Abstract
The activated form of vitamin D3, 1,25-dihydroxyvitamin D3 [1,25(OH)2D3], regulates numerous cellular processes, including inhibition of cancer progression. IL-1β has been reported to facilitate cancer development, especially by inducing an epithelial-to-mesenchymal transition (EMT) in several malignant tumors. However, the underlying mechanism of 1,25(OH)2D3 and IL-1β in colorectal cancer (CRC) still remains largely unknown. To fill in this knowledge gap, we measured cell proliferation and invasion by CCK-8 and Transwell assays after stimulation with 1,25(OH)2D3 and IL-1β. E-cadherin and vimentin were chosen as markers of EMT measured by immunofluorescence, quantitative real-time PCR (qRT-PCR), and Western blot. The expression and function of the vitamin D receptor (VDR) was evaluated by Western blot and luciferase reporter assay. qRT-PCR and RNA-FISH were performed to detect the expression and location of lncTCF7 in vitro. The binding sites of VDR in the lncTCF7 promoter were confirmed by a chromatin immunoprecipitation assay. Based on the above experiments, we found that 1,25(OH)2D3 attenuates IL-1β-induced increased proliferation and invasion in colorectal cancer through enhancing VDR, which inhibits the expression of lncTCF7 by directly binding to its promoter region.
Key words: 1,25(OH)2D3; IL-1β; Vitamin D receptor; Epithelial–mesenchymal transition (EMT); lncTCF7
INTRODUCTION
Colorectal cancer (CRC) is the third most common cancer in the world1. Several studies have reported that vitamin D3 exhibits antitumor activity in multiple cancers, especially CRC2–4. Accumulating evidence shows that increasing vitamin D synthesis reduces the risk and progression of colorectal cancer, and vitamin D deficiency increases the risk of CRC5–7. As the most active form of vitamin D in vivo, 1,25(OH)2D3 regulates up to 5% of genome transcription through the VDR signaling pathway6,8. Long noncoding RNAs (lncRNAs) are defined as transcripts longer than 200 nucleotides that do not code for proteins. Some recent studies have shown that lncRNAs play an important role in cancer process9,10. Jiang and Bikle reported that VDR regulated a variety of lncRNAs, including H19, HOTTIP, and Kcnq1ot1, in skin cancer11, but the link between VDR and lncRNAs in CRC still largely remains unknown.
Inflammation has been reported as one of the hallmarks of cancer development12,13. IL-1β is a member of the interleukin-1 family that has a complex function in inflammation, immunity, and hemopoiesis14,15. IL-1β recently has been shown to induce the upregulation of BIRC3 and methylation of the estrogen receptor ERα gene, leading to EMT and chemoresistance in breast cancer12,16. Searching on the website of GeneCards (http://www.genecards.org/), we found that calcitriol, as one of the VDR agonists, is an IL-1β and TNF-α production inhibitor; thus, we hypothesized that vitamin D3 has the opposite effect of IL-1β, and its underlying mechanism in CRC remains unclear.
In this study, we examined the effect of 1,25(OH)2D3 and IL-1β in CRC cell lines, and we demonstrated that 1,25(OH)2D3 attenuates IL-1β-induced EMT through activating VDR signaling. In addition, we showed that 1,25(OH)2D3 inhibits cancer development partly through the VDR/lncTCF7 axis. These results supplement our knowledge of the mechanism of 1,25(OH)2D3 in CRC.
MATERIALS AND METHODS
Cell Culture
The cell lines DLD1 and LoVo were purchased from the American Type Culture Collection (ATCC, Rockville, MD, USA). DLD1 and LoVo were cultured in DMEM (Gibco, Carlsbad, CA, USA) and F-12K (Gibco) culture media, respectively, at 37°C with 5% CO2. The media were supplemented with 10% fetal bovine serum (FBS), 100 U/ml of penicillin (Keygen Biotech, Beijing, P.R. China), and 100 μg/ml of streptomycin (Keygen Biotech). The plasmid overexpressing lncTCF7 and its empty vector were obtained from GenePharma Co (Shanghai, P.R. China). Lipofectamine 3000 (Invitrogen, Carlsbad, CA, USA) was used for the transfection.
RNA Extraction and qRT-PCR
Total RNA was extracted from cultured cells using TRIzol reagent (Takara, Dalian, P.R. China). RQ1 RNase-Free DNase (Promega, Madison, WI, USA) was used to remove DNA. RNA reverse transcription to cDNA was performed with RevertAid First Strand cDNA Synthesis Kit (Thermo Fisher, Carlsbad, CA, USA), and quantitative real-time PCR (qRT-PCR) analysis was performed with SYBR Green PCR Master Mix (Applied Biosystems, Foster City, CA, USA) according to the manufacturer’s instructions. The primer sequences are shown in Table 1. The results were normalized to the expression of β-actin. The delta Cq (ΔCq) value was used to represent the expression level of lncRNA in qRT-PCR. For each amplicon designed, the ΔCq value was normalized using the equation: ΔCq = Cq (target) − Cq (β-actin).
Table 1.
Primers
| E-cadherin | F: 5′-CGAGAGCTACACGTTCACGG-3′ |
| R: 5′-GGGTGTCGAGGGAAAAATAGG-3′ | |
| Vimentin | F: 5′-TGCCGTTGAAGCTGCTAACTA-3′ |
| R: 5′-CCAGAGGGAGTGAATCCAGATTA-3′ | |
| VDR | F: 5′-GTGGACATCGGCATGATGAAG-3′ |
| R: 5′-GGTCGTAGGTCTTATGGTGGG-3′ | |
| β-Actin | F: 5′-CATGTACGTTGCTATCCAGGC-3′ |
| R: 5′-CTCCTTAATGTCACGCACGAT-3′ | |
| GNAS-AS1 | F: 5′-CCCAGGATGGATAAGGAGTTGA-3′ |
| R: 5′-CTGGTAGCCAGTCACTTCCACTT-3′ | |
| lincRNA-p21 | F: 5′-GCTCGACGCTAGGATCTGAC-3′ |
| R: 5′-GCTTTCCACGACGGTGAC-3′ | |
| MEG3 | F: 5′-CTGCCCATCTACACCTCACG-3′ |
| R: 5′-CTCTCC GCCGTCTGCGCTAGGGGCT-3′ | |
| HOTTIP | F: 5′-CCTAAAGCCACGCTTCTTTG-3′ |
| R: 5′-TGCAGGCTGGAGATCCTACT-3′ | |
| Kcnq1ot1 | F: 5′-CCAGCATAGCCTTATGGA-3′ |
| R: 5′-CACGGACCCTCAGTGAAT-3′ | |
| SRA1 | F: 5′-CTGAGGTCAGTCAGTGGATGG-3′ |
| R: 5′-AGCCTGGTATGGTATGGTTCT-3′ | |
| MALAT1 | F: 5′-GGATTCCAGGAAGGAGCGAG-3′ |
| R: 5′-ATTGCCGACCTCACGGATTT-3′ | |
| SAMMSON | F: 5′-AGACACAGGTGGCTGGTCATG-3′ |
| R: 5′-TGATGACATTGAGCCCACCTT-3′ | |
| lncTCF7 | F: 5′-AGGAGTCCTTGGACCTGAGC-3′ |
| R: 5′-AGTGGCTGGCATATAACCAACA-3′ |
Western Blots
Lysate was extracted from cultured cells with a lysis buffer, and the protein content was assayed by a protein assay (BCA) kit (Thermo Fisher). Proteins were fractionated on 10% SDS-PAGE and transferred onto a PVDF membrane. Rabbit monoclonal antibodies against E-cadherin, vimentin, and VDR (Cell Signaling Technology, Danvers, MA, USA) were used as the primary antibodies. The antibody against GAPDH (Cell Signaling Technology) was used as a loading control. The secondary antibody was then added, and proteins of interest were visualized using Pierce ECL Western Blotting Substrate (Millipore, Boston, MA, USA).
Cell Proliferation
The effect of 1,25(OH)2D3 and IL-1β on proliferation of CRC cell lines was measured with Cell Counting Kit-8 (CCK-8; Dojindo, Kumamoto, Japan). DLD1 and LoVo cells were resuspended, seeded at 1 × 103/100 μl/well in a 96-well plate, and incubated for different times with or without 100 nM 1,25(OH)2D3 in the absence or presence of 10 ng/ml of IL-1β. CCK-8 reagent was added to each well. The plate was incubated for another 2 h at 37°C, and the absorbance at 450 nm was measured.
Transwell Assays
The invasive ability of CRC cells was assayed by a Transwell plate with a filter of 8-μm pores (BD Biosciences, Franklin Lakes, NJ, USA), and the top chamber was precoated with 50 μl of Matrigel (BD Biosciences). A total of 5 × 105 cells in serum-free medium were added into the upper insert. In the lower compartment, 600 μl of medium containing 20% FBS was added. After incubation for 48 h, the cells on the upper surface of the filter were wiped off, and the cells adherent on the undersurface of the filter were fixed in methanol, stained with crystal violet, and counted under a microscope. Three visual fields were selected randomly for cell counting.
Immunofluorescence Analysis
After treatment with 100 nM 1,25(OH)2D3 for 48 h, DLD1 cells were washed with PBS, fixed with 3.7% paraformaldehyde for 10 min at room temperature, then permeabilized with 0.3% Triton X-100 in PBS for 10 min. Subsequently, cells were blocked with 5% bovine serum albumin in PBS for 1 h at room temperature, and then incubated with primary antibody for 1 h at room temperature. After being washed with PBS, the cells were incubated with FITC-conjugated secondary antibodies (Cell Signaling Technology) for 1 h at room temperature. After being washed with PBS, the nuclei were stained with DAPI. The fluorescence was visualized using Fluoview 1000 confocal microscope (Olympus, Tokyo, Japan). Antibody dilutions of 1:200 were used for E-cadherin (Cell Signaling Technology) and 1:100 for vimentin (Cell Signaling Technology).
RNA-FISH
A fragment of a 550-bp lncTCF7 cDNA was amplified from the lncTCF7 plasmid by using a high-fidelity DNA polymerase (Takara) as the DNA template. From this template, a fluorescein-labeled DNA as the lncTCF7 FISH probe was made with PCR Fluorescein Labeling Mix (Roche, Basel, Switzerland). DLD1 cells were cultured on slides treated with or without 100 nM 1,25(OH)2D3 for 48 h. The slide was soaked in proteinase K for 5 min and washed in 2× SSC twice. A FISH hybridization solution (Dingguochangsheng, Beijing, P.R. China) containing 30 ng/μl lncTCF7 FISH probe DNA was applied to the slide, which was subsequently incubated at 37°C for 16 h. The slide was then washed in 0.4× SSC/0.001% NP-40 at 560°C for 5 min followed by a second wash in 2× SSC/0.0001% NP-40 for another 2 min. The slide was covered with a drop of mounting medium containing DAPI and visualized using a Fluoview 1000 confocal microscope (Olympus).
Luciferase Reporter Assay
The promoter region of the human lncTCF7 gene, which contains putative binding sites, was amplified by PCR and then subcloned into the pGL3 vector. Luciferase reporter plasmids were constructed with a series deletion of the lncTCF7 promoter region (lncTCF7-luc) or mutated VDR binding sites (mutVIE). A pRL-TK plasmid expressing Renilla luciferase was used as an internal control. After cotransfection, DLD1 cells were cultured with 100 nM 1,25(OH)2D3 for 24 h. Luciferase activity was detected by a Dual-Luciferase Reporter Assay System (Promega). All of the experiments were performed in triplicate.
Chromatin Immunoprecipitation
DLD1 cells were treated with 100 nM 1,25(OH)2D3 for 24 h. ChIP assays were performed using SimpleChIP® Enzymatic Chromatin IP Kit (Cell Signaling Technology) following the manufacturer’s instructions. Antibody dilutions of 1:50 were used for VDR (Cell Signaling Technology). Primer sequences of the lncTCF7 promoter region were 5′-GTCGCTTAAGCCCAGGAGG-3′ (forward) and 5′-CTATGTTGCCCAGGCTGGA-3′ (reverse).
Tumor Xenograft Model
Male BALB/c nude mice, 4 weeks of age, were purchased from Beijing Vital River Co. (Beijing, P.R. China) and maintained in the Experimental Animal Center of Peking University First Hospital. DLD1 cells (1 × 107 cells) mixed with 50% Matrigel (BD Biosciences) were injected subcutaneously into the right flank of each nude mouse (n = 6/group). The 1,25(OH)2D3 injection method was as described previously8. Tumor volume was measured every 2 days and calculated as width2 × length/2. After 2 weeks, the mice were sacrificed, the tumors were removed, and total mRNA and protein were collected. Animal study was approved by the Medical Ethics Committee of Peking University First Hospital.
Statistical Analysis
Data are presented as mean values ± SEM. Two-tailed Student’s t-test was used for data comparison. Values of p < 0.05 were considered as statistically significant. Each experiment was repeated at least three times independently.
RESULTS
1,25(OH)2D3 Attenuates IL-1β-Induced Increased Proliferation and Invasion in CRC Cells
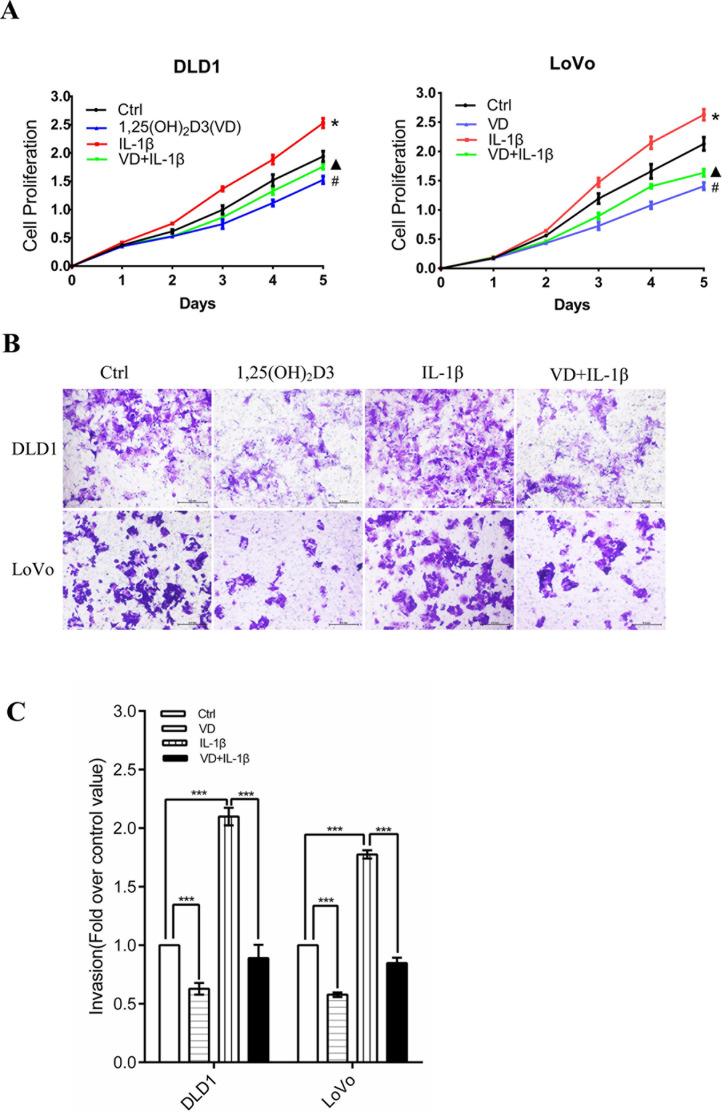
To detect whether 1,25(OH)2D3 and IL-1β affect CRC cell proliferation and invasion, CCK-8 and Transwell assays were performed. CCK-8 assays proved that 1,25(OH)2D3 inhibited the proliferation of DLD1 and LoVo cells, whereas IL-1β facilitated their proliferation (Fig. 1A). Furthermore, 1,25(OH)2D3 also inhibited cell proliferation that was promoted by IL-1β (Fig. 1A). Next, we detected their invasive ability after stimulation by 1,25(OH)2D3 and IL-1β. In a Transwell assay, for both DLD1 and LoVo cell lines, there were 30%–40% decreases in the 1,25(OH)2D3 group compared with the control (Fig. 1B and C). Cell numbers in the IL-1β group increased about twofold (Fig. 1B and C). However, cotreatment with 1,25(OH)2D3 and IL-1β showed that 1,25(OH)2D3 inhibited the effect of IL-1β on cell invasion.
Figure 1.
1,25(OH)2D3 attenuates IL-1β-induced increased proliferation and invasion in colorectal cancer (CRC) cells. Two CRC cell lines, DLD1 and LoVo, were used for the following experiments. (A) Proliferation of CRC cells assessed by CCK-8 assays. 1,25(OH)2D3 (100 nM) inhibits CRC cell proliferation that was promoted by 10 ng/ml of IL-1β. Error bar: mean ± SEM. *, #, ▴ p < 0.05. (B) Cancer cell invasion was measured by Transwell assay. (C) Cells that had moved through the upper chamber were counted. The Ctrl group number was normalized to 1. The values represent the means ± SEM. ***p < 0.001.
1,25(OH)2D3 Inhibits CRC Cell EMT That Was Induced by IL-1β and Promotes the Expression of VDR
Given the effect of 1,25(OH)2D3 and IL-1β on CRC cell proliferation and invasion, we attempted to investigate the underlying molecular mechanisms. Epithelial-to-mesenchymal transition (EMT) plays an important role in cancer metastasis and invasion. VDR is pivotal in the 1,25(OH)2D3 signaling pathway17. Thus, we set out to examine if 1,25(OH)2D3 and IL-1β affect EMT and VDR expression levels.
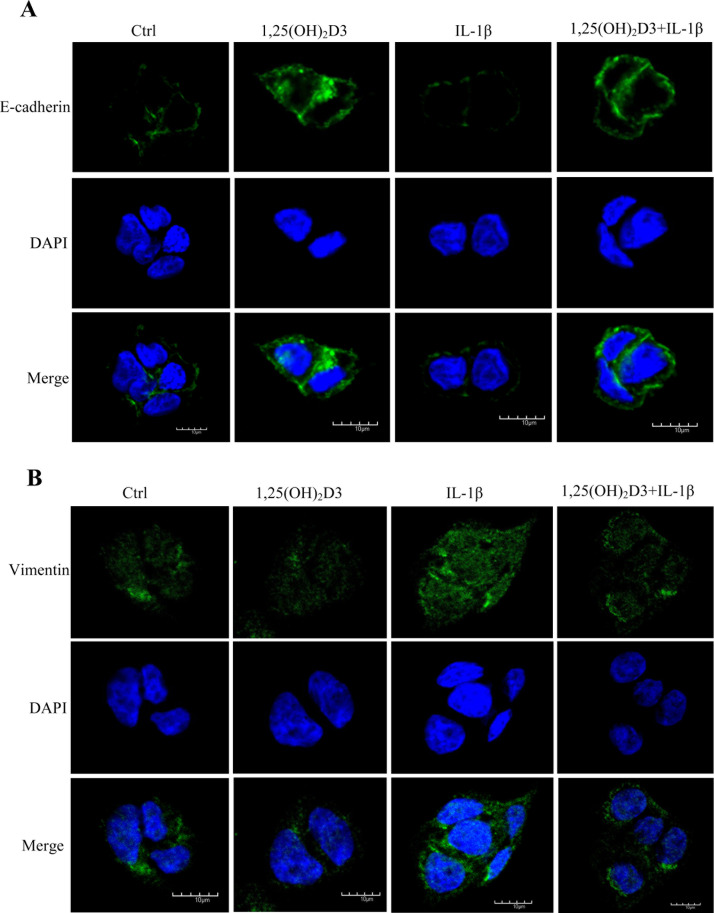
We measured the expression of epithelial and mesenchymal markers by immunofluorescence, qRT-PCR, and Western blot. First, we performed immunofluorescence to detect the expression of E-cadherin and vimentin in DLD1 cells. The results suggested that 1,25(OH)2D3 promoted the expression of E-cadherin and inhibited the expression of vimentin (Fig. 2A and B). IL-1β showed an opposite effect, which could also be inhibited by 1,25(OH)2D3 (Fig. 2A and B).
Figure 2.
1,25(OH)2D3 inhibits CRC cell epithelial-to-mesenchymal transition (EMT) that was induced by IL-1β. (A) Effect of 1,25(OH)2D3 and IL-1β on E-cadherin synthesis in DLD1 cells. DLD1 cells were cultured on slides treated with or without 100 nM 1,25(OH)2D3 in the absence or presence of 10 ng/ml of IL-1β for 48 h before cells were fixed, and immunofluorescence of E-cadherin (green) and nucleus (blue) was performed. (B) Effect of 1,25(OH)2D3 and IL-1β on vimentin synthesis in DLD1 cells. DLD1 cells were cultured on slides treated with or without 100 nM 1,25(OH)2D3 in the absence or presence of 10 ng/ml of IL-1β for 48 h before cells were fixed, and immunofluorescence of vimentin (green) and nucleus (blue) was performed.
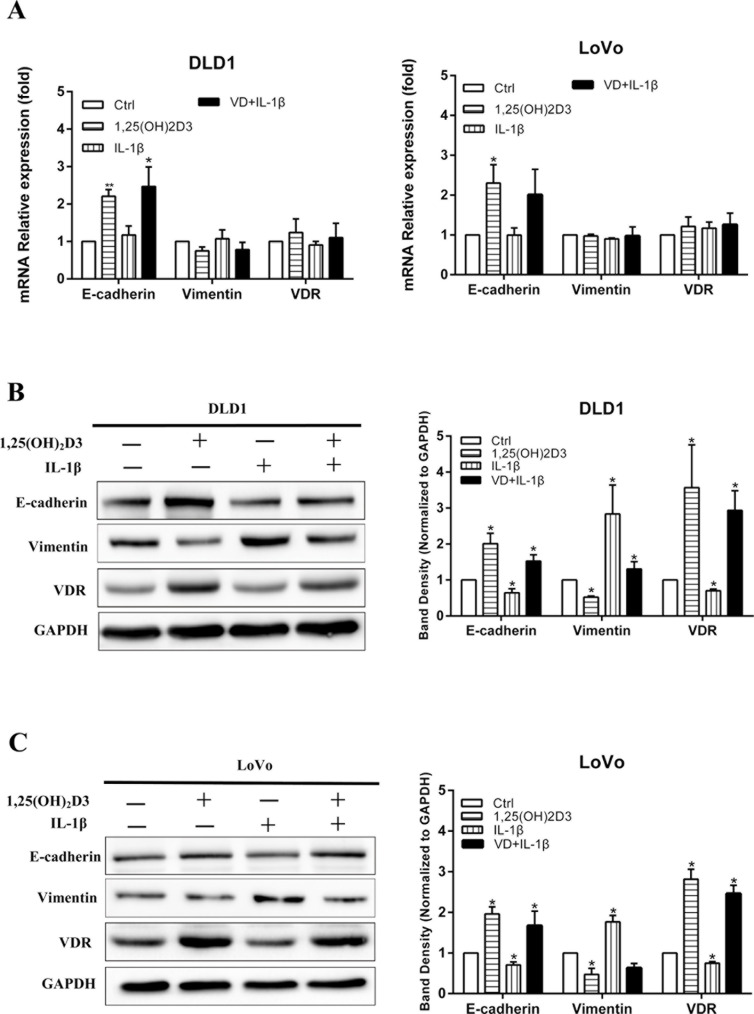
qRT-PCR was performed to detect the mRNA levels of E-cadherin, vimentin, and VDR in DLD1 and LoVo cells after stimulating with 1,25(OH)2D3 and IL-1β. The results showed that 1,25(OH)2D3 increased the expression of E-cadherin but had a nonobvious effect on transcription of vimentin and VDR. On the other hand, IL-1β did not show any influence on E-cadherin, vimentin, or VDR at the transcriptional level (Fig. 3A). Figure 3B and C shows that 1,25(OH)2D3 enhanced the expression of E-cadherin and VDR, but inhibited the expression of vimentin while IL-1β demonstrated opposite effects in CRC cells, and 1,25(OH)2D3 attenuated the effect of IL-1β on EMT and VDR expression.
Figure 3.
1,25(OH)2D3 attenuates IL-1β-induced EMT and increases the expression of vitamin D receptor (VDR). (A) Effect of 1,25(OH)2D3 and IL-1β on mRNA expression of E-cadherin, vimentin, and VDR in DLD1, and LoVo cells were detected by qRT-PCR. β-Actin was used as an internal control. (B, C) The expressions of E-cadherin, vimentin, and VDR in DLD1 and LoVo cells were detected by Western blot after treatment with 1,25(OH)2D3 and IL-1β. GAPDH was used as an internal control. Data represent the mean ± SEM. *p < 0.05, **p < 0.01.
1,25(OH)2D3 Inhibits the Expression of lncTCF7 in CRC Cells
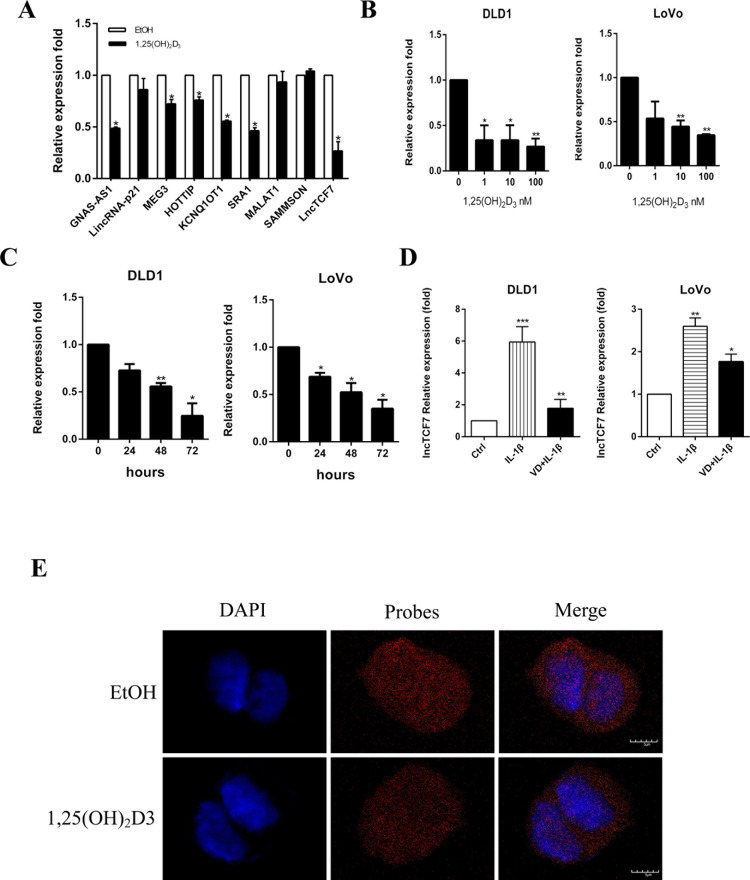
Previous studies have reported that many lncRNAs are involved in cancer progression through the Wnt/β-catenin pathway9,18–20. It has been proven that vitamin D also inhibits colon cancer through the Wnt/β-catenin pathway5. Therefore, we hypothesized that the inhibitory effect of 1,25(OH)2D3 on CRC may be partly through the action of those lncRNAs. We detected the expression level of some lncRNAs in DLD1 cells by qRT-PCR. As demonstrated in Figure 4A, several lncRNAs showed dysregulation after stimulation by 1,25(OH)2D3. Among them, the expression of lncTCF7 changed significantly (Fig. 4A). Moreover, 1,25(OH)2D3 decreased the expression of lncTCF7 in a dose- and time-dependent manner in both DLD1 and LoVo cells (Fig. 4B and C). Because 1,25(OH)2D3 showed the opposite effect with IL-1β, we wondered whether IL-1β could regulate lncTCF7. Figure 4D indicates that IL-1β increased the expression of lncTCF7, and 1,25(OH)2D3 attenuated this effect. To visualize the location of lncTCF7, RNA-FISH was employed. lncTCF7 was localized in both the nuclei and cytoplasm, and more abundant lncTCF7 RNA was seen in the control group compared with the 1,25(OH)2D3 group (Fig. 4E).
Figure 4.
1,25(OH)2D3 inhibits the expression of lncTCF7. (A) Relative mRNA levels of some lncRNAs in DLD1 cells after treatment with 100 nM 1,25(OH)2D3 for 48 h. β-Actin was used as an internal control. (B) The expression level of lncTCF7 in DLD1 and LoVo cells after treatment with increasing doses of 1,25(OH)2D3 for 48 h. β-Actin was used as an internal control. (C) The expression level of lncTCF7 in DLD1 and LoVo cells after treatment with 10 nM 1,25(OH)2D3 for increasing hours. (D) The expression levels of lncTCF7 in DLD1 and LoVo cells were detected by qRT-PCR after treatment with 10 ng/ml IL-1β and 100 nM 1,25(OH)2D3 for 48 h. β-Actin was used as an internal control. (E) LncTCF7 level and localization were studied by RNA-FISH assay on DLD1 cells. After treatment with or without 100 nM 1,25(OH)2D3 for 48 h, DLD1 cells were permeabilized and hybridized with FITC-labeled lncTCF7 probe. Nuclei were stained with DAPI. Red, lncTCF7; blue, nuclei. Scale bar: 5 μm. Data represent the mean ± SEM. *p < 0.05, **p < 0.01, ***p < 0.001.
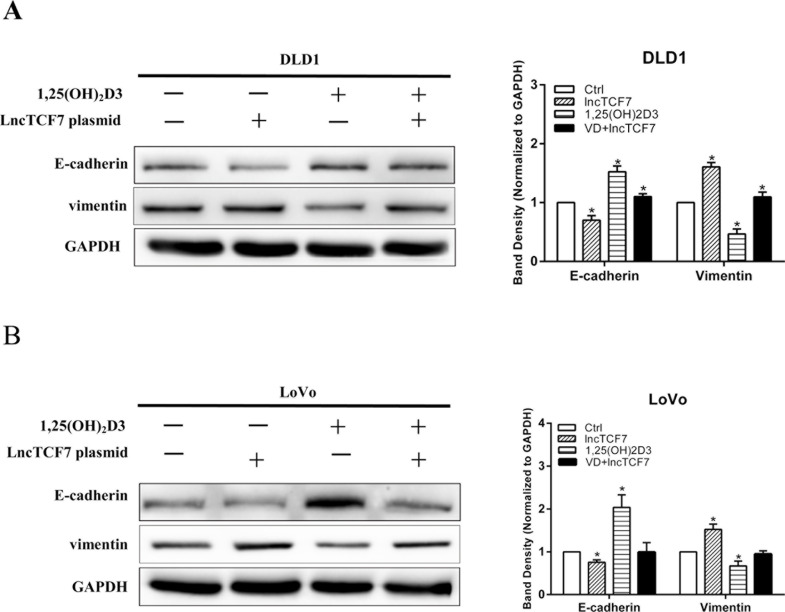
lncTCF7 Facilitates EMT That Was Inhibited by 1,25(OH)2D3
Considering the results that 1,25(OH)2D3 inhibited the expression of lncTCF7, we hypothesized that 1,25(OH)2D3 inhibits EMT in part through decreasing the expression of lncTCF7. Therefore, we overexpressed lncTCF7 by transfecting DLD1 and LoVo cells with lncTCF7 plasmid. Next, the expressions of E-cadherin and vimentin were assayed by Western blot with or without 1,25(OH)2D3 treatment. Compared with the empty plasmid group, E-cadherin expression was reduced and vimentin expression was enhanced in the lncTCF7 group (Fig. 5A and B). We also showed that lncTCF7 partially reversed the expression of E-cadherin and vimentin that was stimulated by 1,25(OH)2D3 (Fig. 5A and B). The results indicate that 1,25(OH)2D3-inhibited EMT was partially reversed by overexpressing lncTCF7.
Figure 5.
lncTCF7 facilitates EMT that was inhibited by 1,25(OH)2D3. DLD1 and LoVo cell lines were transfected with or without lncTCF7 in the absence or presence of 100 nM 1,25(OH)2D3 for 48 h. (A, B) The expressions of E-cadherin and vimentin in DLD1 and LoVo cells were detected by Western blot. GAPDH was used as an internal control. Data represent the mean ± SEM. *p < 0.05.
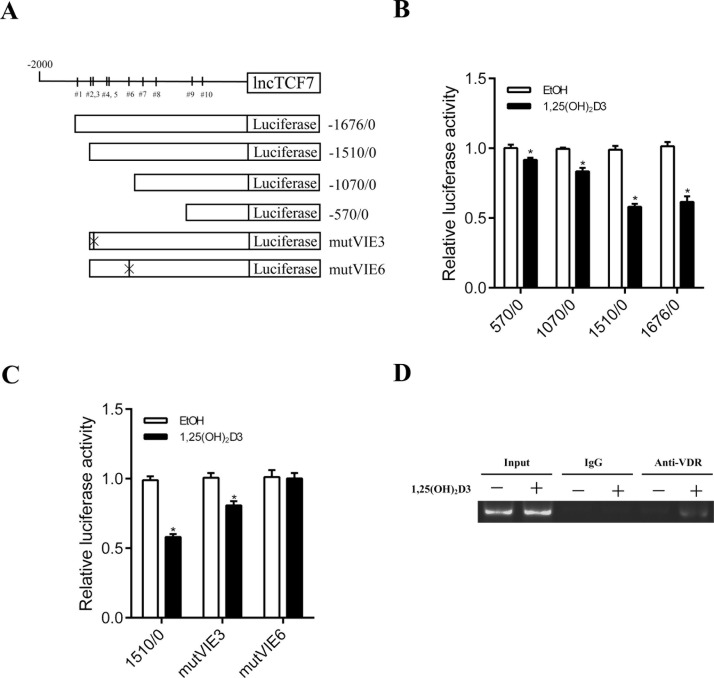
lncTCF7 Is Transcriptionally Inhibited by VDR in Response to 1,25(OH)2D3 Stimulation
To further illuminate how 1,25(OH)2D3 influenced the expression of lncTCF7, we searched for predicted sites that may bind VDR in the lncTCF7 promoter region (http://genome.ufl.edu/mapper). It showed that the promoter region of lncTCF7 harbored 10 potential VDR-inducible elements (VIEs) (Fig. 6A). Luciferase reporter plasmids were constructed with a serial deletion of the lncTCF7 promoter region or mutVIE (Fig. 6A). As shown in Figure 6B, the region between −1510 and −1070 in the lncTCF7 promoter was essential for VDR-mediated regulation. There are five potential VIEs in this region, and we chose two sites with high scores predicting that they are most likely to bind to VDR, VIE3 and VIE6. Mutations in VIE6 markedly increased the reporter activity inhibited by 1,25(OH)2D3 (Fig. 6C). To further prove this finding, a ChIP assay was performed to confirm the interaction between VDR and the promoter region of lncTCF7 in DLD1 cells. ChIP-PCR confirmed that VDR directly bound to the promoter region of lncTCF7 (Fig. 6D). Our study demonstrated that 1,25(OH)2D3 enhanced VDR, which inhibited transcription of lncTCF7 by binding directly to its promoter region.
Figure 6.
lncTCF7 is transcriptionally regulated by VDR in response to 1,25(OH)2D3 stimulation. (A) A schematic representing the 10 predicted VDR binding sites in lncTCF7 promoter region, the reporter construct lncTCF7-Luc, and its truncated and mutated sites. (B) After treatment with 1,25(OH)2D3 for 24 h, transcription activity in DLD1 cells was measured by luciferase assay with a series of deletion mutants of lncTCF7-luc (internal control, pRL-TK). (C) After treatment with 1,25(OH)2D3 for 24 h, relative luciferase activity in DLD1 cells was detected by luciferase assay with VIE mutants of lncTCF7-luc. (D) DLD1 cells stimulated with 100 nM 1,25(OH)2D3 for 24 h was chromatin immunoprecipitated with the specific antibodies. Immunoprecipitated DNAs or chromatin was performed by PCR using a specific primer of the lncTCF7 promoter. Data represent the mean ± SEM. *p < 0.05.
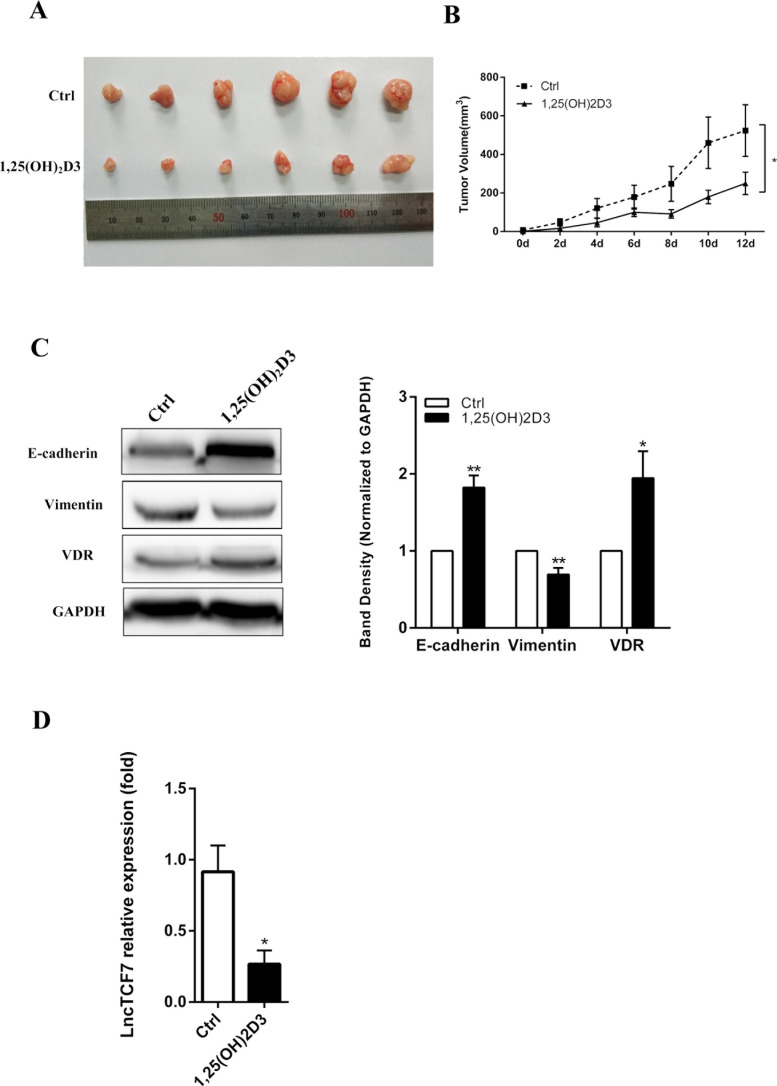
The Effect of the 1,25(OH)2D3–VDR–lncTCF7 Axis In Vivo
Next, we detected whether 1,25(OH)2D3 influenced lncTCF7 in vivo. A total of 1 × 107 DLD1 cells were inoculated into the nude mice. After a palpable tumor could be recognized, 0.5 μg/kg of 1,25(OH)2D3 was injected IP twice a day. As shown in Figure 7A and B, the 1,25(OH)2D3 group led to a significantly smaller tumor volume and slower tumor growth. Then we performed Western blotting to detect whether 1,25(OH)2D3 could regulate EMT-related proteins and VDR. Figure 7C indicates that 1,25(OH)2D3 increased the expression of E-cadherin and VDR and decreased the expression of vimentin. This result proves that 1,25(OH)2D3 inhibited EMT in vivo. Furthermore, the expression of lncTCF7 was detected by qRT-PCR, and there was a significant decrease in the 1,25(OH)2D3 group (Fig. 7D). These data demonstrate the 1,25(OH)2D3–VDR–lncTCF7 axis in vivo.
Figure 7.
The effect of 1,25(OH)2D3–VDR-lncTCF7 axis was detected in vivo. (A) DLD1 line cells were injected subcutaneously into nude mice, (n = 6 for each groups). 1,25(OH)2D3 significantly inhibited the proliferation of DLD1 cells in vivo. (B) Tumor growth curves. Tumor size was calculated every other day after 1,25(OH)2D3 was injected. (C) Total protein of tumors was collected, and Western blotting was performed to investigate the expression of E-cadherin, vimentin, and VDR. (D) Total mRNA of tumors was collected, and qRT-PCR was performed to investigate the expression of lncTCF7. The values represent the means ± SEM. *p < 0.05, **p < 0.01.
DISCUSSION
Research has shown that vitamin D3 and its analogs act as an efficient anticancer agent through several signaling pathways21–23. Searching on the website of GeneCards, we found that calcitriol as one of the vitamin D3 analogs is an IL-1β and TNF-α production inhibitor. IL-1β has been found to promote stem cellness and invasiveness of CRC cells through Zeb1 activation24. Soria et al. reported that coordinated expression of TNF-α and IL-1β had effects on EMT, leading to relapse of breast cancer25. 1,25(OH)2D3 is one of the most activated forms of vitamin D3. Our previous study demonstrated the protective effect of 1,25(OH)2D3 on intestinal epithelial barrier function that was injured by TNF-α26. Therefore, we speculated as to whether 1,25(OH)2D3 will have any impact on IL-1β in CRC. We investigated the proliferation and invasion of CRC cell lines that were stimulated by 1,25(OH)2D3 and IL-1β and found that 1,25(OH)2D3 inhibited IL-1β-induced proliferation and invasion of CRC cells. EMT plays a critical role in tumor progression and invasion27,28. E-cadherin and vimentin are the classical epithelial and mesenchymal markers, respectively29. In previous studies, IL-1β has shown an influence on EMT12. To reveal whether 1,25(OH)2D3 inhibits IL-1β-induced EMT in CRC cells, immunofluorescence analysis, qRT-PCR, and Western blots were performed. As expected, 1,25(OH)2D3 attenuated EMT that was induced by IL-1β.
Vitamin D3 inhibits cancer progression through the VDR pathway, and VDR dysregulation is related to CRC progression and recurrence30. In our previous study, we have found that 1,25(OH)2D3 promoted the expression of VDR in CRC cell lines8. We speculated that 1,25(OH)2D3 attenuates the IL-1β effect, leading to enhancement of the expression of VDR. Therefore, we investigated the expression of VDR after treatment with 1,25(OH)2D3 and IL-1β. Western blots showed that the expression of VDR is decreased after stimulation with IL-1β, but when cotreated with 1,25(OH)2D3 and IL-1β, the expression level of VDR is increased. These results show that 1,25(OH)2D3 inhibits IL-1β’s effect on the expression of VDR, but the mechanism of how 1,25(OH)2D3 attenuates the IL-1β effect on VDR expression needs further research.
It has been reported that lncTCF7 promotes aggressiveness and self-renewal of cancer stem cells in hepatocellular carcinoma31,32. In our previous study, lncTCF7 has shown the ability to promote cell proliferation, migration, and invasion in vitro and tumorigenesis in vivo through activating the Wnt signaling pathway33. Meanwhile, 1,25(OH)2D3 exerts an influence on cancer through the Wnt pathway34. We hypothesized that maybe there was a link between 1,25(OH)2D3 and lncTCF7. We measured the expression of lncTCF7 in CRC cell lines and found that the lncTCF7 level was decreased after stimulation with 1,25(OH)2D3 in dose- and time-dependent manners. lncTCF7 was visualized by RNA-FISH in DLD1 cells, and this showed that 1,25(OH)2D3 downregulated the expression of lncTCF7. We also found that 1,25(OH)2D3 attenuates the effect of IL-1β on lncTCF7 expression.
To further reveal the upstream regulation of lncTCF7, we checked the web (http://genome.ufl.edu/mapper) and found that there are 10 potential sites that may allow VDR to bind to the lncTCF7 promoter region. A luciferase reporter assay and ChIP proved that VDR inhibited the expression of lncTCF7 through directly binding to its promoter region. Furthermore, overexpression of lncTCF7 reversed the inhibition of 1,25(OH)2D3 on EMT in both DLD1 and LoVo cells. In addition, in nude mice inoculation experiments, 1,25(OH)2D3 inhibited cell proliferation and EMT, upregulated VDR expression, and decreased the expression of lncTCF7 in vivo. These results illustrated that 1,25(OH)2D3 inhibits cancer progression partly through decreasing the expression of lncTCF7.
All of the above results prove the existence of a 1,25(OH)2D3–VDR–lncTCF7 signaling axis (Fig. 8) and may provide a novel therapeutic target for colorectal cancer in the future.
Figure 8.
Working model. Shown is a working model depicting how 1,25(OH)2D3 inhibits CRC cell progression. 1,25(OH)2D3 inhibits cancer development through increasing the expression of VDR, which directly bind to the lncTCF7 promoter region. This combination leads to a decreased lncTCF7 level and Wnt depression, thereby repressing CRC cell EMT.
ACKNOWLEDGMENTS
We thank Professor Ding-fang Bu’s excellent technical assistance for RNA-Fish assay and Luciferase reporter assay. This work is supported by the grant on potential therapeutic approaches for surgical infection and the underlying mechanisms from China Health and Medical Development Foundation.
Footnotes
The authors declare no conflicts of interest.
REFERENCES
- 1. Torre L, Bray F, Siegel R, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65(2):87–108. [DOI] [PubMed] [Google Scholar]
- 2. Bikle DD, Jiang Y. The protective role of vitamin D signaling in non-melanoma skin cancer. Cancers (Basel) 2013;5(4):1426–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Okamoto R, Delansorne R, Wakimoto N, Doan NB, Akagi T, Shen M, Ho QH, Said JW, Koeffler HP. Inecalcitol, an analog of 1alpha,25(OH)(2) D(3), induces growth arrest of androgen-dependent prostate cancer cells. Int J Cancer 2012;130(10):2464–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ma Y, Yu WD, Hershberger PA, Flynn G, Kong RX, Trump DL, Johnson CS. 1alpha,25-Dihydroxyvitamin D3 potentiates cisplatin antitumor activity by p73 induction in a squamous cell carcinoma model. Mol Cancer Ther. 2008;7(9):3047–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Pendas-Franco N, Aguilera O, Pereira F, Gonzalez-Sancho JM, Munoz A. Vitamin D and Wnt/beta-catenin pathway in colon cancer: Role and regulation of DICKKOPF genes. Anticancer Res. 2008;28(5a):2613–23. [PubMed] [Google Scholar]
- 6. Deeb KK, Trump DL, Johnson CS. Vitamin D signalling pathways in cancer: Potential for anticancer therapeutics. Nat Rev Cancer 2007;7(9):684–700. [DOI] [PubMed] [Google Scholar]
- 7. Feldman D, Krishnan AV, Swami S, Giovannucci E, Feldman BJ. The role of vitamin D in reducing cancer risk and progression. Nat Rev Cancer 2014;14(5):342–57. [DOI] [PubMed] [Google Scholar]
- 8. Chen S, Bu D, Ma Y, Zhu J, Chen G, Sun L, Zuo S, Li T, Pan Y, Wang X, Liu Y, Wang P. H19 overexpression induces resistance to 1,25(OH)2D3 by targeting VDR through miR-675-5p in colon cancer cells. Neoplasia 2017;19(3):226–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Yang YT, Wang YF, Lai JY, Shen SY, Wang F, Kong J, Zhang W, Yang HY. Long non-coding RNA UCA1 contributes to the progression of oral squamous cell carcinoma by regulating the WNT/beta-catenin signaling pathway. Cancer Sci. 2016;107(11):1581–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Li L, Gu M, You B, Shi S, Shan Y, Bao L, You Y. Long non-coding RNA ROR promotes proliferation, migration and chemoresistance of nasopharyngeal carcinoma. Cancer Sci. 2016;107(9):1215–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jiang YJ, Bikle DD. LncRNA profiling reveals new mechanism for VDR protection against skin cancer formation. J Steroid Biochem Mol Biol. 2014;144(Pt A):87–90. [DOI] [PubMed] [Google Scholar]
- 12. Jiménez-Garduño A, Mendoza-Rodríguez M, Urrutia-Cabrera D, Domínguez-Robles M, Pérez-Yépez E, Ayala-Sumuano J, Meza I. IL-1β induced methylation of the estrogen receptor ERα gene correlates with EMT and chemoresistance in breast cancer cells. Biochem Biophys Res Commun. 2017;490:780–5. [DOI] [PubMed] [Google Scholar]
- 13. Lasry A, Zinger A, Ben-Neriah Y. Inflammatory networks underlying colorectal cancer. Nat Immunol. 2016;17(3):230–40. [DOI] [PubMed] [Google Scholar]
- 14. Apte RN, Voronov E. Is interleukin-1 a good or bad ‘guy’ in tumor immunobiology and immunotherapy? Immunol Rev. 2008;222:222–41. [DOI] [PubMed] [Google Scholar]
- 15. Arima K, Komohara Y, Bu L, Tsukamoto M, Itoyama R, Miyake K, Uchihara T, Ogata Y, Nakagawa S, Okabe H, Imai K, Hashimoto D, Chikamoto A, Yamashita YI, Baba H, Ishimoto T. Down-regulation of 15-PGDH by interleukin-1 beta from activated macrophages leads to poor prognosis in pancreatic cancer. Cancer Sci. 2018;109:462–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mendoza-Rodriguez M, Arevalo Romero H, Fuentes-Panana EM, Ayala-Sumuano JT, Meza I. IL-1beta induces up-regulation of BIRC3, a gene involved in chemoresistance to doxorubicin in breast cancer cells. Cancer Lett. 2017;390:39–44. [DOI] [PubMed] [Google Scholar]
- 17. Verone-Boyle AR, Shoemaker S, Attwood K, Morrison CD, Makowski AJ, Battaglia S, Hershberger PA. Diet-derived 25-hydroxyvitamin D3 activates vitamin D receptor target gene expression and suppresses EGFR mutant non-small cell lung cancer growth in vitro and in vivo. Oncotarget 2016;7(1):995–1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Schmitt AM, Chang HY. Long noncoding RNAs in cancer pathways. Cancer Cell 2016;29(4):452–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Evans JR, Feng FY, Chinnaiyan AM. The bright side of dark matter: lncRNAs in cancer. J Clin Invest. 2016;126(8):2775–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chen LL. Linking long noncoding RNA localization and function. Trends Biochem Sci. 2016;41(9):761–72. [DOI] [PubMed] [Google Scholar]
- 21. Datta Mitra A, Raychaudhuri SP, Abria CJ, Mitra A, Wright R, Ray R, Kundu-Raychaudhuri S. 1alpha,25-Dihydroxyvitamin-D3-3-bromoacetate regulates AKT/mTOR signaling cascades: A therapeutic agent for psoriasis. J Invest Dermatol. 2013;133(6):1556–64. [DOI] [PubMed] [Google Scholar]
- 22. Rosli SN, Shintani T, Hayashido Y, Toratani S, Usui E, Okamoto T. 1alpha,25OH2D3 down-regulates HBp17/FGFBP-1 expression via NF-kappaB pathway. J Steroid Biochem Mol Biol. 2013;136:98–101. [DOI] [PubMed] [Google Scholar]
- 23. Shintani T, Rosli SN, Takatsu F, Choon YF, Hayashido Y, Toratani S, Usui E, Okamoto T. Eldecalcitol (ED-71), an analog of 1alpha,25-dihydroxyvitamin D3 as a potential anti-cancer agent for oral squamous cell carcinomas. J Steroid Biochem Mol Biol. 2016;164:79–84. [DOI] [PubMed] [Google Scholar]
- 24. Li Y, Wang L, Pappan L, Galliher-Beckley A, Shi J. IL-1beta promotes stemness and invasiveness of colon cancer cells through Zeb1 activation. Mol Cancer 2012;11:87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Soria G, Ofri-Shahak M, Haas I, Yaal-Hahoshen N, Leider-Trejo L, Leibovich-Rivkin T, Weitzenfeld P, Meshel T, Shabtai E, Gutman M, Ben-Baruch A. Inflammatory mediators in breast cancer: Coordinated expression of TNFalpha and IL-1beta with CCL2 and CCL5 and effects on epithelial-to-mesenchymal transition. BMC Cancer 2011;11:130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chen S, Zhu J, Chen G, Zuo S, Zhang J, Chen Z, Wang X, Li J, Liu Y, Wang P. 1,25-Dihydroxyvitamin D3 preserves intestinal epithelial barrier function from TNF-alpha induced injury via suppression of NF-kB p65 mediated MLCK-P-MLC signaling pathway. Biochem Biophys Res Commun. 2015;460(3):873–8. [DOI] [PubMed] [Google Scholar]
- 27. Wang Y, Yang T, Zhang Z, Lu M, Zhao W, Zeng X, Zhang W. Long non-coding RNA TUG1 promotes migration and invasion by acting as a ceRNA of miR-335-5p in osteosarcoma cells. Cancer Sci. 2017;108(5):859–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ge Z, Cheng Z, Yang X, Huo X, Wang N, Wang H, Wang C, Gu D, Zhao F, Yao M, Fan J, Qin W. Long noncoding RNA SchLAH suppresses metastasis of hepatocellular carcinoma through interacting with fused in sarcoma. Cancer Sci. 2017;108(4):653–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Jiang FZ, He YY, Wang HH, Zhang HL, Zhang J, Yan XF, Wang XJ, Che Q, Ke JQ, Chen Z, Tong H, Zhang YL, Wang FY, Li YR, Wan XP. Mutant p53 induces EZH2 expression and promotes epithelial-mesenchymal transition by disrupting p68-Drosha complex assembly and attenuating miR-26a processing. Oncotarget 2015;6(42):44660–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Egan JB, Thompson PA, Ashbeck EL, Conti DV, Duggan D, Hibler E, Jurutka PW, Leroy EC, Martinez ME, Mount D, Jacobs ET. Genetic polymorphisms in vitamin D receptor VDR/RXRA influence the likelihood of colon adenoma recurrence. Cancer Res. 2010;70(4):1496–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wu J, Zhang J, Shen B, Yin K, Xu J, Gao W, Zhang L. Long noncoding RNA lncTCF7, induced by IL-6/STAT3 transactivation, promotes hepatocellular carcinoma aggressiveness through epithelial-mesenchymal transition. J Exp Clin Cancer Res. 2015;34:116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wang Y, He L, Du Y, Zhu P, Huang G, Luo J, Yan X, Ye B, Li C, Xia P, Zhang G, Tian Y, Chen R, Fan Z. The long noncoding RNA lncTCF7 promotes self-renewal of human liver cancer stem cells through activation of Wnt signaling. Cell Stem Cell 2015;16(4):413–25. [DOI] [PubMed] [Google Scholar]
- 33. Li T, Zhu J, Wang X, Chen G, Sun L, Zuo S, Zhang J, Chen S, Ma J, Yao Z, Zheng Y, Chen Z, Liu Y, Wang P. Long non-coding RNA lncTCF7 activates the Wnt/beta-catenin pathway to promote metastasis and invasion in colorectal cancer. Oncol Lett. 2017;14(6):7384–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Johnson AL, Zinser GM, Waltz SE. Vitamin D3-dependent VDR signaling delays non-mediated breast tumorigenesis through suppression of beta-catenin activity. Oncotarget 2015;6(18):16304–20. [DOI] [PMC free article] [PubMed] [Google Scholar]