Abstract
The aim of this study was to investigate the underlying mechanisms that transforming growth factor-β (TGF-β)-mediated epithelial-to-mesenchymal transition (EMT) in tumor cells contributes to 5-FU resistance. A series of experiments involving cell viability and caspase activity analyses, siRNA transfection, RNA isolation, and quantitative-PCR (qPCR) assay, cell migration analysis, Western blotting analysis of total protein and membrane protein were performed in this study. Mouse xenograft model was used to determine the effect of the PAR2 inhibitor in vivo. In this study, we found that protease-activated receptor 2 (PAR2) induction in 5-FU therapy is correlated with TGF-β-mediated EMT and apoptosis resistance. PAR2 and TGF-β were both activated in response to 5-FU treatment in vivo and in vitro, and whereas TGF-β inhibition sensitized CRC cells to 5-FU and suppressed cell migration, PAR2 activation eliminated the effect of TGF-β inhibition. Conversely, siRNA-mediated PAR2 depletion or PAR2 inhibition with a specific inhibitor produced a similar phenotype as TGF-β signal inhibition: 5-FU sensitization and cell migration suppression. Moreover, the results of xenograft experiments indicated that the PAR2 inhibitor can enhance cell killing by 5-FU in vivo and suppress EMT signaling. Our results reveal that the TGF-β effects require the coordinating action of PAR2, suggesting that PAR2 inhibition could be a new therapeutic strategy to combat 5-FU resistance in CRC.
Key words: Colorectal cancer (CRC), Protease-activated receptor 2 (PAR2), 5-Fluorouracil (5-FU), Transforming growth factor-β (TGF-β)
INTRODUCTION
Colorectal cancer (CRC) is a common cancer worldwide1, and in China, the 5-year relative survival rate of CRC is approximately 50%2. Currently, the available selection of licensed drugs for advanced CRC chemotherapy is limited and has been restricted to oxaliplatin, 5-fluorouracil (5-FU), irinotecan, and capecitabine for several years3. As one of the most used chemotherapeutic agents for colon cancer, 5-FU can increase the survival probability of CRC patients to a certain extent4, but drug resistance is a primary obstacle that must be overcome in developing CRC therapy strategies because up to half the patients with metastatic CRC show resistance to 5-FU-based chemotherapy4. Our understanding of the mechanisms of 5-FU resistance at a molecular level remains limited, and comprehensive elucidation of these mechanisms could facilitate the development of new therapeutic strategies specific for 5-FU-resistant CRC patients.
Accumulating evidence suggests that epithelial-to-mesenchymal transition (EMT) is related to therapy resistance and tumor metastasis5. In various cancer cells exposed to chemotherapy treatment, EMT is detected and leads to chemotherapy resistance, such as in the case of 5-FU-resistant CRC, sorafenib-resistant hepatocellular cancer, and doxorubicin-resistant breast cancer cells6–8. Therefore, the blocking of EMT-related signaling is a crucial factor affecting therapy sensitivity and the suppression of tumor cell migration and invasion. EMT is associated with the Hedgehog, PI3K, Notch, and TGF-β pathways9,10. In colorectal carcinoma, transforming growth factor-β (TGF-β) pathway hyperactivation might ultimately result in the expression of plasminogen activator inhibitor-1 (PAI-1) and α-smooth muscle actin (α-SMA) in cancer fibroblasts11 through the development of a vicious cycle of repeated TGF-β signaling and an environment that promotes cancer. Intriguingly, the expression of protease-activated receptor 2 (PAR2) was recently reported to mediate TGF-β signaling and affect cytokine-induced apoptosis in CRC cells12, but the effect of PAR2 on TGF-β signaling in response to chemotherapy drugs remains unclear.
In this study, we aimed to elucidate the role of PAR2 in the TGF-β signaling pathway activated by 5-FU therapy in CRC. By analyzing CRC patient samples and multiple CRC cell lines, we showed that PAR2 activation contributes to TGF-β-mediated EMT and apoptosis resistance in response to 5-FU therapy. Notably, our in vivo and in vitro results suggest that PAR2 depletion not only sensitizes CRC cells to 5-FU, but also suppresses 5-FU-induced EMT. Our work represents a considerable advance in the investigation of PAR2’s role in regulating EMT and apoptosis in CRC, and provides a new method for improving the 5-FU therapy performance in CRC.
MATERIALS AND METHODS
Materials
The following materials were from commercial sources: PAR2 agonist, 5-FU, and trypsin (Sigma-Aldrich, Shanghai, P.R. China); TGF-β inhibitor LY2157299 (Selleckchem.com, Hong Kong, P.R. China); PAR2 inhibitor ENMD-547 (Santa Cruz Biotechnology, Dallas, TX, USA); and antibodies against E-cadherin, vimentin, tubulin, Smad3, and phospho-Smad3 (p-Smad3; Cell Signaling Technology, Danvers, MA, USA).
Patient Samples
Tumor tissues samples were collected from 46 patients with CRC before and after receiving 5-FU treatment at the First Affiliated Hospital of Soochow University. No patients underwent radiation treatment prior to the operation. Diagnosis was verified by three independent pathologists. Our study was approved by the Institutional Review Board of the First Affiliated Hospital of Soochow University. Fully informed consent was obtained in written form from every participant.
Cell Culture
HCT116, RKO, HT-29, DLD1, and LoVo cells were obtained from American Type Culture Collection (Manassas, VA, USA) and cultured in McCoy 5A Medium (Thermo Fisher Scientific, Waltham, MA, USA) supplemented with 10% fetal bovine serum (FBS; Thermo Fisher Scientific) at 37°C in a humidified 5% CO2 incubator.
Cell Viability and Caspase Activity Analyses
Cell viability and caspase 3/7 activity were analyzed using a Celltiter Glo kit and Caspase-Glo® 3/7 Assay System (Promega, Madison, WI, USA), respectively, following the manufacturers’ guidelines. Cells were cultured in 96-well plates (0.5 × 104 cells/well) for 24 h and then treated with different combinations of drugs for another 24 h; subsequently, Celltiter Glo solution or proluminescent caspase 3/7 DEVD–aminoluciferin substrate solution was measured and pipetted into each well. Absorbance measurements were recorded as percentages in comparison with control cells.
siRNA and Plasmid Transfection
PAR2 knockdown was performed 24 h before 5-FU treatment by transfecting cells with 200 pmol of human PAR2 siRNA or control scrambled siRNA (Invitrogen, Carlsbad, CA, USA). To generate an expression plasmid encoding full-length PAR2, full-length human PAR2 cDNA was amplified by PCR using the primers 5′-caccatgcggagccccagcgcggcgt-3′ (forward) and 5′-ataggaggtcttaacagtggttgaa-3′ (reverse), and cloned into pcDNA3.1 Directional TOPO expression plasmid (Invitrogen). The transfection of siRNAs or plasmids was conducted using Lipofectamine 2000 (Invitrogen) as described by the manufacturer.
RNA Isolation and Quantitative-PCR (qPCR) Assay
Total RNA was extracted using Tri-Reagent (Sigma-Aldrich, St. Louis, MO, USA) and reverse transcripted (Invitrogen), and qPCR was conducted using a CFX96 Real-time PCR Detection System (Bio-Rad Laboratories, Hercules, CA, USA). The value measured for each gene was normalized relative to that of β-actin gene. The sequences of the primers used in qPCR have been reported previously12.
Cell Migration Analysis
Cell migration assays were performed in a 24-well Transwell system featuring 8-μm pore size membrane inserts (Corning, Corning, NY, USA). Briefly, 2 × 104 cells were transfected, starved, and trypsinized before plating in serum-free medium in the upper chamber. The lower chamber was filled with the same medium including 10% FBS. The cells in the upper chamber were wiped off using cotton swabs, and the cell that had crossed to the opposite side of the membrane, the invaded or migrated cells were fixed with methanol, stained with 5% crystal violet, and quantified under a microscope (Olympus Co., Shinjuku, Tokyo, Japan).
Western Blotting Analysis
Total cell lysates were processed in radioimmunoprecipitation assay buffer that included Boehringer’s protease inhibitor mixture and phosphatase inhibitors (e.g., NaF, PMSF, and sodium orthovanadate). After determining protein concentrations using Bio-Rad’s Bradford assay, proteins were separated using SDS-PAGE, transferred to polyvinylidene fluoride membranes, and Western blotted13.
Mouse Xenograft Model and Treatment
All animal experiments in this study adhered to globally accepted guiding principles of animal use and care in laboratories. Female nude mice (aged 5–6 weeks old; Vital River, P.R. China) were raised under sterile conditions, and 1 × 106 HCT116 cells (suspended in 100 μl of phosphate-buffered saline) were administered subcutaneously into each side of the mice. As soon as tumors were detected, the mice were intraperitoneally injected with 5-FU (25 mg/kg), ENMD-547 (2 mg/kg), or their combination once every 2 days for 10 consecutive days. Tumor dimensions were measured using calipers, and cancer development was quantified using the equation: 0.5 × length × width2. The dissected tumors were fixed with and immunostained for activated caspase-3, E-cadherin, and vimentin. All procedures and experiments involving animals in this study were carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The protocol was approved by the Committee on the Ethics of Animal Experiments of the First Affiliated Hospital of Soochow University. All surgery was performed under sodium pentobarbital anesthesia, and all efforts were made to minimize suffering.
Statistical Analysis
Student’s t-test was used for comparisons of two independent groups; differences were considered significant at a value of p < 0.05.
RESULTS
TGF-β Signaling and PAR2 Expression Were Induced After 5-FU Treatment in CRC Patients
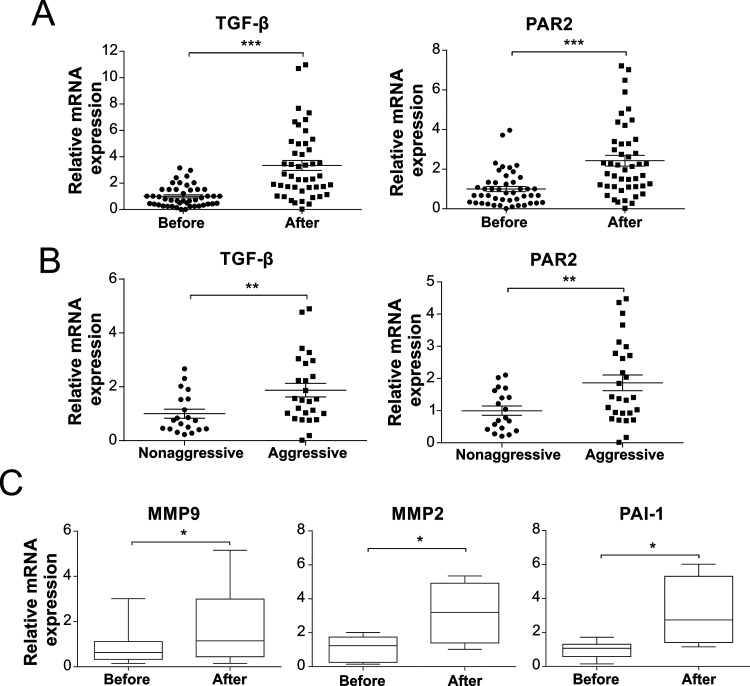
TGF-β and PAR2 levels are reported to be highly correlated with CRC development14,15; thus, we first performed qRT-PCR to examine TGF-β and PAR2 mRNA expression in 46 pairs of randomly selected tumor tissues before and after neoadjuvant chemotherapy. After 5-FU chemotherapy, TGF-β and PAR2 expression levels were significantly higher than before therapy (p < 0.001) (Fig. 1A). Because TGF-β and PAR2 expression is also related to cancer metastasis, we further measured their expression in metastatic cancers. We defined CRC tissues showing metastasis, tumor invasion into bile duct, and venous infiltration as aggressive CRC tissues, and found that TGF-β and PAR2 were markedly upregulated in aggressive CRC tissues compared with the levels in nonaggressive tissues (p < 0.01) (Fig. 1B). We also examined the expression of genes related to migration and invasion, such as genes encoding matrix metalloproteinase-2 (MMP-2), MMP-9, and PAI-1, which are downstream targets of the TGF-β signaling pathway; the expression of these genes accordingly showed considerable elevation after 5-FU treatment (Fig. 1C). Collectively, these results indicated that 5-FU treatment potentially increases TGF-β signaling and PAR2 expression, which might contribute to CRC metastasis.
Figure 1.
Elevated transforming growth factor-β (TGF-β) and protease-activated receptor 2 (PAR2) expression among colorectal cancer CRC patients after 5-fluorouracil (5-FU) therapy. (A) Analysis of TGF-β and PAR2 in RNA extracted from CRC tumor biopsies before chemotherapy (before) and after chemotherapy (after). (B) Comparison of differences in TGF-β and PAR2 expression between aggressive and nonaggressive tumor tissues. (C) Analysis of MMP2, MMP9, and PAI-1 in RNA extracted from CRC tumor biopsies before chemotherapy (before) and after chemotherapy (after). *p < 0.05, **p < 0.01, ***p < 0.001.
5-FU Treatment Reinforced TGF-β and PAR2 Expression in CRC Cells
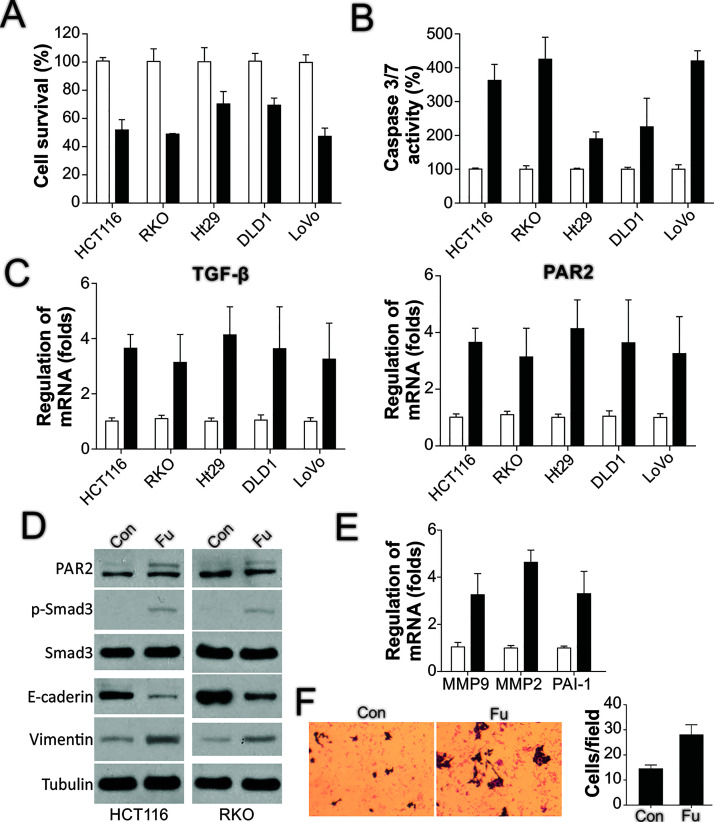
To further confirm in vitro the observed regulatory effect of 5-FU on TGF-β and PAR2, we treated several CRC cell lines with 5-FU and investigated the changes in TGF-β and PAR2. In accord with previous findings, 5-FU treatment led to a loss of cell viability in the cell lines HCT116, RKO, HT-29, DLD1, and LoVo (Fig. 2A). Moreover, caspase 3/7 activity was increased in these cell lines after 5-FU treatment (Fig. 2B), which suggested that 5-FU triggers apoptosis in CRC cells. Furthermore, these findings indicated that PAR2 mRNA levels were increased in these cell lines after 5-FU treatment (Fig. 2C), which suggested that 5-FU can also activate TGF-β signaling and PAR2 activity. Therefore, we examined the 5-FU effect on Smad3, a downstream effector of PAR2 and TGF-β: 5-FU treatment affected PAR2 at the protein level as well (Fig. 2D), and although Smad3 expression was not altered markedly, its phosphorylation was induced after 5-FU treatment (Fig. 2D), which indicated the activation of the TGF-β pathway. Last, because PAR2 and TGF-β activation is correlated with EMT, we examined the expression of two EMT markers, E-cadherin and vimentin, as well as the migration status of the surviving cells after 5-FU treatment. As per our expectation, 5-FU treatment reduced E-cadherin expression but increased vimentin expression (Fig. 2D), and the treatment also induced the expression of the TGF-β downstream targets MMP2, MMP9, and PAI-1 (Fig. 2E). Intriguingly, the surviving HCT116 cells after 5-FU treatment showed enhanced migration activity compared with untreated cells (Fig. 2F). Thus, these results together indicated that 5-FU triggered the activation of TGF-β and PAR2 and enhanced the migration of surviving cells.
Figure 2.
5-FU treatment induces TGF-β and PAR2 expression and cell migration. (A) The indicated CRC cells were treated with 50 μg/ml of 5-FU for 24 h, and then cell viability was detected using the MTS assay. (B) CRC cells were treated as in (A), and then caspase 3/7 activity was analyzed. (C) CRC cells were processed as in (A), and TGF-β (left) and PAR2 (right) expression was examined using RT-PCR. (D) HCT116 and RKO cells were processed as in (A), after which the expression of the indicated proteins was analyzed through Western blotting. (E) HCT116 cells were processed as in (A), and then the expression of target genes was analyzed using RT-PCR. (F) HCT116 cells were processed as in (A), and the migration of the surviving cells after treatment was analyzed.
PAR2 Mediated TGF-β Effects in 5-FU-Induced Apoptosis and EMT
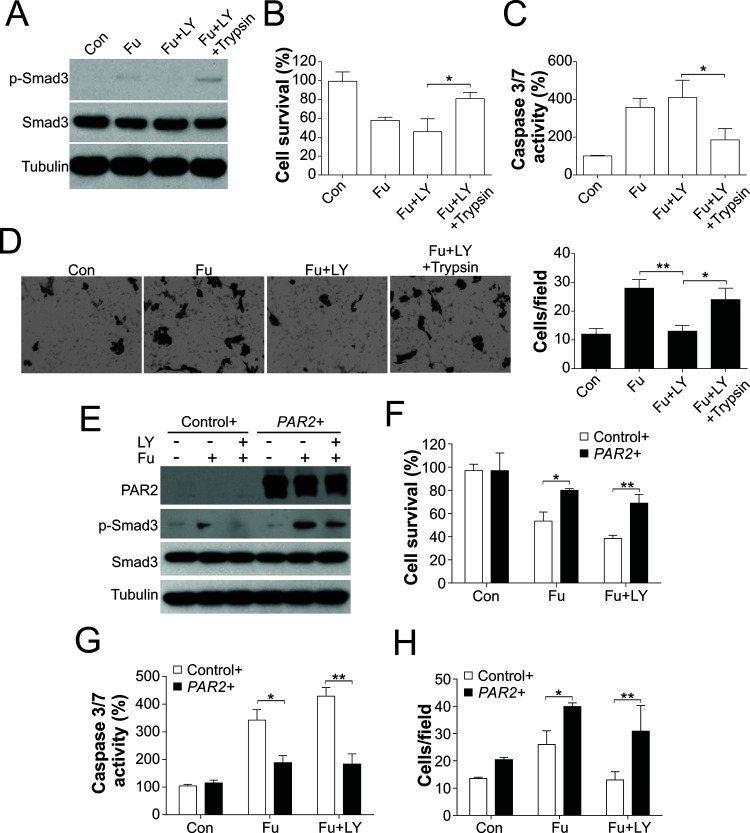
To enhance our understanding of the role of PAR2 and TGF-β signaling in the effects of 5-FU, we treated HCT116 cells with 5-FU together with the TGF-β inhibitor LY2157299. Exposure of cells to LY2157299 blocked the phosphorylation of Smad3 (Fig. 3A), which confirmed the effectiveness of the TGF-β inhibitor. In accord with previous studies, TGF-β inhibition sensitized HCT116 cells to 5-FU treatment (Fig. 3B), increased caspase 3/7 activity (Fig. 3C), and suppressed the migration of the surviving cells after 5-FU treatment (Fig. 3D); these results suggested that TGF-β-modulated EMT limits the tumor cell-killing effect of 5-FU and might contribute to tumor metastasis. However, the effect of LY2157299 was abrogated by a PAR2 agonist. After PAR2 activation with the agonist, the p-Smad3 signal recovered (Fig. 3A), cell viability was increased (Fig. 3B), caspase 3/7 activity was suppressed (Fig. 3C), and cell migration was promoted (Fig. 3D). To further confirm the effect of PAR2, we generated a PAR2 expression plasmid and transfected into HCT116 cells. Consistently, enhanced expression of PAR2 abolished the effect of LY2157299 on p-Smad3 (Fig. 3E), cell viability (Fig. 3F), caspase 3/7 activity (Fig. 3G), and cell migration (Fig. 3H). Furthermore, enhanced expression of PAR2 also enhanced the p-Smad3 and cell migration induced by 5-FU (Fig. 3E and H), but suppressed 5-FU-induced apoptosis (Fig. 3F and G). Collectively, these findings indicated that PAR2-TGF-β signaling contribute to cell migration induced by 5-FU in CRC cells.
Figure 3.
PAR2 coordinates with TGF-β to mediate 5-FU-triggered apoptosis and cell migration. (A) HCT116 cells were treated with 50 μg/ml of 5-FU after pretreatment for 1 day with 100 nmol/L LY2157299 and 100 U/ml trypsin, and then the expression of target proteins was analyzed through immunoblotting. (B) HCT116 cells were processed as in (A), and then cell survival was analyzed using the MTS assay. (C) HCT116 cells were processed as in (A), after which caspase 3/7 activity was analyzed. (D) HCT116 cells were processed as in (A), and the migration of the surviving cells after treatment was analyzed. (E) HCT116 cells transfected with pcDNA3.1 (control+) or pcDNA3.1-PAR2 (PAR2+) were treated with 50 μg/ml of 5-FU after pretreatment for 1 day with 100 nmol/L LY2157299, and then the expression of target proteins was analyzed through immunoblotting. (F) HCT116 cells were processed as in (E), and then cell survival was analyzed using the MTS assay. (G) HCT116 cells were processed as in (E), after which caspase 3/7 activity was analyzed. (H) HCT116 cells were processed as in (E), and the migration of the surviving cells after treatment was analyzed. *p < 0.05, **p < 0.01.
PAR2 Deletion or Inhibition Suppressed 5-FU-Induced EMT and Enhanced the Killing Effect of 5-FU
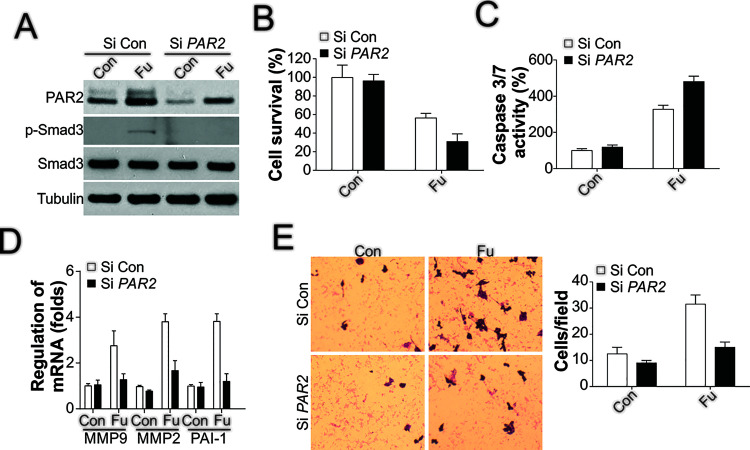
To analyze whether PAR2 expression is critical for TGF-β-triggered migration and invasion of tumor cells, we depleted PAR2 in the HCT116 cell line through transient transfection of siRNA. PAR2 knockdown suppressed the phosphorylation of Smad3 induced by 5-FU treatment (Fig. 4A), which indicated that TGF-β signaling was inhibited, and in the absence of PAR2, HCT116 cells were sensitized to 5-FU treatment (Fig. 4B), and caspase 3/7 activity was increased (Fig. 4C); these effects are all similar to those produced by the TGF-β inhibitor. Next, we tested whether PAR2 depletion affects cell migration. Analysis of the expression of several EMT-related genes revealed that the induction of these genes by 5-FU was compromised by PAR2 siRNA (Fig. 4D). Moreover, PAR2 depletion eliminated the increased migration of the surviving cells after 5-FU treatment (Fig. 4E).
Figure 4.
PAR2 depletion sensitizes HCT116 cells to 5-FU treatment and limits cell migration. (A) HCT116 cells were transfected with PAR2 siRNA overnight and then treated with 50 μg/ml of 5-FU for 1 day; subsequently, immunoblotting was performed to examine the expression of the mentioned proteins. (B) HCT116 cells were processed as in (A), and then cell viability was analyzed using the MTS assay. (C) HCT116 cells were treated as in (A), and then caspase 3/7 activity was analyzed. (D) HCT116 cells were processed as in (A), after which RT-PCR was performed to analyze the expression of the genes of interest. (E) HCT116 cells were treated as in (A), and then the migration of the surviving cells after treatment was analyzed.
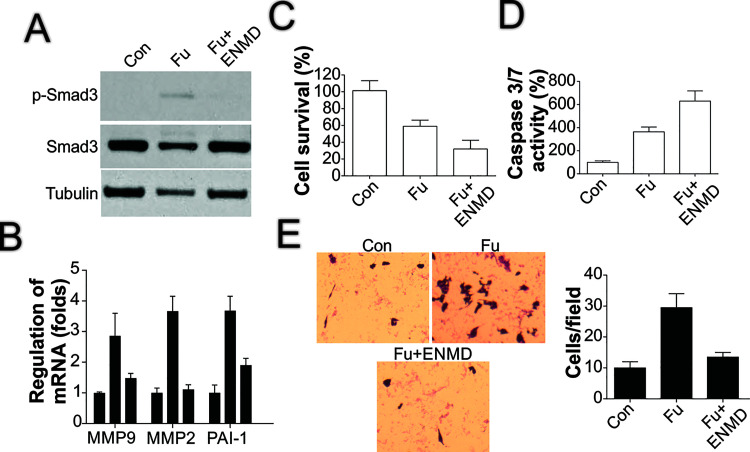
To further confirm the role of PAR2 in the chemotherapeutic effect of 5-FU, we used a PAR2 inhibitor, ENMD-547, together with 5-FU treatment. PAR2 inhibition, like PAR2 depletion, suppressed Smad3 phosphorylation (Fig. 5A) and the induction of TGF-β downstream targets such as MMP2, MMP9, and PAI-1 (Fig. 5B). The combined use of the PAR2 inhibitor with 5-FU also enhanced the killing effect of 5-FU (Fig. 5C) and increased caspase 3/7 activity (Fig. 5D). Furthermore, addition of the PAR2 inhibitor limited the increase in cell migration caused by 5-FU treatment (Fig. 5E). These data collectively suggested that PAR2 mediates the TGF-β-induced chemoresistance and EMT observed in 5-FU treatment.
Figure 5.
PAR2 inhibition produces similar effects as PAR2 depletion. (A) HCT116 cells were treated with 50 μg/ml of 5-FU together with/without 10 μmol/L ENMD-547 pretreatment for 1 day, after which the expression of the indicated proteins was examined through immunoblotting. (B) HCT116 cells were processed as in (A), and the expression of the indicated genes was analyzed using RT-PCR. (C) HCT116 cells were treated as in (A), and then cell survival was examined using the MTS assay. (D) HCT116 cells were processed as in (A), after which caspase 3/7 activity was analyzed. (E) HCT116 cells were processed as in (A), and then the migration of the surviving cells after treatment was analyzed.
PAR2 Inhibition Enhanced the Killing Effect of 5-FU In Vivo
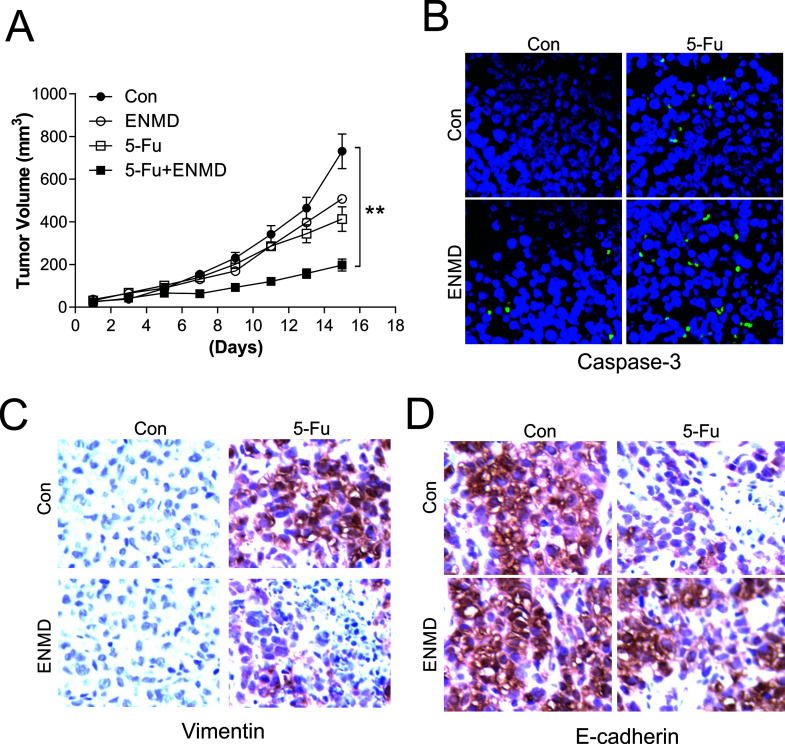
Last, we used a xenograft model to examine the effect of the PAR2 inhibitor in vivo. In nude mice harboring HCT116 tumors and treated with 5-FU and/or ENMD-547, tumor volume was significantly diminished when 5-FU was coapplied with ENMD-547 compared with the volume after treatment with 5-FU alone, although ENMD-547 single treatment did not exert a tumor-suppressive effect (Fig. 6A). Furthermore, we analyzed apoptotic signaling and cell migration by staining for caspase-3, vimentin, and E-cadherin. In agreement with the in vitro data, PAR2 inhibition by ENMD-547 significantly increased the caspase 3 activity induced by 5-FU (Fig. 6B), which indicated that the PAR2 inhibitor can enhance the cell-killing effect of 5-FU. In terms of tumor migration, 5-FU administration caused vimentin upregulation and E-cadherin downregulation, but this effect was reversed when 5-FU was applied in combination with the PAR2 inhibitor (Fig. 6C and D). These findings further confirm the function of PAR2 inhibition in vivo and suggest a new therapy strategy for CRC.
Figure 6.
PAR2 mediates the 5-FU tumor-killing effect and inhibition of cell migration in vivo. (A) Nude mice were subcutaneously injected with 1 × 106 wild-type HCT116 cells and, after 7 days, were intraperitoneally injected, once every 2 days for 10 days, with 50 mg/kg of 5-FU, 1 mg/kg of ENMD-547, or their combination. Tumor volume at specific times after treatment was evaluated; n = 5 per group. (B–D) Mice with HCT116 xenograft tumors were treated as in (A) for 4 consecutive days, and then tissue sections were stained for active caspase 3 (B), vimentin (C), and E-cadherin (D). **p < 0.01.
DISCUSSION
Development of 5-FU resistance has long remained the main obstacle in CRC treatment and a key reason for chemotherapy failure9. Several underlying mechanisms that might account for 5-FU resistance in cancer cells have been recognized, and the most recent work in this area focused our attention on EMT16. The connection between EMT and drug resistance has been investigated in multiple cancers, but the mechanisms involved remain incompletely elucidated. In this study, we found that PAR2 functions as a major coordinator for the TGF-β signaling pathway in regulating 5-FU-induced apoptosis and EMT. Notably, when PAR2 was blocked using its specific inhibitor, ENMD-547, CRC cells were sensitized to 5-FU in vitro and in vivo, and the EMT effects produced by 5-FU were also suppressed. Thus, our findings suggest a new therapy strategy—involving PAR2 targeting—for CRC.
EMT and apoptosis are most basic physiological activities that are independent but are also interconnected in maintaining the homeostasis of our bodies17. Recently, EMT has attracted considerable attention in cancer-related research, and increasing numbers of studies are indicating that EMT plays a critical role in the metastasis and invasion of certain cancer cells. Abnormalities in the regulation of EMT and apoptosis are strongly correlated with multiple pathological activities, such as kidney fibrosis, embryo teratism, and cancer progression18,19. TGF-β is closely associated with the regulation of apoptosis and EMT and plays a key role in pathological activities: TGF-β is regarded as a primary influencing factor in tumor progression, metastasis, and vasculogenesis19,20. A recent study revealed the link between TGF-β signaling and drug resistance14, but the detailed mechanism of TGF-β pathway activation, particularly in CRC, remains poorly understood. Therefore, efforts to elucidate the mechanisms underlying EMT and apoptosis, as well as the mechanisms of TGF-β regulation, could be vital in the identification of novel therapeutic strategies for diverse tumors. In accord with a previous study14, our results showed that the TGF-β pathway was activated in CRC in response to 5-FU treatment and contributed to apoptosis resistance and EMT progression. Interestingly, we also found that PAR2, which functions as a coordinator of TGF-β signaling, modulates the TGF-β signaling pathway and thereby controls the apoptosis and EMT induced by 5-FU in CRC in vivo and in vitro.
PAR2 is a G protein-coupled receptor that has recently been reported to play a crucial role in the development of tumors and various cancers, including CRC15. Zeeh et al. found that PAR2 expression is critical for TGF-β/ALK5 signaling and contributes to metastatic dissemination in pancreatic cancer12. Moreover, TGF-β and PAR2 expression levels might both be elevated and could trigger fibrosis21,22, which facilitates desmoplasia in pancreatic carcinoma23. TGF-β and PAR2 play certain common roles in healthy and pathological stoichiology, including stimulation of desmoplastic progression, invasion, and metastasis23–25. PAR2 and TGF-β were found to be colocalized in pancreatic ductal adenocarcinoma (PDAC), and thus signaling interaction between PAR2 and TGF-β in tumor cells enhanced TGF-β’s carcinogenic effects and PDAC development12. Here we also determined that TGF-β and PAR2 were upregulated in 5-FU-treated CRC patients and cell lines, and our results further suggested that PAR2 mediated TGF-β signaling in 5-FU chemotherapy because the effects of TGF-β inhibition were reversed by PAR2 activation. These findings provide an additional mechanism for inhibiting the activation of TGF-β signaling in CRC chemotherapy.
An increase in the expression of trypsin, the primary internal PAR2-activating factor, has been observed in the context of several tumors, including CRC26,27. In CRC, elevation of PAR2 and trypsin stimulated cell reproduction13, which indicated that trypsin is associated with secretory loops under the condition prevalent in colonic neoplasms, and this leads to their progression. In this context, we also found that PAR2 activation by trypsin promoted EMT but suppressed 5-FU-induced apoptosis, which further confirmed the role of PAR2 in CRC progression. Conversely, certain PAR2 antagonists were reported to suppress CRC cell proliferation by inhibiting the EGFR signaling pathway28. Reduction of PAR2 sensitizes colonic epithelial cells to cytokine-induced apoptosis by suppressing Mcl-1 in vitro29. However, whether PAR2 inhibition produces a similar effect in vivo remains unclear. In this study, we also determined that PAR2 inhibition or depletion sensitized CRC cells to 5-FU treatment. Furthermore, we used a new PAR2 inhibitor, ENMD-547, and observed that it exerted similar effects as PAR2 depletion: sensitization of CRC cells to 5-FU in vitro and in vivo and suppression of EMT signals.
In conclusion, our study has shown for the first time that 5-FU treatment could lead to the activation of TGF-β signaling and PAR2 in CRC both in vivo and in vitro. Specific inhibition or depletion of PAR2 not only enhanced the sensitivity of CRC cells to 5-FU treatment but also reduced the EMT signaling triggered by 5-FU, which could suppress tumor metastasis after chemotherapy. Moreover, we found that a PAR2 inhibitor, ENMD-547, can potentially be used in combination with 5-FU in CRC treatment. However, further investigation is required to address the precise mechanisms and clinical effects of this combination treatment.
ACKNOWLEDGMENTS
This work was supported by Suzhou Science and Technology Project (SYS201618).
Footnotes
The authors declare no conflicts of interest.
REFERENCES
- 1. Rim SH, Seeff L, Ahmed F, King JB, Coughlin SS. Colorectal cancer incidence in the United States, 1999–2004: An updated analysis of data from the National Program of Cancer Registries and the Surveillance, Epidemiology, and End Results Program. Cancer 2009;115(9):1967–76. [DOI] [PubMed] [Google Scholar]
- 2. Li L, Ma BB. Colorectal cancer in Chinese patients: Current and emerging treatment options. Onco Targets Ther. 2014;7:1817–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bracht K, Nicholls AM, Liu Y, Bodmer WF. 5-Fluorouracil response in a large panel of colorectal cancer cell lines is associated with mismatch repair deficiency. Br J Cancer 2010;103(3):340–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Douillard JY, Cunningham D, Roth AD, Navarro M, James RD, Karasek P, Jandik P, Iveson T, Carmichael J, Alakl M, Gruia G, Awad L, Rougier P. Irinotecan combined with fluorouracil compared with fluorouracil alone as first-line treatment for metastatic colorectal cancer: A multicentre randomised trial. Lancet 2000;355(9209):1041–7. [DOI] [PubMed] [Google Scholar]
- 5. Rosano L, Cianfrocca R, Spinella F, Di Castro V, Nicotra MR, Lucidi A, Ferrandina G, Natali PG, Bagnato A. Acquisition of chemoresistance and EMT phenotype is linked with activation of the endothelin A receptor pathway in ovarian carcinoma cells. Clin Cancer Res. 2011;17(8):2350–60. [DOI] [PubMed] [Google Scholar]
- 6. Hiscox S, Jiang WG, Obermeier K, Taylor K, Morgan L, Burmi R, Barrow D, Nicholson RI. Tamoxifen resistance in MCF7 cells promotes EMT-like behaviour and involves modulation of beta-catenin phosphorylation. Int J Cancer 2006;118(2):290–301. [DOI] [PubMed] [Google Scholar]
- 7. Creighton CJ, Li X, Landis M, Dixon JM, Neumeister VM, Sjolund A, Rimm DL, Wong H, Rodriguez A, Herschkowitz JI, Fan C, Zhang X, He X, Pavlick A, Gutierrez MC, Renshaw L, Larionov AA, Faratian D, Hilsenbeck SG, Perou CM, Lewis MT, Rosen JM, Chang JC. Residual breast cancers after conventional therapy display mesenchymal as well as tumor-initiating features. Proc Natl Acad Sci USA 2009;106(33):13820–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zhang N, Yin Y, Xu SJ, Chen WS. 5-Fluorouracil: Mechanisms of resistance and reversal strategies. Molecules 2008;13(8):1551–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Herrmann R. 5-Fluorouracil in colorectal cancer, a never ending story. Ann Oncol. 1996;7(6):551–2. [DOI] [PubMed] [Google Scholar]
- 10. Byers LA, Diao L, Wang J, Saintigny P, Girard L, Peyton M, Shen L, Fan Y, Giri U, Tumula PK, Nilsson MB, Gudikote J, Tran H, Cardnell RJ, Bearss DJ, Warner SL, Foulks JM, Kanner SB, Gandhi V, Krett N, Rosen ST, Kim ES, Herbst RS, Blumenschein GR, Lee JJ, Lippman SM, Ang KK, Mills GB, Hong WK, Weinstein JN, Wistuba II, Coombes KR, Minna JD, Heymach JV. An epithelial-mesenchymal transition gene signature predicts resistance to EGFR and PI3K inhibitors and identifies Axl as a therapeutic target for overcoming EGFR inhibitor resistance. Clin Cancer Res. 2013;19(1):279–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hawinkels LJ, Paauwe M, Verspaget HW, Wiercinska E, van der Zon JM, van der Ploeg K, Koelink PJ, Lindeman JH, Mesker W, ten Dijke P, Sier CF. Interaction with colon cancer cells hyperactivates TGF-beta signaling in cancer-associated fibroblasts. Oncogene 2014;33(1):97–107. [DOI] [PubMed] [Google Scholar]
- 12. Zeeh F, Witte D, Gadeken T, Rauch BH, Grage-Griebenow E, Leinung N, Fromm SJ, Stolting S, Mihara K, Kaufmann R, Settmacher U, Lehnert H, Hollenberg MD, Ungefroren H. Proteinase-activated receptor 2 promotes TGF-beta-dependent cell motility in pancreatic cancer cells by sustaining expression of the TGF-beta type I receptor ALK5. Oncotarget 2016;7(27):41095–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Darmoul D, Marie JC, Devaud H, Gratio V, Laburthe M. Initiation of human colon cancer cell proliferation by trypsin acting at protease-activated receptor-2. Br J Cancer 2001;85(5):772–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Romano G, Santi L, Bianco MR, Giuffre MR, Pettinato M, Bugarin C, Garanzini C, Savarese L, Leoni S, Cerrito MG, Leone BE, Gaipa G, Grassilli E, Papa M, Lavitrano M, Giovannoni R. The TGF-beta pathway is activated by 5-fluorouracil treatment in drug resistant colorectal carcinoma cells. Oncotarget 2016;7(16):22077–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dery O, Corvera CU, Steinhoff M, Bunnett NW. Proteinase-activated receptors: Novel mechanisms of signaling by serine proteases. Am J Physiol. 1998;274(6 Pt 1):C1429–52. [DOI] [PubMed] [Google Scholar]
- 16. Kim AY, Kwak JH, Je NK, Lee YH, Jung YS. Epithelial-mesenchymal transition is associated with acquired resistance to 5-fluorocuracil in HT-29 colon cancer cells. Toxicol Res. 2015;31(2):151–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Song J. EMT or apoptosis: A decision for TGF-beta. Cell Res. 2007;17(4):289–90. [DOI] [PubMed] [Google Scholar]
- 18. Grunert S, Jechlinger M, Beug H. Diverse cellular and molecular mechanisms contribute to epithelial plasticity and metastasis. Nat Rev Mol Cell Biol. 2003;4(8):657–65. [DOI] [PubMed] [Google Scholar]
- 19. Kalluri R, Neilson EG. Epithelial-mesenchymal transition and its implications for fibrosis. J Clin Invest. 2003;112(12):1776–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Thiery JP. Epithelial-mesenchymal transitions in development and pathologies. Curr Opin Cell Biol. 2003;15(6):740–6. [DOI] [PubMed] [Google Scholar]
- 21. Saito A, Osuga Y, Yoshino O, Takamura M, Hirata T, Hirota Y, Koga K, Harada M, Takemura Y, Yano T, Taketani Y. TGF-beta1 induces proteinase-activated receptor 2 (PAR2) expression in endometriotic stromal cells and stimulates PAR2 activation-induced secretion of IL-6. Hum Reprod. 2011;26(7):1892–8. [DOI] [PubMed] [Google Scholar]
- 22. Knight V, Tchongue J, Lourensz D, Tipping P, Sievert W. Protease-activated receptor 2 promotes experimental liver fibrosis in mice and activates human hepatic stellate cells. Hepatology 2012;55(3):879–87. [DOI] [PubMed] [Google Scholar]
- 23. Ikeda O, Egami H, Ishiko T, Ishikawa S, Kamohara H, Hidaka H, Mita S, Ogawa M. Expression of proteinase-activated receptor-2 in human pancreatic cancer: A possible relation to cancer invasion and induction of fibrosis. Int J Oncol. 2003;22(2):295–300. [PubMed] [Google Scholar]
- 24. Ikushima H, Miyazono K. TGFbeta signalling: A complex web in cancer progression. Nat Rev Cancer 2010;10(6):415–24. [DOI] [PubMed] [Google Scholar]
- 25. Padua D, Massague J. Roles of TGFbeta in metastasis. Cell Res. 2009;19(1):89–102. [DOI] [PubMed] [Google Scholar]
- 26. Williams SJ, Gotley DC, Antalis TM. Human trypsinogen in colorectal cancer. Int J Cancer 2001;93(1):67–73. [DOI] [PubMed] [Google Scholar]
- 27. Miyata S, Koshikawa N, Higashi S, Miyagi Y, Nagashima Y, Yanoma S, Kato Y, Yasumitsu H, Miyazaki K. Expression of trypsin in human cancer cell lines and cancer tissues and its tight binding to soluble form of Alzheimer amyloid precursor protein in culture. J Biochem. 1999;125(6):1067–76. [DOI] [PubMed] [Google Scholar]
- 28. Darmoul D, Gratio V, Devaud H, Laburthe M. Protease-activated receptor 2 in colon cancer: Trypsin-induced MAPK phosphorylation and cell proliferation are mediated by epidermal growth factor receptor transactivation. J Biol Chem. 2004;279(20):20927–34. [DOI] [PubMed] [Google Scholar]
- 29. Iablokov V, Hirota CL, Peplowski MA, Ramachandran R, Mihara K, Hollenberg MD, MacNaughton WK. Proteinase-activated receptor 2 (PAR2) decreases apoptosis in colonic epithelial cells. J Biol Chem. 2014;289(49):34366–77. [DOI] [PMC free article] [PubMed] [Google Scholar]