Abstract
Solanum lycopersicum var. cerasiforme accession PI 114490 has broad-spectrum resistance to bacterial spot caused by several species of Xanthomonas. Resistance is quantitatively inherited, and a common quantitative trait locus QTL-11B on chromosome 11 has been identified previously. In this study, the SlPub24 gene was characterized in QTL-11B. SlPub24 in PI 114490 was upregulated by infection with X. euvesicatoria pv. perforans race T3, but its transcription was low in the susceptible line OH 88119 whether or not it was infected by the pathogen. The differential expression of SlPub24 between PI 114490 and OH 88119 was due to great sequence variation in the promoter region. The promoter of SlPub24 in OH 88119 had very low activity and did not respond to pathogen infection. Transgenic lines of OH 88119 overexpressing SlPub24 isolated from PI 114490 showed significantly enhanced resistance, while mutants of Slpub24 generated by CRISPR/Cas9 editing showed more susceptibility to race T3 and to other races. The mutants also showed spontaneous cell death in leaves. The expression of the salicylic acid (SA) pathway gene phenylalanine ammonia-lyase (PAL) and signaling-related genes pathogenesis-related (PR1) and nonexpresser of PR1 (NPR1) were influenced by SlPub24. The content of SA in tomato plants was consistent with the level of SlPub24 expression. Furthermore, SlPUB24 interacted with the cell wall protein SlCWP and could regulate the degradation of SlCWP. The expression levels of SlCWP and SlCWINV1, a cell wall invertase gene, showed opposite patterns during pathogen infection. The activity of SlCWINV1 was lower in mutants than in PI 114490. The results are discussed in terms of the roles of the abovementioned genes, and a potential model for SlPUB24-mediated resistance to bacterial spot is proposed.
Subject terms: Plant molecular biology, Biotic
Introduction
Bacterial spot caused by Xanthomonas euvesicatoria pv. euvesicatoria (race T1), X. vesicatoria (race T2), X. euvesicatoria pv. perforans (races T3 and T4), and X. cynarae pv. gardneri is a widespread disease in tomato production1–3. The disease can cause severe yield loss and fruit quality reduction in tomato4,5. Although the use of resistant varieties is the most effective approach for control of the disease, the existence of multiple species of Xanthomonas and quick shifts of species/races in the same region are among the most important causes of unsuccessful management of the disease4–6. Therefore, sources with more durable and broad-spectrum resistance to the disease are desirable for developing new cultivars.
Several studies have indicated that Solanum lycopersicum var. cerasiforme accession PI 114490 may provide broad-spectrum resistance to all species and races of Xanthomonas causing bacterial spot in tomato7–9. The resistance to races T1–T4 and X. cynarae pv. gardneri in PI 114490 is quantitatively inherited, and several quantitative trait loci (QTLs) have been reported7–13. Classical genetic analyses based on segregation of resistance in F2 and inbred backcross (IBC) populations derived from PI 114490 suggest that its resistance to race T2 is conditioned by two to four loci8. The high correlation between race T1 and race T2 resistance in the IBC population suggests that there are common loci for resistance to both races, while the poor correlation of resistance between races T2 and T3 indicates that resistance to all species and races is not controlled by the same genes in PI 1144908. However, a common locus on chromosome 11 conferring resistance to races T2, T3, and T4 has been identified in the same IBC population13. The common locus conferring resistance to races T3 and T4 has also been confirmed in later studies using the same IBC population and other segregating populations10,12. Recent studies indicate that the common locus on chromosome 11, designated QTL-11B, confers resistance to races T1–T4 and X. cynarae pv. gardneri7,9. All these data suggest that QTL-11B in PI 114490 confers resistance to all races and species. Previous studies have shown that the Solyc11g068940 gene, encoding a plant U-box protein (designated SlPUB24 in this study), in the QTL-11B region of PI 114490 is highly induced by the presence of the race T3 strain14,15, suggesting that it may participate in resistance to race T3.
E3 ubiquitin (Ub) ligases are key regulators in plants for defense during both PAMP-triggered immunity (PTI) and effector-triggered immunity (ETI). Their regulation can be either positive or negative depending on the plant-pathogen system and usually involves the production of hydrogen peroxide (H2O2) and salicylic acid (SA). SPL11 in rice, the first characterized U-box E3 ligase, acts as a negative regulator of plant programmed cell death (PCD) and pathogenic defense. The spl11 mutant shows spontaneous cell death in leaves and confers enhanced resistance to rice blast and bacterial blight16,17. PUB13, the closest ortholog of SPL11 in Arabidopsis, negatively regulates resistance to the biotrophic pathogens Pseudomonas syringae pv. maculicola and Erysiphe cichoracearum but positively regulates resistance to the necrotrophic fungal pathogens Botrytis cinerea and Alternaria brassicicola. The spontaneous cell death and elevated H2O2 accumulation in the pub13 mutant depend on the SA signal18,19. PUB22, PUB23, and PUB24 in Arabidopsis act as negative regulators of PTI in response to several distinct PAMPs. The triple mutant pub22/pub23/pub24 exhibits enhanced resistance to diverse pathogens, accompanied by oxidative burst and plant cell death20,21. PUB17, a U-box ARM repeat E3 ligase conserved in Arabidopsis, Nicotiana benthamiana, tomato and potato22,23, is a positive regulator of cell death and plant disease resistance. Another conserved class of U-box E3 ligases, including CMPG1 in Petroselinum crispum24, PUB20 and PUB21 in A. thaliana25, CMPG1-V in Haynaldia villosa26, NtCMPG1 in N. tabacum, and SlCmpg1 in tomato27, also act as positive regulators of plant disease resistance. The pepper E3 ubiquitin ligase CaRING1 is a positive regulator of resistance and is required for cell death and the SA-dependent defense response to hemibiotrophic Pseudomonas syringae pv. tomato and biotrophic Hyaloperonospora arabidopsidis infections28. In apple fruits, two ubiquitin E3 ligases regulate the immune response with opposing functions. The U-box E3 ligase MdPUB29 is a positive regulator of the defense response to the fungal pathogen Botryosphaeria dothidea, possibly regulating the SA pathway29, while the BTB-BACK domain E3 ligase MdPOB1 ubiquitinates and degrades MdPUB29, resulting in suppression of defense against B. dothidea30.
Here, we reported that U-box E3 ligase protein 24 (SlPUB24) acted as a positive regulator of resistance to bacterial spot in tomato. The knockout mutants also exhibited spontaneous cell death in leaves. The increase in plant pathogen defense was correlated with the SA biosynthesis pathway and signaling. In addition, SlPUB24 targeted the potential cell wall protein SlCWP. A model is proposed to provide additional understanding of the U-box-mediated response to disease.
Results
Sequence variation in SlPub24 between PI 114490 and OH 88119
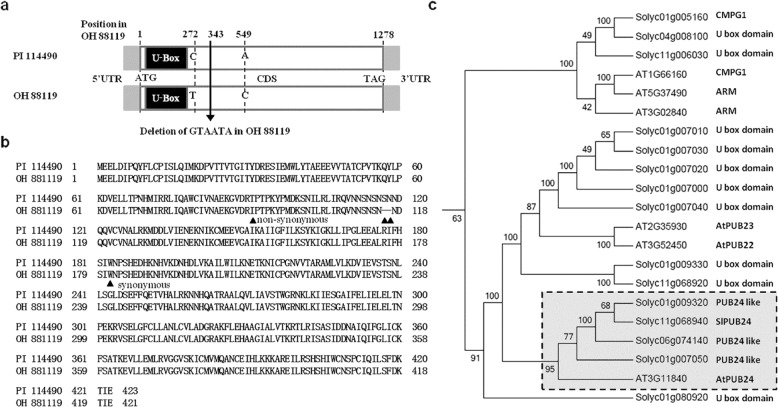
The genomic DNA sequence of the SlPub24 gene was obtained by PCR amplification using gene-specific primers (Table S1). It was 1278 bp in the resistant line PI 114490 and 1272 bp in the susceptible line OH 88119. Full-length cDNA was obtained by RT-PCR and RACE. A 1457-bp fragment and a 1451-bp fragment were obtained for PI 114490 and OH 88119, respectively. Alignment of genomic DNA and cDNA sequences revealed that there was no intron in the gene. There were two single nucleotide polymorphisms (SNPs) and one 6-bp (GTAATA) insertion/deletion (InDel) in the coding region (Fig. 1a) and no sequence variation in the 5′UTR (86 bp) or 3′UTR (93 bp) between PI 114490 and OH 88119. A comparison of deduced amino acid sequences showed that the 6-bp InDel resulted in two amino acid losses in OH 88119, while C272T was a nonsynonymous substitution (T91I), and A549C was a synonymous substitution (Fig. 1b). A U-box domain at the N-terminus of the deduced protein sequence between amino acids 9 and 79 (25–237 bp in cDNA sequence, Fig. 1a) was predicted with SMART (http://smart.embl-heidelberg.de/). Phylogenetic analysis indicated that the deduced protein was closely related to AtPUB24 in Arabidopsis (Fig. 1c), which confirmed the nomenclature of SlPUB24. A BLAST search of SlPUB24 in the tomato genome ITAG release 4.0 (https://solgenomics.net/) identified four genes annotated as U-box domain-containing protein 24 (Fig. 1c), but their coding sequences were quite different (Fig. S1).
Fig. 1. Comparison of SlPub24 sequences between the resistant line PI 114490 and the susceptible line OH 88119, as well as phylogenetic analysis of SlPUB24.
a Diagram shows differences in coding sequences of SlPub24 between PI 114490 and OH 88119. b Alignment of deduced amino acid sequences of SlPUB24 between PI 114490 and OH 88119. ▲ indicates the amino acid substitution or insertion/deletion positions. c Phylogenetic analysis of SlPUB24 with various U-box type E3 ubiquitin ligases in Solanum lycopersicum and Arabidopsis thaliana
SlPUB24 is a ubiquitous protein
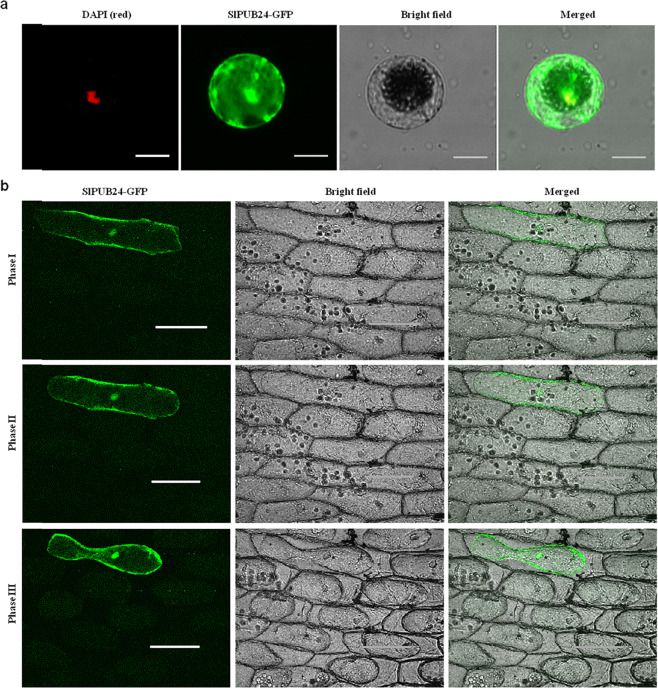
The subcellular localization of the SlPUB24 protein was determined by Agrobacterium-mediated transient expression of the SlPub24 gene in tomato protoplast and onion epidermal cells. The SlPUB24-GFP fusion protein was detected in the cytoplasm, plasma membrane, and nucleus in tomato cells (Fig. 2a), and this localization was confirmed by observation of the SlPUB24-GFP fusion protein during plasmolysis of onion epidermal cells (Fig. 2b). These data suggested that SlPUB24 is a ubiquitous protein.
Fig. 2. Subcellular localization of SlPUB24.
a Subcellular localization of SlPUB24-GFP in tomato protoplasts isolated from tomato line PI 114490. b Dynamic observation of subcellular localization of SlPUB24-GFP in onion epidermal cells infiltrated with 0.3g/ml sucrose solution. Scale bars = 200 μm
Transgenic overexpression of SlPub24 in OH 88119 enhances resistance to X. euvesicatoria pv. perforans race T3
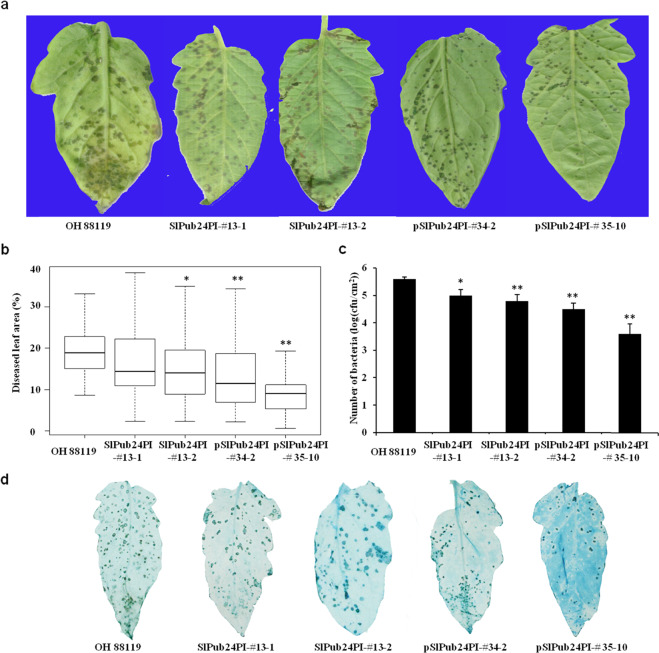
To determine the role of the SlPub24 gene in PI 114490 in resistance to X. euvesicatoria pv. perforans race T3 strain Xv829, two constructs, SlPub24PI (Fig. S2a) and pSlPub24PI (Fig. S2b), were developed for overexpression of the gene in the susceptible tomato line OH 88119. A total of 12 independent transgenic lines for the construct SlPub24PI and 4 independent transgenic lines for the construct pSlPub24PI were obtained. Four transgenic lines, SlPub24PI-#13-1, SlPub24PI-#13-2, pSlPUB24PI-#34-2, and pSlPub24PI-#35-10, were used in the following experiments. All transgenic plants had fewer disease lesions on leaves than OH 88119 plants at 9 days post inoculation (dpi) (Fig. 3a). The percentage of diseased leaf area was significantly (P < 0.05) lower in the three transgenic lines SlPub24PI-#13-2, pSlPub24PI-#34-2, and pSlPub24PI-#35-10 than in OH 88119 (Fig. 3b), and the bacterial populations were significantly (P < 0.05) smaller in all transgenic lines than in OH 88119 (Fig. 3c). The leaves of transgenic plants had less necrotic tissue than OH 88119 (Fig. 3d). The transgenic lines carrying the SlPub24 gene with its native promoter from PI 114490 (construct pSlPub24PI) were more resistant than the transgenic lines carrying only SlPub24 from PI 114490 (construct SlPub24PI), suggesting that the native promoter and UTRs might enhance the resistance.
Fig. 3. Transgenic overexpression of SlPub24 in the susceptible tomato line OH 88119 enhances resistance to Xanthomonas euvesicatoria pv. perforans race T3 strain Xv829.
a Symptoms in leaves of OH 88119 and transgenic plants overexpressing SlPub24 isolated from PI 114490 at 7 days post inoculation (dpi). b Statistical analysis of disease in OH 88119 and transgenic lines at 7 dpi. Error bars represent the SD (n = 30). c Bacterial populations in leaves of OH 88119 and transgenic lines at 9 dpi. Error bars represent the SD (n = 30). d Trypan blue staining of diseased leaves of OH 88119 and transgenic lines (n = 30). The asterisks indicate statistical significance by t test (*P < 0.05, **P < 0.01)
Mutation of SlPub24 in PI 114490 by CRISPR/Cas9 editing increases susceptibility to X. euvesicatoria pv. perforans race T3 and induces spontaneous cell death in leaves
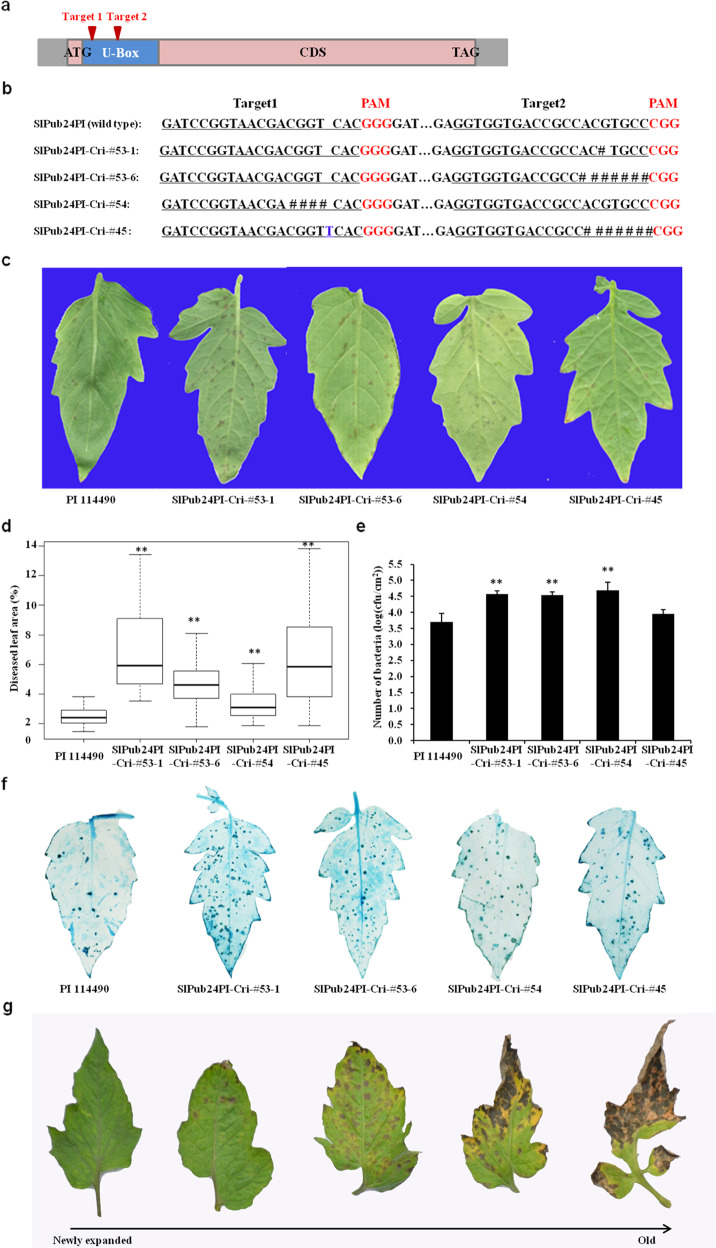
The SlPub24 gene was mutated in PI 114490 at two target sites (Fig. 4a) using the CRISPR/Cas9 editing system to further validate the role of SlPub24 in resistance to X. euvesicatoria pv. perforans race T3. Three mutated lines (SlPub24PI-Cri-#53, SlPub24PI-Cri-#54, and SlPub24PI-Cri-#45) were obtained. The line SlPub24PI-Cri-#53 had two types of mutations in the target 2 region in T1 progenies: a 1-bp deletion in line SlPub24PI-Cri-#53-1 and a 7-bp deletion in line SlPub24PI-Cri-#53-6. Line SlPub24PI-Cri-#54 had a 4-bp deletion in the target 1 region, and line SlPub24PI-Cri-#45 had a T insertion in the target 1 region and a 7-bp deletion in the target 2 region (Fig. 4b). Leaves of mutants showed more disease lesions than those of PI 114490 (Fig. 4c). The percentage of diseased leaf area (Fig. 4d) and bacterial populations in leaves (Fig. 4e) were significantly (p < 0.01) higher in mutants than in PI 114490 at 7 dpi and 9 dpi, respectively. Mutants had more necrotic tissue in leaves than did PI 114490 (Fig. 4f). These results indicated that SlPub24 contributed to resistance to race T3.
Fig. 4. Knockout of SlPub24 in the resistant line PI 114490 by the CRISPR/Cas9 editing system leads to decreased resistance to Xanthomonaseuvesicatoria pv. perforans race T3 strain Xv829 and spontaneous cell death in leaves in mutants.
a Schematic illustration of the two sgRNA target sites (red arrows) in SlPub24. b Mutations identified in four T2 mutant lines. Red font indicates protospacer-adjacent motif (PAM) sequences, and the sgRNA target sequence is underlined. c Symptoms on PI 114490 and mutant leaves at 7 days post inoculation (dpi). d Statistical analysis of disease in PI 114490 and mutants at 7 dpi. Error bars represent the SD (n = 30). e Bacterial population in leaves of PI 114490 and mutants at 9 dpi. Error bars represent the SD (n = 30). f Trypan blue staining of diseased PI 114490 and mutant leaves (n = 30). The asterisks indicate statistical significance by t test (**P < 0.01). g Spontaneous cell death on leaves of mutants at various stages, from newly expanded to old leaves
Spontaneous cell death in leaves was also observed in SlPub24-mutated plants that had not been inoculated with Xv829. Newly expanded leaves were normal, but areas of cell death were observed several days after expansion (Fig. 4g), and the leaves eventually became completely withered.
Differential expression of SlPub24 in PI 114490 and OH 88119 during disease development is caused by promoter sequence variation
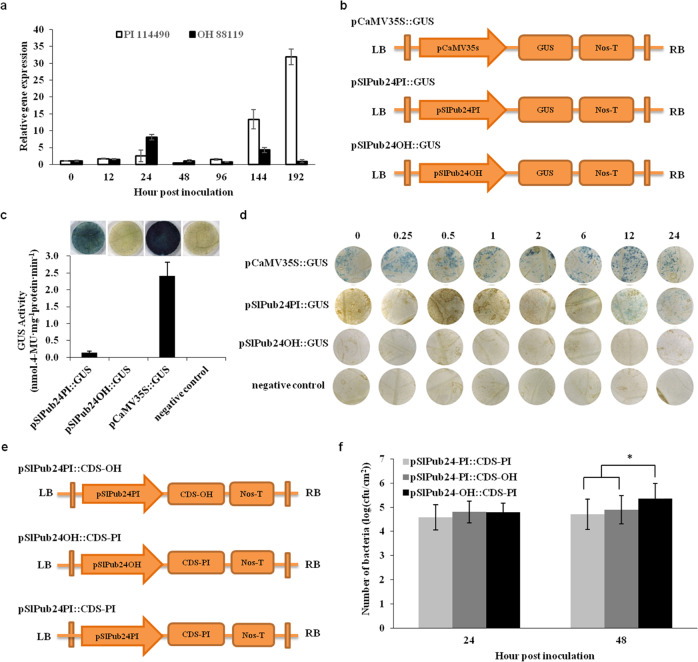
Differential expression of SlPub24 was observed in PI 114490 and OH 88119 during infection by X. euvesicatoria pv. perforans race T3 strain Xv829. In PI 114490, the expression of SlPub24 was at a constant low level from 0 to 96 hpi and then dramatically increased, which was consistent with previous findings14,15. However, the expression of SlPub24 in OH 88119 remained at a low level from 0 to 192 hpi (Fig. 5a).
Fig. 5. Differential expression of SlPub24 in the resistant line PI 114490 and the susceptible line OH 88119 during infection by Xanthomonas euvesicatoria pv. perforans race T3 strain Xv829 is caused by sequence variation in the promoter.
a Expression of SlPub24 in PI 114490 and OH 88119 at various time points after spray inoculation with Xv829. b Schematic diagram of the vector construct. pSlPub24PI::GUS: The SlPub24 promoter isolated from PI 114490 was fused with the GUS reporter. pSlPub24OH::GUS: The SlPub24 promoter isolated from OH 88119 was fused with the GUS reporter. GUS β-glucuronidase, LB left border, NOS-T Nos terminator, RB right border. Agrobacterium tumefaciens GV3101 was used as a negative control, and pCaMV35S::GUS was used as a positive control. c GUS activity in leaves of Nicotiana benthamiana transiently expressing the pSlPub24PI::GUS or pSlPub24OH::GUS constructs. Error bars represent the SD (n = 6). d GUS activity in tomato leaves transiently expressing the pSlPUB24OH::GUS and pSlPUB24PI::GUS constructs at various hours after spray inoculation of Xv829. Agrobacterium strains carrying the constructs were infiltrated into tomato leaves at 48 h before inoculation with Xv829. e Schematic diagram of the vector construct. SlPub24PI::CDS-PI: the SlPup24 coding sequence (CDS) from PI 114490 driven by promoter from PI 114490; SlPub24PI::CDS-OH: the SlPub24 CDS from OH 88119 driven by promoter from PI 114490; SlPub24OH::CDS-PI: the SlPub24 CDS from PI 114490 driven by promoter from OH 88119. f Bacterial population in leaves of OH 88119 plants transiently expressing constructs SlPub24PI::CDS-PI, SlPub24PI::CDS-OH, and SlPub24OH::CDS-PI. Error bars represent the SD (n = 6). The asterisks indicate statistical significance by t test (*P < 0.05)
To investigate why the expression of SlPub24 showed different patterns in PI 114490 and OH 88119, approximately 2.4 kb promoter sequences were obtained from the genomic DNA of these two lines. Substantial sequence variation in the promoter region of SlPub24 was observed between PI 114490 and OH 88119. There were two large InDels, seven small InDels, and 16 SNPs between the two promoter sequences (Table S2). Both promoters were separately fused with GUS (Fig. 5b) to determine their activities. The GUS activity assay in tobacco showed that the promoter activity of SlPub24 from OH 88119 was lower than that of SlPub24 from PI 114490 (Fig. 5c). GUS activity of the construct driven by the promoter isolated from PI 114490 was detected in leaves of OH 88119 at 12 hpi with Xv829, but no GUS activity of the construct driven by the promoter isolated from OH 88119 was detected in leaves of PI 114490 (Fig. 5d). These results suggested that the sequence difference in the promoter region might cause a difference in the activities of the two promoters, resulting in different expression patterns of SlPub24 in response to infection with Xv829 in PI 114490 and OH 88119.
Further comparison of promoter activities was performed by switching the promoters and coding sequences (CDSs) of the SlPub24 gene originating from PI 114490 and OH 88119. Three constructs, pSlPub24PI::CDS-OH, pSlPub24OH::CDS-PI, and pSlPub24PI::CDS-PI (Fig. 5e), were developed to perform transient transformation experiments. The number of bacteria was significantly (P < 0.05) lower when SlPub24 was driven by the promoter from PI 114490 than when it was driven by the promoter from OH 88119 at 48 hpi (Fig. 5f). These results suggested that the SlPub24 gene could confer resistance to X. euvesicatoria pv. perforans race T3 and that the differential expression of SlPub24 during pathogen infection in PI 114490 and OH 88119 might be due to sequence variation in the SlPub24 promoter region.
SlPub24 is associated with resistance to race T3 in the inbred backcross population derived from PI 114490 and other germplasms
The 6-bp InDel in the CDS between PI 114490 and OH 88119 was used as a marker (Table S1) to genotype individual lines of an inbred backcross (IBC) population derived from PI 1144908,12. The responses of each line in the IBC population to race T3 were the same as those in our previous publication12. Single marker-trait association analysis indicated that the marker was significantly (p = 0.0056) associated with resistance to race T3 in the population. The mean disease severity was 3.9 (1–12 scale)13 for lines carrying the PI 114490 allele and 6.4 for lines with the OH 9242 or Fla 7600 allele. The marker explained 12.3% of the phenotypic variation in resistance to race T3 in the population.
Of the 192 tomato lines31 genotyped with markers to detect SlPub24 and Rx4, including PI 114490 and OH 88119, 4 had only the SlPub24 gene, 3 had only the Rx4 gene, and 11 had both the SlPub24 and Rx4 genes (Table 1). Interestingly, nine lines showed evidence of chromosomal crossover events in this region. Seven lines had the PI 114490 marker genotype in the CDS and 5′UTR of SlPub24 but the OH 88119 marker genotype in the promoter region. Two near-isogenic lines (NILs) derived from Hawaii 7998, FG16-804 and FG16-813, had the same genotype as the donor. Line LA1269 had the OH 88119 marker genotype in the CDS but PI 114490 marker genotypes at the promoter and 5′UTR regions, while line LA1218 had the OH 88119 marker genotype in the CDS and 5′UTR but the PI 114490 marker genotype at the promoter (Table 1). Twenty-six lines, including those with either the SlPub24 or Rx4 gene, 9 without both genes, and those with chromosomal crossovers, were subjected to disease evaluation by spray inoculation of race T3 strain Xv829. Lines carrying both the SlPub24 and Rx4 genes exhibited the lowest diseased leaf area (9.3%), followed by lines carrying only Rx4 (11.0%) from PI 128216 or SlPub24 (13.3%) from PI 114490 (Table 1). Lines having chromosomal crossover without either the promoter or CDS from PI 114490 exhibited the same level of susceptibility as lines without both SlPub24 and Rx4 genes. These data suggested that the promoter of SlPub24 from PI 114490 was critical for the function of SlPub24 in disease resistance and confirmed the results of switching the promoters and CDS of the SlPub24 gene originating from PI 114490 and OH 88119.
Table 1.
Marker genotype and response to Xanthomonas euvesicatoria pv. perforans race T3 strain Xv829 in tomato lines
| Germplasm | Marker genotype | Mean diseased leaf area (%) | |||
|---|---|---|---|---|---|
| SlPub24 | Rx4 | ||||
| 284-bp InDel in promoter | 198-bp InDel in 5′-UTR | 6-bp InDel in CDS | 6-bp InDel in CDS | ||
| Money maker | Slpub24Slpub24 | Slpub24Slpub24 | Slpub24Slpub24 | rx4rx4 | 22.2a |
| Liger 87-05 | Slpub24Slpub24 | Slpub24Slpub24 | Slpub24Slpub24 | rx4rx4 | |
| Zhongshu 6 | Slpub24Slpub24 | Slpub24Slpub24 | Slpub24Slpub24 | rx4rx4 | |
| Hunt 100 | Slpub24Slpub24 | Slpub24Slpub24 | Slpub24Slpub24 | rx4rx4 | |
| Ailsa Craig | Slpub24Slpub24 | Slpub24Slpub24 | Slpub24Slpub24 | rx4rx4 | |
| Heinz 1350 | Slpub24Slpub24 | Slpub24Slpub24 | Slpub24Slpub24 | rx4rx4 | |
| Heinz 1706 | Slpub24Slpub24 | Slpub24Slpub24 | Slpub24Slpub24 | rx4rx4 | |
| OH 88119 | Slpub24Slpub24 | Slpub24Slpub24 | Slpub24Slpub24 | rx4rx4 | |
| Rio Grande | Slpub24Slpub24 | Slpub24Slpub24 | Slpub24Slpub24 | rx4rx4 | |
| LA 1218 | SlPub24SlPub24 | Slpub24Slpub24 | Slpub24Slpub24 | rx4rx4 | not included |
| LA 1269 | SlPub24SlPub24 | SlPub24SlPub24 | Slpub24Slpub24 | rx4rx4 | 22.2a |
| LA 2181 | Slpub24Slpub24 | SlPub24SlPub24 | SlPub24SlPub24 | rx4rx4 | 21.2a |
| LA 0395 | Slpub24Slpub24 | SlPub24SlPub24 | SlPub24SlPub24 | rx4rx4 | |
| Ha 7998 | Slpub24Slpub24 | SlPub24SlPub24 | SlPub24SlPub24 | rx4rx4 | |
| FG16-804 | Slpub24Slpub24 | SlPub24SlPub24 | SlPub24SlPub24 | rx4rx4 | |
| LA 2283 | Slpub24Slpub24 | SlPub24SlPub24 | SlPub24SlPub24 | Rx4Rx4 | 15.6ab |
| Fla 8233 | Slpub24Slpub24 | SlPub24SlPub24 | SlPub24SlPub24 | Rx4Rx4 | |
| FG16-813 | Slpub24Slpub24 | SlPub24SlPub24 | SlPub24SlPub24 | Rx4Rx4 | |
| LA 0400 | Slpub24Slpub24 | SlPub24SlPub24 | SlPub24SlPub24 | Rx4Rx4 | not included |
| LA 2183 | Slpub24Slpub24 | SlPub24SlPub24 | SlPub24SlPub24 | Rx4Rx4 | not included |
| PI 114490 | SlPub24SlPub24 | SlPub24SlPub24 | SlPub24SlPub24 | rx4rx4 | 13.3b |
| FG16-802 | SlPub24SlPub24 | SlPub24SlPub24 | SlPub24SlPub24 | rx4rx4 | |
| Ha 7981 | Slpub24Slpub24 | Slpub24Slpub24 | Slpub24Slpub24 | Rx4Rx4 | 11.0b |
| TD-55C-h | Slpub24Slpub24 | Slpub24Slpub24 | Slpub24Slpub24 | Rx4Rx4 | |
| ZF084-1-h | Slpub24Slpub24 | Slpub24Slpub24 | Slpub24Slpub24 | Rx4Rx4 | |
| Black Cherry | SlPub24SlPub24 | SlPub24SlPub24 | SlPub24SlPub24 | Rx4Rx4 | 9.3b |
| LA 0373 | SlPub24SlPub24 | SlPub24SlPub24 | SlPub24SlPub24 | Rx4Rx4 | |
| PI 128216 | SlPub24SlPub24 | SlPub24SlPub24 | SlPub24SlPub24 | Rx4Rx4 | |
| Nongdazhenzhufanqie | SlPub24SlPub24 | SlPub24SlPub24 | SlPub24SlPub24 | Rx4Rx4 | |
| LA 0722 | SlPub24SlPub24 | SlPub24SlPub24 | SlPub24SlPub24 | Rx4Rx4 | not included |
| 11C336 | SlPub24SlPub24 | SlPub24SlPub24 | SlPub24SlPub24 | Rx4Rx4 | not included |
| 11C337 | SlPub24SlPub24 | SlPub24SlPub24 | SlPub24SlPub24 | Rx4Rx4 | not included |
Tomato germplasms with fewer than three plants available for disease evaluation were not included in the least significant difference comparison. Means of diseased leaf area followed by the same letter are not significantly different at P ≤ 0.05 based on Duncan’s multiple range test
SlPub24 also confers resistance to races T1, T2, and T4
Previous studies indicate that the locus QTL-11B from the resistant line PI 114490 confers resistance to races T1–T47,9. To check whether SlPub24 was also resistant to races T1, T2, and T4, PI 114490, OH 88119, and Slpub24 mutants and transgenic lines overexpressing SlPub24 were subjected to disease evaluation. Bacterial populations were significantly smaller in PI 114490 and transgenic lines than in OH 88119 and Slpub24 mutants (Fig. S3), suggesting that SlPub24 also conferred resistance to races T1, T2, and T4.
Expression of SA-related genes and SA content are affected by SlPub24
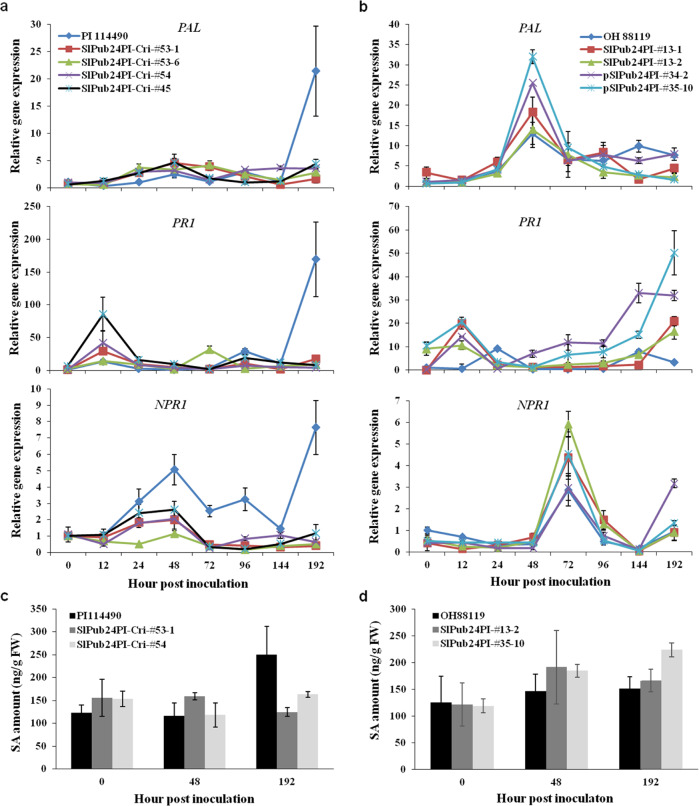
The expression of the SA synthesis-related gene phenylalanine ammonia-lyase (PAL) and the signaling-related genes pathogenesis-related (PR1) and nonexpresser of PR genes 1 (NPR1) in PI 114490, OH 88119, and transgenic plants was measured in this study. The expression levels of PAL, PR1, and NPR1 were low from 0 to 144 hpi and then dramatically increased from 144 to 192 hpi (Fig. 6a), similar to the expression pattern of SlPub24 in PI 114490 (Fig. 5a). However, they were expressed at very low levels in SlPub24-mutated lines from 0 to 192 hpi (Fig. 6a). In transgenic lines overexpressing SlPub24 isolated from PI 114490, the expression of PAL, PR1, and NPR1 was higher at 48, 192, and 72 hpi, respectively, than in OH 88119 (Fig. 6b). The content of SA was consistent with the expression levels of SlPub24, PAL, PR1, and NPR1. The amount of SA in PI 114490 increased at 192 hpi but remained at a constant level in SlPub24-mutated lines (Fig. 6c). Meanwhile, the content of SA was higher in transgenic lines overexpressing SlPub24 than in OH 88119 at 48 and 192 hpi (Fig. 6d). These results suggested that SlPUB24 might affect SA synthesis and signaling, which eventually influence resistance to bacterial spot.
Fig. 6. Relative expression of salicylic acid (SA)-related genes and accumulation of SA in tomato lines at various time points after spray inoculation of Xanthomonas euvesicatoria pv. perforans race T3 strain Xv829.
a Relative expression of PAL, PR1, and NPR1 in the resistant tomato line PI 114490 and SlPub24-mutated lines. b Relative expression of PAL, PR1, and NPR1 in the susceptible tomato line OH 88119 and transgenic lines overexpressing SlPub24 isolated from PI 114490. c SA content in PI 114490 and SlPub24-mutated lines at 0, 48, and 192 h post inoculation (hpi) with Xv829. d SA content in OH 88119 and transgenic lines overexpressing SlPub24 at 0, 48, and 192 hpi of Xv829. PAL phenylalanine ammonia-lyase gene, PR1 pathogenesis-related gene 1, NPR1 nonexpresser of PR gene 1. Error bars represent the SD (n = 3)
SlPUB24 interacts with and promotes degradation of SlCWP
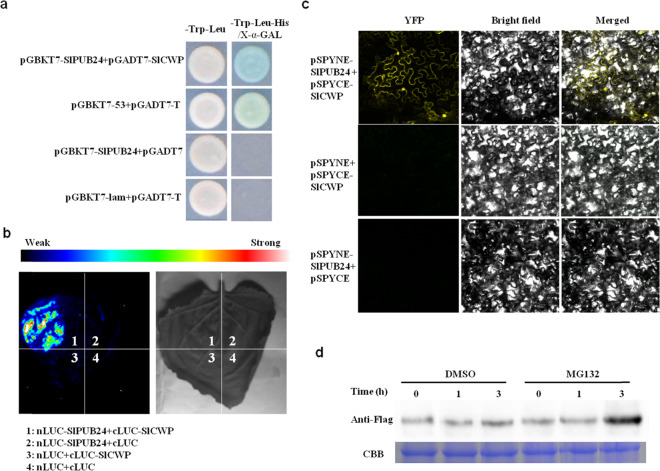
To gain insight into the regulation of SlPUB24, yeast two-hybrid (Y2H) assays were performed to identify proteins that might interact with SlPUB24. After multiple screenings using deficient solid medium, 246 positive colonies associated with 55 genes were obtained, and 48 genes were finally obtained by PCR amplification. Constructs of 29 genes were successfully developed for interaction validation using the full-length CDS, and six genes (Table 2) showed interactions with SlPUB24 in three independent Y2H experiments. One gene, Solyc02g085950 (designated SlCWP), represented by 21 colonies encoding the cell wall protein X77373, was selected for further investigation because it has been reported that cell wall proteins participate in disease defense32. The interaction between SlPUB24 and SlCWP detected by Y2H (Fig. 7a) was verified by bimolecular fluorescence complementation assay (BiFC) and split luciferase complementation assay (SLC) (Fig. 7b, c).
Table 2.
Information on proteins interacting with SlPUB24 in tomato.
| Gene ID | Function annotation |
|---|---|
| Solyc12g005630 | Cytochrome b6-f complex iron-sulfur subunit |
| Solyc03g034220 | Ribulose bisphosphate carboxylase small chain 2B |
| Solyc02g085950 | Cell wall protein X77373 |
| Solyc08g028690 | NAD(P)-binding Rossmann-fold superfamily protein |
| Solyc02g094120 | Sulfite oxidase |
| Solyc06g071050 | Hypersensitive-induced response protein |
Fig. 7. SlPUB24 interacts with SlCWP and regulates the degradation of SlCWP.
a Yeast-two-hybrid assay. The interactions between pGADT7 and pGBKT7-SlPUB24 and between pGADT7-T and pGBKT7-lam were used as negative controls, and the interaction between pGADT7-T and pGBKT7-53 was used as a positive control. b Luciferase complementation image assay. Fluorescence signal intensity represents the interaction of the two proteins. c Bimolecular fluorescence complementation assay. YFP fluorescence was detected by confocal microscope. d The protein levels of SlCWP-Flag in tobacco leaves at 0, 1, and 3 h after treatment with the proteasomal inhibitor MG132 (50 mM) or an equivalent volume of dimethylsulfoxide (DMSO, control) were determined by immunoblot analysis with the Flag antibody. The concentration of total protein was monitored by Coomassie brilliant blue (CBB) staining. Molecular weight of protein: SlCWP = 20.22 kDa
A previous study showed that proteins containing the U-box domain have ubiquitin ligase E3 activity that leads to protein degradation by the 26 S proteasome. MG-132 is a cell-permeable proteasome inhibitor and can block the proteolytic activity of the 26 S proteasome complex33. To further prove the relationship between SlPUB24 and SlCWP, SlPUB24-Myc and SlCWP-Flag were cloned into the vector, and an Agrobacterium-mediated transient expression assay was implemented in N. benthamiana leaves. Immunoblot analysis indicated that SlCWP gradually accumulated and was not degraded by SlPUB24 in tobacco leaves treated with MG132 (Fig. 7d), which indicated that SlPUB24 might promote the degradation of SlCWP.
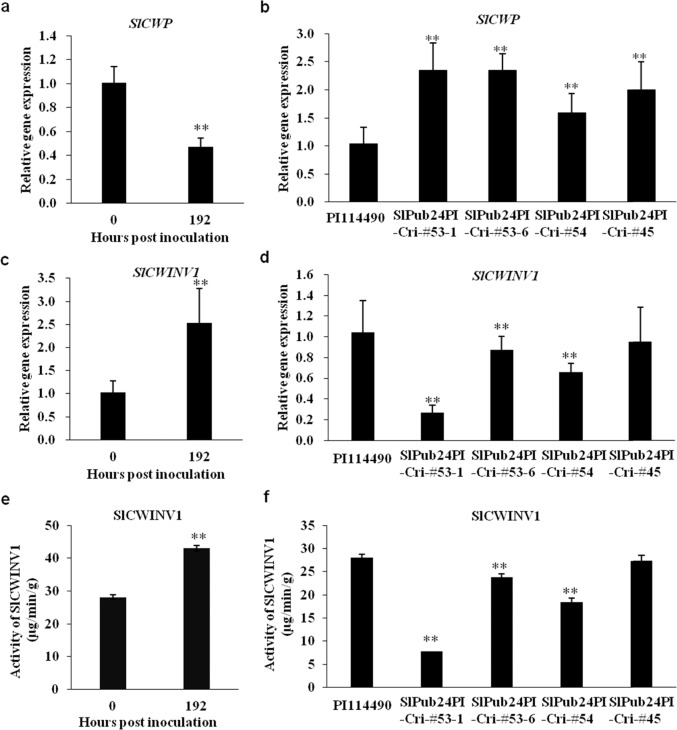
The expression of SlCWP is opposite to that of SlCWIINV1 during the infection of X. euvesicatoria pv. perforans race T3
The relative expression of SlCWP in PI 114490 was significantly lower at 192 hpi than at 0 hpi of Xv829 (Fig. 8a). However, its expression was significantly upregulated at 192 hpi in CRISPR/Cas9-generated mutants of SlPub24 (Fig. 8b). Previous studies have shown that increased invertase activity can enhance the levels of factors (cellulose, xylose, and galactose) involved in cell wall reinforcement34. The bacterium Xv829 can grow in leaves of PI 114490, but no or fewer symptoms occur on the leaf surface of these plants compared to plants of the susceptible line OH 8811912 and bacterial propagation only happens below the epidermal cells in leaves of PI 11449035, suggesting that cell wall defense response might be distinct between resistant and susceptible tomato lines35. Therefore, the expression of the SlCWINV1 (Solyc03g121680) gene encoding the cell wall invertase was examined here. The data showed that the expression of SlCWINV1 was opposite to that of SlCWP. The relative expression of SlCWINV1 in PI 114490 was significantly higher at 192 hpi than at 0 hpi for Xv829 (Fig. 8c), while the gene was downregulated in the mutants (Fig. 8d). SlCWINV1 activity was consistent with gene expression. The activity of SlCWINV1 in PI 114490 was significantly higher at 192 hpi than at 0 hpi (Fig. 8e), whereas the activity of SlCWINV1 was significantly lower in the three mutants, SlPub24PI-Cri#53-1, SlPub24PI-Cri#53-6, and SlPub24PI-Cri#54, than in PI 114490 (Fig. 8f).
Fig. 8. Relative expression of SlCWP and SlCWINV1 as well as enzyme activity of SlCWINV1 in leaves of plants after inoculation with Xanthomonas euvesicatoria pv. perforans race T3 strain Xv829.
a Relative expression of SlCWP in PI 114490 at 0 and 192 h post inoculation (hpi). b Relative expression of SlCWP in PI 114490 and Slpub24 mutants generated by CRISPR/Cas9 editing at 192 hpi. c Relative expression of SlCWINV1 in PI 114490 at 0 and 192 hpi. d Relative expression of SlCWINV1 in PI 114490 and Slpub24 mutants generated by CRISPR/Cas9 editing at 192 hpi. e Activity of SlCWINV1 in PI 114490 at 0 and 192 hpi. f Activity of SlCWINV1 in PI 114490 and Slpub24 mutants generated by CRISPR/Cas9 editing at 192 hpi. Error bars represent the SD (n = 3)
Discussion
As a tomato source with broad-spectrum resistance to bacterial spot, PI 114490 has been used in various breeding programs to develop new lines with partial resistance to different species and races of Xanthomonas8–10,36. Several studies have shown that the common locus QTL-11b on chromosome 11 is responsible for the resistance to multiple species and races of Xanthomonas7,9,10,12,13. The phenotypic variation explained by the locus varies from 12.5 to 29.4% depending on the population of plants and the species of the pathogen used for genetic analyses10,12,13. Based on linkage map position12 and transcriptome data14,15, SlPub24 was identified as a strong candidate gene in the locus for resistance to race T3, and SlPub24 was further investigated in this study. Transgenic overexpression of SlPub24 in the susceptible line OH 88119 increased resistance to race T3 (Fig. 3), while mutation of the gene in the resistant line PI 114490 using the CRISPR/Cas9 editing system decreased resistance to race T3 in mutants (Fig. 4). Furthermore, disease evaluation of mutants and transgenic lines showed that SlPub24 also conferred resistance to races T1, T2, and T4 (Fig. S3). These results suggest that SlPub24 is the gene for resistance to races T1–T4 in the locus QTL-11B.
Gene expression is largely dependent on its promoter activity. Sequence variation in the promoter region, including nucleotide substitution and insertion/deletion of certain fragments, can affect the timing and level of gene expression. Low expression of fw2.2 in tomato plants with large fruits is due to one or more nucleotide substitutions in the promoter region of the gene37,38. The presence of an 11-bp InDel in the promoter region of the SD1 gene disrupts a gibberellin-responsive cis-element, resulting in low expression of the gene in thin-stem tomato plants39. Similarly, insertion of an 11-bp fragment in the promoter region of the Bs3 gene results in the loss of specific recognition by AvrBs3 from the pepper bacterial spot pathogen X. euvesicatoria pv. euvesicatoria40,41, while a deletion of 3 bp in the promoter region of the Xa27 gene causes the loss of specific recognition by AvrXa27 of the rice bacterial blight pathogen X. oryzae pv. oryzae42. In the current study, substantial sequence variation was detected in the promoter regions of SlPub24 between PI 114490 and OH 88119 (Table S2). Promoter activity analysis (Fig. 5c, d) indicated that the promoter of SlPub24 in OH 88119 might have very low activity. Thus, the expression of the gene was low in OH 88119 regardless of whether there was pathogen infection (Fig. 5a). The results of swapping the promoter and CDS regions isolated from PI 114490 and OH 88119 indicated that the CDS of SlPub24 from both PI 114490 and OH 88119 could contribute to resistance to race T3 (Fig. 5f), although sequence variation in the CDS of SlPub24 existed between the two tomato lines. All these data suggested that the expression level of SlPub24 was determined by the activity of its promoter.
Salicylic acid is an important signaling molecule that induces systemic acquired resistance and is associated with pathogen resistance in plants43,44. It has been shown that plants generate SA via the Phenylalanine Ammonia-Lyase (PAL) pathway45–48, and the relative expression of pathogenesis-related (PR) genes and nonexpresser of PR genes 1 (NPR1) are reliable indicators of the activity of SA signaling49. The expression of PAL influences the accumulation of pathogen-induced SA and is associated with disease resistance50,51. Various studies have shown that plant U-box proteins regulate disease resistance through the SA signaling pathway. Overexpression of CMPG1-V, which encodes a U-box E3 ubiquitin ligase in wheat, can improve broad-spectrum resistance to powdery mildew via increased expression of SA-responsive genes26. Overexpression of OsPUB15 in rice causes increased expression of PR genes and enhanced resistance to blast strains in transgenic lines52. Knockdown of OsPUB44 through RNAi significantly suppresses the expression of PAL1 and decreases resistance to Xanthomonas oryzae pv. oryzae in transgenic rice lines53. In this study, the relative expression of the PR1, PAL, and NPR1 genes increased in PI 114490 when plants were infected by race T3 strain Xv829 but remained at a low level in lines with SlPub24 mutated by CRISPR/Cas9 editing (Fig. 6a). In contrast, the transcription of these three genes was lower in OH 88119 than in the transgenic lines overexpressing SlPub24 (Fig. 6b). It should also be noted that the increase in PR1, PAL, and NPR1 expression (Fig. 6a) occurred later than the increase in SlPub24 expression (Fig. 5a) in PI 114490. The SA content in tomato plants was consistent with the expression levels of SlPub24, PAL, PR1, and NPR1 (Fig. 6c, d). These data suggested that SlPUB24 conferred resistance to bacterial spot by regulating the biosynthesis and signaling of SA.
The plant cell wall is the first barrier to pathogen infection, as it can prevent pathogens from entering the cells. It is also the matrix for many proteins involved in pathogen perception. By destroying the cell wall, the pathogen exposes the cell to itself, causing a series of innate immune reactions in plants32. Cell wall invertase (CWI) responds to wounding and pathogen infections. Elevated CWI activity induces resistance to Pseudomonas syringae pv. tomato DC3000 in melatonin-treated Arabidopsis34. CWI can be regulated by specific invertase inhibitor proteins, such as cell wall/vacuolar invertase inhibitors (C/VIFs). AtC/VIF1 showed specific inhibition of VI activity, but AtC/VIF2 inhibited CWI and VI54. A previous study showed that bacteria can enter and propagate in the leaves of PI 114490 plants but are restricted to spongy cell layers due to the formation of wall appositions at the junction between adjacent mesophyll cells35. Thus, the cell wall of PI 114490 might function to prohibit bacterial migration. In this study, the interaction of the cell wall protein SlCWP with SlPUB24 was identified through Y2H and verified by BiFC and SLC (Fig. 7). The expression of SlCWP and SlCWINV1 showed the opposite patterns (Fig. 8), suggesting that SlCWP might inhibit the expression of SlCWINV1 during pathogen infection. However, the expression of SlCWP showed the same pattern as SlPub24. Therefore, it was most likely that SlPUB24 recruited and degraded SlCWP during pathogen infection, removing the inhibition of SlCWP on SlCWINV1. SlCWINV1 plays a role in cell wall reinforcement to form wall appositions to prevent bacterial migration (Fig. 9). Meanwhile, SlPub24 affected the expression of PAL, resulting in changes in SA content and subsequently influencing the expression of PR1 and NPR1, which eventually activated plant resistance.
Fig. 9. Hypothetical SlPUB24 regulatory pathway in response to pathogenic infection with bacterial spot in tomato.
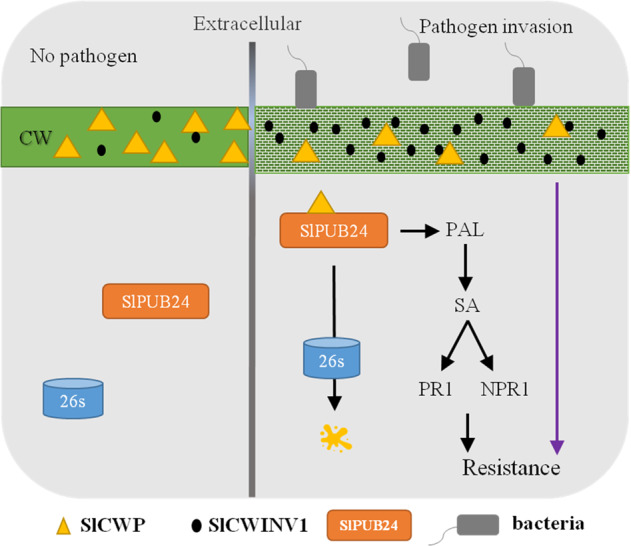
Plant cell walls are normal in the absence of pathogen invasion, and invertase inhibitors dynamically adjust the activity of invertase in the cell wall to stabilize the structure of the cell wall (left). When plants perceive pathogen invasion, SlPUB24 is recruited to the cell wall to interact with the cell wall protein SlCWP for degradation by the 26S proteasome. The reduction in SlCWP may increase the activity of the cell wall invertase SlCWINV1 to strengthen the cell wall for defense against pathogens. SlPub24 also affects salicylic acid (SA) synthesis by regulating the expression of PAL, which results in subsequent alteration of PR1 and NPR1 expression. Through the SA pathway, signals are transmitted to downstream defense-related genes and activate the systemic resistance response
Materials and methods
Plant materials
Solanum lycopersicum var. cerasiforme accession PI 114490 with field resistance8,12 and S. lycopersicum variety OH 88119 without resistance to Xanthomonas euvesicatoria pv. perforans race T34,6 were used for gene isolation, gene expression, and genetic transformation. Nicotiana benthamiana plants were used for Agrobacterium-mediated transient expression analysis. A germplasm collection consisting of 192 tomato lines31 and three near-isogenic lines (NILs), FG16-802 carrying the SlPub24 gene from PI 114490, FG16-804 carrying a QTL on chromosome 11 from Hawaii 7998, and FG18-813 carrying the QTL from Hawaii 7998 and the Rx4 gene from PI 128216, was subjected to genotyping with the marker for selection of the SlPub24 gene, and 26 (Table 1) were selected for disease evaluation. The NILs with partial field resistance to race T3 were kindly provided by Dr. David M. Francis at The Ohio State University. All plants were grown in greenhouses with water and fertilizer supplied unless otherwise described.
Molecular cloning and bioinformatic analysis of SlPub24 and SlCWP
Based on previous map position12 and transcriptome data14, SlPub24 was selected as a candidate gene for the locus QTL-11B on chromosome 11 conferring resistance to race T3. The full-length cDNAs and promoters were amplified from PI 114490 and OH 88119 using gene-specific primers (Table S1). The open reading frame was predicted using ORFfinder in NCBI (https://www.ncbi.nlm.nih.gov/orffinder/). Alignment of deduced amino acid sequences between PI 114490 and OH 88119 was performed using Clustal X (http://www.clustal.org/). Functional domains were predicted using the SMART online tool (http://smart.embl-heidelberg.de/), and phylogenetic trees were created by MEGA X55 using the neighbor-joining method with 1000 bootstrap replicates. Bootstrap values are shown as percentages.
RNA isolation and quantitative RT-PCR analysis
Total RNA was isolated from tomato leaves using the Quick RNA Isolation Kit (Huayueyang Biotechnology Co., Beijing, China) following the manufacturer’s instructions. The concentration of total RNA was determined using a NanoDrop 2000 Spectrophotometer (Thermo Fisher Scientific, Delaware, USA). Single-stranded cDNA was synthesized using the Prime Script™ RT reagent Kit with gDNA Eraser (Takara Bio Inc., Dalian, China) following the manufacturer’s instructions. Quantitative RT-PCR was performed in a 10-μl reaction volume containing 1 μl of diluted cDNA, 5 μl TB Green™ Premix Ex Taq™ (Tli RNaseH Plus) (TaKaRa), 0.25 μl ROX Reference Dye (50×), 0.25 μl each of forward and reverse gene-specific primers (Table S1), and 3.25 μl sterile purified water. The tomato EF1-ɑ (Solyc06g005060) or actin (Solyc11g005330) gene was used as an internal reference gene15,56. Relative expression values were determined using the comparative Ct method (2-ΔΔCt)57.
Determination of SlPub24 promoter activity
Measurement of promoter activity was performed by GUS assay. Promoters of the SlPub24 gene amplified from genomic DNA of PI 114490 and OH 88119 were separately fused with the GUS reporter and cloned into the pCAMBIA1305.1 vector. The resulting constructs were transiently expressed in N. benthamiana leaves using the Agrobacterium-mediated transfection method. A. tumefaciens GV3101 and pCaMV35S::GUS were used as negative and positive controls, respectively. The infiltrated leaves were harvested 3 days after infiltration. Leaf discs (5 mm in diameter) were histochemically stained with 5-bromo-4-chloro-3-indolyl b-D-glucuronide (X-Gluc) for 24 h at 37 °C and then incubated in 70% ethanol for 48 h to remove chlorophyll before photographing. For measurements of GUS activity, 4-methylumbelliferyl beta-D-glucuronide (4-MUG, Sigma-Aldrich, USA) was added as a substrate for the fluorometric assay using the method previously described58, and the 4-MU produced in the GUS reaction was measured by a Thermo Scientific Microplate Reader (Thermo Fisher Scientific, DE, USA). The concentration of total protein extracted from the leaf discs was measured using a Micro BCA Protein Assay Kit (CoWin Biotech Co. Ltd., Jiangsu, China). Final GUS activity was calculated according to the standard curve of 4-MU (Sigma Aldrich) and expressed as nmol.4-MU mg−1 protein min−1.
To compare the promoter activities of the SlPub24 gene during bacterial infection in PI 114490 and OH 88119, the expression of the GUS reporter driven by the 2.4 kb promoter isolated from PI 114490 in OH 88119 plants and driven by the 2.4 kb promoter isolated from OH 88119 in PI 114490 plants was measured at 0, 0.25, 0.5, 1, 2, 6, 12, and 24 h post inoculation (hpi) of X. euvesicatoria pv. perforans race T3 strain Xv829. The promoter of CaMV35S fused with the GUS reporter was cloned into pCAMBIA1305.1 and used as the positive control, while A. tumefaciens GV3101 was used as the negative control. The specific primers for amplification of promoters are listed in Table S1.
Overexpression of SlPub24 in the susceptible line OH 88119
Overexpression of SlPub24 isolated from the resistant line PI 114490 in the susceptible line OH 88119 was performed to determine the role of SlPub24 in resistance to race T3. Two constructs were created for genetic transformation. The first construct, SlPub24PI (Fig. S2a), was developed by inserting the fragment of the coding sequence (CDS) of SlPub24 with a His tag into the vector pBI12159. The CDS fragment was amplified from the cDNA of PI 114490 using gene-specific primers (Table S1). The second construct, pSlPub24PI (Fig. S2b), was generated by inserting a fragment of 3831 bp including the promoter, 5′UTR, CDS, and 3′UTR of the SlPub24 gene into pBI121 with excision of the CaMV 35 S promoter. The DNA fragment was amplified from the genomic DNA of PI 114490 using a pair of specific primers (Table S1). Both destination constructs were confirmed by sequencing, separately transformed into A. tumefaciens strain C58 using electroporation, and then separately transformed into the susceptible tomato line OH 88119 using previously described methods60 with slight modifications. The transgenic tomato lines were verified by PCR using primers (Table S1) specific to each construct.
Mutation of the SlPub24 gene in the resistant line PI 114490 using the CRISPR/Cas9 editing system
The CRISPR/Cas9 vector61 with modification by replacing the Arabidopsis U6 gene promoter with the tomato U6 gene promoter was kindly provided by Dr. Xia Cui at the Institute of Vegetables and Flowers at the Chinese Academy of Agricultural Sciences (Beijing, China). Two target sites (sgRNA1 and sgRNA2) of 20 nucleotides in the U-box domain separated by 57 bp were selected using CRISPR-P (http://cbi.hzau.edu.cn/cgi-bin/CRISPR). The CRISPR/Cas9 construct was generated following a previous description61. The vectors were introduced into A. tumefaciens strain C58 through electroporation and transformed into tomato line PI 114490 using the methods described above. All regenerated T0 lines were subjected to Cas9 detection by PCR using specific primers (Table S1), and only lines containing Cas9 were retained for further detection of mutations in the SlPub24 gene region by sequencing PCR products amplified using a forward primer to the left of sgRNA1 and a reverse primer to the right of sgRNA2 (Table S1). Only homozygous mutants from the T2 generation were used for disease evaluation.
Subcellular localization
The open reading frame of SlPub24 without the termination codon was inserted into the modified pSuper1300 plasmid containing GFP protein at the Xba I and Kpn I (New England BioLabs, MA, USA) sites to generate the vector. The construct was transformed into A. tumefaciens by heat shock and into tomato protoplast and onion epidermal cells by PEG and gene gun, respectively. DAPI staining solution (Huayueyang Biotechnology Co., Beijing, China) was added to the transfected protoplasts for 5–10 min, followed by washing with buffer solution 2–3 times. GFP fluorescence was monitored by excitation at 488 nm, and the DAPI-stained nuclei were observed by excitation at 360 nm with an argon laser using an Olympus BX 51 fluorescence microscope (Olympus Corporation, Tokyo, Japan).
Disease evaluation
X. euvesicatoria pv. euvesicatoria (race T1) strain Xcv110c, X. vesicatoria (race T2) strain Xv1111, X. euvesicatoria pv. perforans race T3 strain Xv829, and X. euvesicatoria pv. perforans race T4 strain scott1 were kindly provided by Dr. Jeffery Jones at the University of Florida. Inoculum preparation and inoculation were performed according to our previous methods11. Both percent diseased leaf area and bacterial population were adopted as parameters for evaluating plant resistance. The percent diseased leaf area was measured using the leaf-by-leaf approach with image analysis software ASSESS V2.011 7 days post inoculation (dpi). Bacterial populations in inoculated leaves of the tomato lines were determined by the dilution plate method11 at 9 dpi. Three inoculated 0.2 cm2 leaf discs from each plant and at least 30 plants for each genotype were sampled to monitor the bacterial population. The data were analyzed using SPSS software (IBM SPSS Statistics, version 20, New York, USA) with one-way analysis of variance (ANOVA) followed by Duncan’s test for multiple comparisons. Probability values less than 5% (P < 0.05) were considered significant.
Cell death detection
Trypan blue staining was performed to visualize cell death in the leaves of the tomato plants after inoculation with Xv829. The inoculated leaves were boiled in trypan blue staining solution (10 ml lactic acid, 10 ml glycerol, 10 g phenol, and 10 mg trypan blue dissolved in 10 ml ddH2O) for 3 min and stained overnight in chloral hydrate solution (2.5 g chloral hydrate dissolved in 1 ml ddH2O). The stained plant leaves were mounted in 70% glycerol for observation. Photographs were taken by a Nikon D3000 Digital SLR camera (Nikon, Tokyo, Japan).
Measurement of SlCWINV1 activity and salicylic acid content
Leaf samples were collected from three plants of each genotype at 0 and 192 h post inoculation with T3 strain Xv829. For the measurement of SlCWINV1 activity, leaf tissues were ground in liquid nitrogen and homogenized in 2 ml/g extraction buffer (50 mM citric acid, 250 mM sorbitol, 10 mM MgCl2, 10 mM KCl, and 1 mM PMSF, pH 6.0). After centrifugation (8500 g, 10 min, 4 °C), the pellets were washed once (10 min) with extraction buffer containing 1% Triton X-100 and twice with extraction buffer only. The cell wall pellets were resuspended in 1 ml/g assay buffer (20 mM triethanolamine, 7 mM citric acid, and 1 mM PMSF, pH 4.6) and used for the determination of SlCWINV1 activity. The activity was monitored by mixing 20–100 μl of invertase preparation, 100 μl of sucrose (100 mM in assay buffer), and the assay buffer up to a volume of 300 μl. After incubation at 37 °C for 30 min, invertase activity was measured by enzymatic determination of the released glucose in a coupled enzymatic-optical assay with hexokinase and glucose-6-phosphate dehydrogenase, according to the Jansen method62. Determination of salicylic acid content was performed by Jiaxing Metware Metabolic Biotechnology Company (Zhejiang, China).
Identification of proteins that interact with SlPUB24 using yeast two-hybrid assay
The yeast two-hybrid (Y2H) assay was performed following the instructions in the Matchmaker GAL4 Two-Hybrid System & Libraries User Manual (Clontech Laboratories, Inc., CA, USA). The full-length SlPub24 open reading frame was amplified from cDNA of PI 114490 using gene-specific primers (Table S1) and cloned into the pGBKT7 bait vector63. The bait vector pGBKT7-SlPUB24 and the pGADT7 prey vector (cDNA library) were cotransformed into the yeast (Saccharomyces cerevisiae) strain AH109 by the PEG/LiOAc method. Self-activation of each protein was inhibited by 3-amino-1,2,4-triazole (3-AT) at different concentrations depending on the gene. The cotransformed yeast strains were first grown on selective medium lacking Leu and Trp (SD/-Leu-Trp) and then transferred to SD/-Trp-Leu-His/X-α-GAL. Protein interactions were determined by the appearance of blue color 3–5 days after incubation at 30 °C.
Plasmid DNA of positive colonies was isolated using a Yeast High-Purity Plasmid Extraction Kit (Aidlab Biotechnologies, Beijing, China). The cDNA inserts were amplified (primers shown in Table S1), and the resulting PCR products were sequenced. The obtained sequences were blasted to the tomato genome sequence database in NCBI (https://www.ncbi.nlm.nih.gov/) and SGN (https://solgenomics.net/) to verify the genes. The full-length sequence of each gene was obtained from SGN and used for gene-specific primer design. Candidate genes were amplified from the cDNA of PI 114490 using gene-specific primers (Table S1), and the yeast two-hybrid method described above was used to verify their interactions with SlPUB24.
Bimolecular fluorescence complementation (BiFC) assay
The ORFs of SlPub24 and SlCWP amplified from cDNA of PI 114490 without stop codons were separately cloned into the pSY-NE and pSY-CE vectors using the Seamless Assembly Cloning Kit (Takara). The fusion vectors were transferred into A. tumefaciens strain GV3101 using heat-shock transformation. Then, strain GV3101 containing fusion proteins was incubated at 28 °C for 12–18 h and resuspended in infiltration buffer (0.1 mM acetosyringone, 10 mM MgCl2, and 10 mM MES) at concentrations of 0.8–1.2 (OD600). Bacteria carrying the pSY-NE construct were mixed with bacteria carrying the pSY-CE construct as well as GV3101 carrying P19 at a 1:1:1 (v/v) ratio and coinfiltrated into the leaves of six-week-old seedlings of N. benthamiana. The yellow fluorescent protein (YFP) signal was detected using an Olympus BX 51 fluorescence microscope (Olympus Corporation) 2 days after infiltration.
Split luciferase complementation (SLC) assay
The SLC assay was performed as previously described64. Constructs of SlCWP-cLUC and SlPUB24-nLUC were cotransformed into N. benthamiana leaves and expressed for 48 h. The abaxial sides of leaves were sprayed with 1 mM beetle luciferin (Promega, WI, USA), and the signal was captured using a Photek camera (HRPCS5, Photek, UK).
Protein degradation assay with MG132 treatment
The plasmids p1300SlPUB24-Myc and p1300SlCWP-Flag were coexpressed with the P19 plasmid by agroinfiltration in the leaves of N. benthamiana. MG132 (50 μM) was infiltrated into the leaf tissue before harvest, and DMSO was used as the control. Tissues were harvested 2 days after infiltration for protein extraction using the Plant Protein Extraction Kit (CWBIO, Beijing, China) following the manufacturer’s instructions. Western blotting was performed as described previously65. Anti-Flag and anti-Myc (CWBIO) were diluted 1:2000. The total protein concentration was monitored by Coomassie brilliant blue (CBB) staining.
Statistical analysis
All samples were analyzed in triplicate, and the data are expressed as the mean ± standard deviation unless noted otherwise. Statistical significance was determined using Student’s t test at the 0.05 (*) and 0.01 (**) levels. All experiments were conducted at least twice with three biological replicates each time.
Supplementary information
Acknowledgements
The authors would like to thank Dr. David M. Francis at the Ohio State University and Tomato Genetic Resources Center at University of California-Davis for providing seeds of tomato lines, Dr. Jeffery B. Jones at the University of Florida for providing strains of Xanthomonas spp., Dr. Zhibiao Ye at Huazhong Agricultural University for providing a tomato cDNA library and all persons who provided the vectors used in this study. The work was partially supported by the National Key Research and Development Program (2016YFD0101007) and the Construction of Beijing Science and Technology Innovation and Service Capacity in Top Subjects (CEFF-PXM2019_014207_000032).
Author contributions
X.L. and W.Y. conceived and designed the research. X.L., G.M., M.W., and Y.Z. performed the experiments. X.L., Z.Q., and Y.Z. analyzed the data. X.L. and W.Y. wrote the manuscript. All authors read and approved the manuscript.
Conflict of interest
The authors declare that they have no conflict of interest.
Supplementary information
Supplementary Information accompanies this paper at (10.1038/s41438-021-00468-4).
References
- 1.Jones JB, Lacy GH, Bouzar H, Stall RE, Schaad NW. Reclassification of the xanthomonads associated with bacterial spot disease of tomato and pepper. Syst. Appl. Microbiol. 2004;27:755–762. doi: 10.1078/0723202042369884. [DOI] [PubMed] [Google Scholar]
- 2.Timisina S, et al. Reclassification of Xanthomonas gardneri (ex Sutic 1957) Jones et al. 2006 as a later heterotypic synonym of Xanthomonas cynarae Trebaol et al. 2000 and description of X. cynarae pv. cynarae and X. cynarae pv. gardneri based on whole genome analyses. Int. J. Syst. Evol. Microbiol. 2019;69:343–349. doi: 10.1099/ijsem.0.003104. [DOI] [PubMed] [Google Scholar]
- 3.Constantin EC, et al. Genetic characterization of strains named as Xanthomonas axonopodis pv. dieffenbachiae leads to a taxonomic revision of the X. axonopodis species complex. Plant Pathol. 2016;65:792–806. doi: 10.1111/ppa.12461. [DOI] [Google Scholar]
- 4.Wang Y, Zhang Y, Gao Z, Yang W. Breeding for resistance to tomato bacterial diseases in China: challenges and prospects. Hortic. Plant J. 2018;4:193–207. doi: 10.1016/j.hpj.2018.08.004. [DOI] [Google Scholar]
- 5.Adhikari P, Adhikari TB, Louws FJ, Panthee DR. Advances and challenges in bacterial spot resistance breeding in tomato (Solanum lycopersicum L.) Int. J. Mol. Sci. 2020;21:1734. doi: 10.3390/ijms21051734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li N, Zhang X, Yang W. Marker-assisted development and characterization of near-isogenic lines carrying the Rx4 gene for hypersensitive resistance to Xanthomonas euvesicatoria pv. perforans race T3 in tomato. Mol. Breed. 2019;39:172. doi: 10.1007/s11032-019-1084-2. [DOI] [Google Scholar]
- 7.Scott JW, Hutton SF, Shekasteband R, Sim SC, Francis DM. Identification of tomato bacterial spot race T1, T2, T3, T4, and Xanthomonas gardneri resistance QTLs derived from PI 114490 populations selected for race T4. Acta Hortic. 2015;1069:53–58. doi: 10.17660/ActaHortic.2015.1069.6. [DOI] [Google Scholar]
- 8.Scott JW, Francis DM, Miller SA, Somodi GC, Jones JB. Tomato bacterial spot resistance derived from PI 114490; Inheritance of resistance to race T2 and relationship across three pathogen races. J. Am. Soc. Hortic. Sci. 2003;128:698–703. doi: 10.21273/JASHS.128.5.0698. [DOI] [Google Scholar]
- 9.Bernal E, Liabeuf D, Francis DM. Evaluating quantitative trait locus resistance in tomato to multiple Xanthomonas spp. Plant Dis. 2020;104:423–429. doi: 10.1094/PDIS-03-19-0669-RE. [DOI] [PubMed] [Google Scholar]
- 10.Hutton SF, et al. Identification of QTL associated with resistance to bacterial spot race T4 in tomato. Theor. Appl. Genet. 2010;121:1275–1287. doi: 10.1007/s00122-010-1387-5. [DOI] [PubMed] [Google Scholar]
- 11.Sun H, Wei J, Zhang J, Yang W. A comparison of disease severity measurements using image analysis and visual estimates using a category scale for genetic analysis of resistance to bacterial spot in tomato. Eur. J. Plant Pathol. 2014;139:125–136. doi: 10.1007/s10658-013-0371-8. [DOI] [Google Scholar]
- 12.Sun H, et al. QTL analysis of resistance to bacterial spot race T3 in tomato. Acta Hortic. Sin. 2011;38:2297–2308. [Google Scholar]
- 13.Yang W, Miller SA, Francis DM, Scott JW, Jones JB. Mining tomato genome sequence databases for molecular markers: application to bacterial resistance and marker assisted selection. Acta Hortic. 2005;695:241–249. doi: 10.17660/ActaHortic.2005.695.26. [DOI] [Google Scholar]
- 14.Du HS, Wang YQ, Yang JJ, Yang WC. Comparative transcriptome analysis of resistant and susceptible tomato lines in response to infection by Xanthomonas perforans race T3. Front. Plant Sci. 2015;6:1173. doi: 10.3389/fpls.2015.01173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Du H, Li W, Wang Y, Yang W. Identification of genes differentially expressed between resistant and susceptible tomato lines during time-course interactions with Xanthomonas perforans race T3. PLoS One. 2014;9:e93476. doi: 10.1371/journal.pone.0093476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zeng LR, et al. Spotted leaf11, a negative regulator of plant cell death and defense, encodes a U-box/armadillo repeat protein endowed with E3 ubiquitin ligase activity. Plant Cell. 2004;16:2795–2808. doi: 10.1105/tpc.104.025171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yin ZC, et al. Characterizing rice lesion mimic mutants and identifying a mutant with broad-spectrum resistance to rice blast and bacterial blight. Mol. Plant Microbe Interact. 2000;13:869–876. doi: 10.1094/MPMI.2000.13.8.869. [DOI] [PubMed] [Google Scholar]
- 18.Li W, et al. The U-Box/ARM E3 ligase PUB13 regulates cell death, defense, and flowering time in Arabidopsis. Plant Physiol. 2012;159:239–250. doi: 10.1104/pp.111.192617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu J, et al. The U-Box E3 ligase SPL11/PUB13 is a convergence point of defense and flowering signaling in plants. Plant Physiol. 2012;160:28–37. doi: 10.1104/pp.112.199430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Trujillo M, Ichimura K, Casais C, Shirasu K. Negative regulation of PAMP-triggered immunity by an E3 ubiquitin ligase triplet in Arabidopsis. Curr. Biol. 2008;18:1396–1401. doi: 10.1016/j.cub.2008.07.085. [DOI] [PubMed] [Google Scholar]
- 21.Stegmann M, et al. The ubiquitin ligase PUB22 targets a subunit of the exocyst complex required for PAMP-triggered responses in Arabidopsis. Plant Cell. 2012;24:4703–4716. doi: 10.1105/tpc.112.104463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.He Q, et al. U-box E3 ubiquitin ligase PUB17 acts in the nucleus to promote specific immune pathways triggered by Phytophthora infestans. J. Exp. Bot. 2015;66:3189–3199. doi: 10.1093/jxb/erv128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang CW, et al. The E3 ubiquitin ligase activity of Arabidopsis PLANT U-BOX17 and its functional tobacco homolog ACRE276 are required for cell death and defense. Plant Cell. 2006;18:1084–1098. doi: 10.1105/tpc.105.039198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kirsch C, Logemann E, Lippok B, Schmelzer E, Hahlbrock K. A highly specific pathogen-responsive promoter element from the immediate-early activated CMPG1 gene in Petroselinum crispum. Plant J. 2001;26:217–227. doi: 10.1046/j.1365-313x.2001.01015.x. [DOI] [PubMed] [Google Scholar]
- 25.Navarro L, et al. The transcriptional innate immune response to flg22. interplay and overlap with Avr gene-dependent defense responses and bacterial pathogenesis. Plant Physiol. 2004;135:1113–1128. doi: 10.1104/pp.103.036749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhu Y, et al. E3 ubiquitin ligase gene CMPG1-V from Haynaldia villosa L. contributes to powdery mildew resistance in common wheat (Triticum aestivum L.) Plant J. 2015;84:154–168. doi: 10.1111/tpj.12966. [DOI] [PubMed] [Google Scholar]
- 27.Gonzalez-Lamothe R, et al. The U-Box protein CMPG1 is required for efficient activation of defense mechanisms triggered by multiple resistance genes in tobacco and tomato. Plant Cell. 2006;18:1067–1083. doi: 10.1105/tpc.106.040998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee DH, Choi HW, Hwang BK. The pepper E3 ubiquitin ligase RING1 gene, CaRING1, is required for cell death and the salicylic acid-dependent defense response. Plant Physiol. 2011;156:2011–2025. doi: 10.1104/pp.111.177568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Han P-L, et al. The apple U-box E3 ubiquitin ligase MdPUB29 contributes to activate plant immune response to the fungal pathogen Botryosphaeria dothidea. Planta. 2019;249:1177–1188. doi: 10.1007/s00425-018-03069-z. [DOI] [PubMed] [Google Scholar]
- 30.Han P-L, et al. BTB-BACK domain E3 ligase MdPOB1 suppresses plant pathogen defense against Botryosphaeria dothidea by ubiquitinating and degrading MdPUB29 protein in apple. Plant Cell Physiol. 2019;60:2129–2140. doi: 10.1093/pcp/pcz106. [DOI] [PubMed] [Google Scholar]
- 31.Liu X, Geng X, Zhang H, Shen H, Yang W. Association and genetic identification of loci for four fruit traits in tomato using InDel markers. Front. Plant Sci. 2017;8:1269. doi: 10.3389/fpls.2017.01269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cantu D, Vicente AR, Labavitch JM, Bennett AB, Powell ALT. Strangers in the matrix: plant cell walls and pathogen susceptibility. Trends Plant Sci. 2008;13:610–617. doi: 10.1016/j.tplants.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 33.Harhouri K, et al. MG132-induced progerin clearance is mediated by autophagy activation and splicing regulation. EMBO Mol. Med. 2017;9:1294–1313. doi: 10.15252/emmm.201607315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhao H, et al. Melatonin regulates carbohydrate metabolism and defenses against Pseudomonas syringae pv. tomato DC3000 infection in Arabidopsis thaliana. J. Pineal Res. 2015;59:109–119. doi: 10.1111/jpi.12245. [DOI] [PubMed] [Google Scholar]
- 35.Wang YQ, Zhang XF, Li N, Liu X. Comparison of cellular responses to Xanthomonas perforans infection between resistant and susceptible tomato accessions. J. Plant Physiol. 2017;209:105–114. doi: 10.1016/j.jplph.2016.11.011. [DOI] [PubMed] [Google Scholar]
- 36.Sim SC, et al. Association analysis for bacterial spot resistance in a directionally selected complex breeding population of tomato. Phytopathology. 2015;105:1437–1445. doi: 10.1094/PHYTO-02-15-0051-R. [DOI] [PubMed] [Google Scholar]
- 37.Frary A, et al. fw2.2: a quantitative trait locus key to the evolution of tomato fruit size. Science. 2000;289:85–88. doi: 10.1126/science.289.5476.85. [DOI] [PubMed] [Google Scholar]
- 38.Nesbitt TC, Tanksley SD. Comparative sequencing in the genus Lycopersicon: implications for the evolution of fruit size in the domestication of cultivated tomatoes. Genetics. 2002;162:365–379. doi: 10.1093/genetics/162.1.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ye, J. et al. Tomato SD1, encoding a kinase interacting protein, is a major locus controlling stem development. J. Exp. Bot.10.1093/jxb/eraa144 (2020). [DOI] [PMC free article] [PubMed]
- 40.Roemer P, et al. Plant pathogen recognition mediated by promoter activation of the pepper Bs3 resistance gene. Science. 2007;318:645–648. doi: 10.1126/science.1144958. [DOI] [PubMed] [Google Scholar]
- 41.Roemer P, et al. Recognition of AvrBs3-like proteins is mediated by specific binding to promoters of matching pepper Bs3 alleles. Plant Physiol. 2009;150:1697–1712. doi: 10.1104/pp.109.139931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Roemer P, Recht S, Lahaye T. A single plant resistance gene promoter engineered to recognize multiple TAL effectors from disparate pathogens. Proc. Natl Acad. Sci. USA. 2009;106:20526–20531. doi: 10.1073/pnas.0908812106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vicente MR-S, Plasencia J. Salicylic acid beyond defence: its role in plant growth and development. J. Exp. Bot. 2011;62:3321–3338. doi: 10.1093/jxb/err031. [DOI] [PubMed] [Google Scholar]
- 44.Klessig DF, Choi HW, Dempsey DMA. Systemic acquired resistance and salicylic acid: past, present, and future. Mol. Plant Microbe Interact. 2018;31:871–888. doi: 10.1094/MPMI-03-18-0067-CR. [DOI] [PubMed] [Google Scholar]
- 45.Dempsey DMA, Vlot AC, Wildermuth MC, Klessig DF. Salicylic acid biosynthesis and metabolism. Arabidopsis Book. 2011;9:e0156–e0156. doi: 10.1199/tab.0156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gao Q-M, Zhu S, Kachroo P, Kachroo A. Signal regulators of systemic acquired resistance. Front. Plant Sci. 2015;6:228. doi: 10.3389/fpls.2015.00228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Khan MIR, Fatma M, Per TS, Anjum NA, Khan NA. Salicylic acid-induced abiotic stress tolerance and underlying mechanisms in plants. Front. Plant Sci. 2015;6:462. doi: 10.3389/fpls.2015.00462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Seyfferth C, Tsuda K. Salicylic acid signal transduction: the initiation of biosynthesis, perception and transcriptional reprogramming. Front. Plant Sci. 2014;5:697. doi: 10.3389/fpls.2014.00697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Herrera-Vasquez A, Salinas P, Holuigue L. Salicylic acid and reactive oxygen species interplay in the transcriptional control of defense genes expression. Front. Plant Sci. 2015;6:171. doi: 10.3389/fpls.2015.00171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kim DS, Hwang BK. An important role of the pepper phenylalanine ammonia-lyase gene (PAL1) in salicylic acid-dependent signalling of the defence response to microbial pathogens. J. Exp. Bot. 2014;65:2295–2306. doi: 10.1093/jxb/eru109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shine MB, et al. Cooperative functioning between phenylalanine ammonia lyase and isochorismate synthase activities contributes to salicylic acid biosynthesis in soybean. N. Phytol. 2016;212:627–636. doi: 10.1111/nph.14078. [DOI] [PubMed] [Google Scholar]
- 52.Wang J, et al. The E3 ligase OsPUB15 interacts with the receptor-like kinase PID2 and regulates plant cell death and innate immunity. BMC Plant Biol. 2015;15:49. doi: 10.1186/s12870-015-0442-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ishikawa K, et al. Bacterial effector modulation of host E3 ligase activity suppresses PAMP-triggered immunity in rice. Nat. Commun. 2014;5:5430. doi: 10.1038/ncomms6430. [DOI] [PubMed] [Google Scholar]
- 54.Link M, Rausch T, Greiner S. In Arabidopsis thaliana, the invertase inhibitors AtC/VIF1 and 2 exhibit distinct target enzyme specificities and expression profiles. FEBS Lett. 2004;573:105–109. doi: 10.1016/j.febslet.2004.07.062. [DOI] [PubMed] [Google Scholar]
- 55.Kumar S, Stecher G, Li M, Knyaz C, Tamura K. MEGA X: molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018;35:1547–1549. doi: 10.1093/molbev/msy096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cui J, et al. Tomato MYB49 enhances resistance to Phytophthora infestans and tolerance to water deficit and salt stress. Planta. 2018;248:1487–1503. doi: 10.1007/s00425-018-2987-6. [DOI] [PubMed] [Google Scholar]
- 57.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(T)(-Delta Delta C) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 58.Hull GA, Devic M. The beta-glucuronidase (gus) reporter gene system: gene fusions; spectrophotometric, fluorometric, and histochemical detection. Methods Mol. Biol. 1995;49:125–141. doi: 10.1385/0-89603-321-X:125. [DOI] [PubMed] [Google Scholar]
- 59.Chen PY, Wang CK, Soong SC, To KY. Complete sequence of the binary vector pBI121 and its application in cloning T-DNA insertion from transgenic plants. Mol. Breed. 2003;11:287–293. doi: 10.1023/A:1023475710642. [DOI] [Google Scholar]
- 60.Fillatti JJ, Kiser J, Rose R, Comai L. Efficient transfer of a glyphosafe tolerance gene into tomato using a binary Agrobacterium tumefaciens vector. Bio-Technol. 1987;5:726–730. [Google Scholar]
- 61.Xing H-L, et al. A CRISPR/Cas9 toolkit for multiplex genome editing in plants. BMC Plant Biol. 2014;14:327. doi: 10.1186/s12870-014-0327-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jansen, A. Modifying post-harvest sucrose loss in sugar beet: assessment of transgenic approaches. PhD thesis. University at Heidelberg, Heidelberg, Germany, (2009).
- 63.Chien CT, Bartel PL, Sternglanz R, Fields S. The two-hybrid systems: a methods to identify and clone genes for proteins that interact with a protein of interest. Proc. Natl Acad. Sci. USA. 1991;88:9578–9582. doi: 10.1073/pnas.88.21.9578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chen H, et al. Firefly luciferase complementation imaging assay for protein-protein interactions in plants. Plant Physiol. 2008;146:368–376. doi: 10.1104/pp.107.111740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.MacPhee DJ. Methodological considerations for improving Western blot analysis. J. Pharmacol. Toxicol. Methods. 2010;61:171–177. doi: 10.1016/j.vascn.2009.12.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.