Abstract
Rose (Rosa spp.) plants flower via two contrasting methods: once flowering (OF) and continuous flowering (CF). Purple branch is a rare continuously flowering variety of Rosa rugosa that is extensively cultivated in China. However, the genetic basis of its CF behavior is unknown. We demonstrated that Purple branch is heterozygous for the TFL1 homolog KSN. One KSN allele with a 9 kb Copia insertion was found to be identical to that from continuously flowering Rosa chinensis Old blush. The other allele was found to be a functional wild-type allele. The overall expression of KSN was closely linked to the floral transition, and it was significantly repressed in continuously flowering Purple branch compared with OF Plena. The promoter region of the normal KSN allele was hypermethylated, and histone methylation at H3H4, H3K9, and H3K27 of the KSN gene locus was modified in continuously flowering Purple branch. Silencing of the DNA methyltransferase genes MET1 and CMT3 and the histone methyltransferase gene SUVR5 in Purple branch led to enhanced KSN expression, but silencing of the histone demethylase gene JMJ12 suppressed KSN expression. Therefore, the CF habit of Purple branch may be due to reduced expression of KSN caused by the halved dose and may be associated with epigenetic modifications together with retrotransposon insertions along the chromosome. Our study revealed a novel mechanism underlying the CF behavior of rose plants.
Subject terms: Flowering, Histone post-translational modifications
Introduction
Rosa rugosa Thunb., a member of the Rosaceae family, which is indigenous to Eastern Asia, was introduced into Europe and North America in the middle of the nineteenth century1,2. In addition to producing fragrant flowers, this popular and economically valuable ornamental species has medicinal properties. R. rugosa flowers are widely used in traditional and folk medicine in China, Japan, and Korea due to the presence of secondary metabolites that exert pharmacological activities3. Essential oil from petals, known as “liquid gold”, is a raw material used in perfumes, cosmetics, aromatherapy, spices, and the nutrition industry4. Unlike modern roses (Rosa hybrida), which flower continuously throughout the year, most R. rugosa varieties flower once a year during the spring. Efforts to produce flowers year-round rely on screening and breeding new continuously flowering R. rugosa varieties.
Flowering, which involves the transition from vegetative growth to reproductive development, is controlled by external environmental cues and endogenous signals through six genetic pathways in Arabidopsis: the photoperiod, vernalization, autonomous, gibberellin, age, and temperature pathways5. Rose plants have three flowering methods: an once flowering (OF) period in the spring; continuous flowering (CF) during favorable growth seasons; and occasional reblooming6,7.
Rose KSN, a homolog of TFL1 of Arabidopsis thaliana, acts as a flowering repressor to control OF/CF. In OF rose cultivars, the expression of KSN is repressed in the winter and early spring, so the plants bloom only in late spring. After blooming, KSN expression is activated and represses further flower formation in the summer6,8,9. Continuously flowering Rosa chinensis Old blush contains a 9 kb Copia retrotransposon insertion in the second intron of KSN, and this insertion blocks KSN expression, enabling continuous flowering9. Old blush is hybridized with European rose plants to generate modern rose plants, in which the recurrent flowering trait has presumably been transferred into the modern rose plants6. Therefore, most continuously flowering rose cultivars are expected to harbor this mutated allele of KSN from Old blush. However, the presence of a null allele has been suggested in the haploid Old blush genome sequence and possibly contributes to its CF behavior10, and some continuously flowering rose plants have no Copia insertion in their KSN gene11,12; thus, the mutation of KSN may not be the only reason for the rose CF trait.
In Arabidopsis, TFL1 maintains the indeterminate growth of the inflorescence meristem by inhibiting AP1 and LFY expression13,14. In tfl1 mutants, indeterminate meristems are rapidly converted into determinate meristems, which produce terminal flowers13,15,16. Functional characterization of FvTFL1 has confirmed its role as a floral repressor that causes OF: mutations in this gene lead to CF in strawberry9,17. In perennial woody plant species, silencing the TFL1 homolog shortens the juvenile period and causes continuous flowering. For example, silencing MdTFL1 in apple (Malus domestica) reduces the juvenile period from 5 years to several months18,19, whereas silencing PcTFL1 in pear (Pyrus communis L.) accelerates flowering by 1–8 months20. In Populus spp., silencing of the TFL1 orthologs PopCEN1/PopCEN2 hastens the first onset of flowering, maintains the indeterminate growth of axillary meristems and accelerates bud dormancy release upon chilling21. In perennial Arabis alpine plants, AaTFL1 regulates the duration of age-dependent vernalization required for AaLFY expression and sets a threshold for flowering22. Therefore, TFL1 (KSN) is functionally conserved as a flowering repressor.
Purple branch is a rare continuously flowering variety of R. rugosa that is cultivated widely in China. R. rugosa Purple branch provides raw materials for the production of essential oils, jams, teas, pies, beverages, and other derivatives, integrating the primary, secondary and tertiary industries. To date, the origin of Purple branch remains unclear, but it is generally considered to have derived from an interspecific cross between R. rugosa Plena and Rosa davurica23,24. Intriguingly, its presumptive parents are OF rose species (Fig. 1); thus, its CF behavior remains unexplained. Elucidating the molecular mechanism of the CF trait in Purple branch will provide a theoretical basis for breeding new varieties with different flowering phenotypes.
Fig. 1. Images of R. rugosa Plena, R. rugosa Purple branch and R. davurica.
R. rugosa Plena and R. davurica are OF species blooming from April to May. R. rugosa Purple branch is a continuously flowering variety that flowers from April to October. The flowering time is represented by the gray boxes
In this paper, we demonstrate that the continuously flowering R. rugosa Purple branch is heterozygous for KSN: it contains one normal transcribed wild-type allele and another transcriptionally blocked allele with a 9-kb Copia insertion, identical to that in R. chinensis Old blush. We show that the CF behavior of Purple branch is associated with reduced KSN expression. This reduction is due to the halved dose of the wild-type KSN allele and is linked to promoter hypermethylation and histone modification at the KSN locus. We thus present a novel mechanism for the production of the CF habit of rose plants.
Materials and methods
Plant materials and growth conditions
R. rugosa Purple branch, R. rugosa Plena, R. davurica and R. chinensis Old blush were grown in the rose resource nursery of Nanjing Agricultural University (Nanjing, China) under natural conditions.
Arabidopsis thaliana wild-type (Columbia), tfl1-14 mutant and transgenic lines were grown in a grown chamber under controlled conditions (22 °C, 40% relative humidity, and 180 μmol m−2 s−1 of photosynthetically active radiation) under long-day conditions (16 h of light/8 h of darkness).
Gene cloning and expression analysis
Genomic DNA from Purple branch, Plena, R. davurica and Old blush leaves was isolated by the CTAB method25. The different fragments of the KSN gene were isolated by PCR using the primers listed in Table S1. For expression analysis, shoot apices of field-grown Purple branch, Plena and R. davurica were collected on 29 March, 11 April and 30 September 2019, flash frozen in liquid nitrogen, and then stored at −80 °C. Total RNA was extracted using a Vazyme FastPure Plant Total RNA Isolation Kit (Polysaccharides & Polyphenolics rich, Vazyme Biotech, Nanjing, China). One microgram of high-quality total RNA was reverse transcribed using TransScript One-Step gDNA Removal and cDNA Synthesis SuperMix (Transgen Biotech, Beijing, China) according to the manufacturers’ instructions. The cDNA was amplified via RT-PCR, and quantitative reverse-transcription PCR (qRT-PCR) of KSN and FT was carried out with ABI QuantStudio 5 instrument (ABI Life Technologies, Carlsbad, USA) using the primers listed in Table S1; RcGAPDH was used as an internal reference26. Each experiment was conducted for three biological replicates, with three technical repeats each.
Methylation-specific PCR (MSP)
Methylation of the KSN promoter was studied using MSP. Less than 500 ng of genomic DNA was treated with sodium bisulfite using an EZ DNA Methylation Kit (Zymo Research, Irvine, USA). The treated DNA was subsequently used as a template for PCR. Approximately 1 kb of the KSN promoter region was amplified using two pairs of primers (Table S1) that were designed via Methyl Primer Express v1.0. The total volume of the PCR was 25 μl, and PCR was performed using TaKaRa EpiTaq HS (for bisulfite-treated DNA) according to the following protocol: 35 cycles of 98 °C for 10 s, 55–60 °C for 30 s, and 72 °C for 30 s, followed by 72 °C for 10 min. The resulting PCR fragments were ligated into a pMD-18-T vector (TaKaRa, Dalian, China), which was then transformed into Escherichia coli (Trans-T1) (Transgen Biotech, Beijing, China), and 10 clones per sample were sequenced using a bioanalyzer. Each experiment was performed for three biological repeats. The methylation percentages of cytosine (C) in CG, CHH and CHG (H=A, C or T) were analyzed using online software (http://katahdin.mssm.edu/kismeth/revpage.pl), and the running parameters were as follows: minimum fraction of positive matches, 0.8; minimum fraction of length, 0.5.
Chromatin immunoprecipitation PCR (ChIP-PCR)
ChIP-PCR was performed as described previously27, with slight modifications. Shoot apices of field-grown Purple branch and Plena were collected in September 2020 and ground in liquid nitrogen in M1 buffer (10 mM phosphate buffer [pH 7.0], 0.1 M NaCl, 10 mM mercaptoethanol, 1 M hexylene glycol, 1× protease inhibitor cocktail [Roche], 5% PVP, and 1 mM PMSF). The suspension was filtered through four layers of Miracloth, after which the filtrate was centrifuged at 12,000 rpm for 10 min. The pelleted chromatin was washed thrice with M2 buffer (M1 buffer plus 10 mM MgCl2 and 0.5% Triton X-100) and once with M3 buffer (10 mM phosphate buffer [pH 7.0], 0.1 M NaCl, 10 mM mercaptoethanol, 1× protease inhibitor cocktail [Roche], and 1 mM PMSF). The chromatin was resuspended in nuclear lysis buffer and sonicated to generate DNA fragments of approximately 500 bp. The lysate was precleared by incubation together with 50 µl of protein-A agarose beads/salmon sperm DNA (Millipore, Billerica, USA) for 1 h. It was then incubated together with IgG, anti-H3K4me3, anti-H3K9me3, and anti-H3K27me2 antibodies (Abcam, Cambridge, UK) overnight. The bound DNA fragments were recovered and purified using columns from a plasmid extraction kit (Qiagen, Hilden, Germany) according to the manufacturer’s instructions. Quantitative real-time PCR was subsequently performed using the bound and input DNA as templates in conjunction with the primers listed in Table S1.
Transient transformation system
The coding regions of MET1, CMT3, JMJ12, and SUVR5 of R. rugosa Purple branch were cloned using the primers listed in Table S1 and then sequenced. The resulting sequences were used for phylogenetic analysis with Arabidopsis thaliana to validate their reliabilities and then submitted to the NCBI database.
To silence specific genes using RNAi, the conserved fragments of MET1 (MW012566), CMT3 (MW012565), JMJ12 (MW012568) and SUVR5 (MW012567) were amplified from cDNA of Purple branch using specific primers (Supplemental Table S1). The 300–400 bp amplicons were then subcloned into a pENTR-D-TOPO vector (Invitrogen, Carlsbad, USA). A pFAST-R03 binary vector (http://www.psb.ugent.be/) was used in the subsequent LR recombination to generate RNAi plasmids.
To analyze the RNAi effects, young shoots of Purple branch were transiently transformed as previously described28. Briefly, young shoots were placed into a 50-mL Agrobacterium solution carrying the gene fragments of interest and vacuum infiltrated for 5 min, and an Agrobacterium solution carrying the empty vector served as a control. After being released from the vacuum, the shoots were washed with deionized water, and the leaves were collected for RNA extraction and q-PCR analysis after 3 days.
Statistical analyses
Statistical analysis of the data was performed via SPSS 17 statistical software. Two groups of data were compared using Kolmogorov–Smirnov (KS) tests (*P < 0.05; **P < 0.01). Multiple groups of data were compared using the Kruskal–Wallis (KW) test, with P < 0.05 considered significant.
Results
Purple branch contains two KSN alleles
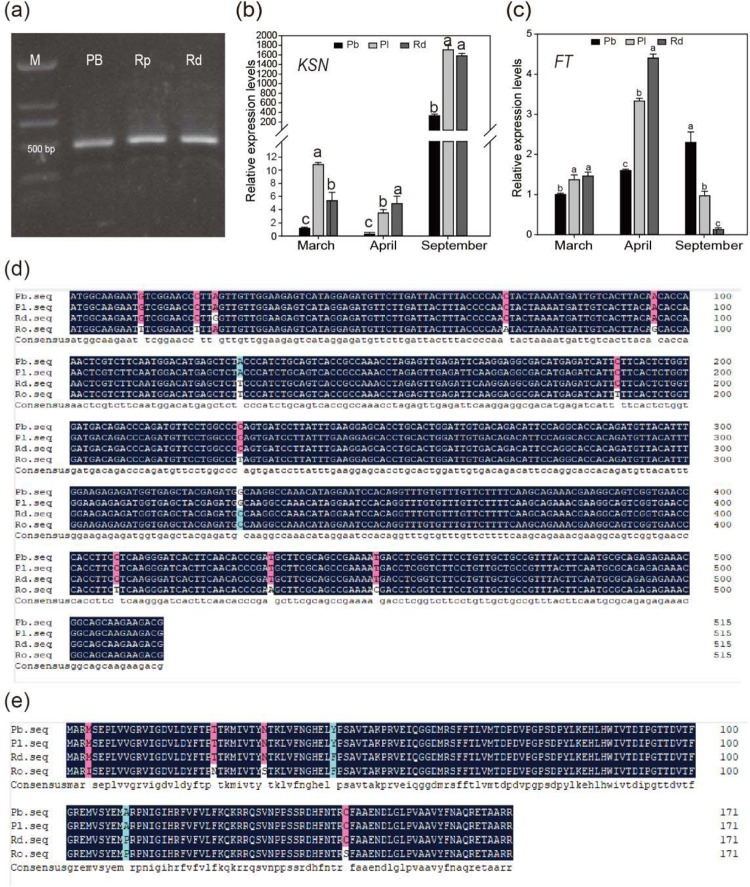
We cloned the KSN promoter and coding region from continuously flowering Purple branch, OF Plena, and R. davurica using the same primers that were previously used to amplify this region in R. chinensis Old blush29. We used the primer pair F1/R1 to amplify the promoter fragment and the primer pair F1/R2 to clone the full-length KSN gene, including both the promoter and coding areas (Fig. 2a). Based on the results of electrophoresis analysis of the PCR products on agarose gels, we obtained two bands from the Purple branch, with primers F1/R1 or F1/R2, and only one band of KSN from Plena and R. davurica (Fig. 2b, d).
Fig. 2. Heterozygous KSN-Copia/KSN-Wt-Pb in continuously flowering Purple branch.
a Schematic representation of the KSN gene in Rosaceae. The promoter is represented by a thick black line, exons are represented by gray boxes, introns are represented by thin black lines, and primers are indicated with arrows. b PCR products separated on an agarose gel by electrophoresis using primers F1/R1 in Purple branch (Pb), Plena (Pl), R. davurica (Rd) and R. chinensis Old blush (Ob). Only one band is amplified in OF Pl and Rd, whereas there are two bands (1 and 2) in CF Pb. c Sequence alignment indicating that Pb promoter 1 is identical to the KSN promoter in Ob and that Pb promoter 2 is identical to the KSN promoter in Pl. d PCR amplification of KSN using primers F1/R2 in Pb, Pl, Rd and Ob. e Sequence alignment of the 2.1 kb product in Pb: the second intron contains a 44 bp insertion compared with the second intron of Pl and Rd. f Schematic representation of the heterozygous KSN-Copia/KSN-Wt-Pb in CF Pb
Subsequent sequence alignment showed that the sequence of one of the KSN alleles from Purple branch was almost the same as that from Plena, except for a few SNPs and a 44 bp insertion in the second intron. The other allele was similar to that from CF R. chinensis Old blush and contained a Copia retrotransposon insertion in the second intron (Figs. 2c, e and S1). These results indicate that the diploid Purple branch is heterozygous for KSN (KSN-Copia/KSN-Wt-Pb) (Fig. 2f). Because neither allele was similar to that of R. davurica, our work contradicts the notion that Purple branch was derived from an interspecific cross between R. rugosa Plena and R. davurica.
KSN expression is lower in Purple branch than in Plena and R. davurica
Because one of the KSN alleles contains a 9 kb Copia insertion in the second intron in Purple branch, we tested whether the other allele contains a 44 bp insertion in the second intron that was transcribed normally. We cloned the coding regions of KSN from the cDNA of Purple branch, Plena, and R. davurica. We obtained a single band from the three different genotypes and sequenced the amplicons (Fig. 3a). Purple branch was identical to Plena (Fig. 3d, e) and contained a few bases that were different from those of R. davurica and R. chinensis Old blush. Thus, continuously flowering Purple branch carrying a wild-type KSN allele can be transcribed normally without interference by the 44 bp insertion.
Fig. 3. Expression analysis of KSN from Purple branch, Plena and R. davurica.
a PCR amplification of KSN using primers F2/R2 from cDNA of Purple branch (Pb), Plena (Pl) and R. davurica (Rd). b, c Expression levels of KSN and FT from Pb, Pl, and Rd in March, April, and September. d, e Show the nucleotide sequence and amino acid sequence alignments of KSN genes from Pb, Pl, Rd, and R. wichurana (Ro). The letters above the bars indicate significant differences, as determined by the KW test (P < 0.05)
To further compare the expression levels of KSN among Purple branch, Plena, and R. davurica and determine the link between KSN and flowering modes, we conducted qRT-PCR on samples collected in March (before blooming), April (during blooming), and September (continuously flowering Purple branch blooms, but OF R. rugosa Plena and R. davurica do not bloom) from all three genotypes. KSN expression was inhibited before flowering, maintained at relatively low levels during flowering, but obviously increased after flowering in September not only in OF R. rugosa Plena and R. davurica but also in continuously flowering Purple branch (Fig. 3b). However, the KSN expression in continuously flowering Purple branch being dramatically lower than that in OF Plena and R. davurica at all three time points may be caused by halved doses, leading to a CF habit. We also analyzed FT expression at the same time points. The expression levels of FT in Purple branch, Plena, and R. davurica were all higher in April than in March, and the levels still increased in September in continuously flowering Purple branch but declined significantly in OF R. rugosa Plena and R. davurica (Fig. 3c). Therefore, KSN expression was negatively associated with the flowering transition in the spring, and the flowering of Purple branch in autumn may be due to the integrative effects of both KSN and FT.
KSN from Purple branch rescues the early-flowering tfl1 mutant phenotype
To determine the functional conservation of the KSN transcript from Purple branch, we expressed this gene under the constitutive 35S promoter in the Arabidopsis tfl1 mutant background. We then examined the flowering phenotype and gene expression levels in the transgenic plants.
The heterologous expression of KSN from Purple branch rescued the early flowering and determinate growth of the inflorescence of tfl1 mutants (Fig. 4a, b). The tfl1-14 mutant produced 5.8 rosette leaves in contrast to the 9 rosette leaves produced by wild-type Arabidopsis at flowering. The flowering of the KSN-OE-1 and KSN-OE-2 lines was delayed to occurring when there were more than 20 rosette leaves. This delay was associated with increased KSN mRNA levels. Seedlings of the KSN-OE-3 line, with relatively high KSN expression levels, were more delayed and produced more than 30 rosette leaves before flowering (Fig. 4b, c).
Fig. 4. Ectopic expression of KSN from continuously flowering Purple branch rescues the early flowering phenotype of the Arabidopsis tfl1 mutant.

a Phenotype of Arabidopsis wild type (WT); tfl1-14 mutants; and the KSN heterologous expression lines KSN-OE-1, KSN-OE-2, and KSN-OE-3. b Rosette leaves of the above genotypes at flowering. c Expression levels of KSN in the above genotypes. The error bars indicate the standard deviations (n = 3). The different letters above the bars indicate significant differences, as determined by the KW test (P < 0.05)
In addition, some transgenic seedlings produced leaf-like floral organs that generated no seeds. These results corroborated those from studies of overexpression of TFL1 from Vitis vinifera, Lotus japonicus and Rosa wichurana in Arabidopsis30–32. Thus, the KSN of Purple branch is the functional homolog of TFL1 in Arabidopsis and other species and acts as a floral repressor.
The KSN promoter in Purple branch is hypermethylated
We hypothesized that the heterozygosity-induced dose decrease may not be the only reason for the inhibition of KSN. The 9 kb insertion may alter the chromatin structure and induce epigenetic suppression or activation. Using MSP, we tested the methylation status of CpG islands in the promoter of the normally transcribed KSN allele in Purple branch. The B fragment (–984 to –502 bp) rather than the A fragment (–501 to –92 bp) was identified as the CpG island for DNA methylation (Fig. 5a, b). The total percentages of the three types of methylation (CG/CHG/CHH) within the B fragment were slightly higher in continuously flowering Purple branch than in OF Plena at all three time points (Fig. 5d), while it was higher only in April within the A fragment in continuously flowering Purple branch (Fig. S2a and S2c). Of the three types of methylation, CG and CHG methylation were most common (Figs. 5c and S2b). The methylation intensity within the promoter was negatively associated with the corresponding level of the KSN transcript presented in Fig. 3. Thus, hypermethylation may have partially contributed to the lower expression of KSN in Purple branch.
Fig. 5. Region B methylation status of the KSN promoter in Purple branch and Plena.
a Schematic representation of fragment B (–984 to –501 bp) and fragment A (–501 to –92 bp) of the KSN promoter. b The methylation status of CG/CHG/CHH sites in region B of KSN promoters from Purple branch (Pb) and Plena (Pl) in March, April, and September. The colored lines above the X-axis show the percentage of methylation at individual cytosine sites. The short bars at the bottom of the graph show the positions of the cytosines. c The pie chart shows the percentage of region B with three types of methylation at different stages in Pb and Pl. d Comparisons of the total methylation percentage of region B in Pb and Pl according to Kismeth software (http://katahdin.mssm.edu/kismeth/revpage.pl). The error bars indicate the standard deviations (n = 3). The asterisks above the bars indicate significant differences, as determined by KS tests (*P < 0.05)
Histone methylation of the KSN locus in Purple branch is modified
To investigate how histone modification regulates KSN expression, we collected shoot apices from field-grown Purple branch and Plena and analyzed KSN expression and the chromatin environment at the KSN locus in continuously flowering Purple branch and OF Plena via ChIP using H3K4me3-specific antibodies (to determine active loci) and H3K9me3- and H3K27me2-specific antibodies (to determine silent loci). We designed specific primers throughout the promoter and the second intron regions (Fig. 6a and Table S1) and performed ChIP-qPCR. KSN expression was obviously lower in Purple branch than in Plena (Fig. S3), which is in line with the previous results shown in Fig. 3. The histone methylation marks H3K9me3 and H3K27me2 increased in continuously flowering Purple branch but remained unchanged or decreased in OF Plena. The H3K4me3 mark decreased in continuously flowering Purple branch but remained unchanged in OF Plena (Fig. 6b). Thus, the enhanced histone methylation at H3K27 and H3K9 and the reduced methylation at H3K4 may play important roles in maintaining the lower expression of the normal KSN allele in Purple branch, leading to its CF behavior.
Fig. 6. Histone methylation of the KSN locus in Purple branch and Plena.
a Schematic representation showing ChIP-qPCR regions A (–638 to –496 bp), B (–387 to –240 bp) and C (494 to 595 bp), marked by black bars below the KSN genomic diagram. b Enrichment of H3K4me3, H3K9me3 and H3K27me2 in different regions of KSN from Purple branch (Pb) and Plena (Pl). IgG was used as a negative control, and ANTI indicates the corresponding antibodies. The error bars indicate the standard deviations (n = 3). The asterisks above the bars indicate significant differences, as determined by KS tests (*P < 0.05; **P < 0.01)
KSN expression increases upon silencing MET1, CMT3, and SUVR5
In Arabidopsis, DNA methylation is mainly performed by members of the C5-MTase families. MET1, which belongs to the METHYLTRANSFERASE (MET) family, maintains CG methylation. CMT3, which belongs to the CHROMOMETHYLTRANSFERASE (CMT) family, maintains CHG methylation33. The cmt3 mutant shows a near-complete loss of CpXpG methylation, and met1 shows a marked reduction in CpG methylation; both types of methylation in turn cause gene silencing34,35. Histone modifications affect various changes in chromatin structure, leading to the promotion or suppression of gene expression36. JMJ12 (REF6), which belongs to the KDM4/JHDM3 group of the JmjC family37, encodes a histone H3 lysine 27 demethylase38, and silencing of RcJMJ12 induced late flowering in Rosa chinensis39. The SET family gene SUVR5 regulates flowering time through H3K9me2 and H3K27me3 and is independent of the vernalization pathway40–42.
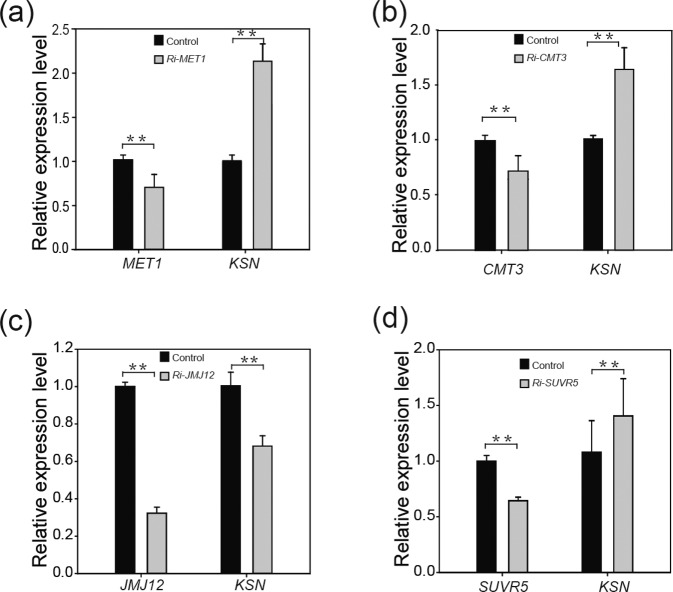
To examine the epigenetic regulation of KSN expression in Purple branch, we cloned the homologs of Arabidopsis MET1, CMT3, JMJ12, and SUVR5 from continuously flowering Purple branch and constructed phylogenetic trees together with their homologs in Arabidopsis to verify their correctness before submitting the sequences to the NCBI database (Fig. S4). We then designed primers (listed in Supplemental Table S1) specific to their conserved regions and silenced the genes in young rose shoots using a transient transformation system described previously28. The subsequent qRT-PCR results showed that silencing MET1, CMT3, JMJ12, and SUVR5 significantly altered the DNA methylation/histone acetylation status at the KSN locus (Figs. S5 and S6), which was followed by a significant increase in KSN transcription in Ri-MET1, Ri-CMT3 and Ri-SUVR5 seedlings compared with a decrease in KSN expression in Ri-JMJ12 seedlings of continuously flowering Purple branch (Fig. 7). Thus, DNA methylation and histone methylation are potentially partially responsible for the repression of KSN in Purple branch.
Fig. 7. Silencing MET1, CMT3, and SUVR5 increases KSN expression in Purple branch.
a, b Expression levels of MET1, CMT3, and KSN in Purple branch seedlings of Ri-MET1 and Ri-CMT3 lines. c, d Expression levels of JMJ12, SUVR5, and KSN in Purple branch seedlings of Ri-JMJ12 and Ri-SUVR5 lines. Gene expression was measured via qRT-PCR for three biological replicates, with three technical repeats each. The RcGADPH gene was used as internal reference. The asterisks above the bars indicate significant differences, as determined by KS tests (**P < 0.01)
Discussion
Previous studies have genotyped KSN in a wide range of rose cultivars and established that some Damask-related roses (e.g., Damask, Moss, Hybrid musk, and Bourbon roses) and Asian roses (e.g., Hybrid rugosa and Hybrid bracteate roses) were CF types but contained no mutated alleles of KSN from R. chinensis12. Similarly, it was also found that continuously flowering R. rugosa Hamanasu from Japan had only a wild KSN allele without any insertion or obvious mutations11. Therefore, the CF behavior of rose plants may not be simply explained by the mutation or insertion of the KSN gene. Interestingly, the present results clearly showed that the KSN allele with the Copia insertion from R. chinensis was substantial in R. rugosa Purple branch (Figs. 2 and 3), which is heterozygous (KSN-Copia/KSN-Wt-Pb) at the KSN locus. The wild-type KSN allele was proven to be functional via heterologous expression in Arabidopsis (Fig. 4). This discrepancy may be caused by different experimental materials or methods, as there are different cultivars and varieties of R. rugosa from different origins. Additionally, the KSN allele carrying the 9 kb insertion was not easily amplified with the normal DNA polymerase and general PCR protocol.
Next, KSN expression was suppressed during the floral initiation period in early spring (April) and dramatically upregulated in the shoot apices after flower initiation in all three species, although KSN expression was still much lower in continuously flowering Purple branch than OF Plena and R. davurica in September (Fig. 3b). In contrast, expression of the floral activator FT significantly increased in April in all three species, decreased in OF Plena and R. davurica but continued climbing in continuously flowering Purple branch (Fig. 3c). Consequently, continuously flowering Purple branch exhibited high expression of both FT and KSN in September. Plant flowering is precisely determined by the equilibrium between floral promoters and repressors. FT, a member of the phosphatidylethanolamine-binding protein (PEBP) family, is a key florigen that integrates the internal and external signals for floral transition43,44. In the shoot apical meristem (SAM), FT interacts with FD to activate floral identity genes such as AP1 and LFY and initiates floral bud formation45,46. Like the flowering promoter FT, TFL1 is also classified as a PEBP member47 but with a function opposite that of FT, acting as a floral repressor. TFL1 interacts with FD to form a TFL1-FD complex, opposing the function of the FT-FD complex in regulating downstream gene expression48. Thus, the floral activator FT may antagonize or suppress the repressor KSN in continuously flowering Purple branch, enabling its flowering in autumn.
Nevertheless, the overall expression of KSN was clearly linked to the alternation of vegetative/reproductive stages of rose plants in the present paper and in six other nonrecurrent flowering species and nine recurrent flowering cultivars49. Furthermore, the expression level of KSN in continuously flowering Purple branch was obviously lower than that in OF Plena and R. davurica at all three time points, which was convincingly associated with the CF trait of Purple branch. Although other factors may be involved in controlling the CF trait, the roles of reduced KSN expression should be predominant and can be mainly attributed to the decreasing dose associated with the heterozygosity of KSN loci in Purple branch (Figs. 2 and 3).
All plants have various transposons (TEs) that can disrupt local gene structure, affect the expression of nearby genes, and induce chromosomal instability. Most TEs are silenced and immobilized under normal conditions. DNA methylation and histone methylation are reversible epigenetic modifications that control transposon activity50–52. The 9 kb Copia-like retrotransposon insertion in R. chinensis Old blush not only blocks transcription of the host KSN allele but also results in a large rearrangement at the CF locus, leading to the complete deletion of the other KSN allele11. Therefore, the diploid Old blush is hemizygous: RoKSNCopia/RoKSNnull. This allowed us to test the DNA and histone methylation status of the KSN locus. We observed hypermethylation of the KSN promoter and enhanced methylation levels of H3K9 and H3K27 at the KSN locus in continuously flowering Purple branch compared with OF varieties without Copia insertions (Figs. 5 and 6). These results implied that the presence of Copia-like retrotransposon insertion may potentially affect the epigenetic status of the KSN locus in continuously flowering Purple branch. Further silencing the DNA methyltransferase genes MET1 and CMT3 and histone methyltransferase gene SUVR5 in young shoots significantly increased the expression of KSN in the Purple branch (Fig. 7), highlighting the importance of epigenetic modification in KSN expression. Taken together, these data suggested that the reduction in KSN expression may be associated with epigenetic modifications, except for the halved dose in continuously flowering Purple branch.
In conclusion, the R. rugosa Purple branch was found to be heterozygous at KSN, and its CF trait was associated with the lower expression of KSN. The reduction in KSN was caused by the halved dose and was also associated with hypermethylation of the promoter region and histone modification at the KSN locus following the 9 kb Copia insertion in the other allele. This suggests a novel mechanism for the production of the CF habit in rose plants.
Supplementary information
Acknowledgements
This work was supported by the National Key R&D Program of China (2019YFD1000400), the NSFC (31972449) and the NSFC-XINJIANG joint foundation (U1803102), which provided grants to Changquan Wang; the NSFC (31801890), which provided a grant to Jinyi Liu; and the Postgraduate Research & Practice Innovation Program of Jiangsu Province, which provided a grant to Mengjuan Bai.
Author contributions
Mengjuan Bai and Jinyi Liu conceived and designed the experiments; Chunguo Fan, Yeqing Chen, Hui Chen, Jun Lu, and Jingjing Sun performed parts of the experiments; and Guogui Ning and Changquan Wang wrote the manuscript.
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
These authors contributed equally: Mengjuan Bai, Jinyi Liu
Supplementary information
Supplementary Information accompanies this paper at (10.1038/s41438-021-00464-8).
References
- 1.Hurst C. Notes on the origin and evolution of our garden roses. J. R. Hortic. Soc. 1941;66:282–289. [Google Scholar]
- 2.Martínez MC, Santiago JL. Narcea-an unknown, ancient cultivated rose variety from northern Spain. Hortic. Res. 2020;7:44. doi: 10.1038/s41438-020-0266-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ng TB, He JS, Niu SM, Zhao L, Liu F. A gallic acid derivative and polysaccharides with antioxidative activity from rose (Rosa rugosa) flowers. J. Pharm. Pharm. 2004;56:537–545. doi: 10.1211/0022357022944. [DOI] [PubMed] [Google Scholar]
- 4.Ueyama Y, Hashimoto S, Nii H, Furukawa K. The essential oil from the flowers of Rosa rugosa Thunb. var. plena Regel. Flavour Frag. J. 2010;5:219–222. doi: 10.1002/ffj.2730050406. [DOI] [Google Scholar]
- 5.Srikanth A, Schmid M. Regulation of flowering time: all roads lead to Rome. Cell Mol. Life. Sci. 2011;68:2013–2037. doi: 10.1007/s00018-011-0673-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bendahmane M, Dubois A, Raymond O, Bris ML. Genetics and genomics of flower initiation and development in roses. J. Exp. Bot. 2013;64:847–857. doi: 10.1093/jxb/ers387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kurokura T, Mimida N, Battey NH, Hytönen T. The regulation of seasonal flowering in the Rosaceae. J. Exp. Bot. 2013;64:4131–4141. doi: 10.1093/jxb/ert233. [DOI] [PubMed] [Google Scholar]
- 8.Randoux M, et al. Gibberellins regulate the transcription of the continuous flowering regulator, RoKSN, a rose TFL1 homologue. J. Exp. Bot. 2012;63:6543–6554. doi: 10.1093/jxb/ers310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Iwata H, et al. The TFL1 homologue KSN is a regulator of continuous flowering in rose and strawberry. Plant J. 2012;69:116–125. doi: 10.1111/j.1365-313X.2011.04776.x. [DOI] [PubMed] [Google Scholar]
- 10.Saint-Oyant LH, et al. A high-quality genome sequence of Rosa chinensis to elucidate ornamental traits. Nat. Plants. 2018;4:473–484. doi: 10.1038/s41477-018-0166-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Horibe T, Yamada K, Otagaki S, Matsumoto S, Kawamura K. Molecular genetic studies on continuous-flowering roses that do not originate from Rosa Chinensis. Acta Hortic. 2015;1064:185–192. doi: 10.17660/ActaHortic.2015.1064.21. [DOI] [Google Scholar]
- 12.Araou, E. Diversité génétique et origine de la remontée de floraison chez le rosier. Rapport de Stage, UMR GenHort, Centre INRA d’Angers-Nantes, France (2011).
- 13.Shannon S, Meeks-Wagner DR. A mutation in the Arabidopsis TFL1 gene affects inflorescence meristem development. Plant Cell. 1991;3:877–892. doi: 10.2307/3869152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Parcy F, Bomblies K, Weigel D. Interaction of LEAFY, AGAMOUS and TERMINAL FLOWER1 in maintaining floral meristem identity in Arabidopsis. Development. 2002;129:2519–2527. doi: 10.1242/dev.129.10.2519. [DOI] [PubMed] [Google Scholar]
- 15.Bradley D, Ratcliffe O, Vincent C, Carpenter R, Coen E. Inflorescence commitment and architecture in Arabidopsis. Science. 1997;275:80–83. doi: 10.1126/science.275.5296.80. [DOI] [PubMed] [Google Scholar]
- 16.Alvarez J, Guli CL, Yu XH, Smyth DR. TERMINAL FLOWER: a gene affecting inflorescence development in Arabidopsis thaliana. Plant J. 2010;2:103–116. doi: 10.1111/j.1365-313X.1992.00103.x. [DOI] [Google Scholar]
- 17.Koskela EA, et al. Mutation in TERMINAL FLOWER1 reverses the photoperiodic requirement for flowering in the wild strawberry Fragaria vesca. Plant Physiol. 2012;159:1043–1054. doi: 10.1104/pp.112.196659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Flachowsky H, et al. The MdTFL1 gene of apple (Malus× domestica Borkh.) reduces vegetative growth and generation time. Tree Physiol. 2012;32:1288–1301. doi: 10.1093/treephys/tps080. [DOI] [PubMed] [Google Scholar]
- 19.Naozumi M, et al. Four TFL1/CEN-like genes on distinct linkage groups show different expression patterns to regulate vegetative and reproductive development in apple (Malus x domestica Borkh.) Plant Cell Physiol. 2009;50:394–412. doi: 10.1093/pcp/pcp001. [DOI] [PubMed] [Google Scholar]
- 20.Freiman A, et al. Development of a transgenic early flowering pear (Pyrus communis L.) genotype by RNAi silencing of PcTFL1-1 and PcTFL1-2. Planta. 2012;235:1239–1251. doi: 10.1007/s00425-011-1571-0. [DOI] [PubMed] [Google Scholar]
- 21.Mohamed R, et al. Populus CEN/TFL1 regulates first onset of flowering, axillary meristem identity and dormancy release in Populus. Plant J. 2010;4:674–688. doi: 10.1111/j.1365-313X.2010.04185.x. [DOI] [PubMed] [Google Scholar]
- 22.Wang R, et al. Aa TFL1 confers an age-dependent response to vernalization in perennial Arabis alpina. Plant Cell. 2011;4:1307–1321. doi: 10.1105/tpc.111.083451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sun Fanxia. A new member of the rose family-Purple bruch. Rural Practical Science and Technology Information. 2007;1:16. [Google Scholar]
- 24.Gang Ding, Xianshui Meng, Guizhi Dong. Purple bruch and its bud variety. China Flower Bonsai. 2007;12:14–15. [Google Scholar]
- 25.Xu Q, Wen X, Deng X. A simple protocol for isolating genomic DNA from chestnut rose (Rosa roxburghii tratt) for RFLP and PCR analyses. Plant Mol. Biol. Rep. 2004;22:301–302. doi: 10.1007/BF02773140. [DOI] [Google Scholar]
- 26.Liu J, et al. MIKC C-type MADS-box genes in Rosa chinensis: the remarkable expansion of ABCDE model genes and their roles in floral organogenesis. Hortic. Res. 2018;5:1–15. doi: 10.1038/s41438-017-0012-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu X, et al. AGAMOUS terminates floral stem cell maintenance in Arabidopsis by directly repressing WUSCHEL through recruitment of Polycomb Group proteins. Plant Cell. 2011;23:3654–3670. doi: 10.1105/tpc.111.091538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lu J, Bai M, Ren H, Liu J, Wang C. An efficient transient expression system for gene function analysis in rose. Plant Methods. 2017;13:116. doi: 10.1186/s13007-017-0268-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Iwata H, Gaston AL, Remay A, Thouroude T, Jeauffre J. The TFL1 homologue KSN is a regulator of continuous flowering in rose and strawberry. Plant J. 2011;69:116–125. doi: 10.1111/j.1365-313X.2011.04776.x. [DOI] [PubMed] [Google Scholar]
- 30.Carmona MJ, Calonje M, Martínez-Zapater JM. The FT/TFL1 gene family in grapevine. Plant Mol. Biol. 2007;63:637–650. doi: 10.1007/s11103-006-9113-z. [DOI] [PubMed] [Google Scholar]
- 31.Guo X, Zhao Z, Chen J, Hu X, Luo D. A putative CENTRORADIALIS/TERMINAL FLOWER 1-like gene, Ljcen1, plays a role in phase transition in Lotus japonicus. J. Plant Physiol. 2006;163:436–444. doi: 10.1016/j.jplph.2005.04.037. [DOI] [PubMed] [Google Scholar]
- 32.Randoux M, et al. RoKSN, a floral repressor, forms protein complexes with RoFD and RoFT to regulate vegetative and reproductive development in rose. N. Phytol. 2014;202:161–173. doi: 10.1111/nph.12625. [DOI] [PubMed] [Google Scholar]
- 33.Cao D, et al. Genome-wide identification of cytosine-5 DNA methyltransferases and demethylases in Solanum lycopersicum. Gene. 2014;550:230–237. doi: 10.1016/j.gene.2014.08.034. [DOI] [PubMed] [Google Scholar]
- 34.Lindroth AM, et al. Requirement of CHROMOMETHYLASE3 for maintenance of CpXpG methylation. Science. 2001;292:2077–2080. doi: 10.1126/science.1059745. [DOI] [PubMed] [Google Scholar]
- 35.Cao X, et al. Role of the DRM and CMT3 methyltransferases in RNA-directed DNA methylation. Curr. Biol. 2003;13:2212–2217. doi: 10.1016/j.cub.2003.11.052. [DOI] [PubMed] [Google Scholar]
- 36.Jenuwein T, Allis CD. Translating the histone code. Science. 2001;293:1074–1080. doi: 10.1126/science.1063127. [DOI] [PubMed] [Google Scholar]
- 37.Huang Y, Chen D, Liu C, Shen W, Ruan Y. Evolution and conservation of JmjC domain proteins in the green lineage. Mol. Genet Genomics. 2016;291:33–49. doi: 10.1007/s00438-015-1089-4. [DOI] [PubMed] [Google Scholar]
- 38.Lu F, Cui X, Zhang S, Jenuwein T, Cao X. Arabidopsis REF6 is a histone H3 lysine 27 demethylase. Nat. Genet. 2011;43:715–719. doi: 10.1038/ng.854. [DOI] [PubMed] [Google Scholar]
- 39.Dong Y, Lu J, Liu J, Jalal A, Wang C. Genome-wide identification and functional analysis of JmjC domain-containing genes in flower development of Rosa chinensis. Plant Mol. Biol. 2020;102:417–430. doi: 10.1007/s11103-019-00955-2. [DOI] [PubMed] [Google Scholar]
- 40.Thorstensen T, Grini PE, Aalen RB. SET domain proteins in plant development. Biochim. Biophys. Acta. 2011;1809:407–420. doi: 10.1016/j.bbagrm.2011.05.008. [DOI] [PubMed] [Google Scholar]
- 41.Krichevsky A, Kozlovsky SV, Gutgarts H, Citovsky V. Arabidopsis co-repressor complexes containing polyamine oxidase-like proteins and plant-specific histone methyltransferases. Plant Signal. Behav. 2007;2:174–177. doi: 10.4161/psb.2.3.3726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Krichevsky A, et al. C2H2 zinc finger-SET histone methyltransferase is a plant-specific chromatin modifier. Dev. Biol. 2007;303:259–269. doi: 10.1016/j.ydbio.2006.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lin MK, et al. FLOWERING LOCUS T protein may act as the long-distance florigenic signal in the cucurbits. Plant Cell. 2007;19:1488–1506. doi: 10.1105/tpc.107.051920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tamaki S, Matsuo S, Wong HL, Yokoi S, Shimamoto K. Hd3a protein is a mobile flowering signal in rice. Science. 2007;316:1033–1036. doi: 10.1126/science.1141753. [DOI] [PubMed] [Google Scholar]
- 45.Fornara F, de Montaigu A, Coupland G. SnapShot: control of flowering in Arabidopsis. Cell. 2010;141:550.e1–550.e2. doi: 10.1016/j.cell.2010.04.024. [DOI] [PubMed] [Google Scholar]
- 46.Srikanth A, Schmid M. Regulation of flowering time: all roads lead to Rome. Cell Mol. Life Sci. 2011;68:2013–2037. doi: 10.1007/s00018-011-0673-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Karlgren A, et al. Evolution of the PEBP gene family in plants: functional diversification in seed plant evolution. Plant Physiol. 2011;156:1967–1977. doi: 10.1104/pp.111.176206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hanano S, Goto K. Arabidopsis TERMINAL FLOWER1 is involved in the regulation of flowering time and inflorescence development through transcriptional repression. Plant Cell. 2011;3:3172–3184. doi: 10.1105/tpc.111.088641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang LN, Liu YF, Zhang YM, Fang RX, Liu QL. The expression level of Rosa Terminal Flower 1 (RTFL1) is related with recurrent flowering in roses. Mol. Biol. Rep. 2011;39:3737–3746. doi: 10.1007/s11033-011-1149-8. [DOI] [PubMed] [Google Scholar]
- 50.Lippman Z, et al. Role of transposable elements in heterochromatin and epigenetic control. Nature. 2004;430:471–476. doi: 10.1038/nature02651. [DOI] [PubMed] [Google Scholar]
- 51.Mirouze M, et al. Selective epigenetic control of retrotransposition in Arabidopsis. Nature. 2009;461:427–430. doi: 10.1038/nature08328. [DOI] [PubMed] [Google Scholar]
- 52.Tsukahara S, et al. Bursts of retrotransposition reproduced in Arabidopsis. Nature. 2009;461:423–426. doi: 10.1038/nature08351. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.