Abstract
Aim The article reviews the current usage of biocides during this lockdown period for sanitizing our living areas due to the pandemic and discusses the pros and cons. Subject COVID-19 spread like wildfire to over 200 countries of the world across all continents. The causative agent, novel coronavirus (SARS-CoV-2) is being counter attacked by a thorough application of disinfectants and sterilants. However, the virus mutated over 30 times during this global pandemic, creating panic and leading to enhanced pathogenicity and consequently to more stringent sanitation measures for controlling it. However, excessive use of different types of biocides for disinfecting surfaces is highly alarming in several cases. Extensive application of biocides affects the microbial flora, leading to an abrupt decrease in the number and diversity of beneficial microbes that may directly affect the functioning of nutrient cycles. Results The increased concentration of biocides in agricultural land via surface water or pond water indirectly affect the soil and water ecosystem, soil aggregation and fertility. This will also lead to the flourishing of resistant strains due to loss of competition from the other species, which fail to persist after prolonged use of biocides. Conclusion It is necessary to realize the environmental impacts of biocides and sterilants. It is the right time to stop their entry into the agricultural ecosystem by following adequate management strategies and complete neutralization.
Keywords: Biocides, COVID-19, Microbiome, Agroecosystem
Introduction
The pandemic COVID-19 is caused by “novel coronavirus” the acute respiratory syndrome coronavirus 2 (SARS-CoV-2). First, it was identified as a respiratory illness in Wuhan City (China); WHO declared COVID-19 as a global health emergency on 30 January 2020 (Chakraborty and Maity 2020; Gallegos 2020; Liu et al. 2020). COVID-19 spreads mainly by droplets or aerosols from the coughing or sneezing of both symptomatic and asymptomatic COVID-19 infected person and by fomites (Chan et al. 2020; Huang et al. 2020). Indirect contact via surfaces and clothes touched by the infected people and may remain active for several days (Conway and Lipner 2020; Casanova et al. 2008). The droplets are released by sneezing and coughing and cannot be airborne, so they settle down on objects and surfaces surrounding the containment/buffer area. People who come in contact with these surfaces and then touch their nose, mouth or eyes become infected with COVID-19.
Only methods to prevent the disease is by stopping direct contact through use of masks and proper machinery for the infected and people dealing with them. Direct contact may be minimized by the use of appropriate biocides in the form of sprays, handwashes, gels, cleaning liquids, to eliminate the virus from the commonly used solid surfaces, medical devices, our body/clothes, etc. Two types of products have been authorized; (i) products for human hygiene consisting of alcoholic gels or solutions and (ii) products for disinfection of surfaces. Although disinfectant and sanitization is one of the safest ways to keep away from SARS-CoV-2, indiscriminate uses of biocides are increasing our vulnerability to other diseases during the pandemic. However, many of the approved sanitizers and disinfectants have negative impacts on the respiratory or immune system and reducing resistance to the disease (Table 1). In this article, we have explained the impact of different types of sanitizers and disinfectants on human health and on environment (due to substantial changes in existing microbiota) and consequently socio-economical impact during post-COVID-19 era.
Table 1.
Categorization of sanitizers based on the EPA’s design for the Environment Program to protect global people from the dangerous pandemic caused by coronavirus originated from Wuhan in China in December 2019.
| Sanitizers | |||
|---|---|---|---|
| Nature of sanitizer | Main ingredients and structure | Method of application | Virus eliminating mechanism |
| Good (having no negative impact on human) | Ethyl alcohol C2H5OH (60%) | Rubbed it in hand for 10–20 s | Antimicrobial activity of alcohols can be attributed to their ability to denature and coagulate proteins |
| Isopropyl alcohol (CH3)2 CHOH (70%) | Rubbed it in hand for 10–20 s | Virus cell are lysed, and their cellular metabolism are disrupted | |
| Soft soap potassium stearate (C17H35COOK) | Washing with water for 10 s | Soap breaks down virus’s fat membrane | |
| Glycerol (CH2OH)3 | It is also rubbed well with hand | The infectious material falls apart during rubbing | |
| Nature | Composition or formula | Effect on body | Using procedure |
|---|---|---|---|
| Toxic | Benzalkonium chloride (BAC), | Irritant and can cause asthmatic reactions | Composition of handwash and hand sanitizer |
| Quaternary ammonium salt ammonium carbonate and bicarbonate (NH4HCO3) | Adversely affect the respiratory system and changes the neuro-development | It is also used in hand sanitizer that rubbed on skin for removing virus |
| Disinfectants | |||
|---|---|---|---|
| Nature of disinfectant | Name and structural formula | Composition | Method and surface of application |
| Good (no negative impact on human) | Hydrogen peroxide H2O2 | Hydrogen peroxide + peroxy acetic acid | Hard non-porous (HN); food contact post-rinse required (FCR) |
| Sodium bisulphate NaHSO4 | Dilute with water | Spray on hard surface | |
| Ethanol CH3CH2OH | 1.60% ethanol 2.ethanol + ammonium salt(quaternary) | Disinfecting wipes, spray on hard surface | |
| Isopropanol (CH3)2CHOH | 1.70% isopropanol 2.isopropanol + quaternary ammonium salt | Disinfecting wipes, spray on hard surface | |
| Thymol (C10H14O) | Thymol, a component of the botanical thyme oil | sprays to convenient disinfecting wipes | |
| Citric acid (C6H8O7) | (Citric acid +water) (citric acid + vinegar) | Hard non-porous surface. Spray wipes | |
| L-lactic acid (C3H6O3) | L-lactic acid + dodecylbenzenesulfonic acid mixture | Hard non-porous surface | |
| Dodecylbenzenesulfonic acid (C12H25C6H4.SO3H) | Dodecylbenzenesulfonic acid + L- lactic acid | Solid non-porous surface | |
| Octanoic acid (C8H16O2) | Dilutable | Hard non-porous Surface | |
| Nature of disinfectant | Category of compound | Name and formula | Effect on body |
|---|---|---|---|
| Toxic (having negative impact) | Chlorinated compounds | 1.Sodium hypochlorite (NaOCl) | irregular heartbeat, severe injury to heart, liver, kidneys, and lungs, cancer, muscle tremors |
| 2.Hypochlorous acid (HClO2) | |||
| 3.Sodium chloride (NaCl) | |||
| 4.Chlorine monooxide (Cl2O) | |||
| 5.Sodium dichloro-s-triazinetrione Hexachloro benzene (C3H4Cl2N3NaO) | |||
| Phenolic compound | 1.Cresols (C7H8O) | Inhibitory effects on genotoxicity of several mutagens | |
| 2.Hexachlorobenzene (C6Cl6) | |||
| 3.Chlorophenols(C6H5OCl) | |||
| Ammonium compound | 1.Quaternary ammonium compounds (quats) | Mild skin and respiratory irritation up to severe caustic burns on skin | |
| 2.Ammonium carbonate(NH4)2CO3 | |||
| 3.Ammoniumbicarbonate (NH4HCO3) | |||
| Peracid | Peroxyacetic acid peracetic acid | Considered to pose an asthma risk | |
| Iodized compound | ZZZ Disinfectant | Causes severe skin burns and eye damage | |
| Silver compound | 1.Silver concentrations between 10–100 µg/L | ||
| 2. Silver ion + citric acid solution | Liver and kidney damage, irritation of the eyes, skin. respiratory, changes in blood cells | ||
| Organic acids | Glycolic acid (HOCH2CO2H) Octanoic acid (C8H16O2) | Glycholic acid: redness, irritation, scarring, and discolorationOctanoic acid: nausea, bloating and diarrhoea | |
| Aldehydic compound | Glutaraldehyde (C5H8O2) | Cause of cancer | |
| Peroxy compound |
Potassium peroxymonosulfate (KHSO5·KHSO4·K2SO4) |
Cause urticaria, contact dermatitis and asthma |
Need for judicious application of biocides to combat COVID-19
Viruses are categorized by their structure as enveloped and non-enveloped viruses. Influenza virus and coronaviruses are enveloped and are easiest to destroy through proper sanitization (Maillard 2004). The US Environmental Protection Agency (EPA) has listed various disinfectant/antimicrobial agents effective for killing viruses, e.g. SARS-CoV-2 (Table 1) where each product has a label with instructions about the quantity to be used for a given surface area and the protocol to be used for disinfection. The guidelines state that the surface should be wet or dry while application of the biocides, and the time and quantity for application should be minimally done for maximum effect. One should understand that all disinfectants might not work on all surfaces, and application of excessive amounts does not help in increasing the percentage of disinfection.
The disinfectants might also be irritable to human if they come in contact with skin, eyes/nose, etc., and therefore, proper protection for skin (wearing gloves/ long sleeved dress and long pants) and for face (mask) should be used. During the process of disinfection, children, pets and other unwanted traffic should be kept away, for the entire duration of treatment. After use, the masks, gloves, wipes, etc., should be appropriately discarded so that it does not come in contact with mankind. A list of biocides used for surface disinfection to prevent COVID-19 mentioned in Table1 has been adapted from the EPA weblink (https://www.epa.gov/pesticide-registration/list-n-disinfectants-use-against-sars-cov-2). The list also shows the required contact time, applicable surface types, formulation type, and dilutable or not. Human coronaviruses such as Severe Acute Respiratory Syndrome (SARS) coronavirus, Middle East Respiratory Syndrome (MERS) coronavirus or endemic human coronaviruses (HCoV) can persist on inanimate surfaces like metal, glass or plastic for up to 9 days, but can be efficiently inactivated by surface disinfection procedures with 62–71% ethanol, 0.5% hydrogen peroxide or 0.1% sodium hypochlorite within 1 min. Other biocidal agents such as 0.05–0.2% benzalkonium chloride or 0.02% chlorohexidine digluconate are less effective (Kampf et al. 2020).
Impact on agroecosystem and subsequent genotoxicity
This natural ecosystem having both biotic and abiotic factors associated with it is often modified by human beings, to some extent, by the use of fertilizers and pesticides on the one hand and by growing on selective types of crops on a given piece of land, on the other. Use of agriculture related machinery is also another modification inflicted by man on the ecology of a given territory. The natural biogeochemical cycles that allow the cycling of minerals and elements, e.g. sulphur, nitrogen, phosphorous, in particular biosphere, is often disrupted by human affairs, e.g. forceful irrigation, ploughing, tilling, harvesting, etc. The use of chemicals in the form of pesticides/ fertilizers also affects the general microbiota, causing radical changes in the composition and functioning of the biosphere, leading to far-reaching changes in the ecosystem.
Agroecology is the association of agricultural practices and ecological consequences and includes the study of complete food production, economy, environmental issues, and resulting social implications (Charles and Wezel 2015). Migration of huge quantity biocides used during the COVID-19 era is deposited to the agricultural land, pond and river through surface water which must have a negative impact on the agroecology of the countries, especially in regions with heavy impact (Kumar et al. 2020; DeMarini et al. 1982). This may be compared with the results of green revolution (1960s) in Punjab, which India had undertaken to lift poverty and hunger. The heavy usage of chemicals during green revolution had far-reaching impact on the ecology and on economy (Nelson et al. 2019).
Current issues regarding the extensive application of biocides in human localities to prevent diseases may have severe effects on soil ecology in future by reducing the plant soil rhizospheric microflora, if they enter nearby farmlands. Biocides vanquish the beneficial non-target organisms essential for the recycling of micro- and macronutrients, consequently decreasing the soil fertility. The soil microbial diversity is an important criterion to sustain continuous production in agricultural land. The total mass of the microflora and microfauna beneath the soil is 20 times more than total human in earth (Torsvik et al. 1990). Thus, the varieties of microorganisms are integrated in successive food chains in the soil food web and subsequently lose the good microbiota (Fig. 1). Therefore, post-COVID-19 is a challenge for researchers to maintain the structural and functional dynamics of the soil ecosystem. More research is therefore needed to find means of remediation, before the biocides used elsewhere can enter agricultural lands or escape form the point of application and pollute water bodies, which in turn pollute agricultural fields.
Fig. 1.
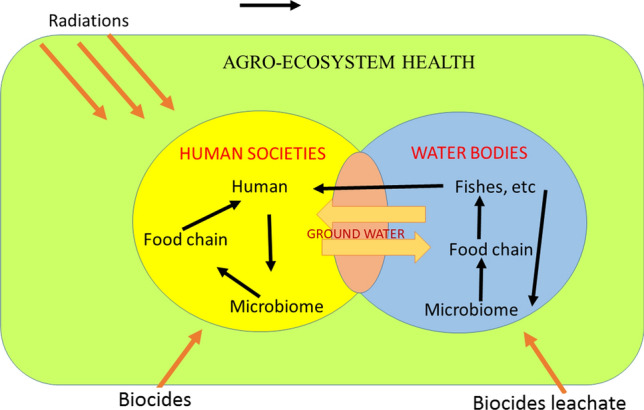
Graphical representation of impact of biocides on agroecosystem
Their deposition in surface water can create strong genotoxic effects by interacting with cellular machinery and interrupting DNA replication processes. Several countries used sodium hypochlorite (NaOCl) for surface sanitization of public areas (https://www.mohfw.gov.in/pdf/). Sodium hypochlorite is a cytotoxic chemical and affects a living cell by changing the pH and/or due to its oxidizing properties. It can exert clastogenic effects in the chromosome and induces sister chromatid exchanges or chromosome breakage as shown by scientists using Chinese hamster lung fibroblasts (CHL) and Human HE2144 fibroblasts (Sasaki et al. 1980; Fukuda et al. 1989). Substances such as sodium hydroxide (NaOH) is generated when sodium hypochlorite reacts with water, and this strong alkali elicits mutagenic effects due to high, non-physiological pH values again causing genotoxic effects in mammalian cell (Fischer et al. 2016).
Consequences of using indiscriminate biocides: future concerns and recommendations
Health workers and governments around the world are working to slow down the spread of COVID-19 where the large-scale disinfection efforts are becoming common. Using methods ranging from simple hand-wiping to mobile spray cannons, workers and volunteers are attempting to halt the transfer of the virus by touch. While there are questions about the efficacy of some of the broader spraying tactics, disinfecting frequently touched surfaces can help stop the spread of the virus.
It should be well understood that all chemical disinfectants are biocides, meaning that they have been made to kill and destroy organisms from smaller to higher groups. Therefore, even commonly used cleaning agents such as disinfectants and sterilants may directly or indirectly harm workers in health care facilities and elsewhere. For example, formaldehyde (a broad spectrum sterilant) is highly an effective antimicrobial, but has been marked as a carcinogen and is a known skin sensitizer. Also, another common disinfectant, glutaraldehyde, is a strong irritant for skin, eyes and our respiratory system.
The United nations organization (UNO) has clearly stated that for headquarter buildings simple disinfectant is sufficient to clean an area and has stated that walls and soft furnishings do not need to be cleaned unless clearly soiled, and also that widespread spraying or ‘fogging’ has not been recommended. Mostly suggestions on disinfection of common areas, e.g. schools, mosques, streets and buildings/club houses is that the highly touched areas should be wiped down effectively, rather than spraying of biocides into the air. However, Internet is filled with news of sanitation workers spraying antiseptic solution on streets (Manila, Philippines) or buildings (worldwide) to help prevent the spread of COVID-19, and Indian health workers went to the extent of spraying chlorinated disinfectant on a group of migrants, (who suffered eye and skin irritations) fearing the spread of coronavirus from cities to smaller towns and villages.
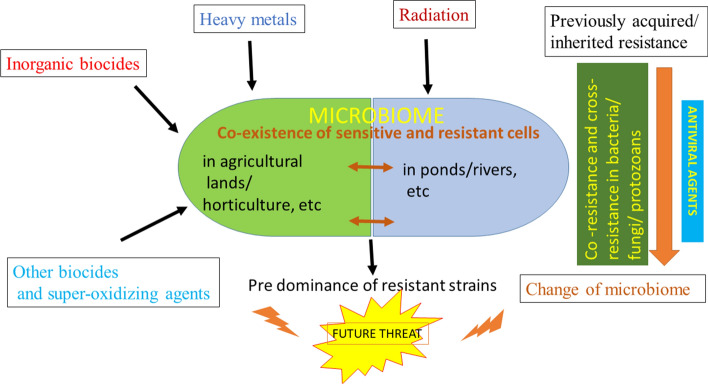
Consistent use of biocides in the form of antiviral agents over a long period of time would disturb the entire terrestrial and aquatic microbiome which not only contains viruses, but also bacteria, fungal species, protozoans, etc., and that may not be necessarily pathogenic. Imbalance caused by use of indiscriminate amounts of biocides to ward off COVID-19 may promote the selection, survival and prevalence of the very resistant microbes, by eventually destroying the susceptible ones (Fig. 2).
Fig. 2.
Anticipated role of prolonged and overuse of antiviral agents to combat COVID-19. All forms of microorganisms are predicted to change genotypically and phenotypically, allowing dominance of resistant strains. Recommendation is to use biocides only as per instruction and after careful study of the area of application
The emergence of bacterial resistance to biocides and the possible linkage between biocide and antibiotic resistance has been a major topic of discussion and concern (Maillard 2005; Kaweeteerawat 2017). The emergence of bacterial resistance to biocides to low (inhibitory) concentrations has been widely reported, mainly from laboratory studies, but also from environmental investigations. Low to intermediate levels of resistance have been observed in most cases, although from time to time high-level resistance has been reported, with bisphenoltriclosan (Sasatsu et al. 1993; Heath et al. 1998, 2000) or with the chemo-sterilant glutaraldehyde (Griffiths et al. 1997; Fraud et al. 2001) and other oxidizing agents (Walsh et al. 2001).
There is now a better understanding of the overall mechanisms that enable bacteria to withstand exposure to low concentrations of a biocide (Dukan et al. 1996). Cross-resistance and co-resistance have been postulated as the major instigation for development of antibiotic resistant strains. Cross-resistance against any particular agent/biocide/drug is caused when the microbe becomes tolerant to similarly acting agents by employing the same strategy. Reports suggest that mutations in genes and ‘hot spots’ result in resistance to quinolones amongst clinical isolates (Paul et al 2019). Susceptible bacterium change to resistant strains after prolonged exposure to an agent or a group of antimicrobials due to acquired mutations leading to the alteration of permeability in the LPS layer of cellular envelope or by reduction in the pore size of porin channel (Nikaido 2003; Tkachenko et al. 2007). Sometimes antibiotic resistance is caused due to over expression of efflux pumps leading to lower cellular levels of antimicrobial agents (Levy 2002; Piddock 2006; Thorrold et al. 2007). Apart from bacteria, viral strains too undergo mutations rapidly, thus becoming increasingly resistant to biocides and medication. The same virus that caused the epidemic in China in 2019–2020 mutated over 30 times and has now spread to over 200 countries in the world, where their altered genotype has made them more suited to prevail in varied climatic conditions, worsening the consequences of the pandemic (Helmy et al. 2020; Dawood 2020; Roy et al. 2020; Paul and Mandal 2019; Chen et al. 2020).
‘Co-resistance’ indicates resistance to more than one class of biocides by the same bacterial strain due to their presence on the same extra-chromosomal DNA element (plasmid) and may be transferred and expressed together in a new host. Currently, antibiotic resistance resulting due to frequent use of biocides (organic disinfectant/ heavy metals) in livestock is gaining attention, and there is also an apprehension of spreading drug resistance to other organisms belonging to other habitats, showing different phenotype/ genotype. A recent report showed that commonly used herbicides can increase or decrease the minimum inhibitory concentration (MIC) of different antibiotics (Kurenback et al. 2018).
The above studies on co-resistance strongly indicate the chances of increased drug resistant strains resulting due to the persistent and prolonged introduction of antiviral agents (biocides) to the environment. Such strains may then dominate the total microbiota of the Earth and become increasingly resistant via genetic exchange and other strategies of cross-resistance (Fig. 3). On the other hand, the biocide-susceptible but ecologically beneficial microorganisms that not only are involved in the global biogeochemical cycles, but also compete against pathogenic microbes inhabiting various microbiomes, would either perish or change their phenotype. This is again an alarming situation because the microbiome maintains the fine balance of life and dead, by playing vital roles in the biosphere. Nitrogen, Sulphur, carbonates and phosphates are recycled due to the beneficial microorganisms which now would face severe challenges after exposure to high volumes of sterilants and antimicrobial agents.
Fig. 3.
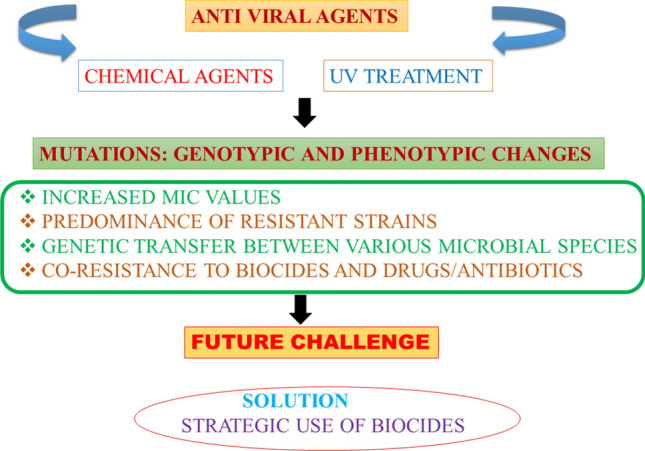
Anticipated role of prolonged and overuse of antiviral agents to combat COVID-19. All forms of microorganisms are predicted to change genotypically and phenotypically, allowing dominance of resistant strains. Recommendation is to use biocides only as per instruction and after careful study of the area of application
Conclusion for future biology
Selective pressure inflicted upon microbes results in their evolution to alter their phenotype and form biocide-resistant strains. The stress induced at concentrations lesser than the lethal dose might trigger SOS response allowing mutations in some cells to become biocide-resistant. The take home lesson for us from these experiences is that one should evaluate whether certain localities have undergone repeated exposure of biocides, due to which radical changes in the genomes of pathogens and the microbiome (terrestrial and aquatic) have been encountered. An important pre-requisite to understanding the effect of industrially manufactured biocides in the environment is to construct a database of production quanta, consumption pattern and their diverse uses (Roman et al. 2012; Choi et al. 2020). Since attempts made in this direction are only a few, a consolidated strategy for use of biocides and assessments of exposure to them has become necessary.
Before using biocides for preventing widespread viral/ bacterial attacks, speculation should be done about the immediate and future impact of such an application. A well-designed strategic action should be followed or treatment pattern should be laid down for a given area depending on the existing biocide load of the area. Additionally, for the application of biocides, one should follow strict guidelines. This will not only impede microbial growth and proliferation, but will also discourage the evolution and dissemination of drug resistant groups of microorganisms.
Acknowledgement
The authors thanks AUUP, NOIDA and IIT Kharagpur for provision of resources
Funding
None.
Compliance with ethical standards
Conflicts of interest
The authors declare that they have no conflicts of interest.
Contributor Information
Debarati Paul, Email: drdebaratipaul@gmail.com, Email: dpaul@amity.edu.
Santi M. Mandal, Email: mandalsm@gmail.com, Email: smmandal@iitkgp.ac.in
References
- Casanova L, Alfano-Sobsey E, Rutala WA, Weber DJ, Sobsey M. Virus transfer from personal protective equipment to healthcare employees' skin and clothing. Emerg Infect Dis. 2008;14:1291–1293. doi: 10.3201/eid1408.080085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakraborty I, Maity P. COVID-19 outbreak: migration, effects on society, global environment and prevention. STOTEN. 2020;728:138882. doi: 10.1016/j.scitotenv.2020.138882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan J, Yuan S, Kok K. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. The Lancet. 2020 doi: 10.1016/S0140-6736(20)30154-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charles F, Wezel A. Agroecology and Agricultural Change. International Encyclopedia of the Social & Behavioral Sciences. 2015 doi: 10.1016/B978-0-08-097086-8.91026-2. [DOI] [Google Scholar]
- Chen J, Wang R, Wang M, Wei GW. Mutations strengthened SARS-CoV-2 infectivity. J Mol Biol. 2020;432:5212–5226. doi: 10.1016/j.jmb.2020.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi YH, Kang MS, Huh DA, Chae WR, Moon KW. Priority setting for management of hazardous biocides in korea using chemical ranking and scoring method. Int J Environ Res Public Health. 2020;17:1970. doi: 10.3390/ijerph17061970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conway J, Lipner SR. Recommendations for physician white coats and clothing practices for prevention of COVID-19 transmission. Dermatol Ther. 2020 doi: 10.1111/dth.14103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawood AA. Mutated COVID-19 may foretell a great risk for mankind in the future. New microbes and new infections. 2020;35:100673. doi: 10.1016/j.nmni.2020.100673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeMarini DM, Plewa MJ, Brockman HE. Use of four short-term tests to evaluate the mutagenicity of municipal water. J Toxicol Environ Health. 1982;9:127–140. doi: 10.1080/15287398209530148. [DOI] [PubMed] [Google Scholar]
- Dukan S, Touati D. Hypochlorous acid stress in Escherichia coli: resistance, DNA damage, and comparison with hydrogen peroxide stress. J Bacteriol. 1996;178:6145–6150. doi: 10.1128/jb.178.21.6145-6150.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer M, Renevey N, Thür B, Hoffmann D, Beer M. Hoffmann B Efficacy assessment of nucleic acid decontamination reagents used in molecular diagnostic laboratories. PLoS ONE. 2020;11:e0159274. doi: 10.1371/journal.pone.0159274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraud S, Maillard JY, Russell AD. Comparison of the mycobactericidal activity of ortho-phthalaldehyde, glutaraldehyde and other dialdehydes by a quantitative suspension test. J Hosp Infect. 2001;48:214–221. doi: 10.1053/jhin.2001.1009. [DOI] [PubMed] [Google Scholar]
- Fukuda MN, Sasaki H, Lopez L, Fukuda M. Survival of recombinant erythropoietin in the circulation: the role of carbohydrates. Blood. 1989;73:84–89. doi: 10.1182/blood.V73.1.84.84. [DOI] [PubMed] [Google Scholar]
- Gallegos A (2020) WHO declares public health emergency for novel coronavirus. Medscape Medical news. (https://www.medscape.com/viewarticle/924596)
- Griffiths PA, Babb JR, Bradley CR. Glutaraldehyde-resistant Mycobacterium chelonae from endoscope washer disinfectors. J Appl Microbiol. 1997;82:519–526. doi: 10.1046/j.1365-2672.1997.00171.x. [DOI] [PubMed] [Google Scholar]
- Heath RJ, Li J, Roland GE. Inhibition of the Staphylococcus aureus NADPH-dependent enoyl-acyl carrier protein reductase by triclosan and hexachlorophene. J Bio Chem. 2000;275:4654–4659. doi: 10.1074/jbc.275.7.4654. [DOI] [PubMed] [Google Scholar]
- Heath RJ, Yu YT, Shapiro MA. Broad spectrum antimicrobial biocides target the FabI component of fatty acid synthesis. J Biol Chem. 1998;273:30316–30320. doi: 10.1074/jbc.273.46.30316. [DOI] [PubMed] [Google Scholar]
- Helmy YA, Fawzy M, Elaswad A, Sobieh A, Kenney SP, Shehata AA. The COVID-19 Pandemic: a comprehensive review of taxonomy, genetics, epidemiology, diagnosis, treatment, and control. J Clin Med. 2020;9:1225. doi: 10.3390/jcm9041225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C, Wang Y, Li X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. The Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kampf G, Todt D, Pfaender S, Steinmann E. Persistence of coronaviruses on inanimate surfaces and its inactivation with biocidal agents. J Hosp Infect. 2020 doi: 10.1016/j.jhin.2020.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar GD, Mishra A, Dunn L, Townsend A, Oguadinma IC, Bright KR, Gerba CP. Biocides and novel antimicrobial agents for the mitigation of coronaviruses. Front Microbiol. 2020;11:1351. doi: 10.3389/fmicb.2020.01351.PMID:32655532;PMCID:PMC7324725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurenbach B, Hill AM, Godsoe W, Hamelsveld SV, Heinemann JA. Agrichemicals and antibiotics in combination increase antibiotic resistance evolution. Peer J. 2018;6:e5801. doi: 10.7717/peerj.5801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy SB. Active efflux, a common mechanism for biocide and antibiotic resistance. J Appl Microbiol. 2002;92:65–71. doi: 10.1046/j.1365-2672.92.5s1.4.x. [DOI] [PubMed] [Google Scholar]
- Liu J, Liao X, Qian S. Community transmission of severe acute respiratory syndrome coronavirus 2. Shenzhen, China: Emerg. Infect. Dis; 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaweeteerawat C, Na Ubol P, Sangmuang S, Aueviriyavit S, Maniratanachote R. Mechanisms of antibiotic resistance in bacteria mediated by silver nanoparticles. J Toxicol Environ Health A. 2017;80:1276–1289. doi: 10.1080/15287394.2017.1376727. [DOI] [PubMed] [Google Scholar]
- Maillard JY. Virus susceptibility to biocides: an understanding. Rev Med Microbiol. 2001;12:63–74. doi: 10.1097/00013542-200104000-00001. [DOI] [Google Scholar]
- Maillard JY. Viricidal activity of biocides. In: Fraise AP, Lambert PA, Maillard JY, editors. Principles and practice of disinfection, preservation and sterilization. 4. Oxford: Blackwell Sci; 2004. pp. 272–323. [Google Scholar]
- Maillard JY. Antimicrobial biocides in the healthcare environment: efficacy, usage, policies, and perceived problems. Ther Clin Risk Manag. 2005;1:307–320. [PMC free article] [PubMed] [Google Scholar]
- Nelson AE, Ravichandran K, Antony U. The impact of the green revolution on indigenous crops of India. J Ethnic Foods. 2019;6:8. doi: 10.1186/s42779-019-0011-9. [DOI] [Google Scholar]
- Nikaido H. Molecular basis of bacterial outer membrane permeability revisited. Microbiol Mol Biol Rev. 2003;67:593–656. doi: 10.1128/MMBR.67.4.593-656.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul D, Chakraborty R, Mandal SM. Biocides and health-care agents are more than just antibiotics: Inducing cross to co-resistance in microbes. Ecotoxicol Env Safety. 2019;174:601–610. doi: 10.1016/j.ecoenv.2019.02.083. [DOI] [PubMed] [Google Scholar]
- Paul D, Mandal SM. Microbial Adaptation and Resistance to Pesticides. In: Mandal S, Paul D, editors. Bacterial Adaptation to Co-resistance. Singapore: Springer; 2019. [Google Scholar]
- Piddock LJV. Clinically relevant chromosomally encoded multidrug resistance efflux pumps in bacteria. Clin Microbiol Rev. 2006;19:382–402. doi: 10.1128/CMR.19.2.382-402.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roman C, Scripcariu L, Diaconescu R, Grigoriu A. Information technologies in public health management: a database on biocides to improve quality of life. Iranian J Public Health. 2012;41:21–26. [PMC free article] [PubMed] [Google Scholar]
- Roy C, Manda SM, Mondol SK, Mukherjee S, Ghosh W, Chakraborty R. Trends of mutation accumulation across global SARS-CoV-2 genomes: implications for the evolution of the novel coronavirus. Genomics. 2020 doi: 10.1016/j.ygeno.2020.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki M, Sugimura K, Yoshida MA, Abe S. Cytogenetic effects of 60 chemicals on cultured human and Chinese hamster ceUs. Kromosomo II. 1980;20:574–584. [Google Scholar]
- Sasatsu M, Shimizu K, Noguchi N. Triclosan-resistant Staphylococcus aureus. The Lancet. 1993;341:756. doi: 10.1016/0140-6736(93)90526-M. [DOI] [PubMed] [Google Scholar]
- Thorrold CA, Letsoalo MEA, Dusé G, Marais E. Effux pump activity in fluoroquinolone and tetracycline resistant Salmonella and E. coli implicated in reduced susceptibility to household antimicrobial cleaning agents. Int J Food Microbiol. 2007;113:315–320. doi: 10.1016/j.ijfoodmicro.2006.08.008. [DOI] [PubMed] [Google Scholar]
- Tkachenko O, Shepard J, Aris VM, Joy A, Bello A, Londono I, Marku J, Soteropoulos P, Peteroy-Kelly MA. A triclosan-ciprofloxacin cross-resistant mutant strain of Staphylococcus aureus displays an alteration in the expression of several cell membrane structural and functional genes. Res Microbiol. 2007;158:651–658. doi: 10.1016/j.resmic.2007.09.003. [DOI] [PubMed] [Google Scholar]
- Torsvik VJ, Goksoyr J, Daae FL. High diversity in DNA of soil bacteria. Appl Environ Microbiol. 1990;56:782–787. doi: 10.1128/aem.56.3.782-787.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh SE, Maillard JY, Russell AD. Possible mechanisms for the relative efficacies of ortho-phthalaldehyde and glutaraldehyde against glutaraldehyde-resistant Mycobacterium chelonae. J Appl Microbiol. 2001;91:80–92. doi: 10.1046/j.1365-2672.2001.01341.x. [DOI] [PubMed] [Google Scholar]