Abstract
EFSA received a mandate from the European Commission to assess the effectiveness of some of the control measures against diseases included in the Category A list according to Regulation (EU) 2016/429 on transmissible animal diseases (‘Animal Health Law’). This opinion belongs to a series of opinions where these control measures will be assessed, with this opinion covering the assessment of control measures for African Swine Fever (ASF). In this opinion, EFSA and the AHAW Panel of experts reviewed the effectiveness of: (i) clinical and laboratory sampling procedures, (ii) monitoring period and (iii) the minimum radius of the protection and surveillance zone, and the minimum length of time the measures should be applied in these zones. The general methodology used for this series of opinions has been published elsewhere; nonetheless, specific details of the model used for the assessment of the laboratory sampling procedures for ASF are presented here. Here, also, the transmission kernels used for the assessment of the minimum radius of the protection and surveillance zones are shown. Several scenarios for which these control measures had to be assessed were designed and agreed prior to the start of the assessment. In summary, several sampling procedures as described in the diagnostic manual for ASF were considered ineffective and a suggestion to exclude, or to substitute with more effective procedures was made. The monitoring period was assessed as non‐effective for several scenarios and a longer monitoring period was suggested to ensure detection of potentially infected herds. It was demonstrated that the surveillance zone comprises 95% of the infections from an affected establishment, and therefore is considered effective. Recommendations provided for each of the scenarios assessed aim to support the European Commission in the drafting of further pieces of legislation, as well as for plausible ad hoc requests in relation to ASF.
Keywords: African Swine Fever, Suids, disease control measures, vector borne disease
Summary
This opinion is part of a series of opinions, in which the three‐first Terms of Reference (ToR) of a mandate received from the European Commission (EC) have been considered. The background and specific details of this mandate can be found in the opinion. The ToRs in this mandate request an assessment of the effectiveness of:
the clinical and laboratory examination in their capacity to detect disease (or estimate the disease prevalence within an establishment), either in suspect or confirmed animals in a single establishment, or in establishments within restricted zones (ToR 1);
the effectiveness of the duration of the monitoring period (for different scenarios) in the control of suspected and confirmed outbreaks (ToR 2);
the size and duration of the restriction zones, in their capacity for mitigating disease spread (ToR 3).
In order to harmonise the approach to these assessments, the methodology used in this series of opinions, covering all Category A diseases, was agreed on, and published in a separate technical report (EFSA, 2020a).
A qualitative assessment of the existing clinical examination procedures for African swine fever virus (ASF) was carried out. For assessing the effectiveness of the laboratory examination, the within‐herd dynamics of African swine fever virus (ASFV) were modelled for five different scenarios (simulating spread with current, as well as historic, ASFV strains of different properties as regards e.g. virulence), using a stochastic SEIR epidemic model. More specifically, the number of dead pigs and prevalence of infectious and seropositive pigs, respectively, at different time points post‐introduction of the virus to the herd as predicted by the model were used for the assessment. Further, scripts were written that allowed the calculation of the median time (days) to reach a 10% seroprevalence and infection prevalence, respectively, in a herd, as well as time to detection of a potential ASF outbreak in a herd given a surveillance scheme based on weekly sampling of at least two dead post weaning pigs for virus detection. The assessment confirmed the effectiveness for early detection in the event of a suspicion (within 13 days post‐infection) of the collection of samples from at least five pigs (dead or with clinical signs if a sufficient number of dead pigs is not found) for detection of virus. In contrast, a random sample aiming at 10% seroprevalence would lead to a delayed detection in all scenarios and was assessed as not effective. Further, reducing the seroprevalence to be detected was not considered effective either for early detection. For surveillance purposes aiming at early detection in the absence of a suspicion, a weekly sampling of at least two dead post weaning pigs or pigs older than 2 months in each epidemiological unit was assessed as effective, and would lead to virus detection at median times of between 10 and 14 days post‐infection assuming a 3% baseline mortality.
To answer ToR 2, and to assess the minimum length of time measures should be implemented in the protection and surveillance zones (ToR 3.2), an extensive literature search (ELS) was carried out. This ELS aimed to assess the average, shortest and longest period between the earliest point of infection of a pig herd with an ASFV virus, and the time of reporting of a suspicion by the competent authority. The average time to the reporting of a suspicion report was used then to assess the effectiveness of the length of the monitoring period. For some of the scenarios, the existing length of the monitoring period for ASF (15 days) was considered sufficient. However, for other scenarios, the length of the monitoring period is considered effective only for outbreaks occurring in small farms. Because of the initial low mortality, the detection of an outbreak in large herds could be delayed. In those cases, extending the length of the monitoring period to 23 days is recommended. To assess the effectiveness of the minimum length of time, the measures should be applied in the protection and surveillance zones, the average and the longest time assessed via the ELS were used, respectively. Based on this, the minimum duration of the protection zone (15 days) and the surveillance zone (30 days), according to existing legislation) was considered effective.
To assess the effectiveness of the minimum radius to be implemented in the protection and surveillance zones (ToR 3.1), transmission kernels were used. However, in the absence of kernels estimated for ASF, available kernels for Classical swine fever (CSF) were used. These kernels were built using data from previous outbreaks and represent the relative risk of transmission to each individual establishment from the affected establishment. Assuming the transmission occurs from an affected establishment, the probability of ASF transmission beyond the protection zone and surveillance zone was 2 and 0.2%, respectively. The minimum radius was thus considered highly effective if/when focusing on the control of the spread of the disease among and between domestic pig herds. It is important to note, however, that the transmission kernels presented cover only some of the risk pathways associated with spread from the index case and that these probabilities do not take into account the risk of transmission due to wild boar, or movements of live animals and products off the establishment prior to confirmation.
1. Introduction
1.1. Background and Terms of Reference as provided by the requestor
Regulation (EU) 2016/429 on transmissible animal diseases (‘Animal Health Law’), hereinafter referred to as AHL, requires the Commission to lay down detailed rules on the disease control measures against listed diseases as referred to in point (a), (b) and (c) of its Article 9 (category A, B and C diseases). The Commission is empowered to adopt delegated acts supplementing the rules laid down in Part III of Regulation (EU) 2016/429 on transmissible animal diseases (Animal Health Law) on disease control measures for listed diseases as referred to in point (a), (b) and (c) of its Article 9 (category A, B and C diseases). Therefore, the Commission has developed and adopted a Delegated Regulation laying down rules for the prevention and control of certain diseases (‘the Delegated Regulation’). The rules laid down in the Delegated Regulation are in respect of terrestrial animals largely replicating the rules currently in force concerning the disease control measures in the event of animal diseases with serious effects on the livestock as they have proven to be effective in preventing the spread of those diseases within the Union. Consequently, many animal disease control measures laid down in existing Directives will be, to the extent that not already done by the Animal Health Law, replaced by the rules provided in the Delegated Regulation. At the same time, these rules have been aligned with the international standards from the World Organisation for Animal Health (OIE), wherever these existed. However, certain disease control measures proposed in the Delegated Regulation, in particular in its Annexes, were considered as outdated i.e. possibly not based on most recent scientific evidence at the time of development. Their review is considered as necessary. Moreover, for those category A diseases for which rules were not established before or were not detailed enough, certain disease control and risk mitigating measures are, due to the lack of scientific basis, extrapolated from other diseases, for which rules existed in the past. Finally, for some other diseases the evidence and scientific knowledge, was not available to the Commission and to the Member States at the time of developing the Delegated Regulation due to the time constraints. The following diseases are examples of the later: infection with Rift Valley fever (RVF), infection with Mycoplasma mycoides subsp. Mycoides SC (Contagious bovine pleuropneumonia) (CBPP), Contagious caprine pleuropneumonia (CCPP), Sheep pox and goat pox, infection with peste des petits ruminants virus (PPR), African horse sickness (AHS), Glanders. In this regard, the existing rules will cease to apply as from the date of application of the Animal Health Law and its complementing legislation including the Delegated Regulation, i.e. from 21 April 2021. Certain of the proposed measures for the prevention and control of category A diseases of terrestrial animals should therefore be assessed in order to ensure that they are effective and updated based on the latest scientific knowledge in this new set of legislation. This is particularly important in the case of those diseases that are less common or have been never reported in the Union.
1.1.1. ToR 1: Sampling of animals and establishments for the detection of category A diseases in terrestrial animals
Based on available scientific information, assess the effectiveness of existing sampling procedures to detect or rule out the presence of each category A disease of terrestrial animals and, in case of absence of effective procedures, develop them, in order to complete the rules provided for in Annex I to the Delegated Regulation. In particular, provide for disease‐specific procedures for the sampling of:
ToR1.1 Animals for clinical examinations to ensure the detection of the relevant category A disease during the performance of official investigations in establishments that are affected or suspected to be affected by category A diseases and visits in establishments located in restricted zones in accordance with Articles 6(2), 13(3)(c), 14(1) and 26(2) of the Delegated Regulation.
ToR 1.2 Animals for laboratory examinations to ensure the detection of the relevant category A disease during the performance of official investigations in establishments that are affected or suspected to be affected by category A diseases and visits in establishments located in restricted zones in accordance with Articles 6(2), 12(3), 13(3)(c), 14(1), 26(2) of the Delegated Regulation.
ToR 1.3 Establishments to ensure the detection of the relevant category A disease for the performance of visits in establishments located in protection zones larger than 3 km and establishments located in the surveillance zone in accordance with Articles 26(5) and 41 of the Delegated Regulation.
ToR 1.4 Animals for clinical and laboratory examinations to ensure the detection of the relevant category A disease for the movement of animals from restricted zones in accordance with Articles 28(5), 43(5), 56(1)(c) of the Delegated Regulation.
ToR 1.5 Animals for laboratory examinations to ensure the detection of the relevant category A disease before and after being introduced in the affected establishments for repopulation, in accordance with Article 59(2), (3) and (9) of the Delegated Regulation.
1.1.2. ToR 2: Monitoring period
1.1.2.1 ToR 2.1 Assess the effectiveness of the length of the monitoring periods set out in Annex II of the Delegated Regulation for each category A disease of terrestrial animals. In this regard, it is important to take into consideration that the monitoring period was introduced as a management tool, which represents a time frame of reference assigned to each category A disease for the competent authority to apply certain control measures and to carry out investigations in the event of suspicion and confirmation of category A diseases in terrestrial animals.
This assessment should be carried out with respect to the following situations:
the records analysis carried out by the competent authority in the framework of the epidemiological enquiry referred to in Article 57 of Regulation (EU) 2016/429, in the event of suspicion of a category A disease (Article 8(4) of the Delegated Regulation);
the derogation from killing in the event of an outbreak of a category A disease in establishments keeping animals of listed species in two or more epidemiological units (Article 13(1) of the Delegated Regulation);
the tracing carried out by the competent authority to identify establishments and other locations epidemiologically linked to an establishment affected by a category A disease (Article 17(2) of the Delegated Regulation);
the exemption applied to certain products from the prohibitions laid down in Annex VI taking into account the date they were produced (Article 27(3)(c) of the Delegated Regulation);
the specific conditions for authorising movements of semen from approved germinal product establishments in the protection and surveillance zones (Article 32(c) and 48(c) of the Delegated Regulation);
the repopulation of establishments affected by a category A disease (Article 57(1)(b) and 59(4)(b) of the Delegated Regulation).
1.1.2.2 ToR 2.2 Propose the length of what should be the monitoring period in those diseases for which the time is assessed as not effective.
1.1.3. ToR 3: Minimum radius of restricted zones and duration of the disease control measures in restricted zones
ToR 3.1 Assess the effectiveness to control the spread of the disease of the minimum radius of the protection and surveillance zones set out in Annex V of the Delegated Regulation for each category A disease of terrestrial animals.
ToR 3.2 Assess the effectiveness to control the spread of the disease of the minimum periods during which the competent authority should apply the restriction measures in the protection and surveillance zones as set out in Annex X and XI for each category A disease of terrestrial animals.
1.1.4. ToR 4: Prohibitions in restricted zones and risk‐mitigating treatments for products of animal origin and other materials
ToR 4.1 Assess the effectiveness to control the spread of disease of prohibitions set out in Annex VI of the Delegated Regulation with respect to the risk associated for each category A disease, to the listed activities and commodities.
ToR 4.2 Review the available scientific information on risk‐mitigating treatments that are effective to control the presence of category A disease agents in products of animal origin and other relevant materials. Based on this:
provide an opinion on the effectiveness of the risk‐mitigating treatments for products of animal origin and other materials produced or processed in the restricted zone set out in Annex VII and VIII, and
if relevant, suggest new treatments or procedures that can be effective to mitigate or to eliminate such risk.
1.2. Interpretation of the Terms of Reference
To address the ToRs of the mandate, EFSA proposed and agreed with the European Commission the following:
The publication of fourteen individual opinions, one per each of the diseases included in the list of category A diseases for terrestrial animals, with each of these opinions providing the answer to ToRs 1, 2 and 3. The current document is one of the 14 opinions covering ToRs 1, 2 and 3 for African Swine Fever (ASF).
The publication of a unique opinion covering ToR 4 for all diseases listed (i.e. ToR 4 is not covered in this opinion).
To address ToR 1 (effectiveness of sampling procedures), EFSA agreed with the EC on 21 scenarios (based on different articles of the Delegated Act) for which the effectiveness of the sampling procedures will be assessed (Annex C). Although these scenarios will be assessed independently, some of these scenarios may be merged if the assessment processes are the same.
To address ToR 2 (effectiveness of the monitoring period), 7 scenarios previously agreed with the contractor were defined (Annex D). The assessment of the effectiveness of the monitoring period will be done by assessing its ability to ensure that specific actions can be carried out without posing a risk of disease spread, if the monitoring period is calculated backwards or forwards from a specific date. If the length of the monitoring period estimated by EFSA is longer than the existing monitoring periods, the existing monitoring period will be considered non effective. If the length of the monitoring period estimated by EFSA is shorter than the existing monitoring period, this existing monitoring period will be considered effective from a disease control point of view. No assessment of the plausible unnecessary economic burden that may be placed on the stakeholders as a result of an excessive length of the monitoring periods will be done by EFSA.
The assessment of the minimum duration and the length of the radius of the protection and surveillance zones (ToR 3) will be done independently. The setting of these two zones (protection and surveillance zones) surrounding an affected establishment and the control measures implemented in each one of the zones are based on the general principle that the probability of disease spread is larger the closer the establishment is to an affected establishment. The validity of this statement will not be assessed in this manuscript; nonetheless the limitations that this assumption may have in the control of certain diseases will, when relevant, be discussed.
-
The following scenarios in ToR 1 (Annex C) were not relevant for ASF, and therefore were not included in the assessment:
scenario 4 because there are no non‐listed species for which ASFV sampling is recommended,
scenario 7 because the minimum radius of the protection zone for ASF is 3 km,
scenarios 10, 11, 16 and 17 because they refer to poultry, and
scenario 14 as it refers to ungulates.
The duration of the monitoring period for ASF as described in Annex II of the Delegated Regulation is 15 days.
The minimum length of the radius of the protection zone (PZ) and surveillance zone (SZ) for ASF as described in Annex V of the Delegated regulation are 3 and 10 km, respectively.
The minimum duration of the measures in the PZ and SZ for ASF as described in Annex X and XI of the Delegated Regulation are 15 and 30 days respectively.
2. Epidemiology and geographical distribution of African swine fever
2.1. Epidemiology
African swine fever (ASF) is a severe contagious haemorrhagic disease affecting all breeds of domestic swine and Eurasian wild boar. The agent is the ASF virus (ASFV), a double‐stranded DNA virus and sole member of the Asfarviridae family, genus Asfivirus (Galindo and Alonso, 2017).
ASF was first reported in the early 1900s from eastern and southern Africa (Mulumba‐Mfumu et al., 2019). Historically, the virus was introduced into Europe on two occasions, in 1957 and 1960, respectively, both times to the Iberian Peninsula (Costard et al., 2009). The second introduction led to the establishment of the disease on the Iberian Peninsula for three decades, with further spread within Europe and to South America and the Caribbean. Successful eradication in Europe was achieved in the 1990s (with the exception of Sardinia where it has remained endemic) until a third spill over event from the African continent to Europe occurred in 2007. This time ASFV was introduced to Georgia from where it spread through the Caucasus and the Russian Federation, reaching Poland and the Baltic states in 2014, and from there spreading further towards west and south. Since 2018, ASF has also spread widely in China and large parts of Asia (Dixon et al., 2020).
African wild suids, in particular warthogs, are the natural hosts of ASFV and can become infected via a biological vector – soft ticks of the Ornithodoros genus (Acari; Argasidae).1 This creates an ongoing sylvatic cycle that can spill over into domestic swine environments in Africa. In African wild suids, infection with ASFV results in asymptomatic infection (Jori and Bastos, 2009; Jori et al., 2013). However, in domestic swine and wild boar, infection can cause acute haemorrhagic fever with case fatality rates up to 100% in naïve populations infected with virulent strains (Blome et al., 2020).
Transmission of ASFV occurs through direct contact with infected animals (wild or domestic swine), ingestion of contaminated materials (e.g. swill feeding) or through indirect contact with fomites (bedding, vehicles, equipment, clothes, footwear, etc.) or soft ticks bites (Guinat et al., 2016).
ASF is a notifiable disease due to its serious economic impact to the pork and farming industry. As there is no vaccine nor treatment available, early detection is key, as well as responding quickly to outbreaks. A key component of early detection is passive surveillance, which relies on farmers, veterinarians, hunters and other professionals from the pig and wildlife industries to report suspicions of ASF in swine or wild boar immediately to veterinary authorities (Dixon et al., 2020).
Clinical signs and outcomes of ASF vary depending on species susceptibility, infectious dose and strain virulence. The incubation period is between 3 and 14 days. In acute forms, the clinical signs are high fever (> 40°C), watery and bloody diarrhoea, vomiting, haemorrhaging with bluish–purple spots on the body, ocular and nasal discharge, bloody froth from the mouth, nervous signs and abortion in pregnant sows. Death occurs 4–7 days after symptom onset in both domestic swine and wild boar (Gallardo et al., 2018), while in peracute forms, sudden death with few signs can occur. Case fatality rates in these two forms can reach up to 100%. In subacute forms, signs are less pronounced (depression, anorexia, weight loss), the disease course is longer (up to 30 days) and the mortality lower (30–70%) (Cruciere, 2003). The ASFV strains of genotype II currently circulating in Eastern and Central Europe as well as in Asia are highly virulent, and typically cause an acute to peracute form of the disease (Pikalo et al., 2019), although a limited number of genotype II viruses of lower virulence have also been isolated from ASF‐infected wild boar (Gallardo et al., 2018; Zani et al., 2018).
A chronic form of the disease typically associated with unspecific and rather mild clinical signs, may also occur. Such chronic infections have been generally associated with infection with attenuated genotype I strains that historically circulated in the Iberian Peninsula (Pikalo et al., 2019). Pigs with chronic infections will eventually succumb to the disease. To what extent pigs that survive an infection may remain infectious over time and be able to transmit the infection and thus play a role, as so‐called carriers, in the epidemiology of the disease is debated.
There are a wide number of validated diagnostic techniques available, including virus detection tests, antigen detection and polymerase chain reaction (PCR) techniques. Antibody tests are also available in the form of ELISAs, lateral flow devices and confirmatory tests, used for surveillance. For early detection of the virus, the reference technique is PCR on blood or organ (spleen, lymph nodes, tonsil, kidney) samples.
2.2. Geographical distribution of African swine fever
African swine fever entered EU in 2014 and since then, ASFV has been spreading through Eastern Europe and slowly expanding mainly in a south‐westerly direction (EFSA, 2020b). In the years 2015–2020, cases of ASF have been reported in 13 MSs – Belgium, Bulgaria, Czechia, Estonia, Germany, Greece, Hungary, Sardinia (Italy), Latvia, Lithuania, Poland, Romania and Slovakia – and many other countries in Europe, Asia and Africa (Figure 1).
Figure 1.
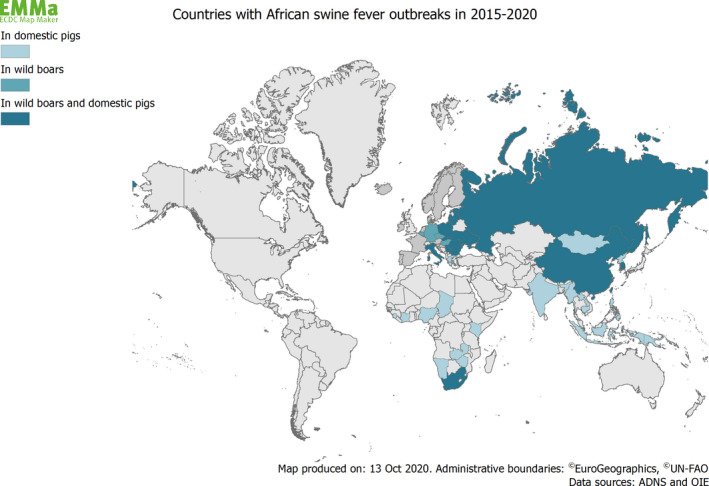
Map of countries with reported outbreaks of African swine fever between 2015 and 2020 (Data sources: ADNS and OIE). It should be noted that the disease is present in most countries in sub‐Saharan Africa, in spite of the absence of outbreak reports during the period in question (Mulumba‐Mfumu et al., 2019)
3. Data and methodologies
3.1. Methodology used in ToR 1
Although the general methodology applied to all opinions covering the assessment of control measures for the Category A diseases produced under this mandate has been published elsewhere (EFSA, 2020a), specific details of the methodology related to the ASF opinion are presented below.
Mathematical model and transmission scenarios considered
For the purpose of ToR 1 (i.e. to assess the effectiveness of available sampling procedures), the within‐herd dynamics of African swine fever virus (ASFV) were modelled for five different scenarios (simulating spread with current, as well as historic, ASFV strains of different properties as regards virulence), using a stochastic SEIR epidemic model (Keeling and Rohani, 2008). For scenarios with viruses of high virulence as those currently circulating in Europe, the pig population is divided into three classes: susceptible (i.e. uninfected; S), exposed (i.e. infected, but not yet infectious; E) and infectious (I). The survival rate of pigs or wild boar infected with such ASFV strains is very low, i.e. < 10% (Guinat et al., 2016) and may thus be ignored for the purpose of the model. For strains of lower virulence, the pig population is divided into four classes: susceptible (i.e. uninfected; S), exposed (i.e. infected, but not yet infectious; E), infectious (I) and recovered (R). Disease‐associated mortality was assumed to occur at a constant rate during the infectious period.
The force of infection is given by,
where β is the transmission rate, I(t) is the number of infectious pigs and N(t) is the total number of pigs at time t. This formulation assumes homogeneous mixing (i.e. individuals uniformly and randomly contact each other) and frequency‐dependent transmission (i.e. the number of contacts is independent of the population size) (Keeling and Rohani, 2008). The durations of the latent (time between infection by ASFV and the pigs becoming infectious) and infectious periods were assumed to follow gamma distributions with means μE and μI and shape parameters kE and kI, respectively (i.e. with variances μE 2/kE and μI 2/kI). This was incorporated in the model by subdividing the latent and infectious classes into kE and kI stages each of mean duration μE/kE and μI/kI, respectively (Anderson and Watson, 1980).
The number of pigs in each class in the model takes integer values, while transitions between compartments are stochastic processes. The number of transitions of each type during a short time interval δt was drawn from a binomial distribution with the number of pigs in the appropriate class n and transition probability q (the appropriate per capita rate multiplied by δt) as parameters.
The initial herd size was assumed to be 50, 200 or 1,000 pigs. Parameter estimates are given in Table 1. Those for moderately virulent strains (case fatality equal or below 50%) used in scenarios 1 and 2 (Malta 1978 and The Netherlands 1986, respectively) were estimated from transmission experiments (de Carvalho Ferreira et al., 2012, 2013). For scenarios 3–5 (scenarios based on Georgia 2007), these parameters were extracted from a study, which inferred transmission parameters from data on nine outbreaks in the Russian Federation (Guinat et al., 2018). A case fatality of 100% was used in these three scenarios with different reproduction rates – R0 – (low, medium and high, for scenarios 3, 4 and 5, respectively), although with similar beta transmission parameters for scenarios 4 and 5 (2.2).
Table 1.
Parameters used for modelling the transmission of African swine fever virus; five different scenarios were considered based on viruses of different virulence (moderate case fatality in scenarios 1–2 and high in scenarios 3–5), and different reproduction ratios (R0) (a low, medium and high R0 was used for scenarios 3, 4 and 5, respectively)
| Disease scenario | R0 | β | μE | kE | μI | kI | Case fatality (%) |
|---|---|---|---|---|---|---|---|
| 1. Malta 1978 | 20.4 | 2.8 | 5.0 | 10† | 7.3 | 10† | 25 |
| 2. The Netherlands 1986 | 8.1 | 0.9 | 5.0 | 10† | 9.0 | 20† | 50 |
| 3. Georgia 2007, low | 4.8 | 0.7 | 6.1 | 18 | 6.9 | 20 | 100 |
| 4. Georgia 2007, medium | 13.2 | 2.2 | 9.7 | 28 | 6.0 | 25 | 100 |
| 5. Georgia 2007, high | 17.4 | 2.2 | 9.0 | 23 | 7.9 | 22 | 100 |
Assumed values based on ranges reported in de Carvalho Ferreira et al. (2013).
R0 – reproduction ratio.
β – transmission rate.
μE – mean latent period.
kE – shape parameter for gamma‐distributed latent period.
μI – mean infectious period.
kI – shape parameter for gamma‐distributed infectious period.
Within‐herd dynamics of ASFV
The within‐herd dynamics of ASFV is shown in Figure 2. Here, the median (solid line) and 95% prediction interval (shading) for the number of (from left to right): exposed, infectious, and recovered pigs, and for the cumulative number of dead pigs, are shown for the five scenarios considered in Table 1 (rows); these scenarios differ in terms of the R0, beta transmission parameters and disease‐associated mortality rate considered (see details in Table 1).
Figure 2.
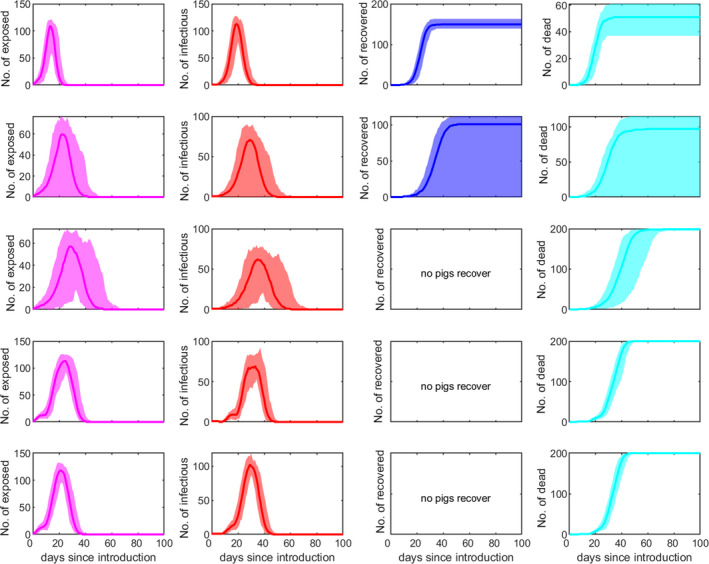
Within‐herd dynamics of ASFV in pigs. The plots show the median (solid line) and 95% prediction interval (shading) for the number of exposed pigs (magenta), infectious pigs (red), recovered pigs (blue) and cumulative number of dead pigs (cyan) for the five scenarios considered in Table 1; these scenarios differ in terms of R0, transmission parameters and disease‐associated mortality (rows; see Table 1 for details)
Detection of African swine fever virus
For the mathematical model, and in all scenarios, the prevalence of virus‐positive pigs was assumed to correspond to the prevalence of infectious pigs (when parameterising the model, virus positivity was assumed to correspond to infectiousness).
Some considerations taken when assessing sampling procedures are:
For moderately virulent strains, pigs in the recovered class were assumed to be seropositive.
-
For highly virulent strains, 10% of infectious pigs were assumed to seroconvert prior to death. This is based on:
the latent and infectious period distributions in the model;
a gamma distribution for the time to seroconversion with a shape parameter of 17.8 and a mean of 20.2 days; and
the latent and infectious periods and time to seroconversion are independent of one another.
- When sampling dead pigs, the probability of detection, pD, was computed using the hypergeometric distribution (i.e. sampling without replacement), so that
where M is the total number of dead pigs, and is equal to
K is the number of dead pigs that are infected (and detectable), being equal to
and SS is the number of dead pigs sampled with mB being the baseline mortality. Baseline mortality (proportion of pigs dying as a result of non‐ASFV reasons each week) was assumed to be 0.25%, based on 3% post‐weaning mortality during a 3‐month production cycle. The sensitivity and specificity of the diagnostic test used to confirm infection were assumed to be 100%.
3.2. Methodology used in ToR 2
To estimate the time lag between infection and reporting of an ASF suspicion (ToR 2), an extensive literature search (ELS) was outsourced by EFSA (OC/EFSA/ALPHA/2020/02 – LOT 2). The aim of this ELS was to answer the epidemiological question of: ‘what is the average, shortest and longest period of time for an outbreak of ASF to be reported (measured as the number of days from the earliest point of infection with ASFV, to the time of declaration of a suspicion by the competent authority after the clinical investigation by an official veterinarian)?’. To answer this question, an ELS on case reports, papers describing outbreaks or epidemics of ASF, and any other relevant grey literature or data was carried out. For the inclusion criteria in the ELS, the earliest point of infection had to have been estimated by carrying out an epidemiological investigation. Papers and other sources of data where the earliest point of infection was determined purely by subtracting a known incubation period from the date of the suspicion of the outbreak were excluded. The ELS was restricted to studies conducted in Europe or describing results obtained in Europe. If none or very few articles were retrieved (less or equal to 5) in the first search, the search was extended to the rest of the world. The general protocol used for the ELS is shown in Annex 5 of the Methodology report (EFSA, 2020a).
3.3. Methodology used in ToR 3
Methodology for assessing the effectiveness of the minimum radius of the protection and surveillance zones
The assessment of radius size of restricted zones (ToR 3), to prevent further disease spread at a given probability, was performed by using disease transmission kernels (EFSA, 2020a). As studies investigating the transmission of African swine fever virus between farms included transmission associated with infected wild boars, studies investigating Classical Swine Fever transmission using kernels were identified in the published literature instead, and used for this assessment (the protection and surveillance zones as described in the Animal Health Law only aim to reduce transmission between domestic pigs, and not that due to wild boar). The functional form, parameter estimates and the 95% confidence or credible intervals for the parameters (where provided) of the best‐fitting kernel were extracted from each study.
For each kernel, the probability of transmission beyond given distances (if transmission were to occur from an infected establishment) was computed using the estimates, and the lower and upper 95% confidence limits for the parameters. In addition, the distances, at which a threshold probability of transmission beyond that distance is reached, were also calculated for each kernel using the estimates, and the lower and upper 95% confidence limits.
Methodology for assessing the effectiveness of the duration of the protection and surveillance zones
To estimate the duration of measures in the protection and surveillance zones, the outputs obtained from the ELS described in Section 3.2 were used. Further details can be found in the Methodology report (EFSA, 2020a).
3.4. Uncertainty
A description of the methodology followed to deal with uncertainty is provided in a Methodology report published by EFSA (EFSA, 2020a).
4. Assessment
4.1. Assessment of sampling procedures (ToR 1)
4.1.1. Assessment of sampling procedures in the event of suspicion or confirmation of African Swine Fever (ASF)
4.1.1.1. In the event of a suspicion of ASF in an establishment where animals of the listed species are kept
The purpose of this section is to assess the effectiveness of disease‐specific sampling procedures of animals of listed species in a suspected establishment, based on clinical examination (TOR 1.1) and laboratory examination (TOR 1.2), in their ability to detect ASFV in kept animals if the disease is present in that establishment, or to rule it out if not present (Art. 6 (2)). For further details, see Annexes B and C.
1st Scenario of sampling procedures
ToR 1.1 and ToR 1.2 in accordance with Mandate
Article 6(2) of the Delegated Regulation
Commission Implemented Regulation 2018/1882 on listed species
The following elements of the scenario were taken into consideration for the assessment:
It concerns an event of suspicion of ASF in an establishment with kept animals of the listed species;
The listed species for ASF as provided in Commission Implemented Regulation 2018/1882 are those belonging to the Suidae family;
Subsequent to the suspicion, the competent authority shall immediately conduct an investigation to confirm or rule out the presence of the disease;
The official veterinarian must perform a clinical examination and collect samples for further laboratory examination (see Annex C for details on guidelines on how the clinical and laboratory examination must be carried out).
Summary of sampling procedures
The procedure of clinical examination of pigs in suspect herds is enacted in Directive 2002/60/EC and the ASF Diagnostic Manual (Commission Decision 2003/422/EC, hereinafter called ‘Diagnostic Manual’) and is described in detail in Annex C.
In summary, sick and anorectic pigs, pigs recently introduced from suspected sources, pigs kept in subunits recently visited by external visitors and pigs recently recovered from the disease have to be examined.
If dead or moribund pigs are detected in a suspect establishment, post‐mortem examinations must be carried out, on at least five of these pigs and in particular on pigs that have shown very evident signs of disease or had high fever before death and died recently. If deemed necessary, the post‐mortem examination of three to four pigs in contact with dead or moribund pigs should be carried out. Samples from pigs that have been subjected to post‐mortem examination (organs or tissues) must be collected for virological testing. Organ and tissue samples should be collected from at least five pigs.
Blood samples for laboratory testing must also be collected if the competent authority considers that the observed clinical signs or lesions, that may suggest ASF, are not sufficient to confirm an outbreak of ASF. Blood samples for laboratory tests must be taken from:
the suspected pigs and
from other pigs in each subunit in which the suspected pigs are kept
The minimum number of samples to be taken for serological tests must allow for the detection of 10% seroprevalence with 95% confidence in the subunit in question. The number of samples to be taken for virological tests should be decided by the competent authority.
If the inspection in the suspect holding has not indicated the presence of pigs with very evident signs of disease or that had high fever before death and died recently, the competent authority shall carry out further examinations in the suspect holding. Another option foresees the clinical examination of pigs selected at random in the subunits for which a risk of introduction of the ASF virus has been identified or is suspected. The minimum number of pigs to be examined must allow the detection of fever, if it occurs, at a prevalence of 10% with 95% confidence in these subunits.
Assessment
Pursuant to the Diagnostic Manual, the establishment can be officially declared an ASF suspect holding due to clinical or pathological findings in pigs or based on epidemiological findings (direct or indirect risky contacts with potential sources of infection).
In case of circulation of highly virulent ASFV (such as those strains currently circulating in Europe), the infected pigs will show signs of the disease following the incubation period. A few days after the appearance of clinical signs, the infected pigs start to die at an increasing rate (Figure 2).
Below we present more specific results obtained from the model, where the number of infectious and dead pigs observed for the different scenarios considered in Table 1 are shown.
In Tables 2 and 3 below, the infection prevalence (median and 95% prediction interval of the number of infectious pigs) at 13 and 23 days post‐infection, respectively, and the number of dead pigs (due to ASF) observed in the preceding week (as estimated using the SEIR model described above) are presented. The results are shown per scenario, and assuming three different herd sizes (50, 200 and 1,000). The selection of 13 and 23 days was based on the results for ToR 2, where the average time between infection and the report of a suspicion is assessed (see Section 4.2).
Table 2.
Predicted median (95% prediction interval) prevalence (%) of African swine fever virus infectious pigs at 13 days post‐introduction to a pig herd, and number of dead infected pigs in the preceding week based on a stochastic SEIR epidemic model
| Scenario | Infection prevalence (%) at 13 dpi | No. dead infected pigs | ||||
|---|---|---|---|---|---|---|
| Herd size | Herd size | |||||
| 50 | 200 | 1,000 | 50 | 200 | 1,000 | |
| 1. Malta 1978 | 52 (0, 69) | 21 (6, 38) | 5 (2, 10) | 4 (0, 8) | 4 (0, 12) | 4 (0, 13) |
| 2. The Netherlands 1986 | 12 (0, 27) | 4 (0, 10) | 0.7 (0, 1.7) | 1 (0, 5) | 2 (0, 7) | 2 (0, 5) |
| 3. Georgia 2007, low | 8 (0, 23) | 2 (0, 5) | 0.4 (0.1, 1.1) | 1 (0, 3) | 1 (0, 4) | 1 (0, 4) |
| 4. Georgia 2007, medium | 13 (4, 23) | 4 (1, 6) | 0.7 (0.3, 1.2) | 1 (0, 2) | 1 (0, 2) | 1 (0, 2) |
| 5. Georgia 2007, high | 15 (8, 27) | 4 (2, 8) | 0.8 (0.3, 1.4) | 1 (0, 2) | 1 (0, 2) | 1 (0, 2) |
Table 3.
Predicted median (95% prediction interval) prevalence (%) of African swine fever virus infectious pigs at 23 days post‐introduction to a pig herd and number of dead infected pigs in the preceding week based on a stochastic SEIR epidemic model
| Scenario | Infection prevalence (%) at 23 dpi | No. dead infected pigs | ||||
|---|---|---|---|---|---|---|
| Herd size | Herd size | |||||
| 50 | 200 | 1,000 | 50 | 200 | 1,000 | |
| 1. Malta 1978 | 16 (0, 37) | 47 (26, 68) | 65 (51, 69) | 5 (0, 10) | 27 (17, 38) | 106 (57, 143) |
| 2. The Netherlands 1986 | 49 (0, 67) | 25 (0, 51) | 5 (0, 13) | 7 (0, 15) | 14 (0, 32) | 16 (0, 38) |
| 3. Georgia 2007, low | 33 (0, 71) | 10 (1, 25) | 2 (0.3, 6) | 6 (0, 17) | 7 (0, 18) | 7 (0, 21) |
| 4. Georgia 2007, medium | 50 (19, 72) | 18 (8, 29) | 4 (1, 7) | 9 (3, 17) | 11 (4, 19) | 12 (3, 21) |
| 5 Georgia 2007, high | 67 (45, 88) | 29 (12, 46) | 7 (3, 12) | 10 (5, 17) | 12 (5, 21) | 11 (5, 20) |
The results from the mathematical model presented in Table 2 show that, on average 4–7 infectious animals would be present in an infected pig herd 13 days post‐introduction of a highly virulent virus such as those currently circulating in Europe (scenarios 3–5); further, as shown in Table 3, after 23 days more than 15 infectious pigs would be present (Table 3, scenario 3).2
In these two tables, the number of dead pigs in the herd the preceding week is also shown for the two infection dates considered (13 and 23 days). Although the average number of dead ASF infected pigs is above 5 in all scenarios, it must be noticed that it is also possible that no dead pigs may be found in the herd (independently of herd size), at 23 days post‐infection and even for scenarios where a high virulence of the strain is assumed (Table 3, scenario 3, where a low R0 is assumed).
This relatively low initial mortality, and the fact that other characteristic clinical signs or pathological changes may not be present in infected animals at an early stage of the epidemic (further, sick animals may have been removed prior to the inspection), lead to the conclusion that in a suspect establishment, ASF cannot be ruled out based purely on clinical examination.
In the event that no moribund or dead pigs were identified in the herd, the diagnostic manual (Chapter IV A point 3) foresees, as an option, the clinical examination of randomly selected animals. The minimum number of pigs in each subunit to be examined must allow the detection of fever, if it occurs, at a prevalence of 10% with 95% confidence. Table 4 shows the number of days needed to achieve a 10% prevalence of infection in a herd/subunit with the 95% prediction interval. In this table, it is shown that a minimum of 6 and a maximum of 45 days could elapse before a 10% prevalence of infection is observed. Considering that not all infected animals may develop clinical signs (including fever), it can be concluded that aiming to detect infection based on the random sampling of animals for clinical examination could lead to a very late detection.
Table 4.
Predicted median (and 95% prediction interval) time (days post introduction) to 10% infection prevalence of African swine fever virus in a pig herd
| Scenario | Time to 10% infection prevalence | ||
|---|---|---|---|
| Herd size | |||
| 50 | 200 | 1,000 | |
| 1. Malta 1978 | 8 (6, 10) | 11 (9, 16) | 15 (14, 17) |
| 2. The Netherlands 1986 | 12 (7, 18) | 18 (13, 27) | 27 (22, 33) |
| 3 Georgia 2007, low | 14 (9, 22) | 23 (18, 38) | 33 (27, 45) |
| 4 Georgia 2007, medium | 12 (10, 21) | 22 (19, 25) | 28 (25, 33) |
| 5 Georgia 2007, high | 12 (9, 14) | 19 (15, 22) | 25 (23, 29) |
In the event that the suspicion is raised due to moribund or dead pigs found in the herd, the minimum number of dead pigs and pigs showing clinical signs to be sampled, to detect ASF with a 95% confidence, are shown in Table 5. This table was created using the RiBESS+ tool3 and takes into account the minimum number of dead pigs that will be present at the farm the week preceding the sampling. For the scenarios where sufficient numbers of dead pigs were not observed, the presence of at least one clinically infected animal was assumed. The design prevalence used was calculated using the mathematical model for each of the scenarios considered, and for the different herd sizes; a Uniform distribution (0.9, 0.99) of the test sensitivity of the virological diagnostic test was used for the calculations. Three herd sizes were considered 50, 200 and 1,000 pigs. The calculations were repeated assuming sampling took place 13 (A.) and 23 (B.) days post‐infection.
Table 5.
Number of sampled animals by category (dead and showing clinical signs) needed to achieve 95% confidence in the detection of African swine fever virus in an infected herd (RiBESS+ analysis)
| A. 13 days post‐infection | ||||||
|---|---|---|---|---|---|---|
| Scenario | No. of pigs | |||||
| 50 | 200 | 1,000 | ||||
| Dead | Clinical | Dead | Clinical | Dead | Clinical | |
| 1. Malta, 1978 | 3 | 0 | 3 | 0 | 4 | 0 |
| 2. The Netherlands, 1986 | 2 | 1 | 3 | 0 | 4 | 0 |
| 3. Georgia, 2007 low) | 2 | 1 | 3 | 1 | 4 | 1 |
| 4. Georgia, 2007 (medium) | 2 | 1 | 3 | 1 | 4 | 1 |
| 5. Georgia, 2007 (high) | 2 | 1 | 3 | 1 | 4 | 1 |
| B. 23 days post‐infection | ||||||
| Scenario | No. of pigs | |||||
| 50 | 200 | 1,000 | ||||
| Dead | Clinical | Dead | Clinical | Dead | Clinical | |
| 1. Malta, 1978 | 2 | 0 | 2 | 0 | 2 | 0 |
| 2. The Netherlands, 1986 | 2 | 0 | 3 | 0 | 3 | 0 |
| 3. Georgia, 2007 (low) | 2 | 0 | 3 | 0 | 3 | 0 |
| 4. Georgia, 2007 (medium) | 2 | 0 | 3 | 0 | 3 | 0 |
| 5. Georgia, 2007 (high) | 2 | 0 | 3 | 0 | 3 | 0 |
Based on the results shown in Table 5, it can be concluded that the collection of samples (tissue or blood) from at least five pigs (dead or with clinical signs if a sufficient number of dead pigs is not found) enables to detect the virus with at least 95% confidence 13 days post infection, even when the number of dead pigs due to ASF may not have reached the expected level of five pigs per week yet.
Based on the diagnostic manual for ASF, in the event that the observed clinical signs or lesions, that may suggest ASF, are not sufficient to confirm an outbreak of ASF, blood samples for laboratory tests must be taken from the suspected pigs and from other pigs in each subunit in which the suspected pigs are kept; among the latter, the minimum number of samples to be taken for serological tests must allow for the detection of 10% seroprevalence with 95% confidence. The number of days needed to achieve a 10% seroprevalence was investigated using the ASF mathematical model previously described and are shown in Table 6. Table 6 shows the time (median and 95% prediction interval) to reach 10% seroprevalence in a herd for each of the scenarios considered, and for three different herd sizes. The number of dead pigs observed in the preceding week is also shown.
Table 6.
Predicted median (95% prediction interval) time (days post introduction) to 10% seroprevalence of African swine fever virus in a pig herd and number of dead infected pigs in the preceding week based on a stochastic SEIR epidemic model
| Scenario | Time to 10% seroprevalence | Number of dead infected pigs in the preceding week | ||||
|---|---|---|---|---|---|---|
| Herd size | Herd size | |||||
| 50 | 200 | 1,000 | 50 | 200 | 1,000 | |
| 1. Malta, 1978 | 14 (12, 18) | 18 (15, 22) | 22 (20, 24) | 5 (1, 9) | 17 (9, 26) | 88 (68, 115) |
| 2. The Netherlands, 1986 | 21 (17, 29) | 27 (23, 36) | 36 (32, 43) | 7 (2, 14) | 27 (17, 39) | 136 (110, 162) |
| 3. Georgia, 2007 (low) | 38 (28, 50) | 53 (45, 64) | –† | 15 (3, 24) | 33 (12, 52) | –† |
| 4. Georgia, 2007 (medium) | 34 (28, 41) | 43 (40, 48) | 54 (48, 59) | 22 (10, 30) | 61 (38, 92) | 148 (71, 355) |
| 5. Georgia, 2007 (high) | 33 (28, 37) | 42 (36, 47) | 51 (46, 54) | 23 (13, 29) | 68 (38, 94) | 226 (79, 474) |
Was never reached before all animals were dead.
The results of the model analysis demonstrate that a 10% prevalence of seropositive animals in a herd will not be reached before 30 days (median) after introduction of an ASFV strain of high virulence (Table 6, scenarios 3–5). Furthermore, reducing the seroprevalence to be detected, would not lead to an early detection in these scenarios either, as the average seroprevalence observed at day 23 post‐introduction only reached > 5% in small herds (range 3–7%) and never > 3% (range 0.2–2.9%) in herds with 200 pigs or more (overall median 1.8%; results not shown). The larger the herd, the longer the time it will take to reach a 10% prevalence. The model also shows that at the time a 10% seroprevalence is reached in the herd, the number of dead pigs in the preceding week would be ≥ 5, regardless of scenario and herd size. Thus, sampling of dead pigs would always allow for an earlier confirmation of the disease than serological testing aiming at a 10% seroprevalence.
For virus strains of lower virulence, such as those that circulated in Europe between the 1960s and 1990s (Scenarios 1 and 2), within‐herd seroprevalences as high as 80% have been reported (Ordas et al., 1981). In case of suspicion of circulation of a virus strain of lower virulence, the random sampling of pigs according to the present guidelines would allow to detect the presence of a seropositive animal in a herd after (on average) 14–36 days post introduction4 (Table 6, scenarios 1–2). However, according to the model output, also for viruses of lower virulence, sampling of dead pigs would allow for an earlier confirmation of the disease than serological testing aiming at detecting a 10% seroprevalence.
Development of new procedures
At an early stage of the epidemic, ASF cannot be ruled out based on clinical or pathognomonic examination; this should be highlighted in any new guidelines.
Randomly selecting pigs for the detection of fever (assuming a 10% prevalence and with a 95% confidence) should not be recommended if the aim is early detection; likewise the random sampling of pigs aiming at detecting a 10% seroprevalence, would lead to a late detection and it is not recommended. Regardless of the virulence of the ASFV strain in question (i.e. for either highly virulent strains as those currently circulating or strains of lower virulence), sampling of dead pigs and pigs with clinical signs would lead to an earlier detection.
The procedure foreseeing the testing of randomly selected animals in suspect holdings (Commission Decision 2003/422/EC Chapter IV A point 5) could be excluded and substituted with targeted sampling of dead and moribund animals.
4.1.1.2. For the purposes of the epidemiological enquiry as referred to Article 57 of Regulation (EU)2016/429 in an establishment affected and officially confirmed with ASF
The purpose of this section is to assess the effectiveness of disease‐specific sampling procedures, based on laboratory examination (ToR 1.2), in their ability to detect the disease in the event of preventive killing and in their ability to support the epidemiological investigation (disease detection, prevalence estimation, virus identification etc.) in kept animals of listed species in an affected establishment, before or when they are killed or found dead. The purposes of the epidemiological enquiry are described in Article 57 of Regulation (EU)2016/429. For further details, see Annexes B and C.
2nd Scenario of sampling procedures
ToR 1.2 in accordance with Mandate
Article 12(3) and the Art. 7 (4) (Preventive killing) of the Delegated Regulation
Article 57 of the Regulation (EU) 2016/429
The following elements of the scenario were taken into consideration for the assessment:
It concerns an affected establishment officially confirmed;
Kept animals of listed species found dead or before/when they are killed are sampled;
Competent authority collects samples for laboratory examination;
-
The purposes of the sampling are:
-
supporting the epidemiological enquiry to:
identify the likely origin of the disease;
calculate the likely length of time that the disease is present;
identify establishments where the animals could have contracted the disease and movements from the affected establishment that could have led to the spread of the disease; and
obtain information on the likely spread of the listed disease in the surrounding environment, including the presence and distribution of disease vectors
confirming/ruling out disease in the event of preventive killing.
-
Summary of sampling procedures
According to the Chapter IV B of the Diagnostic Manual, in a herd where pigs are killed following confirmation of the disease, blood samples for serological tests have to be collected at random from pigs of every subunit of the holding when they are killed. The minimum number of samples to be taken from each subunit of the holding must enable the detection of 10% seroprevalence of the infection with 95% confidence in the subunit in question.
Samples for virological tests must also be taken in accordance with the instructions of the competent authority, which will take into account the range of tests that can be performed, the sensitivity of the laboratory tests that will be used and the epidemiological situation.
In those areas where the presence of ASFV‐infected competent vectors (soft ticks) have been previously demonstrated, appropriate collections of soft ticks for virological tests must be taken in accordance with the instructions of the competent authority and Annex III to Directive 2002/60/EC.
Assessment
Laboratory testing aiming at detection of 10% seroprevalence of the infection with 95% confidence in the subunit in question cannot be considered effective in detecting the infected subunits of an affected herd (see assessment under Section 4.1.1.1).
Development of new procedures
The procedure foreseeing the serological testing of randomly selected animals in other subunits of an affected holding (Commission Decision 2003/422/EC Chapter IV B) with the aim of disease detection could be excluded and substituted with targeted sampling of dead or moribund animals, or those with any clinical signs (e.g. fever, reluctance to move and/or showing signs of anorexia). The sampling should be performed before killing of pigs. Nonetheless, serological testing of the same animals could be recommended as a new procedure to better understand how long the virus may have been circulating in the herd.
Alternative methods for sampling and testing of pigs in outbreak holdings could be introduced to speed up and support the epidemiological investigation by increasing the number of samples collected and tested at the outbreak farm with limited extra labour needed. It has been demonstrated that blood samples collected from pigs or wild boar with cotton swabs can effectively be used for virus DNA and antibody detection with traditional laboratory tests like PCR and ELISA, respectively (Petrov et al., 2014; Sauter‐Louis et al., 2020), but also with pen side tests like lateral flow devices (LFD) for ASFV antibody and antigen detection (Carlson et al., 2018). Testing larger numbers of pigs in the herd may give a better understanding of the development of the epidemic in an affected herd and help to establish the likely route of introduction of the virus.
4.1.1.3. For granting a specific derogation from killing animals of the categories described in article 13.2 of the Delegated Regulation in an ASF affected establishment
3rd Scenario of sampling procedure
ToR 1.1 and ToR 1.2 in accordance with Mandate
Article 13(3)c of the Delegated Regulation
The following elements of the scenario were taken into consideration during for the assessment:
It concerns an affected establishment where infection is officially confirmed;
-
In the establishment where there are kept animals of listed species of the following specific categories animal categories based on article 13(2):
animals kept in a confined establishment
animals kept for scientific purposes or purposes related to conservation of protected or endangered species
animals officially registered in advance as rare breeds
animals with a duly justified high genetic, cultural or educational value
the competent authority may grant specific derogation from killing all the animals of listed species belonging to any of the above categories in an affected establishment, provided that specific conditions are fulfilled;
The animals should be subjected to clinical surveillance, including laboratory examinations;
Sampling procedures should ensure that the animals do not pose a risk of transmission of the category A disease if left alive.
The purpose of this section is to assess the effectiveness of disease‐specific sampling procedures based on clinical (ToR 1.1) and laboratory (ToR 1.2) examinations of the animals of listed species belonging to the categories described in article 13(2) of an affected establishment, in order to grant a specific derogation from killing these animals, while ensuring that they do not pose a risk for the transmission of the disease. For further details, see Annexes B and C.
Summary of sampling procedures
There are no sampling procedures to grant a derogation from killing of animals in an affected establishment.
Assessment
Animals in an affected establishment and for which a specific derogation from killing has been granted should be subjected to clinical surveillance, including laboratory examinations. Sampling procedures should ensure that the animals do not pose a risk of transmission if left alive.
Animals of the holding that are negative for antibodies and for virus do not pose any risk of transmission of ASF. Recovered animals with antibody‐positive results only do not pose a risk of transmission but should be monitored for viraemia and virus excretion for a period of 1 year to exclude risk of transmission due to intermittent or persistent virus excretion.
Development of new procedures
Blood sampling of all surviving animals for virus detection by relevant diagnostic test should be performed with 3 months interval over 1‐year period. During that period, the animals must be kept in isolation and under strict biosafety conditions, including protection against competent arthropod vectors.
Sentinel animals comingled with surviving animals could also be used to exclude any virus transmission. Sentinel animals have to be tested with the same interval and methods as surviving animals.
4.1.1.4. For wild animals of the listed species within the ASF affected establishment and its surroundings
The purpose of this section is to assess the effectiveness of disease‐specific sampling procedures, based on clinical (ToR 1.1) and laboratory (ToR 1.2) examinations of the wild animals of listed species within the affected establishment and in its surroundings. The purpose of the sampling procedures is to ensure the detection of the virus, if the virus is present in these wild species. For further details, see Annexes B and C.
5th Scenario of sampling procedures
TOR 1.1 and TOR 1.2 in accordance with Mandate
Article 14(1) of the Delegated Regulation
Article 57 of the Regulation (EU) 2016/429
Commission Implemented Regulation 2018/1882 on listed species
The following elements of the scenario should be taken into consideration during for the assessment:
It concerns to an affected and officially confirmed establishment;
Wild animals of listed species may exist within the establishment and in the surroundings of the establishment;
The competent authority may establish these sampling procedures in addition to other measures;
The purpose of the sampling procedures in wild animals of listed species is to ensure the detection of the virus, if the virus is present in these wild species.
Summary of sampling procedures
No guidelines for the sampling of wild animals of listed species are described for animals within an establishment and its surroundings.
Assessment
In case wild boars have entered the territory of the affected establishment (e.g. pastures), there is a risk of dispersal of the virus into the wild boar population in the surroundings of the affected establishment. Contrarily, infection may have originated in the wild boar population, being wild boars the source of infection for pigs in the establishment. The sampling procedures should ensure the detection of the infection in wild boars caught within the establishment and found dead or hunted in its surroundings to support the management of the related risks.
Development of new procedures
If incursion of wild boars to the territory of the establishment has occurred and those animals have been caught and culled, blood and tissue samples should be collected for laboratory examination and virus and antibody detection with relevant diagnostic tests performed.
Enhanced passive surveillance (wild boar carcass search) in the area surrounding the establishment should be implemented. All wild boar found dead should be tested for virus and antibodies. If hunting is ongoing in the surrounding, the shot animals should also be tested.
4.1.1.5. For animals of listed species in the non‐affected establishments located in a protection zone
The purpose of this section is to assess the effectiveness of disease‐specific sampling procedures based on clinical (ToR 1.1) and laboratory (ToR 1.2) examinations of the animals of listed species in establishments located in the protection zone. The purpose of the sampling procedures is to ensure the detection of the virus, if the virus is present in these animals. For further details, see Annexes B and C.
6th Scenario of sampling procedures
ToR 1.1 and ToR 1.2 in accordance with Mandate
Article 26(2) of the Delegated Regulation
The following elements of the scenario should be taken into consideration during for the assessment:
It concerns the protection zone with radius up to 3 km;
Official veterinarians must visit at least once all the non‐affected establishments with kept animals of listed species located in the protection zone;
Among others, they must perform a clinical examination of kept animals of listed species and if necessary, collection of samples for laboratory examination;
The purpose of sampling procedures is to confirm or rule out the presence of a category A disease.
Summary of sampling procedures
According to the Chapter IV F of the Diagnostic Manual, sick and anorectic pigs, pigs recently introduced from suspect sources, pigs kept in subunits recently visited by external visitors and pigs recently recovered from the disease have to be examined clinically.
If dead or moribund pigs are detected in an establishment, post‐mortem examinations must be carried out on at least five of these pigs and in particular on pigs that have shown very evident signs of disease or had high fever before death and died recently. If deemed necessary, the post‐mortem examination of three to four pigs in‐contact with dead or moribund pigs should be carried out.
If the inspection in the holding has not indicated the presence of the pigs referred above, the competent authority shall carry out further examinations in the holding. The clinical examination on pigs selected at random in the subunits of the holding with the minimum number of pigs to be examined, allowing the detection of fever if it occurs at a prevalence of 10% with 95% confidence in these subunits, must be carried out.
Blood samples for serological tests must be taken from all holdings in the protection zone. The minimum number of blood samples to be taken must allow for the detection of 10% seroprevalence with 95% confidence in pigs in each subunit in the holding.
Aside, based on the Strategic approach to the management of African Swine Fever for the EU – Rev. February 2020, SANTE/7113/2015 – Rev 12, continuous/weekly sampling should be applied in domestic pig establishments located in areas covered by Decision 2014/709/EU. In this working document, sampling of at least two dead post‐weaning pigs or pigs older than 2 months in each epidemiological unit for virus detection is prescribed during the period the restricted zone in question.
For herds with mortality rates < 2 pigs/week in the target age group (e.g. small herds, breeding/multiplier herds) all post weaning pigs or pigs older than 2 months dying during the period in which the protection zone is in force should be tested.
Assessment
For points already discussed in Section 4.1.1.1, the assessment remains as per Section 4.1.1.1.
In relation to the continuous sampling of at least two dead pigs, the predicted time (median and 95% confidence) to detect ASFV when testing two dead pigs weekly is shown in Table 7. The simulated time to detection is also shown in Figure 3.
Table 7.
Median (95% prediction interval) time to detecting African swine fever virus when testing two dead pigs per week
| Scenario | Herd size | ||
|---|---|---|---|
| 50 | 200 | 1,000 | |
| Malta 1978 | 11 (3, 17) | 11 (3, 18) | 12 (5, 20) |
| The Netherlands 1986 | 10 (2, 19) | 11 (2, 20) | 14 (4, 24) |
| Georgia 2007, low | 11 (6, 16) | 11 (6, 16) | 12 (7, 27) |
| Georgia 2007, medium | 10 (5, 14) | 10 (5, 17) | 12 (6, 24) |
| Georgia 2007, high | 11 (7, 17) | 11 (7, 18) | 14 (7, 25) |
Figure 3.
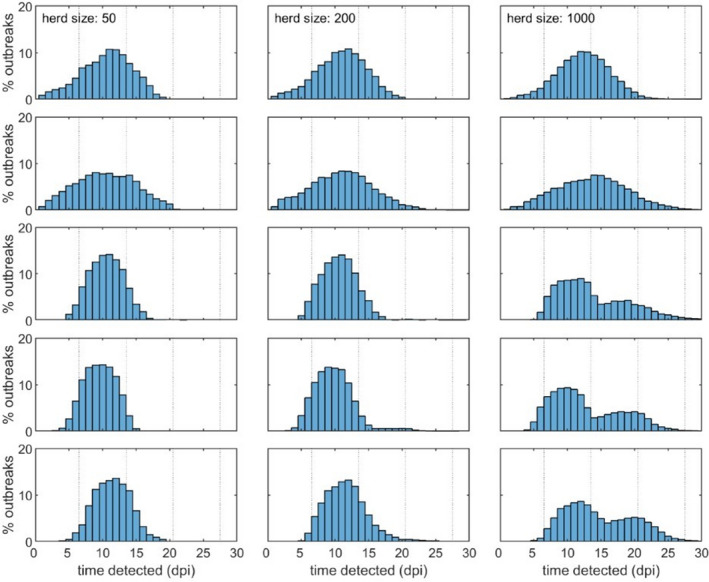
Simulated time to detection (days post‐introduction) of ASFV in a pig herd when testing two dead pigs each week. The plots show the time to detection in a herd of 50 (left), 200 (middle) or 1,000 (right) pigs for five scenarios which differ in R0, transmission parameters and disease‐associated mortality (rows; see Table 1 for details). The vertical dotted lines demarcate the weekly sampling periods
This sampling scheme will detect the virus in an affected herd at median times of 10–14 days post‐infection and, with 95% confidence, assuming a 3% baseline mortality over the whole production cycle in the target age groups (see Table 7).
Development of new procedures
No new sampling procedures are needed. Nonetheless, as mentioned in Section 4.1.1.1, the testing of randomly selected animals could be substituted with the sampling of dead animals (passive surveillance). Further, the weekly collection of tissue samples from at least two dead post weaning pigs or pigs older than 2 months in each epidemiological unit as described in the ‘Strategic approach to the management of African Swine Fever for the EU’ (SANTE/7113/2015) to be applied for the sampling of establishments in restricted zones located in areas covered by Decision 2014/709/EU, could be applied also for the purpose of this scenario.
4.1.1.6. For non‐affected establishments located in a surveillance zone
The purpose of this section is to assess the effectiveness of disease‐specific sampling procedures, based on clinical (ToR 1.1) and laboratory (ToR 1.2) examinations of the animals of listed species, for the sampling of the establishments located within the surveillance zone. The purpose of the sampling procedure is to ensure disease detection if the virus is present in establishments within the surveillance zone. For further details, see Annexes B and C.
8th Scenario of sampling procedures
ToR 1.3 in accordance with Mandate
Article 41 of the Delegated Regulation
The following elements of the scenario were taken into consideration for the assessment:
Ιt concerns the surveillance zone;
Sample of the establishments of kept animals of listed species in the surveillance zone;
Official veterinarians carry out visits to all establishments among others perform clinical examination of kept animals of listed species and if necessary, collection of samples for laboratory examination;
The purpose of sampling procedure is to ensure the detection of the disease if the disease is present in any of the establishments.
Summary of sampling procedures
According to the Chapter IV G of the Diagnostic Manual, sick and anorectic pigs, pigs recently introduced from suspected sources, pigs kept in subunits recently visited by external visitors and pigs recently recovered from the disease have to be examined.
If dead or moribund pigs are detected in a holding, post‐mortem examinations must be carried out, on at least five of these pigs and in particular on pigs that have shown very evident signs of disease or have had high fever before death and died recently. If deemed necessary, the post‐mortem examination of three to four pigs in contact with dead or moribund pigs should be carried out.
Blood samples for serological tests must be taken from holdings, where sampling is deemed necessary by the competent authority and from all semen collection centres. The minimum number of blood samples to be taken must allow for the detection of 10% seroprevalence with 95% confidence in pigs in each subunit in the holding.
Assessment
See Sections 4.1.1.1 and 4.1.1.5.
Development of new procedures
The weekly sampling of at least two dead pigs could also be carried out in all establishments within the surveillance zone according to Section 4.1.1.5 and as described in the ‘Strategic approach to the management of African Swine Fever for the EU’ (SANTE/7113/2015).
4.1.2. Assessment of sampling procedures to grant derogations for animal movements
4.1.2.1. From non‐affected establishments located in the protection zone to slaughterhouses located within the protection zone or in the surveillance zone or outside the restricted zone
9th Scenario of sampling procedures
ToR 1.4 in accordance with Mandate
Article 28(5) of the Delegated Regulation
Article 29 of the Delegated Regulation
The following elements of the scenario were taken into consideration for the assessment:
It concerns the protection zone;
Grant derogation for movement of kept animals of listed species from a non‐affected establishment in the protection zone;
Animals to be moved to a slaughterhouse located within the protection zone or in the surveillance zone or outside the restricted zone;
Clinical examinations and laboratory examination of animals kept in the establishment, including those animals to be moved.
The purpose of this section is to assess the effectiveness of disease‐specific sampling procedures based on clinical and/or laboratory examinations of the animals of an establishment in a protection zone, in order to grant a derogation from prohibitions in the movement of animals, and allow for the animals to be moved to a slaughterhouse located within the protection zone or in the surveillance zone or outside the restricted zone (Art29). For further details, see Annexes B and C.
Summary of sampling procedures
Pursuant to the ASF diagnostic manual (Commission Decision 2003/422/EC) Chapter IV D, within the 24‐h period before moving the pigs:
-
a)
the first, sick and anorectic pigs, pigs recently introduced, pigs kept in subunits recently visited by external visitors and pigs recently recovered from the disease have to be examined clinically. This should include taking the body temperature.
-
b)
a clinical examination of pigs must be carried out in each subunit in which the pigs to be moved are kept. In case of pigs older than 3–4 months, this examination must include taking the temperature of a proportion of pigs allowing for the detection of fever if it occurs at a prevalence of 20% with 95% confidence in the subunits in question.
Blood samples for serological tests or blood or organ samples such as tonsil, spleen or lymph nodes for virological tests must be taken at slaughter from pigs proceeding from each of the subunits from which pigs have been moved. The minimum number of samples to be taken must allow for the detection of 10% seroprevalence or virus prevalence with 95% confidence in each subunit.
Assessment
For points already discussed in Section 4.1.1.1, the assessment remains as per Section 4.1.1.1.
For the option of sampling in order to detect a 10% virus prevalence, the results of the model analysis show that days needed to achieve a 10% prevalence of infection in a herd/subunit is at a minimum of 6 and a maximum of 45 days (Table 4). Considering that animals to be moved to the slaughterhouse have been clinically examined prior to dispatch and only animals with no clinical suspicion are permitted to move, it is highly unlikely that the prevalence of the infection among the animals submitted to slaughterhouse could be 10%.
As a conclusion, the virological testing according to the present guidelines with the aim of detecting presence of virus at the slaughterhouse cannot be considered effective.
Development of new procedures
The weekly sampling of two dead pigs if carried out in protection and surveillance zones according to Section 4.1.1.5 and as described in the ‘Strategic approach to the management of African Swine Fever for the EU’ (SANTE/7113/2015), would lead to an early detection of the disease, and therefore is recommended as it would allow for the safe movement of animals.
4.1.2.2. From non‐affected establishments located in the protection zone to a plant approved for processing or disposal of animal by‐products in which the animals are immediately killed
The purpose of this section is to assess the effectiveness of disease‐specific sampling procedures based on clinical and/or laboratory examinations of the animals of an establishment in a protection zone, in order to grant derogation from prohibitions in the movement of these animals to a plant approved for processing or disposal of animal by‐products in which the kept animals are immediately killed (Art37). For further details, see Annexes B and C.
12th Scenario of sampling procedures
ToR 1.4 in accordance with Mandate
Article 28(5) and article 37 of the Delegated Regulation
The following elements of the scenario were taken into consideration for the assessment:
It concerns the protection zone;
To grant derogation for movement of kept animals of listed species from a non‐affected establishment in the protection zone;
The animals to be moved to a plant approved for processing or disposal of animal by‐products in which the kept animals are immediately killed;
Clinical examinations and laboratory examinations of animals kept in the establishment, including those animals to be moved.
Summary of sampling procedures
No specific sampling procedures are described.
Assessment
See Sections 4.1.1.1 and 4.1.1.5.
Development of new procedures
Same as for 4.1.2.1.
4.1.2.3. From an establishment in a surveillance zone to a slaughterhouse located within or outside the restricted zone and from an establishment outside the surveillance zone to a slaughterhouse situated in the surveillance zone
The purpose of this section is to assess the effectiveness of disease‐specific sampling procedures based on clinical and/or laboratory examinations of the animals of listed species in order to grant derogation from prohibitions and allow for these animals to be moved: a) from an establishment in a surveillance zone to a slaughterhouse located within or outside the restricted zone, b) from an establishment outside the surveillance zone to a slaughterhouse situated in the surveillance zone. For further details, see Annexes B and C.
13th Scenario of sampling procedures
ToR 1.4 in accordance with Mandate
Article 43(5) and article 44 of the Delegated Regulation
The following elements of the scenario were taken into consideration for the:
It concerns kept animals of listed species of the establishments in the surveillance zone;
To grant derogation for movement from an establishment in the surveillance zone to be moved to a slaughterhouse within the restricted zone or outside the restricted zone;
To grant derogation for movement from an establishment outside the surveillance zone to a slaughterhouse situated in the surveillance zone;
Clinical examinations and laboratory examination of animals kept in the establishment, including those animals to be moved.
Summary of sampling procedures
No specific sampling procedures are described.
Assessment
See Sections 4.1.1.1 and 4.1.1.5.
Development of new procedures
Same as for Section 4.1.2.1.
4.1.2.4. From an establishment in a surveillance zone to an establishment belonging to the same supply chain, located in or outside the surveillance zone
The purpose of this section is to assess the effectiveness of disease‐specific sampling procedures based on clinical and/or laboratory examinations of kept ungulates of listed species in order to grant derogation and allow to be moved from an establishment in the surveillance zone to an establishment belonging to the same supply chain, located in or outside the surveillance zone, in order to complete the production cycle before slaughter. For further details, see Annexes B and C.
15th Scenario of sampling procedures
ToR 1.4 in accordance with Mandate
Article 43(5) and article 45(2) of the Delegated Regulation
The following elements of the scenario were taken into consideration for the assessment:
It concerns the surveillance zone;
Grant derogation for movement of kept animals of listed species;
from the surveillance zone;
To be moved to an establishment belonging to the same supply chain, located in or outside the surveillance zone, to complete the production cycle before slaughter;
Clinical examinations and laboratory examination of animals kept in the establishment, including those animals to be moved.
Summary of sampling procedures
No specific sampling procedures are described.
Assessment
Procedures suggested for establishments in the surveillance zone as described in Section 4.1.1.6 should already be in place assuring early detection of the disease in the establishments within the zone. Clinical and laboratory examination of animals to be moved would provide additional confidence in disease freedom in these animals.
Development of new procedures
The continuous passive surveillance as described in Section 4.1.1.6 should be complemented with clinical examination of all animals at dispatch.
In the absence of dead post weaning pigs or pigs older than 2 months in each epidemiological unit, for continuous passive surveillance in the establishment during the period before dispatch this could be replaced by sampling of live animals and testing of blood samples for virus (with relevant diagnostic test, e.g. PCR testing). However, to allow early detection, every animal in the group to be dispatched must be tested for virus.
4.1.2.5. From an establishment located in the restricted zone to move within the restricted zone when restriction measures are maintained beyond the period set out in Annex XI of the Delegated Regulation
The purpose of this section is to assess the effectiveness of disease‐specific sampling procedures based on clinical and/or laboratory examinations of the animals of an establishment located in the restricted zone of an outbreak in order to allow their move within the restricted zone, when restriction measures are maintained beyond the period set out in Annex XI of the Delegated Regulation. For further details, see Annexes B and C.
18th Scenario of sampling procedures
ToR 1.4 in accordance with Mandate
Article 56(1) of the Delegated Regulation
The following elements of the scenario were taken into consideration for the assessment:
It concerns the restricted zone when restriction measures are maintained beyond the period set out in Annex XI;
To grant derogation for movement of kept animals of listed species from an establishment within the restricted zone;
Clinical examinations and laboratory examination of animals kept in the establishment, including those animals to be moved.
Summary of sampling procedures
Clinical examination for African swine fever has to be carried out by an official veterinarian in accordance with the checking and sampling procedures laid down in Part A of Chapter IV of the Annex to Commission Decision 2003/422/EC (see Section 4.1.1.1) on the date of shipment, or at least twice a year with an interval of at least 4 months provided additional safety measures have been followed.
According to implementing Decision 2014/178/EU, the pigs have to be subjected to laboratory testing for ASF carried out with negative results on samples taken in accordance with the sampling procedures as laid down in the plan for the eradication ASF. The present eradication plans implemented in EU affected countries foresee weekly sampling of at least two dead 60 days or older pigs for ASF virus detection.
Additionally, the pigs of the holding must have been subjected at least twice a year, with an interval of at least 4 months, to inspections by the competent veterinary authority, which included sampling for laboratory testing in accordance with the sampling procedures laid down in Part A of Chapter IV of the Diagnostic Manual (see Section 4.1.1.1).
Assessment
See Sections 4.1.1.1 and 4.1.1.5.
Development of new procedures
Same as for Section 4.1.2.1.
4.1.3. Assessment of sampling procedures for repopulation purposes
4.1.3.1. For the animals that are kept for the repopulation prior to their introduction
19th Scenario of sampling procedures
ToR 1.5 in accordance with Mandate
Article 59(2) of the Delegated Regulation
The following elements of the scenario were taken into consideration for the assessment:
It concerns the repopulation of a previous affected establishment;
Animals intended to repopulation shall be sampled prior to their introduction into the establishment of destination;
The samples shall be collected from a representative number of animals to be introduced of each consignment from each establishment or from a representative number of animals of each consignment (if animals are all to be introduced at different times or from different establishments of origin);
Laboratory examinations;
The purpose sampling procedures is to rule out the presence of the disease.
The purpose of this section is to assess the effectiveness of disease‐specific sampling procedures based on laboratory examinations of the animals that are kept for the repopulation prior to their introduction to rule out the presence of the disease. For further details, see Annexes B and C.
Summary of sampling procedures
There are no specific procedures for sampling of animals intended for repopulation.
Assessment
If the animals intended for repopulation originate from a holding located in a surveillance zone, the procedures in place for movement of pigs to other establishments from such holdings will apply. See Section 4.1.1.5.
If the pigs intended for repopulation come from a disease‐free area, there are no requirements for prior testing of pigs before being moved and general regulations in place for moving live pigs will apply.
In areas where biological vectors are present, ASFV presence in soft tick vector species should be ruled out before introducing animals for repopulation.
Development of new procedures
As per Section 4.1.1.5.
4.1.3.2. In the event of unusual mortalities or clinical signs being notified during the repopulation
The purpose of this section is to assess the effectiveness of disease‐specific sampling procedures based on laboratory examinations of the animals that have been repopulated, in the event of unusual mortalities or clinical signs being notified during the repopulation; to rule out the presence of the disease. For further details, see Annexes B and C.
20th Scenario of sampling procedure
ToR 1.5 in accordance with Mandate
Article 59(9) of the Delegated Regulation
The following elements of the scenario were taken into consideration for the assessment:
It concerns the repopulated establishment;
Unusual mortalities or clinical signs during the repopulation;
The official veterinarians shall without delay collect samples for laboratory examination;
The purpose of sampling procedures is to rule out the presence of the disease.
Summary of sampling procedures
According to Part E of Chapter IV of the Annex to the Decision 2003/422/EC after any reintroduction of pigs, the competent authority shall ensure that in case of any disease or death of the pigs in the holding due to unknown reasons, the pigs in question are immediately tested for ASF.
Assessment
Assuming that the animals in question will be tested for the presence of ASFV, the present procedures can be considered effective for early detection of ASF in the repopulated animals.
Development of new procedures
None.
4.1.3.3. For animals that have been repopulated
The purpose of this section is to assess the effectiveness of disease‐specific sampling procedures based on laboratory examinations of the animals that have been repopulated, on the last day of the monitoring period calculated forward from the date on which the animals were placed in the repopulated establishment. In case the repopulation takes place in several days, the monitoring period will be calculated forward from the last day in which the last animal is introduced in the establishment. For further details, see Annexes B and C.
21st Scenario of sampling procedure
ToR 1.5 in accordance with Mandate
Article 59(5) of the Delegated Regulation
The following elements of the scenario were taken into consideration for the assessment:
It concerns the repopulated establishment;
Animals that have been used for repopulation;
Laboratory examinations;
Sampling procedures to rule out the presence of the disease.
Summary of sampling procedures
According to Part E of Chapter IV of the Annex to Decision 2003/422/EC, when pigs are reintroduced into a holding, blood samples must be collected at the earliest 45 days after the reintroduction of the pigs.
In the holding where the disease occurrence has not been linked to vectors:
In case sentinel pigs are reintroduced, blood samples for serological tests must be taken at random from a number of pigs that allow for the detection of 10% seroprevalence with 95% confidence in each subunit of the holding,
In case of total repopulation, blood samples for serological tests must be taken at random from a number of pigs that allow for the detection of 20% seroprevalence with 95% confidence in each subunit of the holding.
When pigs are reintroduced into a holding where the disease occurrence has been linked to vectors:
In case sentinel pigs are reintroduced, blood samples for serological tests must be taken at random from a number of pigs that allow for the detection of 5% seroprevalence with 95% confidence in each subunit of the holding,
In case of total repopulation, blood samples for serological tests must be taken at random from a number of pigs that allow for the detection of 10% seroprevalence with 95% confidence in each subunit of the holding and that procedure must be repeated at the earliest 60 days after total repopulation.
Assessment
See Section 4.1.1.5.
Development of new procedures
As per Section 4.1.1.5.
4.2. Assessment of the length of the monitoring period
The concept of the monitoring period was introduced as a management tool for the investigation and control of suspected and confirmed outbreaks of Category A diseases in terrestrial animals. This tool aimed to standardise the methodology by which relevant authorities responded to suspected and confirmed cases of these diseases. In this regard, a disease‐specific monitoring period was set for each of the 14 diseases included in the Category A list. Throughout the EU legislation, the monitoring period is used as an aid in the control of these diseases, although the specific purpose in which the monitoring period is used varies depending on the articles of the legislation.
The length of the monitoring period for each disease is set out in Annex II of the Commission Delegated Regulation (EU) 2020/687 supplementing the rules laid down in Part III of Regulation (EU) 2016/429 (Animal Health Law).
Annex D in this Opinion describes the seven scenarios for which an assessment of the length of the monitoring period for ASF had been requested.
For the assessment of this ToR, the methodology described in Section 2.3 of the Technical Report published by EFSA was followed. In essence, in order to assess the length of the monitoring period, the purpose of this monitoring period for each of the scenarios was ascertained.
To answer all scenarios except scenario 5, an extensive literature search (ELS) on the average, shortest and longest period of time between the earliest point of infection of domestic pigs with a ASF virus, and the time of reporting of a suspicion by the competent authority, was carried out. The time period between reporting of a suspicion and the notification of the disease was also assessed. Several outcomes were designed for the ELS as shown in the protocol, and the results are presented below.
To answer scenario 5, a literature search was conducted by EFSA on the seroconversion period in domestic pigs, as well as the time when antibodies are no longer detectable in blood, with the outputs being discussed with relevant experts.
4.2.1. Results
Period between the earliest point of infection and suspicion report
A search was carried out identifying 457 references published after 1/1/2000. Among these references, 31 were selected to be included in the qualitative review. The full selection process is displayed in Figure 4.
Figure 4.
PRISMA diagram ASF Monitoring period ELS
The majority of the references reported dates instead of periods (26 references out of 31); these dates were used to calculate the periods of interest. Information on the main outcome of interest, the period between the earliest point of infection and the suspicion report, was retrieved in nine references and is summarised in Table 8.
Table 8.
Summary of African Swine Fever literature extraction for the outcome ‘period between earliest point of infection and suspicion report’
| Reference | Country | Outbreak year | Period between earliest point of infection and suspicion report (days) |
|---|---|---|---|
| Animal Health ‐ Regulatory Committee (2014) | Lithuania | 2014 | 181 |
| Nurmoja et al. (2020) | Estonia | 2015–2017 | 11 (7–20)2 |
| Animal Health ‐ Regulatory Committee (2016) | Lithuania | 2016 | 3; 93 |
| OIE Standing Group of Experts on African swine fever in Europe (2017) | Romania | 2017 | 54 |
| Lamberga et al. (2020) | Latvia | 2017–2018 | 13; 225 |
| Animal Health ‐ Regulatory Committee (2018) | Romania | 2018 | 54 |
| Zani et al. (2019) | Bulgaria | 2018 | 235 |
| Nielsen et al. (2017) | Denmark | NA | 13–196 |
| Andraud et al. (2019) | France | NA | 11; 156 |
Based on laboratory findings of seropositive animals.
Median (min–max) estimated based on the number of sick or dead animals and the presence of PCR positive and/or seropositive animals at the time of suspicion.
Unclear introduction routes, ‘investigations ongoing’.
Based on date of introduction of infected animals.
Based on the date of death of index case and considering the maximum survival time of 10 days after the infection.
Based on transmission model and disease/mortality thresholds for detection.
Based on the results from Table 8, the shortest period was 3 days, and was observed in the context of an outbreak that took place in 2016 in a small backyard farm (three pigs) in Lithuania. However, the way the introduction date was estimated is not indicated in the reference.
The longest delay was 23 days, and was based on data collected in a Bulgarian backyard farm during the 2018 outbreak (Zani et al., 2019). The estimation was made by back‐calculating the introduction date from the date of death of the index case and considering a maximum survival time of 10 days after the infection. The average period was calculated as 13 days.
Seroconversion period
In experimental studies with ASFV from North‐eastern or Southern Estonia (genotype II), seroconversion in wild boar or domestic pigs was detected from 10 days post inoculation (dpi) using ELISA (Nurmoja et al., 2017; Gallardo et al., 2018). In the study by Nurmoja et al. (2017), a doubtful result in ELISA was, however, observed at 9 dpi, and the latest seroconversion in inoculated wild boar was reported at 13 dpi (Nurmoja et al., 2017). Using immunoperoxidase test (IPT), seroconversion was observed in inoculated and in‐contact pigs between 8.5 ± 1.29 dpi and 13.12 ± 2.23 (min. and max.) days post exposure (dpe), respectively (Gallardo et al., 2018).
In an experimental study in domestic pigs with an ASFV from Lithuania (genotype II) seroconversion was observed (in two in‐contact pigs) from 18 days dpe using ELISA. Using IPT, one inoculated and five in‐contact pigs yielded positive results between 17 and 21 dpi/dpe (Gallardo et al., 2015).
In domestic pigs infected with The Netherlands'86 virus strain (genotype I), seroconversion was observed from 10 dpi using ELISA (Petrov et al., 2018). ASFV p73‐specific antibodies were detected by ELISA in sera from all but one initially infected pig 4–9 days after the individual onset of clinical signs, with this onset taking place in most animals between 4 and 6 dpi, leading to a detectable serological response from 10 to 14 dpi.
The authors in this paper believed that failure in the initial challenge may have explained this longer time to first detectable serological response, as some of the pigs may have got infected through contact to sick pen‐mates. These animals developed fever at a later stage and tested negative after the initial challenge for a prolonged period of time. All pigs were found antibody‐positive using ELISA from 29 dpi (Petrov et al., 2018).
4.2.2. Assessment
Considering the results presented above, an assessment of the effectiveness of the monitoring period for ASF, depending on the purpose of that period in the different scenarios shown in Annex D, was carried out. For ASF, the length of the existing monitoring period is 15 days.
Scenarios 1, 2 and 3
1st Scenario of monitoring period
ToR 2 in accordance with article 8 and Annex II of the Delegated Regulation
Article 57 of the Regulation (EU) 2016/429
Aim: to assess the effectiveness of the length of the Monitoring Period, as the time period calculated backwards from the date of the notification of the suspicion of a category A disease in an establishment with kept animals of listed species, for the purposes of the epidemiological enquiry in the event of a suspicion of an ASF outbreak
2nd Scenario of monitoring period
ToR 2 in accordance with article 17(2) and Annex II of the Delegated Regulation
Article 57 of the Regulation (EU) 2016/429
Aim: to assess the effectiveness of the length of the Monitoring Period, as the time period calculated backwards from the date of notification of the suspicion of a category A disease in an establishment with kept animals of listed species, for the purposes of the epidemiological enquiry in the event of confirmation of an ASF outbreak
3rd Scenario of monitoring period
ToR 2 in accordance with article 13(b) and Annex II of the Delegated Regulation
Aim: to assess the effectiveness of the length of the Monitoring Period, as the time period calculated backwards from the date of confirmation of a ASF outbreak in an epidemiological unit in which the disease has not been confirmed, in order to provide derogations from killing the animals in this unit, if this unit has been completely separated, and handled by different personnel during this monitoring period
For the first three scenarios, the main purpose of the use of the monitoring period is to be able to carry a full epidemiological investigation (i.e. in scenarios 1 and 2, at the time of the suspicion and confirmation, respectively), or part of the epidemiological investigation (i.e. scenario 3 where the aim is to identify any possible epidemiological links between the affected establishment and any separated non‐affected epidemiological units). The length of the monitoring period should then dictate how far back or forward the activities related to tracing (and other activities needed during an epidemiological investigation) should go (checks for production records, animal movement records etc.). This monitoring period is the time where the infection could have been present unknowingly in an establishment, and due to the regular activities carried out in this establishment, could have spread to other epidemiological units. In the case of scenario 3, if no epidemiological links between the establishment that has been confirmed positive and the other epidemiological units are found during the investigation (and only if other conditions described in the legislation are met), a derogation from killing the animals in the separated non‐affected epidemiological units could be granted.
The period of time when the disease could have been present, unknowingly, in an establishment, equates then to the time period between the entry of the ASFV into the establishment, and the reporting of the suspicion. Once the suspicion has been officially reported, control measures are implemented, and further spread is in this way prevented.
Based on the ELS carried out and presented above, the average length of the time between infection and the suspicion report was estimated as 13 days based on articles where an epidemiological investigation was carried out. Although the existing monitoring period is longer that the average calculated using this methodology, it is important to take into account that most references displaying short periods between introduction and the suspicion report referred to either:
a very small farm (e.g. Lithuania, 2016 in backyard farms of 2–3 pigs)
establishments where high awareness was expected (e.g. Romania, 2017–2018: region bordering Ukraine)
In affected countries, most ASF outbreaks in the domestic pig sector are found in backyard establishments. In these establishments, many different types of housing arrangements are plausible, leading to a faster or slower transmission of the virus, resulting in large differences in the period between entry and suspicion of the disease as seen in this literature search. Therefore, the length of the monitoring period is considered effective only for outbreaks occurring in small farms, where the death of a small number of pigs would represent a large percentage of the pigs in the establishment and therefore would be more evident, or in case of high degree of awareness in the area. At the early stages of an outbreak, the proportion of dead pigs would be very small in large herds and could be missed. In those cases, extending the length of the monitoring period to the longest length of 23 days shown in the results is recommended.
This would be particularly relevant for the third scenario, where the identification or not of potential links between the affected farm and the unaffected epidemiological unit may lead to the derogation of killing of the animals in the unaffected unit.
Scenario 4
4th Scenario of monitoring period
ToR 2 in accordance with article 27(3)c and Annex II of the Delegated Regulation
Aim: to assess the effectiveness of the length of the Monitoring Period, as the time period calculated backwards from the date of notification of the suspicion of the ASF outbreak in the protection zone. Products or other materials likely to spread the disease, must had been obtained or produced, before this time period in order to be exempted from prohibitions of movements
The main purpose of the monitoring period in scenario 4 is to ensure that certain products or materials, likely to spread the disease, that have been produced in a non‐affected establishment located in the protection zone of an affected establishment, can be moved safely and without posing a risk of disease spread. In this scenario, and in contrast with the previous three scenarios, the establishment of concern is neither a suspect establishment nor an affected establishment. For the assessment of this scenario, we assume that the earliest plausible point of infection of these products or materials in the establishment of concern would be the earliest plausible point of infection of the establishment that originated the protection zone. If these products have been obtained or produced before the earliest point of infection of the affected establishment, then they could be exempted from prohibitions to be moved, as long as other conditions specified in the legislation are met (e.g. the products must have been clearly separated during the production process, storage and transport, from products not eligible for dispatch outside the restricted zone).
As discussed for scenarios 1–3, as the proportion of dead pigs at the early stages of an outbreak would be very small in large herds, extending the length of the monitoring period to the longest length of 23 days shown in the results is recommended. Alternatively, the sampling procedures described in Section 4.1.1.5 could be implemented.
Scenario 5
5th Scenario of monitoring period
ToR 2 in accordance with article 32 (c), article 48(c) and Annex II of the Delegated Regulation
The purpose of this section is to assess the effectiveness of the length of the Monitoring Period, as the time period calculated forwards from the date of semen collection from animals of listed species kept in approved germinal product establishments in the protection or in the surveillance zone, to prove that the donor animal has tested favourable on a sample taken not earlier than 7 days after the monitoring period
The aim of the monitoring period is to ensure that semen from animals in a non‐affected establishment (located in a protection or surveillance zone) that has been collected and frozen after the earliest time of infection of the affected establishment that originated the protection zone, is safe to be moved without posing a risk of disease spread. In this scenario, EFSA is requested to assess the length of time, after the semen was taken, when the animal should be tested in order to allow that semen to be moved. Here, it is assumed that the earliest point of infection of the animal would be on, or after the earliest point of infection of the affected establishment that originated the protection zone, and the latest date the semen could have become contaminated would be the date the semen was collected.
In the case of an ASF outbreak, based on the existing legislation, the pigs would have to be tested not earlier than the time in days of the monitoring period plus 7 days (15 + 7= 22 days) counted after the semen was taken.
Due to the high case fatality of ASF, the likelihood of infected domestic pigs surviving and going undetected after 22 days would be very low (as also discussed above). Aside, there is uncertainty regarding detection of ASFV in semen; no studies have been found documenting this. According to Penrith and Vosloo (2009), sexual transmission of ASFV in pigs has not been documented but the authors mention that ASFV is shed in genital secretions. Greig and Plowright (1970) did indeed detect infectious ASFV in vaginal swabs obtained from ASFV‐infected pigs. They also sampled preputial swabs from boars but results from these swabs are not presented/mentioned in their paper.
Despite this, and assuming that missing an infected establishment as described above would be plausible, below we summarise the assessment in the case that domestic pigs need to be sampled via serology in order to assess the infection status of the animal at the time the semen was taken (indicating whether the semen was infected or not). A negative serological test, if carried out at the right time, would indicate that the animal has never been exposed to the agent, and therefore, it will indicate that the semen is free of the agent too.
Taken into account the results presented in Section 2.1.2, the existing length of time requested by the Delegated Regulation (22 days) prior to the sampling of the animal is considered effective, since it would be sufficient to ensure that a pig infected with ASF virus on the day the semen was taken, would have detectable antibodies by either ELISA or IPT.
Scenarios 6 and 7
6th Scenario of monitoring period
ToR 2 in accordance with article 57 (1) and Annex II of the Delegated Regulation
Aim: to assess the effectiveness of the length of the Monitoring Period, as the time period calculated forward from the date of the final cleaning and disinfection in an affected establishment, after which the repopulation of the establishment may be allowed by the competent authority (assuming relevant control of insects and rodents was carried out)
7th Scenario of monitoring period
ToR 2 in accordance with article 59 (4) and Annex II of the Delegated Regulation
Aim: to assess the effectiveness of the length of the Monitoring Period, as the time period calculated forward from the date the first animal was introduced for the purpose of repopulation, during this monitoring period, all animals of the listed species intended for repopulation should be introduced
In scenarios 6 and 7, the monitoring period is used in the context of repopulation. In scenario 6, the monitoring period is used to ensure that the repopulation process is not put at risk due to the disease still being present unknowingly in establishments within the surrounding area of the establishment to be repopulated (if an establishment tested positive to ASF virus within a distance equal or lower to the radius of the surveillance zone, the repopulation process could not take place). Repopulation can only take place after a number of days equal to the monitoring period has elapsed since the final cleaning, disinfection and disinfestation (in areas where soft ticks of the genus Ornithodoros are present) of the affected establishment.
In this regard the number of days of the monitoring period for ASF counted from the day of the final cleaning and disinfection must ensure enough time for any potentially infected surrounding establishment to be reported as a suspicion. Considering the results presented above, and taking into account that a good level of awareness is expected due to the disease having been present in the area, the EFSA AHAW Panel considers the existing length of the monitoring period (15 days) effective, as it would allow for the identification of any potentially infected establishment in the surrounding area prior to the repopulation taking place.
In scenario 7, the monitoring period must be counted forwards from the date in which the first animal is introduced into the establishment to be repopulated, with all the animals intended for repopulation of this establishment being introduced within the length of time of this monitoring period.
The aim of the monitoring period in this scenario is to ensure the early detection of any potentially recently infected animal intended for repopulation once they have been moved into the repopulated establishment. Although the preferred option is that all animals are introduced into the establishment to be repopulated at the same time, this is not always feasible. The first clinical and laboratory sampling of the repopulated animals takes place once all the animals are in situ. By restricting the period of time during which animals may be introduced into the establishment, the period of time during which the disease could be unknowingly spreading within the establishment is reduced. Assuming that the latest point of infection of the first pig or batch of pigs introduced into the repopulated establishment is the day when the animals are moved, clinically ill pigs would be observed at the first visit, if this visit is carried out a number of days equal to the incubation period. The EFSA AHAW Panel considers the existing length of the monitoring period (15 days) effective as it would allow for early detection of potentially infected pigs at the first visit following re‐stocking.
4.3. Assessment of the minimum radius and time periods of the protection and surveillance zones set in place subsequent to a disease outbreak
4.3.1. Assessment of the minimum radius
The purpose of this section is to assess the effectiveness to control the spread of ASF by implementing a protection and surveillance zones of a minimum radius, as set out in Annex V of the Delegated Regulation, surrounding the establishment where the disease has been confirmed. Based on this regulation, the minimum radius of the protection and surveillance zone for ASF should be of 3 and 10 km, respectively (see Annex E).
Results
To answer this ToR, transmission kernels have been used to analyse outbreak data for two epidemics of Classical Swine Fever in Europe. As transmission kernels have not been estimated for African swine fever, it was decided to use available kernels for CSF for this purpose. Three publications were found describing kernel functions for CSF based on the two European outbreaks, namely Backer et al. (2009) and Boender et al. (2014) using data from the CSF epidemic in the Netherlands in 1997–1998 and (Gamado et al., 2017) using UK data from the year 2000.
All studies used the same functional form for the kernel, namely,
where k is the kernel, r is the distance to an infected farm, d0 is the distance at which the kernel is reduced by 50% and α is the parameter controlling how rapidly the kernel declines with distance.
Parameters were estimated using data from the 1997–1998 epidemic in the Netherlands (Backer et al., 2009; Boender et al., 2014) and the 2000 epidemic in the UK (Gamado et al., 2017) (Table 9).
Table 9.
Kernels for the transmission of classical swine fever virus in Europe used as a proxy for African swine fever
| Epidemic | Parameters* | Reference | |
|---|---|---|---|
| d0 (km) | α | ||
| The Netherlands 1997–1998 | 1.0 | 2.2 | Backer et al. (2009) |
| The Netherlands 1997–1998 | 0.55 (0.42, 0.73) | 2.27 (2.15, 2.40) | Boender et al. (2014) |
| UK 2000 | 0.28 (0.04, 5.53) | 1.71 (0.94, 3.80) | Gamado et al. (2017) |
95% confidence intervals are shown in brackets if they were reported in the original reference.
For the three kernels in Table 9, the probability of transmission beyond given distances (if transmission were to occur from an infected establishment) was computed using the estimates, lower 95% confidence limits and upper 95% confidence limits, including beyond the proposed radius for the protection and surveillance zones (3 km and 10 km, respectively) (Figure 5). In addition, the distances at which a threshold probability of transmission beyond that distance is reached were also calculated for each kernel using the estimates, lower 95% confidence limits and upper 95% confidence limits (Figure 6). The corresponding values computed using the estimates are summarised in Tables 10 and 11.
Figure 5.
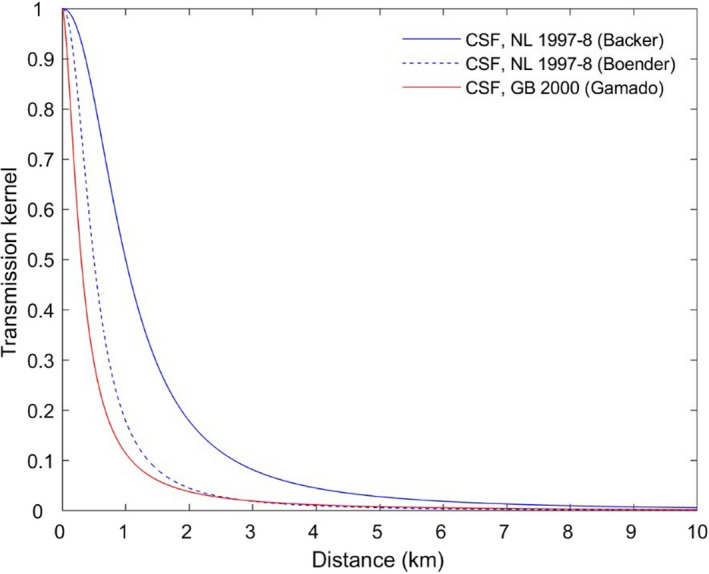
Kernels for the transmission of Classical swine fever used as a proxy for African swine fever for assessing the minimum radius of the protection and surveillance zones
Figure 6.
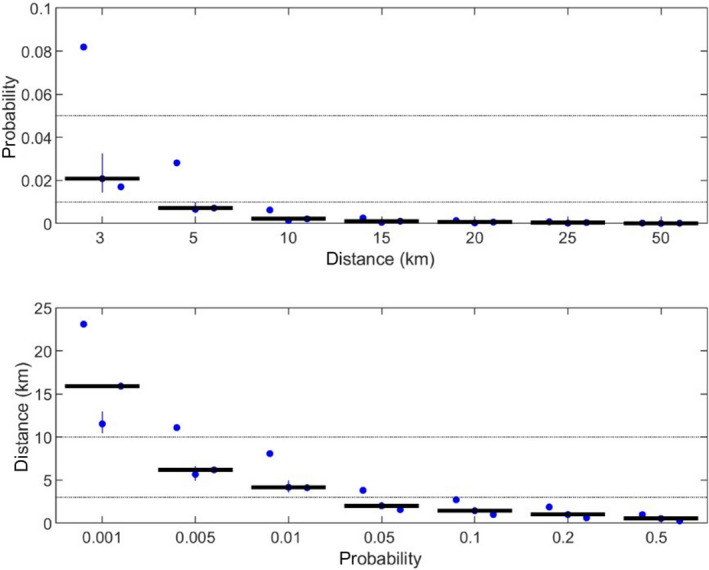
Assessment of the radius of the protection and surveillance zone for African swine fever virus assuming the same transmission kernels as for CSF. The top panel shows the probability of transmission beyond a given distance (if transmission were to occur from an infected establishment) computed using the estimates (blue circles) and the lower and upper 95% confidence limits (error bars) for each kernel (and in the same order as) in Table 9. The thick black line indicates the median probability for all kernels. The black dotted lines indicate threshold probabilities of 0.05 and 0.01. The bottom panel shows the distances at which a threshold probability of transmission beyond that distance is reached when calculated using the estimates (circles) and lower and upper 95% confidence limits (error bars) for each kernel. The thick black line indicates the median distance for all kernels. The black dotted lines indicate distances of 3 and 10 km (i.e. the proposed radius of the protection and surveillance zones, respectively)
Table 10.
Probability of transmission of African swine fever virus beyond different distances assuming the same transmission kernels as for CSF
| Distance (km) | |||||||
|---|---|---|---|---|---|---|---|
| 3 | 5 | 10 | 15 | 20 | 25 | 50 | |
| Median | 0.02 | 0.007 | 0.002 | 0.001 | 0.001 | 0.001 | < 0.001 |
| Minimum | 0.02 | 0.006 | 0.001 | 0.001 | < 0.001 | < 0.001 | < 0.001 |
| Maximum | 0.08 | 0.03 | 0.006 | 0.003 | 0.001 | 0.001 | < 0.001 |
Table 11.
Distances (km) at which the probability of transmission of African swine fever virus beyond that distance reaches a threshold level
| Threshold probability of transmission | |||||||
|---|---|---|---|---|---|---|---|
| 0.001 | 0.005 | 0.01 | 0.05 | 0.1 | 0.2 | 0.5 | |
| Median | 15.9 | 6.2 | 4.2 | 2.0 | 1.4 | 1.0 | 0.6 |
| Minimum | 11.5 | 5.7 | 4.1 | 1.6 | 1.0 | 0.6 | 0.3 |
| Maximum | 23.1 | 11.1 | 8.1 | 3.8 | 2.7 | 1.9 | 1.0 |
Assessment
Based on the kernel results above, if transmission occurs from an infected farm, the median relative probability of transmission beyond the protection (3 km) and surveillance zones (10 km) is 2 and 0.2%, respectively. In several articles of the AHL, a threshold of 95% is used for different purposes; if we use this threshold as to determine whether or not the minimum radius is efficient, the assessment based on the data presented will lead to the conclusion that the minimum radius is highly effective if/when focusing on the control of the spread of the disease among and between domestic pig herds. Using the same threshold, hypothetical protection and surveillance zones with radii of 2 and 4 km, respectively, would also be considered effective. Nonetheless, it is crucial to note that these probabilities do not take into account the risk of transmission due to wild boar.
In the absence of estimated transmission kernels for ASF, this assessment was based on kernels for the transmission of CSF. Despite the similarities suggested by the names, and, to a large extent, shared typical clinical presentation, these two diseases are caused by completely different viruses, something that may be a source of bias for this assessment. However, given that (1) the two diseases share main transmission routes in the European context, and (2) CSF is considered more contagious with higher levels of shedding in all secretions, and thus more prone to indirect and local spread than ASF (Schulz et al., 2017), any bias caused by using the CSF kernel is thus likely to underestimate the effectiveness of the minimum radius for ASF.
4.3.2. Assessment of the minimum period
The purpose of this section is to assess the effectiveness to control the spread of disease of the minimum periods during which the competent authority should apply the restriction measures in the protection and surveillance zones as set out in Annex X and XI (for ASF).
The length of the minimum period of the protection zone and surveillance zone is 15 and 30 days, respectively (see Annex E). In the protection zone, all farms are visited for a clinical inspection. This aims to quickly identify infected farms where infection has started before control measures were implemented. The movement control applies for 30 days, ensuring that possibly infected pigs in both protection and surveillance zones are not moved to uninfected farms.
From Table 8 in Section 4.2.1, it follows that the median time between introduction and suspicion is 13 days. The maximum period between introduction and suspicion is 23 days. Consequently, a period of 30 days for the movement ban is effective to detect infected pig farms and to prevent the movement of infected pigs from the surveillance zone.
4.3.3. Uncertainty analysis
Although several sources of uncertainty were identified during the scientific assessment (see Annex F), their impact on the outputs of the assessment could not be quantified.
5. Conclusions and Recommendations
| Sampling procedure | Laboratory guidelines based on Commission Decision 2003/422/EC if not stated otherwise | Conclusions | Recommendations |
|---|---|---|---|
| 4.1.1.1 In the event of a suspicion of ASF in an establishment where animals of the listed species are kept |
Chapter IV A. 4. Samples of the organs or tissues from pigs that have been subjected to post‐mortem examination must be collected for virological tests
5. Blood samples for laboratory tests If further clinical signs or lesions that may suggest ASF are detected in a suspected holding, but the competent authority deems that these findings are not sufficient to confirm an outbreak of ASF and that laboratory tests are therefore necessary, blood samples for laboratory tests must be taken from the suspected pigs and from other pigs in each subunit in which the suspected pigs are kept, in accordance with the following procedures: the minimum number of samples to be taken for serological tests must allow for the detection of 10% seroprevalence with 95% confidence in the subunit in question; the number of samples to be taken for virological tests will be in accordance with the instructions of the competent authority, which will take into account the range of tests that can be performed, the sensitivity of the laboratory tests that will be used and the epidemiological situation. Chapter V B. For detection of the ASF virus, antigen or genome from dead or humanely destroyed pigs, tonsils, lymph nodes (gastrohepatic, renal, submandibular and retropharyngeal), spleen, kidney and lung tissues are the most suitable samples (1). In case of autolysed carcases, an entire long bone or the sternum is the specimen of choice. Anticoagulated blood and/or clotted blood samples must be collected from pigs showing signs of fever or other signs of disease, in accordance with the instructions of the competent authority. (1) It is recommended to collect also samples of ileum, as they may be useful for the diagnosis of classical swine fever. SANTE 7113/2015 2.1.5. Sampling for laboratory investigations will be performed a) In case of clinical signs resembling ASF (e.g. fever or haemorrhagic lesions). If necessary, sampling should be repeated to exclude ASF when specific clinical signs occur. b) Each week, in the form of virological testing of at least the first two deaths (post weaning pigs or pigs older than 2 months) in each production unit. c) When ante or post‐mortem signs raise suspicion at home slaughtering at least within the area covered by Commission Decision 2014/709/EU. OIE Disease card: Identification of the agent A complete set of field samples should be submitted and especially: • blood collected during the early febrile stage in EDTA (0.5%) • spleen, lymph nodes, tonsil, lungs, kidney and bone marrow kept at 4°C |
At an early stage of the epidemic the disease cannot be ruled out based on clinical or pathognomonic examination. Random examination of pigs, in pig subunits, aiming at the detection of fever (if it occurs) assuming a prevalence of 10% with 95% confidence will lead to a late disease detection. Virological examination of tissue samples, or blood samples, from at least five dead pigs (and pigs with clinical signs if less than two dead pigs found) would allow early virus detection (13 days post infection, with 95% confidence). Random serological sampling of pigs (in suspect herds where no clinical signs are found) to detect a 10% prevalence with 95% confidence, would lead to a late detection of the virus (more than 30 days since introduction) in outbreaks caused by highly virulent strains. Further in these outbreaks reducing the seroprevalence to be detected, would not lead to early detection either. In case of both highly and low virulent strains, sampling of dead and clinical animals would lead to an earlier detection. |
Randomly selecting pigs for the detection of fever (assuming a 10% prevalence and with a 95% confidence) should not be recommended if the aim is early detection. Target investigation and sampling of dead and moribund animals should be used for early detection as described in the diagnostic manual. In the event of finding no clinical signs in the suspect establishment, the investigation of random blood samples (collected to detect 10% prevalence with 95% confidence) is not recommended for early detection. |
|
Serological tests Serum collected within 8–21 days after infection in convalescent animals |
|||
| 4.1.1.2. For the purposes of the epidemiological enquiry as referred to Article 57 of Regulation (EU)2016/429 in an ASF officially confirmed establishment |
Annex Chapter IV B. SAMPLING PROCEDURES IN A HOLDING WHEN PIGS ARE KILLED FOLLOWING CONFIRMATION OF DISEASE 1. In order that the manner of introduction of the ASF virus into an infected holding and the length of time elapsed since its introduction may be established, when pigs are killed on a holding following confirmation of an outbreak in accordance with Article 5(1)(a) of Directive 2002/60/EC, blood samples for serological tests must be taken at random from the pigs when they are killed. 2. The minimum number of pigs to be sampled must allow for the detection of 10% seroprevalence with 95% confidence in pigs in each subunit of the holding*. Samples for virological tests must also be taken in accordance with the instructions of the competent authority, which will take into account the range of tests that can be performed, the sensitivity of the laboratory tests that will be used and the epidemiological situation. In those areas where the presence of vectors infected with the ASF virus has been previously demonstrated, appropriate collections of soft ticks for virological tests must also be taken in accordance with the instructions of the competent authority and Annex III to Directive 2002/60/EC. 3. However, in case of secondary outbreaks, the competent authority may decide to derogate from points 1 and 2 and establish other sampling procedures, taking into account the epidemiological information already available on the source and means of virus introduction into the holding and the potential spread of disease from the holding. * However, if the derogation provided in Article 6(1) of Directive 2002/60/EC has been applied, sampling must concern the subunits of the holding where pigs have been killed, without prejudice to the further examinations and sampling to be carried out on the remaining pigs in the holding, which shall be carried out in accordance with the instructions of the competent authority. |
If the aim is disease confirmation, serological testing of randomly selected pigs in other subunits of an affected establishment would not be of help with early detection in these subunits. |
Target investigation and sampling of dead and moribund animals should be used for early detection in other units of the affected establishment and should replace the random serological sampling. Also, serological sampling of these dead or moribund animals is advised. Testing larger numbers of pigs in the herd may give a better understanding of the development of the epidemic in an affected herd, and help to establish the likely route of introduction of the virus. |
| 4.1.1.3. For granting a specific derogation from killing animals of the categories of article 13.2 of the Delegated Regulation in an ASF‐affected establishment | No specific sampling procedures in the legislation | Recovered animals with antibody‐positive results only do not pose a risk of transmission but should be monitored for possible reactivation of viraemia and virus excretion. |
Recovered pigs should be monitored for viraemia and virus excretion for a period of one year, with sampling taking place every three months. If sentinel pigs are used, they should be sampled with the same regime as for recovered pigs. During that period, the animals must be kept in isolation and under strict biosafety conditions, including protection against vector bites. |
| 4.1.1.4. For wild animals of the listed species within the ASF affected establishment and its surroundings. |
SANTE 7113/2015 3.1.4. Measures to be taken in infected areas to eradicate the disease a) Surveillance (key measure): i) Principle of sampling should be based on enhanced passive surveillance: all found carcasses and sick wild boar have to be tested for ASF using PCR. ii) Active patrolling to find carcasses (trained staff) in order to reinforce passive surveillance. iii) It is recommended that samples be delivered as soon as possible to the laboratory max within 48–72 h from the sampling. l) Testing for ASF of all wild boar found dead and culled. All hunted wild boar tested for ASF virus detection using PCR and for Ab detection. |
Wild suids within the affected establishment would be considered as kept animals and thus any sampling procedure would be the same as for domestic pigs. | Enhanced passive surveillance (wild boar carcass search) in the area surrounding the establishment should be implemented. All wild boar found dead should be tested for virus and antibodies. If hunting is ongoing in the surrounding, the shot animals should also be tested. |
| 4.1.1.5. For animals of listed species in the non‐affected establishments located in a protection zone |
Chapter IV F. 2. The minimum number of blood samples to be taken must allow for the detection of 10% seroprevalence with 95% confidence in pigs in each subunit in the holding. However, the derogation provided for in Article 10(5) and Article 11(4) of Directive 2002/60/EC may only be granted if the competent authority ensures that the number of blood samples taken allow for the detection of 5% seroprevalence with 95% confidence in each subunit in the holding. |
Weekly sampling of at least two dead post weaning pigs or pigs older than 2 months in each epidemiological unit would lead to virus detection at median times of between 10 and 14 days post‐infection with 95% confidence, assuming a 3% baseline mortality. Random serological sampling of pigs would lead to a later detection of the virus. |
The testing of randomly selected animals could be excluded and substituted with the sampling of dead animals (passive surveillance). The weekly sampling of at least two dead post weaning pigs or pigs older than 2 months in each epidemiological unit as described in the ‘Strategic approach to the management of African Swine Fever for the EU’ (SANTE/7113/2015), should be applied for the sampling of establishments in restricted zones located in areas covered by Decision 2014/709/EU. |
| 4.1.1.6. For non‐affected establishments located in a surveillance zone |
Chapter IV G In addition, blood samples for serological tests must be taken from pigs: — in any other holding where sampling is deemed necessary by the competent authority, — in all semen collection centres. 2. Whenever blood sampling for serological tests is carried out in holdings located in the surveillance zone, the number of blood samples to be taken in these holdings must be in accordance with section F(2), first sentence. (2. The minimum number of blood samples to be taken must allow for the detection of 10% seroprevalence with 95% confidence in pigs in each subunit in the holding.) However, the derogation provided for in Article 10(5) and Article 11(4) of Directive 2002/60/EC may only be granted if the competent authority ensures that in each holding in the zone blood samples for serological tests are taken. The minimum number of blood samples to be taken must allow for the detection of 5% seroprevalence with 95% confidence in each subunit in the holding. |
See Section 4.1.1.1 and 4.1.1.5. | The weekly sampling of two dead pigs could also be carried out in all establishments within the surveillance zone according to Section 4.1.1.5 and as described in the ‘Strategic approach to the management of African Swine Fever for the EU’ (SANTE/7113/2015). |
| 4.1.2.1. From non‐affected establishments located in the protection zone to slaughterhouses located within the protection zone or in the surveillance zone or outside the restricted zone |
Council Directive 2002/60/EC Article 10 3. The competent authority may authorise the removal of pigs from the holding concerned, on condition that: (e) if the pigs are to be slaughtered or killed, a sufficient number of samples is then taken from the pigs in accordance with the diagnostic manual in order that the presence of African swine fever virus in these holdings can be confirmed or ruled out; Chapter IV D 4. When the pigs referred to in point 3 are slaughtered or killed, blood samples for serological tests or blood or organ samples such as tonsil, spleen or lymph nodes for virological tests must be taken from pigs proceeding from each of the subunits from which pigs have been moved. The minimum number of samples to be taken must allow for the detection of 10% seroprevalence or virus prevalence with 95% confidence in each subunit. The type of samples to be taken and the test to be used will be in accordance with the instructions of the competent authority, which will take into account the range of tests that can be performed, the sensitivity of these tests and the epidemiological situation. 5. However, if clinical signs or post‐mortem lesions suggesting ASF are detected when the pigs are slaughtered or killed, by way of derogation from point 4, the provisions for sampling laid down in section C shall apply (C. SAMPLING PROCEDURES WHEN PIGS ARE KILLED AS A PREVENTIVE MEASURE ON A SUSPECTED HOLDING). 6. The derogation provided for in Article 10(5) and Article 11(4) of Directive 2002/60/EC may be granted if the competent authorities ensure that an intensive sampling and testing scheme is also applied on the groups of pigs to be checked or sampled referred to in points 2, 3 and 4. In the context of this scheme, the minimum number of blood samples to be taken must allow for the detection of 5% seroprevalence with 95% confidence in the group of pigs in question. |
See Section 4.1.1.1 The virological testing with the aim of detecting the virus at the slaughterhouse in randomly selected slaughter pigs assuming a virus prevalence of 10% with 95% confidence cannot be considered effective. |
The weekly sampling of at least two dead pigs if carried out in protection and surveillance zones according to Section 4.1.1.5 and as described in the ‘Strategic approach to the management of African Swine Fever for the EU’ (SANTE/7113/2015), would lead to an early detection of the disease, and therefore is recommended as it would allow for the safe movement of animals. |
| 4.1.2.2 From non‐affected establishments located in the protection zone to a plant approved for processing or disposal of animal by‐products in which the animals are immediately killed |
See Section 4.1.2.1 . Council Directive 2002/60/EC Article 10 3. The competent authority may authorise the removal of pigs from the holding concerned, on condition that: (e) if the pigs are to be slaughtered or killed, a sufficient number of samples is then taken from the pigs in accordance with the diagnostic manual in order that the presence of African swine fever virus in these holdings can be confirmed or ruled out. |
See Sections 4.1.1.1 and 4.1.1.5 | Same as for 4.1.2.1 |
| 4.1.2.3. From an establishment in a surveillance zone to a slaughterhouse located within or outside the restricted zone and from an establishment outside the surveillance zone to a slaughterhouse situated in the surveillance zone |
Chapter IV A. 4. Samples of the organs or tissues from pigs that have been subjected to post‐mortem examination must be collected for virological tests Chapter IV D. 4. When the pigs referred to in previous are slaughtered or killed, blood samples for serological tests or blood or organ samples such as tonsil, spleen or lymph nodes for virological tests must be taken from pigs proceeding from each of the subunits from which pigs have been moved. The minimum number of samples to be taken must allow for the detection of 10% seroprevalence or virus prevalence with 95% confidence in each subunit. The type of samples to be taken and the test to be used will be in accordance with the instructions of the competent authority, which will take into account the range of tests that can be performed, the sensitivity of these tests and the epidemiological situation |
See Sections 4.1.1.1 and 4.1.1.5 | Same as for 4.1.2.1 |
| 4.1.2.4. From an establishment in a surveillance zone to an establishment belonging to the same supply chain, located in or outside the surveillance zone | No specific sampling procedures prescribed. | No sampling procedure prescribed. However, procedures suggested for establishments in the surveillance zone should already be in place (Section 4.1.1.5). The continuous passive surveillance as described in Section 4.1.1.6. |
Sampling procedure in 4.1.1.5 should be complemented with clinical examination of all animals at dispatch. In the absence of at least 2 dead post weaning pigs or pigs older than 2 months during the period before dispatch this could be complemented by sampling of live animals. However, to allow early detection every animal in the group to be dispatched must be tested. |
| 4.2.1.5 From an establishment located in the restricted zone to move within the restricted zone when restriction measures are maintained beyond the period set out in Annex XI of the Delegated Regulation |
Article 3 /…/ 1. the pigs have been subjected to laboratory testing for African swine fever carried out with negative results on samples taken in accordance with the sampling procedures as laid down in the plan for the eradication of African swine fever referred to in the second paragraph of Article 1 of this Decision within a period of 15 days prior to the date of the movement/…/ 2. the pigs come from a holding: (a) that has been subjected at least twice a year, with an interval of at least 4 months, to inspections by the competent veterinary authority, which/…/(ii) included a clinical examination and sampling in accordance with the checking and sampling procedures laid down in Part A of Chapter IV of the Annex to Decision 2003/422/EC; (c) in which the pigs over the age of 60 days have been subjected to the laboratory testing for African swine fever referred to in paragraph 1. |
See Sections 4.1.1.1 and 4.1.1.5 | Same as for 4.1.2.1 |
| 4.1.3.1 For the animals that are kept for the repopulation prior to their introduction | No guidelines described for ruling out the presence of disease prior to the introduction. |
If the pigs intended for repopulation come from a disease‐free area, there are no requirements for prior testing of pigs before being moved, and general regulations in place for moving live pigs will apply. If pigs originate from a holding located in a surveillance zone the procedures in place for movement of pigs to other establishments from such holdings will apply. See Section 4.1.1.5. |
Same as for 4.1.1.5 In areas where biological vectors are present, ASFV presence in soft tick vector species should be ruled out before introducing animals for repopulation. |
| 4.1.3.2 In the event of unusual mortalities or clinical signs being notified during the repopulation |
Blood samples for serological tests Chapter IV A. 4. Samples of the organs or tissues from pigs that have been subjected to post‐mortem examination must be collected for virological tests Chapter E 3. After any reintroduction of pigs, the competent authority shall ensure that in case of any disease or death of the pigs in the holding due to unknown reasons, the pigs in question are immediately tested for ASF. |
Decision 2003/422/EC lays down the procedures after any reintroduction of pigs. The competent authority shall ensure that in case of any disease or death of the pigs in the holding due to unknown reasons, the pigs in question are immediately tested for ASF. | None. |
| 4.1.3.3 For animals that have been repopulated |
E.1. When pigs are reintroduced into a holding in accordance with Article 13(3) (disease has not been linked to vectors), of Directive 2002/60/EC, the following sampling procedures must be applied:
2. When pigs are reintroduced into a holding in accordance with Article 13(4) (disease has been linked to vectors) of Directive 2002/60/EC, the following sampling procedures must be applied:
Then, the procedure laid down in the third indent above must be repeated at the earliest 60 days after total repopulation. 3. After any reintroduction of pigs, the competent authority shall ensure that in case of any disease or death of the pigs in the holding due to unknown reasons, the pigs in question are immediately tested for ASF. |
See Section 4.1.1.5 | Same as for 4.1.1.5 |
| ToR 2 | ||
|---|---|---|
| Description | Conclusions | Recommendations |
|
4.2 Assessment of the length of the monitoring period of ASF |
For scenarios 1–4, the length of the monitoring period is considered effective only for outbreaks occurring in small farms, where the death of a small number of pigs would represent a large percentage of the pigs in the establishment and therefore would be more evident, or in case of high degree of awareness in the area. The length of the monitoring period is considered effective for scenarios 5–7. |
At the early stages of an outbreak, the proportion of dead pigs would be very small in large herds and could be missed. In those cases, extending the length of the monitoring period to the longest length of 23 days shown in the results is recommended for scenarios 1–4. Alternatively, the sampling procedures described in Section 4.1.1.5 could be implemented in order to allow derogations for the movement of products. |
| ToR 3 | ||
|---|---|---|
| Description | Conclusions | Recommendations |
|
4.3.1 Assessment of the minimum radius |
It was observed that if transmission occurs from an infected farm, the median relative probability of transmission beyond the protection (3 km) and surveillance zones (10 km) is 2 and 0.2%, respectively. The minimum radius is considered highly effective if/when focusing on the control of the spread of the disease among and between domestic pig herds (more than 95% of the transmission, if transmission occurs will take place within the protection zone). It is crucial to note that these probabilities do not take into account the risk of transmission due to wild boar. |
None. |
|
4.3.2 Assessment of the minimum period |
The duration of measures in the protection and surveillance zones are considered effective. | It is recommended to maintain the duration of the protection (15 days) and surveillance zones (30 days). |
Abbreviations
- ASF
African swine fever
- ASFV
African swine fever virus
- AHS
African horse sickness
- CSF
Classical swine fever
- CSFV
Classical swine fever virus
- CBPP
Contagious bovine pleuropneumonia
- CCPP
Contagious caprine pleuropneumonia
- DNA
deoxyribonucleic acid
- dpe
days post exposure
- dpi
days post inoculation
- ELISA
enzyme‐linked immunosorbent assay
- ELS
extensive literature search
- FMD
Foot and mouth disease
- HPAI
Highly Pathogenic Avian Influenza
- IPT
immunoperoxidase test
- LSD
lumpy skin disease virus
- NCD
Newcastle disease virus
- OIE
World Organization for Animal Health
- PCR
polymerase chain reaction
- PZ
protection zone
- RP
rinderpest virus
- RT‐PCR
reverse transcription polymerase chain reaction
- RVFV
Rift Valley fever virus
- SEIR
Susceptible‐Exposed‐Infectious‐Removed
- SPGP
Sheep pox and goat pox
- SZ
surveillance zone
- ToR
Terms of Reference
Annex A – Definitions in EU legislation
1.
| Terms | Definitions |
|---|---|
| Clinical examination | The clinical examination comprises: (i) an initial general evaluation of the animal health status of the establishment which comprises all the animals of listed species kept in the establishment; and (ii) an individual examination of the animals included in the sample referred to in point (a). The sampling of animals for clinical examination is carried out in accordance with point A.1 of Annex I for terrestrial animals (Delegated Regulation article 3) |
| Confined establishment | Means any permanent, geographically limited establishment, created on a voluntary basis and approved for the purpose of movements, where the animals are: (a) kept or bred for the purposes of exhibitions, education, the conservation of species or research; (b) confined and separated from the surrounding environment; and (c) subject to animal health surveillance and biosecurity measures (AHL: Regulation 2016/429 article 4(48)) |
| Epidemiological unit | Means a group of animals with the same likelihood of exposure to a disease agent (AHL: Regulation 2016/429 article 4(39)) |
| Establishment | Means any premises, structure, or, in the case of open‐air farming, any environment or place, where animals or germinal products are kept, on a temporary or permanent basis, except for: (a) households where pet animals are kept; (b) veterinary practices or clinics (AHL: Regulation 2016/429 article 4(27)) |
| Health status | Means the disease status as regards the listed diseases relevant for a particular listed species with respect to: (a) an animal; (b) animals within: (i) an epidemiological unit; (ii) an establishment; (iii) a zone; (iv) a compartment; (v) a Member State; (vi) a third country or territory (AHL: Regulation 2016/429 article 4(34)) |
| Infected zone | Means a zone in which restrictions on the movements of kept and wild animals or products and other disease control and biosecurity measures may be applied with the view to preventing the spread of a category A disease in the event of official confirmation of the disease in wild animals. (Delegated Regulation article 2(15)) |
| Kept animals | Means animals which are kept by humans, including, in the case of aquatic animals, aquaculture animals (AHL: Regulation 2016/429 article 4(5)) |
| Outbreak | Means the officially confirmed occurrence of a listed disease or an emerging disease in one or more animals in an establishment or other place where animals are kept or located (AHL: Regulation 2016/429 article 4 (40) |
| Protection zone | Means a zone around and including the location of an outbreak, where disease control measures are applied in order to prevent the spread of the disease from that zone (AHL: Regulation 2016/429 article 4(42)) |
| Listed diseases |
Means diseases listed in accordance with Article 5(1) (AHL: Regulation 2016/429 article 4 (18)) List of the diseases (AHL: Regulation 2016/429, Annex II) |
| Listed species |
Means an animal species or group of animal species listed in accordance with Article 8(2), or, in the case of emerging diseases, an animal species or group of animal species which meets the criteria for listed species laid down in Article 8(2); (AHL: Regulation 2016/429 article 4(20)) List of species and groups of species (Commission Implemented Regulation 2018/1882) |
| Monitoring periods | It is appropriate to follow a single approach for the measures to apply in the event of a category A disease. However, the epidemiology of diseases should be taken into account to establish the appropriate moment for the competent authority to apply control measures and to carry out investigations if there is suspicion or confirmation of those diseases. Therefore, ‘monitoring periods’ should be provided, as reference time frames for each category A disease affecting terrestrial animals based on incubation periods and other relevant elements that may affect the spread of the disease. (Delegated Regulation whereas 10). |
| Restricted zone | Means a zone in which restrictions on the movements of certain animals or products and other disease control measures are applied, with a view to preventing the spread of a particular disease into areas where no restrictions are applied; a restricted zone may, when relevant, include protection and surveillance zones (AHL: Regulation 2016/429 article 4(41)) |
| Surveillance zone | Means a zone which is established around the protection zone, and where disease control measures are applied in order to prevent the spread of the disease from the protection zone (AHL: Regulation 2016/429 article 4(43)) |
| Wild animals | Means animals which are not kept animals (AHL: Regulation 2016/429 article 4(8)) |
| Zone | Means: (a) for terrestrial animals, an area of a Member State, third country or territory with a precise geographical delimitation, containing an animal subpopulation with a distinct health status with respect to a specific disease or specific diseases subject to appropriate surveillance, disease control and biosecurity measures (AHL: Regulation 2016/429 article 4 (35)) |
Annex B – Scenarios of ToR 1
1.
| ToRs | Legislation | Scenario | Description of the Scenario | Elements of the Scenario |
|---|---|---|---|---|
|
ToR 1.1 ToR 1.2 |
6(2) of the Delegated Regulation | 1st Scenario | To assess the effectiveness of disease‐specific sampling procedures of animals of listed species in a suspected establishment, based on clinical examination (TOR 1.1) and laboratory examination (TOR 1.2), in their ability to detect a category A disease in kept animals if the disease is present in that establishment, or to rule it out if not present (Art. 6 (2)). |
|
| ToR 1.2 |
Art. 12(3), Art. 7 (4) (Preventive killing) of the Delegated Regulation, and Art. 57 Reg.2016/429 |
2nd Scenario | To assess the effectiveness of disease‐specific sampling procedures, based on laboratory examination (ToR 1.2), in their ability to detect the disease in the event of preventive killing, and in their ability to support with the epidemiological investigation (disease detection, prevalence estimation, virus identification etc.) in kept animals of listed species in an affected establishment, before or when they are killed or found dead. The purposes of the epidemiological enquiry are described in Article 57 of Regulation (EU)2016/429. |
for the purposes of:
|
|
ToR 1.1 ToR 1.2 |
Article 13(3)c of the Delegated Regulation | 3rd Scenario | To assess the effectiveness of disease‐specific sampling procedures based on clinical (ToR 1.1) and laboratory (ToR 1.2) examinations of the animals of listed species belonging to the categories described in article 13(2)) of an affected establishment, in order to grant a specific derogation from killing these animals, while ensuring that they do not pose a risk for the transmission of the disease. |
|
|
ToR 1.1 ToR 1.2 |
Article 14(1) of the Delegated Regulation Art. 57 Reg.2016/429 |
4th Scenario | To assess the effectiveness of disease‐specific sampling procedures based on clinical (ToR 1.1) and laboratory (ToR 1.2) examinations of the animals of non‐listed species kept in an affected establishment, in their ability to ensure the detection of the virus if the virus is present in these species. |
|
|
ToR 1.1 ToR 1.2 |
Article 14(1) of the Delegated Regulation Art. 57 Reg.2016/429 |
5th Scenario | To assess the effectiveness of disease‐specific sampling procedures based on clinical (ToR 1.1) and laboratory (ToR 1.2) examinations of the wild animals of listed species within the affected establishment and in its surroundings. The purpose of the sampling procedures is to ensure the detection of the virus, if the virus is present in these wild species |
|
|
ToR 1.1 ToR 1.2 |
Article 26(2) of the Delegated Regulation | 6th Scenario | To assess the effectiveness of disease‐specific sampling procedures based on clinical (ToR 1.1) and laboratory (ToR 1.2) examinations of the animals of listed species in establishments located in the protection zone. The purpose of the sampling procedures is to ensure the detection of the virus, if the virus is present in these animals. |
|
| ToR 1.3 | Article 26(5) of the Delegated Regulation point A.3 of Annex I | 7th Scenario | To assess the effectiveness of disease‐specific sampling procedures, based on clinical (ToR 1.1) and laboratory (ToR 1.2) examinations of the animals of listed species, for the sampling of establishments located in a protection zone when the radius is larger than 3 km. The purpose of the sampling procedure is to ensure disease detection of the virus if the virus is present in establishments within the protection zone |
|
| ToR 1.3 | Article 41 of the Delegated Regulation | 8th Scenario | To assess the effectiveness of disease‐specific sampling procedures, based on clinical (ToR 1.1) and laboratory (ToR 1.2) examinations of the animals of listed species, for the sampling of the establishments located within the surveillance zone. The purpose of the sampling procedure is to ensure disease detection if the virus is present in establishments within the surveillance zone |
|
| Derogations to allow animal movements | ||||
| ToR 1.4 |
Article 28(5) of the Delegated Regulation Article 29 of the Delegated Regulation |
9th Scenario | To assess the effectiveness of disease‐specific sampling procedures based on clinical and/or laboratory examinations of the animals of an establishment in a protection zone, in order to grant a derogation from prohibitions in the movement of animals, and allow for the animals to be moved to a slaughterhouse located within the protection zone or in the surveillance zone or outside the restricted zone (Art29) |
|
| ToR 1.4 |
Article 28(5) and Article 30(1) of the Delegated Regulation |
10th Scenario | To assess the effectiveness of disease‐specific sampling procedures based on clinical and/or laboratory examinations, to grant a derogation from prohibitions in the movement of day‐old‐chicks located in the protection zone and hatched from eggs originating in the restricted zone or outside the restricted zone. The sampling procedures should ensure that the movement of these day‐old‐chicks to an establishment located in the same Member State but if possible, outside the restricted zone |
|
| ToR 1.4 |
Article 28(5) and Article 30(2) of the Delegated Regulation |
11th Scenario | To assess the effectiveness of disease‐specific sampling procedures based on clinical and/or laboratory examinations, to grant a derogation from prohibitions in the movement of ready‐to‐lay poultry located in the protection zone to establishments located in the same MS and if possible within the restricted zone. |
clinical examinations and laboratory examination of animals kept in the establishment, including those animals to be moved |
| ToR 1.4 |
Article 28(5) and Article 37 of the Delegated Regulation |
12th Scenario | To assess the effectiveness of disease‐specific sampling procedures based on clinical and/or laboratory examinations of the animals of an establishment in a protection zone, in order to grant derogation from prohibitions in the movement of these animals to a plant approved for processing or disposal of animal by‐products in which the kept animals are immediately killed (Art37) |
clinical examinations and laboratory examinations of animals kept in the establishment, including those animals to be moved |
| ToR 1.4 |
Article 43(5) and Article 44 of the Delegated Regulation |
13th Scenario | To assess the effectiveness of disease‐specific sampling procedures based on clinical and/or laboratory examinations of the animals of listed species in order to grant derogation from prohibitions and allow for these animals to be moved: a) from an establishment in a surveillance zone to a slaughterhouse located within or outside the restricted zone, b)from an establishment outside the surveillance zone to a slaughterhouse situated in the surveillance zone |
|
| ToR 1.4 |
Article 43(5) and Article 45(1) of the Delegated Regulation |
14th Scenario | To assess the effectiveness of disease‐specific sampling procedures based on clinical and/or laboratory examinations of kept ungulates of listed species in order to grant a derogation and allow for the animals to be moved from an establishment in the surveillance zone to pastures situated within the surveillance zone |
|
| ToR 1.4 |
Article 43(5) and Article 45(2) of the Delegated Regulation |
15th Scenario | To assess the effectiveness of disease‐specific sampling procedures based on clinical and/or laboratory examinations of kept ungulates of listed species in order to grant derogation and allow to be moved from an establishment in the surveillance zone to an establishment belonging to the same supply chain, located in or outside the surveillance zone, in order to complete the production cycle before slaughter |
|
| ToR 1.4 |
Article 43(5) and Article 46(1) of the Delegated Regulation |
16th Scenario | To assess the effectiveness of disease‐specific sampling procedures based on clinical and/or laboratory examinations to grant derogation of movements of day‐old‐chicks hatched from establishment located in the surveillance zone, from eggs originating within the surveillance zone and eggs originating outside the restricted zone, to an establishment located in the same Member State where they were hatched |
|
| ToR 1.4 |
Article 43(5) and Article 46(2) of the Delegated Regulation |
17th Scenario | To assess the effectiveness of disease‐specific sampling procedures based on clinical and/or laboratory examinations, to grant a derogation from prohibitions in the movement of ready‐to‐lay poultry located in the surveillance zone to establishments located in the same MS. |
|
| ToR 1.4 | Article 56(1)c of the Delegated Regulation | 18th Scenario | To assess the effectiveness of disease‐specific sampling procedures based on clinical and/or laboratory examinations of the animals of an establishment located in the restricted zone of an outbreak in order to allow their move within the restricted zone, when restriction measures are maintained beyond the period set out in Annex XI |
|
| Repopulation | ||||
| ToR 1.5 | Article 59(2),(3) of the Delegated Regulation | 19th Scenario | To assess the effectiveness of disease‐specific sampling procedures based on laboratory examinations of the animals that are kept for the repopulation prior to their introduction to rule out the presence of the disease. |
|
| ToR 1.5 | Article 59(9) of the Delegated Regulation | 20th Scenario | To assess the effectiveness of disease‐specific sampling procedures based on laboratory examinations of the animals that have been repopulated, in the event of unusual mortalities or clinical signs being notified during the repopulation; to rule out the presence of the disease. |
|
| ToR 1.5 | Article 59(5) of the Delegated Regulation | 21st Scenario | To assess the effectiveness of disease‐specific sampling procedures based on laboratory examinations of the animals that have been repopulated, on the last day of the monitoring period calculated forward from the date on which the animals were placed in the repopulated establishment. In case the repopulation takes place in several days, the monitoring period will be calculated forward from the last day in which the last animal is introduced in the establishment. |
|
Annex C – Existing sampling procedures for ASF
1.
Sampling scenarios for ASF – Based on Commission Decision 2003/422/EC if not stated otherwise
| Scenario | Description of the Scenario | Clinical guidelines | Laboratory guidelines |
|---|---|---|---|
| 1st | To assess the effectiveness of disease‐specific sampling procedures of animals of listed species in a suspected establishment, based on clinical examination (TOR 1.1) and laboratory examination (TOR 1.2), in their ability to detect a category A disease in kept animals if the disease is present in that establishment, or to rule it out if not present (Art. 6 (2)). |
Chapter IV A. 2. Inspection of production and health records; inspection of each subunit of the holding Clinical examination must include taking the body temperature and must primarily concern the following pigs/group of pigs: sick or anorexic pigs; pigs recently introduced from confirmed outbreaks or from other suspected sources; pigs kept in subunits recently visited by external visitors who had recent close contact with ASF‐suspected or infected pigs or for whom other particularly risky contacts with a potential source of the ASF virus have been identified; pigs already sampled and serologically tested for ASF, in case the results of these tests do not allow ASF to be ruled out, and in‐contact pigs; pigs recently recovered from the disease. If the inspection in the suspected holding has not indicated the presence of the pigs or group of pigs referred to in the above subparagraph, the competent authority, without prejudice to other measures that may be applied in the holding in question in accordance with Directive 2002/60/EC and taking into account the epidemiological situation, shall: carry out further examinations in the holding in question, or ensure that blood samples for laboratory tests are taken from the pigs in the holding in question. 3. The clinical examination in the holding in question must be carried out on pigs selected at random in the subunits for which a risk of introduction of the ASF virus has been identified or is suspected. The minimum number of pigs to be examined must allow for the detection of fever if it occurs at a prevalence of 10% with 95% confidence in these subunits. 4. If dead or moribund pigs are detected in a suspected holding, post‐mortem examinations must be carried out, preferably on at least five of these pigs and in particular on pigs that have: shown very evident signs of disease before death, high fever, died recently. If these examinations have not shown lesions suggesting ASF but, due to the epidemiological situation, further investigations are deemed necessary: a clinical examination, and blood sampling be carried out in the subunit where the dead or moribund pigs were kept; and post‐mortem examinations may be carried out on three to four in‐contact pigs, particularly if these pigs are showing clinical signs. Irrespective of the presence or absence of lesions suggesting ASF, samples of the organs or tissues from pigs that have been subjected to post‐mortem examination must be collected for virological tests. These samples must preferably be collected from recently dead pigs. |
Chapter IV A. 4. Samples of the organs or tissues from pigs that have been subjected to post‐mortem examination must be collected for virological tests
5. Blood samples for laboratory tests If further clinical signs or lesions that may suggest ASF are detected in a suspected holding, but the competent authority deems that these findings are not sufficient to confirm an outbreak of ASF and that laboratory tests are therefore necessary, blood samples for laboratory tests must be taken from the suspected pigs and from other pigs in each subunit in which the suspected pigs are kept, in accordance with the following procedures: the minimum number of samples to be taken for serological tests must allow for the detection of 10% seroprevalence with 95% confidence in the subunit in question; the number of samples to be taken for virological tests will be in accordance with the instructions of the competent authority, which will take into account the range of tests that can be performed, the sensitivity of the laboratory tests that will be used and the epidemiological situation. Chapter V B. For detection of the ASF virus, antigen or genome from dead or humanely destroyed pigs, tonsils, lymph nodes (gastrohepatic, renal, submandibular and retropharyngeal), spleen, kidney and lung tissues are the most suitable samples(1). In case of autolysed carcases, an entire long bone or the sternum is the specimen of choice. Anticoagulated blood and/or clotted blood samples must be collected from pigs showing signs of fever or other signs of disease, in accordance with the instructions of the competent authority. (1) It is recommended to collect also samples of ileum, as they may be useful for the diagnosis of classical swine fever. SANTE 7113/2015 2.1.5. Sampling for laboratory investigations will be performed
OIE Disease card: Identification of the agent
Serological tests Serum collected within 8–21 days after infection in convalescent animals |
| 2nd | To assess the effectiveness of disease‐specific sampling procedures, based on laboratory examination (ToR 1.2), in their ability to detect the disease in the event of preventive killing, and in their ability to support with the epidemiological investigation (disease detection, prevalence estimation, virus identification, etc.) in kept animals of listed species in an affected establishment, before or when they are killed or found dead. The purposes of the epidemiological enquiry are described in Article 57 of Regulation (EU)2016/429. |
Council Directive 2002/60/EC Article 8 Member States shall ensure that the epidemiological inquiry in relation to suspected cases or outbreaks of African swine fever is carried out on the basis of questionnaires, prepared within the framework of the contingency plans referred to in Article 21. Such an inquiry shall deal at least with: (a) the length of time during which African swine fever virus may have existed on the holding before the disease was notified or suspected; (b) the possible origin of African swine fever on the holding and the identification of other holdings in which pigs may have become infected or contaminated from the same source; (c) the movement of persons, vehicles, pigs, carcases, semen, meat or any material which could have carried the virus to or from the holdings in question; (d) the possibility that vectors or feral pigs cause the disease to spread. If the results of this inquiry suggest that African swine fever may have spread from or to holdings located in other Member States, the Commission and the Member States concerned shall be immediately informed. |
Annex Chapter IV B. SAMPLING PROCEDURES IN A HOLDING WHEN PIGS ARE KILLED FOLLOWING CONFIRMATION OF DISEASE 1. In order that the manner of introduction of the ASF virus into an infected holding and the length of time elapsed since its introduction may be established, when pigs are killed on a holding following confirmation of an outbreak in accordance with Article 5(1)(a) of Directive 2002/60/EC, blood samples for serological tests must be taken at random from the pigs when they are killed. 2. The minimum number of pigs to be sampled must allow for the detection of 10% seroprevalence with 95% confidence in pigs in each subunit of the holding*. Samples for virological tests must also be taken in accordance with the instructions of the competent authority, which will take into account the range of tests that can be performed, the sensitivity of the laboratory tests that will be used and the epidemiological situation. In those areas where the presence of vectors infected with the ASF virus has been previously demonstrated, appropriate collections of soft ticks for virological tests must also be taken in accordance with the instructions of the competent authority and Annex III to Directive 2002/60/EC. 3. However, in case of secondary outbreaks, the competent authority may decide to derogate from points 1 and 2 and establish other sampling procedures, taking into account the epidemiological information already available on the source and means of virus introduction into the holding and the potential spread of disease from the holding. * However, if the derogation provided in Article 6(1) of Directive 2002/60/EC has been applied, sampling must concern the subunits of the holding where pigs have been killed, without prejudice to the further examinations and sampling to be carried out on the remaining pigs in the holding, which shall be carried out in accordance with the instructions of the competent authority. |
| 3rd | To assess the effectiveness of disease‐specific sampling procedures based on clinical (ToR 1.1) and laboratory (ToR 1.2) examinations of the animals of listed species belonging to the categories described in article 13(2)) of an affected establishment, in order to grant a specific derogation from killing these animals, while ensuring that they do not pose a risk for the transmission of the disease. |
Council Directive 2002/60/EC Article 5 2. In cases where an outbreak has been confirmed in a laboratory, a zoo, a wild life park or a fenced area where pigs are kept for scientific purposes or purposes related to conservation of species or conservation of rare breeds, the Member State concerned may decide to derogate from paragraphs 1(a) and 1(e), provided that basic Community interests are not adversely affected. |
No specific sampling procedures in the legislation |
| 4th | To assess the effectiveness of disease‐specific sampling procedures, based on clinical (ToR 1.1) and laboratory (ToR 1.2) examinations of the animals of non‐listed species kept in an affected establishment, in their ability to ensure the detection of the virus if the virus is present in these species. | N/A; There are no non‐listed species that can be infected with ASF. | N/A; There are no non‐listed species that can be infected with ASF. |
| 5th | To assess the effectiveness of disease‐specific sampling procedures, based on clinical (ToR 1.1) and laboratory (ToR 1.2) examinations of the wild animals of listed species within the affected establishment and in its surroundings. The purpose of the sampling procedures is to ensure the detection of the virus, if the virus is present in these wild species | No guidelines described for animals within establishment and its surroundings; there are guidelines for detection of the virus in feral pigs. |
SANTE 7113/2015 3.1.4. Measures to be taken in infected areas to eradicate the disease a) Surveillance (key measure):
l) Testing for ASF of all wild boar found dead and culled. All hunted wild boar tested for ASF virus detection using PCR and for Ab detection. |
| 6th | To assess the effectiveness of disease‐specific sampling procedures based on clinical (ToR 1.1) and laboratory (ToR 1.2) examinations of the animals of listed species in establishments located in the protection zone. The purpose of the sampling procedures is to ensure the detection of the virus, if the virus is present in these animals. |
Council Directive 2002/60/EC Article 10 1.(a) A census of all the holdings shall be carried out as soon as possible; after the establishment of the protection zone these holdings shall be visited by an official veterinarian within not more than seven days in order to conduct a clinical examination of the pigs and to check the register and the pig identification marks referred to in Articles 4 and 5 of Directive 92/102/EEC. 4. The measures in the protection zone shall continue to be applied at least until: (a) cleansing, disinfection and, if necessary, disinsectisation in the infected holdings have been carried out; (b) pigs on all holdings have undergone clinical and laboratory examinations carried out in accordance with the diagnostic manual in order to detect the possible presence of African swine fever virus. The examinations referred to in point (b) shall not take place until 45 days have elapsed since the completion of preliminary cleansing, disinfection and, if necessary, disinsectisation measures on the infected holdings. Chapter IV F. 1. In order that the measures referred to in Article 10 of Directive 2002/60/EC may be lifted in a protection zone, in all holdings in the zone: — a clinical examination must be carried out in accordance with the procedures laid down in Section A(2) and (3) |
Chapter IV F. 2. The minimum number of blood samples to be taken must allow for the detection of 10% seroprevalence with 95% confidence in pigs in each subunit in the holding. However, the derogation provided for in Article 10(5) and Article 11(4) of Directive 2002/60/EC may only be granted if the competent authority ensures that the number of blood samples taken allow for the detection of 5% seroprevalence with 95% confidence in each subunit in the holding. |
| 7th | To assess the effectiveness of disease‐specific sampling procedures, based on clinical (ToR 1.1) and laboratory (ToR 1.2) examinations of the animals of listed species, for the sampling of establishments located in a protection zone when the radius is larger than 3 km. The purpose of the sampling procedure is to ensure disease detection of the virus if the virus is present in establishments within the protection zone | N/A; protection zone radius for ASF is 3 km | N/A; protection zone radius for ASF is 3 km |
| 8th | To assess the effectiveness of disease‐specific sampling procedures, based on clinical (ToR 1.1) and laboratory (ToR 1.2) examinations of the animals of listed species, for the sampling of the establishments located within the surveillance zone. The purpose of the sampling procedure is to ensure disease detection if the virus is present in establishments within the surveillance zone |
Council Directive 2002/60/EC Article 10 4. The measures in the surveillance zone shall continue to be applied at least until: (a) cleansing, disinfection and, if necessary, disinsectisation in the infected holdings have been carried out; (b) pigs on all holdings have undergone clinical and, where necessary, laboratory examinations as laid down in the diagnostic manual in order to detect the eventual presence of African swine fever virus. The examinations referred to in point (b) shall not take place until 40 days have elapsed since the completion of preliminary cleansing, disinfection and, if necessary, disinsectisation measures on the infected holdings. Chapter IV G 1. In order that the measures referred to in Article 11 of Directive 2002/60/EC may be lifted in a surveillance zone, a clinical examination must be carried out in all holdings in the zone in accordance with the procedures laid down in Section A(2). |
Chapter IV G In addition, blood samples for serological tests must be taken from pigs:
2. Whenever blood sampling for serological tests is carried out in holdings located in the surveillance zone, the number of blood samples to be taken in these holdings must be in accordance with Section F(2), first sentence. (2. The minimum number of blood samples to be taken must allow for the detection of 10% seroprevalence with 95% confidence in pigs in each subunit in the holding.) However, the derogation provided for in Article 10(5) and Article 11(4) of Directive 2002/60/EC may only be granted if the competent authority ensures that in each holding in the zone blood samples for serological tests are taken. The minimum number of blood samples to be taken must allow for the detection of 5% seroprevalence with 95% confidence in each subunit in the holding. |
| Derogations to allow animal movements | |||
| 9th | To assess the effectiveness of disease‐specific sampling procedures based on clinical and/or laboratory examinations of the animals of an establishment in a protection zone, in order to grant a derogation from prohibitions in the movement of animals, and allow for the animals to be moved to a slaughterhouse located within the protection zone or in the surveillance zone or outside the restricted zone (Art29) |
Council Directive 2002/60/EC Article 10 1.(f) Pigs may not be removed from the holding in which they are kept for at least 40 days after the completion of the preliminary cleansing and disinfection, and, if necessary, disinsectisation of the infected holdings. After 40 days, the competent authority may authorise the removal of pigs from the said holding to be directly transported to:
3. The competent authority may authorise the removal of pigs from the holding concerned, on condition that: (a) an official veterinarian has carried out a clinical examination of the pigs in the holding and in particular of those to be moved, including the taking of the body temperature in accordance with the procedures laid down in the diagnostic manual and a check of the register and the pig identification marks referred to in Articles 4 and 5 of Directive 92/102/EEC; Chapter IV D D. CHECKING AND SAMPLING PROCEDURES BEFORE AUTHORISATION IS GIVEN TO MOVE PIGS FROM HOLDINGS LOCATED IN PROTECTION OR SURVEILLANCE ZONES AND IN CASE THESE PIGS ARE SLAUGHTERED OR KILLED (ARTICLES 10 AND 11 OF DIRECTIVE 2002/60/EC) 1. Without prejudice to the provisions of Article 11(1)(f), second subparagraph, of Directive 2002/60/EC, in order that authorisation may be given to move pigs from holdings located in protection or surveillance zones in accordance with Article 10(3) of the said Directive, the clinical examination to be carried by an official veterinarian must: — be carried out within the 24‐h period before moving the pigs, — be in accordance with the provisions laid down in A(2). 3. In case of pigs to be moved to a slaughterhouse, to a processing plant or to other places to be then killed or slaughtered, in addition to the investigations to be carried out in accordance with point 1, a clinical examination of pigs must be carried out in each subunit in which the pigs to be moved are kept. In case of pigs older than three to four months, this examination must include taking the temperature of a proportion of pigs.The minimum number of the pigs to be checked must allow for the detection of fever if it occurs at a prevalence of 20% with 95% confidence in the subunits in question. |
Council Directive 2002/60/EC Article 10 3. The competent authority may authorise the removal of pigs from the holding concerned, on condition that: (e) if the pigs are to be slaughtered or killed, a sufficient number of samples is then taken from the pigs in accordance with the diagnostic manual in order that the presence of African swine fever virus in these holdings can be confirmed or ruled out; Chapter IV D 4. When the pigs referred to in point 3 are slaughtered or killed, blood samples for serological tests or blood or organ samples such as tonsil, spleen or lymph nodes for virological tests must be taken from pigs proceeding from each of the subunits from which pigs have been moved. The minimum number of samples to be taken must allow for the detection of 10% seroprevalence or virus prevalence with 95% confidence in each subunit. The type of samples to be taken and the test to be used will be in accordance with the instructions of the competent authority, which will take into account the range of tests that can be performed, the sensitivity of these tests and the epidemiological situation. 5. However, if clinical signs or post‐mortem lesions suggesting ASF are detected when the pigs are slaughtered or killed, by way of derogation from point 4, the provisions for sampling laid down in section C shall apply (C. SAMPLING PROCEDURES WHEN PIGS ARE KILLED AS A PREVENTIVE MEASURE ON A SUSPECTED HOLDING). 6. The derogation provided for in Article 10(5) and Article 11(4) of Directive 2002/60/EC may be granted if the competent authorities ensure that an intensive sampling and testing scheme is also applied on the groups of pigs to be checked or sampled referred to in points 2, 3 and 4. In the context of this scheme, the minimum number of blood samples to be taken must allow for the detection of 5% seroprevalence with 95% confidence in the group of pigs in question. |
| 12th | To assess the effectiveness of disease‐specific sampling procedures based on clinical and/or laboratory examinations of the animals of an establishment in a protection zone, in order to grant derogation from prohibitions in the movement of these animals to a plant approved for processing or disposal of animal by‐products in which the kept animals are immediately killed (Art37) |
Council Directive 2002/60/EC Same as previous scenario Article 10 1.(f) Pigs may not be removed from the holding in which they are kept for at least 40 days after the completion of the preliminary cleansing and disinfection, and, if necessary, disinsectisation of the infected holdings. After 40 days, the competent authority may authorise the removal of pigs from the said holding to be directly transported to:
3. The competent authority may authorise the removal of pigs from the holding concerned, on condition that: (a) an official veterinarian has carried out a clinical examination of the pigs in the holding and in particular of those to be moved, including the taking of the body temperature in accordance with the procedures laid down in the diagnostic manual and a check of the register and the pig identification marks referred to in Articles 4 and 5 of Directive 92/102/EEC; |
Same as previous scenario Council Directive 2002/60/EC Article 10 3. The competent authority may authorise the removal of pigs from the holding concerned, on condition that: (e) if the pigs are to be slaughtered or killed, a sufficient number of samples is then taken from the pigs in accordance with the diagnostic manual in order that the presence of African swine fever virus in these holdings can be confirmed or ruled out; |
| 13th | To assess the effectiveness of disease‐specific sampling procedures based on clinical and/or laboratory examinations of the animals of listed species in order to grant derogation from prohibitions and allow for these animals to be moved : a) from an establishment in a surveillance zone to a slaughterhouse located within or outside the restricted zone, b)from an establishment outside the surveillance zone to a slaughterhouse situated in the surveillance zone |
Chapter IV D. 3. In case of pigs to be moved to a slaughterhouse, to a processing plant or to other places to be then killed or slaughtered, a clinical examination of pigs must be carried out in each subunit in which the pigs to be moved are kept. In case of pigs older than three to four months, this examination must include taking the temperature of a proportion of pigs. The minimum number of the pigs to be checked must allow for the detection of fever if it occurs at a prevalence of 20% with 95% confidence in the subunits in question. |
Blood samples for serological tests Chapter IV A. 4. Samples of the organs or tissues from pigs that have been subjected to post‐mortem examination must be collected for virological tests Chapter IV D. 4. When the pigs referred to in previous are slaughtered or killed, blood samples for serological tests or blood or organ samples such as tonsil, spleen or lymph nodes for virological tests must be taken from pigs proceeding from each of the subunits from which pigs have been moved. The minimum number of samples to be taken must allow for the detection of 10% seroprevalence or virus prevalence with 95% confidence in each subunit. The type of samples to be taken and the test to be used will be in accordance with the instructions of the competent authority, which will take into account the range of tests that can be performed, the sensitivity of these tests and the epidemiological situation. |
| 15th | To assess the effectiveness of disease‐specific sampling procedures based on clinical and/or laboratory examinations of kept ungulates of listed species in order to grant derogation and allow to be moved from an establishment in the surveillance zone to an establishment belonging to the same supply chain, located in or outside the surveillance zone, in order to complete the production cycle before slaughter |
Council Directive 2002/60/EC Article 11 1.(f) Pigs may not be removed from the holding in which they are kept for at least 30 days after the completion of the preliminary cleansing, disinfection and, if necessary, disinsectisation of the infected holdings. After 30 days, subject to the conditions set out in Article 10(3), the competent authority may authorise the removal of the pigs from the said holding to be directly transported to:
Article 6 1. Where the presence of African swine fever is confirmed in holdings which consist of two or more separate production units and in order that the fattening of pigs may be completed, the competent authority may decide to derogate from the provisions of Article 5(1)(a) as regards healthy pig production units on a holding which is infected provided that the official veterinarian confirms that the structure, size and distance apart of these production units and the operations carried out there are such that the production units provide completely separate facilities for housing, keeping and feeding, so that the virus cannot spread from one production unit to another. Article 5 2. In cases where an outbreak has been confirmed in a laboratory, a zoo, a wild life park or a fenced area where pigs are kept for scientific purposes or purposes related to conservation of species or conservation of rare breeds, the Member State concerned may decide to derogate from paragraphs 1(a) and 1(e), provided that basic Community interests are not adversely affected |
No specific sampling procedures in the legislation |
| 18th | To assess the effectiveness of disease‐specific sampling procedures based on clinical and/or laboratory examinations of the animals of an establishment located in the restricted zone of an outbreak in order to allow their move within the restricted zone, when restriction measures are maintained beyond the period set out in Annex XI |
Implementing Decision 2014/178/EU Article 3 By way of derogation from the prohibition provided for in point (a) of Article 2, the Member States concerned may authorise the dispatch of live pigs from a holding located in the areas listed in Part II of the Annex to other areas in the territory of the same Member State provided that the pigs have been resident for a period of at least 30 days or since birth on the holding and no live pigs have been introduced into that holding during a period of at least 30 days prior to the date of the movement and 1. and a clinical examination for African swine fever has been carried out by an official veterinarian in accordance with the checking and sampling procedures laid down in Part A of Chapter IV of the Annex to Commission Decision 2003/422/EC (1) on the date of shipment, or 2. the pigs come from a holding: (a) that has been subjected at least twice a year, with an interval of at least 4 months, to inspections by the competent veterinary authority, which: (i) followed the guidelines and procedures laid down in Chapter IV of the Annex to Decision 2003/422/EC; (ii) included a clinical examination and sampling in accordance with the checking and sampling procedures laid down in Part A of Chapter IV of the Annex to Decision 2003/422/EC. |
Article 3 /…/ 1. the pigs have been subjected to laboratory testing for African swine fever carried out with negative results on samples taken in accordance with the sampling procedures as laid down in the plan for the eradication of African swine fever referred to in the second paragraph of Article 1 of this Decision within a period of 15 days prior to the date of the movement/…/ 2. the pigs come from a holding: (a) that has been subjected at least twice a year, with an interval of at least 4 months, to inspections by the competent veterinary authority, which/…/(ii) included a clinical examination and sampling in accordance with the checking and sampling procedures laid down in Part A of Chapter IV of the Annex to Decision 2003/422/EC; (c) in which the pigs over the age of 60 days have been subjected to the laboratory testing for African swine fever referred to in paragraph 1. |
| Repopulation | |||
| 19th | To assess the effectiveness of disease‐specific sampling procedures based on laboratory examinations of the animals that are kept for the repopulation prior to their introduction to rule out the presence of the disease | No guidelines described for ruling out the presence of disease prior to the introduction. | No guidelines described for ruling out the presence of disease prior to the introduction. |
| 20th | To assess the effectiveness of disease‐specific sampling procedures based on laboratory examinations of the animals that have been repopulated, in the event of unusual mortalities or clinical signs being notified during the repopulation; to rule out the presence of the disease | No specific guidelines described in legislation |
Blood samples for serological tests Chapter IV A. 4. Samples of the organs or tissues from pigs that have been subjected to post‐mortem examination must be collected for virological tests Chapter E 3. After any reintroduction of pigs, the competent authority shall ensure that in case of any disease or death of the pigs in the holding due to unknown reasons, the pigs in question are immediately tested for ASF. |
| 21st | To assess the effectiveness of disease‐specific sampling procedures based on laboratory examinations of the animals that have been repopulated, on the last day of the monitoring period calculated forward from the date on which the animals were placed in the repopulated establishment. In case the repopulation takes place in several days, the monitoring period will be calculated forward from the last day in which the last animal is introduced in the establishment | No specific guidelines described in legislation |
E.1. When pigs are reintroduced into a holding in accordance with Article 13(3) (disease has not been linked to vectors), of Directive 2002/60/EC, the following sampling procedures must be applied:
2. When pigs are reintroduced into a holding in accordance with Article 13(4) (disease has been linked to vectors) of Directive 2002/60/EC, the following sampling procedures must be applied:
Then, the procedure laid down in the third indent above must be repeated at the earliest 60 days after total repopulation. 3. After any reintroduction of pigs, the competent authority shall ensure that in case of any disease or death of the pigs in the holding due to unknown reasons, the pigs in question are immediately tested for ASF. |
Annex D – Scenarios of ToR 2
1.
| ToRs | Legislation | Scenario | Description of the Scenario | Elements of the Scenarios |
|---|---|---|---|---|
| ToR 2 |
Article 8 of the Delegated Regulation Article 57 of 2016/429 Regulation Annex II of the Delegated Regulation |
1st Scenario | To assess the effectiveness of the length of the Monitoring Period, as the time period calculated backwards from the date of the notification of the suspicion of a category A disease in an establishment with kept animals of listed species, for the purposes of the epidemiological enquiry in the event of a suspicion. |
|
| ToR 2 |
Article 17(2) and Article 57 of 2016/429 Regulation Annex II of the Delegated Regulation |
2nd Scenario | To assess the effectiveness of the length of the Monitoring Period, as the time period calculated backwards from the date of notification of the suspicion of a category A disease in an establishment with kept animals of listed species, for the purposes of the epidemiological enquiry in the event of confirmation of the disease. |
The aim of the epidemiological enquire is the same as above. |
| ToR 2 |
Article 13(b) of the Delegated Regulation Annex II of the Delegated Regulation |
3rd Scenario | To assess the effectiveness of the length of the Monitoring Period, as the time period calculated backwards from the date of confirmation of a category A disease in an establishment with kept animals of listed species, during which the epidemiological units in which the disease has not been confirmed were kept completely separated and handled by different personnel, in order to provide derogations from killing. |
|
| ToR 2 |
Article 27(3)c of the Delegated Regulation Annex II of the Delegated Regulation |
4th Scenario | To assess the effectiveness of the length of the Monitoring Period, as the time period calculated backwards from the date of notification of the suspicion of the latest outbreak of a category A disease in the protection zone. Products or other materials likely to spread the disease, must had been obtained or produced, before this time period in order to be exempted from prohibitions of movements. |
|
| ToR 2 |
Article 32(c) of the Delegated Regulation Article 48(c) of the Delegated Regulation Annex II of the Delegated Regulation |
5th Scenario | To assess the effectiveness of the length of the Monitoring Period, as the time period calculated forwards from the date of semen collection from animals of listed species kept in approved germinal product establishments in the protection or in the surveillance zone, to prove that the donor animal has tested favourable on a sample taken not earlier than 7 days after the monitoring period. |
|
| ToR 2 |
Article 57(1)b of the Delegated Regulation Annex II of the Delegated Regulation |
6th Scenario | To assess the effectiveness of the length of the Monitoring Period, as the appropriate time period calculated forwards from the date after the final cleaning and disinfection and when relevant control of insects and rodents was carried out in an affected establishment, after which the repopulation of the establishment may be allowed by the competent authority. |
|
| ToR 2 |
Article 59(4)b of the Delegated Regulation Annex II of the Delegated Regulation |
7th Scenario | To assess the effectiveness of the length of the Monitoring Period, as the appropriate time period calculated forwards the date when the first animal was introduced, during which all the animals of listed species intended for repopulation should be introduced. |
|
Annex E – Minimum radius and minimum period of duration of protection and surveillance zones
1.
| Category A diseases | Minimum radius of Protection zone Annex V |
Minimum radius of Surveillance zone Annex V |
Minimum period of duration of measures in the protection zone (Article 39(1)) Annex X | Additional period of duration of surveillance measures in the protection zone Article 39(3)) Annex X |
Minimum period of duration of measures in the surveillance zone (as referred to in Articles 55 and 56 of this Regulation) Annex XI |
|---|---|---|---|---|---|
| Foot and mouth disease (FMD) | 3 km | 10 km | 15 days | 15 days | 30 days |
| Infection with rinderpest virus (RP) | 3 km | 10 km | 21 days | 9 days | 30 days |
| Infection with Rift Valley fever virus (RVFV) | 20 km | 50 km | 30 days | 15 days | 45 days |
| Infection with lumpy skin disease virus (LSD) | 20 km | 50 km | 28 days | 17 days | 45 days |
| Infection with Mycoplasma mycoides subsp. mycoides SC (Contagious bovine pleuropneumonia) (CBPP) | Establishment | 3 km | 45 days | Not applicable | 45 days |
| Sheep pox and goat pox (SPGP) | 3 km | 10 km | 21 days | 9 days | 30 days |
| Infection with peste des petits ruminant virus (PPR) | 3 km | 10 km | 21 days | 9 days | 30 days |
| Contagious caprine pleuropneumonia (CCPP) | Establishment | 3 km | 45 days | Not applicable | 45 days |
| African horse sickness (AHS) | 100 km | 150 km | 12 months | Not applicable | 12 months |
| Infection with Burkholderia mallei (Glanders) | Establishment | Establishment | 6 months | Not applicable | Not applicable |
| Classical swine fever (CSF) | 3 km | 10 km | 15 days | 15 days | 30 days |
| African swine fever (ASF) | 3 km | 10 km | 15 days | 15 days | 30 days |
| Highly pathogenic avian influenza (HPAI) | 3 km | 10 km | 21 day | 9 days | 30 days |
| Infection with Newcastle disease virus (NCD) | 3 km | 10 km | 21 days | 9 days | 30 days |
Annex F – Uncertainty
1.
| Source or location of the uncertainty | # | Nature or cause of uncertainty as described by the experts | Impact of the uncertainty on the assessment |
|---|---|---|---|
| ToR 1 | 1 | The model used to answer the ToR is based on the assumption of homogeneous mixing, that may not hold for certain production systems | The effectiveness of the sampling strategies could be over or underestimated. |
| 2 | Estimates for the models parameters for moderately virulence scenarios are extracted from data come from experimental infections which may not reflect field transmission. Furthermore, data for high virulent scenarios come from a limited (n=9) number of outbreaks occurring in a single country (Russian Federation) | The effectiveness of the sampling strategies could be over or underestimated. | |
| 3 | The sensitivity of virus detection is assumed to be 100% for pigs in the I compartment; the sensitivity of serological tests is assumed to be 100% for pigs in the R compartment. | The effectiveness of the sampling strategies could be over or underestimated. | |
| ToR 2 | 4 | Data to estimate time from infection and suspicion could be only extracted from nine references (although these came from multiple countries and were all recent). | The effectiveness of the proposed zone size could be over or underestimated. |
| 5 | Data on the time needed from infection until a virus or antibody response is detectable originated from experimental challenges which may not be reflective of field conditions | The effectiveness of the proposed zone size could be over or underestimated. | |
| ToR 3 | 6 | No transmission kernels are available for ASFV. Transmission kernels for CSFV were used as a proxy. Although transmission routes are likely to be similar for the two viruses, CSFV is more prone to indirect transmission via fomites because of higher levels of excretion | The effectiveness of the proposed zone size could be over or underestimated. |
| 7 | CSF kernels were available for two epidemics (NL 1998–9 and UK 2000) and may not be representative of transmission in other regions due to differences in farm density, management practices, etc. | The effectiveness of the proposed zone size could be over or underestimated. |
Suggested citation: EFSA AHAW PAnel (EFSA Panel on Animal Health and Welfare) , Nielsen SS, Alvarez J, Bicout DJ, Calistri P, Depner K, Drewe JA, Garin‐Bastuji B, Gonzales Rojas JL, Gortázar Schmidt C, Herskin M, Michel V, Miranda Chueca MÁ, Pasquali P, Roberts HC, Sihvonen LH, Spoolder H, Ståhl K, Velarde A, Viltrop A, Winckler C, De Clercq K, Klement E, Stegeman JA, Gubbins S, Antoniou S‐E, Broglia A, Van der Stede Y, Zancanaro G and Aznar I, 2021. Scientific Opinion on the assessment of the control measures of the category A diseases of Animal Health Law: African Swine Fever. EFSA Journal 2021;19(1):6402, 82 pp. 10.2903/j.efsa.2021.6402
Requestor: European Commission
Question number: EFSA‐Q‐2020‐00197
Panel members: Søren Saxmose Nielsen, Julio Alvarez, Dominique Joseph Bicout, Paolo Calistri, Klaus Depner, Julian Ashley Drewe, Bruno Garin‐Bastuji, José Luis Gonzales Rojas, Christian Gortázar Schmidt, Mette Herskin, Virginie Michel, Miguel Ángel Miranda Chueca, Paolo Pasquali, Helen Clare Roberts, Liisa Helena Sihvonen, Hans Spoolder, Karl Ståhl, Antonio Velarde, Arvo Viltrop and Christoph Winckler.
Acknowledgements: The Panel wishes to thank the following for the support provided to this scientific output: Sandra Blome from Friedrich‐Loeffler‐Institut (Federal Research Institute for Animal Health), Anette Botner from Statens Serum Institute and Ann Sofie Olesen from University of Copenhagen, and Elisabeth Dorbek‐Kolin from EFSA.
Adopted: 18 December 2020
Notes
EFSA Story maps: African Swine Fever https://efsa.maps.arcgis.com/apps/MapJournal/index.html?appid=db62d00222644945862b40fe6277831a
Average of the prevalence observed for each of the three herd sizes.
Minimum and maximum average observed considering scenarios 1 and 2.
References
- Anderson D and Watson R, 1980. On the spread of a disease with gamma distributed latent and infectious periods. Biometrika, 67, 191 10.2307/2335333 [DOI] [Google Scholar]
- Andraud M, Halasa T, Boklund A and Rose N, 2019. Threat to the French swine industry of african swine fever: surveillance, spread, and control perspectives. Frontiers Veterinary Science, 6, 248 10.3389/fvets.2019.00248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Animal Health ‐ Regulatory Committee , 2014. Presentations 21 August 2014: African swine fever ‐ Lithuania. Available online: https://ec.europa.eu/food/animals/health/regulatory_committee/presentations_en
- Animal Health ‐ Regulatory Committee , 2016. Presentations 5‐6 July 2016 : African swine fever ‐ Lithuania. Available online: https://ec.europa.eu/food/animals/health/regulatory_committee/presentations_en
- Animal Health ‐ Regulatory Committee , 2018. Presentations 17‐18 January 2018: African swine fever ‐ Romania. Available online: https://ec.europa.eu/food/animals/health/regulatory_committee/presentations_en
- Backer JA, Hagenaars TJ, van Roermund HJW and de Jong MCM, 2009. Modelling the effectiveness and risks of vaccination strategies to control classical swine fever epidemics. Journal of the Royal Society, Interface, 6, 849–861. 10.1098/rsif.2008.0408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blome S, Franzke K and Beer M, 2020. African swine fever – a review of current knowledge. Virus Research, 287, 198099 10.1016/j.virusres.2020.198099 [DOI] [PubMed] [Google Scholar]
- Boender GJ, van den Hengel R, van Roermund HJW and Hagenaars TJ, 2014. The influence of between‐farm distance and farm size on the spread of classical swine fever during the 1997–1998 epidemic in The Netherlands. PLoS ONE, 9, e95278 10.1371/journal.pone.0095278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson J, Zani L, Schwaiger T, Nurmoja I, Viltrop A, Vilem A, Beer M and Blome S, 2018. Simplifying sampling for African swine fever surveillance: assessment of antibody and pathogen detection from blood swabs. Transboundary and Emerging Diseases, 65, e165–e172. 10.1111/tbed.12706 [DOI] [PubMed] [Google Scholar]
- de Carvalho Ferreira HC, Weesendorp E, Elbers ARW, Bouma A, Quak S, Stegeman JA and Loeffen WLA, 2012. African swine fever virus excretion patterns in persistently infected animals: a quantitative approach. Veterinary Microbiology, 160, 327–340. 10.1016/j.vetmic.2012.06.025 [DOI] [PubMed] [Google Scholar]
- de Carvalho Ferreira HC, Backer JA, Weesendorp E, Klinkenberg D, Stegeman JA and Loeffen WLA, 2013. Transmission rate of African swine fever virus under experimental conditions. Veterinary Microbiology, 165, 296–304. 10.1016/j.vetmic.2013.03.026 [DOI] [PubMed] [Google Scholar]
- Costard S, Wieland B, de Glanville W, Jori F, Rowlands R, Vosloo W, Roger F, Pfeiffer DU and Dixon LK, 2009. African swine fever: how can global spread be prevented? Philosophical Transactions of the Royal Society B: Biological Sciences, 364, 2683–2696. 10.1098/rstb.2009.0098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruciere C, 2003. Peste porcine africaine In: Lefèvre P‐C, Blancou J. and C R. (eds.). Principales maladies infectieuses et parasitaires du bétail : Europe et régions chaudes, Lavoisier: pp. 735–746. [Google Scholar]
- Dixon LK, Stahl K, Jori F, Vial L and Pfeiffer DU, 2020. African Swine fever epidemiology and control. Annual Review of Animal Biosciences, 8, 221–246. 10.1146/annurev-animal-021419-083741 [DOI] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority), Alvarez J, Roberts HC, Stahl K, Viltrop A, Clercq KD, Klement E, Stegeman JA, Gubbins S, Antoniou S‐E, Zancanaro G and Aznar I, 2020a. Technical report on the methodological approach used for the assessment of the control measures for Category A diseases in the context of the new Animal Health Law. EFSA Supporting Publications 2020;17:1988E. 10.2903/sp.efsa.2020.EN-1988 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), Miteva A, Papanikolaou A, Gogin A, Boklund A, Bøtner A, Linden A, Viltrop A, Schmidt CG, Ivanciu C, Desmecht D, Korytarova D, Olsevskis E, Helyes G, Wozniakowski G, Thulke H‐H, Roberts H, Abrahantes JC, Ståhl K, Depner K, González Villeta LC, Spiridon M, Ostojic S, More S, Vasile TC, Grigaliuniene V, Guberti V and Wallo R, 2020b. Epidemiological analyses of African swine fever in the European Union (November 2018 to October 2019). EFSA Journal 2020;18(1):5996, 107 pp. 10.2903/j.efsa.2020.5996 [DOI] [Google Scholar]
- Galindo I and Alonso C, 2017. African swine fever virus: a review. Viruses, 9 10.3390/v9050103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallardo C, Nieto R, Soler A, Pelayo V, Fernandez‐Pinero J, Markowska‐Daniel I, Pridotkas G, Nurmoja I, Granta R, Simon A, Perez C, Martin E, Fernandez‐Pacheco P and Arias M, 2015. Assessment of African Swine fever diagnostic techniques as a response to the epidemic outbreaks in Eastern European Union Countries: how to improve surveillance and control programs. Journal of Clinical Microbiology, 53, 2555–2565. 10.1128/JCM.00857-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallardo C, Nurmoja I, Soler A, Delicado V, Simón A, Martin E, Perez C, Nieto R and Arias M, 2018. Evolution in Europe of African swine fever genotype II viruses from highly to moderately virulent. Veterinary Microbiology, 219, 70–79. 10.1016/j.vetmic.2018.04.001 [DOI] [PubMed] [Google Scholar]
- Gamado K, Marion G and Porphyre T, 2017. Data‐driven risk assessment from small scale epidemics: estimation and model choice for spatio‐temporal data with application to a classical swine fever outbreak. Frontiers in Veterinary Science, 4 10.3389/fvets.2017.00016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greig A and Plowright W, 1970. The excretion of two virulent strains of African swine fever virus by domestic pigs. The Journal of Hygiene, 68, 673–682. 10.1017/s0022172400042613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guinat C, Gogin A, Blome S, Keil G, Pollin R, Pfeiffer DU and Dixon L, 2016. Transmission routes of African swine fever virus to domestic pigs: current knowledge and future research directions. Veterinary Record, 178, 262 10.1136/vr.103593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guinat C, Porphyre T, Gogin A, Dixon L, Pfeiffer DU and Gubbins S, 2018. Inferring within‐herd transmission parameters for African swine fever virus using mortality data from outbreaks in the Russian Federation. Transboundary and Emerging Diseases, 65, e264–e271. 10.1111/tbed.12748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jori F and Bastos ADS, 2009. Role of wild suids in the Epidemiology of African Swine Fever. EcoHealth, 6, 296–310. 10.1007/s10393-009-0248-7 [DOI] [PubMed] [Google Scholar]
- Jori F, Vial L, Penrith ML, Pérez‐Sánchez R, Etter E, Albina E, Michaud V and Roger F, 2013. Review of the sylvatic cycle of African swine fever in sub‐Saharan Africa and the Indian ocean. Virus Research, 173, 212–227. 10.1016/j.virusres.2012.10.005 [DOI] [PubMed] [Google Scholar]
- Keeling MJ and Rohani P, 2008. Modeling infectious diseases in humans and animals. Biometrics, 64, 993 10.1111/j.1541-0420.2008.01082_7.x [DOI] [Google Scholar]
- Lamberga K, Olševskis E, Seržants M, Berzinš A, Viltrop A and Depner K, 2020. African swine fever in two large commercial pig farms in LATVIA ‐ estimation of the high risk period and virus spread within the farm. Veterinary Sciences, 7 10.3390/vetsci7030105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulumba‐Mfumu LK, Saegerman C, Dixon LK, Madimba KC, Kazadi E, Mukalakata NT, Oura CAL, Chenais E, Masembe C, Ståhl K, Thiry E and Penrith ML, 2019. African swine fever: update on Eastern, Central and Southern Africa. Transboundary and Emerging Diseases, 66, 1462–1480. 10.1111/tbed.13187 [DOI] [PubMed] [Google Scholar]
- Nielsen JP, Larsen TS, Halasa T and Christiansen LE, 2017. Estimation of the transmission dynamics of African swine fever virus within a swine house. Epidemiology and Infection, 145, 2787–2796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nurmoja I, Schulz K, Staubach C, Sauter‐Louis C, Depner K, Conraths FJ and Viltrop A, 2017. Development of African swine fever epidemic among wild boar in Estonia ‐ two different areas in the epidemiological focus. Scientific Reports, 7, 12562 10.1038/s41598-017-12952-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nurmoja I, Mõtus K, Kristian M, Niine T, Schulz K, Depner K and Viltrop A, 2020. Epidemiological analysis of the 2015–2017 African swine fever outbreaks in Estonia. Preventive Veterinary Medicine, 181 10.1016/j.prevetmed.2018.10.001 [DOI] [PubMed] [Google Scholar]
- OIE Standing Group of Experts on African swine fever in Europe , 2017. African swine fever situation in Romania. Available online: http://web.oie.int/RR-Europe/eng/Regprog/en_GF_TADS%20-%20Standing%20Group%20ASF.htm
- Ordas A, Sanchez‐Botija C and Diaz S, 1981. Epidemiological studies on ASF [African swine fever] in Spain. Proceedings of the FAO/CEE Expert Consultation on African Swine Fever Research, Sassari (Italy), 23 Sep 1981.
- Penrith M‐L and Vosloo W, 2009. Review of African swine fever : transmission, spread and control: review article. 80, 5 10.4102/jsava.v80i2.172 [DOI] [PubMed] [Google Scholar]
- Petrov A, Schotte U, Pietschmann J, Dräger C, Beer M, Anheyer‐Behmenburg H, Goller KV and Blome S, 2014. Alternative sampling strategies for passive classical and African swine fever surveillance in wild boar. Veterinary Microbiology, 173, 360–365. 10.1016/j.vetmic.2014.07.030 [DOI] [PubMed] [Google Scholar]
- Petrov A, Forth JH, Zani L, Beer M and Blome S, 2018. No evidence for long‐term carrier status of pigs after African swine fever virus infection. Transboundary and Emerging Diseases, 65, 1318–1328. 10.1111/tbed.12881 [DOI] [PubMed] [Google Scholar]
- Pikalo J, Zani L, Hühr J, Beer M and Blome S, 2019. Pathogenesis of African swine fever in domestic pigs and European wild boar – Lessons learned from recent animal trials. Virus Research, 271, 197614 10.1016/j.virusres.2019.04.001 [DOI] [PubMed] [Google Scholar]
- Sauter‐Louis C, Forth JH, Probst C, Staubach C, Hlinak A, Rudovsky A, Holland D, Schlieben P, Göldner M, Schatz J, Bock S, Fischer M, Schulz K, Homeier‐Bachmann T, Plagemann R, Klaaß U, Marquart R, Mettenleiter TC, Beer M, Conraths FJ and Blome S, 2020. Joining the club: first detection of African swine fever in wild boar in Germany. Transboundary and Emerging Diseases, n/a.. 10.1111/tbed.13890 [DOI] [PubMed] [Google Scholar]
- Schulz K, Staubach C and Blome S, 2017. African and classical swine fever: similarities, differences and epidemiological consequences. Veterinary Research, 48, 84 10.1186/s13567-017-0490-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zani L, Forth JH, Forth L, Nurmoja I, Leidenberger S, Henke J, Carlson J, Breidenstein C, Viltrop A, Höper D, Sauter‐Louis C, Beer M and Blome S, 2018. Deletion at the 5’‐end of Estonian ASFV strains associated with an attenuated phenotype. Scientific Reports, 8, 6510 10.1038/s41598-018-24740-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zani L, Dietze K, Dimova Z, Forth JH, Denev D, Depner K, Alex and rov T, 2019. African swine fever in a Bulgarian backyard farm‐a case report. Veterinary Sciences, 6 10.3390/vetsci6040094 [DOI] [PMC free article] [PubMed] [Google Scholar]


