces ‘waiting impulsivity’ on the human four-choice serial reaction time task: cross-species translational significance. Neuropsychopharmacology. 2014;39:1519–1526. doi: 10.1038/npp.2013.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Patton JH, Stanford MS, Barratt E. Factor structure of the Barratt impulsiveness scale. J. Clin. Psychol. 1995;51:768–774. doi: 10.1002/1097-4679(199511)51:6<768::AID-JCLP2270510607>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 51.Bevilacqua L, et al. A population-specific HTR2B stop codon predisposes to severe impulsivity. Nature. 2010;468:1061–1066. doi: 10.1038/nature09629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dalley JW, Roiser JP. Dopamine, serotonin and impulsivity. Neuroscience. 2012;215:42–58. doi: 10.1016/j.neuroscience.2012.03.065. [DOI] [PubMed] [Google Scholar]
- 53.Sheehan, D. et al. The Mini-International Neuropsychiatric Interview (MINI): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J. Clin. Psychiatryhttps://pubmed.ncbi.nlm.nih.gov/9881538/ (1998). [PubMed]
- 54.Carpenter LL, et al. Tryptophan depletion during continuous CSF sampling in healthy human subjects. Neuropsychopharmacology. 1998;19:26–35. doi: 10.1016/S0893-133X(97)00198-X. [DOI] [PubMed] [Google Scholar]
- 55.Brosch T, Schiller D, Mojdehbakhsh R, Uleman JS, Phelps EA. Neural mechanisms underlying the integration of situational information into attribution outcomes. Soc. Cogn. Affect. Neurosci. 2013;8:640–646. doi: 10.1093/scan/nst019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Swainson R, et al. Probabilistic learning and reversal deficits in patients with Parkinson’s disease or frontal or temporal lobe lesions: possible adverse effects of dopaminergic medication. Neuropsychologia. 2000;38:596–612. doi: 10.1016/S0028-3932(99)00103-7. [DOI] [PubMed] [Google Scholar]
- 57.Bland AR, et al. EMOTICOM: a neuropsychological test battery to evaluate emotion, motivation, impulsivity, and social cognition. Front. Behav. Neurosci. 2016;10:1–17. doi: 10.3389/fnbeh.2016.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hartley CA, et al. Serotonin transporter polyadenylation polymorphism modulates the retention of fear extinction memory. Proc. Natl. Acad. Sci. USA. 2012;109:5493–5498. doi: 10.1073/pnas.1202044109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gillan CM, et al. Functional neuroimaging of avoidance habits in obsessive-compulsive disorder. Am. J. Psychiatry. 2015;172:284–293. doi: 10.1176/appi.ajp.2014.14040525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Passamonti L, et al. Effects of acute tryptophan depletion on prefrontal-amygdala connectivity while viewing facial signals of aggression. Biol. Psychiatry. 2012;71:36–43. doi: 10.1016/j.biopsych.2011.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Savulich G, et al. Moral emotions and social economic games in paranoia. Front. Psychiatry. 2018;9:1–10. doi: 10.3389/fpsyt.2018.00615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Skandali N, et al. Dissociable effects of acute SSRI (escitalopram) on executive, learning and emotional functions in healthy humans. Neuropsychopharmacology. 2018;43:2645–2651. doi: 10.1038/s41386-018-0229-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Beck AT, Steer RA, Ball R, Ranieri WF. Comparison of beck depression inventories-IA and-II in psychiatric outpatients. J. Pers. Assess. 1996;67:588–597. doi: 10.1207/s15327752jpa6703_13. [DOI] [PubMed] [Google Scholar]
- 64.Levenson MR, Kiehl KA, Fitzpatrick CM. Assessing psychopathic attributes in a noninstitutionalized Population. J. Pers. Soc. Psychol. 1995;68:151–158. doi: 10.1037/0022-3514.68.1.151. [DOI] [PubMed] [Google Scholar]
- 65.Fernstrom, J. D. Diet-induced changes in plasma amino acid pattern: effects on the brain uptake of large neutral amino acids, and on brain serotonin synthesis. In Baumann P (ed) Transport Mechanisms of Tryptophan in Blood Cells, Nerve Cells, and at the Blood-Brain Barrier. Journal of Neural Transmission, vol 15. 10.1007/978-3-7091-2243-3_5 (Springer, Vienna, 2019). [DOI] [PubMed]
- 66.Tooby, J. & Cosmides, L. The evolutionary psychology of the emotions and their relationship to internal regulatory variables. Handb. Emot. 114–137. https://books.google.com/books/about/Handbook_of_Emotions_Third_Edition.html?id=DFK1QwlrOUAC (2008).
- 67.Wagner U, N’Diaye K, Ethofer T, Vuilleumier P. Guilt-specific processing in the prefrontal cortex. Cereb. Cortex. 2011;21:2461–2470. doi: 10.1093/cercor/bhr016. [DOI] [PubMed] [Google Scholar]
- 68.Bastin C, Harrison BJ, Davey CG, Moll J, Whittle S. Feelings of shame, embarrassment and guilt and their neural correlates: a systematic review. Neurosci. Biobehav. Rev. 2016;71:455–471. doi: 10.1016/j.neubiorev.2016.09.019. [DOI] [PubMed] [Google Scholar]
- 69.Deakin JFW. The origins of ‘5-HT and mechanisms of defence’ by Deakin and Graeff: a personal perspective. J. Psychopharmacol. 2013;27:1084–1089. doi: 10.1177/0269881113503508. [DOI] [PubMed] [Google Scholar]
- 70.Dale E, et al. Effects of serotonin in the hippocampus: how SSRIs and multimodal antidepressants might regulate pyramidal cell function. CNS Spectr. 2016;21:143–161. doi: 10.1017/S1092852915000425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.McCormick C, Rosenthal CR, Miller TD, Maguire EA. Hippocampal damage increases deontological responses during moral decision making. J. Neurosci. 2016;36:12157–12167. doi: 10.1523/JNEUROSCI.0707-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Schafer M, Schiller D. The hippocampus and social impairment in psychiatric disorders. Cold Spring Harb. Symp. Quant. Biol. 2019;LXXXIII:1–14. doi: 10.1101/sqb.2018.83.037614. [DOI] [PubMed] [Google Scholar]
- 73.Wolkenstein L, Schönenberg M, Schirm E, Hautzinger M. I can see what you feel, but I can’t deal with it: impaired theory of mind in depression. J. Affect. Disord. 2011;132:104–111. doi: 10.1016/j.jad.2011.02.010. [DOI] [PubMed] [Google Scholar]
- 74.Bell C, Hood S, Nutt D. Acute tryptophan depletion. Part II: clincal effects and implications. Aust. N. Z. J. Psychiatry. 2005;39:565–574. doi: 10.1080/j.1440-1614.2005.01628.x. [DOI] [PubMed] [Google Scholar]
- 75.Pilluta M, Murnighan J. Unfairness, anger, and spite: emotional rejections of ultimatum offers. Organ Behav. Hum. Decis. Process. 1996;68:208–224. doi: 10.1006/obhd.1996.0100. [DOI] [Google Scholar]
- 76.da Cunha-Bang S, et al. Serotonin 1B receptor binding is associated with trait anger and level of psychopathy in violent offenders. Biol. Psychiatry. 2016;82:267–274. doi: 10.1016/j.biopsych.2016.02.030. [DOI] [PubMed] [Google Scholar]
- 77.Frankle W, et al. Brain serotonin transporter distribution in subjects with impulsive aggressivity: a positron emission study with [11C]McN 5652. Am. J. Psychiatry. 2005;162:915–923. doi: 10.1176/appi.ajp.162.5.915. [DOI] [PubMed] [Google Scholar]
- 78.Ren J, et al. Anatomically defined and functionally distinct dorsal raphe serotonin sub-systems. Cell. 2018;175:472–487.e20. doi: 10.1016/j.cell.2018.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.van Donkelaar EL, et al. Mechanism of acute tryptophan depletion: is it only serotonin. Mol. Psychiatry. 2011;16:695–713. doi: 10.1038/mp.2011.9. [DOI] [PubMed] [Google Scholar]
- 80.Winstanley CA, Dalley JW, Theobald DEH, Robbins TW. Fractioning impulsivity: contrasting effects of central 5-HT depletion on different measures of impulsive behaviour. Neuropsychopharmacology. 2004;29:1331–1343. doi: 10.1038/sj.npp.1300434. [DOI] [PubMed] [Google Scholar]
- 81.Cuthbert BN, Insel TR. Toward the future of psychiatric diagnosis: the seven pillars of RDoC. BMC Med. 2013;11:126. doi: 10.1186/1741-7015-11-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.