Abstract
Background
Noise aversion is a common behavioural disorder in dogs; affected dogs show fear behaviours in response to noise stimuli. Pharmacological treatment is effective for many dogs; clinical reports suggest anxiolytic treatment lowers the need for treatment over time. We aimed to evaluate the effect of dexmedetomidine oromucosal gel for dogs with noise aversion over a series of noise events. Furthermore, we evaluated burden of care for owners of dogs with noise aversion via questionnaire.
Methods
Owners of enrolled dogs completed records for 10 noise events indicating whether their dog received dexmedetomidine gel and pretreatment and post-treatment anxiety scores; adverse events were noted. Owners were queried about burden of care.
Results
Twenty-two client-owned dogs completed recordings for 10 events. Logistic regression results showed a significant effect for time of event with decreased probability of receiving treatment for subsequent events (OR=0.75, P=0.0017). Within an event, significant improvement in anxiety was seen (median improvement 11 points; paired Wilcoxon; P<0.0001). We found overall burden of care was manageable, yet many owners agreed with statements regarding frustration (42 per cent), stress (46 per cent), guilt (42 per cent) and sadness (75 per cent) about their dog’s condition.
Conclusions
Repeated use of dexmedetomidine gel for noise events resulted in decreased need for administration. Burden of care is important to discuss with clients.
Keywords: canine, fear, anxiety, storm
Introduction
Noise aversion is among the most common behavioural disorders in dogs. While estimates vary, at least 30–40 per cent of dogs are reported to show noise aversion,1 with up to 50 per cent showing some form of fear response to noise over their lifetime.2 The signs of noise aversion displayed differ for individual dogs but generally include some combination of hiding, panting, trembling, pacing, owner-seeking behaviour and escape behaviour. The most common triggers for noise aversion are fireworks, gunshots and thunder; however, dogs may react to other triggers such as household alarms, construction, traffic and vacuums. Many dogs respond to multiple triggers2 3; a recent study showed high correlations in reactivity among noise triggers, particularly thunder, fireworks and gunshots.1 The burden of care for these dogs can be substantial; dogs with severe noise aversion can cause damage to themselves and to property during their attempts to escape.4 The fear and anxiety associated with noise aversion is a welfare concern for dogs, making treatment critical. Treatment for noise aversion includes environmental and behavioural modification, as well as pharmacological therapy.
Frequently used pharmacologicak agents include episodic treatments such as trazodone,5 benzodiazepines6 7 and clonidine8; these have been used alone or in combination with a baseline (daily) medication such as a selective serotonin reuptake inhibitor. The efficacy of these medications for noise aversion has only been systematically studied for alprazolam (in combination with clomipramine6). A retrospective study5 and case report9 have reported on the efficacy of trazodone, but no clinical trials have been published using trazodone specifically for noise aversion. Recently, two medications received FDA (Food and Drug Administration) approval for the treatment of noise aversion in dogs: an oromucosal gel formulation of dexmedetomidine (Sileo, Zoetis) and a partial benzodiazepine receptor agonist, imepitoin (Pexion, Boehringer-Ingelheim Vetmedica). In placebo-controlled clinical trials, these medications were shown to decrease signs of reactivity during a particular noise event (fireworks).10 11 Dexmedetomidine is a centrally acting alpha-2 agonist with anxiolytic and (at higher doses) sedative effects mediated through action in the locus coeruleus.12 The gel formulation provides anxiolysis without sedation and can be repeated, as needed, every two hours, up to four times (for a total of five doses), as needed during a noise event. Since its approval, there has been anecdotal information to suggest that over time, dogs have less need for treatment with dexmedetomidine gel. From a learning theory perspective, this would make sense; as dogs experience a reduction in anxiety during a noise event, they gradually learn that the previously aversive noise is not a threat, resulting in a lowered or absent reaction to the noise.13 While this explanation is reasonable, it has not been evaluated systematically in a group of affected dogs. The primary objective of this pilot study was to evaluate whether repeated administration of dexmedetomidine gel, over a series of consecutive noise events, would reduce the need for future treatment in dogs with noise reactivity. A secondary objective was to gather preliminary information on the burden of care assessed by owners of dogs with noise aversion.
We hypothesised that when given doses of dexmedetomidine gel over a series of 10 noise events, dogs would decrease their response to the noise, resulting in a reduction in the need for pharmacological treatment. We further predicted that dexmedetomidine gel would decrease the signs of anxiety postadministration and that it would be easy to administer with few adverse effects. Finally, we predicted that owners will report a burden of care that impacts their quality of life and their relationship with their dog.
Materials and methods
All dog owners provided written informed consent to participate, and dogs remained with their owners during and following the study. We recruited 24 dogs with a history of noise aversion of at least six months’ duration. To be eligible, dogs had to be exposed to their noise triggers at least once per week; for thunder, a longer interval was allowed as timing of thunder is unable to be predicted. Owners were recruited from the area around North Carolina State University (NCSU) through flyers emailed to the College of Veterinary Medicine (CVM) community, targeted vet clinics near Ft. Bragg artillery range and through social media postings (Facebook and Twitter). Interested owners completed a survey that served as a prescreening; owners of potentially eligible dogs were then contacted by email or phone to set-up a screening visit.
At screening, owners completed a Noise Aversion Survey (which included questions about severity of signs and overall burden of care), a Burden-of-Care Survey (which included more specific details about burden of care and was based on prior work evaluating caregiver burden in general health conditions ((unpublished)) and provided informed consent for the study. Owners were told that the study was to evaluate the way dogs respond to treatment with dexmedetomidine gel but remained naïve to the hypothesis of the study. Dogs received routine physical examinations, and previous medical records were evaluated. To be eligible, dogs needed to be generally healthy and free of serious gingival disease with no known history of sensitivity to alpha-2 agonists. Owners were queried about any medications their dogs were receiving and any medications owners had previously tried for treating their dog’s noise aversion. Dogs were excluded if they were receiving behavioural medication for any reason or nutraceutical for the purpose of treating noise aversion (supplements for other conditions such as joint pain were allowed if they had been at a stable dose). Dogs were also excluded if they had begun a new behavioural modification programme during the last month, and owners were instructed not to begin any new behavior modification programmes for the duration of the study.
During the screening visit, individual noise triggers to be followed during the study were identified. Eligible triggers included: traffic, construction, fireworks, storms/thunder, vacuum, gunshots and household appliances; other triggers were allowed if they were described by the owner, consistently elicited a fearful response from their dog, and occurred at least once per week. After the screening visit, noise events were defined as any occurrence of noise triggers that had previously been identified as a trigger for that dog and that occurred during a 24-hour period, whether as a single, continuous or recurring noise.
Dogs were prescribed dexmedetomidine gel at the label dose for their weight to be administered into the buccal pouch. Owners were provided with enough dexmedetomidine gel for at least 10 noise events and received instructions and a demonstration for the use of dexmedetomidine gel. They were also provided with instructions about dose adjustment (lowering) if needed, and reversal information was provided for the referring veterinarian. Owners were instructed that if their dog was given a score of >2 for responsiveness or ability to walk (described under outcome measures), they were to reduce the dose by one dot for the next noise event when treatment was administered.
For each noise event, owners were then asked to administer dexmedetomidine gel to their dog as directed if their dog was showing signs of noise aversion. Before administration, owners were asked to rate the intensity of the noise trigger and score each dog’s signs of anxiety using a scale consisting of 14 items, each scored from 0 (none) to 4 (continuously). Items included: trembling, vocalising, pacing, seeking people, trying to hide, trying to escape, freezing, cowering, hypervigilance, salivation, panting, refusing food/treats, inappropriate urination and inappropriate defecation; total score could range from 0 to 56. Sixty minutes after the application of dexmedetomidine gel, they completed a second questionnaire about the severity and duration of the noise event and their dog’s anxiety using the same scale. They also scored ease of administration and the dogs’ postadministration responsiveness and ability to walk on a four-point scale. If needed, additional doses of dexmedetomidine could be repeated every two hours up to a total of five doses (with a questionnaire completed after each dose). However, if an identified triggering noise event occurred and the dog was not showing signs of noise aversion, owners were asked to still complete the questionnaire about the noise event (severity and duration) and indicate that dexmedetomidine gel was not given. The study continued for each dog until they had a total of 10 noise events (with no requirement for the number of doses of dexmedetomidine gel that needed to be given). Owners were contacted every two weeks to confirm continued study participation, report the number of noise episodes experienced, gather information on any adverse events and determine if additional dexmedetomidine gel was needed to complete the study. Once 10 events were recorded, owners returned to the NCSU-CVM to return their paperwork and complete an end-of-study survey where they were asked about their impressions of treatment including severity of signs and current burden of care.
Outcome measures for the effect of repeated treatment included the need for treatment over time and overall response to treatment. Secondary measures included reduction in the signs of anxiety with administration of dexmedetomidine gel, level of alertness postdosing, ease of administration and any adverse events.
Statistics
Descriptive statistics were used to describe the study population. In order to examine the probability of the patient receiving a dose for an event, a logistic regression model was used. One model was fit using only the number of the event to determine if dexmedetomidine gel was administered less often as events continued. A second model was fit for the event number within each type of noise stimulus, censoring observations for a stimulus after any event in which it was present simultaneously with another to avoid potential biasing. A third logistic model was fit to determine if there was an effect specifically for thunder, subsetting the data from the second (censored) model to only those that had thunder as the event. A final model was fit to examine the probability of the patient receiving a dose for thunder to include all noise events where thunder was recorded without censoring. All models included a random intercept for the patient. A Bonferroni adjusted cut-off value of P<0.01 was used for significance in these analyses.
Scores for anxiety were summed across the 14 items on the anxiety scale for each predose and postdose ratings. A paired Wilcoxon test was used to compare predose and postdose scores for anxiety behaviours due to a lack of normality. To evaluate the effect of event number on anxiety scores, a linear mixed effect model was fit with the predose anxiety score as the response and the event number as the lone predictor with a random intercept included for each subject. Tabulations and proportions are shown for burden of care (before and after the study), ease of administration and adverse effects on responsiveness and walking.
Results
Animals
Twenty-four client owned dogs enrolled in the study. One owner was lost to follow-up due to ongoing health concerns (for the owner), and one owner discontinued treatment due to perceived lack of effect; this left 22 dogs who completed the study with data recorded for 10 events each. Enrolled dogs were an average age (±SD) of 6.5 years (±3.5 years); average weight (±SD) of 19.5 kg (±11.1 kg); and male:female ratio of 13:11. Many dogs were reactive to more than one type of noise; types of noises and the number of dogs reactive to each noise are shown in table 1. The median score for severity of signs was 3 (range 3–4; scale 1=no symptoms to 4=severe symptoms) and median score for burden of care was 2 (range 1–4; scale 1=no burden to 5=severe burden).
Table 1.
Number of dogs with each noise trigger identified at baseline
| Noise | Number of dogs |
| Loud party | 6 |
| Construction | 11 |
| Fireworks | 21 |
| Gunshots | 16 |
| Sirens | 3 |
| Sports events | 2 |
| Thunder | 20 |
| Traffic | 6 |
| Vacuum | 6 |
| Other: gym noise | 1 |
| Other: dishwasher/washing machine | 2 |
| Other: fire alarm | 2 |
| Other: neighbours (music and dryer) | 3 |
| Other: kids banging | 1 |
Owners were able to select noise triggers that their dog responded to from a list provided or to indicate an additional (unlisted) noise trigger using ‘Other’; dogs could respond to more than one noise trigger.
Treatment
Dogs were treated according to the product insert unless adverse effects or sedation were seen. Dogs received an average of 5 µg/kg (±2 µg/kg; range: 4–8 µg/kg) per dose. Five dogs required dose adjustments. Two dogs had dose reductions: one dog went from 0.5 ml dexmedetomidine oromucosal gel (8 µg/kg) to 0.25 ml (4 µg/kg), the other went from 1.25 ml dexmedetomidine oromucosal gel (4 µg/kg) to 1.0 ml (3 µg/kg) and then to 0.75 ml (3 µg/kg). Two dogs had dose increases: one dog increased from 1.0 ml dexmedetomidine oromucosal gel (4 µg/kg) to 1.25 ml (5 µg/kg), the other increased from 0.75 ml dexmedetomidine oromucosal gel (4 µg/kg) to 1.0 ml (5 µg/kg) but then withdrew from the study due to perceived lack of efficacy. Finally, one dog received an accidental overdose of 2.0 ml (6 µg/kg); no adverse reaction was reported.
Time effect
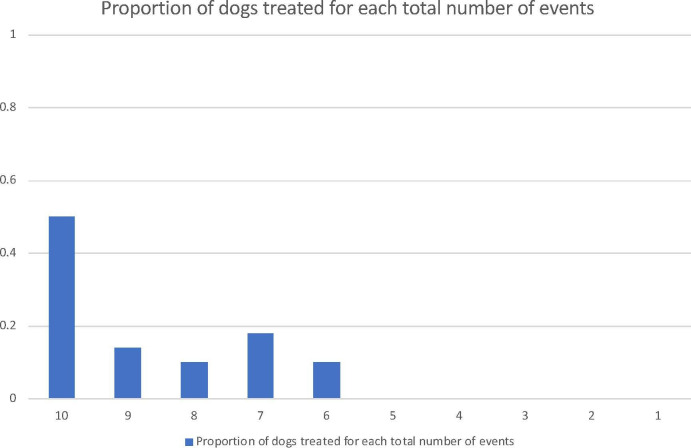
A time effect was found for the probability of dosing in the predicted direction. With each subsequent event, the odds of the owner dosing the patient decreased by over 25 per cent (table 2). Dogs received dexmedetomidine treatment for a range of 6–10 of their 10 recorded events each; 11 of 22 dogs (50 per cent) did not require treatment for at least one of the 10 events. The proportion of dogs who received treatment for each total number of events is shown in figure 1.
Table 2.
Logistic regression model of the overall effect of event number (time) on the probability of dogs being administered a dose of dexmedetomidine oromucosal gel
| Estimate | se | Z value | P(X>Z|) | |
| Intercept | 4.31 | 0.83 | 5.17 | 2.38×10–7 |
| Event number | −0.28 | 0.09 | −3.138 | 0.0017 |
Odds of being administered a dose equal e−0.28 = 0.75 (25 per cent decrease with each subsequent event).
Figure 1.
Proportion of dogs who received treatment for each total number of events. Eleven of 22 dogs received treatment for all 10 events, while 11/22 dogs received treatment for between six and nine total events. No dogs received treatment for less than five events.
Time effect within a given noise stimulus
No time effect was found within stimuli for the probability of dosing; during subsequent events of a particular stimulus, owners were not significantly less likely to dose their dog (table 3). Since the above model made no attempt to control for the type of event, it saw all 10 events in sequence within each patient. The model for individual stimuli, however, saw each stimulus repeated fewer times, thus making an effect more difficult to demonstrate.
Table 3.
Logistic regression model of the effect of event number (time) on the probability of dogs being administered a dose of dexmedetomidine oromucosal gel for individual noise stimuli
| Estimate | se | Z value | P(X>Z|) | |
| Intercept | 5.00 | 1.87 | 2.67 | 0.008 |
| Event number | −0.389 | 0.192 | −2.025 | 0.043 |
The negative estimate shows that the probability of dosing decreases with subsequent events; however, this decrease is not statistically significant. This may be due to the larger se in this model and the fewer occurrences of each stimulus.
Time effect within a particular stimulus (thunder)
No time effect was found for the probability of dosing during thunder; during subsequent events, owners were not significantly less likely to dose their dog (table 4). The magnitude of the estimate is again similar to that of the overall model but with a larger se that may be related to sample size of the observations.
Table 4.
Logistic regression model of the effect of event number (time) on the probability of dogs being administered a dose of dexmedetomidine oromucosal gel for only events involving thunder
| Estimate | se | Z value | P(X>Z|) | |
| Intercept | 3.34 | 1.02 | 3.29 | 0.001 |
| Event number | −0.17 | 0.15 | −1.18 | 0.238 |
The prior model censored thunder events if they occurred with another stimulus. For example, if there was a noise event that involved both thunder and fireworks, future events involving thunder were not included. Using the uncensored data, with all events that involved thunder included, the same model was fit with results shown in table 5. While still not significant using our adjusted P value, these results show a decreased probability of subsequent dosing when the stimulus type was thunder.
Table 5.
Logistic regression model of the effect of event number (time) on the probability of dogs being administered a dose of dexmedetomidine oromucosal gel for only events involving thunder (without censoring)
| Estimate | se | Z value | P(X>Z|) | |
| Intercept | 5.48 | 2.05 | 2.68 | 0.007 |
| Event number | −0.37 | 0.17 | −2.11 | 0.035 |
In this model, all events where thunder occurred were included in the model (uncensored data).
Effect on anxiety scale scores
Dexmedetomidine gel successfully decreased the overall score for patients’ anxiety behaviours (V=14 028, n=177, P<0.0001). The quartiles range from 7 to 18 points of change on the 14-question questionnaire with a median difference of 11 points. The linear mixed effects model (to evaluate the effect of event number on predose anxiety scores) found a significant effect (P=0.005) with an average decrease of 0.43 points for each subsequent event (se=0.151).
Sedation and responsiveness
The majority of dogs remained fully responsive and able to walk normally one hour after each dose of dexmedetomidine gel. The cross-tabulation of responsiveness and ability to walk are shown in table 6. Three dogs (over five events) received a score of >2 for responsiveness and/or ability to walk, triggering a dose reduction of 0.25 ml for subsequent dosing (however, the owner of one dog did not reduce the dose). Other adverse effects reported included licking lips postdose (two dogs) and hypersalivation (one dog).
Table 6.
Cross-tabulation of walking and responsiveness scores postdosing
| Responsiveness | Ability to walk | |||
| 1 | 2 | 3 | 4 | |
| 1 | 81 | 11 | 0 | 0 |
| 2 | 14 | 36 | 3 | 0 |
| 3 | 0 | 1 | 1 | 0 |
| 4 | 0 | 0 | 0 | 0 |
Not all owners reported both responsiveness and ability to walk scores for all recorded events. Scoring rubric for responsiveness: 1=fully responsive (dog responds as usual to your call); 2=responsive to your call but slow to respond because of lack of motivation or the dog is normally tired at this time of day; 3=the dog is slow to respond due to abnormal lack of alertness; 4=the dog is unresponsive to your call, abnormally drowsy or sleepy. Scoring rubric for ability to walk: 1=stands and walks normally across the room; 2=slow to stand but walks normally; 3=reluctant to stand and hesitates to move/walk is uncoordinated; 4=unable to stand or walk.
Burden of care and impressions of treatment
At baseline, at least 25 per cent of owners indicated agreement with statements indicating some frustration and guilt due to their dog’s health (noise aversion; table 7). Greater than 50 per cent of the owners indicated agreement for one item: ‘In the past four weeks, caring for my dog’s health has made me sad’. Results for statements with at least 25 per cent of owners responding as ‘Agree’ or ‘Strongly agree’ are shown in table 7. Most owners reported relatively low levels of agreement with statements regarding overall burden and impacts on their relationship with their dog (full results in online supplemental materials).
Table 7.
Proportion of responses for burden of care survey items with at least 25 per cent agreement from owners
| Strongly disagree | Disagree | Neither agree nor disagree | Agree | Strongly agree | Total disagree | Total agree | |
| In the past four weeks, caring for my dog’s health has been tiring | 0.20 | 0.25 | 0.25 | 0.29 | 0 | 0.46 | 0.29 |
| In the past four weeks my dog has interrupted my sleep because of its health problems | 0.25 | 0.37 | 0.04 | 0.25 | 0.08 | 0.62 | 0.33 |
| In the past four weeks I have done less physical activity with my dog (such as walking) because of my dog’s health | 0.46 | 0.21 | 0.04 | 0.25 | 0.04 | 0.67 | 0.29 |
| In the past four weeks I have felt embarrassed by my dog’s health | 0.42 | 0.29 | 0.04 | 0.17 | 0.08 | 0.71 | 0.25 |
| In the past four weeks my dog’s health has frustrated me | 0.25 | 0.17 | 0.17 | 0.33 | 0.08 | 0.42 | 0.42 |
| In the past four weeks I have felt aggravated because of my dog’s health | 0.37 | 0.21 | 0.12 | 0.21 | 0.08 | 0.58 | 0.29 |
| In the past four weeks, caring for my dog’s health has been stressful | 0.21 | 0.25 | 0.08 | 0.37 | 0.08 | 0.46 | 0.46 |
| In the past four weeks, caring for my dog’s health has made me sad | 0.12 | 0.08 | 0.04 | 0.37 | 0.37 | 0.21 | 0.75 |
| In the past four weeks I have worried that my dog will need more care in the future | 0.21 | 0.12 | 0.42 | 0.21 | 0.04 | 0.33 | 0.25 |
| In the past four weeks because of my dog’s health I have been afraid of what the future may hold for my dog | 0.42 | 0.12 | 0.21 | 0.21 | 0.04 | 0.54 | 0.25 |
| In the past four weeks I have felt guilty that I should have been doing more to care for my dog | 0.25 | 0.29 | 0.04 | 0.37 | 0.04 | 0.54 | 0.42 |
vr.106046supp001.pdf (53.1KB, pdf)
At the end of the study, owners were asked again about their dog’s severity of signs and how severity and burden of care had changed during the study. The median score for severity of signs remained at 3; however, the range was now 2–4 (vs 3–4 at baseline) with nine owners indicating a score of 2 (vs zero owners indicating a score of 2 at baseline). On a scale of 1=much better to 7=much worse, the median rating for noise aversion compared with 10 days ago was 2 (range 1–2) and the median rating for burden of care compared with 10 days ago was 2.5 (range 1–4).
Discussion
This study found that treatment with dexmedetomidine gel over a series of noise events reduced the need for treatment on subsequent events. This supports our hypothesis that dogs may become more tolerant of noise events when they are not associated with anxiety, thus needing less frequent dosing on repeated exposure. This observation fits with an exposure treatment theory: when the dog is exposed to an anxiety-provoking stimulus and is able to remain calm, the dog will gradually become desensitised to the stimulus. In this model, the dog’s appetitive (positive) response opposes the aversive (negative) response and works when the appetitive response is stronger than the aversive response. Similarly, an inhibitory learning framework may also be applied in which the dog learns a new association (safety) for the previously anxiety-provoking stimulus.13 Multiple neurotransmitters (norepinephrine, serotonin and dopamine) and brain regions (amygdala, hippocampdus an locus ceurelus) are involved in the response to noise triggers.14 There is a risk for sensitisation (rather than habituation or desensitisation) if the noise trigger evokes a fear response; this is mitigated by appropriate recognition of fear by dog owners, anxiolytic treatment and counterconditioning.
Many dogs were reactive to multiple noise stimuli, including construction, fireworks, gunfire and thunder. This is not uncommon in dogs with noise aversion1 2; however, we were interested in whether the effect of dosing over repeated events would have more effect for one stimulus versus another. When the data were analysed for individual stimuli, there was no significant effect on the probability of dosing for subsequent events. Similar results were found when analysing only the effect of treatment during repeated thunder as the stimulus. For both analyses, the magnitude of the estimated effect was very similar to that of the overall time effect; the larger se in these models may be indicative of a need for a larger sample for each stimulus rather than a lack of effect. When all thunder events were included in the analysis (ie, the data were not censored), there was more evidence that the probability of dosing decreased.
Dexmedetomidine gel, when administered, was effective at reducing dogs’ scores on the anxiety scale. The anxiety scale asked owners about their dog’s intensity of performing a series of 14 behaviours that are associated with anxiety.10 There was a highly significant change (improvement) in score for the postdose assessment compared with the predose assessment. In addition, the median decrease (11 points) provide strong support for this effect. The effectiveness of dexmedetomidine gel has been shown previously in a randomised, placebo-controlled trial,10 so this finding is not surprising but is supportive of the positive effect. Furthermore, there was a significant decrease in average predose anxiety score over subsequent events; this supports the assertion that dogs have a decrease in their response to anxiety triggers over repeated dosing. While the average decrease was small, across events this represented a clinically relevant decrease in anxiety score. Owners also rated improvement in their dog’s severity of signs, overall noise aversion and burden of care. In addition, and as shown previously,10 administration of dexmedetomidine gel was not associated with adverse events; the majority of dogs were fully responsive and able to walk normally one-hour postdosing. This is important as habituation and counterconditioning are facilitated by experiencing the noise event while relaxed but fully aware. Only three dogs received scores above 2 for responsiveness and/or ability to walk; two of these dogs received dose reductions, while the third was maintained at her dose with no scores above 2 for subsequent events. No dogs were scored as unresponsive to their owner’s call or unable to stand for any dosing event, and the treatment was well tolerated overall.
There are several limitations of the study that warrant discussion. First, this was an open-label trial; all owners were aware that they were giving their dog active treatment. Owners were naïve to the study hypothesis, however, to avoid influencing their administration of the treatment. Owners were aware that the study was evaluating the effect of repeated doses of dexmedetomidine gel on noise aversion in their dog and that they were being asked to record any ‘events’ regardless of whether they treated their dog or not. While not explicitly describing the hypothesis of the study, it remains possible that owners would guess the hypothesis and change their behaviour. This possibility is tempered by the fact that owners joined the trial actively looking for a treatment for their dog’s noise aversion and would be unlikely to skip treatment if treatment was needed. The open-label nature of the study may, however, have affected their predose and postdose anxiety scale assessments. The significant decrease in anxiety scores was dramatic; thus, even with this limitation, it is likely that the scores represent a true decrease in anxiety behaviours following treatment. Second, as dogs could be treated for multiple noise triggers, it was not possible to determine whether the effect of repeated dosing was more evident for a particular trigger. This does, however, closely resemble the clinical condition, where dogs are often reactive to more than one noise trigger.1 15
Conclusions
Overall, this study found that repeated use of dexmedetomidine gel for noise events resulted in a decreased need for administration over time. The number of events varied for each patient, with a range of 6–10 treated events for each dog. This is important for discussing with owners: repeated use may result in a decrease in need for the treatment, yet individual results may vary. However, it is promising that 50 per cent of the dogs in the study did not require treatment for at least one event. We did not find that the response was different for individual stimuli, including thunder; however, the estimate of the effect size was of similar magnitude to the overall effect. In the future, selecting dogs with reactivity to one stimulus, thunder for example, and following only thunder events may provide further evidence regarding the effect of repeated use. An additional study could also provide consistent dosing for a set number of events (five, for example) and then begin to evaluate whether dogs required treatment for subsequent events.
As a secondary objective, this study also found that, for the owners who participated in this study, there was a moderate burden of care associated with their dog’s noise aversion. While none of the owners in this study indicated that their dog’s noise aversion strongly impacted their relationship with their dog, there was agreement for statements about frustration and guilt. The highest proportion of owner agreement was shown for sadness; owners indicated that caring for their dog’s health had made them sad over the last four weeks. This finding regarding sadness may be a reflection of the decreased welfare of dogs with noise aversion. The fear and anxiety experienced by dogs with noise aversion can be profound; particularly when triggers occur regularly, this is detrimental to a dog’s quality of life. Indeed, a recent study investigating caregiver burden in owners of pets with behavioural problems found that owners remarked on feeling ‘sad that (they were) unable to resolve their [dog’s] fear and anxiety’.16 This is important for veterinarians to recognise when counselling owners of dogs with noise reactivity. Treatment for noise aversion is critical, and support for owners of dogs with this condition is needed, particularly acknowledging the frustration and sadness inherent in caring for a dog with anxiety and noise aversion. Improvement in owner ratings for severity and burden of care further support the importance of treating noise aversion in dogs.
Acknowledgments
The authors would like to thank the clients and dogs who participated in the study. We would also like to thank Amy Pike for her contributions to the original study design and outcome assessments. Finally, we would like to thank Dr Margaret Gober Thompson for sharing the Caregiver Burden questionnaire with us for this project.
Footnotes
Funding: This work was sponsored by Zoetis.
Competing interests: SC is an employee of Zoetis, and MEK is an employee of Orion Corporation. MG has been a paid consultant in veterinary behavioural medicine.
Ethics approval: All procedures were performed with approval from the North Carolina State University College of Veterinary Medicine Institutional Animal Care and Use Committee (Protocol 18–153-O).
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: Data may be obtained from a third party and are not publicly available. Please contact the corresponding author (MEG) with any enquiries.
References
- 1. Tiira K, Sulkama S, Lohi H. Prevalence, comorbidity, and behavioral variation in canine anxiety. J Vet Behav 2016;16:36–44. 10.1016/j.jveb.2016.06.008 [DOI] [Google Scholar]
- 2. Blackwell EJ, Bradshaw JWS, Casey RA. Fear responses to noises in domestic dogs: prevalence, risk factors and co-occurrence with other fear related behaviour. Appl Anim Behav Sci 2013;145:15–25. 10.1016/j.applanim.2012.12.004 [DOI] [Google Scholar]
- 3. Overall KL, Dunham AE, Frank D. Frequency of nonspecific clinical signs in dogs with separation anxiety, thunderstorm phobia, and noise phobia, alone or in combination. J Am Vet Med Assoc 2001;219:467–73. 10.2460/javma.2001.219.467 [DOI] [PubMed] [Google Scholar]
- 4. Overall KL, Dunham AE, Juarbe-Diaz SV. Phenotypic determination of noise reactivity in 3 breeds of working dogs: a cautionary tale of age, breed, behavioral assessment, and genetics. J Vet Behav 2016;16:113–25. 10.1016/j.jveb.2016.09.007 [DOI] [Google Scholar]
- 5. Gruen ME, Sherman BL. Use of trazodone as an adjunctive agent in the treatment of canine anxiety disorders: 56 cases (1995-2007). J Am Vet Med Assoc 2008;233:1902–7. 10.2460/javma.233.12.1902 [DOI] [PubMed] [Google Scholar]
- 6. Crowell-Davis SL, Seibert LM, Sung W, et al. Use of clomipramine, alprazolam, and behavior modification for treatment of storm phobia in dogs. J Am Vet Med Assoc 2003;222:744–8. 10.2460/javma.2003.222.744 [DOI] [PubMed] [Google Scholar]
- 7. Herron ME, Shofer FS, Reisner IR. Retrospective evaluation of the effects of diazepam in dogs with anxiety-related behavior problems. J Am Vet Med Assoc 2008;233:1420–4. 10.2460/javma.233.9.1420 [DOI] [PubMed] [Google Scholar]
- 8. Ogata N, Dodman NH. The use of clonidine in the treatment of fear-based behavior problems in dogs: an open trial. J Vet Behav 2011;6:130–7. 10.1016/j.jveb.2010.10.004 [DOI] [Google Scholar]
- 9. Gruen ME, Sherman BL. Animal behavior case of the month: thunderstorm phobia. J Am Vet Med Assoc 2012;241:1293–5. 10.2460/javma.241.10.1293 [DOI] [PubMed] [Google Scholar]
- 10. Korpivaara M, Laapas K, Huhtinen M, et al. Dexmedetomidine oromucosal gel for noise-associated acute anxiety and fear in dogs-a randomised, double-blind, placebo-controlled clinical study. Vet Rec 2017;180:356. 10.1136/vr.104045 [DOI] [PubMed] [Google Scholar]
- 11. Engel O, Müller HW, Klee R, et al. Effectiveness of imepitoin for the control of anxiety and fear associated with noise phobia in dogs. J Vet Intern Med 2019;33:2675–84. 10.1111/jvim.15608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tanaka M, Yoshida M, Emoto H, et al. Noradrenaline systems in the hypothalamus, amygdala and locus coeruleus are involved in the provocation of anxiety: basic studies. Eur J Pharmacol 2000;405:397–406. 10.1016/S0014-2999(00)00569-0 [DOI] [PubMed] [Google Scholar]
- 13. Blakey SM, Abramowitz JS. The effects of safety behaviors during exposure therapy for anxiety: critical analysis from an inhibitory learning perspective. Clin Psychol Rev 2016;49:1–15. 10.1016/j.cpr.2016.07.002 [DOI] [PubMed] [Google Scholar]
- 14. Keller NE, Hennings AC, Dunsmoor JE. Behavioral and neural processes in counterconditioning: past and future directions. Behav Res Ther 2020;125:103532. 10.1016/j.brat.2019.103532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Separation BKC. Confinement or noises: what is Scaring that dog? Vet Clin N Am-Small 2018;48:367. [DOI] [PubMed] [Google Scholar]
- 16. Buller K, Ballantyne KC. Living with and loving a pet with behavioral problems: Pet owners’ experiences. J Vet Behav 2020;37:41–7. 10.1016/j.jveb.2020.04.003 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
vr.106046supp001.pdf (53.1KB, pdf)