Abstract
Baicalein, an active ingredient separated from Astragalus membranaceus, has shown its anticancer ability in various cancers. However, its effect on nasopharyngeal carcinoma has not been explored yet. The present study aimed to investigate the effect of baicalein on the growth, proliferation, apoptosis, and cell cycle of human nasopharyngeal carcinoma cells, as well as transplanted nude mouse xenograft. The results showed that baicalein inhibited the growth and proliferation of CNE1 and CNE2 cells in a time- and concentration-dependent manner. It also caused a significant increase in the number of cells in the G0/G1 phase and a decrease in the G2/M phase, thereby reducing the number of cells entering mitosis and inhibiting the proliferation of tumor cells. Baicalein also significantly induced apoptosis of CNE1 and CNE2 cells. Western blots showed that baicalein decreased the expression of Bcl-xl and Mcl-1 and increased the expression of Bax, Bad, and caspase 3, 8, and 9. In CNE1- and CNE2-transplanted tumors of mice, baicalein significantly inhibited tumor growth. In conclusion, baicalein could inhibit the growth and proliferation of human nasopharyngeal carcinoma cells, change their cell cycle, and induce apoptosis. Baicalein also effectively limits both CNE1- and CNE2-transplanted tumors in nude mice. Downregulation of Bcl-xl and Mcl-1 proteins and upregulation of Bax and Bad may be involved in the mechanism.
Key words: Baicalein, Nasopharyngeal carcinoma (NPC), Apoptosis, Cell cycle, Xenograft
INTRODUCTION
Nasopharyngeal carcinoma (NPC) is a serious malignant tumor mainly occurring in China1. At present, radiotherapy can increase the local control rate of nasopharyngeal carcinoma. Intensity-modulated radiotherapy produced satisfactory survival outcomes for patients with acceptable late toxicities, but not for distant control, especially for patients with N3 stage2. However, about two thirds of patients have been in the middle and late stages when they receive treatment, as patients usually present in a more advanced stage of disease. As a result, the 10-year overall survival for locoregionally advanced nasopharyngeal carcinoma was about 49.5%3. Although the chemotherapy drugs have achieved high efficiency, the long-term effects are still not ideal, and there are more serious toxic and side effects. In addition, the multidrug resistance of tumor to chemotherapy restricts the efficacy of chemotherapy drugs. To improve the therapy effect, combination therapy of radiotherapy and chemotherapy with alternative treatment has received more and more attention. Therefore, for nasopharyngeal carcinoma, especially advanced nasopharyngeal carcinoma, in addition to improving the level of radiotherapy and chemotherapy technology we should consider multidisciplinary comprehensive treatment in order to achieve the goal of “cure.”
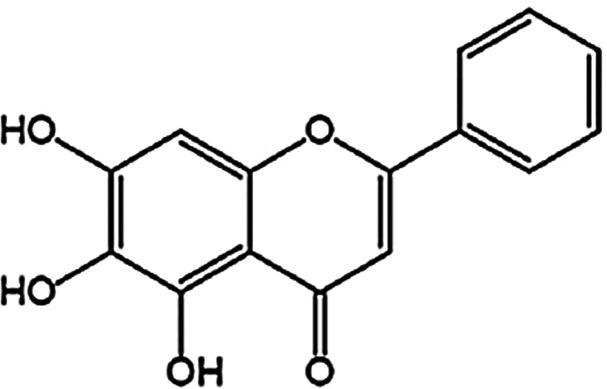
It has been recognized that the reversal of multidrug resistance and radiosensitization of some carcinomas by Chinese traditional medicine can make up for the disadvantages of radiotherapy and chemotherapy and achieve a better antitumor effect4. Astragalus membranaceus is a type of East Asia yellow peony that is rich in flavonoids. It has been approved to treat hyperlipidemia, hypertension, dysentery, viral hepatitis, viral myocarditis, bronchial asthma, chronic bronchitis, allergic rhinitis, inflammation, and tumors as a Chinese traditional medicine5. The chemical constituents of A. membranaceus were found to contain flavonoids, saponins, and polysaccharides. As a key ingredient of Chinese herbal medicines such as Huangqi Tang, Xiaochaihu Tang, and Xiaochaihu Tang of Japan, it is used to prevent and treat many tumors6. Baicalein is an active ingredient separated from A. membranaceus. The molecular formula of baicalein is C15H10O5, as shown in Figure 1 7. As the main bioactive component of A. membranaceus, baicalein has demonstrated its antitumor activity in both animal and cell studies8. In recent years, baicalein has received increasing attention as it can inhibit proliferation of cancer cells, or induce apoptosis and autophagic cell death of cancer cells with low toxicity9–11. However, the effect and mechanism of baicalein in the treatment of nasopharyngeal carcinoma has not yet been explored.
Figure 1.
Structure of baicalin.
Hence, the present study was performed to investigate the effect of baicalein on the growth, proliferation, apoptosis, and cell cycle of human nasopharyngeal carcinoma cells. We also examined its effect on the expression of apoptosis-associated protein and the growth of nasopharyngeal carcinoma xenografts to explore the underlying mechanism. The findings in this study may provide experimental basis for the clinical application of baicalein in the treatment of nasopharyngeal carcinoma.
MATERIALS AND METHODS
Nasopharyngeal Carcinoma Cells
Human nasopharyngeal cancer cells CNE1 and CNE2 were purchased from Biomart Co. Ltd (Shanghai, P.R. China) and inoculated in our laboratory in Jinhua Central Hospital. Cells were cultured in regular RPMI-1640 medium, which is made by fully dissolving RPMI-1640 culture dry powder (Beyotime, Haimen, Jiangsu, P.R. China) with distilled water. Penicillin and streptomycin (Beyotime) were added into the medium to reach a concentration of 100 U/ml. The pH was adjusted to 7.2 with NaHCO3 (Sinopharm Chemical Reagent, Beijing, P.R. China). The RPMI-1640 medium was then sterilized, subpackaged, and stored at −20°C for use. Newborn bovine serum (Beyotime) was added to reach a concentration of 10% before use. Cells were cultured in a cell culture incubator (Thermo Fisher Scientific, Waltham, MA, USA) under 37°C with 90% relative humidity and 5% CO2. The RPMI-1640 medium was regularly changed once a day.
Drug Treatment and Cytotoxicity Test by MTT Method
Logarithmic growth phase cells were seeded in 96-well plates (6 × 103/well). After 8–12 h of cell adherence, RPMI-1640 medium was added to reach 200 μl/well in total. Baicalein (purity 98%; Sigma-Aldrich, St. Louis, MO, USA) was added to the culture medium to different concentrations (12.5 μM, 25 μM, 50 μM, 100 μM). Cisplatin (2 mg/L; DDP; Sigma-Aldrich) was added in a well to different concentrations (10 μM, 20 μM, 30 μM, 40 μM) as positive control groups. Next, cells were grown in an incubator (Thermo Fisher Scientific) for 6 days. The medium was changed once a day with different concentrations of baicalein or DDP. Four replicates were set for each concentration at each time point. On MTT test day, 4 μl of 5 μg/L MTT solution (Beyotime) was first added into the wells. The cells were continually incubated with MTT solution for 4 h. After that, the culture medium in each well was all removed, and 150 μl of dimethyl sulfoxide was added to the wells. The mixture was gently vibrated for 10 min. Finally, the absorbance under the wavelength of 570 nm was measured on a spectrophotometer (Tecan, Shanghai, P.R. China) and the percent growth of cells was calculated as follows: % growth of cells = 1 − (absorbance of control well − absorbance of experimental well)/absorbance of control well × 100%.
Clonogenicity Assay
Cells (5 × 103) were suspended with DMEM/10% FBS in 150-μl Mix agar. They were then plated in 30-mm plates overlying a 1% agar–DMEM/10% FBS (1:1) bottom layer. Next, cells were kept in medium containing 40 μM DDP or different concentrations of baicalein. The medium was changed once a day with different concentrations of baicalein or DDP. Ten days later, the number of remaining colonies was counted.
Detection of Apoptosis by Flow Cytometer
After incubation of CNE1 and CNE2 nasopharyngeal carcinoma cells with baicalein (12.5 μM, 25 μM, 50 μM, 100 μM) or 40 μM DDP for 48 h, cells were digested and stained with propidium iodide and annexin V conjugated to green-fluorescent FITC dye (Sigma-Aldrich). Apoptosis rate was measured on a flow cytometer (Thermo Fisher Scientific).
Detection of Cell Cycle by Flow Cytometer
After incubation of CNE1 and CNE2 nasopharyngeal carcinoma cells with baicalein (12.5 μM, 25 μM, 50 μM, 100 μM) or DDP (40 μM) for 48 h, cells were digested and fixed with 70% ethanol and stained with propidium iodide (Sigma-Aldrich). The relative rates of cells in the G0/G1 phase, S phase, or G2/M phase were detected on a flow cytometer (Thermo Fisher Scientific).
Western Blot
After incubation of CNE1 nasopharyngeal carcinoma cells with baicalein (12.5 μM, 25 μM, 50 μM, 100 μM) or DDP (40 μM) for 48 h, or with IC50 concentration of baicalein (20.95 μM) or DDP (2.49 μM) for 12 h, cells were washed three times with 0.01 M PBS at 4°C. Then 300 μl of protein lysate (50 mmol/L NaCl, 0.5 mmol/L Tris-HCl pH 7.5, 1% Triton X-100, 150 mmol/L EDTA, 0.5 mmol/L PMSF) was added to the wells. The Western blot kit package was purchased from Beyotime. The lysate was further lysed on ice at 4°C for 30 min before it was centrifuged at 12,000 rpm for 10 min. Next, the supernatant was assayed for protein concentration, and the protein concentration was adjusted to 3 μg/μl. The protein was then separated in 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis and transferred to polyvinylidene difluoride (PVDF) membrane (Bio-Rad, Hercules, CA, USA). Afterward, the PVDF membrane was incubated with a mixture of Tris-buffered saline (TBS) and Tween 20 and nonfat milk for 1 h. They were then incubated with corresponding primary antibodies at 4°C overnight. The PVDF membrane was then washed with a mixture of TBS and Tween 20 four times before it was incubated with corresponding secondary antibodies (1:5,000) on a shaker under 25°C for 1 h. Finally, bands were visualized with an Electro-Chemi-Luminescence (ECL) Substrate Kit (Rahn AG, Zurich, Switzerland) and quantified with Quantity One software (Bio-Rad).
Establishment of Nasopharyngeal Carcinoma Xenograft Model
BALB/C-nu/nu nude mice (18–22 g) were housed in the animal center of Jinhua Central Hospital under SPF conditions and a constant temperature of 24°C. After CNE1 and CNE2 cells in logarithmic growth phase were digested with trypsin, a cell suspension of 5 × 107 cells/ml was prepared. Each nude mouse was inoculated subcutaneously with 0.2 ml of cell suspension. When tumors grew to 1 × 0.8 × 0.4 cm3, nude mice were randomly divided into five groups: control, DDP, 1.0 mg/kg BAI, 2.0 mg/kg BAI, and 3.0 mg/kg BAI, with 10 mice in each group. The DDP group was used as positive control. DDP was intraperitoneally injected at 6 mg/kg every 3 days. Baicalein was given by intraperitoneal injection once a day. The control group was injected intraperitoneally with the same volume of saline. During the treatment, the tumor size, body weight, activity, diet, and defecation of each mouse were measured and recorded every 3 days. The animals were treated for 12 days. Three days after the last injection was completed, mice were sacrificed. The tumor tissue was collected and weighed. The long diameter (a) and the short diameter (b) of the tumor were gauged to calculate the tumor volume (V) using the formula V = πab 2/6. The tumor inhibition rate (TIR), relative tumor volume (RTV) as well as relative tumor proliferation rate (T/C) were calculated as follows: Tumor inhibition rate (%) = (average tumor weight in control mice − average tumor weight in experimental mice)/average tumor weight in control mice × 100%; RTV = V1/V0 (V1, final tumor volume; V0, initial tumor volume); T/C = average RTV of treatment group/average RTV of control group × 100%.
Statistical Analysis
The SPSS13.0 statistical analysis software (SPSS Inc., Chicago, IL, USA) was used to analyze the data with one-way analysis of variance (ANOVA) method. Data were expressed as mean ± SEM. A value of p < 0.05 was considered to be statistically significant.
RESULTS
The Inhibition of CNE1 and CNE2 Proliferation by Baicalein and DDP
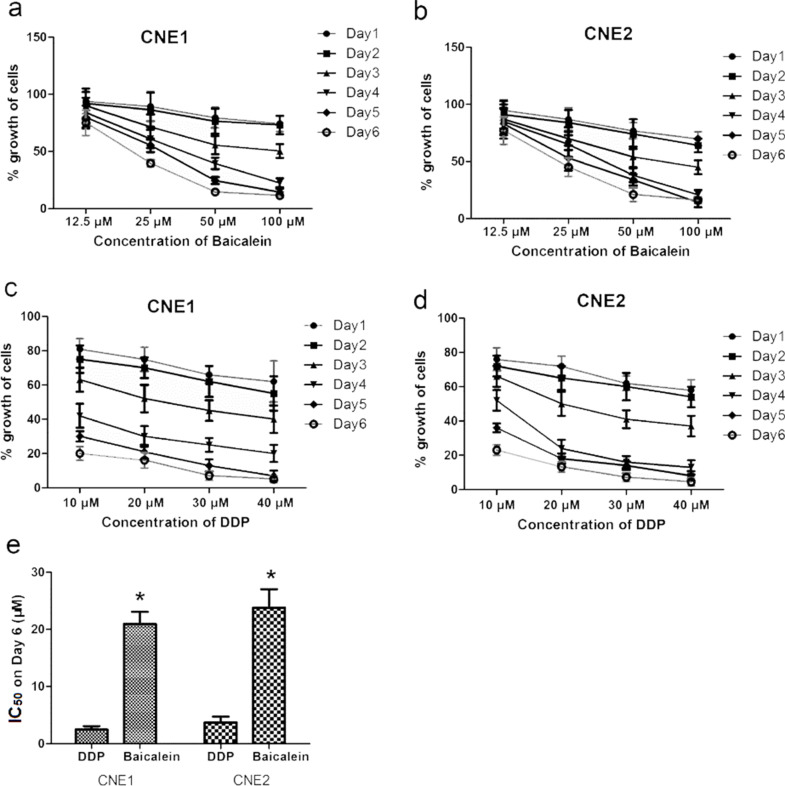
The inhibition of CNE1 and CNE2 proliferation by baicalein and DDP is shown in Figure 2a–d. The results of MTT colorimetric assay showed that different dosages of baicalein and DDP significantly decreased the cell growth of CNE1 and CNE2 cells. In each group, the cell growth percentage was decreased as the dosage increased. The inhibition effect was both time and concentration dependent. Figure 2e shows the IC50 value of baicalein and DDP. The IC50 of DDP for CNE1 and CNE2 was 2.49 μM and 3.73 μM, respectively. The IC50 of baicalein for CNE1 and CNE2 was 20.95 μM and 23.82 μM, respectively.
Figure 2.
Effects of baicalein and cisplatin (DDP) on the growth of CNE1 and CNE2 cells. The results of MTT colorimetric assay. (a) CNE1 treated by baicalein; (b) CNE2 treated by baicalein; (c) CNE1 treated by DDP; (d) CNE2 treated by DDP. (e) The IC50 values of baicalein and DDP. Cells were treated with different dosages of baicalein or 40 μM DDP for 6 days. MTT colorimetric assay was done every day. The mortality was calculated as: (absorbance of control well—absorbance of experimental well)/absorbance of control well × 100%. Data are expressed as mean ± SEM. *p < 0.05 compared to DDP.
The Effect of Baicalein on the Cell Cycle of CNE1 and CNE2
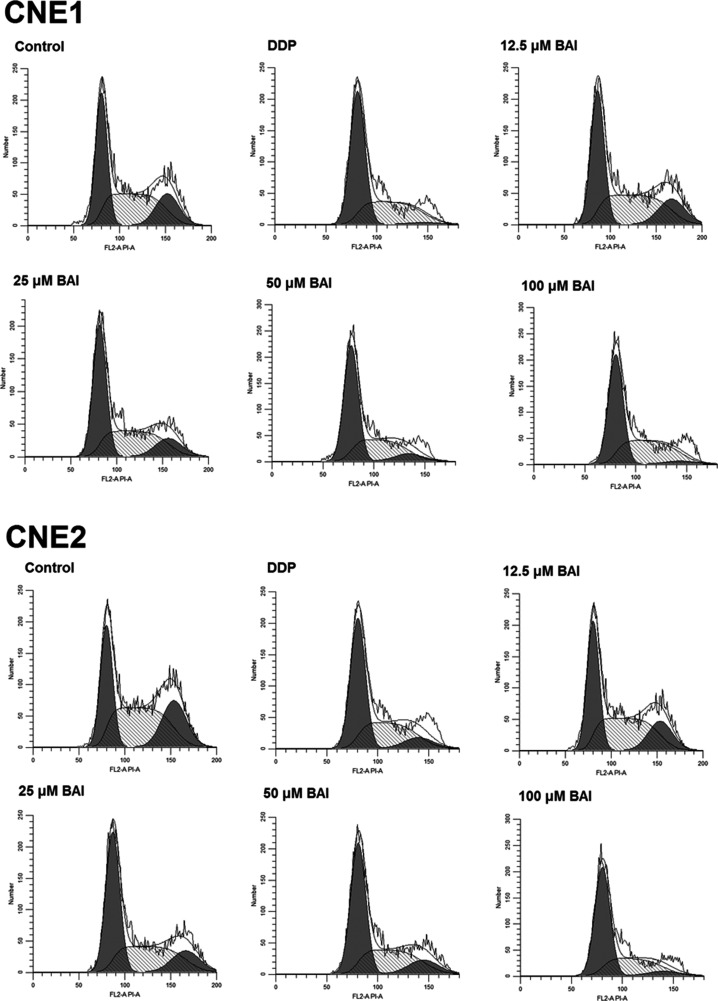
The effect of baicalein on the cell cycle change of CNE1 and CNE2 cells was analyzed and shown in Tables 1 and 2 and Figure 3. The cell cycle arrest of 40 μM DDP and 25 μM, 50 μM, 100 μM baicalein groups in CNE1 and CNE2 cells occurred in the G0/G1 phase (p < 0.05 compared to control), as demonstrated by the significantly higher proportion of cells in this phase. The proportions of cells in the G2/M phase in these groups were significantly decreased compared with that of control (p < 0.05). The cell cycle images from flow cytometry are shown in Figure 3.
Table 1.
Effects of Baicalein on Cell Cycle of CNE1 Cells (% of Total Cells)
| Groups | G0/G1 | S | G2/M |
|---|---|---|---|
| Control | 41.6 ± 4.2 | 40.1 ± 4.3 | 18.3 ± 2.7 |
| Cisplatin (DDP) | 58.5 ± 3.6* | 39.6 ± 4.5 | 1.9 ± 0.5* |
| 12.5 μM baicalein | 42.6 ± 3.9 | 43.2 ± 5.5 | 14.2 ± 2.6 |
| 25 μM baicalein | 50.3 ± 4.5* | 38.6 ± 4.1 | 11.1 ± 1.2* |
| 50 μM baicalein | 55.6 ± 5.4* | 39.4 ± 3.8 | 5.0 ± 1.3* |
| 100 μM baicalein | 56.9 ± 5.2* | 38.7 ± 3.9 | 4.4 ± 1.2* |
p < 0.05 compared to control.
Table 2.
Effects of Baicalein on Cell Cycle of CNE2 Cells (% of Total Cells)
| Groups | G0/G1 | S | G2/M |
|---|---|---|---|
| Control | 39.6 ± 3.2 | 38.4 ± 3.9 | 22.0 ± 2.1 |
| Cisplatin (DDP) | 57.6 ± 3.5* | 35.7 ± 3.1 | 6.7 ± 1.2* |
| 12.5 μM baicalein | 41.3 ± 3.9 | 41.6 ± 4.2 | 17.1 ± 2.3 |
| 25 μM baicalein | 49.3 ± 3.6* | 38.5 ± 3.2 | 12.2 ± 1.5* |
| 50 μM baicalein | 52.3 ± 2.9* | 37.6 ± 4.1 | 10.1 ± 1.6* |
| 100 μM baicalein | 55.9 ± 3.1* | 39.6 ± 4.3 | 4.5 ± 1.3* |
p < 0.05 compared to control.
Figure 3.
The cell cycle images from flow cytometry.
The Effect of Baicalein on Colony and Apoptosis of CNE1 and CNE2
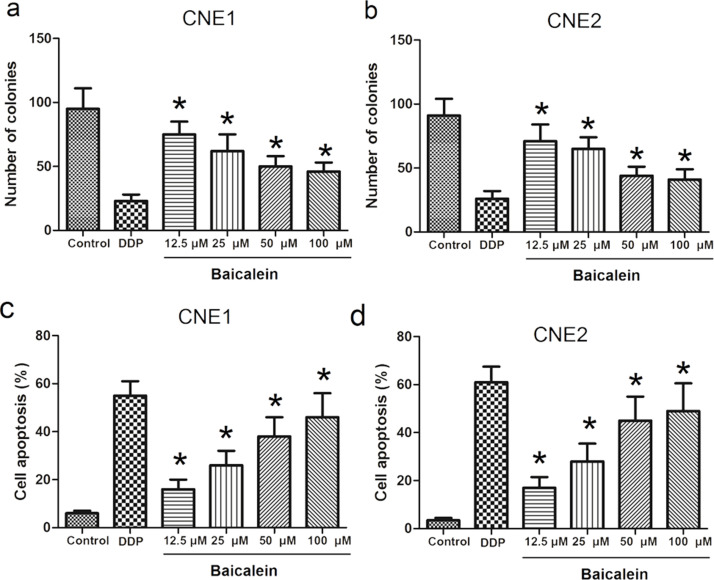
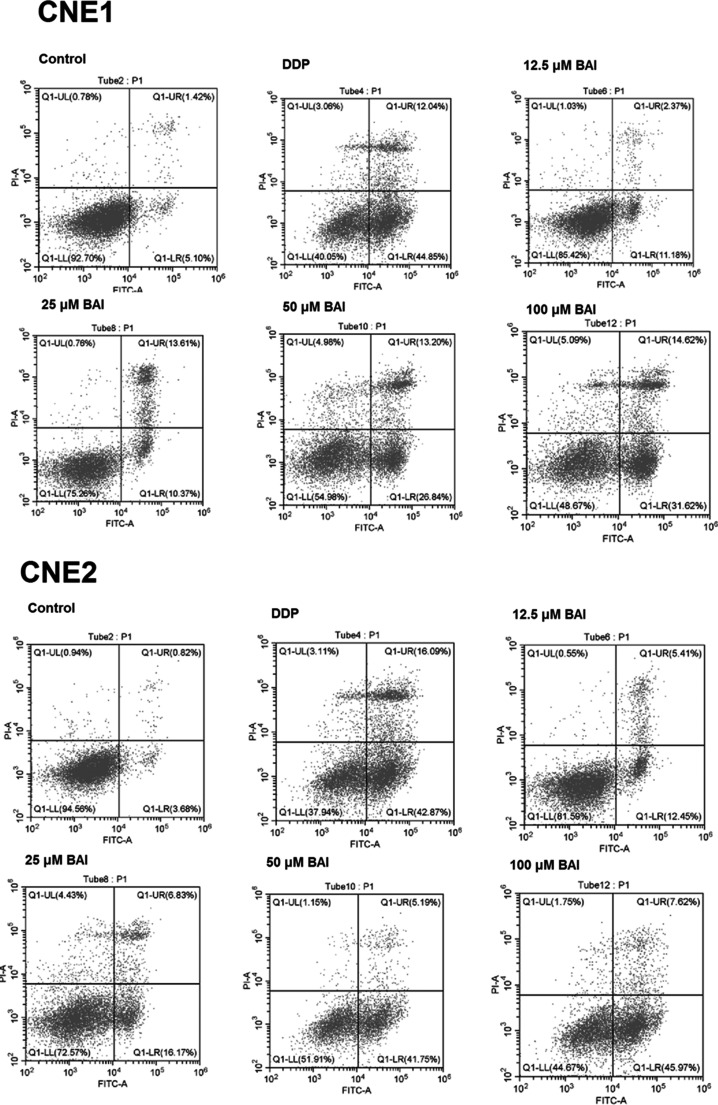
As shown in Figure 4a and b, compared to control, the number of colonies in DDP and baicalein groups were significantly decreased (p < 0.05) in CNE1 and CNE2 cells. Figure 4c and d shows the apoptotic rates in CNE1 and CNE2 cells. The cell apoptosis images from flow cytometry are shown in Figure 5. As shown in Figure 4c, the apoptotic rate in DDP group was 55.3 ± 6.1%. In baicalein groups, the apoptotic rates were 16.2 ± 4.5%, 26.5 ± 6.2%, 38.2 ± 8.1%, and 46.6 ± 10.3% in 12.5 μM, 25 μM, 50 μM, and 100 μM groups, respectively. As shown in Figure 4d, in CNE2 cells the apoptotic rates were 61.6 ± 6.5%, 17.5 ± 4.5%, 28.4 ± 7.4%, 45.6 ± 10.1%, 49.3 ± 11.6% in DDP and 12.5 μM, 25 μM, 50 μM, 100 μM in baicalein groups. The apoptotic rates in DDP and baicalein groups were all significantly different from control (P<0.05).
Figure 4.
The effect of baicalein on colony and apoptosis of CNE1 and CNE2. CNE1 and CNE2 cells were treated with 40 μM DDP or different dosages of baicalein for 48 h, and then the colony and apoptosis of CNE1 and CNE2 were analyzed. (a) Number of colonies of CNE1; (b) number of colonies of CNE2; (c) apoptosis of CNE1; (d) apoptosis of CNE2). Data are expressed as mean ± SEM. *p < 0.05 compared to control.
Figure 5.
The cell apoptosis images from flow cytometry.
Effect of Baicalein on Expression of Apoptosis-Related Proteins in CNE1 and CNE2
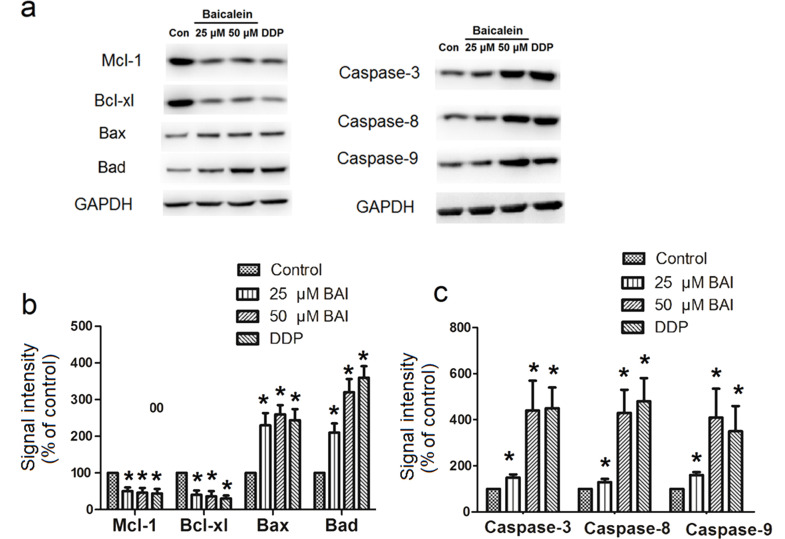
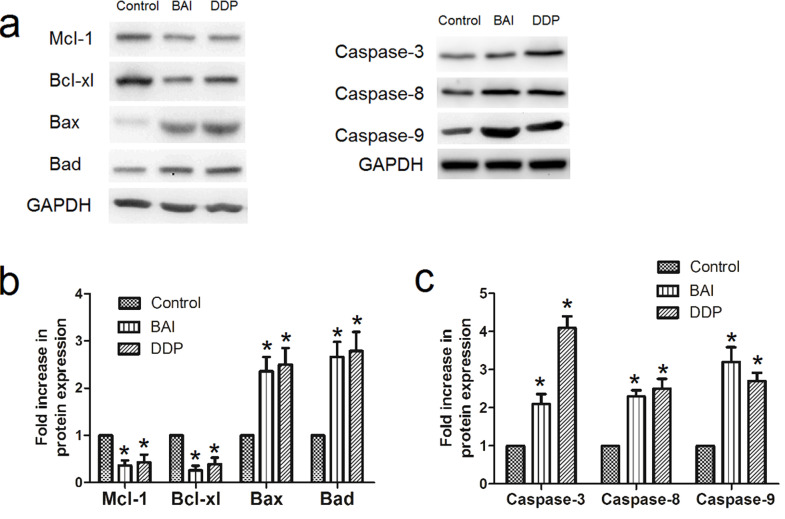
Western blot analysis of apoptosis-related proteins (Mcl-1, Bcl-xl, Bax, Bad, caspase 3, 8, and 9) in the extracted cell proteins was performed at 48 h after CNE1 cells were treated with 25 μM, 50 μM baicalein or 40 μM DDP (Fig. 6), or at 12 h after CNE1 cells were treated with IC50 baicalein (20.95 μM) or IC50 DDP (2.49 μM) (Fig. 7). The representative Western blot bands are shown in Figures 6a and 7a, while the statistical analysis results of the fold increases of protein expression are shown in Figures 6b and c and 7b and c. The expression of both Bcl-xl and Mcl-1 was decreased by baicalein or DDP compared to control (p < 0.05). Expression of Bax, Bad, caspase 3, 8, and 9, on the other hand, was significantly increased by baicalein or DDP compared to control (p < 0.05).
Figure 6.
Effect of 48 h treatment of baicalein on expression of apoptosis-related proteins in CNE1 and CNE2. Western blot analysis of apoptosis-related proteins (Mcl-1, Bcl-xl, Bax, Bad, caspase 3, 8, and 9) in the extracted proteins from cells was performed after CNE1 cells were treated with 25 μM, 50 μM baicalein, or 40 μM DDP for 48 h. (a) Examples of Western blots; (b) fold increase in Mcl-1, Bcl-xl, Bax, Bad; (c) fold increase in caspase 3, 8, and 9. Data are expressed as mean ± SEM. *p < 0.05 compared to control.
Figure 7.
Effect of 12-h treatment of baicalein on expression of apoptosis-related proteins in CNE1 and CNE2. Western blot analysis of apoptosis-related proteins (Mcl-1, Bcl-xl, Bax, Bad, caspase 3, 8, and 9) in the extracted proteins from cells was performed after CNE1 cells were treated with IC50 concentration of baicalein (20.95 μM) or DDP (2.49 μM) for 12 h. (a) Examples of Western blots; (b) fold increase in Mcl-1, Bcl-xl, Bax, Bad; (c) fold increase in caspase 3, 8, and 9. Data are expressed as mean ± SEM. *p < 0.05 compared to control.
Effect of Baicalein on Nasopharyngeal Carcinoma Xenografts
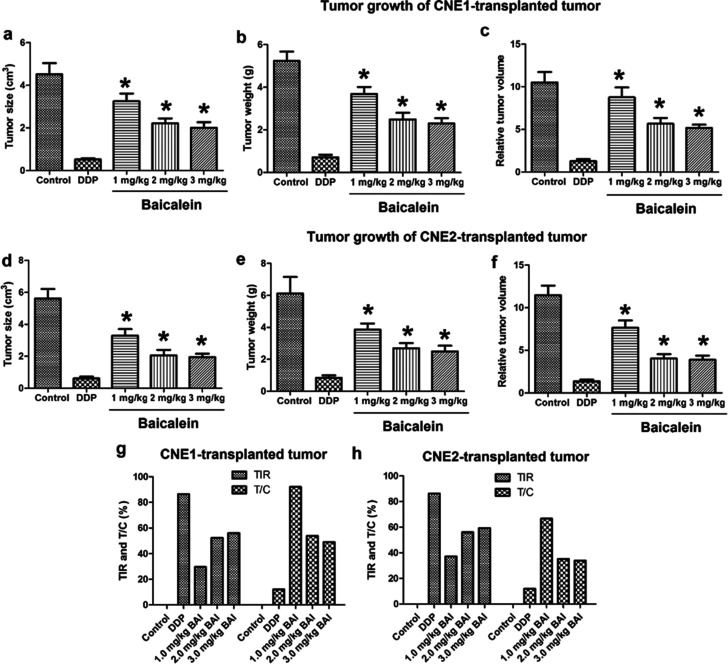
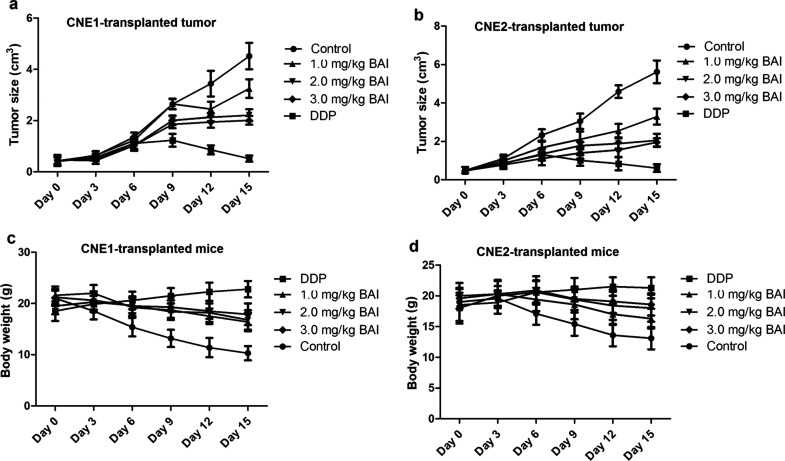
After the nasopharyngeal carcinoma xenograft model was established with CNE1 or CNE2 cells, mice were treated with 6 mg/kg DDP (positive control) or different dosages of baicalein. On the fifth day, the tumor tissue was collected and weighed. Figure 8a–f shows the changes in tumor size, tumor weight, and relative tumor volume, and Figure 8g and h shows the tumor inhibition rates (TIR) and T/C of nasopharyngeal carcinoma xenografts. There was no death in each group during the experiment. With the increase in the concentration of baicalein, the tumor size and weight growth were both dose-dependently inhibited (p < 0.05 compared to control). According to the criteria for antitumor drug screening, only drugs with a TIR of >30% and T/C <60% are of significance. As shown in Figure 8g, the TIRs of the CNE1-transplanted tumor by 1.0 mg/kg BAI, 2.0 mg/kg BAI, and 3.0 mg/kg BAI are 29.64%, 52.39%, and 56.00%, respectively. As shown in Figure 8h, the TIRs of the CNE2-transplanted tumor by 1.0 mg/kg BAI, 2.0 mg/kg BAI, and 3.0 mg/kg BAI are 37.09%, 56.05%, and 59.30%, respectively. Figure 9 shows the tumor growth curve and mice body weight curves. The tumor size changes are shown in Figure 9a and b, which indicates that tumor size grew more slowly in the BAI and DDP groups. The body weight changes are shown Figure 9c and d, which shows that the body weight dropped more slowly in BAI and DDP groups.
Figure 8.
Effect of baicalein on tumor growth of CNE1- or CNE2-transplanted tumor. After the nasopharyngeal carcinoma xenograft model was established with CNE1 or CNE2 cells, mice were treated with 40 μM DDP (positive control) or different dosages of baicalein. On the fifth day, the tumor tissue was collected and weighed. (a) Tumor size of CNE1-transplanted tumor; (b) tumor weight of CNE1-transplanted tumor; (c) relative tumor volume of CNE1-transplanted tumor; (d) tumor size of CNE2-transplanted tumor; (e) tumor weight of CNE2-transplanted tumor; (f) relative tumor volume of CNE2-transplanted tumor; (g) tumor inhibition rates (TIR) and T/C of CNE1-transplanted tumor; (h) TIR and T/C of CNE2-transplanted tumor. *p < 0.05 compared to control.
Figure 9.
The tumor growth curve and mice body weight curves. The tumor growth curve and mice body weight were measured every 3 days. (a) Tumor size growth curve of CNE1-transplanted tumor; (b) the tumor size growth curve of CNE2-transplanted tumor; (c) the body weight of mice with CNE1-transplanted tumor; (d) the body weight of mice with CNE2-transplanted tumor.
DISCUSSION
According to a review, of all the patients with nasopharyngeal carcinoma in the world, about 80% of them are in China, especially in southern China12. Nasopharyngeal carcinoma has been a major malignant tumor in Guangxi and Guangdong provinces of China. The pathogenesis of nasopharyngeal carcinoma is closely related to Epstein–Barr virus13. Because of this epidemiological feature, the incidence is higher in children and adults. Unfortunately, both radiation and chemotherapy have serious toxic and side effects, which seriously affect the treatment efficacy and life quality.
At present, radiation treatment is still a primary method for nasopharyngeal carcinoma treatment. Although radiotherapy alone has an ideal therapeutic effect on early nasopharyngeal carcinoma patients, patients with advanced nasopharyngeal carcinoma should receive a combination of radiation therapy and chemotherapy14. However, besides eliminating tumor cells, chemotherapy could produce toxic effects on normal tissues. It also induces resistance to tumor cells and high rate of side effects. Therefore, finding an inexpensive, safe therapeutic drug with fewer side effects has become a considerable adjuvant approach.
The effect of baicalein to treat cancer has been confirmed in many studies. For example, baicalein could inhibit the cyclin D1 protein expression and delay cancer cell growth in bladder10. In pancreatic cancer, it caused apoptosis by decreasing the antiapoptotic protein Mcl-111. In another study, baicalein could target c-Myc gene through the Wnt signaling pathway to promote apoptosis15. In the present study, the results of MTT colorimetric assay showed that different dosages of baicalein significantly decreased the proliferation of CNE1 and CNE2 cells. The inhibition effect was both time and concentration dependent. This result indicates that baicalein had the ability to inhibit the proliferation of these two cell lines. The effect of baicalein on the cycle change of these two cell lines was analyzed by flow cytometry. It was shown that 25 μM, 50 μM, 100 μM of baicalein significantly increased the rate of cells in the G0/G1 phase, but decreased the rate of cells in the G2/M phase. These results suggested that baicalein had the ability to reduce the number of cells entering the mitosis and inhibit the proliferation of tumor cells.
Apoptosis, also known as “programmed cell death,” is a series of changes in cell morphology caused by various biochemical events, including changes in cell membrane symmetry, cell shrinkage, nuclear fragmentation, and chromatin condensation. In recent years, the role of Bcl-xl in apoptosis inhibition and tumorigenesis promotion, which belongs to Class I members in the Bcl-2 family, has attracted much attention. Mcl-1 is an important antiapoptotic protein. Both Mcl-1 and Bcl-2 have the function of promoting cell survival in an independent way16. Mcl-1 can combine with Bak, Bax, and other Bcl-2 family proteins to prevent apoptosis, as the release of these factors may activate caspase 9 and 3, resulting in apoptosis. The caspase family is a protease system that directly leads to the disintegration of apoptotic cells and can enzymatically inactivate apoptosis inhibitors. The stimulation of apoptotic signals activates caspases, induces oligomerization of caspase 9 precursors, and forms apoptotic bodies. The formation of apoptotic bodies activates caspase 9 and 3, which will initiate a cascade of caspase-induced apoptosis. In the present experiment, the Western blot analysis of apoptosis-related proteins (Mcl-1, Bcl-xl, Bax, Bad, caspase 3, 8, and 9) in the extracted cell proteins was performed at 48 h after CNE1 cells were treated with 25 μM, 50 μM baicalein or 40 μM DDP, or at 12 h after CNE1 cells were treated with IC50 baicalein (20.95 μM) or IC50 DDP (2.49 μM). Similar to DDP, baicalein significantly decreased the expression of Bcl-xl and Mcl-1 compared to control. Expression of other two Bcl family proteins, Bax and Bad, on the other hand, were significantly increased by baicalein. Expression of caspase 3, 8, and 9 was also significantly increased by baicalein. The expressions of both caspase 8 and 9 were increased, suggesting that two apoptotic pathways were involved in the proapoptotic effect of baicalein.
The nude mouse xenograft model has been applied in the study of tumor cell cycle progression and motility, tumor growth, and radiosensitization in nasopharyngeal carcinoma17–20. An important indicator for observing transplanted tumor models in nude mice is the tumor inhibition rate. In this experiment, with the increase in the concentration of baicalein, the increase in the tumor volume and weight was dose-dependently inhibited. The tumor inhibition rate of baicalein on CNE1 or CNE2-transplanted tumors was significantly increased with its dosage. These results indicate that baicalein was effective to limit both CNE1- and CNE2-transplanted tumors. The tumor inhibition rate in CNE2-transplanted tumors seems higher, which suggests that CNE2-transplanted tumor may be more sensitive to baicalein. Moreover, the tumor size grows more slowly in BAI groups, while the body weight drops more slowly in BAI groups, indicating that baicalein achieved satisfactory results in tumor growth control.
In conclusion, the results showed that baicalein could inhibit the growth and proliferation of human nasopharyngeal cancer cells, change their cell cycle, and induce their apoptosis. Based on the preliminary animal experiments, baicalein also exhibits antitumor activity. The mechanism may involve the downregulation of Bcl-xl and Mcl-1 proteins and upregulation of Bax and Bad proteins. Although it seems that the tumor inhibition ability of baicalein is not as good as DDP, considering the benefits of baicalein, including fewer side effects and lower cost, it could be an alternative medicine to treat nasopharyngeal carcinoma.
ACKNOWLEDGMENT
The authors declare no conflicts of interest.
Footnotes
The authors declare no conflicts of interest.
REFERENCES
- 1. Cao SM, Simons MJ, Qian CN. The prevalence and prevention of nasopharyngeal carcinoma in China. Chin J Cancer 2011;30(2):114–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wu LR, Liu YT, Jiang N, Fan YX, Wen J, Huang SF, Guo WJ, Bian XH, Wang FJ, Li F, Song D, Wu JF, Jiang XS, Liu JY, He X. Ten-year survival outcomes for patients with nasopharyngeal carcinoma receiving intensity-modulated radiotherapy: An analysis of 614 patients from a single center. Oral Oncol. 2017;69:26–32. [DOI] [PubMed] [Google Scholar]
- 3. Huang PY, Zeng Q, Cao KJ, Guo X, Guo L, Mo HY, Wu PH, Qian CN, Mai HQ, Hong MH. Ten-year outcomes of a randomised trial for locoregionally advanced nasopharyngeal carcinoma: A single-institution experience from an endemic area. Eur J Cancer 2015;51(13):1760–70. [DOI] [PubMed] [Google Scholar]
- 4. Qi F, Zhao L, Zhou A, Zhang B, Li A, Wang Z, Han J. The advantages of using traditional Chinese medicine as an adjunctive therapy in the whole course of cancer treatment instead of only terminal stage of cancer. Biosci Trends 2015;9(1):16–34. [DOI] [PubMed] [Google Scholar]
- 5. Li-Weber M. New therapeutic aspects of flavones: the anticancer properties of Scutellaria and its main active constituents Wogonin, Baicalein and Baicalin. Cancer Treat Rev. 2009;35(1):57–68. [DOI] [PubMed] [Google Scholar]
- 6. Zheng N, Dai J, Cao H, Sun S, Fang J, Li Q, Su S, Zhang Y, Qiu M, Huang S. Current understanding on antihepatocarcinoma effects of xiao chai hu tang and its constituents. Evid Based Complement Alternat Med. 2013;529458(10):18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chen K, Zhang S, Ji Y, Li J, An P, Ren H, Liang R, Yang J, Li Z. Baicalein inhibits the invasion and metastatic capabilities of hepatocellular carcinoma cells via down-regulation of the ERK pathway. PLoS One 2013;8(9):e72927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bie B, Sun J, Guo Y, Li J, Jiang W, Yang J, Huang C, Li Z. Baicalein: A review of its anti-cancer effects and mechanisms in hepatocellular carcinoma. Biomed Pharmacother. 2017;93:1285–91. [DOI] [PubMed] [Google Scholar]
- 9. Chao JI, Su WC, Liu HF. Baicalein induces cancer cell death and proliferation retardation by the inhibition of CDC2 kinase and survivin associated with opposite role of p38 mitogen-activated protein kinase and AKT. Mol Cancer Ther. 2007;6(11):3039–48. [DOI] [PubMed] [Google Scholar]
- 10. Wu JY, Tsai KW, Li YZ, Chang YS, Lai YC, Laio YH, Wu JD, Liu YW. Anti-bladder-tumor effect of baicalein from Scutellaria baicalensis Georgi and its application in vivo. Evid Based Complement Alternat Med. 2013;579751(10):17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Takahashi H, Chen MC, Pham H, Angst E, King JC, Park J, Brovman EY, Ishiguro H, Harris DM, Reber HA, Hines OJ, Gukovskaya AS, Go VL, Eibl G. Baicalein, a component of Scutellaria baicalensis, induces apoptosis by Mcl-1 down-regulation in human pancreatic cancer cells. Biochim Biophys Acta 2011;8(74):10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Yu WM, Hussain SS. Incidence of nasopharyngeal carcinoma in Chinese immigrants, compared with Chinese in China and South East Asia: Review. J Laryngol Otol. 2009;123(10):1067–74. [DOI] [PubMed] [Google Scholar]
- 13. Zhang G, Zong J, Lin S, Verhoeven RJ, Tong S, Chen Y, Ji M, Cheng W, Tsao SW, Lung M, Pan J, Chen H. Circulating Epstein–Barr virus microRNAs miR-BART7 and miR-BART13 as biomarkers for nasopharyngeal carcinoma diagnosis and treatment. Int J Cancer 2015;136(5):18. [DOI] [PubMed] [Google Scholar]
- 14. Lee AW, Ma BB, Ng WT, Chan AT. Management of nasopharyngeal carcinoma: Current Practice and future perspective. J Clin Oncol. 2015;33(29):3356–64. [DOI] [PubMed] [Google Scholar]
- 15. He N, Zhang Z. Baicalein suppresses the viability of MG-63 osteosarcoma cells through inhibiting c-MYC expression via Wnt signaling pathway. Mol Cell Biochem. 2015;405(1–2):187–96. [DOI] [PubMed] [Google Scholar]
- 16. Carrington EM, Zhan Y, Brady JL, Zhang JG, Sutherland RM, Anstee NS, Schenk RL, Vikstrom IB, Delconte RB, Segal D, Huntington ND, Bouillet P, Tarlinton DM, Huang DC, Strasser A, Cory S, Herold MJ, Lew AM. Anti-apoptotic proteins BCL-2, MCL-1 and A1 summate collectively to maintain survival of immune cell populations both in vitro and in vivo. Cell Death Differ. 2017;24(5):878–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. He J, Cai L, Chen Y, He Y, Wang M, Tang J, Guan H, Wang J, Peng X. Antitumor and radiosensitizing effects of SKLB-163, a novel benzothiazole-2-thiol derivative, on nasopharyngeal carcinoma by affecting the RhoGDI/JNK-1 signaling pathway. Radiother Oncol. 2018;5(18):007. [DOI] [PubMed] [Google Scholar]
- 18. Zhou L, Chen HM, Qu S, Li L, Zhao W, Liang ZG, Yu BB, Chen KH, Lu QT, Lin GX, Zhu XD. Reduced QSOX1 enhances radioresistance in nasopharyngeal carcinoma. Oncotarget 2017;9(3):3230–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zhong J, Shi P, Chen Y, Huang R, Xiao Y, Zheng X, Zheng D, Peng L. Diffusion kurtosis imaging of a human nasopharyngeal carcinoma xenograft model: Initial experience with pathological correlation. Magn Reson Imaging 2018;47:111–7. [DOI] [PubMed] [Google Scholar]
- 20. Feng X, Zhang C, Zhu L, Zhang L, Li H, He L, Mi Y, Wang Y, Zhu J, Bu Y. DEPDC1 is required for cell cycle progression and motility in nasopharyngeal carcinoma. Oncotarget 2017;8(38):63605–19. [DOI] [PMC free article] [PubMed] [Google Scholar]