Abstract
KIFC1 (kinesin family member C1) plays a critical role in clustering of extra centrosomes in various cancer cells and thus could be considered as a promising therapeutic target. However, whether KIFC1 is involved in the procession of renal cell carcinoma (RCC) still remains unclear. In this study, we found that KIFC1 was upregulated in RCC tissues and is responsible for RCC tumorigenesis (p < 0.001). The high expression of KIFC1 correlates with aggressive clinicopathologic parameters. Kaplan–Meier analysis suggested that KIFC1 was associated with poor survival prognosis in RCC. Silencing KIFC1 dramatically resulted in inhibition of proliferation, delayed the cell cycle at G2/M phase, and suppressed cell invasion and migration in vitro. The antiproliferative effect of KIFC1 silencing was also observed in xenografted tumors in vivo. miR-338-3p could directly bind to the 3′-untranslated region (3′-UTR) of KIFC1, and ectopic miR-338-3p expression mimicked the inhibitory functions of KIFC1 silencing on RCC cells through inactivation of the PI3K/AKT signaling pathway. Therefore, these results revealed that KIFC1 may be a novel biomarker and an effective therapeutic target for the treatment of RCC.
Key words: KIFC1, miR-338-3p, Renal cell carcinoma (RCC), Proliferation, Poor prognosis
INTRODUCTION
Renal cell carcinoma (RCC) is the most lethal kidney malignancy, accounting for more than 100,000 deaths each year worldwide1. In addition to severe stimulating factors like diabetes, smoking, hypertension, and obesity, approximately 2%–5% of RCCs resulted from hereditary renal cancer syndromes2. The histologic subtypes of RCC are divided into papillary, collecting duct, clear cell, and chromophobe, of which clear cell RCC (ccRCC) is the most common subtype (approximately 70%–75%) found in patients3. Furthermore, about 20%–35% of RCC patients are diagnosed with metastatic disease; 20%–40% of RCC patients, who have received an early nephrectomy, are still found to have suffered from the recurrence of metastatic disease, and the lung organ is the favorite metastatic site4,5. Metastatic RCC is related to chemotherapy and radiotherapy resistance and is usually accompanied with poor prognosis according to previous studies6. Therefore, there is an urgency to explore novel therapeutic strategies for targeting specific molecules in the treatment of RCC.
KIFC1 (HSET; kinesin family member C1) as a member of the kinesin-14 family is a C-terminal kinesin motor protein7. KIFC1 is considered as a nonessential factor in normal somatic cells; however, recent studies have demonstrated that high KIFC1 expression plays a critical role in clustering of extra centrosomes in malignant cancer cells like those from the lung, breast, and ovary8–10. During mitosis, most healthy human cells harbor one pair of centrosomes and are located at the poles of the bipolar spindle11. In cancer development, supernumerary centrosomes appear simultaneously, resulting in centrosome amplification, which is a distinct hallmark of cancer cells12. Centrosome amplification directly contributes to the genetic instability of most cancer cells and is associated with advanced tumor progression13. Researchers have demonstrated that abundant KIFC1 expression is essential for cancer cell survival and metastasis by mediating excess centrosomes to produce viable progeny cells and avoiding cell death induced by aneuploidy14. However, there are no reports regarding the role of KIFC1 in the occurrence and development of RCC.
In the present study, to address the hypothesis that high KIFC1 expression is associated with RCC progression, we performed a retrospective analysis and demonstrated that increased KIFC1 was a vital driver of RCC and a prognostic marker to predict poor prognosis in human RCC. In addition, further biological function studies indicated that the inhibition of KIFC1 could dramatically decrease cell proliferation and colony formation, delay the cell cycle, inhibit cell invasion and migration in vitro, and suppress tumorigenesis in vivo. In addition, miR-338-3p could directly target KIFC1 and is involved in KIFC1-mediated cell proliferation and migration in RCC cells through the PI3K/AKT signaling pathway. Our study may provide valuable information to gain better understanding of molecular mechanisms of KIFC1 on RCC development.
MATERIALS AND METHODS
Clinical Specimen Collection
A total of 58 clinical RCC tissue samples and paired adjacent normal tissues were obtained from patients who underwent nephrectomy surgery in the Department of Urology at the Second Affiliated Hospital of Xi’an Jiaotong University (Xi’an, P.R. China) from June 2005 to August 2010. None of the selected patients had received any preoperative treatment such as radiotherapy or chemotherapy. The fresh tissues were either embedded with paraffin or stored at −80°C for further analysis. The clinicopathologic parameters such as gender, age, Fuhrman grade, TNM stage, and tumor size were collected from each patient. The types of isolated tumors were all confirmed as ccRCC according to the detection of pathologic findings. The tumor staging was evaluated by the criteria of the 2010 AJCC TNM classification system, and tumor grading was based on the Fuhrman’s nuclear grading system15. Informed consent was obtained from all patients, and this study was approved by the Human Ethics Committee of Xi’an Jiaotong University. Follow-up data regarding disease-free survival (DFS) and overall survival (OS) have been obtained from the patient records. Date of last follow-up in this cohort was September 2015, and median follow-up for survivors was 47 months (range: 1–60).
Immunohistochemical Staining
Immunohistochemical staining for KIFC1 protein was performed as follows. The embedded tissue samples were cut into 2-μm sections, and then attached to glass slides and dried at 37°C. Subsequently, all sections were deparaffinized with xylene and a graded concentration of ethanol solutions for 15 min; 0.3% H2O was added to the sections for 10 min before being washed away with diluted water. The sections were antigen retrieved with 10 mM citrate buffer (PH 6.0) and irradiated in a microwave for 5 min. Serum dilution was used to block the nonspecific binding of possible endogenous proteins at 37°C for 30 min. The diluted primary antibody solution anti-KIFC1 (1:1,000; Abcam, Cambridge, MA, USA) was added to the sections and incubated at 4°C overnight. The slides were then washed three times with PBS prior to incubation with biotinylated secondary antibody (1:5,000; Abcam). The immunoreactions were detected by staining with DAB (TianGen, Beijing, P.R. China). For all cases, the expression scores were blindly evaluated by two independent pathologists and then divided according to the percentage of KIFC1+ cells in the tissue samples in 10 randomly selected fields: 0∼5%, negative (0); 6∼10%, weak (1+); 11∼60%, moderate (2+); >60%, strong expression (3+). Tissue samples were then divided into three groups according to the KIFC1 expression levels: negative expression (0), low expression (1+), and high expression (2+ or 3+).
Cell Lines and Cell Culture
Human RCC cell lines 786-O, 769-P, and OS-RC-2, and normal proximal tubule epithelial cell line HK-2 were obtained from the Second Affiliated Hospital of Xi’an Jiaotong University. Cells were cultured at 37°C with 5% CO2 in DMEM (Sigma-Aldrich, St. Louis, MO, USA) containing 10% FBS (Gibco, Grand Island, NY, USA) and 1% penicillin/streptomycin (Sigma-Aldrich).
Cell Transfection
The small interference RNA (siRNA) sequences were designed for KIFC1: sense 5′-UCG AAA UGA GAA AUC UCG GAG-3′, antisense 5′-CCG AGA UUU CUC AUU UCG AAU-3′. The negative control siRNA (siRNA-NC) were random sequences that have no homology with any known mammalian gene. KIFC1 siRNA (si-KIFC1) and negative control (siRNA-NC) were synthesized by GenePharma Company (Shanghai, P.R. China). To overexpress miR-338-3p, miR-338-3p mimic and mimic negative control (miR-NC) were purchased from GenePharma. 786-O, 769-P, and OS-RC-2 cells were seeded at 1 × 105 cells per well in six-well plates and incubated overnight at 37°C with 5% CO2. Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA) was used to transiently transfect cells with si-KIFC1, siRNA-NC, miR mimic, or miR-NC (GenePharma). The efficiency of transfection was evaluated by real-time quantitative PCRRT-qPCR and Western blot analysis after 48 h of transfection.
Real-Time Quantitative PCR
Total RNA from the tissue samples and RCC cell lines were extracted using TRIzol reagent (Invitrogen), and then reverse transcribed to cDNA with High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA, USA) according to the manufacturer’s instructions. RT-qPCR was performed using the SYBR Green Super Mix (Bio-Rad Laboratories, Hercules, CA, USA) on the AB 7500 Fast Real-Time PCR System (Applied Biosystems, Beijing, P.R. China) with the following conditions: 2 min at 50°C, 10 min at 95°C, 38 cycles of 15 s at 95°C, and 60 s at 60°C. β-Actin was used as the reference control. The KIFC1 and β-actin primer sequences were designed as follows: KIFC1, 5′- AGC CTC CTT CAG ATT GGT GC-3′ (sense) and 5′-GCT GCC CTC AGA AAT ACC GA-3′ (antisense); β-actin, 5′-GAG GCG TGA TGG TGG GCA-3′ (sense) and 5′-CAA ACA TCA TCT GGT CAT CTT CTC-3′ (antisense). For miRNA expression assay, quantification of miR-338-3p expression was performed using TaqMan assays (Applied Biosystems, Foster City, CA, USA) according to the manufacturer’s instructions. U6 snRNA was used as the reference control. After the amplification, the Ct values were normalized by the subtraction of the Ct value of β-actin or U6, and the relative mRNA expression and miRNA were calculated according to the 2−ΔΔCt method.
Western Blot
Whole-cell lines or tissue proteins were extracted with RIPA lysis buffer (Solarbio, Beijing, P.R. China) containing 0.2 mM phenylmethylsulfonyl fluoride (PMSF), according to the standard methods. Protein concentrations were determined by the Bradford assay (Bio-Rad Laboratories). Cell lysate samples (40 μg) were separated by 15% SDS-PAGE and transferred to a polyvinylidene difluoride filter (Immobilon; Millipore, Bedford, MA, USA). After blocking with 5% milk, the filter was incubated overnight with appropriate primary antibodies (Santa Cruz Biotechnology, Santa Cruz, CA, USA) at 4°C overnight, and then incubated with HRP-conjugated secondary antibodies (Amersham, Piscataway, NJ, USA) at 4°C for 1 h. The bands were detected by enhanced chemiluminescence (Amersham) and then performed using the Quantity One software (Bio-Rad Laboratories).
Cell Proliferation Assay
Cells were seeded at a density of 5 × 103 cells per well in 96-well plates for 5 days after transfection. MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) solution (Life Sciences, Little Chalfont, UK) was added into each well to a final concentration of 5 mg/ml and incubated at 37°C for 4 h. The MTT formazan precipitate was collected, dissolved in dimethyl sulfoxide, and measured using an ELISA reader (Bio-Rad Laboratories) at a wavelength of 490 nm.
Colony Formation Assay
Cells were plated into six-well plates (200 cells per well) after transfection and incubated for 14 days with the medium replaced every 3 days. After that, cells were washed with PBS three times, fixed with methanol, and stained with Giemsa for 15 min. The number of colonies containing more than 50 cells was counted and photographed using a microscope (Olympus, Tokyo, Japan).
Cell Cycle Assay
Cells were harvested 36 h after transfection with KIFC1 or control siRNA and fixed in 70% ice-cold ethanol overnight. Then cells were washed with PBS and with propidium iodide (50 μg/ml) at room temperature in the dark for 30 min. Cell cycle distribution analysis was examined with flow cytometry (BD Bioscience, San Jose, CA, USA).
Transwell Invasion Assay
The invasion ability of RCC cells was tested in Matrigel-coated cell culture chambers (8-μm pore size; Millipore). First, RCC cells were transfected with KIFC1 or control siRNA and cultured in 24-well dishes for 48 h. Then RCC cells that were resuspended in 200 μl of serum-free DMEM containing 1.5 × 105 cells were placed into the upper Matrigel chamber. Medium with 10% FBS was added into the lower chambers as chemoattractant. After 24 h of incubation, cells remaining on the upper membrane were carefully removed. Cells that had invaded through the filter were stained with 0.1% crystal violet and manually counted using a microscope from 10 randomly selected fields. Data are presented as the mean value for triplicate experiments.
Transwell Migration Assay
The migration ability of RCC cells transfected with KIFC1 or control siRNA was tested in Corning Transwell insert chambers (Millipore). Briefly, 24 h after transfection, 1 × 105 RCC cells that were resuspended in 200 μl of serum-free DMEM were placed into the upper chamber without Matrigel. Medium with 10% FBS was added into the lower chambers as chemoattractant. After 24 h of incubation, cells remaining on the upper membrane were carefully removed. Cells that had invaded through the filter were manually counted using a microscope from 10 randomly selected fields. Data are presented as the mean value for triplicate experiments.
Tumorigenicity Assay
BALB/C nude mice (4 weeks old) were purchased from the Animal Center of The Fourth Military Medical University (Xi’an, P.R. China). Cultured 786-O, 769-P, and OS-RC-2 cells were harvested with trypsin-EDTA, washed, and resuspended in PBS. Animals were randomly divided into three groups (n = 10) for subcutaneous injection of control, si-KIFC1, or siRNA-NC at the dorsal midline along with 5 × 106 cells (in 100 ml of PBS) per site. Tumor sizes (mm3), V = (width2 × length), were measured using calipers every 7 days. The mice were euthanized after 35 days postinoculation, and the dissected tumors were collected and prepared for analyzing. The animal experiment was reviewed and approved by the Animal Care and Use Committee of Xi’an Jiaotong University.
Luciferase Reporter Assay
The wild type (WT) or mutant (MT) 3′-untranslated region (3′-UTR) of human KIFC1 that was a putative target of miR-338-3p was synthesized and cloned into the pGL3-promoter vector (Promega, Madison, WI, USA) to construct the WT 3′-UTR KIFC1 and MT 3′-UTR KIFC1 plasmids. The MT 3′-UTR KIFC1 target site was generated by nucleotide replacement. For luciferase activity assay, 786-O cells were seeded into 12-well plates overnight before transfection, and then cotransfected with WT 3′-UTR KIFC1, or MT 3′-UTR KIFC1, and miR-338-3p mimic, or miR-NC for 36 h. Relative luciferase activity was determined using the Dual-Luciferase Reporter Assay System (Promega). Renilla luciferase activity was normalized to firefly luciferase activity.
Statistical Analysis
Statistical analyses were performed using SPSS 12.0 software. Each experiment was repeated in triplicate, unless otherwise indicated. All data are presented as mean ± standard deviation (SD). DFS and OS curves were calculated using the Kaplan–Meier method and compared by log-rank test. Comparisons between groups were done using the unpaired Student’s t-test. A value of p < 0.05 was considered statistically significant.
RESULTS
KIFC1 Is Overexpressed in RCC Tissue Samples and Cell Lines
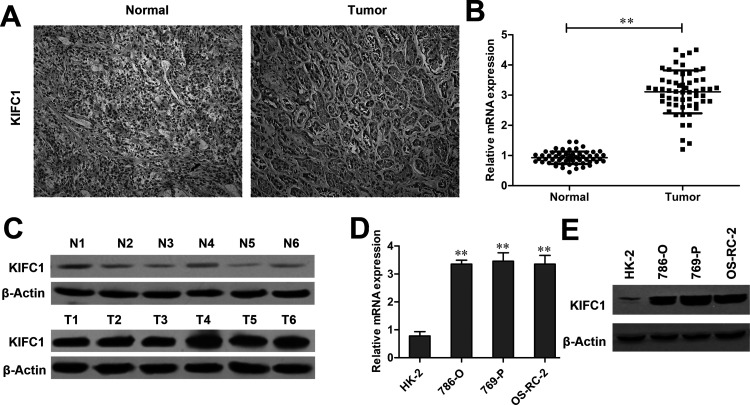
A total of 58 patients with RCC were included in this study, and all of the cases were diagnosed as ccRCC. The expression of KIFC1 was first evaluated in RCC tissues and paired adjacent tissues by immunohistochemistry. Of all 58 patients, immunohistochemical staining revealed that the KIFC1 expression was positively detected in 48 (82.76%) RCC cases, whereas only 9 (15.52%) adjacent normal cases were found with low KIFC1 expression (p < 0.001) (Table 1 and Fig. 1A). Immunohistochemical analyses also showed that, among these 48 KIFC1+ expression cases, 37 cases were detected with high KIFC1 expression (2+ or 3+), and 11 cases were found with low KIFC1 expression (1+). Furthermore, in accordance with the immunohistochemical staining, RT-qPCR and Western blot experiments revealed that the mRNA and protein levels of KIFC1 were significantly upregulated in RCC tissues compared with adjacent normal tissues (p < 0.01) (Fig. 1B and C). The expression level of KIFC1 was then detected in the RCC cell lines (786-O, 769-P, and OS-RC-2) and control cell line (HK-2). As shown in Figure 1D and E, KIFC1 expression was remarkably elevated in 786-O, 769-P, and OS-RC-2 cell lines compared to the HK-2 cell line at both transcript levels and translate levels (p < 0.01, respectively).
Table 1.
Expression of KIFC1 in RCC Tumors and Adjacent Normal Tissues
| Total | KIFC1 (−) | KIFC1 (+) | p Value | |
|---|---|---|---|---|
| Tumors | 58 | 10 (17.24%) | 48 (82.76%) | 0.001 |
| Normal tissues | 58 | 49 (84.48%) | 9 (15.52%) |
Figure 1.
Kinesin family member C1 (KIFC1) is upregulated in renal cell carcinoma (RCC) samples and cell lines. (A) Immunohistochemical image of an RCC sample containing both normal and tumor tissues stained with anti-KIFC1 antibody. Tumor tissues showed stronger staining than normal tissues. (B) Relative mRNA levels of KIFC1 extracted from 58 RCC tissue samples and their paired adjacent normal tissues. (C) Western blot analyses of KIFC1 expression in six representative tumors and paired adjacent tissues. (D) Real-time quantitative PCR (RT-qPCR) and (E) Western blot analyses of KIFC1 expression in 786-O, 769-P, OS-RC-2, and HK-2 cell lines. The experiments were triplicate repeats. Data shown as mean ± standard deviation (SD). **p < 0.01 when compared to the control group.
KIFC1 Overexpression Is Associated With RCC Clinicopathological Parameters and Poor Prognosis
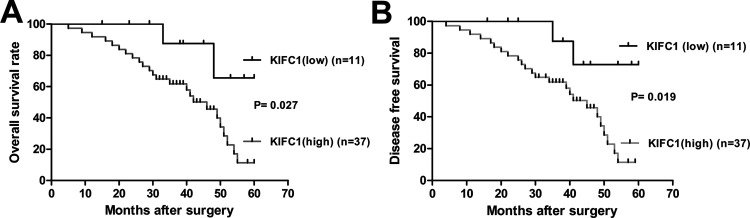
The correlations of KIFC1 expression with various clinicopathological characters were analyzed in the RCC patients mentioned above. As shown in Table 2, the expression level of KIFC1 was significantly associated with Fuhrman stage (p < 0.001), pT stage (p < 0.001), lymph node metastasis (p < 0.05), distant metastasis (p < 0.05), advanced pTNM stage (p < 0.001), and tumor size (p < 0.05), but was not related to patients’ age or gender. Thus, KIFC1 was likely to be involved in the development and progression of RCC. Furthermore, the prognostic value of KIFC1 for OS and DFS in 48 KIFC1+ expression cases was evaluated by comparing the patients with low and high KIFC1 expression. The Kaplan–Meier survival analysis indicated that patients with high KIFC1 expression had significantly lower OS rates than those with low KIFC1 expression cases (p = 0.027) (Fig. 2A). Among them, 81.82% (9/11) patients survived in the KIFC1 low group, while in the KIFC1 high group the survival rate was only 35.15% (13/37). DFS curves also showed that, compared to the KIFC1 low group, the recurrence rate was significantly increased in the high KIFC1 expression patients (p = 0.019) (Fig. 2B). Hence, KIFC1 expression seemed to be a valuable predictive factor for prognosis of RCC patients.
Table 2.
KIFC1 Expression and Clinicopathologic Factors in RCC Patients
| Variables | Cases (N = 48) | KIFC1 Expression | p Value | |
|---|---|---|---|---|
| Low (n = 11) | High (n = 37) | |||
| Age (years) | 0.578 | |||
| >50 | 27 | 6 | 21 | |
| ≤50 | 21 | 5 | 16 | |
| Gender | 0.623 | |||
| Male | 33 | 7 | 26 | |
| Female | 15 | 4 | 11 | |
| Fuhrman grade | <0.001 | |||
| Grade 1/2 | 26 | 2 | 24 | |
| Grade 3/4 | 22 | 9 | 13 | |
| pT stage | <0.001 | |||
| T1/2 | 20 | 6 | 20 | |
| T3/4 | 28 | 5 | 17 | |
| Lymph nodes | <0.05 | |||
| N0 | 15 | 5 | 10 | |
| N1/2 | 33 | 6 | 27 | |
| Metastasis | <0.05 | |||
| M0 | 16 | 7 | 9 | |
| M1 | 32 | 4 | 28 | |
| TNM stage | <0.001 | |||
| I/II | 10 | 6 | 4 | |
| III/IV | 38 | 5 | 33 | |
| Tumor size (cm) | <0.05 | |||
| >5 | 20 | 10 | 10 | |
| ≤5 | 28 | 1 | 27 | |
Values of p < 0.05 are statistically significant.
Figure 2.
Kaplan–Meier survival curves for overall survival (OS) and disease-free survival (DFS) stratified by KIFC1 expression. (A) OS for high and low KIFC1 expression patients. (B) DFS for high and low KIFC1 expression patients. Patients with high KIFC1 expression have poorer OS and DFS.
KIFC1 Knockdown Inhibits RCC Cell Proliferation and Causes G2/M Cell Cycle Arrest
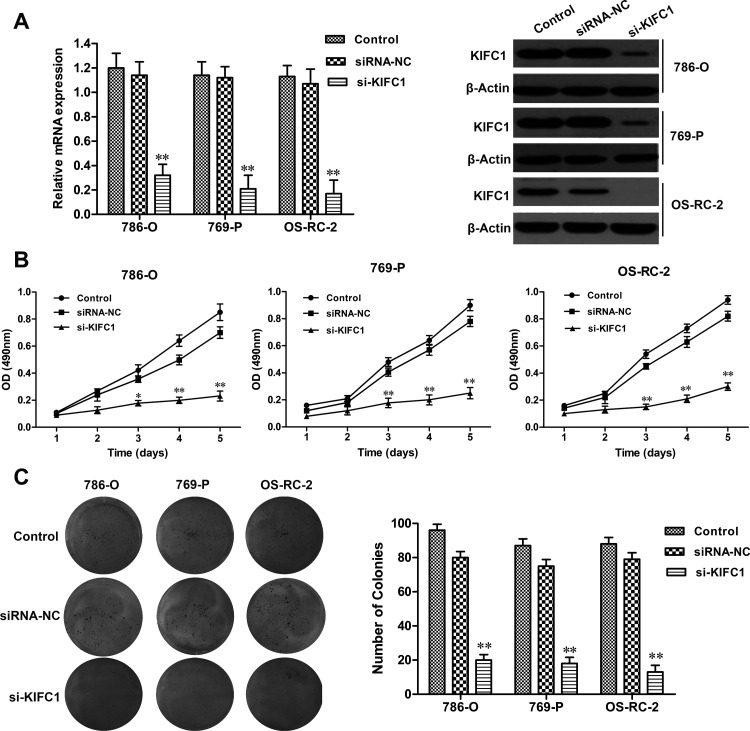
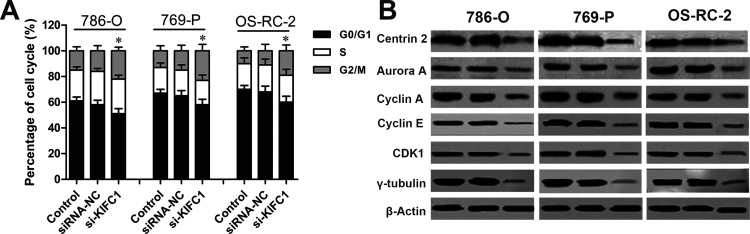
Considering the clinical significance of KIFC1, the crucial role of KIFC1 in the development of RCC was investigated by the siRNA method. The efficacy of siRNA for KIFC1 knockdown in 786-O, 769-P, and OS-RC-2 cells was confirmed by RT-qPCR and Western blot (p < 0.01, respectively) (Fig. 3A). Next, the effect of KIFC1 on RCC cell proliferation was assessed by MTT assay. We observed that KIFC1 knockdown significantly decreased the proliferation rate in 786-O, 769-P, and OS-RC-2 cell lines, when compared to the siRNA-NC group (p < 0.01, respectively) (Fig. 3B). Furthermore, consistent with MTT results, colony formation assay showed that KIFC1 knockdown significantly reduced the colony formation capacity in 786-O, 769-P, and OS-RC-2 cell lines compared to the siRNA-NC group (p < 0.01, respectively) (Fig. 3C). In addition, flow cytometry assay revealed that KIFC1 knockdown in 786-O, 769-P, and OS-RC-2 cells significantly delayed the duration of the G2 and M phases when compared to the NC group (p < 0.05, respectively), but had no obvious effect on the S or G0/G1 phases (Fig. 4A). Several crucial cell cycle regulatory factors in RCC cells were examined with KIFC1 knockdown. As shown in Figure 4B, the expression levels of centrin-2, aurora A, cyclin A, cyclin E, γ-tubulin, and cyclin-dependent kinase 1 (CDK1) were detected and found to be significantly reduced in RCC cells with KIFC1 silencing compared with those transfected with siRNA-NC. Overall, these findings suggested that KIFC1 expression had a crucial impact on the proliferation and growth of RCC cells.
Figure 3.
Effect of KIFC1 knockdown on RCC cell proliferation. (A) The efficiency of KIFC1 small interference RNA (siRNA) on KIFC1 knockdown in 786-O, 769-P, and OS-RC-2 cell lines. (B) Inhibition of RCC cell proliferation by KIFC1 siRNA detected by MTT assay. (C) Inhibition of RCC cell colony formation by KIFC1 siRNA. The experiments were triplicate repeats. Data shown as mean ± SD. *p < 0.05, **p < 0.01 compared to siRNA-NC.
Figure 4.
Effect of KIFC1 knockdown on RCC cell cycle. (A) Cell cycle distribution analysis in RCC cells with KIFC1 knockdown was detected by flow cytometry. (B) The expression levels of selected cell cycle regulatory factors in RCC cells with KIFC1 knockdown were detected by Western blot. The experiments were triplicate repeats. Data shown as mean ± SD. *p < 0.05 compared to siRNA-NC.
KIFC1 Knockdown Suppresses RCC Cell Migration and Invasion
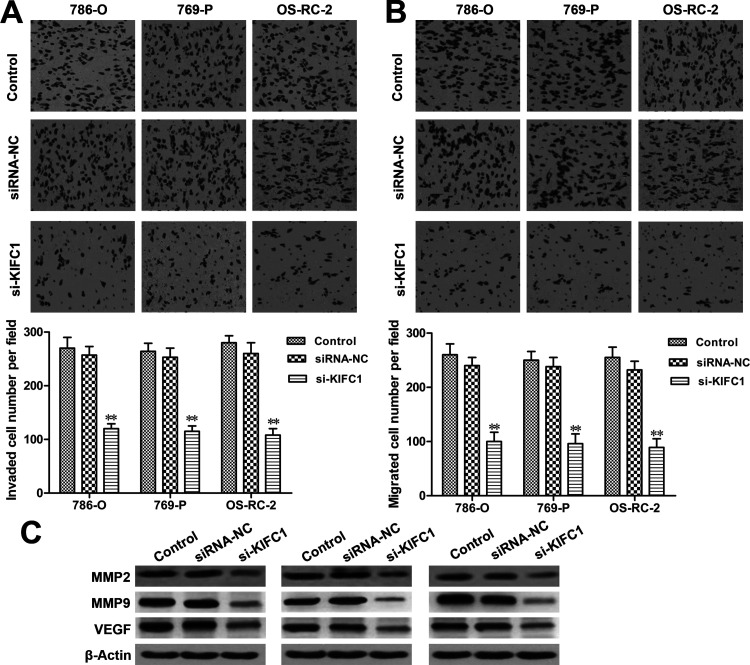
Migration and invasion assays were employed to investigate the effect of KIFC1 on RCC cell migration and invasion. Our results showed that silencing KIFC1 significantly inhibited the invading and migrating abilities of 786-O, 769-P, and OS-RC-2 cells when compared to the siRNA-NC group (p < 0.01, respectively) (Fig. 5A and B). Furthermore, the expression levels of MMP-2, MMP-9, and VEGF, which are thought to be critically involved in the processes of tumor invasion, migration, and metastasis, were also investigated in our study. As shown in Figure 5C, the expression levels of MMP-2, MMP-9, and VEGF were downregulated in the KIFC1 siRNA-transfected cells, when compared with control siRNA-transfected cells. Taken together, these results suggested that KIFC1 silencing could significantly attenuate RCC cell metastasis.
Figure 5.
KIFC1 siRNA decreased RCC cell migration and invasion. (A) Reduction of cell invasion in RCC cells with KIFC1 knockdown was detected by invasion assay. (B) Reduction of cell migration in RCC cells with KIFC1 knockdown was detected by migration assay. (C) The expression levels of MMP-2, MMP-9, and VEGF in RCC cells with KIFC1 knockdown were detected by Western blot. The experiments were triplicate repeats. Data shown as mean ± SD. **p < 0.01 compared to siRNA-NC.
Silencing KIFC1 Decreased the Tumorigenic Potential of RCC Cell Lines In Vivo
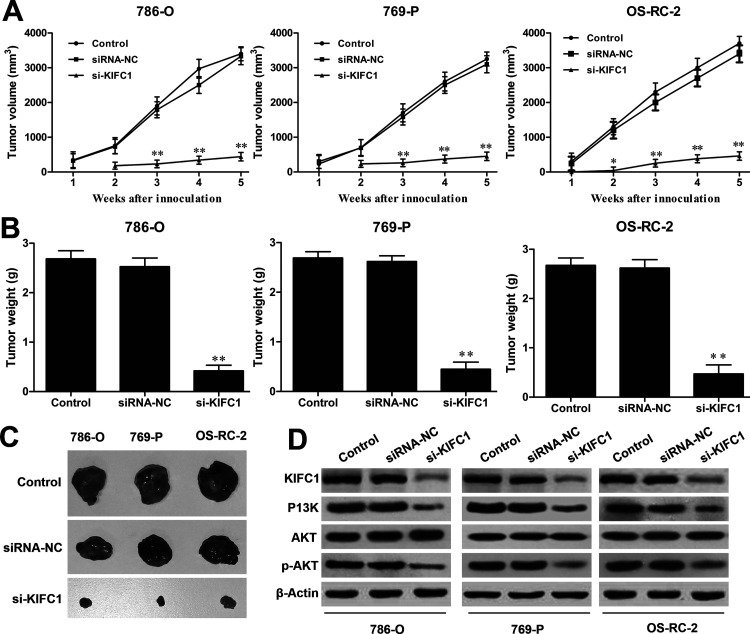
Since knocking down KIFC1 plays an inhibitory role on tumor progression in vitro, it was very important to assess its effect in vivo. 786-O, 769-P, and OS-RC-2 cells transfected with si-KIFC1 or siRNA-NC were subcutaneously injected into nude mice. Over 35 days, the average volume of the tumors in mice of the si-KIFC1 group grew slowly compared to the siRNA-NC group (Fig. 6A). Tumor tissues were isolated from the sacrificed mice at the 35th day, and as shown in Figure 6B and C, both the weight and volume of the tumors were much smaller in the si-KIFC1 group compared to the siRNA-NC group, which indicated that KIFC1 knockdown could significantly inhibit RCC tumor growth in vivo. Western blot also revealed that the expression levels of KIFC1, PI3K, and phosphorylated (p)-AKT were reduced in the xenografted tumor tissues with si-KIFC1 transfection (Fig. 6D), which indicated that KIFC1 might regulate tumor growth via the PI3K/AKT signaling pathway in RCC progression.
Figure 6.
Effect of KIFC1 knockdown on tumorigenesis in nude mice. (A) Time course of tumor volume was assessed by serial microcaliper measurements from the nude mice treated with 786-O, 769-P, and OS-RC-2 cells transfected with si-KIFC1 or siRNA-NC. Tumor weights (B) and representative tumor images (C) of xenografted tumors from each group were measured at 35 days after injection. (D) The expression levels of KIFC1, PI3K, p-AKT, and AKT were determined by Western blot assay. The experiments were triplicate repeats. Data shown as mean ± SD. *p < 0.05, **p < 0.01 compared to siRNA-NC.
KIFC1 Is a Direct Target of miR-338-3p in RCC
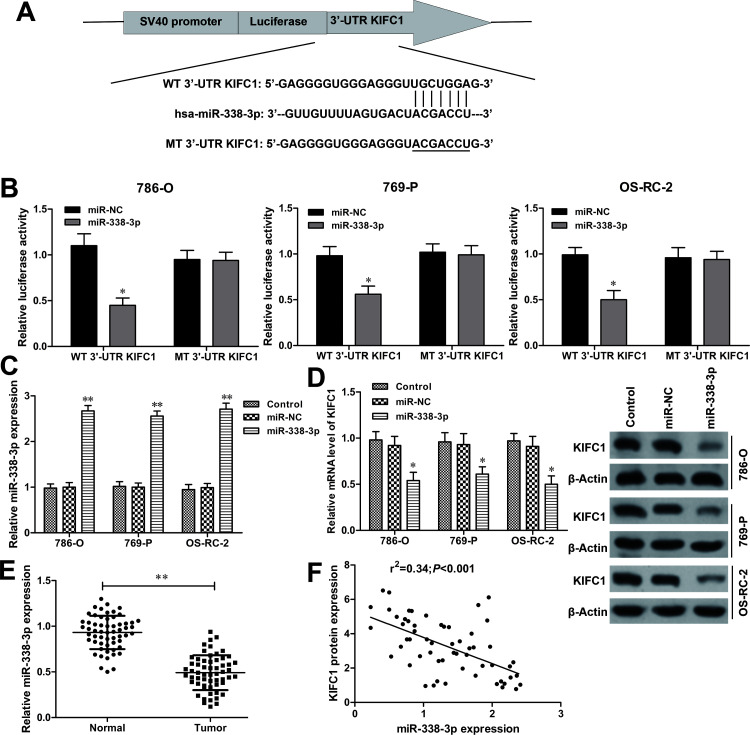
The potential miRNAs that may target KIFC1 were predicted by bioinformatic databases (TargetScan and microRNA.org), and miR-338-3p was identified as a putative candidate (Fig. 7A). To verify whether KIFC1 could be regulated by miR-338-3p, luciferase reporter assay was performed in RCC cells. The luciferase activity was dramatically reduced in RCC cells with WT 3′-UTR KIFC1 and miR-338-3p mimic cotransfection (all p < 0.05) (Fig. 7B). However, no obvious effect was found in the MT 3′-UTR KIFC1 transfection group. To further determine the direct interaction between miR-338-3p and KIFC1, miR-338-3p was overexpressed in RCC cells by miR-338-3p mimic transfection (all p < 0.01) (Fig. 7C). RT-qPCR and Western blot assay revealed that ectopic expression of miR-338-3p significantly suppressed the mRNA and protein levels of KIFC1 in RCC cells (Fig. 7D). In addition, miR-338-3p expression was further measured in the collected 58 paired RCC tissues. RT-qPCR showed that miR-338-3p was significantly downregulated in RCC tissues when compared to adjacent normal tissues (p < 0.01) (Fig. 7E). Furthermore, Spearman’s rank test showed that miR-338-3p expression was negatively correlated with KIFC1 expression in RCC tissue samples (r 2 = 0.34, p < 0.001) (Fig. 7F). Therefore, these findings suggested that KIFC1 was directly regulated by miR-338-3p in RCC cells.
Figure 7.
KIFC1 is a direct target of miR-338-3p in RCC. (A) The putative binding site of miR-338-3p on the 3′-untranslated region (3′-UTR) of KIFC1 was predicted by the bioinformatic databases (TargetScan and microRNA.org). (B) Luciferase activity of RCC cells cotransfected with wild type (WT) 3′-UTR KIFC1 or mutant (MT) 3′-UTR KIFC1 and miR-338-3p mimics or miR-NC were detected by luciferase reporter assay. (C) The efficiency of miR-338-3p mimics or miR-NC was measured by RT-qPCR in RCC cells. (D) The mRNA and protein expression levels of KIFC1 in RCC cells with miR-338-3p mimics or miR-NC transfection were assessed by RT-qPCR and Western blot assays. (E) The expression levels of miR-338-3p in 58 paired RCC tissue samples were detected by RT-qPCR. (F) Spearman’s correlation analysis of KIFC1 mRNA and miR-338-3p expression levels in human RCC tissue samples. The experiments were triplicate repeats. Data shown as mean ± SD. *p < 0.05, **p < 0.01 compared to miR-NC.
Restoration of miR-338-3p Inhibits RCC Cell Proliferation and Invasion
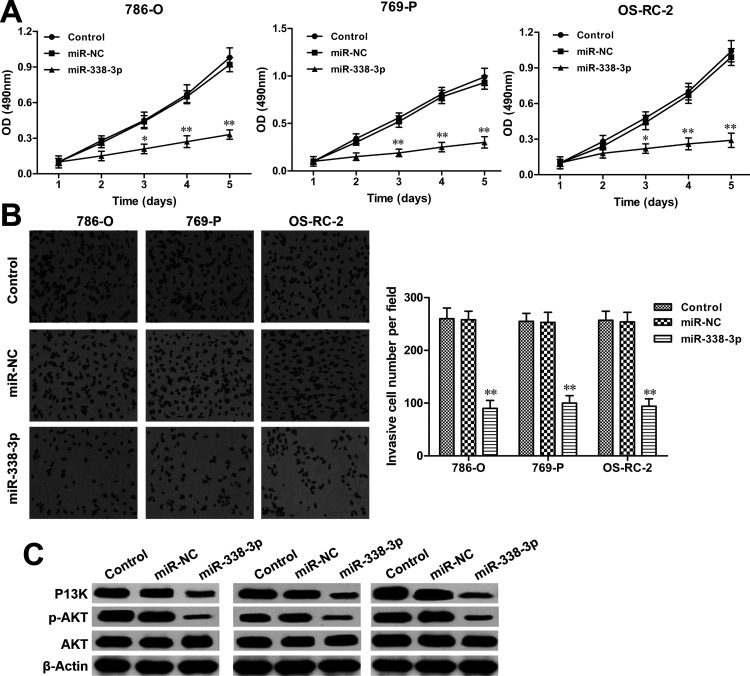
To further explore the regulatory mechanism underlying the miR-338-3p/KIFC1 axis on RCC progression, the MTT assay was performed to investigate the role of miR-338-3p on RCC cell proliferation. The results showed that restoration of miR-338-3p markedly decreased cell proliferation in 786-O, 769-P, and OS-RC-2 cells when compared with miR-NC transfection groups (all p < 0.01) (Fig. 8A). Furthermore, the Transwell invasion assay revealed that forced expression of miR-338-3p significantly inhibited the invasion abilities of RCC cells after miR-338-3p mimic transfection (all p < 0.01) (Fig. 8B). In addition, the expression levels of p-AKT and PI3K were also reduced by miR-338-3p overexpression in RCC cells (Fig. 8C). These findings could be mimicked by KIFC1 knockdown in RCC cells. Therefore, these data suggested that the inhibitory effect of KIFC1 silencing on cell proliferation, invasion, and migration was partially regulated by miR-338-3p in RCC cells.
Figure 8.
Restoration of miR-338-3p inhibits RCC cell proliferation and invasion. (A) Cell proliferative rates of RCC cells with miR-338-3p mimic or miR-NC transfection were determined by MTT assay. (B) Cell invasion abilities of RCC cells with miR-338-3p mimic or miR-NC transfection were measured by Transwell invasion assay. (C) The activation of PI3K/AKT signaling in RCC cells with miR-338-3p mimic or miR-NC transfection was detected by Western blot assay. The experiments were triplicate repeats. Data shown as mean ± SD. *p < 0.05, **p < 0.01 compared to miR-NC.
DISCUSSION
In the current study, 58 RCC tissue samples isolated from the clinic were all diagnosed with the ccRCC subtype. The KIFC1 expression level was determined by RT-qPCR and Western blot and found that significantly abundant KIFC1 expression was detected in tumor tissues compared to adjacent normal tissues. Immunohistochemical staining also verified that KIFC1 was overexpressed in tumor tissues, whereas negligible or lower KIFC1 expression was exhibited in adjacent normal tissues. To explore whether KIFC1 expression plays a key role in the progression of RCC, the correlation between KIFC1 expression levels and the clinicopathological parameters of 48 positive KIFC1 patients with RCC were analyzed. We observed that RCC tissue with a high level of KIFC1 expression was significantly associated with higher Fuhrman grade, higher pT stage, advanced pTNM stage, and larger tumor size compared to RCC tissue with a low level of KIFC1 expression, indicating that its expression might attribute to the intrinsic aggressiveness of RCC. Moreover, overexpressing KIFC1 was correlated with poor prognoses in RCC patients. These results were consistent with previous studies, which demonstrated that elevated KIFC1 expression predicted aggressive tumor progression in ovarian adenocarcinomas and breast cancer8,9.
Since KIFC1 was clarified to be upregulated in RCC tissue samples, it is necessary to further unravel the mechanism underlying the regulatory functions of KIFC1 involved in the progression of RCC. The small interference RNA technique was employed to silence KIFC1 expression in 786-O, 769-P, and OS-RC-2 cell lines. Interestingly, KIFC1 knockdown in RCC cells could significantly suppress cell proliferation and colony formation capacity and cause cell cycle arrest at the G2/M phase. Furthermore, Transwell invasion and migration assays also demonstrated the inhibitory effects of KIFC1 silencing on cell invasion and migration in vitro. Consistently, through the si-KIFC1 xenograft model in nude mice, we found that KIFC1 silencing significantly inhibited tumorigenesis in vivo. Therefore, it was suggested that KIFC1 might directly accelerate the malignant potential in RCC.
In order to avoid supernumerary centrosome duplication in a single cell cycle, centrosome duplication is tightly orchestrated by DNA replication, mitosis, and cytokinesis16. The proper order of centrosome duplication is thought to be completed by cell cycle signaling pathways including cyclin D1, cyclin E, cyclin A, and CDKs, while inhibitors of CDK2, p21, or p27 may block centrosome duplication17,18. In the current study, the cell cycle assay showed that KIFC1 knockdown in cells delayed them at the G2/M phase, directly leading to slower mitoses, which conceivably mitigated the chromosomal instability in malignant cells caused by traverse mitosis19,20. Signaling pathway regulators like cyclin A and cyclin E are crucial for triggering entry into mitosis by binding to CDK1 during cancer cell cycle. Therefore, CDK1 activation is committed to control proper cell division and the onset of mitosis. In other words, G2/M transition is directly controlled by the CDK1–cyclin complexes, which are also referred to as M phase promoting factor16. From the present study, we noticed that KIFC1 knockdown in RCC cells could negatively regulate G2/M phase progression by significantly suppressing the expression of cyclin A, cyclin E, and CDK1.
Furthermore, the mitotic kinases including aurora A, B, and C have been found to be overexpressed in diverse cancers and intimately associated with the mitotic errors during oncogenesis21–23. Centrin-2 and γ-tubulin, as the key markers of the centrosome activity during mitosis24, were also downregulated by KIFC1 silencing in RCC cells. These findings were in agreement with previous studies, which also pointed out that overexpression of KIFC1 was suggested to play key roles in accelerating the mitosis phase by clustering multiple centrosome amplifications with genomic instability in cancer cells, and resulted in dysregulation of cell cycle kinetics8,9,25.
Metastasis is an important characteristic of RCC26,27. The mechanism underlying RCC metastases remains elusive; it is an urgent need to uncover this mystery. The present study elucidated that KIFC1 knockdown in RCC cells could inhibit cell invasion and migration abilities through reducing PI3K/AKT signaling and the expressions of MMP-2, MMP-9, and VEGF. PI3K/AKT, an intracellular signaling pathway, plays crucial roles in the development of diverse malignancies28. The activation of PI3K/AKT signaling triggers a series of target genes that mediate cell proliferation and apoptosis, as well as invasion and migration29. It is known that MMPs are crucially involved in the processes of malignant cell progression and aggressiveness30. Among these MMPs, the expressions and activities of MMP-2 and MMP-9 are directly correlated with metastatic abilities and progression of various cancers31–33. VEGF is another key mediator in tumor cell invasion and metastasis, as well as angiogenesis34. Previous reports had demonstrated that downregulation of VEGF had an antimetastatic effect on RCC cells35. Our current study verified that the activation of PI3K/AKT signaling and the expressions of MMP-2, MMP-9, and VEGF were intimately involved in KIFC-regulated RCC progression. Therefore, KIFC1 was suggested to regulate RCC invasion and migration through the PI3K/AKT signaling pathway.
However, there seems to be a controversial view: although KIFC1 expression is found in some somatic and germ cells and participates in various bioprocesses such as vesicular and organelle trafficking, spermiogenesis, oocyte development, and double-strand DNA transportation36,37, the truth is that KIFC1 is only present in certain cell types including fibroblast cells and germ cells and is redundant and dispensable in most somatic cells that do not undergo acentrosomal cell division38. In tumor cells, to modulate the well-ordered centrosome clustering, KIFC1 has the ability to delay anaphase to obtain extra time during mitosis of cancer cells and greatly assist tumor malignancy12. These findings demonstrate that KIFC1 is indispensable and important for cancer cell survival and undoubtedly has great potential as a therapeutic target for cancers.
miRNAs are noncoding, small RNAs that can posttranscriptionally regulate hundreds of gene expressions through binding to the 3′-UTR of their target mRNAs; thus, miRNAs have been reported to be involved in a wide range of biological processes39. Recently, a number of miRNAs have been demonstrated to play crucial roles in the progression of tumorigenesis through regulating the expression of targeting oncogenes or tumor suppressors40. miR-338-3p has been implicated in the initiation and development of various types of cancers. Sui et al. suggested that miR-338-3p functions as a tumor suppressor that inhibits thyroid cancer progression through targeting AKT341. Zhang et al. indicated that miR-338-3p inhibits the metastasis of RCC by downregulation of activin receptor-like kinase 5 (ALK5)42. Furthermore, Tong et al. also illustrated that miR-338-3p suppresses cell proliferation and invasion of RCC through targeting sex-determining region Y-box 4 (SOX4)41. Accordingly, identifying the molecular targets of miR-338-3p will contribute to the better understanding of the mechanisms underlying the progression of RCC. In the current study, the luciferase report assay and RT-qPCR confirmed that KIFC1 was a novel target of miR-338-3p. Restoration of miR-338-3p could mimic the inhibitory role of KIFC1 silencing on RCC proliferation and invasion. Furthermore, there was an inverse relationship between miR-338-3p and KIFC1 expression in clinical RCC tissue samples. Therefore, we suspected that upregulation of KIFC1 by miR-338-3p might result in the proliferation and invasion properties of RCC.
Taken together, we identified that KIFC1 was overexpressed in RCC tissues and cell lines, and the high KIFC1 expression in tumor tissues was associated with the poor prognosis of RCC patients. KIFC1, negatively regulated by miR-338-3p, served as an oncogene in the RCC cell proliferation and aggressiveness through activating the PI3K/AKT signaling pathway. Collectively, this study showed potential future prospects of using KIFC1 gene therapy as an effective RCC treatment method.
ACKNOWLEDGMENT
We would like to thank Dr. Chong for his thoughtful suggestions and contributions to this work.
Footnotes
The authors declare no conflicts of interest.
REFERENCES
- 1. Ljungberg B, Campbell SC, Choi HY, Jacqmin D, Lee JE, Weikert S, Kiemeney LA. The epidemiology of renal cell carcinoma. Eur Urol. 2011;60(4):615–21. [DOI] [PubMed] [Google Scholar]
- 2. Adeniran AJ, Shuch B, Humphrey PA. Hereditary renal cell carcinoma syndromes: Clinical, pathologic, and genetic features. Am J Surg Pathol. 2015;39(12):e1–18. [DOI] [PubMed] [Google Scholar]
- 3. Olshan AF, Kuo TM, Meyer A-M, Nielsen ME, Purdue MP, Rathmell WK. Racial difference in histologic subtype of renal cell carcinoma. Cancer Med. 2013;2(5):744–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lam JS, Shvarts O, Leppert JT, Figlin RA, Belldegrun AS. Renal cell carcinoma 2005: New frontiers in staging, prognostication, and targeted molecular therapy. J Urol. 2005;173(6):1853–62. [DOI] [PubMed] [Google Scholar]
- 5. Volpe A, Patard JJ. Prognostic factors in renal cell carcinoma. World J Urol. 2010;28(3):319–27. [DOI] [PubMed] [Google Scholar]
- 6. Lam JS, Leppert JT, Figlin RA, Belldegrun AS. Role of molecular markers in the diagnosis and therapy of renal cell carcinoma. Urology 2005;66(5 Suppl):1–9. [DOI] [PubMed] [Google Scholar]
- 7. Kwon M, Godinho SA, Chandhok NS, Ganem NJ, Azioune A, Thery M, Pellman D. Mechanisms to suppress multipolar divisions in cancer cells with extra centrosomes. Genes Dev. 2008;22(16):2189–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mittal K, Choi da H, Klimov S, Pawar S, Kaur R, Mitra AK, Gupta MV, Sams R, Cantuaria G, Rida PC, Aneja R. A centrosome clustering protein, KIFC1, predicts aggressive disease course in serous ovarian adenocarcinomas. J Ovarian Res. 2016;9(1):17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pannu V, Rida PC, Ogden A, Turaga RC, Donthamsetty S, Bowen NJ, Rudd K, Gupta MV, Reidm D, Cantuaria G, Walczak CE, Aneja R. HSET overexpression fuels tumor progression via centrosome clustering-independent mechanisms in breast cancer patients. Oncotarget 2015;6(8):6076–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Grinberg-Rashi H, Ofek E, Perelman M, Skarda J, Yaron P, Hajduch M, Jacob-Hirsch J, Amariglio N, Krupsky M, Simansky DA, Ram Z, Pfeffer R, Galernter I, Steinberg DM, Ben-Dov I, Rechavi G, Izraeli S. The expression of three genes in primary non-small cell lung cancer is associated with metastatic spread to the brain. Clin Cancer Res. 2009;15(5):1755–61. [DOI] [PubMed] [Google Scholar]
- 11. Rieder CL, Faruki S, Khodjakov A. The centrosome in vertebrates: More than a microtubule-organizing center. Trends Cell Biol. 2001;11(10):413–9. [DOI] [PubMed] [Google Scholar]
- 12. Chan JY. A clinical overview of centrosome amplification in human cancers. Int J Biol Sci. 2011;7(8):1122–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Godinho SA, Picone R, Burute M, Dagher R, Su Y, Leung CT, Polyak K, Brugge JS, Thery M, Pellman D. Oncogene-like induction of cellular invasion from centrosome amplification. Nature 2014;510(7503):167–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Xiao YX, Yang WX. KIFC1: A promising chemotherapy target for cancer treatment? Oncotarget 2016;7(30):48656–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Edge SB, Compton CC. The American Joint Committee on Cancer: The 7th edition of the AJCC staging manual and the future of TNM. Ann Surg Oncol. 2010;17(6):1471–4. [DOI] [PubMed] [Google Scholar]
- 16. Bendris N, Lemmers B, Blanchard JM. Cell cycle, cytoskeleton dynamics, and beyond: The many functions of cyclins and CDK inhibitors. Cell Cycle 2015;14(12):1786–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rhind N, Russell P. Signaling pathways that regulate cell division. Cold Spring Harb Perspect Biol. 2012;4(10):a005942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Fukasawa K. Centrosome amplification, chromosome instability, and cancer development. Cancer Lett. 2005;230(1):6–19. [DOI] [PubMed] [Google Scholar]
- 19. Gisselsson D. Mitotic instability in cancer: Is there method in the madness? Cell Cycle 2005;4(8):1007–10. [DOI] [PubMed] [Google Scholar]
- 20. Gisselsson D, Hoglund M. Connecting mitotic instability and chromosome aberrations in cancer—Can telomeres bridge the gap? Semin Cancer Biol. 2005;15(1):13–23. [DOI] [PubMed] [Google Scholar]
- 21. Fu J, Bian M, Jiang Q, Zhang C. Roles of Aurora kinases in mitosis and tumorigenesis. Mol Cancer Res. 2007;5(1):1–10. [DOI] [PubMed] [Google Scholar]
- 22. Meraldi P, Honda R, Nigg EA. Aurora kinases link chromosome segregation and cell division to cancer susceptibility. Curr Opin Genet Dev. 2004;14(1):29–36. [DOI] [PubMed] [Google Scholar]
- 23. Ducat D, Zheng Y. Aurora kinases in spindle assembly and chromosome segregation. Exp Cell Res. 2004;301(1):60–7. [DOI] [PubMed] [Google Scholar]
- 24. Salisbury JL, Suino KM, Busby R, Springett M. Centrin-2 is required for centriole duplication in mammalian cells. Curr Biol. 2002;12(15):1287–92. [DOI] [PubMed] [Google Scholar]
- 25. Kim N, Song K. KIFC1 is essential for bipolar spindle formation and genomic stability in the primary human fibroblast IMR-90 cell. Cell Struct Funct. 2013;38(1):21–30. [DOI] [PubMed] [Google Scholar]
- 26. Xu M, Gu M, Zhang K, Zhou J, Wang Z, Da J. miR-203 inhibition of renal cancer cell proliferation, migration and invasion by targeting of FGF2. Diagn Pathol. 2015;10(1):24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Keizman D, Maimon N, Mishaeli M, Kuchuk I, Gottfried M. [The current approach to metastatic renal cell carcinoma]. Harefuah 2015;154(8):535–9. [PubMed] [Google Scholar]
- 28. Liu GL, Yang HJ, Liu B, Liu T. Effects of microRNA-19b on the proliferation, apoptosis, and migration of Wilms’ tumor cells via the PTEN/PI3K/AKT signaling pathway. J Cell Biochem. 2017;118(10):3424–34. [DOI] [PubMed] [Google Scholar]
- 29. Fresno Vara JA, Casado E, de Castro J, Cejas P, Belda-Iniesta C, Gonzalez-Baron M. PI3K/Akt signalling pathway and cancer. Cancer Treat Rev. 2004;30(2):193–204. [DOI] [PubMed] [Google Scholar]
- 30. Ren F, Tang R, Zhang X, Madushi WM, Luo D, Dang Y, Li Z, Wei K, Chen G. Overexpression of MMP family members functions as prognostic biomarker for breast cancer patients: A systematic review and meta-analysis. PLoS One 2015;10(8):e0135544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Araujo RF Jr, Lira GA, Vilaca JA, Guedes HG, Leitao MC, Lucena HF, Ramos CC. Prognostic and diagnostic implications of MMP-2, MMP-9, and VEGF-alpha expressions in colorectal cancer. Pathol Res Pract. 2015;211(1):71–7. [DOI] [PubMed] [Google Scholar]
- 32. Kim SH, Kim K, Lee JS, Koo BS, Kim JH, Chang JH, Choi EC. Correlations of oral tongue cancer invasion with matrix metalloproteinases (MMPs) and vascular endothelial growth factor (VEGF) expression. J Surg Oncol. 2006;93(4):330–7. [DOI] [PubMed] [Google Scholar]
- 33. Johansson N, Ahonen M, Kahari VM. Matrix metalloproteinases in tumor invasion. Cell Mol Life Sci. 2000;57(1):5–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Li J, Li L, Li Z, Gong G, Chen P, Liu H, Wang J, Liu Y, Wu X. The role of miR-205 in the VEGF-mediated promotion of human ovarian cancer cell invasion. Gynecol Oncol. 2015;137(1):125–33. [DOI] [PubMed] [Google Scholar]
- 35. Powles T, Kayani I, Sharpe K, Lim L, Peters J, Stewart GD, Berney D, Sahdev A, Chowdhury S, Boleti E, Shamash J, Reynolds AR, Jones R, Blank C, Haanen J, Bex A. A prospective evaluation of VEGF-targeted treatment cessation in metastatic clear cell renal cancer. Ann Oncol. 2013;24(8):2098–103. [DOI] [PubMed] [Google Scholar]
- 36. Rath O, Kozielski F. Kinesins and cancer. Nat Rev Cancer 2012;12(8):527–39. [DOI] [PubMed] [Google Scholar]
- 37. Farina F, Pierobon P, Delevoye C, Monnet J, Dingli F, Loew D, Quanz M, Dutreix M, Cappello G. Kinesin KIFC1 actively transports bare double-stranded DNA. Nucleic Acids Res. 2013;41(9):4926–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Yang WX, Sperry AO. C-terminal kinesin motor KIFC1 participates in acrosome biogenesis and vesicle transport. Biol Reprod. 2003;69(5):1719–29. [DOI] [PubMed] [Google Scholar]
- 39. Hammond SM. An overview of microRNAs. Adv Drug Deliv Rev. 2015;87(1):3–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Perge P, Nagy Z, Igaz I, Igaz P. Suggested roles for microRNA in tumors. Biomol Concepts 2015;6(2):149–55. [DOI] [PubMed] [Google Scholar]
- 41. Tong Z, Meng X, Wang J, Wang L. MicroRNA-338-3p targets SOX4 and inhibits cell proliferation and invasion of renal cell carcinoma. Exp Ther Med. 2017;14(5):5200–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Zhang X, Wang C, Li H, Niu X, Liu X, Pei D, Guo X, Xu X, Li Y. miR-338-3p inhibits the invasion of renal cell carcinoma by downregulation of ALK5. Oncotarget 2017;8(38):64106–13. [DOI] [PMC free article] [PubMed] [Google Scholar]