Abstract
MicroRNA-338-3p (miR-338-3p) has been reported to be a tumor suppressor in multiple cancer types. However, the biological role of miR-338-3p and its underlying mechanism in multiple myeloma (MM) remain unclear. In the present study, we investigated the biological role and potential of miR-338-3p in MM. We found that miR-338-3p was significantly decreased in newly diagnosed and relapsed MM tissues and cell lines. Overexpression of miR-338-3p in MM cells significantly inhibited proliferation and promoted apoptosis, caspase 3, and caspase 8 activity. Bioinformatics algorithm analysis predicted that cyclin-dependent kinase 4 (CDK4) was a direct target of miR-338-3p, and this was experimentally verified by a dual-luciferase reporter assay. Furthermore, overexpression of miR-338-3p inhibited CDK4 expression on mRNA and protein levels. Of note, the restoration of CDK4 expression markedly abolished the effect of miR-338-3p overexpression on cell proliferation, apoptosis, caspase 3, and caspase 8 activities in MM cells. Taken together, the present study is the first to demonstrate that miR-338-3p functions as a tumor suppressor in MM through inhibiting CDK4. This finding implies that miR-338-3p is a potential therapeutic target for the treatment of MM.
Key words: Multiple myeloma (MM), MicroRNAs, miR-338-3p, CDK4
INTRODUCTION
Multiple myeloma (MM) is a malignancy of plasma blasts/plasma cells (PCs), characterized by the accumulation of clonal malignant plasma in the bone marrow1. Although there have been advances in diagnosis and treatment in recent years, including proteasome inhibitors, immunomodulatory medicine, and autologous stem cell transplantation, MM remains largely incurable by current therapeutic strategies2,3. Therefore, there is an urgent need to develop new targeted therapeutic approaches for the treatment of MM.
MicroRNAs (miRNAs) are a class of small (18–25 nucleotides in length), noncoding RNAs that function as negative regulators of protein-coding genes by binding with complementary sequences within the 3′-untranslated region (3′-UTR) of the target mRNA to induce mRNA degradation or translational repression4. Increasing evidence has confirmed that miRNAs play crucial roles in various biological processes, such as embryonic development, hematopoiesis, inflammation regulation, immune responses, and tumorigenesis5,6. miRNAs are known to be involved in the initiation, development, and progression of many cancers through the regulation of oncogenes or tumor suppressors7,8. Aberrant miRNAs have been reported to contribute to the development and progression of MM9,10, thus eliciting interest for these molecules as diagnosis markers and antitumor therapeutic agents in MM diagnosis and treatment.
miR-338-3p has been reported to be downregulated and to function as a tumor-suppressive miRNA in several types of cancers11–17. Nevertheless, the biological function of miR-338-3p and the molecular mechanisms in MM are still unclear. Therefore, the aims of this study were to investigate the expression and biological role of miR-338-3p in MM progression and to explore the potential molecular mechanism of miR-338-3p in MM development.
MATERIALS AND METHODS
Sample Collection
Twenty-eight MM tissues and 15 healthy donor samples were harvested at the Second Affiliated Hospital of Jilin University during January 2014 to December 2016. All patients were diagnosed according to the World Health Organization diagnostic criteria for MM. The normal PCs (nPCs) from healthy donors were used as controls. This study was performed with written informed consent from the study participants and the approval of the Ethics Committee of Jilin University (Changchun, P.R. China), based on the Declaration of Helsinki, before collection of the specimens.
Cell Culture and Transfection
Four human MM cell lines (NCI-H929, MM1S, U266, and RPMI-8266) were brought from the Cell Bank of the Chinese Academy of Sciences (Shanghai, P.R. China). All cell lines and the nPCs were grown in RPMI-1640 (Gibco, Carlsbad, CA, USA) supplemented with 10% FBS (Invitrogen, Carlsbad, CA, USA) and 1% penicillin–streptomycin (Sigma-Aldrich, St. Louis, MO, USA) under a humidified atmosphere at 37°C with 5% CO2.
miR-338-3p mimic or corresponding negative control (miR-NC) was purchased from Genechem (Shanghai, P.R. China). Cyclin-dependent kinase 4 (CDK4) code sequence (without 3′-UTR) was amplified by PCR and inserted into pCDNA3.1 vector (Invitrogen) and named as pcDNA3.1-CDK4, which was used as the CDK4 overexpression plasmid. MM1S cells were seeded at a concentration of 2 × 105 per well in 24-well plates for 24 h, and then transfected with the indicated nucleotides or plasmids using Lipofectamine 2000 (Invitrogen) according to the manufacturer’s instructions.
Quantitative Reverse Transcriptase PCR
Total RNA was isolated from MM tissues and cultured cells using TRIzol reagent (Invitrogen) according to the manufacturer’s protocol. Two micrograms of total RNA was used for reverse transcription using a Reverse Transcription Kit (Takara Biochemical, Tokyo, Japan) in an ABI-7900HT machine following the manufacturer’s instructions. Hsa-miR-338-3p primer and U6 snRNA qPCR primer were purchased from Genecopoeia (Guangzhou, P.R. China). The primers of CDK4 and GAPDH were used in this study as described previously18. The relative expression of miR-338-3p and CDK4 mRNA was normalized to U6 snRNA or GAPDH and calculated using the 2−ΔΔCt method.
Cell Proliferation, Colony Formation, Cell Cycle, and Apoptosis Assays
For the proliferation assay, transfected cells were seeded in a 96-well plate at a density of 5 × 103 cells/well in 100-μl volume and cultured for 24–72 h. At the indicated times (24, 48, and 72 h), cell counting kit-8 (CCK-8) reagents (Dojindo, Kumamoto, Japan) were added to each well, and the cells were cultured for an additional 4 h. The OD value at 450 nm was measured using a microplate reader (BioTek, Winooski, VT, USA).
For cell cycle assay, the transfected cells were harvested and seeded in six-well plates at a concentration of 2 × 105/well. The cells were fixed in 70% ethanol at 4°C overnight and then stained with 50 μg/ml propidium iodide (Keygen, Nanjing, P.R. China). Cell cycle arrest was analyzed using fluorescence-activated cell sorting FACSCalibur) and Cell Quest software (both from Becton-Dickinson, San Jose, CA, USA).
Cell apoptosis was determined using an Annexin-V-Fluos Staining Kit (Roche, Basel, Switzerland) in the FACSCalibur. Cell apoptosis ratio was analyzed by the Cell Quest software.
Bioinformatics Prediction and Dual-Luciferase Reporter Assay
Three miRNA databases (TargetScan, Pictar, and Miranda) were used to predict the putative target genes of miR-338-3p. The 3′-UTR sequence of CDK4 predicted to bind with miR-338-3p, together with a corresponding mutated sequence within the predicted target sites, was synthesized and inserted into the pGL3 dual-luciferase expression vector (Promega, Madison, WI, USA) and called WT-CDK4-3′UTR and MT-CDK4-3′UTR, respectively. Subsequently, MM1S cells were plated into 24-well plates and transfected with miR-338-3p mimic or miR-NC mimic, together with WT-CDK4-3′UTR or MT-CDK4-3′UTR vector using the Lipofectamine 2000 reagent (Invitrogen). After 48 h, cells were harvested, and luciferase activity was measured using a Dual-Luciferase Reporter Assay System (Promega) according to the manufacturer’s instructions. pRL-TK expressing Renilla luciferase was cotransfected as an internal control.
Western Blot
Whole proteins were lysed in RIPA buffer (Beyotime, Jiangsu, P.R. China) supplemented with protease and phosphatase inhibitors (Beyotime). The protein concentrations were quantified with BCA Protein Assay Kit (Tiangen, Beijing, P.R. China). An equal amount of 40 μg of protein was separated by 10% SDS-PAGE and transferred to nitrocellulose membranes (Amersham Bioscience, Piscataway, NJ, USA). Membranes were incubated with primary antibody against CDK4 and GAPDH (both from Santa Cruz Biotechnology, Santa Cruz, CA, USA) overnight at 4°C, followed by incubation with HRP-conjugated goat anti-mouse IgG (Santa Cruz Biotechnology) at 1:5,000 dilution. The protein bands were detected using an enhanced chemiluminescence detection reagent (ECL; Thermo Scientific, Rockford, IL, USA) and were quantified using Quantity One software 4.5.0 basic (Bio-Rad, Hercules, CA, USA).
Statistical Analysis
All the statistical data are shown as mean ± standard deviation (SD) of at least three independent experiments. All statistical analyses were performed with SPSS 19.0 software (SPSS, Inc., Chicago, IL, USA). The difference between two groups was analyzed with unpaired Student’s t-test. One-way ANOVA was applied for three groups. Difference was considered significant with a value of p < 0.05 or p < 0.01.
RESULTS
miR-338-3p Was Significantly Decreased in MM Samples and Cell Lines
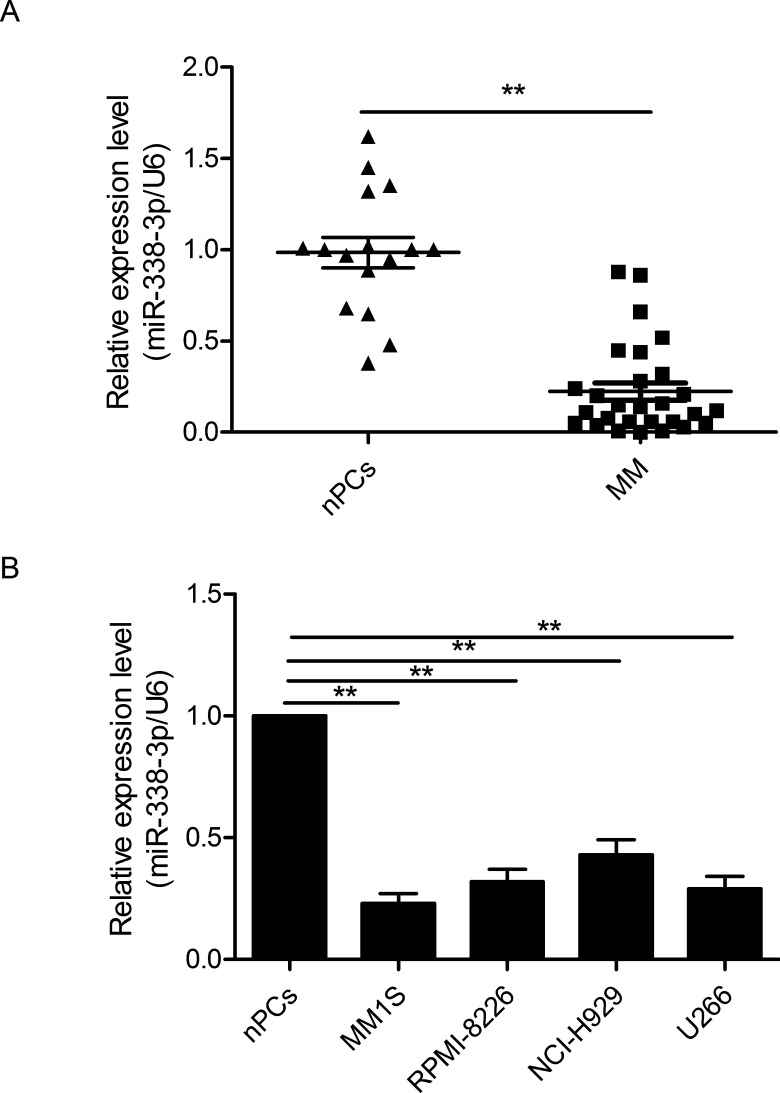
To investigate the expression status of miR-338-3p in MM, we first performed RT-qPCR to determine the miR-338-3p expression in 28 diagnosed MM samples and 15 healthy donors’ nPCs. The data showed that the mean level of miR-338-3p in MM tissues was significantly higher than that in nPCs (p < 0.01) (Fig. 1A). Moreover, we assessed miR-338-3p expression in four MM cell lines and nPCs. As shown in Figure 1B, all MM cell lines (NCI-H929, MM1S, U266, and RPMI-8266) exhibited high expression compared with nPCs (p < 0.01). These results indicated that miR-338-3p may be involved in the progression of MM.
Figure 1.
MicroRNA-338-3p (miR-338-3p) is downregulated in multiple myeloma (MM) tissues and cell lines. (A) Relative miR-338-3p expression levels in 25 MM tissues and normal plasma cells of 18 healthy donors [normal plasma blasts/plasma cell (nPCs)] were determined by quantitative reverse transcriptase PCR (RT-qPCR). (B) The expression of miR-338-3p in four MM cell lines was significantly decreased compared to that in the nPCs. U6 snRNA was used as an internal control. **p < 0.01.
miR-338-3p Inhibits Cell Proliferation and Cell Cycle Progression and Induces Cell Apoptosis
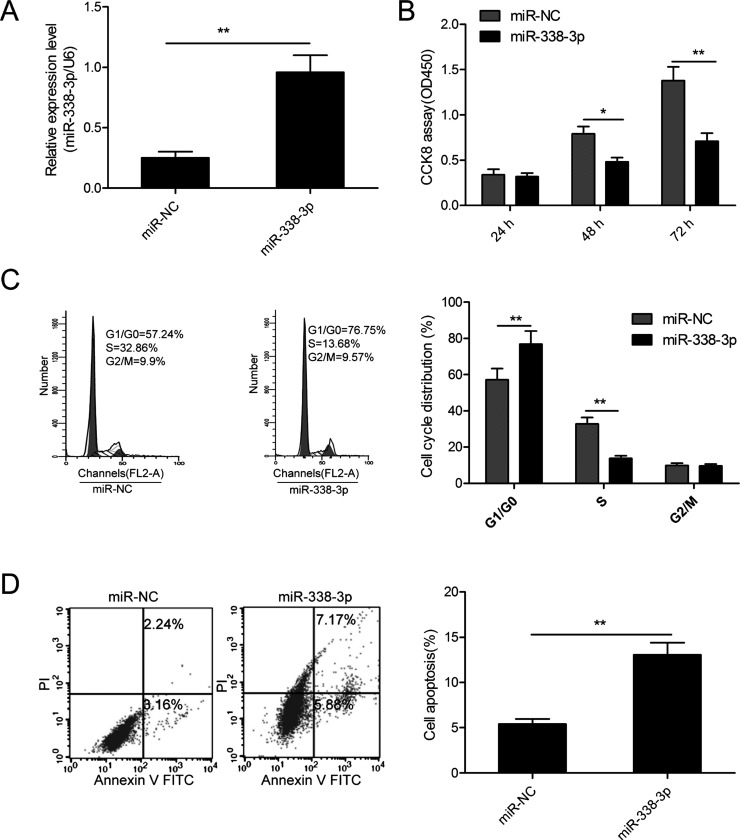
To investigate the potential role of miR-338-3p in MM, MM1S cells were transfected with miR-338-3p mimic or miR-NC to restore miR-338-3p expression. As measured by RT-qPCR, we found that miR-338-3p expression was effectively upregulated in MM1S cells after transfection with miR-338-3p mimic compared to cells transfected with miR-NC (Fig. 2A). The CCK-8 assay showed that restoration of miR-338-3p significantly inhibited the cell proliferation of MM1S cells (Fig. 2B). Since the cell cycle is closely associated with proliferation, we also investigated the effect of miR-338-3p on the cell cycle in MM1S cells. We found that restoration of miR-338-3p significantly increased the proportion of cells in the G0/G1 phase and reduced the proportion of the S phase (Fig. 2C). Next, we detected cell apoptosis in MM1S cells transfected with miR-338-3p mimic or miR-NC. As determined by flow cytometric analysis, the overexpression of miR-338-3p significantly promoted the cell apoptosis ratio in MM1S cells (Fig. 2D). These results implied that miR-338-3p functioned as a tumor suppressor in MM.
Figure 2.
miR-338-3p inhibits cell proliferation and cell cycle progression and induces cell apoptosis. (A) Relative miR-338-3p expression levels in MM1S cells transfected with miR-338-3p mimic or corresponding negative control (miR-NC). (B) Cell proliferation, (C) cycle progression, and (D) apoptosis were determined in MM1S cells transfected with miR-338-3p mimic or miR-NC. *p < 0.05; **p < 0.01.
CDK4 Is a Direct Target Gene of miR-338-3p in MM Cells
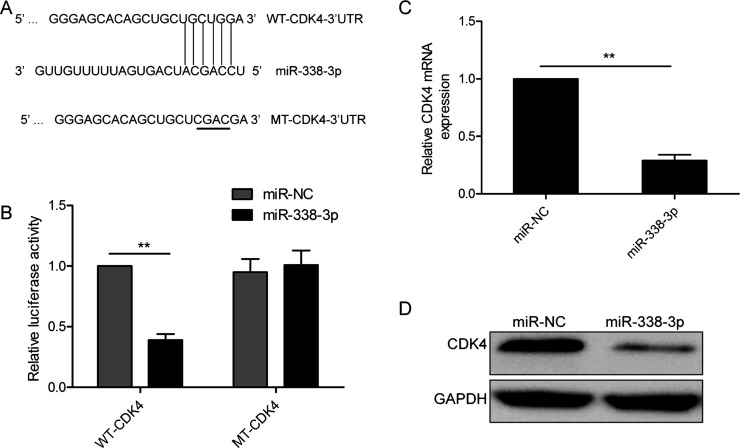
To explore the mechanism of miR-338-3p in MM progression, we used three miRNA databases (TargetScan, Pictar, and Miranda) to search for candidate targets of miR-338-3p and found a putative miR-338-3p-binding site located in the 3′-UTR of CDK4 mRNA (Fig. 3A). To confirm whether miR-338-3p directly binds to CDK4, a luciferase reporter assay was performed to confirm that miR-338-3p could bind to the 3′-UTR of CDK4. Reporter assays revealed that the overexpression of miR-338-3p obviously inhibited the luciferase activity of wild-type (WT) CDK4 3′-UTR, but had no influence on that of mutant (MT) CDK4 3′-UTR (p > 0.05) (Fig. 3B). Furthermore, we found that miR-338-3p overexpression markedly suppressed the mRNA and protein expressions of CDK4 in MM1S cells (Fig. 3C and D).
Figure 3.
Cyclin-dependent kinase 4 (CDK4) is a direct target gene of miR-338-3p in MM cells. (A) miR-338-3p and its putative binding site on the wild-type (WT) and mutant (MT) CDK4 3′-UTR. The replaced site is underlined. (B) Relative dual-luciferase activity in MM1S cells cotransfected with WT-CDK4-3′UTR or MT-CDK4-3′UTR and miR-338-3p mimic or miR-NC. CDK4 expression on (C) mRNA and (D) protein levels was determined in MM1S cells transfected with miR-338-3p mimic or miR-NC. GAPDH was used as an internal control. **p < 0.01.
miR-338-3p Expression Was Inversely Correlated With CDK4 in MM Tissues
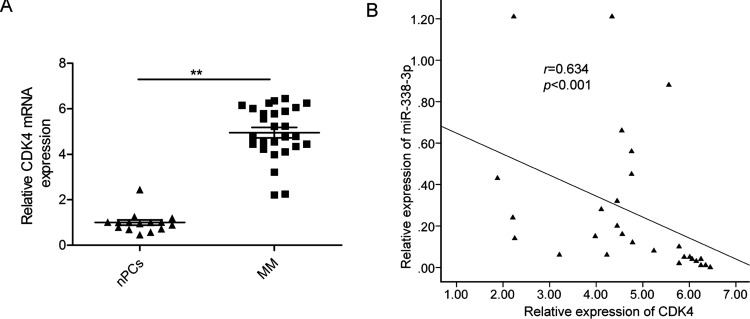
To further investigate the relationship between miR-338-3p and CDK4, we first examined the CDK4 mRNA expression level in MM tissues and healthy donors’ nPCs by RT-qPCR. We found that CDK4 mRNA level was upregulated in MM tissues compared with nPCs (Fig. 4A). Furthermore, we found that the CDK4 mRNA level in the MM tissues was inversely correlated with miR-338-3p expression (p < 0.05) (Fig. 4B).
Figure 4.
miR-338-3p expression was inversely correlated with CDK4 in MM tissues. GAPDH was used as an internal control. (A) Relative CDK4 mRNA expression levels in MM tissues and nPCs were determined by RT-qPCR. p < 0.05; **p < 0.01. (B) The correlation between the expression levels of CDK4 and miR-338-3p was observed in 25 human MM tissues (n = 48).
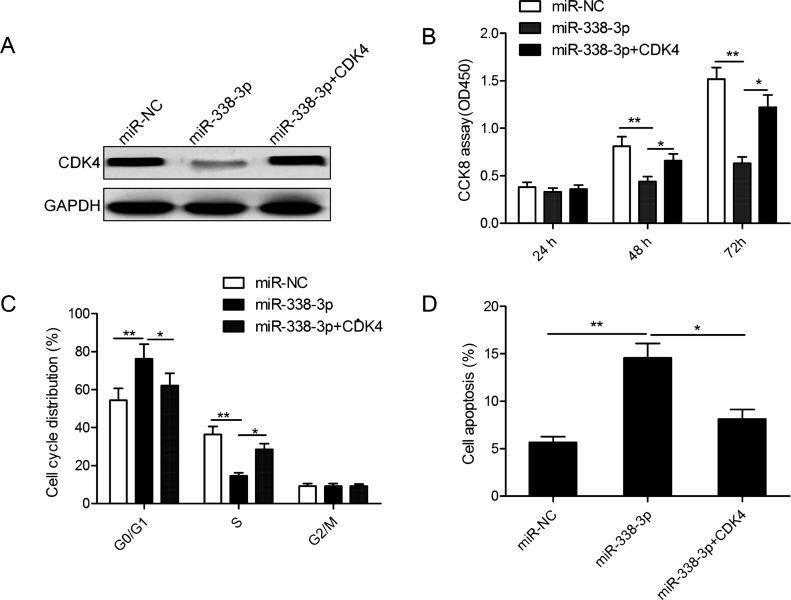
Overexpression of CDK4 Partially Rescued the miR-338-3p-Induced Biological Effects on MM Cells
To further confirm whether miR-338-3p exerted its biological function by targeting CDK4, we restored CDK4 expression by transfection with the overexpression construct plasmid (pCDNA3.1-CDK4) in miR-338-3p-overexpressing MM1S cells (Fig. 5A). In addition, our data demonstrated that CDK4 restoration partially abrogated the effect of miR-338-3p, resulting in a significant decrease in proliferation, and promoted G1 phase arrest and apoptosis in miR-338-3p-overexpressing MM1S cells (Fig. 5B–D).
Figure 5.
Overexpression of CDK4 partially rescued the miR-338-3p-induced biological effects on MM cells. (A) The CDK4 protein expression was detected in MM1S cells cotransfected with either miR-338-3p or miR-NC and with/without CDK4 overexpression vector. (B) Cell proliferation, (C) cell cycle progression, and (D) apoptosis were determined in MM1S cells cotransfected with either miR-338-3p or miR-NC and with/without CDK4 overexpression vector. *p < 0.05; **p < 0.01.
DISCUSSION
Numerous miRNAs have been reported to be involved in MM initiation, development, and progression, suggesting that miRNAs could be used as a valuable diagnostic and prognostic biomarker and be attractive therapeutic targets of MM. For example, Yang et al. reported that miR-410 promoted cell proliferation and cell cycle progression and inhibited cell apoptosis both in vitro and in vivo by targeting KLF1019. Yu et al. showed that ectopic expression of miR-497 in MM cells dramatically suppressed cell proliferation and clonogenicity and induced cell arrest at the G0/G1 stage and apoptosis in vitro, as well as tumor growth in vivo by regulating PBX320. Zhao et al. showed that miR-144-3p overexpression significantly inhibited MM growth in vitro and in vivo by regulating c-MET21. The present study verified that miR-338-3p expression was downregulated in MM tissues and cell lines. The in vitro experiments showed that restoration of miR-338-3p repressed the cell proliferation and cell cycle progression, as well as promoted cell apoptosis. To explore the underlying mechanisms, we identified CDK4 as a target of miR-338-3p in MM cells. These results suggested that miR-338-3p might be a potential target of MM.
miR-338-3p, located on the seventh intron of the apoptosis-associated tyrosine kinase gene, was originally found to contribute to basolateral polarity formation in the epithelial cells22. It has been shown that miR-338-3p expression was downregulated and functioned as a tumor suppressor in non-small lung cancer11, ovarian cancer12, hepatocellular carcinoma14, gastric cancer13, thyroid cancer15, colorectal cancer17, and breast cancer16. However, the biological function of miR-338-3p and its related mechanism involved in the progression of MM have not been fully elucidated. The present study demonstrated that the expression of miR-338-3p was significantly downregulated in MM tissues and cell lines compared with the nPCs. Function assays demonstrated that overexpression of miR-338-3p significantly inhibited cell proliferation and cycle progression and promoted cell apoptosis of MM cells. These results indicated that miR-338-3p may serve as a tumor suppressor in MM.
It is well known that miRNAs exert biological functions by regulating their target genes23. To explore the molecular mechanisms responsible for the growth inhibitory effects of miR-338-3p in MM, we first used three bioinformatics analysis tools (TargetScan, Pictar, and Miranda) to predict miR-338-3p target genes. Among the candidate target genes, we focused on CDK4, an oncogene, which was reportedly overexpressed in MM tissues24, as a potential target of miR-338-3p. CDK4 could regulate the cell cycle in the G1/S stage by binding with cyclin D125,26. It has been shown that CDK4 expression is significantly upregulated in MM tissues, and downregulation of CDK4 by its inhibitor impaired tumor growth of MM27,28. CDK4 has been reported to be a target of miR-338-3p in hepatic stellate cells18; however, the interaction between miR-338-3p and CDK4 has not been experimentally validated in MM. In the present study, we demonstrated that miR-338-3p negatively regulates CDK4 by binding the 3′-UTR of CDK4 using a luciferase reporter assay. Also, we found that overexpression of miR-338-3p in human MM1S cells significantly inhibited CDK4 expression at both the mRNA and protein levels. These results suggest that CDK4 is a target gene in MM, which is in accordance with previous studies that demonstrated miR-338-3p targets CDK4 in hepatic stellate cells18. Moreover, our results showed that CDK4 mRNA expression was upregulated and inversely correlated with miR-338-3p in MM tissues. We also demonstrated that CDK4 restoration partially abrogated the effect of miR-338-3p, resulting in a significant decrease in proliferation, and promoted the G1 phase arrest and apoptosis in miR-338-3p-overexpressing MM1S cells. These results indicated that miR-338-3p may exert an anticancer role in MM cells by targeting CDK4.
Collectively, the present study provided evidence that miR-338-3p expression was downregulated in MM tissues and cell lines and that miR-338-3p was able to inhibit cell proliferation and cell progression, as well as promote cell apoptosis by repressing CDK4. These results suggested that miR-338-3p might be a novel therapeutic target for the treatment of patients with advanced MM.
ACKNOWLEDGMENT
The authors declare no conflicts of interest.
Footnotes
The authors declare no conflicts of interest.
REFERENCES
- 1. Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63(1):11–30. [DOI] [PubMed] [Google Scholar]
- 2. Keats JJ, Chesi M, Egan JB, Garbitt VM, Palmer SE, Braggio E, Van Wier S, Blackburn PR, Baker AS, Dispenzieri A, Kumar S, Rajkumar SV, Carpten JD, Barrett MT, Fonseca R, Stewart AK, Bergsagel PL. Clonal competition with alternating dominance in multiple myeloma. Blood 2012;120(5):1067–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Palumbo A, Anderson K. Multiple myeloma. N Engl J Med. 2011;364(11):1046–60. [DOI] [PubMed] [Google Scholar]
- 4. Bartel DP. MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell 2004;116(2):281–97. [DOI] [PubMed] [Google Scholar]
- 5. Malan-Muller S, Hemmings SM, Seedat S. Big effects of small RNAs: A review of microRNAs in anxiety. Mol Neurobiol. 2013;47(2):726–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. McManus MT. MicroRNAs and cancer. Semin Cancer Biol. 2003;13(4):253–8. [DOI] [PubMed] [Google Scholar]
- 7. Farazi TA, Spitzer JI, Morozov P, Tuschl T. miRNAs in human cancer. J Pathol. 2011;223(2):102–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Garzon R, Marcucci G. Potential of microRNAs for cancer diagnostics, prognostication and therapy. Curr Opin Oncol. 2012;24(6):655–9. [DOI] [PubMed] [Google Scholar]
- 9. Abdi J, Qiu L, Chang H. Micro-RNAs, new performers in multiple myeloma bone marrow microenvironment. Biomark Res. 2014;2:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Yang YZ, Zhang XY, Wan Q, Li J. [Role of Exosomal miRNA in multiple myeloma progression and its possible mechanism—Review]. Zhongguo Shi Yan Xue Ye Xue Za Zhi 2017;25(1):301–5. [DOI] [PubMed] [Google Scholar]
- 11. Zhang P, Shao G, Lin X, Liu Y, Yang Z. MiR-338-3p inhibits the growth and invasion of non-small cell lung cancer cells by targeting IRS2. Am J Cancer Res. 2017;7(1):53–63. [PMC free article] [PubMed] [Google Scholar]
- 12. Zhang Y, Shi B, Chen J, Hu L, Zhao C. MiR-338-3p targets pyruvate kinase M2 and affects cell proliferation and metabolism of ovarian cancer. Am J Transl Res. 2016;8(7):3266–73. [PMC free article] [PubMed] [Google Scholar]
- 13. Chen JT, Yao KH, Hua L, Zhang LP, Wang CY, Zhang JJ. MiR-338-3p inhibits the proliferation and migration of gastric cancer cells by targeting ADAM17. Int J Clin Exp Pathol. 2015;8(9):10922–8. [PMC free article] [PubMed] [Google Scholar]
- 14. Nie H, Li J, Yang XM, Cao QZ, Feng MX, Xue F, Wei L, Qin W, Gu J, Xia Q, Zhang ZJ. Mineralocorticoid receptor suppresses cancer progression and the Warburg effect by modulating the miR-338-3p-PKLR axis in hepatocellular carcinoma. Hepatology 2015;62(4):1145–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sui GQ, Fei D, Guo F, Zhen X, Luo Q, Yin S, Wang H. MicroRNA-338-3p inhibits thyroid cancer progression through targeting AKT3. Am J Cancer Res. 2017;7(5):1177–87. [PMC free article] [PubMed] [Google Scholar]
- 16. Jin Y, Zhao M, Xie Q, Zhang H, Wang Q, Ma Q. MicroRNA-338-3p functions as tumor suppressor in breast cancer by targeting SOX4. Int J Oncol. 2015;47(4):1594–602. [DOI] [PubMed] [Google Scholar]
- 17. Xue Q, Sun K, Deng HJ, Lei ST, Dong JQ, Li GX. MicroRNA-338-3p inhibits colorectal carcinoma cell invasion and migration by targeting smoothened. Jpn J Clin Oncol. 2014;44(1):13–21. [DOI] [PubMed] [Google Scholar]
- 18. Duan B, Hu J, Zhang T, Luo X, Zhou Y, Liu S, Zhu L, Wu C, Liu W, Chen C, Gao H. miRNA-338-3p/CDK4 signaling pathway suppressed hepatic stellate cell activation and proliferation. BMC Gastroenterol. 2017;17(1):12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Yang N, Chen J, Zhang H, Wang X, Yao H, Peng Y, Zhang W. LncRNA OIP5-AS1 loss-induced microRNA-410 accumulation regulates cell proliferation and apoptosis by targeting KLF10 via activating PTEN/PI3K/AKT pathway in multiple myeloma. Cell Death Dis. 2017;8(8):e2975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Yu T, Zhang X, Zhang L, Wang Y, Pan H, Xu Z, Pang X. MicroRNA-497 suppresses cell proliferation and induces apoptosis through targeting PBX3 in human multiple myeloma. Am J Cancer Res. 2016;6(12):2880–9. [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 21. Zhao Y, Xie Z, Lin J, Liu P. MiR-144-3p inhibits cell proliferation and induces apoptosis in multiple myeloma by targeting c-Met. Am J Transl Res. 2017;9(5):2437–46. [PMC free article] [PubMed] [Google Scholar]
- 22. Tsuchiya S, Oku M, Imanaka Y, Kunimoto R, Okuno Y, Terasawa K, Sato F, Tsujimoto G, Shimizu K. MicroRNA-338-3p and microRNA-451 contribute to the formation of basolateral polarity in epithelial cells. Nucleic Acids Res. 2009;37(11):3821–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Cora D, Re A, Caselle M, Bussolino F. MicroRNA-mediated regulatory circuits: Outlook and perspectives. Phys Biol. 2017;14(4):045001. [DOI] [PubMed] [Google Scholar]
- 24. Urashima M, Teoh G, Ogata A, Chauhan D, Treon SP, Hoshi Y, DeCaprio JA, Anderson KC. Role of CDK4 and p16INK4A in interleukin-6-mediated growth of multiple myeloma. Leukemia 1997;11(11):1957–63. [DOI] [PubMed] [Google Scholar]
- 25. Mayer EL. Inhibition of cyclin-dependent kinases 4 and 6 in breast cancer. Clin Adv Hematol Oncol. 2015;13(4):215–7. [PubMed] [Google Scholar]
- 26. Basso AD, Doll RJ. Inhibition of cyclin-dependent kinases—A review of the recent patent literature. Recent Pat Anticancer Drug Discov. 2006;1(3):357–67. [DOI] [PubMed] [Google Scholar]
- 27. Baughn LB, Di Liberto M, Wu K, Toogood PL, Louie T, Gottschalk R, Niesvizky R, Cho H, Ely S, Moore MA, Chen-Kiang S. A novel orally active small molecule potently induces G1 arrest in primary myeloma cells and prevents tumor growth by specific inhibition of cyclin-dependent kinase 4/6. Cancer Res. 2006;66(15):7661–7. [DOI] [PubMed] [Google Scholar]
- 28. Ely S, Di Liberto M, Niesvizky R, Baughn LB, Cho HJ, Hatada EN, Knowles DM, Lane J, Chen-Kiang S. Mutually exclusive cyclin-dependent kinase 4/cyclin D1 and cyclin-dependent kinase 6/cyclin D2 pairing inactivates retinoblastoma protein and promotes cell cycle dysregulation in multiple myeloma. Cancer Res. 2005;65(24):11345–53. [DOI] [PubMed] [Google Scholar]