Abstract
miR-150 has been demonstrated to inhibit tumor progression in various human cancers, including colorectal cancer, ovarian cancer, and thyroid cancer. However, the role of miR-150 in melanoma remains to be determined. In this study, we found that miR-150 was underexpressed in melanoma tissues and cell lines. Through transfection of miR-150 mimics, we found that miR-150 significantly inhibited the proliferation, migration, and invasion of melanoma cells. In mechanism, we found that MYB was a target of miR-150 in melanoma cells. Overexpression of miR-150 significantly inhibited mRNA and protein levels of MYB in melanoma cells. Moreover, there was an inverse correlation between the expression of miR-150 and MYB in melanoma tissues. We also showed that MYB was upregulated in melanoma tissues and cell lines. Through functional experiments, we found that restoration of MYB in miR-150-overexpressed melanoma cells rescued the proliferation, migration, and invasion. Therefore, our findings demonstrated that miR-150 suppressed the proliferation, migration, and invasion of melanoma cell by downregulating MYB.
Key words: miR-150, MYB, Proliferation, Invasion, Melanoma
INTRODUCTION
Melanoma is one of the most malignant skin cancers and contributes to a large proportion of skin cancer-related deaths around the world each year1,2. Although some advance has been achieved on the treatment of melanoma in past years, the incidence rate and the mortality of melanoma patients are still very high and increasing every year. Because of tumor metastasis, the outcome and 5-year survival rate of melanoma patients are quite poor3. Therefore, there is an urgent need to explore the underlying molecular mechanism of melanoma progression.
MicroRNAs (miRNAs) are a class of short noncoding RNA molecules that have a length of about 22 nucleotides4. miRNAs are shown to regulate gene expression through binding to the complementary sites of the 3′-UTR of target mRNAs4. miRNAs are widely expressed in all kinds of tissues or cell types. Increasing evidence shows that miRNAs exert important functions in a diversity of biological processes, such as cell survival, apoptosis, and metastasis5,6. Because of their key functions, miRNA expression is closely related with human cancers including melanoma7. Many miRNAs are dysregulated in cancers. Accumulating reports indicate that miRNAs are promising diagnostic and prognostic biomarkers and therapeutic targets for various cancers8. Therefore, understanding the underlying mechanism of miRNA function is particularly important for the intervention of melanoma.
Previous studies indicated that miR-150 inhibits the aggressiveness of lung squamous cell carcinoma cells9 and colorectal cancer10. However, the function of miR-150 in melanoma remains elusive. In this study, we found that miR-150 was downregulated in melanoma tissues and cell lines. Overexpression of miR-150 inhibited the proliferation, migration, and invasion of melanoma cells. Mechanistically, we found that MYB was a direct target of miR-150 in melanoma cells. We showed that restoration of MYB reversed the effects of miR-150 on melanoma cells. Therefore, our data demonstrated that miR-150 suppressed melanoma progression via targeting MYB.
MATERIALS AND METHODS
Clinical Specimens and Cell Lines
Fifty-one pairs of malignant melanoma and the tumor adjacent normal tissues were acquired from patients undergoing a surgical procedure and were histopathologically diagnosed at Shanxian Central Hospital. For all samples, informed consent approved by the Independent Ethical Committees of Shanxian Central Hospital was obtained. Tissue samples were stored at −80°C.
Human melanoma cell lines, including MeWo, MHEM, A375, WM-115, and WM35, were purchased from the Chinese Academy of Sciences (Beijing, P.R. China) and were grown in RPMI-1640 medium (Gibco, Rockville, MD, USA) supplemented with 10% fetal bovine serum (FBS; Gibco). Primary human epidermal melanocytes (PEM; PromoCell, Beijing, P.R. China) from adult skin were maintained in serum- and PMA-free melanocyte growth medium M2 (PromoCell). All cells were cultured in a 37°C humidified atmosphere with 5% CO2.
Oligonucleotide and Transfection
The miR-150 mimics, inhibitors, and corresponding negative controls (NCs) were synthesized by GenePharma (Shanghai, P.R. China) and transfected into cells using Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA).
Cell Proliferation Assay
Cell proliferation was detected by Cell Counting Kit-8 (CCK-8; 7 Sea Biotech, Shanghai, P.R. China). Cells were grown in a 96-well plate with 1 × 104 cells per well and incubated in 37°C with 5% CO2 until cell confluence rate reached 70%. After transfection with plasmid for 48 h, cells were incubated for 24, 48, and 72 h. CCK-8 solution (10 μl) was seed into each well. The absorbance at 450 nm was measured with SUNRISE Microplate Reader (Tecan, Männedorf, Switzerland).
Transwell Invasion Assay
A total of 1 × 104 cells were transfected with miR-150 mimics or inhibitor for 48 h. The transfected cells were then suspended in 500 μl of serum-free medium and seeded into a Transwell membrane (Corning Incorporated; Corning, NY, USA) precoated with Matrigel (BD Biosciences, San Jose, CA, USA). The lower chamber was filled with 500 μl of growth medium containing 10% FBS, which served as a chemoattractant. The cells were cultured at 37°C for 24 h. The noninvaded cells on the top well were gently scraped off. Subsequently, 0.4% crystal violet (Sigma-Aldrich, St. Louis, MO, USA) was used to stain the invaded cells on the lower filter side. Cell invasion was evaluated under a microscope (Olympus, Tokyo, Japan).
Xenograft Assay
For tumor growth in vivo, 5 × 106 A375 cells transfected with miR-150 or controls were subcutaneously injected into the flanks of 6-week-old BALB/c nude mice from HFK Biosciences. Tumor weights were determined 4 weeks postinjection. Animal experiments were performed in accordance with relevant guidelines and regulations of the Institutional Animal Care and Use Committees at Shanxian Central Hospital.
Reverse Transcription and Real-Time PCR
Total RNA was extracted from cultured cells using TRIzol reagent (Invitrogen) according to the manufacturer’s protocol, and cDNA was synthesized from total RNA by a PrimerScript RT Reagent kit (TaKaRa, Shiga, Japan). miRNA from total RNA was reverse transcribed using the Prime-Script miRNA cDNA Synthesis Kit (TaKaRa). Real-time (RT)-PCR was performed with the SYBR Green Premix Ex Taq II (TaKaRa) on Applied Biosystems Step One Plus Real-Time PCR System (Applied Biosystems, Carlsbad, CA, USA). GAPDH was used as the endogenous control for the detection of mRNA expression level, and U6 was used as endogenous control for miRNA expression analysis.
Luciferase Reporter Assay
The wild-type (WT) or mutant (Mut) 3′-UTR of MYB was designed and prepared by GenePharma Co., Ltd. and then cotransfected into the cells with miR-150. Relative luciferase activity was calculated 48 h posttransfection by the Dual-Luciferase Reporter Assay (Promega, Madison, WI, USA).
Statistical Analysis
Statistical analysis was performed using the SPSS 17.0 (SPSS Inc., Chicago, IL, USA) and done by analysis of variance (ANOVA) or Student’s t-test. Statistical significance was defined as a value of p < 0.05.
RESULTS
miR-150 Was Downregulated in Melanoma
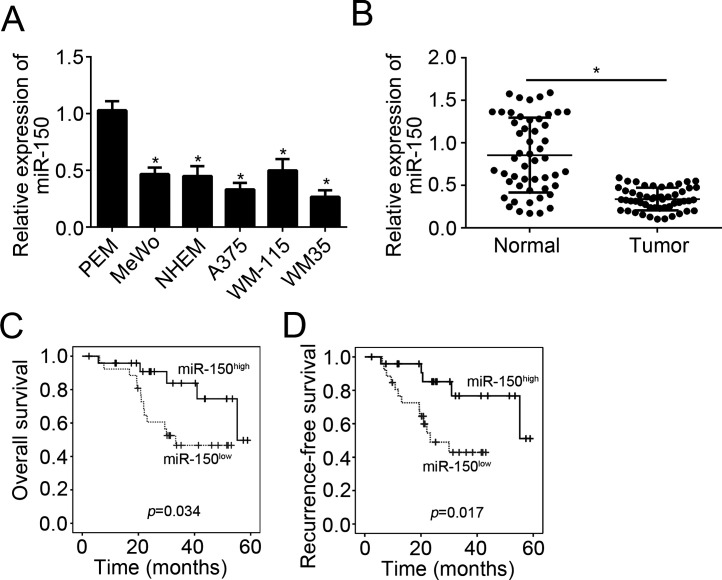
To investigate the role of miR-150, we first analyzed its expression patterns in melanoma tissues and cell lines using qRT-PCR. We found that expression of miR150 was significantly downregulated in melanoma cell lines (MeWo, MHEM, A375, WM-115, and WM35 cells) compared with primary human epidermal melanocytes (PEM) (Fig. 1A). Moreover, we obtained 51 pairs of melanoma tissues and adjacent normal tissues and found that miR-150 was also downregulated in melanoma tissues compared to normal tissues (Fig. 1B). To determine whether miR-150 could serve as a prognostic biomarker, we performed Kaplan–Meier analysis according to miR-150 expression. The results indicated that higher expression of miR-150 in patients with melanoma was associated with better prognosis (Fig. 1C and D).
Figure 1.
MicroRNA-150 (miR-150) was downregulated in melanoma. (A) qRT-PCR results indicated that miR-150 was downregulated in melanoma cell lines, including MeWo, NHEM, A375, WM-115, and WM35 compared with PEM cells. (B) Relative expression of miR-150 in 51 pairs of melanoma tissues and normal tissues were analyzed by qRT-PCR. (C, D) Overall survival and recurrence survival were determined by Kaplan–Meier analysis according to miR-150 expression in melanoma tissues. *p < 0.05.
miR-150 Suppressed the Proliferation, Migration, and Invasion of Melanoma Cells
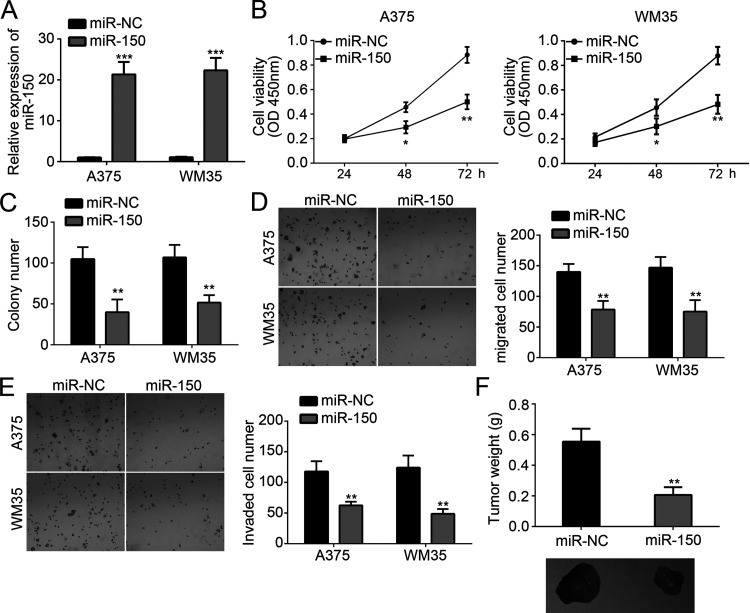
To explore the function of miR-150 in melanoma cells, we overexpressed miR-150 in A375 and WM35 cells through transfection with miR-150 mimics. By qRT-PCR, we found that miR-150 was significantly upregulated in A375 and WM35 cells transfected with miR-150 mimics compared to miR-NC (Fig. 2A). Then we performed CCK-8 and colony formation assay to evaluate the effect of miR-150 on cellular proliferation. Results indicated that overexpression of miR-150 significantly inhibited the proliferation and colony numbers (Fig. 2B and C). Tumor metastasis contributes to cancer malignance. Therefore, we measured the effect of miR-150 on tumor metastasis by Transwell assay. The results demonstrated that overexpression of miR-150 significantly reduced the numbers of migrated and invaded cells (Fig. 2D and E). Furthermore, we performed xenograft experiments by injecting A375 cells into nude mice. Four weeks postinjection, we measured the tumor weights and found that miR-150 overexpression also suppressed tumor growth in vivo (Fig. 2F). Taken together, these findings indicated that miR-150 served as a tumor suppressor in melanoma.
Figure 2.
miR-150 suppressed the proliferation, migration, and invasion of melanoma cells. (A) Relative expression of miR-150 in A375 and WM35 cells transfected with miR-150 mimics or negative control (NC). (B, C) Cellular proliferation was determined by Cell Counting Kit-8 (CCK-8) and colony formation assays. (D, E) Transwell assay was used to detect the migration and invasion of A375 and WM35 cells transfected with miR-150 mimics or control. (F) Tumor weights were measured 4 weeks postinjection. *p < 0.05, **p < 0.01, and ***p < 0.001.
MYB Was a Target of miR-150
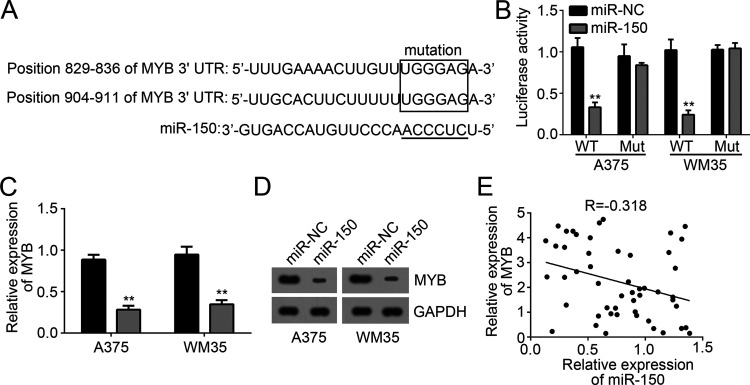
miRNAs targeted mRNAs to regulate gene expression. In order to search the target genes of miR-150 in melanoma, we made a prediction by informatics analysis through the TargetScan tool. Among all target genes, MYB ranked top. We found that there were three potential associating sites of miR-150 in the 3′-UTR of MYB mRNA (Fig. 3A). Therefore, we chose MYB for the following experiments. To validate their interaction, we conducted luciferase activity reporter assay with WT-MYB-3′-UTR or Mut-MYB-3′-UTR reporter plasmid. We found that overexpression of miR-150 significantly suppressed the luciferase activity in A375 and WM35 cells transfected with WT-MYB-3′-UTR but not Mut-MYB-3′-UTR plasmid (Fig. 3B). Moreover, we found that overexpression of miR-150 markedly reduced the mRNA and protein levels of MYB in A375 and WM35 cells transfected with miR-150 mimics (Fig. 3C and D). Then we analyzed the correlation between the expression levels of miR-150 and MYB in melanoma tissues. We found that there was a negative relationship between their expression levels in melanoma tissues (Fig. 3E). Taken together, our findings demonstrated that MYB was a target of miR-150 in melanoma cells.
Figure 3.
MYB was a target of miR-150. (A) Predicted binding sites of miR-150 in the 3′-UTR of MYB mRNA. (B) Luciferase activity assay indicated that overexpression of miR-150 in A375 and WM35 cells transfected with wild type (WT)-MYB-3′-UTR region reduced the luciferase activity. (C, D) mRNA and protein levels of MYB were measured by qRT-PCR and Western blot in A375 and WM35 cells transfected with miR-150 mimics. (E) qRT-PCR was used to determine the coexpression relationship between MYB and miR-150 in melanoma tissues. **p < 0.01.
MYB Was Overexpressed in Melanoma
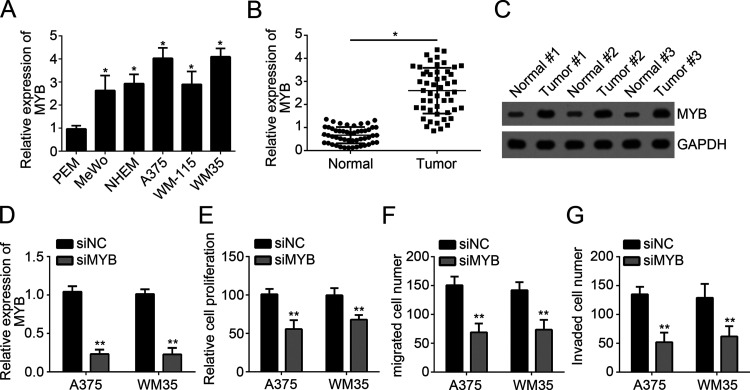
To further explore the role of MYB in melanoma, we analyzed its expression patterns in melanoma cell lines and tissues by qRT-PCR. Our results indicated that MYB was highly expressed in melanoma cell lines (MeWo, MHEM, A375, WM-115, and WM35 cells) compared to normal cells (PEM) (Fig. 4A), In addition, qRT-PCR analysis indicated that MYB was also upregulated in melanoma tissues compared to adjacent normal tissues (Fig. 4B). Western blot also achieved similar results (Fig. 4C). To explore the function of MYB, we silenced MYB in A375 and WM35 cells with specific siRNA against MYB (Fig. 4D). Then we performed CCK-8 and Transwell assays. The results showed that MYB knockdown significantly inhibited the proliferation, migration, and invasion of A375 and WM35 cells (Fig. 4E–G), which indicated that MYB may be an oncogene in melanoma.
Figure 4.
MYB was overexpressed in melanoma. (A) Relative expression of MYB in melanoma cell lines. (B) qRT-PCR analysis for the expression of MYB in melanoma tissues and paired normal tissues. (C) Western blot was used to analyze the protein levels of MYB in pairs of melanoma tissues and normal tissues. (D) MYB expression was downregulated in A375 and WM35 cells transfected with siMYB. (E) CCK-8 assay was used to measure cell proliferation in A375 and WM35 cells transfected with siMYB or siNC. (F, G) Transwell assay was utilized to determine the migration and invasion in A375 and WM35 cells transfected with siMYB or siNC. *p < 0.05 and **p < 0.01.
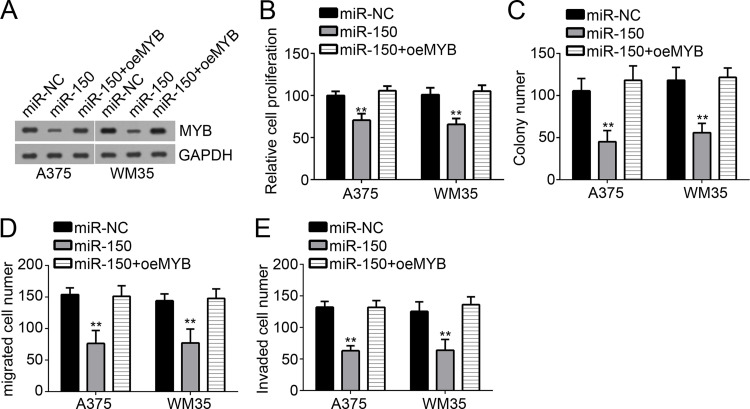
Restoration of MYB Reversed the Effects of miR-150
Finally, in order to determine whether miR-150 suppressed melanoma progression by targeting MYB, we restored MYB expression in miR-150-overexpressing A375 and WM35 cells by transfection with MYB ectopic-expressing plasmid. Western blot showed that MYB was significantly upregulated in miR-150-overexpressing A375 and WM35 cells (Fig. 5A). Then we performed CCK-8 and colony formation assays. We found that overexpression of miR-150 inhibited the proliferation of A375 and WM35 cells, while restoration of MYB rescued cellular proliferation (Fig. 5B and C). We also conducted Transwell assay to assess cell migration and invasion. We found that overexpression of MYB significantly rescued the numbers of migrated and invaded A375 and WM35 cells (Fig. 5C and D). Taken together, our findings demonstrated that miR-150 repressed the proliferation, migration, and invasion of melanoma cells by directly targeting MYB.
Figure 5.
Restoration of MYB reversed the effects of miR-150. (A) Western blot was used to detect the protein levels of MYB in A375 and WM35 cells. (B, C) CCK-8 and colony formation assays were used to determine the proliferation of A375 and WM35 cells. (D, E) The migration and invasion of A375 and WM35 cells were measured by Transwell assay. **p < 0.01.
DISCUSSION
Melanoma is one of the most malignant skin cancers and contributes to a large proportion of skin cancer-related deaths around the world each year1,2. However, the mechanism of melanoma development and progression remains largely unknown. Therefore, it is urgently required to determine the pathogenesis of melanoma. miRNAs have been demonstrated to be important regulators in various cancers, including melanoma11,12. Thus, defining the relationship between miRNA and melanoma will contribute to the intervention of melanoma. In this study, we explored the expression patterns, functions, and molecular mechanism of miR-150 in melanoma cells.
miRNAs are widely recognized as vital regulators in all kinds of tumors, including gastric cancer13, papillary thyroid carcinoma14, non-small cell lung cancer15, colon carcinoma16, osteosarcoma5, hepatocellular carcinoma17, and melanoma18. Accumulating evidence demonstrates that miRNAs delicately control gene expression to participate in the regulation of many biological processes, such as proliferation, apoptosis, migration, and invasion. For instance, Liu et al. reported that miRNA-200c inhibits epithelial–mesenchymal transition, invasion, and migration of lung cancer by targeting HMGB119. Zhang et al. reported that miR-143 inhibits cell proliferation and invasion by targeting DNMT3A in gastric cancer20. Wu et al. showed that miRNA-206 prevents the pathogenesis of hepatocellular carcinoma by modulating expression of met proto-oncogene and cyclin-dependent kinase 621. Sakr et al. indicated that miR-150-5p and miR-133a suppress glioma cell proliferation and migration through targeting membrane type 1 matrix metalloproteinase22. Sun et al. reported that miRNA-150 suppresses cell proliferation and metastasis in hepatocellular carcinoma by inhibiting the GAB1–ERK axis23. In this study, we found that miR-150 was significantly downregulated in melanoma tissues and cell lines. Through CCK-8, colony formation, and Transwell assays, we found that overexpression of miR-150 significantly suppressed the proliferation, migration, and invasion of melanoma cells. Our data suggested that miR-150 served as a tumor suppressor in melanoma.
MYB is a proto-oncogene protein and belongs to a member of the MYB family of transcription factors. MYB is originally recognized to play an essential role in the regulation of hematopoiesis and tumorigenesis24. Evidence indicates that dysregulation of MYB could lead to the development of acute myeloid leukemia25. Recently, more evidence has demonstrated that MYB is also involved in other cancers, including pancreatic cancer26, glioma27, breast cancer28, cylindroma29, cervical cancer30, and melanoma31. In addition, Liang et al. reported that miRNA-103a inhibits gastric cancer cell proliferation, migration, and invasion by targeting c-Myb32. Yu et al. reported that miRNA-424 is downregulated in hepatocellular carcinoma and suppresses cell migration and invasion through c-Myb33. However, the function and mechanism of MYB in melanoma need to be further explored. In our study, we found that MYB was a direct target of miR-150 in melanoma cells. We showed that overexpression of miR-150 significantly reduced the expression of MYB in melanoma cells. Moreover, through qRT-PCR, we found that there was an inverse correlation between the expression levels of MYB and miR-150 in melanoma tissues. In addition, through functional experiments, we found that restoration of MYB significantly rescued the proliferation, migration, and invasion of melanoma cells inhibited by miR-150.
In summary, our data demonstrated that miR-150 was a tumor suppressor in melanoma. In addition, upregulation of miR-150 suppressed the proliferation, migration, and invasion of melanoma cells through targeting MYB. Our study suggested that miR-150 may be a promising therapeutic target for melanoma treatment.
ACKNOWLEDGMENT
The authors declare no conflicts of interest.
Footnotes
The authors declare no conflicts of interest.
REFERENCES
- 1. Maddodi N, Setaluri V. Role of UV in cutaneous melanoma. Photochem Photobiol. 2008;84(2):528–36. [DOI] [PubMed] [Google Scholar]
- 2. Sarkar D, Leung EY, Baguley BC, Finlay GJ, Askarian-Amiri ME. Epigenetic regulation in human melanoma: Past and future. Epigenetics-Us 2015;10(2):103–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Fattore L, Costantini S, Malpicci D, Ruggiero CF, Ascierto PA, Croce CM, Mancini R, Ciliberto G. MicroRNAs in melanoma development and resistance to target therapy. Oncotarget 2017;8(13):22262–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zhang K, Guo L. MiR-767 promoted cell proliferation in human melanoma by suppressing CYLD expression. Gene 2018;641:272–8. [DOI] [PubMed] [Google Scholar]
- 5. Dong XC, Lv B, Li YS, Cheng QH, Su C, Yin GY. MiR-143 regulates the proliferation and migration of osteosarcoma cells through targeting MAPK7. Arch Biochem Biophys. 2017;630:47–53. [DOI] [PubMed] [Google Scholar]
- 6. Nabhan M, Louka ML, Khairy E, Tash F, Ali-Labib R, El-Habashy S. MicroRNA-181a and its target Smad 7 as potential biomarkers for tracking child acute lymphoblastic leukemia. Gene 2017;628:253–8. [DOI] [PubMed] [Google Scholar]
- 7. Liu P, Hu YT, Ma L, Du M, Xia L, Hu ZS. miR-425 inhibits melanoma metastasis through repression of PI3K-Akt pathway by targeting IGF-1. Biomed Pharmacother. 2015;75:51–7. [DOI] [PubMed] [Google Scholar]
- 8. Mirzaei H, Gholamin S, Shahidsales S, Sahebkar A, Jaafari MR, Mirzaei HR, Hassanian SM, Avan A. MicroRNAs as potential diagnostic and prognostic biomarkers in melanoma. Eur J Cancer 2016;53:25–32. [DOI] [PubMed] [Google Scholar]
- 9. Suetsugu T, Koshizuka K, Seki N, Mizuno K, Okato A, Arai T, Misono S, Uchida A, Kumamoto T, Inoue H. Downregulation of matrix metalloproteinase 14 by the antitumor miRNA, miR-150-5p, inhibits the aggressiveness of lung squamous cell carcinoma cells. Int J Oncol. 2018;52(3):913–24. [DOI] [PubMed] [Google Scholar]
- 10. Fan H, Liu X, Zheng WW, Zhuang ZH, Wang CD. MiR-150 alleviates EMT and cell invasion of colorectal cancer through targeting Gli1. Eur Rev Med Pharmacol Sci. 2017;21(21):4853–9. [PubMed] [Google Scholar]
- 11. Liu SN, Suo J, Wang CX, Sun X, Wang DG, He L, Zhang Y, Li W. Downregulation of tissue miR-338-3p predicts unfavorable prognosis of gastric cancer. Cancer Biomark. 2018;21(1):117–22. [DOI] [PubMed] [Google Scholar]
- 12. Peng J, Liu HL, Liu CH. MiR-155 promotes uveal melanoma cell proliferation and invasion by regulating NDFIP1 expression. Technol Cancer Res Treat. 2017;16(6):1160–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Guo B, Zhao TH, Wang T, Li Q, Wang XF, Wang WJ, Song TS, Huang C. MicroRNA-302b-3p suppresses cell proliferation through AKT pathway by targeting IGF-1R in human gastric cancer. Cell Physiol Biochem. 2017;42(4):1701–11. [DOI] [PubMed] [Google Scholar]
- 14. Yin YL, Hong SB, Yu S, Huang YR, Chen SW, Liu YJ, Zhang Q, Li YB, Xiao HP. MiR-195 inhibits tumor growth and metastasis in papillary thyroid carcinoma cell lines by targeting CCND1 and FGF2. Int J Endocrinol. 2017;2017:6180425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zhang XQ, He XJ, Liu YB, Zhang HQ, Chen H, Guo SX, Liang YG. MiR-101-3p inhibits the growth and metastasis of non-small cell lung cancer through blocking PI3K/AKT signal pathway by targeting MALAT-1. Biomed Pharmacother. 2017;93:1065–73. [DOI] [PubMed] [Google Scholar]
- 16. Wang JY, Jiang JB, Li Y, Wang YL, Dai Y. MicroRNA-299-3p suppresses proliferation and invasion by targeting VEGFA in human colon carcinoma. Biomed Pharmacother. 2017;93:1047–54. [DOI] [PubMed] [Google Scholar]
- 17. Zhou K, Luo XY, Wang Y, Cao DC, Sun G. MicroRNA-30a suppresses tumor progression by blocking Ras/Raf/MEK/ERK signaling pathway in hepatocellular carcinoma. Biomed Pharmacother. 2017;93:1025–32. [DOI] [PubMed] [Google Scholar]
- 18. Saleiban A, Faxalv L, Claesson K, Jonsson JI, Osman A. miR-20b regulates expression of proteinase-activated receptor-1 (PAR-1) thrombin receptor in melanoma cells. Pigment Cell Melanoma Res. 2014;27(3):431–41. [DOI] [PubMed] [Google Scholar]
- 19. Liu PL, Liu WL, Chang JM, Chen YH, Liu YP, Kuo HF, Hsieh CC, Ding YS, Chen WW, Chong IW. MicroRNA-200c inhibits epithelial-mesenchymal transition, invasion, and migration of lung cancer by targeting HMGB1. PLoS One 2017;12(7):e0180844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zhang Q, Feng Y, Liu P, Yang J. MiR-143 inhibits cell proliferation and invasion by targeting DNMT3A in gastric cancer. Tumour Biol. 2017;39(7):1010428317711312. [DOI] [PubMed] [Google Scholar]
- 21. Wu H, Tao J, Li X, Zhang T, Zhao L, Wang Y, Zhang L, Xiong J, Zeng Z, Zhan N, Steer CJ, Che L, Dong M, Wang X, Niu J, Li Z, Yan G, Chen X, Song G. MicroRNA-206 prevents the pathogenesis of hepatocellular carcinoma by modulating expression of met proto-oncogene and cyclin-dependent kinase 6 in mice. Hepatology 2017;66(6):1952–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sakr M, Takino T, Sabit H, Nakada M, Li ZC, Sato H. miR-150-5p and miR-133a suppress glioma cell proliferation and migration through targeting membrane-type-1 matrix metalloproteinase. Gene 2016;587(2):155–62. [DOI] [PubMed] [Google Scholar]
- 23. Sun W, Zhang ZC, Wang JL, Shang RZ, Zhou L, Wang X, Duan JL, Ruan B, Gao Y, Dai B, Qu SB, Liu W, Ding R, Wang L, Wang DS, Dou KF. MicroRNA-150 suppresses cell proliferation and metastasis in hepatocellular carcinoma by inhibiting the GAB1-ERK axis. Oncotarget 2016;7(10):11595–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Clarke M, Volpe G, Sheriff L, Walton D, Ward C, Wei W, Dumon S, Garcia P, Frampton J. Transcriptional regulation of SPROUTY2 by MYB influences myeloid cell proliferation and stem cell properties by enhancing responsiveness to IL-3. Leukemia 2017;31(4):957–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ramaswamy K, Forbes L, Minuesa G, Gindin T, Brown F, Kharas MG, Krivtsov AV, Armstrong SA, Still E, de Stanchina E, Knoechel B, Koche R, Kentsis A. Peptidomimetic blockade of MYB in acute myeloid leukemia. Nature Commun. 2018;9:110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Azim S, Zubair H, Srivastava SK, Bhardwaj A, Zubair A, Ahmad A, Singh S, Khushman M, Singh AP. Deep sequencing and in silico analyses identify MYB-regulated gene networks and signaling pathways in pancreatic cancer. Sci Rep. 2016;6:28446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zhang BP, Yuan F, Liu J, Li Y, Zhou FC, Liu XX, Hao Z, Li QS, Zheng YR, Wang WZ. Hsa-miR-495 acts as a tumor suppressor gene in glioma via the negative regulation of MYB. Mol Med Rep. 2016;14(1):977–82. [DOI] [PubMed] [Google Scholar]
- 28. Li YH, Jin K, van Pelt GW, van Dam H, Yu X, Mesker WE, ten Dijke P, Zhou FF, Zhang L. c-Myb enhances breast cancer invasion and metastasis through the Wnt/beta-catenin/Axin2 pathway. Cancer Res. 2016;76(11):3364–75. [DOI] [PubMed] [Google Scholar]
- 29. Rajan N, Andersson M, Sinclair N, Fehr A, Hodgson K, Lord C, Kazakov D, Vanecek T, Ashworth A, Stenman G. Overexpression of MYB drives proliferation of CYLD-defective cylindroma cells. J Invest Dermatol. 2016;136(9):S186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Du XJ, Lin L, Zhang LJ, Jiang J. microRNA-195 inhibits the proliferation, migration and invasion of cervical cancer cells via the inhibition of CCND2 and MYB expression. Oncol Lett. 2015;10(4):2639–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hijiya N, Zhang J, Ratajczak MZ, Kant JA, Deriel K, Herlyn M, Zon G, Gewirtz AM. Biologic and therapeutic significance of Myb expression in human-melanoma. Proc Natl Acad Sci USA 1994;91(10):4499–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Liang JL, Liu XF, Xue HP, Qiu B, Wei B, Sun K. MicroRNA-103a inhibits gastric cancer cell proliferation, migration and invasion by targeting c-Myb. Cell Prolif. 2015;48(1):78–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Yu L, Ding GF, He CZ, Sun L, Jiang YF, Zhu LY. MicroRNA-424 is down-regulated in hepatocellular carcinoma and suppresses cell migration and invasion through c-Myb. PLoS One 2014;9(3):e91661. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]