Abstract
MicroRNA-132 (miR-132) has been demonstrated to be a tumor suppressor in several types of tumors. However, the expression and the role of miR-132 in human thyroid cancer are still poorly understood. The aim of the present study was to examine the potential roles and molecular mechanism of miR-132 in thyroid cancer. We found that miR-132 expression levels were significantly downregulated in thyroid cancer tissues and cell lines. Function assays showed that overexpression of miR-132 in TPC1 cells inhibited cell proliferation, migration, and invasion. Forkhead box protein A1 (FOXA1) was identified as a direct target of miR-132 in thyroid cancer cells. Knockdown of FOXA1 in TPC1 cells significantly inhibited cell proliferation, migration, and invasion, which mimicked the suppressive effect induced by miR-132 overexpression. Restoration of FOXA1 expression partially reversed the suppressive effect induced by miR-132 overexpression. Taken together, these results suggested that miR-132 acts as a tumor suppressor in thyroid cancer through targeting FOXA1.
Key words: MicroRNAs, miR-132, Thyroid cancer, FOXA1
INTRODUCTION
Thyroid cancer is the most common endocrine malignancy and one of the most rapidly growing cancers over the last several decades1. Despite the advances in diagnostic and therapeutic approaches that have greatly improved the prognosis of thyroid cancer, up to 30% of patients present with locoregional recurrence or distant metastases within 10 years2. Therefore, further investigations into the molecular mechanisms that are involved in thyroid cancer progression and metastasis are essential for effective treatment of this disease.
MicroRNAs (miRNAs) are a class of endogenous, single-stranded, short (18–25 nucleotides in length), highly conserved noncoding RNAs that control the expression of a large number of genes by binding to the 3′-untranslated region (3′-UTR) of target mRNAs, leading to mRNA cleavage/degradation or translational repression3,4. Increasing evidence has suggested that miRNAs function as tumor suppressors or oncogenes in various types of cancer by regulating proliferation, metastasis, and angiogenesis5,6. Recent advances in the understanding of the molecular mechanisms of thyroid cancer have revealed that multiple miRNAs are involved in the occurrence and development of thyroid cancer7–9.
MicroRNA-132 (miR-132), located in the intron of a noncoding gene on chromosome 17 in humans10, has been reported to function as a tumor suppressor in several malignant tumors, such as ovarian cancer11, glioma12, lung cancer13, hepatocellular carcinoma14, and breast cancer15. However, the role and molecular mechanism of miR-132 in thyroid cancer remain unclear. Therefore, in this study, we investigate the potential role and molecular mechanisms of miR-132 in thyroid cancer.
MATERIALS AND METHODS
Patients and Tissue Samples
Thirty paired human thyroid cancer specimens and adjacent noncancerous tissues (ANTs) were obtained from patients who underwent surgical resection in the First Hospital of Jilin University (Changchun, P.R. China) between September 2013 and December 2015. All tissue samples were snap frozen in liquid nitrogen immediately following surgery until use. All samples were histologically classified by two clinical pathologists. This study was approved by the ethics committees of the First Hospital of Jilin University (Changchun, P.R. China), and informed consent was obtained from all patients.
Reagents, Cell Culture, and Transfection
Chemically synthesized miR-132 mimic (miR-132) or negative control (miR-Ctrl) was purchased from Ambion (Austin, TX, USA). The RNAi plasmids targeting human FOXA1 mRNA (si-FOXA1) or scramble control (si-Ctrl) were bought from GenePharma (Shanghai, P.R. China). The FOXA1 overexpression plasmid was provided by Xue Wang (Jilin University, Changchun; P.R. China). Human thyroid cancer cell lines (TPC1 and GLAG-66) and a human normal thyroid follicular epithelial cell line (Nthy-ori 3-1) were purchased from the Type Culture Collection of the Chinese Academy of Sciences (Shanghai, P.R. China) and were cultured in DMEM (Gibco-BRL, Gaithersburg, MD, USA) supplemented with 10% FBS (Gibco-BRL), l-glutamine, and penicillin/streptomycin in a 5% CO2 atmosphere. Transfection was performed using Lipofectamine 3000 reagent (Invitrogen, Life Technologies, Carlsbad, CA, USA) according to the manufacturer’s instructions.
Real-Time Quantitative Reverse Transcription PCR
Total RNA, including miRNAs, was isolated from tissues and cultured cells using TRIzol® reagent (Invitrogen) according to the manufacturer’s instructions and reversely transcribed to cDNA using reverse transcriptase Moloney murine leukemia virus (TaKaRa, Tokyo, Japan). Quantitative real-time polymerase chain reaction (PCR) was performed using SYBR Premix Ex Taq (TaKaRa) following the manufacturer’s protocol under ABI 7900 Sequence Detection System (Life Technologies, Mount Vernon, NY, USA). The primers of miR-132 and U6 were brought from Applied Biosystems (Foster City, CA, USA). The primers of FOXA1 and GAPDH were used in this study as described previously16. miR-132 expression was normalized to U6 internal control, and FOXA1 mRNA expression was normalized to GAPDH internal control using the 2−ΔΔCt method.
Cell Proliferation
Cell proliferation was analyzed using the cell counting kit 8 (CCK-8; Dojindo, Kumamoto, Japan) according to the manufacturer’s instructions. In brief, transfected cells were seeded in 96-well plates at a density of 2 × 103 cells/well in 100 μl of DMEM. At indicated times (24, 48, and 72 h), 10 μl of CCK-8 (Dojindo) was added to each well and cultured for an additional 2 h. Then the OD value was measured in a spectrophotometer (SpectraMax Plus; Molecular Devices, Sunnyvale, CA, USA) at 450 nm.
Migration and Invasion Assays
The Transwell migration and invasion assay was performed using Transwell inserts (Corning, New York, NY, USA). Briefly, 2 × 104 transfected cells in 100 μl of serum-free medium were added to the upper chamber of an insert (8-μm pore size; Millipore) coated with (for invasion assay) or without (for migration assay) Matrigel (BD Biosciences, Franklin Lakes, NJ, USA), and 600 μl of medium containing 10% FBS was added to the lower chamber. After incubation at 37°C for 24 h (for migration assay) or 48 h (for invasion assay), the migrated or invaded cells were fixed in 70% ethanol for 30 min and stained with 1% crystal violet for 10 min. The number of migrated or invaded cells was counted and photographed using an IX71 inverted microscope (Olympus, Tokyo, Japan) at 200× magnification in at least five fields.
Luciferase Reporter Assay
A full-length human FOXA1 3′-UTR with a wild-type and mutant target sequence for miR-132 was amplified by PCR and inserted downstream of the Renilla psi-CHECK2 (Promega, Madison, WI, USA) at the NotI and XhoI sites. For the luciferase reporter assay, the luciferase reporter gene vector with wild-type FOXA1 (Wt-FOXA1) or mutant FOXA1 (Mut-FOXA1) was cotransfected with miR-132 mimic or miR-Ctrl into TPC1 cells using Lipofectamine 3000 (Invitrogen) according to the manufacturer’s protocol. Cells were harvested at 48 h after transfection, and luciferase activity was measured using the Dual-Luciferase Reporter Assay System (Promega Corp.).
Western Blot
Total protein was isolated from cultured cells with RIPA lysis buffer [1% NP-40, 0.1% sodium dodecyl sulfate (SDS), 100 μg/ml phenylmethylsulfonyl fluoride, 0.5% sodiumdeoxycholate, in PBS] containing proteinase inhibitor (Sigma-Aldrich, USA) on ice for 30 min. Protein concentrations were measured using a BCA Assay Kit (Pierce, Rockford, IL, USA). Thirty micrograms of protein mixed with 2× SDS loading buffer [125 mmol/L Tris-HCl, 4% SDS, 20% glycerol, 100 mmol/L dithiothreitol (DTT), and 0.2% bromophenol blue] was loaded per lane and was separated by 10% SDS-polyacrylamide gel electrophoresis (PAGE) at 120 V for 2 h, and then transferred onto the polyvinylidene difluoride (PVDF) membranes (Millipore, Bedford, MA, USA). The membranes were blocked with 4% nonfat dry milk in TBST (20 mM Tris-HCl, pH 7.5, 150 mM NaCl, 0.1% Tween 20) for 2 h at room temperature, followed by incubation with antibody against FOXA1 (1:500; Santa Cruz, Santa Cruz, CA, USA) or GAPDH (1:5,000; Santa Cruz) at 4°C overnight. Finally, the membrane was washed with TBST and incubated with horseradish peroxidase (HRP)-conjugated goat anti-mouse IgG (1:5,000; Santa Cruz) at room temperature for 2 h. Protein bands were detected using the enhanced chemiluminescence (ECL) reagents (Pierce; Thermo Fisher Scientific Inc., Waltham, MA, USA) and exposed on an X-ray film (Pierce). GAPDH was used as a loading control.
Statistical Analysis
All statistical analyses were performed using the SPSS 19.0 statistical software package (Chicago, IL, USA), and the data are expressed as the means ± standard deviation (SD). Student’s t-test or one-way ANOVA was used for comparison of differences between groups. A value of p < 0.05 was considered significant in this study.
RESULTS
miR-132 Expression Was Downregulated in Human Osteosarcoma Tissues and Cell Lines
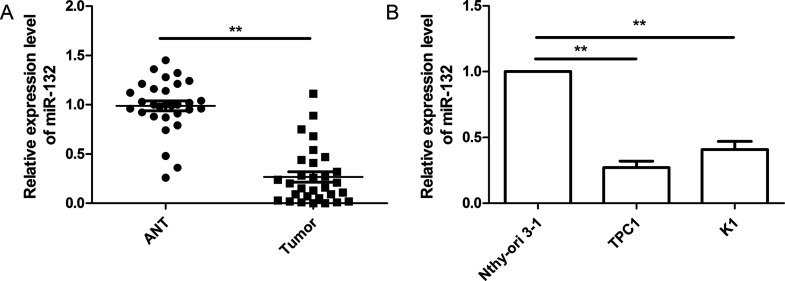
The expression level of miR-132 was quantified in thyroid cancer tissues and ANTs by real-time quantitative reverse transcription PCR (RT-qPCR). Compared with the ANTs, the expression level of miR-132 was significantly downregulated in human thyroid cancer tissues (p < 0.01) (Fig. 1A). Furthermore, miR-132 expression was determined by RT-qPCR in two thyroid cancer cell lines (TPC1 and GLAG-66) and a human thyroid follicular epithelial cell line (Nthy-ori 3-1). As shown in Figure 1B, miR-132 expression was significantly decreased in thyroid cancer cell lines compared to the normal thyroid cell line (Nthy-ori 3-1; p < 0.05). TPC1 cells showed the lower expression of miR-132 compared to GLAG-66 cells (Fig. 1B); thus, TPC1 cells were selected for further experiments. The data above suggest that miR-132 decreases in both thyroid cancer tissues and cell lines.
Figure 1.
MicroRNA-132 (miR-132) expression was downregulated in thyroid cancer tissues and cell lines. (A) Relative miR-132 expression level was determined by real-time quantitative reverse transcription polymerase chain reaction (RT-qPCR) in 30 thyroid cancer tissues and adjacent noncancerous tissues (ANTs). (B) Relative miR-132 expression level was determined by RT-qPCR in two thyroid cancer cell lines (TPC1 and GLAG-66), compared to those in the human normal thyroid follicular epithelial cell line (Nthy-ori3-1). U6 was used as the internal control. **p < 0.01.
miR-132 Inhibits Cell Proliferation, Migration, and Invasion of Thyroid Cancer Cells
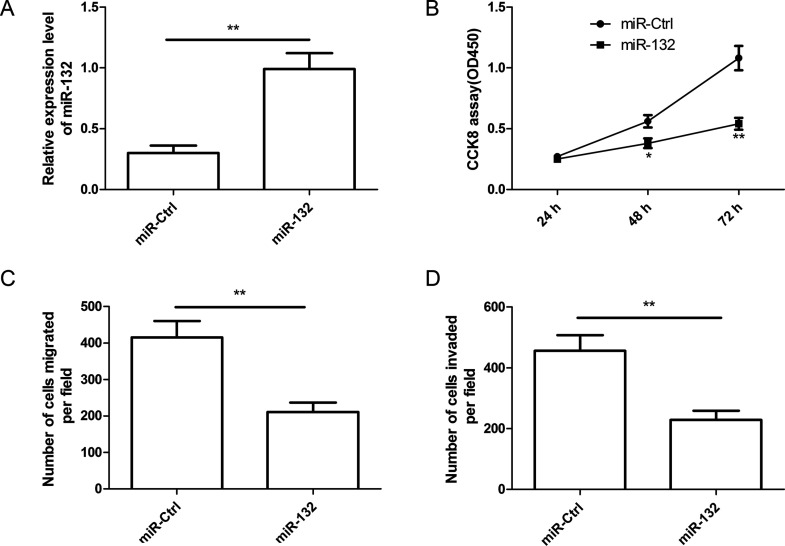
To explore the potential role of miR-132 in thyroid cancer cell growth and metastasis, we transfected miR-132 mimic into TPC1 cells, and then performed cell proliferation, migration, and invasion assays at the indicated times. RT-qPCR confirmed that TPC1 cells transfected with miR-132 mimic successfully increased miR-132 expression level compared to cells transfected with miR-Ctrl (Fig. 2A). The CCK-8 assay showed that miR-132 overexpression in TPC1 cells significantly inhibited cell proliferation (Fig. 2B). In addition, we also found that miR-132 overexpression in TPC1 cells significantly inhibited cell migration (Fig. 2C) and invasion (Fig. 2D). Collectively, these results suggested that miR-132 can efficiently inhibit cell proliferation, migration, and invasion of TPC1 cells.
Figure 2.
miR-132 inhibited cell proliferation, migration, and invasion in thyroid cancer cells. (A) Relative expression level of miR-132 was detected in TPC1 cells transfected with miR-132 mimic or miR-Ctrl by RT-qPCR. U6 was used as the internal control. (B–D) Cell proliferation, migration, and invasion were determined in TPC1 cells transfected with miR-132 mimic or miR-Ctrl. *p < 0.05, **p < 0.01.
FOXA1 Was a Direct Target of miR-132
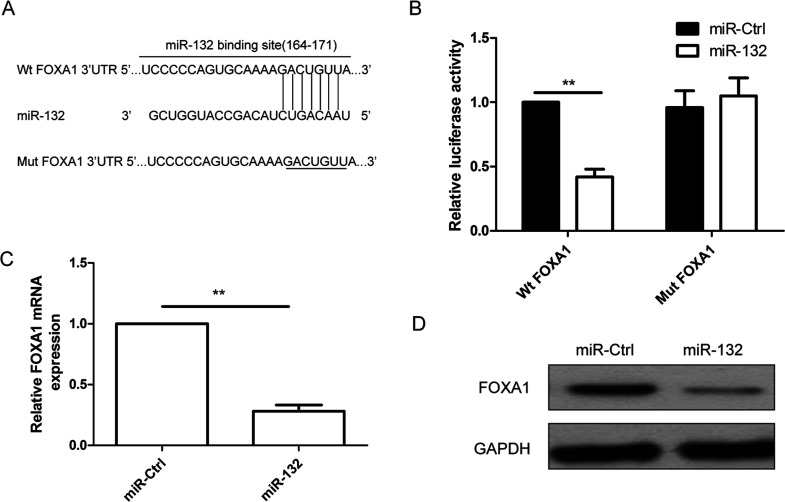
FOXA1 is predicted to be a potential target of miR-132 since TargetScan software demonstrated a putative binding site (164–171) to miR-132 in the 3′-UTR region of FOXA1 (Fig. 3A). To identify whether FOXA1 is a direct target of miR-132, Wt and Mut FOXA1 3′-UTR were cloned into reporter plasmids, respectively, and were transfected into the TPC1 cells along with the miR-132 mimic or miR-Ctrl. As validated by the luciferase reporter assay, the luciferase activity of Wt FOXA1 3′-UTR was significantly suppressed in the cells transfected with the miR-132 mimic relative to the cells transfected with the miR-Ctrl (p < 0.01) (Fig. 3B); however, miR-132 mimic did not affect the luciferase activity of mutant FOXA1-3′-UTR. RT-qPCR and Western blot were then used to determine expression levels of FOXA1 in TPC1 cells transfected with the miR-132 mimic or miR-Ctrl. The results showed that FOXA1 expression on the mRNA level (Fig. 3C) and protein level (Fig. 3D) was significantly decreased in cells transfected with miR-132 mimic compared to cells transfected with miR-Ctrl. These results suggest that FOXA1 is a direct target of miR-132 in thyroid cancer cells.
Figure 3.
FOXA1 was a direct target of miR-132 in thyroid cancer cells. (A) The predicted binding sites for miR-132 in the 3′-untranslated region (3′-UTR) of FOXA1 (positions 164–171) and the mutations in the binding sites are shown. (B) Luciferase activities were determined in TPC1 cells 48 h after cotransfection with reporter vectors carrying either wild-type FOXA1 3′-UTR (Wt-FOXA1) or mutated (Mut-FOXA1), and miR-132 mimic or miR-Ctrl. FOXA1 expression at the mRNA level (C) and protein level (D) was detected in TPC1 cells transfected with miR-132 mimic or miR-Ctrl. GAPDH was used as the internal control. **p < 0.01.
FOXA1 Deletion Inhibited Cell Proliferation, Migration, and Invasion in Thyroid Cancer Cells
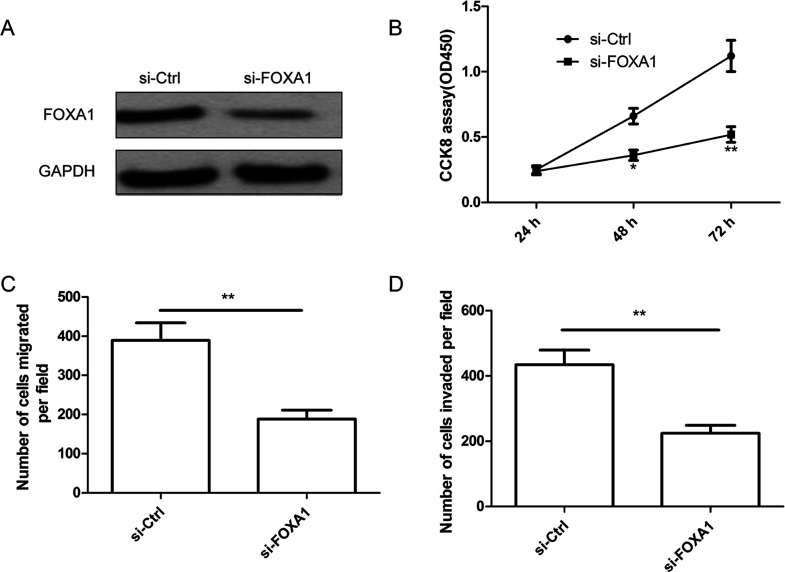
To determine the role of FOXA1 in thyroid cancer cells, TPC1 cells were transfected with si-FOXA1 or si-Ctrl. As shown in Figure 4A, the FOXA1 protein expression was downregulated in cells transfected with si-FOXA1 compared with cells transfected with si-Ctrl. In addition, we also found that knockdown of FOXA1 significantly inhibited cell proliferation, migration, and invasion of TPC1 cells (Fig. 4B–D), which mimicked the effect of miR-132 overexpression on thyroid cancer cells.
Figure 4.
FOXA1 deletion inhibited cell proliferation, migration, and invasion in thyroid cancer cells. (A) FOXA1 protein expression was determined in TPC1 cells transfected with si-FOXA1 or si-Ctrl by Western blot. GAPDH was used as the internal control. Cell proliferation (B), migration (C), and invasion (D) were determined in TPC1 cells transfected with si-FOXA1 or si-Ctrl. *p < 0.05, **p < 0.01.
miR-132 Inhibited Cell Proliferation, Migration, and Invasion via Targeting FOXA1 in Thyroid Cancer Cells
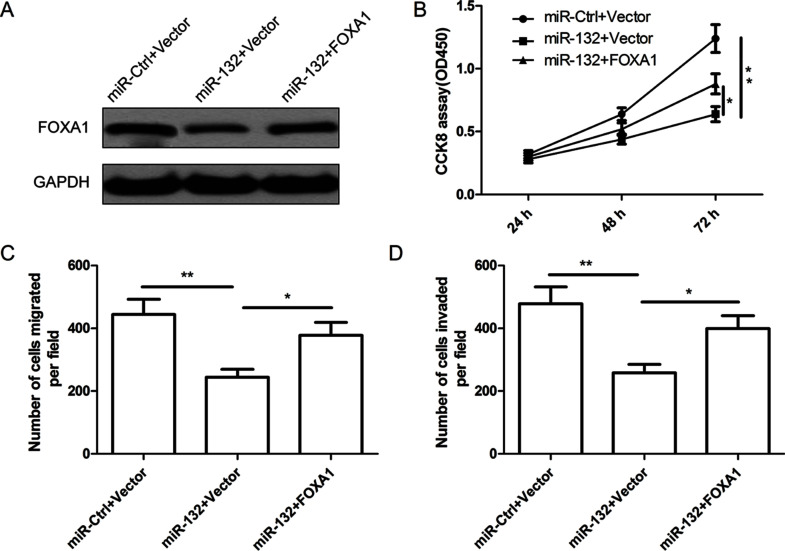
To further illustrate whether miR-132 affects human thyroid cancer cell proliferation, migration, and invasion through FOXA1, FOXA1 overexpression plasmid or blank vector, together with miR-132 mimic or miR-Ctrl, was cotransfected into the TPC1 cells, and their effects on proliferation, migration, and invasion in the above cells were determined. As shown in Figure 5A, FOXA1 expression was upregulated in TPC1-miR-132 cells after transfection with FOXA1 overexpression plasmid. In addition, we found that FOXA1 overexpression was able to counteract the inhibitory effect induced by miR-132 overexpression on cell proliferation (Fig. 5B), migration (Fig. 5C), and cell invasion (Fig. 5D) in thyroid cancer cells.
Figure 5.
miR-132 inhibits cell proliferation, migration, and invasion in thyroid cancer cells via targeting FOXA1 expression. (A) FOXA1 protein expression was detected in TPC1 cells transfected with miR-132 mimic or miR-Ctrl, together with overexpression FOXA1 plasmids, or vector. GAPDH was used as the internal control. Cell proliferation (B), migration (C), and invasion (D) were determined in TPC1 cells transfected with miR-132 mimic or miR-Ctrl, together with overexpression FOXA1 plasmids or vector. *p < 0.05, **p < 0.01.
DISCUSSION
Accumulating evidence has suggested that miRNAs play crucial roles in the initiation and progression of thyroid cancers and that exploration of cancer-specific miRNAs and their downstream targets may contribute to the identification of novel diagnosis biomarkers and therapeutic targets for human thyroid cancers7–9. In this study, we initially evaluated the expression of miR-132 in 30 paired samples of thyroid cancer tissues and ANTs. Our data showed that the expression of miR-132 was impaired in HCC tissues. Furthermore, miR-132 was reduced in two thyroid cancer cell lines compared with a normal thyroid cell line. Function assays revealed that miR-132 suppressed cell proliferation, migration, and invasion of thyroid cancer cells. These results indicate that miR-132 may play a critical role in the initiation and progression of thyroid cancers.
Pathologically, miR-132 may play a role in inflammation development, cell transformation, and vascular smooth muscle dysfunction17–19. It has been suggested that miR-132 functioned as a tumor suppressor in multiple cancers11–15. However, another study showed that miR-132 upregulation promotes gastric cancer cell growth through suppression of FoxO1 translation, suggesting it is an oncogene in gastric cancer20. These conflicting results suggested that miR-132 functioned as either a tumor suppressor or oncogene in human cancers, which might be dependent on the type of cancer cells. In this study, with a series of in vitro experiments, we confirmed that miR-132 expression was downregulated in thyroid cancer tissues and cell lines and that miR-132 overexpression inhibited cell proliferation, migration, and invasion of thyroid cancer cells. These results suggested that miR-132 served as a tumor suppressor in thyroid cancer.
To further investigate the underlying mechanisms by which miR-132 exerts its biological effects on thyroid cancer cells, it is necessary to identify its downstream functional targets. In this study, FOXA1 was predicted as a direct target of miR-132 by the public database TargetScan. Using dual-luciferase reporter assay, RT-qPCR, and Western blot analysis, we further confirmed that FOXA1 was a target of miR-132 in thyroid cancer cells. FOXA1, a member of the human Forkhead-box family, has been implicated in congenital disorders, diabetes, and carcinogenesis21. Recently, a report demonstrated that the expression of FOXA1 was upregulated in human anaplastic thyroid carcinoma (ATC) and that silencing of FOXA1 in an ATC cell line causes G1 growth arrest and reduction of cell proliferation22, suggesting its oncogenic role in thyroid cancer. In addition, FOXA1 has been identified as a target gene of several miRNAs, including miR-19416, miR-21223, miR-1724, and miR-129025. Here we showed that FOXA1 was a target of miR-132 in thyroid cancer cells. FOXA1 knockdown showed the same regulatory effect as upregulation of miR-132 in TPC1 cells with decreased cell proliferation, migration, and invasion. In addition, overexpression of FOXA1 in TPC1 reversed the inhibitory effect of miR-132 overexpression on proliferation, migration, and invasion. These results suggested that miR-132 exerts it tumor-suppressing functions in thyroid cancer by targeting FOXA1.
In summary, the results presented here first demonstrate that the expression level of miR-132 was decreased in thyroid cancer tissues and cell lines and that miR-132 inhibited cell proliferation, migration, and invasion of thyroid cancer by repressing FOXA1. These results suggested that miR-132 functioned as tumor suppressor by targeting FOXA1 and that miR-132 may serve as a potential therapeutic candidate for thyroid cancer.
ACKNOWLEDGMENT
The authors declare no conflicts of interest.
Footnotes
The authors declare no conflicts of interest.
REFERENCES
- 1. Brown RL, de Souza JA, Cohen EE. Thyroid cancer: Burden of illness and management of disease. J Cancer 2011;2:193–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lang BH, Wong KP, Wan KY, Lo CY. Significance of metastatic lymph node ratio on stimulated thyroglobulin levels in papillary thyroid carcinoma after prophylactic unilateral central neck dissection. Ann Surg Oncol. 2012;19(4):1257–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Fabian MR, Sonenberg N, Filipowicz W. Regulation of mRNA translation and stability by microRNAs. Annu Rev Biochem. 2010;79:351–79. [DOI] [PubMed] [Google Scholar]
- 4. Bartel DP. MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell 2004;116(2):281–97. [DOI] [PubMed] [Google Scholar]
- 5. McManus MT. MicroRNAs and cancer. Semin Cancer Biol. 2003;13(4):253–8. [DOI] [PubMed] [Google Scholar]
- 6. Farazi TA, Spitzer JI, Morozov P, Tuschl T. miRNAs in human cancer. J Pathol. 2011;223(2):102–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mazeh H, Mizrahi I, Halle D, Ilyayev N, Stojadinovic A, Trink B, Mitrani-Rosenbaum S, Roistacher M, Ariel I, Eid A, Freund HR, Nissan A. Development of a microRNA-based molecular assay for the detection of papillary thyroid carcinoma in aspiration biopsy samples. Thyroid 2011;21(2):111–8. [DOI] [PubMed] [Google Scholar]
- 8. Pallante P, Visone R, Croce CM, Fusco A. Deregulation of microRNA expression in follicular-cell-derived human thyroid carcinomas. Endocr Relat Cancer 2010;17(1):F91–104. [DOI] [PubMed] [Google Scholar]
- 9. Lodewijk L, Prins AM, Kist JW, Valk GD, Kranenburg O, Rinkes IH, Vriens MR. The value of miRNA in diagnosing thyroid cancer: A systematic review. Cancer Biomark. 2012;11(6):229–38. [DOI] [PubMed] [Google Scholar]
- 10. Nudelman AS, DiRocco DP, Lambert TJ, Garelick MG, Le J, Nathanson NM, Storm DR. Neuronal activity rapidly induces transcription of the CREB-regulated microRNA-132, in vivo. Hippocampus 2010;20(4):492–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tian H, Hou L, Xiong YM, Huang JX, Zhang WH, Pan YY, Song XR. miR-132 targeting E2F5 suppresses cell proliferation, invasion, migration in ovarian cancer cells. Am J Transl Res. 2016;8(3):1492–501. [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 12. Wang H, Li XT, Wu C, Wu ZW, Li YY, Yang TQ, Chen GL, Xie XS, Huang YL, Du ZW, Zhou YX. miR-132 can inhibit glioma cells invasion and migration by target MMP16 in vitro. Onco Targets Ther. 2015;8:3211–8. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 13. Li Y, Zu L, Wang Y, Wang M, Chen P, Zhou Q. miR-132 inhibits lung cancer cell migration and invasion by targeting SOX4. J Thorac Dis. 2015;7(9):1563–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Liu K, Li X, Cao Y, Ge Y, Wang J, Shi B. MiR-132 inhibits cell proliferation, invasion and migration of hepatocellular carcinoma by targeting PIK3R3. Int J Oncol. 2015;47(4):1585–93. [DOI] [PubMed] [Google Scholar]
- 15. Zhang ZG, Chen WX, Wu YH, Liang HF, Zhang BX. MiR-132 prohibits proliferation, invasion, migration, and metastasis in breast cancer by targeting HN1. Biochem Biophys Res Commun. 2014;454(1):109–14. [DOI] [PubMed] [Google Scholar]
- 16. Zhu X, Li D, Yu F, Jia C, Xie J, Ma Y, Fan S, Cai H, Luo Q, Lv Z, Fan L. miR-194 inhibits the proliferation, invasion, migration, and enhances the chemosensitivity of non-small cell lung cancer cells by targeting forkhead box A1 protein. Oncotarget 2016;7(11):13139–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Magill ST, Cambronne XA, Luikart BW, Lioy DT, Leighton BH, Westbrook GL, Mandel G, Goodman RH. microRNA-132 regulates dendritic growth and arborization of newborn neurons in the adult hippocampus. Proc Natl Acad Sci USA 2010;107(47):20382–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Shaked I, Meerson A, Wolf Y, Avni R, Greenberg D, Gilboa-Geffen A, Soreq H. MicroRNA-132 potentiates cholinergic anti-inflammatory signaling by targeting acetylcholinesterase. Immunity 2009;31(6):965–73. [DOI] [PubMed] [Google Scholar]
- 19. Mulik S, Xu J, Reddy PB, Rajasagi NK, Gimenez F, Sharma S, Lu PY, Rouse BT. Role of miR-132 in angiogenesis after ocular infection with herpes simplex virus. Am J Pathol. 2012;181(2):525–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Li W, Zhang J, Chen T, Yin P, Yang J, Cao Y. miR-132 upregulation promotes gastric cancer cell growth through suppression of FoxO1 translation. Tumour Biol. 2016;37(12):15551–7. [DOI] [PubMed] [Google Scholar]
- 21. Katoh M, Katoh M. Human FOX gene family (Review). Int J Oncol. 2004;25(5):1495–500. [PubMed] [Google Scholar]
- 22. Nucera C, Eeckhoute J, Finn S, Carroll JS, Ligon AH, Priolo C, Fadda G, Toner M, Sheils O, Attard M, Pontecorvi A, Nose V, Loda M, Brown M. FOXA1 is a potential oncogene in anaplastic thyroid carcinoma. Clin Cancer Res. 2009;15(11):3680–9. [DOI] [PubMed] [Google Scholar]
- 23. Tu H, Wei G, Cai Q, Chen X, Sun Z, Cheng C, Zhang L, Feng Y, Zhou H, Zhou B, Zeng T. MicroRNA-212 inhibits hepatocellular carcinoma cell proliferation and induces apoptosis by targeting FOXA1. Onco Targets Ther. 2015;8:2227–35. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 24. Xu Z, Zhang C, Cheng L, Hu M, Tao H, Song L. The microRNA miR-17 regulates lung FoxA1 expression during lipopolysaccharide-induced acute lung injury. Biochem Biophys Res Commun. 2014;445(1):48–53. [DOI] [PubMed] [Google Scholar]
- 25. Endo Y, Toyama T, Takahashi S, Yoshimoto N, Iwasa M, Asano T, Fujii Y, Yamashita H. miR-1290 and its potential targets are associated with characteristics of estrogen receptor alpha-positive breast cancer. Endocr Relat Cancer 2013;20(1):91–102. [DOI] [PubMed] [Google Scholar]